Abstract
The potential gain in efficacy of pulmonary administration over IV administration of some antibiotics such as ciprofloxacin (CIP) may be limited by the short residence time of the drug at the site of infection after nebulization. Complexation of CIP with copper reduced its apparent permeability in vitro through a Calu-3 cell monolayer and greatly increased its pulmonary residence time after aerosolisation in healthy rats. Chronic P. aeruginosa lung infections in cystic fibrosis patients result in airway and alveolar inflammation that may increase the permeability of inhaled antibiotics and alter their fate in the lung after inhalation compared to what was seen in healthy conditions. The objective of this study was to compare the pharmacokinetics and efficacy of CIP-Cu2+ complex-loaded microparticles administered by pulmonary route with a CIP solution administered by IV to model rats with chronic lung infection. After a single pulmonary administration of microparticles loaded with CIP-Cu2+ complex, pulmonary exposure to CIP was increased 2077-fold compared to IV administration of CIP solution. This single lung administration significantly reduced the lung burden of P. aeruginosa expressed as CFU/lung measured 24 h after administration by 10-fold while IV administration of the same dose of CIP was ineffective compared to the untreated control. This better efficacy of inhaled microparticles loaded with CIP-Cu2+ complex compared with CIP solution can be attributed to the higher pulmonary exposure to CIP obtained with inhaled CIP-Cu2+ complex-loaded microparticles than that obtained with IV solution.
Keywords: Pulmonary administration, Lung infections, Pseudomonas aeruginosa, Inhaled antibiotics, Pharmacokinetics, Pharmacodynamics
Graphical abstract
Highlights
-
•
CIP-copper complexes were administered pulmonary to model rats chronically infected with P. aeruginosa.
-
•
This administration increased pulmonary exposure to CIP by 3698 compared with IV administration of CIP.
-
•
This increase in pulmonary exposure to ciprofloxacin was 3.5 times greater than that observed in healthy rats.
-
•
This increased pulmonary exposure to CIP resulted in greater efficacy in reducing Pseudomonas lung burden than IV CIP.
1. Introduction
Effective therapy using pulmonary delivery of some antibiotics such as ciprofloxacin (CIP) can be limited by their short residence time at the site of infection. However, a novel complexed form of CIP may help overcome this obstacle while maintaining in vitro antimicrobial activity against biofilms (Lamy et al., 2018; Tewes et al., 2019). Pulmonary delivery of antibiotics from a solution has demonstrated clinical benefit in the treatment of chronic bacterial lung infections, as it can allow for significantly higher drug concentrations at the site of infection with reduced systemic levels compared to IV administration (Zhou et al., 2015). This is mainly true for antibiotics having low lung-blood barrier permeability, such as the currently used tobramycin, colistin methanesulfonate and aztreonam lysine. However, nebulization of a pure antibiotic solution is less attractive for antibiotics that diffuse readily across the pulmonary epithelial barrier and equilibrate rapidly with blood concentrations, as in the case of CIP (Endermann et al., 2011; Gontijo et al., 2014; Stass et al., 2013a, Stass et al., 2013b) or levofloxacin (Gaspar et al., 2016). Thus, for antimicrobial molecules with relatively high permeability across the lung-blood barrier, advanced formulations can be developed to retain these molecules in the airway after inhalation by slowing their flux across this barrier. These advanced formulations typically work by controlling the rate of drug release from the formulation (Cipolla et al., 2016; Gaspar et al., 2016; Günday Türeli et al., 2017; McShane et al., 2018; Nurbaeti et al., 2019; Serisier et al., 2013), or by reducing the apparent permeability of the drug across the epithelial barrier (Griffith et al., 2014; Lamy et al., 2019, Lamy et al., 2018; Tewes et al., 2016). To increase the pulmonary residence time of CIP after aerosolization, we recently developed a formulation containing a cupric complex of CIP that is positively charged at physiological pH (Brillault et al., 2017; Lamy et al., 2018; Tewes et al., 2016). This CIP‑copper complex (CIP-Cu2+) significantly decreased the apparent permeability of CIP across a pulmonary epithelium model consisting of Calu-3 cells (Brillault et al., 2017). This decrease in permeability observed in vitro was reflected in vivo in healthy rats by a reduction in the systemic absorption rate of CIP and sustained high concentrations of CIP in the lung epithelial lining fluid (ELF) after pulmonary administration of CIP-Cu-loaded microparticles (Lamy et al., 2019, Lamy et al., 2018). Additionally, the in vitro antibacterial activities of CIP and CIP-Cu2+ evaluated against Pseudomonas aeruginosa (P. aeruginosa) grown in planktonic cultures (Brillault et al., 2017; Tewes et al., 2016) or as biofilms (Tewes et al., 2019) were found to be equivalent. Thus, due to the sustained high concentrations of CIP observed in the rat lungs and the maintenance of antibacterial activity observed in vitro, better in vivo efficacy of inhaled CIP-Cu2+ complex can be expected compared to CIP alone. However, it is not always clear whether the high concentrations of antibiotics obtained in the lung after inhalation result in more effective killing of P. aeruginosa (Tiddens et al., 2014). These high antibiotic concentrations could be due to the deposition of the aerosol primarily in the airways, meaning that less antibiotic would be available to the rest of the lung. This may be especially true for less ventilated diseased areas, which may receive a lower dose of drug than healthier lung areas. In this case, IV antibiotic therapy should result in more effective antibiotic concentrations in the diseased areas and should treat the infection better than inhalation therapy.
Thus, the aim of this study was to compare, from a pharmacokinetic (PK) and efficacy (PD) point of view, a formulation of microparticles loaded with a CIP-Cu2+ complex administered intratracheally (IT) with a solution of CIP administered IV to rat models of chronic lung infection.
