Abstract
Background & Aims
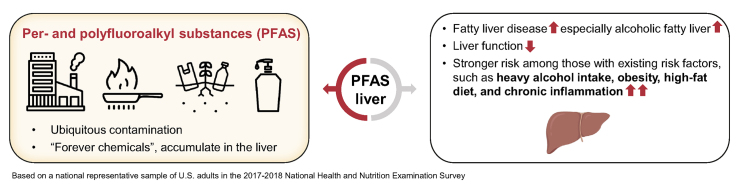
Per- and polyfluoroalkyl substances (PFAS) are widespread pollutants with demonstrated hepatotoxicity. Few studies have examined the association between PFAS and fatty liver disease (FLD) risk in an adult population.
Methods
In this cross-sectional study of participants from the 2017–2018 National Health and Nutrition Examination Survey, serum PFAS were measured, and FLD cases were ascertained by vibration-controlled transient elastography. Logistic regression models were used to examine the association between circulating PFAS levels and FLD risk. Analyses were stratified into non-alcoholic FLD and alcoholic FLD risk groups by alcohol intake status, as well as controlling for other risk factors, including personal demographics, lifestyle factors, and related health factors.
Results
Among 1,135 eligible participants, 446 had FLD. For FLD risk, the multivariable-adjusted odds ratio per log-transformed SD increase (ORSD) in perfluorohexane sulfonate (PFHxS) was 1.13 (95% CI 1.01–1.26). The association between PFHxS and FLD appeared stronger among individuals with obesity or high-fat diets (both pinteraction <0.05). When limiting the analysis to 212 heavy drinkers (≥2 drinks/day for women and ≥3 drinks/day for men), significantly higher risk of alcoholic FLD was found for higher levels of perfluorooctanoic acid (ORSD 1.79; 95% CI 1.07–2.99), PFHxS (ORSD 2.06; 95% CI 1.17–3.65), and perfluoroheptane sulfonic acid (ORSD 1.44; 95% CI 1.00–2.07), and marginally significant higher risk for total PFAS (ORSD 2.12; 95% CI 0.99–4.54). In never or light drinkers, we did not observe any significant association between PFAS and non-alcoholic FLD. Significant positive associations were found for PFAS with aspartate aminotransferase, gamma-glutamyl transaminase, total bilirubin, and albumin (β ranged from 0.008 to 0.101, all p <0.05).
Conclusions
Higher serum PFAS was moderately associated with FLD risk and worse liver function in the general population, and among those with independent risk factors, including heavy alcohol intake, obesity, or high-fat diets, PFAS increased the risk. These results suggest synergistic effects on hepatic steatosis between PFAS exposures as measured through biomonitoring data and lifestyle risk factors in a nationally representative US population.
Impact and Implications
The per- and polyfluoroalkyl substances (PFAS) may convey higher risk for chronic liver disease in humans. Among 1,135 US adults in the 2017–2018 National Health and Nutrition Examination Survey, we found that higher serum PFAS was associated with higher fatty liver disease risk and worse liver function, especially among those with liver disease risk factors, including heavy alcohol intake, obesity, or high-fat diets. Continuously monitoring PFAS in the population and examining how they potentiate risk to the liver are essential.
Keywords: Per- and polyfluoroalkyl substances, PFAS, PFOS, PFOA, PFHxS, Fatty liver disease, NAFLD, AFLD, Liver function, NHANES
Graphical abstract
Highlights
-
•
PFAS may convey higher risk for chronic liver disease in humans.
-
•
We found that higher serum PFAS was associated with higher fatty liver disease risk and worse liver function.
-
•
This was especially evident in those with liver disease risk factors, including heavy alcohol intake, obesity, or high-fat diets.
-
•
Continuously monitoring PFAS in the population and examining how they potentiate risk to the liver are essential.
Introduction
The burden of liver diseases worldwide is estimated to increase substantially in the next several decades.1 The prevalence of non-alcoholic fatty liver disease (NAFLD) and alcoholic fatty liver disease (AFLD) is rising with an increasing trend for consequent end-stage chronic liver diseases.2,3 In the United States, for example, NAFLD and AFLD are the top contributors to the burden of liver disease mortality.4 Although excess alcohol intake, obesity, and diabetes are the leading causes of fatty liver disease (FLD), exposure to environmental contaminants may also contribute to this multifactorial disease.5,6
The per- and polyfluoroalkyl substances (PFAS) are a group of structurally stable chemicals that are widely used to make fluoropolymer coatings and products that resist heat, oil, stains, grease, and water.7,8 In recent decades, alerts have been raised regarding their ubiquitous contamination, persistence, and potentially adverse effects on environmental and human health.9 Review and meta-analysis of human population and experimental studies have demonstrated that PFAS are associated with hepatotoxicity and worse liver functions, and have also suggested the possibility of a higher risk of liver cancer.10,11 However, although growing evidence implicates higher PFAS in abnormal liver biomarkers, for example, alanine aminotransferase (ALT), gamma-glutamyl transaminase (GGT), and bilirubin,[11], [12], [13], [14] the specific association with FLD, especially AFLD, and with advanced liver disease requires additional exploration.
To date, only one epidemiological study reported a null association between the total level of eight blood polyfluoroalkyl chemicals and NAFLD that was defined using the hepatic steatosis index (HSI) and US fatty liver index (USFLI).15 No study has yet evaluated associations between individual PFAS and vibration-controlled transient elastography (VCTE)-diagnosed NAFLD or AFLD risk in adults. Specifically, VCTE has been approved by the US Food and Drug Administration for the non-invasive detection of more advanced diseases in patients who have FLD or who are at risk for FLD.16 National efforts to phase out long-chain PFAS,17 especially perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), are underway in the United States and several other countries, yet exposures persist, and these compounds are still used in international commerce. Continuously tracking and re-examining the exposure levels of PFAS and their potential associations with liver functions is likely to generate useful public health information.