2. Materials and methods
2.1. Materials
P. aeruginosa PAO1 strain (from ATCC 15692, Manassas, USA), was used to prepare bacteria-loaded agar beads. Ciprofloxacin base (CIP) powder (purity ≥98,0%), copper hydroxide Cu(OH)2, hyaluronic acid (HA) sodium salt from Streptococcus equi, calcium hydroxide Ca(OH)2, formic acid and ammonium carbonate (NH4)2CO3 were purchased from Sigma Aldrich (Saint-Quentin Fallavier, France).
2.2. Formulation of CIP-Cu2+ loaded amorphous CaCO3 particles
CIP-Cu-loaded amorphous CaCO3 particles were prepared by spray drying as previously described (Lamy et al., 2018; Tewes et al., 2016). Briefly, an aqueous solution made of CIP (1.6 g/L), calcium hydroxide (0.8 g/L), hyaluronic acid (0.4 g/L), copper hydroxide (0.45 g/L) and formic acid (0.1% v/v) was mixed, just before the spray drying process, with a second aqueous solution of ammonium carbonate (2.4 g/L) in a Y-shaped tube connected to the peristaltic pump (rate 30%) of a B-290 Mini Spray-Dryer (Büchi, Switzerland). The spray dryer operated in open suction mode and the settings were as follows: 15 L/min for spray airflow, 630 L/h for drying airflow and 120 °C for temperature inlet. Under these conditions, amorphous spherical hollow particles with a mass median aerodynamic diameter (MMAD) of <5 μm and a CIP loading of 41.94 ± 10.4 wt% are obtained (Lamy et al., 2019, Lamy et al., 2018; Tewes et al., 2016). Nine milligrams of these microparticles completely dissolve in <0.25 h in 200 ml of simulated lung epithelial lining fluid with a pH of 7 (Lamy et al., 2018).
2.3. Preparation of the P. aeruginosa-loaded agar beads
Sterile and P. aeruginosa-loaded agar beads were prepared using an adapted version of the method described by Growcott et al. (Growcott et al., 2011). A fresh suspension of the P. aeruginosa strain PAO1 (ATCC 15692, Manassas, USA), prepared in Muller Hinton II broth (MHB, BD, Le Pont de Clair, France), was cultured to exponential growth phase and then adjusted to an OD600 of 0.3 with a plate reader (infinite M200, Tecan) using 100 μL placed in a 96-well plate. Two milliliters of this suspension were washed and concentrated in 1 mL of sterile phosphate buffer saline (PBS) by centrifugation. The bacteria were embedded into agar beads by mixing 1 mL of this concentrated suspension with 9 mL of molten agar (48 °C) at 2% w/v (VWR, Fontenay-sous-Bois, France). This agar suspension was then quickly emulsified for 5 min into 150 mL of warmed (48 °C) paraffin oil (Sigma Aldrich, France) containing 0.01% v/v of sorbitan monostearate (SPAN® 60, Sigma Aldrich). Then, the emulsion was cooled by placing ice chips around the beaker for 1 h and the beads were collected by centrifugation at 5,000 g for 10 min and then washed 4 times with PBS pH 7.4. Bead concentration was determined using a Neubauer hemocytometer. The number of bacteria per bead was determined from measurements of colony-forming units (CFU/ml) carried out after homogenization of the beads in PBS and plating of a tenfold serial dilution of this suspension on MH agar. Beads having between 12 and 20 CFU/beads were used. The size distribution of blank beads prepared in the same condition was evaluated in PBS by laser light diffraction measurement (Microtrac® X100 particle size analyzer).
2.4. In vivo experiments
In vivo experiments were carried out in compliance with EC Directive 2010/63/EU after agreement by the Ethic Committee (COMETHEA) and registration with the French Ministry of Higher Education and Research (n°201612221658962). Male Sprague-Dawley rats weighing between 300 and 350 g were purchased from Janvier Laboratories (Le Genest-St.-Isle, France). Animals were housed one per cage, subjected to 12 h/12 h light and darkness cycles with access to food and water ad libitum.
2.4.1. Development of the chronic lung infection model
On the day of infection, agar beads loaded with P. aeruginosa were dispersed in sterile physiological saline (0.9% NaCl) to obtain a range of inoculum concentration between 6 × 106 to 0.5 × 106 CFU/mL. Then, 0.1 ml of the agar bead suspension (6 × 105 to 0.5 × 105 CFU) was instilled into isoflurane-anesthetized rats using a curved cannula with a rounded tip inserted between the vocal cords. The evolution of the animals weigh was followed every day and 1 mL of physiological serum was injected subcutaneously the first 3 days after the infection to prevent dehydration. At various days post-infection (3, 4, 6, 7), animals (n = 18) were euthanized using overdose of pentobarbital (Dolethal® - 150 mg/kg) and their lungs were removed and homogenized in PBS. Serial dilutions of these homogenates were spread on MH agars to assess the progression of the infection.
2.4.2. Pharmacokinetic study
Rats were dosed with CIP at 2.5 mg/kg by intravenous (IV) injection of a CIP solution (n = 21) or by intratracheal (IT) administration of CIP-Cu-loaded particles (n = 20) 6 days after the inoculation of 1 × 105 P. aeruginosa per animal embedded in agar beads. The IT administrations were performed under isoflurane anesthesia and the CIP-Cu2+ powder was administered using a Dry Powder Insufflator DP-4 (Penn-Century Inc., Philadelphia, USA) as previously described (Gaspar et al., 2016; Lamy et al., 2018; Marchand et al., 2010; Nurbaeti et al., 2019). Then, at predetermined times after dosing of CIP to animals (0.5, 2, 4, 6, and 18 h), bronchoalveolar lavage (BAL) fluid and intracardiac blood were sampled to allow the assay of CIP and urea using the HPLC methods described below. The BALs were carried out on animals under isoflurane anesthesia and immobilized in a supine position with cervical hyperextension. One mL of saline at 37 °C was injected into the airways via a polyethylene catheter (0.58 mm i.d. and 0.96 mm o.d.; Harvard, Les Ulis, France) inserted into the trachea (50 mm deep). The BAL fluid (300 to 800 μL) was obtained by aspiration, centrifuged at 500 g for 5 min, and the supernatant was stored at −20 °C. The CIP concentrations in the pulmonary epithelial lining fluid (ELF) were calculated from the concentration measured in the BAL fluids using the ratio of BAL/ELF urea concentrations as a correction factor for the dilution of ELF during the lavage (Gontijo et al., 2014; Lamy et al., 2018; Marchand et al., 2010). The concentration of protein in the ELF is 10-fold lower than in plasma, and thus the total CIP concentrations in the ELF were considered unbound concentrations, as previously reported (Lamy et al., 2018). The profiles of total concentrations of CIP in ELF and unbound CIP in plasma as a function of time were normalized by the CIP dose and plotted as mean ± SD. Non-compartmental pharmacokinetic parameters and mean areas under the curves showing unbound CIP concentrations in plasma and ELF as a function of time (AUC) from 0.5 h to 18 h were estimated from mean plasma and ELF concentrations (AUCunbound plasma and AUCELF) using WinNonLin 7.0 software (Pharsight, USA).
2.4.3. Efficacy study
At the time of dosing, then 4 and 24 h after dosing, the animals (n = 27) were euthanized and their lungs were removed and homogenized in PBS. Serial dilutions of these homogenates were plated on MH agars to evaluate the progression of the infection. Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software Inc.). All data were plotted as means ± SD and analysed with a one-way analysis of variance (ANOVA) and Tukey's multiple comparisons test. p < 0.05 was considered statistical significance.
2.5. Ciprofloxacin assay
The CIP concentrations were determined by a reverse-phase HPLC coupled to a fluorimeter (λexit: 280 nm, λem: 460 nm) as previously described (Lamy et al., 2019, Lamy et al., 2018). In short, the chromatography was carried out using an XTerra MS C18 column (5 μm, 100 × 2.1 mm, Waters, Saint- Quentin-en-Yveline, France) for the assay of CIP in BAL, or a Phenomenex C18 column (5 μm, 150 × 2.1 mm) for the assay of CIP in plasma. Mobile phases flowing at a flow rate of 0.25 ml/min, made of 0.1% formic acid in water, acetonitrile and sodium heptane sulfonate mixed in a ratio 80/19/1 (v/v/v) for the assay in BAL fluid and in a ratio 86/14/1 (v/v/v) for the assay in plasma were used. For assay in BAL, seven calibration standards (from 1.56 ng/mL to 10 ng/mL) and 3 levels of control (3.125, 2.5, 7.5 ng/mL) were prepared in saline solution (0.9% NaCl). For assay in plasma, six calibration standards (from 5 ng/mL to 100 ng/mL) and 3 levels of control (10, 25, 75 ng/mL) were prepared in blank plasma. Interday variability was assessed in both media to be <20% for low-concentration controls and 15% for medium- and high-concentration controls.
A 1/X2–weighted linear regression was applied for the calculation. The CIP concentrations in ELF were calculated from BAL concentrations by using the dilution factor induced by the lung lavage. This dilution factor was evaluated by measuring the BAL/plasma urea concentration ratios. Urea in BAL was measured using a liquid chromatography in tandem with mass spectrometry (LC-MS/MS) and a Cobas® 8000 modular analyzer was used for measurement in plasma, as previously described (Gontijo et al., 2014).
3. Results
3.1. Influence of the inoculum value on the stability of the chronic agar bead infection model
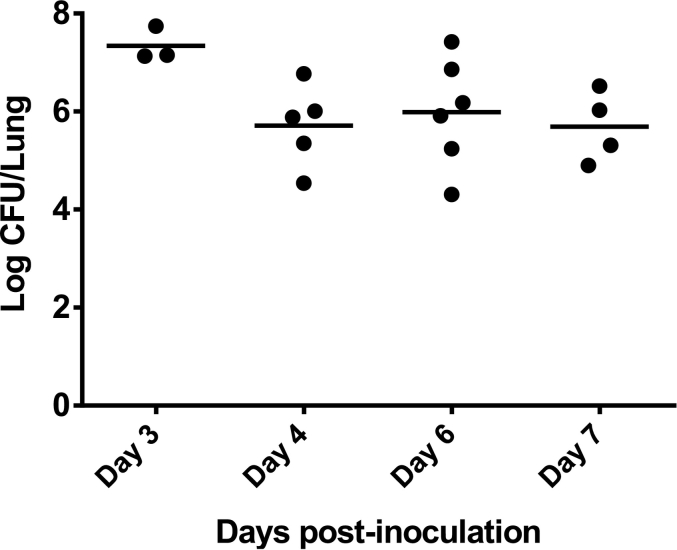
Preliminary experiments showed that the maximal inoculum that could be instilled into the lungs of rats with <30% mortality rate over 7 days was 6 × 105 CFU per animal. Bead size was another parameter that affected the mortality rate of the animals. Infection with beads with a volume-weighted median diameter (D50) of <120 μm increased the mortality rate. Therefore, beads with a D50 between 120 and 150 μm were used in the study. To determine from which day a stable chronic infection can be obtained, the evolution of the pulmonary bacterial burden as a function of time was monitored after pulmonary instillation of the same inoculum of 6 × 105 CFU per animal (Fig. 1).
Fig. 1.
P. aeruginosa lung burden measured several days after intratracheal inoculation of 6 × 105 CFU to rats (n = 18) using P. aeruginosa-loaded agar beads with a median diameter between 120 and 150 μm.
With this inoculum of 6 × 105 CFU per animal, mean lung bacterial burden values remained stable around 5.80 ± 0.90 log10 CFU from day 4 to day 7 post-infection. However, their variabilities were large (RSD between 12% and 18%) and may require a large number of animals to observe a significant effect after treatment.