Herein, we aimed to evaluate the associations between serum PFAS levels and VCTE-diagnosed FLD, including NAFLD and AFLD, among community-dwelling adults aged ≥20 years in the United States. We further examined the associations between overall and individual serum PFAS and liver function biomarkers.
Patients and methods
Study population
We used data from the 2017–2018 National Health and Nutrition Examination Survey (NHANES), a nationally representative survey of the civilian noninstitutionalised US population conducted by the Centers for Disease Control and Prevention every 2 years. In NHANES 2017–2018, a total of 8,704 participants completed both the interview and medical examination. We excluded participants aged <20 years (n = 3,439), without valid serum PFAS (n = 3,861), without VCTE assessments (n = 92), or with missing values in demographic covariates (n = 69). We further excluded participants with positive HBV or HCV infection (n = 101) or using steatogenic medications (n = 7). A total of 1,135 participants were included in the final analysis (Fig. S1). The NHANES protocol was approved by the National Center for Health Statistics Research Ethics Review Board. Informed consent was obtained from all participants. The study population was limited to the 2017–2018 survey because it was the first survey cycle to include VCTE.
Assessment of PFAS
Online solid-phase extraction coupled to high-performance liquid chromatography–turboionspray ionisation–tandem mass spectrometry was used by NHANES for the quantitative detection of serum PFAS in a random one-third subsample of participants who were 12 years of age or older as described on the NHANES website.18 The lower limit of detection was 0.10 ng/ml. PFAS detected in <80% of participants were not included in this study (see Supplementary methods and Table S1), leaving PFOS, PFOA, perfluorohexane sulfonate (PFHxS), perfluorononanoic acid (PFNA), perfluoroheptane sulfonic acid (PFHpS), and perfluorodecanoate (PFDA) for analysis. Total PFAS was calculated as the sum of these six substances.
Assessment of liver function markers
Clinical biomarkers measured through the NHANES standard biochemistry profile included ALT, GGT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, high-sensitivity C-reactive protein (hs-CRP), and albumin.18 These biomarkers were a priori selected as either commonly used in the diagnosis and evaluation of treatment of liver function19 or commonly associated with FLD-related metabolic status.
Assessment of FLD
In 2017–2018, VCTE was used to assess the amount of fat tissue in the liver in NHANES participants aged 12 years and older.18,[20], [21], [22] The controlled attenuation parameter score provides a measure of the portion of the liver affected by fatty change at the time of the procedure. Consistent with previous studies, we defined FLD with a controlled attenuation parameter score ≥285 dB/m and a high likelihood of advanced fibrosis with liver stiffness measurements ≥8.6 kPa.23,24
Assessment of covariates
Histories of cancer, diabetes, hypertension, and liver diseases were self-reported. Height, weight, and waist circumference were measured in the Medical Examination Center, and BMI was calculated as weight (kg) divided by height squared (m2). Dietary intakes, including alcohol intake, were assessed using 2-day 24-h dietary recalls. A high-fat diet was defined as ≥35% total energy from fat intake. High chronic inflammation was defined as hs-CRP >3.0 mg/L. Hepatitis B core antibody and hepatitis C antibody were measured using the VITROS immunodiagnostic products. Detailed information on data collection can be found on the NHANES website.18,25
Statistical analysis
Statistical analyses were conducted by following the NHANES guidelines, considering the survey’s complex sampling design. Comparison of characteristics between high FLD risk and other participants was performed using Student’s t test for continuous variables and the Χ2 test for categorical variables. For the comparison of baseline levels of PFAS and liver function markers, weighted geometric means with SDs were calculated and p values derived from the weighted linear logistic regression using log-transformed values.
To explore the association between PFAS and the risk of FLD, we categorised PFAS levels into tertile categories based on the distribution of PFAS among non-FLD and calculated the odds ratios (ORs) with 95% CIs using a crude model and a multivariable logistic regression model, adjusting for age group (20 to <40, 40 to <60, or ≥60 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other), ever smoker (yes or no), ever drink alcohol (yes or no), total physical activity (min/day), BMI (kg/m2), history of diabetes (yes or no), cancer (yes or no), hypertension (yes or no), aspirin use (yes or no), and high-fat diet (yes or no). The p values for linear trend and the ORs per log-transformed SD were also calculated.
Considering the alcohol-attributable aetiology of AFLD, we separately analysed the association between PFAS and NAFLD in never and light drinkers (<2 drinks/day for women and <3 drinks/day for men), and the association between PFAS and AFLD in heavy drinkers (≥2 drinks/day for women and ≥3 drinks/day for men).
We also performed stratified analyses by sex, race/ethnicity, smoking status, alcohol drinking status, obesity, waist circumference, high-fat diet, and levels of ALT and C-reactive protein (CRP). Values of p for interaction were calculated by testing the product of log-transformed PFAS and the stratified factors in the multivariable logistic models. For sensitivity analyses, we excluded participants with a history of cancer or with albuminuria (urine albumin–creatinine ratio ≥25 mg/g for women and ≥17 mg/g for men). The albuminuria sensitivity test was performed because of the enhanced PFAS excretion in albuminuria, with known inverse association of albuminuria to serum PFAS.26,27
To examine the associations between PFAS and liver function markers, both the PFAS levels and liver function markers were log-transformed. Linear regression analyses were performed using crude models and multivariable models. All the analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), and a two-sided p <0.05 was statistically significant.
Results
Table 1 lists the characteristics by the presence of FLD among the participants in the NHANES 2017–2018. The prevalence of non-FLD was 61.1% in our study population. Those with FLD were older and more likely to be male, Hispanic, and ever smokers; they also tended to have higher BMI and waist circumference, and histories of diabetes, hypertension, and aspirin use (all p <0.05).
Table 1.