3.2. Pharmacokinetic of ciprofloxacin in rat models of chronic P. aeruginosa lung infection
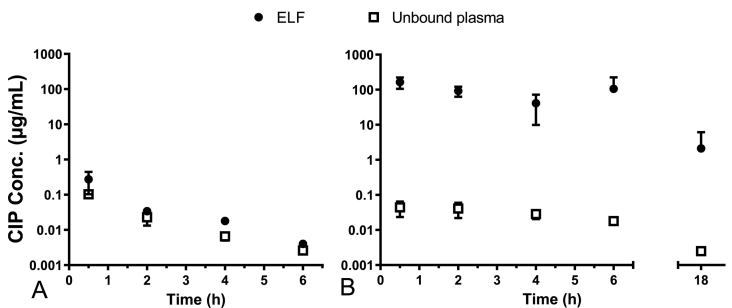
The pharmacokinetics of CIP in rat models of chronic lung infection with P. aeruginosa was determined after IV administration of a CIP solution (Fig. 2A) and after IT administration of dry amorphous CaCO3 particles loaded with CIP-Cu2+ complex (Fig. 2B).
Fig. 2.
Mean unbound CIP concentrations normalized by the CIP dose measured in ELF (black circle) and in plasma (open square) versus time after IV administration of a CIP solution (A), and after IT administration of CIP-Cu-loaded particles (B). The CIP dose was 2.5 mg/kg for all animals. The unbound plasma fraction was fixed at 59% of the total CIP concentration according to Roosendaal et al. (Roosendaal et al., 1987). Data points represent averaged values ± standard deviation (SD) of 3–7 individual measurements.
Half an hour after IV administration, the CIP maximum concentration in ELF (Cmax-ELF = 0.27 ± 0.12 μg/mL) was measured. This Cmax value was 2.6 times higher than the plasma CIP concentration measured at the same time (Fig. 2A), suggesting rapid diffusion of CIP from plasma to the lungs. During the 2- to 6-h period after administration, CIP concentrations both in the lung ELF and plasma decreased with corresponding elimination half-lives (t1/2) of 1.4 hand 1.3 h. Lung exposure to a drug can be assessed using the ratio of the area under the ELF concentration-time curve (AUCELF) normalized by the plasma AUC of the unbound drug (AUCunbound plasma). After IV administration of a CIP solution to rats with a chronic pulmonary infection model, the AUCELF to AUCunbound plasma ratio was 1.78.
After IT administration of dry particles loaded with CIP-Cu2+ complex, the ELF CIP concentration measured half an hour after the administration was of 164 ± 58 μg/mL, that is 607 times higher than the Cmax-ELF value measured after IV administration of a CIP solution (Fig. 2B). From then on, CIP concentrations in ELF decreased with a t1/2 of 2.9 h. Consequently, at 18 h after inhalation, the CIP concentration in the ELF was 2.1 ± 4.0 μg/mL, which is, despite the large variability, still 7.8 times higher than the CIP Cmax-ELF obtained after half an hour after IV administration of the CIP solution. The initial plasma concentration of unbound CIP (0.04 ± 0.02 μg/mL) measured half an hour after IT administration was 2.5 times lower than that measured after IV administration (0.10 ± 0.01 μg/mL). The plasma (t1/2) was equal to 4.1 h. Thus, plasma elimination of CIP was 3.25 times slower after IT administration of particles loaded with CIP-Cu2+ complex than after IV administration of a CIP solution. The AUCELF to AUCunbound plasma ratio was 3698 after IT administration of particles loaded with CIP-Cu2+ complex. Thus, pulmonary exposure to CIP was (3698/1.78) = 2077 times higher after IT administration of CIP-Cu loaded particles than after IV administration of a CIP solution.
3.3. Efficacy of the inhaled CIP-Cu2+ particles versus an IV CIP solution in a chronic lung infection model
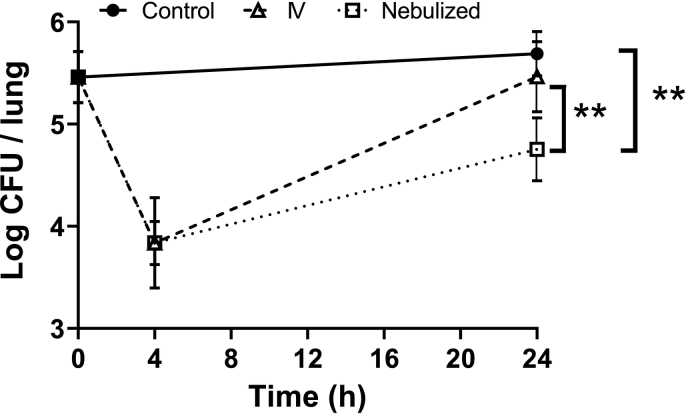
The progression of the P. aeruginosa burden in the lungs of the animals was evaluated 4 and 24 h after the administrations of CIP (Fig. 3). All treated animals received the same dose of CIP (2.5 mg/kg) either by IV administration of a CIP solution or by IT administration of CaCO3 microparticles loaded with CIP-Cu2+ complex. The two treatments induced a decrease in the pulmonary bacterial burden from 5.45 ± 0.25 log10 CFU to 3.84 ± 0.44 log10 CFU and 3.84 ± 0.21 log10 CFU, 4 h after the administration of the solution of CIP and CIP-Cu2+ powder, respectively. Then, for both treatments, this decrease in P. aeruginosa burden observed at the 4-h time point was followed by an increase measured at the 24-h time point after administration. However, this increase was less intense for the group treated with IT administration of microparticles loaded with CIP-Cu2+ complex compared with IV administration of CIP. Consequently, the pulmonary burden of P. aeruginosa measured at 24 h was significantly lower after IT administration (4.75 ± 0.31 log10 CFU) than for the untreated control (5.69 ± 0.22 log10 CFU) and IV group (5.46 ± 0.34 log10 CFU), respectively. No significant difference was observed at 24 h between the untreated control group and the IV group.
Fig. 3.
Mean log colony forming unit (CFU) per lung versus time profiles in group receiving either no treatment (Control, black circle), a CIP solution by IV administration (open triangle), or CIP-Cu2+ microparticles by IT administration (open square). The CIP dose was 2.5 mg/kg for all animals. Data points represent averaged values ± standard deviation (SD) of 4–5 individual measurements. Statistical analyses were performed using a one-way ANOVA and Tukey's multiple comparisons test (** mean P ≤ 0.01).
4. Discussion
The chronic infection model obtained by instillation of P. aeruginosa-loaded agar beads used in this study has been shown to mimic the chronic infection and inflammation that affect patients with cystic fibrosis (CF) (Bragonzi et al., 2005; Growcott et al., 2011; Kukavica-Ibrulj and Levesque, 2008; Torres et al., 2019). The airway and alveolar inflammation seen during chronic P. aeruginosa infections can lead to increased permeability of the alveolar and airway epithelium, sometimes with the formation of exudates (Azghani et al., 2000; Ulrich et al., 2010). This increased permeability could alter the fate of inhaled molecules, which could have a low pulmonary residence time in infected lungs, whereas they had low permeability and high pulmonary residence time under healthy conditions.
The pulmonary exposure to free CIP observed after IV administration in rats with chronic lung infection, was similar to that previously observed in healthy rats (Gontijo et al., 2014), with a ratio of AUCELF to AUCunbound plasma around 2 in both cases. Furthermore, the mean plasma elimination half-lives (t1/2) of CIP measured after IV administration were comparable between healthy rats (1.1 h) (Gontijo et al., 2014) and model rats with chronic P. aeruginosa lung infection (1.3 h). Both CIP plasma t1/2 were also similar to the t1/2 measured in the lung ELF after IV CIP administration in healthy (1.2 h) or infected (1.4 h) rats. CIP is a molecule with a relatively high permeability across the pulmonary barrier (Brillault et al., 2017; Ong et al., 2013), and concentrations of unbound CIP molecules already equilibrate rapidly across this barrier under noninflammatory conditions (Lamy et al., 2018; Stass et al., 2013a, Stass et al., 2013b). Thus, a potential alteration in lung barrier integrity due to inflammation should not result in a major change in CIP permeation rate, since CIP permeability is already high in healthy state. Similar pulmonary exposure to CIP was obtained in healthy rats and rats with chronic lung infection after IV administration of CIP solutions, suggesting similar high permeability of the blood-lung barrier in healthy and infected animals. In healthy rats, similar CIP pulmonary exposures are obtained after IV or pulmonary administration (Gontijo et al., 2014). Similar observation were made with levofloxacin, another fluoroquinolone with close structure to CIP (Griffith et al., 2014). Therefore, the impact of the mode of administration (IT versus IV) on pulmonary exposure to CIP should be minimal. For this reason and for ethical reasons to limit the use of animals, it was decided not to perform a group of animals with pulmonary administration of CIP solution in this study. Another study that evaluated the pharmacokinetics of CIP after IV administration in rats with chronic P. aeruginosa lung infection showed that plasma exposure to CIP was twice as high in infected rats as in healthy rats due to lower plasma clearance in the infected animals (Torres et al., 2017). In the present study, lung infection had no effect on plasma CIP exposure. However, it is difficult to compare the two studies because they were performed with different types of P. aeruginosa-loaded beads (agar versus alginate-based) and with different doses of CIP (2.5 mg/kg versus 20 mg/kg).
Interestingly, lung exposure to CIP measured after inhalation of particles loaded with CIP-Cu2+ complex in model rats with chronic P. aeruginosa lung infection was characterized by a ratio of AUCELF to AUCunbound, plasma of 3698, which is 3.5 times higher than in healthy animals for which the ratio was 1069 (Lamy et al., 2018). This increased pulmonary exposure also resulted in an elevated CIP t1/2 in ELF of 2.9 h (compared to 1.4 h after IV administration), but also in an increased plasma CIP t1/2 of 4.1 h (compared to1.3 h after IV). Thus, plasma elimination of CIP was 3.6 times slower after IT administration of particles loaded with CIP-Cu2+ complex than after IV administration of a CIP solution. This suggests that the rate of absorption of CIP from the lung to the blood was reduced enough to become slower than the intrinsic plasma CIP t1/2 and thus became the limiting step controlling the plasma elimination of CIP, characterizing a flip-flop pharmacokinetics (Yáñez et al., 2011).
The targeting advantage that was obtained by administering the CIP by IT administration of the microparticles loaded with CIP-Cu2+ compared to the IV administration of the CIP solution can be evaluated by measuring the drug targeting index (DTI), defined as the ratio of AUCELF to AUCunbound plasma following IT administration divided by the same ratio following IV administration (Yapa et al., 2014). The DTI obtained by IT administration of microparticles loaded with CIP-Cu2+ complex was 2077 (3698/1.78). This value, well above unity, indicates that a high degree of targeting to the lungs (higher lung exposure and minimal plasma exposure) was achieved after inhalation administration of CIP-Cu2+ loaded microparticles compared to IV administration.
Similarly, the inhaled levofloxacin solution marketed under the brand name Quinsair® contains magnesium, a metal cation like copper, which complexes levofloxacin and increases its apparent aqueous solubility and decreases its apparent epithelial permeability (Surber et al., 2017, Surber et al., 2013). The PK of Quinsair® have been characterized in a mouse model of pulmonary infection (Sabet et al., 2009). The authors reported that aerosolized administration achieved a 9-fold higher AUC in lung tissue when compared with the dose-normalized intraperitoneal administration of levofloxacin.