Characteristics of participants with available data on PFAS and FLD in the NHANES 2017–2018.
| Non-FLD |
FLD |
p value | |
|---|---|---|---|
| n = 689 | n = 446 | ||
| Age, year | 45.1 (1.1) | 50.5 (0.9) | <0.001 |
| Female, % | 54.9 | 45.5 | 0.03 |
| Race/ethnicity, % | 0.02 | ||
| Non-Hispanic White | 66.5 | 63.9 | |
| Non-Hispanic Black | 10.4 | 6.8 | |
| Hispanic | 14 | 20.6 | |
| Other | 9.1 | 8.7 | |
| Ever smoker, % | 34.9 | 45.3 | 0.004 |
| Ever drink alcohol, % | 93.6 | 90.6 | 0.19 |
| Physical activity, min/day | 858.2 (71.7) | 865.9 (73.6) | 0.91 |
| BMI, kg/m2 | 27.1 (0.4) | 33.9 (0.4) | <0.001 |
| Waist circumference, cm | 93.6 (1.0) | 111.7 (1.2) | <0.001 |
| History of cancer, % | 8.8 | 9.1 | 0.87 |
| Diagnosed diabetes, % | 5.1 | 18.2 | <0.001 |
| History of hypertension, % | 20.2 | 42.7 | <0.001 |
| History of liver diseases, % | 2.2 | 5.5 | 0.05 |
| Aspirin user, % | 16.2 | 28.6 | <0.001 |
| High-fat diet, %∗ | 55.1 | 54.9 | 0.08 |
Values are weighted mean (SD) for continuous variables and weighted percentage for categorical variables; p values were derived from Student’s t test for continuous variables and the Chi-square test for categorical variables.
FLD, fatty liver disease; NHANES, National Health and Nutrition Examination Survey; PFAS, per- and polyfluoroalkyl substances.
High-fat diet was defined as ≥35% total energy from fat intake.
Table 2 presents the geometric mean levels of PFAS and liver function biomarkers by FLD status. The levels of all the PFAS except PFDA were higher in participants with FLD than in those without FLD although only PFHpS reached statistical significance (0.26 ± 0.02 vs. 0.22 ± 0.02 ng/ml; p = 0.02). All liver function biomarkers except total bilirubin differed marginally or significantly between the two groups. Participants with FLD had higher ALT, GGT, ALP, and hs-CRP, but lower albumin (all p <0.05), than participants without FLD.
Table 2.
Serum levels of PFAS and liver function biomarkers according to FLD risk in the NHANES 2017–2018.
| Non-FLD |
FLD risk |
p value | |
|---|---|---|---|
| n = 689 | n = 446 | ||
| PFAS | |||
| PFOS, ng/ml | 4.52 (0.18) | 4.64 (0.25) | 0.66 |
| PFOA, ng/ml | 1.49 (0.06) | 1.49 (0.09) | 0.98 |
| PFHxS, ng/ml | 1.10 (0.04) | 1.18 (0.07) | 0.13 |
| PFNA, ng/ml | 0.43 (0.03) | 0.44 (0.04) | 0.57 |
| PFHpS, ng/ml | 0.22 (0.02) | 0.25 (0.02) | 0.02 |
| PFDA, ng/ml | 0.21 (0.01) | 0.19 (0.01) | 0.06 |
| Total PFAS, ng/ml | 8.60 (0.31) | 8.97 (0.40) | 0.38 |
| Liver function biomarkers | |||
| AST, U/L | 19.86 (0.37) | 21.02 (0.46) | 0.01 |
| ALT, U/L | 17.80 (0.56) | 23.77 (0.89) | <0.001 |
| ALP, IU/L | 70.36 (1.74) | 78.43 (1.95) | 0.01 |
| GGT, U/L | 19.18 (0.67) | 29.26 (1.37) | <0.001 |
| Total bilirubin, mg/dl | 6.84 (0.25) | 6.97 (0.31) | 0.71 |
| hs-CRP, mg/L | 1.54 (0.10) | 3.04 (0.29) | <0.001 |
| Albumin, g/dl | 4.12 (0.03) | 4.06 (0.03) | 0.04 |
Values are weighted geometric mean (SD); p values were derived from the weighted linear logistic regression using log-transformed values. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; hs-CRP, high-sensitivity C-reactive protein; NAFLD, non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoate; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
PFAS and FLD, according to alcohol intake status
In the crude models, PFHxS and PFHpS were positively associated with FLD risk; however, after adjustment, only PFHxS was significantly associated with a higher FLD risk. The OR of per log-transformed SD increase (ORSD) in PFHxS was 1.13 (95% CI 1.01–1.26; p trend = 0.03; Table 3).
Table 3.
Associations between serum PFAS and fatty liver disease risk, stratified by alcohol intake status.