In CF patients, P. aeruginosa grows principally in pulmonary mucus and ELF (Bjarnsholt et al., 2009). Thus, pulmonary ELF should be considered the primary antibiotic target site for the treatment of pneumonia caused by this bacterium (Onufrak et al., 2016; Rodvold et al., 2011; Wenzler et al., 2016). Several studies have shown that maximizing CIP exposure at the site of infection is essential to reduce the increasing rate of antibiotic resistance (Martinez et al., 2012; Onufrak et al., 2016) or to prevent biofilm formation by this pathogen (Bjarnsholt et al., 2009). Hence, for CIP, the ability to target the lungs to achieve high concentrations in the ELF while minimizing systemic exposure has a major advantage in terms of maximizing efficacy and reducing the potential for resistance development and is essential to ensure optimal treatment of these pulmonary infections.
P. aeruginosa lung burden, measured 24 h after inoculation, was significantly lower after IT administration of CIP-Cu-loaded particles (4.75 ± 0.31 log10 CFU) than in the untreated control group (5.69 ± 0.22 log10 CFU) and the CIP IV group (5.46 ± 0.34 log10 CFU), respectively. It has been demonstrated previously that CIP or CIP-Cu2+ are equally effective against P. aeruginosa cultured either planktonically or as biofilms (Tewes et al., 2020, Tewes et al., 2019, Tewes et al., 2016). Also, the same efficacy between the 2 modes of administration (IV and IT) was observed 4 h after inoculation (Fig. 3). Thus, the better efficacy observed at 24 h with inhaled CIP-Cu2+ loaded particles compared to IV CIP solution could be attributed to the longer residence time of CIP in the lungs obtained with the CIP-Cu2+ complex.
In summary, the present study shows that in a rat model of chronic P. aeruginosa lung infection, pulmonary administration of CIP-Cu-loaded microparticles resulted in an approximately 3700-fold increase in lung CIP residence time compared with intravenous administration of CIP solution. This increase in lung residence time of CIP is responsible for improved efficacy of CIP 24 h after administration compared with intravenous administration of CIP solution. Similarly, another study in which CPI was administered by pulmonary aerosolization on days 4 and 6 post-infection to rats showed better efficacy of microparticles loaded with CPI-Cu (4-log reduction in CFU/lung) than particles made of CPI-HCl (similar CFU/lung counts to untreated control) (Tewes et al., 2020). However, other formulations of CIP or fluoroquinolones that have been developed to treat pulmonary P. aeruginosa infection by inhalation have also elaborated strategies to successfully increase the pulmonary residence time of fluoroquinolones, sometimes without significant clinical results (Brillault and Tewes, 2020). For comparison purposes, some of the PK and formulation parameters of these recently developed formulations, namely Cipro-DPI (Ciprofloxacin PulmoSphere®), Quinsair® (Aeroquin™), and Linhaliq® (Apulmiq®), are presented in Table 1. However, this study has some limitations that do not allow it to go beyond a basic comparison. Indeed, a single dose was evaluated whereas the dosage regimens used in the clinic generally involve repeated administrations. Moreover, to better evaluate this formulation, other doses (higher than 2.5 mg/kg) should be tested.
Table 1.
Comparison of the tested formulation with marketed or clinically tested CIP or fluoroquinolone formulations for pulmonary inhalation. (BAL: bronchoalveolar lavage; ELF: pulmonary epithelial lining fluid).
| Formulations | Drug Loading | Pulmonary exposure in rats | references | |
|---|---|---|---|---|
| Present study | Dry powder made of CIP-Cu-loaded amorphous CaCO3 microparticles. | 41.9 ± 10.4 wt% |
|
Present study |
| Quinsair® | 246 mg of levofloxacin hemihydrate solubilized in 2.4 mL of aqueous solution containing Mg2+ to reduce the transport of levofloxacin across the lung-blood barrier when administered by nebulization. Commercialized. | 100 mg/mL of levofloxacin |
|
Geller et al. (2010); Griffith et al. (2014); Surber et al. (2017) |
| Cipro-DPI | Crystalline zwitterionic form (uncharged) of CIP used to slow the dissolution rate compared to CIP-HCl. Crystals of CIP are coated with a porous layer of phospholipids. | 65 wt% |
|
Endermann et al. (2011); Weers and Tarara (2009) |
| Linhaliq® | CIP-HCl encapsulated in liposomes, with a lipid/CIP mass ratio of 2. Mixed with free CIP-HCl. pH of about 3.5 or less | Liposome suspension at 45 mg/mL CIP mixed in different proportions with a free CIP solution at 18 mg/mL. |
|
Cipolla et al., 2016, Cipolla et al., 2014 |
5. Conclusion
After a single pulmonary administration of microparticles loaded with CIP-Cu2+ complex to rat models of chronic P. aeruginosa lung infection, pulmonary exposure to CIP was increased 2077-fold compared to IV administration of CIP solution. This single lung administration significantly reduced the lung burden of P. aeruginosa expressed as CFU/lung measured 24 h after administration by 10-fold while IV administration of the same dose of CIP was ineffective compared to the untreated control. This better efficacy of inhaled microparticles loaded with CIP-Cu2+ complex compared with CIP solution can be attributed to the higher pulmonary exposure to CIP obtained with inhaled particles than that obtained with IV solution.
CRediT authorship contribution statement
Frederic Tewes: Writing – original draft, Conceptualization, Methodology, Resources, Writing – review & editing, Funding acquisition, Project administration. Barbara Lamy: Investigation, Methodology. Julian Laroche: Investigation, Methodology. Isabelle Lamarche: Investigation, Methodology. Sandrine Marchand: Conceptualization, Methodology, Resources, Writing – review & editing, Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work has benefited from the facilities and expertise of PREBIOS platform (Université de Poitiers). Analytical assays for urea in plasma using a Cobas® 8000 modular analyzer were supported by the University hospital of Poitiers, France.
Data availability
Data will be made available on request.
References
- Azghani A., Miller E., Peterson B. Virulence factors from Pseudomonas aeruginosa increase lung epithelial permeability. Lung. 2000;178:261–269. doi: 10.1007/s004080000031. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T., Jensen P.Ø., Fiandaca M.J., Pedersen J., Hansen C.R., Andersen C.B., Pressler T., Givskov M., Høiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- Bragonzi A., Worlitzsch D., Pier G.B., Timpert P., Ulrich M., Hentzer M., Andersen J.B., Givskov M., Conese M., Döring G. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J. Infect. Dis. 2005;192:410–419. doi: 10.1086/431516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillault J., Tewes F. Control of the lung residence time of highly permeable molecules after nebulization: example of the fluoroquinolones. Pharmaceutics. 2020;12:387. doi: 10.3390/pharmaceutics12040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillault J., Tewes F., Couet W., Olivier J. In vitro biopharmaceutical evaluation of ciprofloxacin/metal cation complexes for pulmonary administration. Eur. J. Pharm. Sci. 2017;97:92–98. doi: 10.1016/j.ejps.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Cipolla D., Shekunov B., Blanchard J., Hickey A. Lipid-based carriers for pulmonary products: Preclinical development and case studies in humans. Adv. Drug Deliv. Rev. Improv. Effic. Inhaled Drugs Sev. Lung Diseas.: Emerg. Pulmon. Deliv. Strateg. 2014;75:53–80. doi: 10.1016/j.addr.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Cipolla D., Blanchard J., Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics. 2016;8:6. doi: 10.3390/pharmaceutics8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endermann R., Labischinski H., Ladel C., Petersen U., Newton B. 2011. Treatment of bacterial diseases of the respiratory organs. US 8,034,817 B2. [Google Scholar]
- Gaspar M.C., Grégoire N., Sousa J.J.S., Pais A.A.C.C., Lamarche I., Gobin P., Olivier J.-C., Marchand S., Couet W. Pulmonary pharmacokinetics of levofloxacin in rats after aerosolization of immediate-release chitosan or sustained-release PLGA microspheres. Eur. J. Pharm. Sci. 2016;93:184–191. doi: 10.1016/j.ejps.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Geller D.E., Flume P.A., Griffith D.C., Morgan E., White D., Loutit J., Dudley M.N. Pharmacokinetics (PK) of aerosol MP-376 (aeroquin; levofloxacin inhalation solution) in CF patients. J. Cyst. Fibros. 2010;9:S23. doi: 10.1016/S1569-1993(10)60088-4. [DOI] [Google Scholar]
- Gontijo A.V.L., Brillault J., Grégoire N., Lamarche I., Gobin P., Couet W., Marchand S. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 1. Ciprofloxacin, moxifloxacin, and grepafloxacin. Antimicrob. Agents Chemother. 2014;58:3942–3949. doi: 10.1128/AAC.02818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D.C., Dudley M.N., Surber M.W., Bostian K.A., Rodny O. 2014. Aerosol Fluoroquinolone Formulations for Improved Pharmacokinetics. US8815838B2. [Google Scholar]
- Growcott E.J., Coulthard A., Amison R., Hardaker E.L., Saxena V., Malt L., Jones P., Grevot A., Poll C., Osborne C. Characterisation of a refined rat model of respiratory infection with Pseudomonas aeruginosa and the effect of ciprofloxacin. J. Cyst. Fibros. 2011;10:166–174. doi: 10.1016/j.jcf.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Günday Türeli N., Torge A., Juntke J., Schwarz B.C., Schneider-Daum N., Türeli A.E., Lehr C.-M., Schneider M. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur. J. Pharm. Biopharm. 2017;117:363–371. doi: 10.1016/j.ejpb.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Kukavica-Ibrulj I., Levesque R.C. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab. Anim. 2008;42:389–412. doi: 10.1258/la.2007.06014e. [DOI] [PubMed] [Google Scholar]
- Lamy B., Tewes F., Serrano D.R., Lamarche I., Gobin P., Couet W., Healy A.M., Marchand S. New aerosol formulation to control ciprofloxacin pulmonary concentration. J. Control. Release. 2018;271:118–126. doi: 10.1016/j.jconrel.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Lamy Barbara, Serrano Dolores Remedios, O’Connell Peter, Couet W., Marchand S., Healy A.M., Tewes F. Use of leucine to improve aerodynamic properties of ciprofloxacin-loaded maltose microparticles for inhalation. Eur. J. Pharmaceut. Res. 2019;1:2–11. [Google Scholar]
- Marchand S., Gobin P., Brillault J., Baptista S., Adier C., Olivier J.-C., Mimoz O., Couet W. Aerosol Therapy with Colistin Methanesulfonate: a Biopharmaceutical issue Illustrated in Rats. Antimicrob. Agents Chemother. 2010;54:3702–3707. doi: 10.1128/AAC.00411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.N., Papich M.G., Drusano G.L. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 2012;56:2795–2805. doi: 10.1128/AAC.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane P.J., Weers J.G., Tarara T.E., Haynes A., Durbha P., Miller D.P., Mundry T., Operschall E., Elborn J.S. Ciprofloxacin Dry Powder for Inhalation (ciprofloxacin DPI): Technical design and features of an efficient drug–device combination. Pulm. Pharmacol. Ther. 2018;50:72–79. doi: 10.1016/j.pupt.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Nurbaeti S.N., Brillault J., Tewes F., Olivier J.-C. Sustained-release microparticle dry powders of chloramphenicol palmitate or thiamphenicol palmitate prodrugs for lung delivery as aerosols. Eur. J. Pharm. Sci. 2019;138 doi: 10.1016/j.ejps.2019.105028. [DOI] [PubMed] [Google Scholar]