| Tertile 1 (lowest) | Tertile 2 | Tertile 3 (highest) | p trend | Per log SD | |
|---|---|---|---|---|---|
| PFOS | |||||
| Median (ng/ml) | 2.1 | 4.9 | 10.3 | ||
| Case/non-case n | 142/237 | 155/222 | 149/230 | ||
| Crude model | 1 (reference) | 1.06 (0.70–1.60) | 1.20 (0.72–2.02) | 0.66 | 1.03 (0.90–1.17) |
| Adjusted model | 1 (reference) | 1.17 (0.75–1.80) | 1.22 (0.66–2.23) | 0.80 | 1.02 (0.86–1.21) |
| Never/light drinkers (n = 923) | 1 (reference) | 0.77 (0.41–1.47) | 0.85 (0.42–1.74) | 0.62 | 0.95 (0.77–1.17) |
| Heavy drinkers (n = 212) | 1 (reference) | 4.17 (1.08–16.2) | 4.72 (0.73–30.6) | 0.17 | 1.47 (0.84–2.57) |
| PFOA | |||||
| Median (ng/ml) | 0.8 | 1.5 | 2.7 | ||
| Case/non-case n | 148/249 | 169/226 | 129/214 | ||
| Crude model | 1 (reference) | 1.26 (0.81–1.98) | 0.91 (0.62–1.33) | 0.98 | 1.00 (0.82–1.21) |
| Adjusted model | 1 (reference) | 1.26 (0.79–2.03) | 1.07 (0.63–1.83) | 0.67 | 1.04 (0.86–1.27) |
| Never/light drinkers | 1 (reference) | 1.10 (0.66–1.82) | 0.94 (0.56–1.57) | 0.53 | 0.93 (0.75–1.16) |
| Heavy drinkers | 1 (reference) | 2.37 (0.62–9.03) | 2.07 (0.50–8.58) | 0.03 | 1.79 (1.07–2.99) |
| PFHxS | |||||
| Median (ng/ml) | 0.5 | 1.2 | 2.4 | ||
| Case/non-case n | 121/230 | 191/248 | 134/211 | ||
| Crude model | 1 (reference) | 1.43 (0.85–2.39) | 1.26 (0.89–1.77) | 0.09 | 1.07 (0.98–1.15) |
| Adjusted model | 1 (reference) | 1.45 (0.80–2.60) | 1.34 (0.84–2.13) | 0.03 | 1.13 (1.01–1.26) |
| Never/light drinkers | 1 (reference) | 1.28 (0.62–2.66) | 1.07 (0.63–1.80) | 0.76 | 1.02 (0.90–1.15) |
| Heavy drinkers | 1 (reference) | 2.41 (0.97–5.96) | 6.12 (0.76–49.1) | 0.01 | 2.06 (1.17–3.65) |
| PFNA | |||||
| Median (ng/ml) | 0.2 | 0.5 | 0.9 | ||
| Case/non-case n | 155/244 | 152/235 | 139/210 | ||
| Crude model | 1 (reference) | 0.98 (0.70–1.37) | 0.95 (0.61–1.49) | 0.56 | 1.04 (0.90–1.20) |
| Adjusted model | 1 (reference) | 0.91 (0.57–1.45) | 0.92 (0.54–1.54) | 0.66 | 1.04 (0.89–1.21) |
| Never/light drinkers | 1 (reference) | 0.75 (0.44–1.28) | 0.75 (0.42–1.33) | 0.96 | 1.00 (0.84–1.21) |
| Heavy drinkers | 1 (reference) | 1.74 (0.56–5.43) | 1.89 (0.49–7.27) | 0.40 | 1.21 (0.78–1.90) |
| PFHpS | |||||
| Median (ng/ml) | 0.1 | 0.2 | 0.6 | ||
| Case/non-case n | 117/231 | 167/248 | 162/210 | ||
| Crude model | 1 (reference) | 1.22 (0.85–1.74) | 1.63 (1.12–2.39) | 0.06 | 1.11 (0.99–1.24) |
| Adjusted model | 1 (reference) | 1.37 (0.83–2.26) | 1.24 (0.72–2.12) | 0.51 | 1.05 (0.91–1.20) |
| Never/light drinkers | 1 (reference) | 1.13 (0.77–1.65) | 1.03 (0.64–1.67) | 0.94 | 1.00 (0.87–1.14) |
| Heavy drinkers | 1 (reference) | 3.18 (0.67–15.0) | 3.90 (0.92–16.5) | 0.05 | 1.44 (1.00–2.07) |
| PFDA | |||||
| Median (ng/ml) | 0.2 | 0.3 | 0.5 | ||
| Case/non-case n | 313/445 | 79/112 | 54/132 | ||
| Crude model | 1 (reference) | 1.02 (0.63–1.66) | 0.58 (0.36–0.94) | 0.08 | 0.81 (0.63–1.05) |
| Adjusted model | 1 (reference) | 1.22 (0.67–2.21) | 0.86 (0.52–1.42) | 0.20 | 0.90 (0.76–1.06) |
| Never/light drinkers | 1 (reference) | 1.17 (0.64–2.16) | 0.86 (0.44–1.69) | 0.15 | 0.85 (0.68–1.06) |
| Heavy drinkers | 1 (reference) | 1.26 (0.25–6.40) | 0.89 (0.27–2.90) | 0.39 | 1.23 (0.77–1.97) |
| Total PFAS | |||||
| Median (ng/ml) | 4.5 | 9.1 | 17.2 | ||
| Case/non-case n | 142/234 | 161/225 | 143/230 | ||
| Crude model | 1 (reference) | 1.05 (0.67–1.67) | 1.04 (0.64–1.68) | 0.37 | 1.06 (0.92–1.24) |
| Adjusted model | 1 (reference) | 1.18 (0.70–2.00) | 1.08 (0.59–1.99) | 0.36 | 1.09 (0.91–1.32) |
| Never/light drinkers | 1 (reference) | 0.66 (0.39–1.10) | 0.78 (0.39–1.55) | 0.87 | 0.98 (0.79–1.22) |
| Heavy drinkers | 1 (reference) | 7.51 (1.81–31.2) | 3.30 (0.84–13.1) | 0.05 | 2.12 (0.99–4.54) |
Logistic regression models were used.
In 212 heavy drinkers, PFOA, PFHxS, and PFHpS were positively associated with AFLD risk (Table 3). The multivariable-adjusted ORSD was 1.79 (95% CI 1.07–2.99; p trend = 0.03) for PFOA, 2.06 (95% CI 1.17–3.65; p trend = 0.01) for PFHxS, and 1.44 (95% CI 1.00–2.07; p trend = 0.05) for PFHpS. Total PFAS were marginally associated with a higher AFLD risk (ORSD 2.12; 95% CI 0.99–4.54; p trend = 0.05). In contrast, in 923 never or light drinkers, no statistically significant association was found between PFAS and NAFLD risk (Table 3). Detailed stratified results by alcohol status are presented in Table S2.
For advanced liver fibrosis, PFOA and PFHxS were associated with more severe fibrosis stages only in heavy drinkers (Table S3). In the ordinal logistic regression analysis, The ORSD was 1.75 (95% CI 1.10–2.79) for PFOA and 1.61 (95% CI 1.10–2.35) for PFHxS. For total PFAS, the positive association was marginally significant (ORSD 1.74; 95% CI 0.98–3.11).
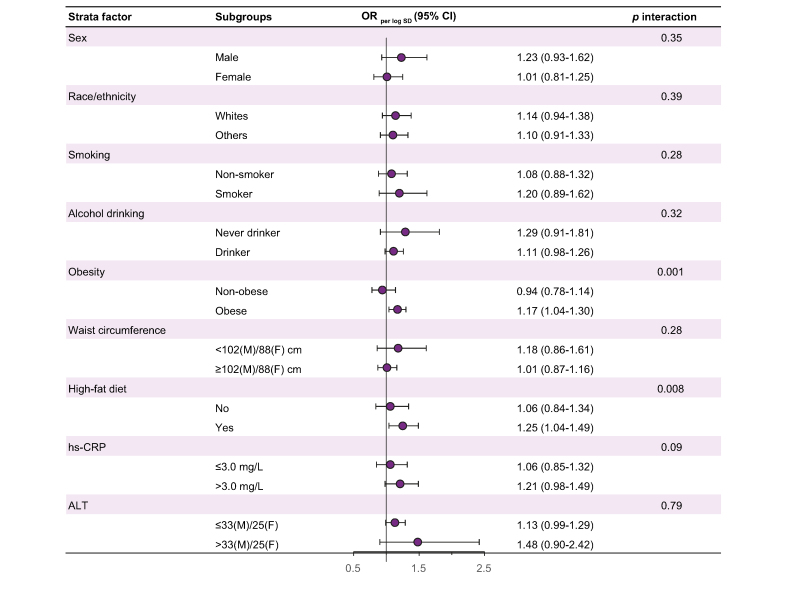
When we further examined the association between PFAS (e.g. PFHxS) and total FLD risk in the stratified analyses, stronger associations were found among participants with obesity (ORSD 1.20; 95% CI 1.03–1.41; p interaction = 0.001) and high-fat diet (ORSD 1.29; 95% CI 0.99–1.67; p interaction = 0.008) and marginally with high chronic inflammation (ORSD 1.21; 95% CI 0.98–1.49; p interaction = 0.09; Fig. 1). No significant interaction was observed between PFHxS and sex, race/ethnicity, smoking status, waist circumference, and ALT (p interaction >0.05 for all; Fig. 1), nor was there between other PFAS and the stratification factors (p interaction >0.05 for all except PFOA and chronic inflammation; Table S4).
Fig. 1.
Stratified results for associations between serum PFHxS and fatty liver disease risk.
Model was adjusted for age group (20 to <40, 40 to <60, or ≥60 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other), ever smoker (yes or no), ever drank alcohol (yes or no), physical activity (min/day), BMI (kg/m2), history of diabetes (yes or no), cancer (yes or no), hypertension (yes or no), aspirin use (yes or no), and high-fat diet (yes or no), while excluding the corresponding strata factor in each analysis. Logistic regression models were used. ALT, alanine aminotransferase; F, female; hs-CRP, high-sensitivity C-reactive protein; M, male; OR, odds ratio; PFHxS, perfluorohexane sulfonate.
For PFHxS and total FLD, after excluding 98 participants with a history of cancer, the point estimate became slightly higher (e.g. ORSD 1.20; 95% CI 1.04–1.37; p trend = 0.01). After excluding 164 participants with albuminuria, the point estimate also became higher (e.g. ORSD 1.25; 95% CI 1.06–1.47; p trend = 0.009; Table S5).
PFAS and liver function biomarkers
The multivariable-adjusted model of the association between PFAS and the liver function biomarkers showed positive linear associations with AST, GGT, bilirubin, and albumin, but not with ALT, ALP, or hs-CRP. Significant (p <0.05) positive associations were found for PFOS with total bilirubin (β = 0.058), PFOA with GGT (β = 0.090) and bilirubin (β = 0.101), PFHxS with AST (β = 0.045), PFNA with GGT (β = 0.078), and total PFAS with bilirubin (β = 0.084). Total and all individual PFAS substances were positively associated with albumin (β ranged from 0.008 to 0.027, all p <0.05; Table 4). When limiting the analysis to participants considered obese, PFOA and total PFAS were inversely associated with hs-CRP (β = -0.204 and -0.204, respectively), and PFHpS was positively associated with ALT (β = 0.089; all p <0.05; Table S6). Additional analyses suggested positive associations between PFAS and HDL, total cholesterol, and haemoglobin A1c levels, and inverse associations with insulin (Table S7).
Table 4.
Linear regression coefficients β (denoted by significance) of log-transformed PFAS and liver function biomarkers.