- Ong H.X., Traini D., Bebawy M., Young P.M. Ciprofloxacin is actively Transported across bronchial lung epithelial cells using a Calu-3 air interface cell model. Antimicrob. Agents Chemother. 2013;57:2535–2540. doi: 10.1128/AAC.00306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onufrak N.J., Forrest A., Gonzalez D. Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing. Clin. Ther. 2016;38:1930–1947. doi: 10.1016/j.clinthera.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvold K.A., George J.M., Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid. Clin. Pharmacokinet. 2011;50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I.A., van den Berghe-van Raffe M., Vink-van den Berg J.C., Michel M.F. Comparative activities of ciprofloxacin and ceftazidime against Klebsiella pneumoniae in vitro and in experimental pneumonia in leukopenic rats. Antimicrob. Agents Chemother. 1987;31:1809–1815. doi: 10.1128/AAC.31.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet M., Miller C.E., Nolan T.G., Senekeo-Effenberger K., Dudley M.N., Griffith D.C. Efficacy of Aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009;53:3923–3928. doi: 10.1128/AAC.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serisier D.J., Bilton D., Soyza A.D., Thompson P.J., Kolbe J., Greville H.W., Cipolla D., Bruinenberg P., Gonda I., Investigators, the O.-2 Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax. 2013;68:812–817. doi: 10.1136/thoraxjnl-2013-203207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stass H., Nagelschmitz J., Willmann S., Delesen H., Gupta A., Baumann S. Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: a phase I study. Clin. Drug Investig. 2013;33:419–427. doi: 10.1007/s40261-013-0082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stass H., Weimann B., Nagelschmitz J., Rolinck-Werninghaus C., Staab D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized, dose-escalation study. Clin. Ther. 2013;35:1571–1581. doi: 10.1016/j.clinthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Surber M.W., Bostian K.A., Dudley M.N., Lomovskaya O., Griffith D.C. 2013. Aerosolized Fluoroquinolones and Uses Thereof. US8524735B2. [Google Scholar]
- Surber, M.W., Bostian, K.A., Griffith, D.C., Dudley, M.N., Rodny, O., 2017. Aerosol fluoroquinolone formulations for improved pharmacokinetics. MX345158B. patent EP2344129A1.
- Tewes F., Brillault J., Lamy B., O’Connell P., Olivier J.-C., Couet W., Healy A.M. Ciprofloxacin-loaded inorganic–organic composite microparticles to treat bacterial lung infection. Mol. Pharm. 2016;13:100–112. doi: 10.1021/acs.molpharmaceut.5b00543. [DOI] [PubMed] [Google Scholar]
- Tewes F., Bahamondez-Canas T.F., Smyth H.D.C. Efficacy of Ciprofloxacin and its copper complex against Pseudomonas aeruginosaBiofilms. AAPS PharmSciTech. 2019;20:205. doi: 10.1208/s12249-019-1417-9. [DOI] [PubMed] [Google Scholar]
- Tewes F., Bahamondez-Canas T.F., Moraga-Espinoza D., Smyth H.D.C., Watts A.B. In vivo efficacy of a dry powder formulation of ciprofloxacin-copper complex in a chronic lung infection model of bioluminescent Pseudomonas aeruginosa. Eur. J. Pharm. Biopharm. 2020;152:210–217. doi: 10.1016/j.ejpb.2020.05.014. [DOI] [PubMed] [Google Scholar]
- Tiddens H.A.W.M., Bos A.C., Mouton J.W., Devadason S., Janssens H.M. Inhaled antibiotics: dry or wet? Eur. Respir. J. 2014;44:1308–1318. doi: 10.1183/09031936.00090314. [DOI] [PubMed] [Google Scholar]
- Torres B.G.S., Helfer V.E., Bernardes P.M., Macedo A.J., Nielsen E.I., Friberg L.E., Dalla Costa T. Population pharmacokinetic modeling as a tool to characterize the decrease in ciprofloxacin free interstitial levels caused by pseudomonas aeruginosa biofilm lung infection in wistar rats. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02553-16. e02553–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres B.G.S., Awad R., Marchand S., Couet W., Tewes F. In vitro evaluation of Pseudomonas aeruginosa chronic lung infection models: are agar and calcium-alginate beads interchangeable? Eur. J. Pharm. Biopharm. 2019;143:35–43. doi: 10.1016/j.ejpb.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Ulrich M., Worlitzsch D., Viglio S., Siegmann N., Iadarola P., Shute J.K., Geiser M., Pier G.B., Friedel G., Barr M.L., Schuster A., Meyer K.C., Ratjen F., Bjarnsholt T., Gulbins E., Döring G. Alveolar inflammation in cystic fibrosis. J. Cyst. Fibros. 2010;9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weers J.G., Tarara T. Pulmonary delivery of a fluoroquinolone. CA2724009A1. patent US20090992371. 2009 [Google Scholar]
- Wenzler E., Fraidenburg D.R., Scardina T., Danziger L.H. Inhaled antibiotics for gram-negative respiratory infections. Clin. Microbiol. Rev. 2016;29:581–632. doi: 10.1128/CMR.00101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez J.A., Remsberg C.M., Sayre C.L., Forrest M.L., Davies N.M. Flip-flop pharmacokinetics – delivering a reversal of disposition: challenges and opportunities during drug development. Ther. Deliv. 2011;2:643–672. doi: 10.4155/tde.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapa S., Li J., Patel K., Wilson J.W., Dooley M.J., George J., Clark D., Poole S., Williams E., Porter C.J.H., Nation R.L., McIntosh M.P. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 2014;58:2570–2579. doi: 10.1128/AAC.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.T., Leung S.S.Y., Tang P., Parumasivam T., Loh Z.H., Chan H.-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv. Drug Deliv. Rev. 2015;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.