| AST | ALT | ALP | GGT | Bilirubin | hs-CRP | Albumin | |
|---|---|---|---|---|---|---|---|
| PFOS | |||||||
| Crude model | 0.050† | 0.051∗ | 0.010 | 0.062 | 0.149† | -0.168∗ | 0.018† |
| Adjusted model | 0.029 | 0.026 | 0.004 | 0.019 | 0.058∗ | -0.06 | 0.017† |
| PFOA | |||||||
| Crude model | 0.074∗ | 0.071∗ | 0.013 | 0.092∗ | 0.175∗ | -0.182∗ | 0.028† |
| Adjusted model | 0.057 | 0.057 | 0.015 | 0.090∗ | 0.101∗ | -0.049 | 0.027∗ |
| PFHxS | |||||||
| Crude model | 0.069† | 0.084∗ | -0.005 | 0.072 | 0.161† | -0.169∗ | 0.027† |
| Adjusted model | 0.045∗ | 0.048 | <0.001 | 0.034 | 0.057 | -0.048 | 0.023† |
| PFNA | |||||||
| Crude model | 0.042∗ | 0.038 | 0.037∗ | 0.079∗ | 0.068 | -0.057 | 0.012∗ |
| Adjusted model | 0.041 | 0.045 | 0.031 | 0.078∗ | 0.035 | -0.010 | 0.015∗ |
| PFHpS | |||||||
| Crude model | 0.024 | 0.055∗ | 0.008 | 0.044 | 0.078∗ | -0.018 | 0.009∗ |
| Adjusted model | 0.003 | 0.018 | -0.004 | -0.009 | 0.021 | 0.012 | 0.008∗ |
| PFDA | |||||||
| Crude model | 0.022 | 0.004 | -0.009 | 0.052 | 0.050 | -0.254∗ | 0.014∗ |
| Adjusted model | 0.023 | 0.036 | -0.007 | 0.095 | 0.040 | -0.154 | 0.015∗ |
| Total PFAS | |||||||
| Crude model | 0.069† | 0.080∗ | 0.012 | 0.097∗ | 0.188† | -0.199∗ | 0.026† |
| Adjusted model | 0.045 | 0.051 | 0.010 | 0.059 | 0.084∗ | -0.069 | 0.026† |
Adjusted model included age group (20 to <40, 40 to <60, or ≥60 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other), ever smoker (yes or no), ever drink alcohol (yes or no), physical activity (min/day), BMI (kg/m2), history of diabetes (yes or no), cancer (yes or no), hypertension (yes or no), aspirin use (yes or no), and high-fat diet (yes or no). ∗p <0.05. †p <0.001. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; hs-CRP, high-sensitivity C-reactive protein; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoate; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Discussion
This study in a 2017–2018 US nationally representative survey found that serum PFAS levels were associated with higher FLD risk and altered liver function in adults aged 20 years or older. Specifically, a higher PFHxS level was associated with a significantly higher FLD risk, especially among individuals who had obesity, high-fat diet, and chronic inflammation. When stratified by alcohol intake status in an otherwise fully adjusted model, total PFAS, PFOA, PFHxS, and PFHpS were associated with a significantly higher AFLD risk and more severe fibrosis stage in heavy drinkers, whereas no association was found for NAFLD in never or light drinkers. Serum AST, GGT, bilirubin, and albumin were positively associated with PFAS, whereas hs-CRP was inversely associated with PFAS.
Comparison with previous studies
Few studies to date have examined the interplay between PFAS and alcohol on FLD. We for the first time reported that in heavy drinkers who consumed ≥2 or 3 drinks/day, PFAS, especially PFOA, PFHxS, and PFHpS, were associated with a higher risk of VCTE-diagnosed AFLD, not NAFLD. In heavy drinkers, PFAS, especially PFOA and PFHxS, were also associated with advanced liver fibrosis risk. Stronger associations of PFAS with total FLD were found in participants with obesity, high-fat diets, and chronic inflammation, which are potential risk factors for chronic liver diseases. This finding is potentially consistent with previous NHANES work showing that as serum PFAS exposure ranges decline over time in successive NHANES surveys, the association of serum PFAS to liver transaminases is most easily seen in obese individuals.28
Our results indicated that a higher level of serum PFHxS was associated with higher odds of total FLD, although in the stratified analysis, this association remained only for AFLD but not for NAFLD. A previous study in children with NAFLD found that PFAS, especially PFHxS, was positively associated with the biopsy severity of NAFLD disease.29 PFHxS is the third most commonly detected PFAS, following PFOS and PFOA, and one of the most long-lasting PFAS in humans.30 Global action in phasing our PFHxS is urged.31 We did not find statistically significant associations for the total level or other individual types of PFAS with total FLD or NAFLD in this study. This finding is consistent with a previous study using NAFLD cases identified by HSI and USFLI scores in the 1999–2014 NHANES, which found that the sum of eight blood PFAS levels was not associated with NAFLD risk (multivariable-adjusted OR 0.99; 95% CI 0.90–1.08).15 Compared with that study, we included more comprehensive analyses of individual PFAS and used VCTE-diagnosed NAFLD to minimise misclassification of NAFLD risk. Collectively, the current epidemiological studies on PFAS and NAFLD risk based on biopsy or non-invasive measures of liver stiffness such as VCTE is limited. Evaluation of populations with known higher exposures than the general public as revealed by NHANES data may assist this process.32
Consistent with our results, studies in the earlier NHANES cycles found that higher levels of certain PFAS were associated with biomarkers of liver injury; for example, PFOS, PFOA, PFHxS, and PFNA were associated with ALT, GGT, and bilirubin.[12], [13], [14] A recent dose–response systematic review of both rodent and human studies and meta-analysis on human liver enzymes detected associations between PFOA, PFOS, and PFNA and ALT, AST, and GGT, suggesting a contribution of PFAS to the growing human NAFLD burden.11 This review mentioned insufficient evidence for PFHxS and other less common PFAS, whereas our study associates PFHxS directly to liver steatosis and adds to the evidence that PFAS with long half-lives are hepatotoxic.
Biological mechanisms
The liver is considered an important target organ for exposure to exogenous chemicals and for PFAS accumulation.33,34 Mechanistic research regarding the PFAS contribution to liver malfunction and steatosis led to the following hypotheses. First, PFAS may alter hepatic lipid, amino acid, and carbohydrate metabolism.11,[35], [36], [37], [38], [39] In the stratified analysis, we found that the positive association between PFHxS and FLD risk was stronger in people with obesity and also those with a high-fat diet. Similarly, in the 2011–2014 NHANES, PFOA, PFHxS, and PFNA were associated with higher serum liver function biomarkers but only among obese participants,14 whereas the transaminase associations pertain to the entire NHANES population in earlier survey cycles characterised by the higher serum PFAS.13,40 We thus hypothesised that PFAS-mediated lipid perturbations could contribute to an elevated FLD risk, especially in a metabolically unhealthy stage. Second, PFAS may directly interact with the liver and alter hepatic metabolism even in the absence of comorbid risk factors. Changes in the cytochrome P450 pathway, cytokeratin C18 biomarkers, peroxisome proliferator-activated receptors α and γ, and fatty acid transporters fatty acid translocase (Cd36) levels were associated with PFAS exposures.32,33,41 Third, based on our finding of a more robust association between PFAS and AFLD in heavy drinkers, PFAS could interact with the alcohol metabolism in the liver, although very few studies to date provide direct evidence on possible mechanisms.
Last, PFAS exposure may alter the inflammatory response, and the definitive mechanisms of immunotoxicity remain controversial. Interestingly, PFAS may exert anti-inflammatory effects, for example, decreasing cytokine release from cells through activation of NF-κB and limiting leucocyte chemotaxis. PFAS exposure was associated with lower levels of CRP in previous studies.17,42 Based on 2005–2012 NHANES data, PFAS was positively associated with bilirubin (anti-inflammatory) and inversely associated with CRP (pro-inflammatory),18 which was consistent with our findings. Meanwhile, we found that hs-CRP was higher in NAFLD. Higher hs-CRP was commonly reported as a risk factor for NAFLD,43 and mildly increased bilirubin has been inversely associated with NAFLD risk in a few epidemiological studies.[44], [45], [46] These paradoxical observations require further studies into the interplay of PFAS, inflammation, immunity, oxidative stress, and steatosis.47 In vitro data may provide useful and complementary insights in this regard.
Owing to the cross-sectional design of this study, we cannot rule out the possibility that impaired liver function might reversely affect PFAS excretion, especially in heavy alcohol drinkers. PFAS can also be found in consumed beverages including alcoholic beverages as well as in foods.48 Although longitudinal study also supports the relationship of PFAS exposure to undesirable changes in serum transaminases,49 to date, no prospective study has investigated PFAS and incident FLD risk score or confirmed biopsy results. Another knowledge gap in the mechanistic research is how different PFAS might impact inflammation and degree of steatosis in the liver. The mechanisms underlying the association between PFAS exposure and liver health would benefit from further exploration.
Public health implications
Countries such as the United States have initiated strategic plans to gradually phase out PFAS, especially long-chain PFAS.17 From 1999–2000 to 2017–2018, the blood PFOS and PFOA levels of the US population based on NHANES declined by more than 70%.35 However, these chemicals remain in use worldwide, reside in innumerable available products,7 and are detectable in nearly all US adults and may persist in vivo for an extended period, with estimated half-lives of 2–6 years in the human body.30 Our study showed that the association with liver function persists at lower-level exposures of PFAS in the population over the past decade. Owing to the obesity epidemic and increasing prevalence of diabetes, steatosis may be the most prevalent pathology associated with end-stage liver diseases including cirrhosis and liver cancer, as well as chronic liver disease mortality. Continuously monitoring PFAS in the population and examining how they potentiate risk to the liver is therefore essential.
Moreover, our study for the first time showed different risk stratification for FLD in people who are heavy alcohol drinkers, had obesity, had high-fat diet, or had chronic inflammation. Special care and prevention could be suggested to these high-risk populations.
Strengths and limitations
This is the first study on the association between PFAS and FLD risk measured and diagnosed by VCTE. Strengths included a large representative sample, a comprehensive panel of liver function biomarkers, well-validated detection and quantification of steatosis using VCTE,21,23,24 and stratification analyses considering personal demographics, lifestyle factors, and liver-related metabolic factors. However, limitations of this study merit consideration. First, as discussed before, this is a cross-sectional study with inherited study design limitations. Second, residual confounders associated with PFAS exposures, for example, environmental exposure to other liver toxicants,6,15 were not controlled in the models. Third, NHANES is a representative sample of the US population, yet owing to the differences in PFAS exposure levels and the FLD epidemic worldwide, these results may not be able to be generalised to other populations with diverse racial/ethnic groups, or different aetiologies or causes of liver disease.
Conclusions
In the general population, PFAS, especially PFHxS, were moderately associated with FLD risk and impaired liver functions. Importantly, among those with independent lifestyle risk factors for hepatic steatosis, such as heavy alcohol intake, obesity, high-fat diet, and chronic inflammation, PFAS compounded that risk. PFAS, especially PFOA, PFHxS, and PFHpS, appeared to be a risk factor for potentially alcohol-attributable FLD risk and advanced liver fibrosis in heavy drinkers, suggesting that there might be synergistic effects on FLD between PFAS and lifestyle risk factors, especially alcohol intake.
Financial support
Dr. Xuehong Zhang is supported by National Cancer Institute U01 CA259208-01A1, American Cancer Society Research Scholar Grant (RSG NEC-130476), and American Cancer Society Interdisciplinary Team Award (PASD-22-1003396-01).
Authors' contributions
Conceptualised and designed the study: Xinyuan Zhang, LZ, Xuehong Zhang. Analysed the data: LZ. Reviewed the data analyses: Xinyuan Zhang and CD. Drafted the manuscript: Xinyuan Zhang. Revised the manuscript and made critical intellectual contribution to the study: all authors.
Data availability statement
The NHANES data used in this study are publicly available (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm).
Conflicts of interest
AD has provided volunteer and paid consultation support for populations with PFAS-contaminated water that seek medical monitoring.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100694.
Values presented from the models are odds ratio (95% CI). Adjusted model included age group (20 to <40, 40 to <60, or ≥60 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other), ever smoker (yes or no), ever drink alcohol (yes or no), physical activity (min/day), BMI (kg/m2), history of diabetes (yes or no), cancer (yes or no), hypertension (yes or no), aspirin use (yes or no), and high-fat diet (yes or no). Ever drink alcohol was not adjusted among heavy drinkers. Values of p trend were calculated using log-transformed continuous exposure variables. PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoate; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J., Shield K.D. Global burden of alcohol use disorders and alcohol liver disease. Biomedicines. 2019;7:99. doi: 10.3390/biomedicines7040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik J.M., Golabi P., Biswas R., Alqahtani S., Venkatesan C., Younossi Z.M. Nonalcoholic fatty liver disease and alcoholic liver disease are major drivers of liver mortality in the United States. Hepatol Commun. 2020;4:890–903. doi: 10.1002/hep4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanyal A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 6.Cave M., Appana S., Patel M., Falkner K.C., McClain C.J., Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118:1735–1742. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glüge J., Scheringer M., Cousins I.T., DeWitt J.C., Goldenman G., Herzke D., et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS) Environ Sci Process Impacts. 2020;22:2345–2373. doi: 10.1039/d0em00291g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha B., Schymanski E.L., Ruttkies C., Cousins I.T., Wang Z. Exploring open cheminformatics approaches for categorizing per- and polyfluoroalkyl substances (PFASs) Environ Sci Process Impacts. 2019;21:1835–1851. doi: 10.1039/c9em00321e. [DOI] [PubMed] [Google Scholar]
- 9.Evich M.G., Davis M.J.B., McCord J.P., Acrey B., Awkerman J.A., Knappe D.R.U., et al. Per- and polyfluoroalkyl substances in the environment. Science. 2022;375 doi: 10.1126/science.abg9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodrich J.A., Walker D., Lin X., Wang H., Lim T., McConnell R., et al. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello E., Rock S., Stratakis N., Eckel S.P., Walker D.I., Valvi D., et al. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect. 2022;130 doi: 10.1289/EHP10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C.Y., Lin L.Y., Chiang C.K., Wang W.J., Su Y.N., Hung K.Y., et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol. 2010;105:1354–1363. doi: 10.1038/ajg.2009.707. [DOI] [PubMed] [Google Scholar]
- 13.Gleason J.A., Post G.B., Fagliano J.A. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res. 2015;136:8–14. doi: 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Jain R.B., Ducatman A. Selective associations of recent low concentrations of perfluoroalkyl substances with liver function biomarkers: NHANES 2011 to 2014 data on US adults aged ≥20 years. J Occup Environ Med. 2019;61:293–302. doi: 10.1097/JOM.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 15.Li W., Xiao H., Wu H., Pan C., Deng K., Xu X., et al. Analysis of environmental chemical mixtures and nonalcoholic fatty liver disease: NHANES 1999–2014. Environ Pollut. 2022;311 doi: 10.1016/j.envpol.2022.119915. [DOI] [PubMed] [Google Scholar]
- 16.Kwo P.Y., Cohen S.M., Lim J.K. ACG clinical guideline: evaluation of abnormal liver Chemistries. Am J Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 17.Genser B., Teles C.A., Barreto M.L., Fischer J.E. Within- and between-group regression for improving the robustness of causal claims in cross-sectional analysis. Environ Health. 2015;14:60. doi: 10.1186/s12940-015-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omoike O.E., Pack R.P., Mamudu H.M., Liu Y., Strasser S., Zheng S., et al. Association between per and polyfluoroalkyl substances and markers of inflammation and oxidative stress. Environ Res. 2021;196 doi: 10.1016/j.envres.2020.110361. [DOI] [PubMed] [Google Scholar]
- 19.Vilar-Gomez E., Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Sasso M., Beaugrand M., de Ledinghen V., Douvin C., Marcellin P., Poupon R., et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui M.S., Vuppalanchi R., Van Natta M.L., Hallinan E., Kowdley K.V., Abdelmalek M., et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–163.e152. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cusi K., Isaacs S., Barb D., Basu R., Caprio S., Garvey W.T., et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD) Endocr Pract. 2022;28:528–562. doi: 10.1016/j.eprac.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Heredia N.I., Zhang X., Balakrishnan M., Daniel C.R., Hwang J.P., McNeill L.H., et al. Physical activity and diet quality in relation to non-alcoholic fatty liver disease: a cross-sectional study in a representative sample of U.S. adults using NHANES 2017–2018. Prev Med. 2022;154 doi: 10.1016/j.ypmed.2021.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilar-Gomez E., Nephew L.D., Vuppalanchi R., Gawrieh S., Mladenovic A., Pike F., et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. 2022;75:1491–1506. doi: 10.1002/hep.32207. [DOI] [PubMed] [Google Scholar]
- 25.Johnson C.L., Paulose-Ram R., Ogden C.L., Carroll M.D., Kruszon-Moran D., Dohrmann S.M., et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 26.Jain R.B., Ducatman A. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ Res. 2019;174:143–151. doi: 10.1016/j.envres.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Lin P.D., Cardenas A., Hauser R., Gold D.R., Kleinman K.P., Hivert M.F., et al. Per- and polyfluoroalkyl substances and kidney function: follow-up results from the Diabetes Prevention Program trial. Environ Int. 2021;148 doi: 10.1016/j.envint.2020.106375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain R.B., Ducatman A. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci Total Environ. 2019;653:74–81. doi: 10.1016/j.scitotenv.2018.10.362. [DOI] [PubMed] [Google Scholar]
- 29.Jin R., McConnell R., Catherine C., Xu S., Walker D.I., Stratakis N., et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: an untargeted metabolomics approach. Environ Int. 2020;134 doi: 10.1016/j.envint.2019.105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Fletcher T., Mucs D., Scott K., Lindh C.H., Tallving P., et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75:46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockholm convention on persistent organic pollutants Report of the Persistent Organic Pollutants Review Committee on the work of its fourteenth meeting. (Addendum) Risk profile on perfluorohexane sulfonic acid (PFHxS), its salts and PFHxS-related compounds; 2018. http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC14/Overview/tabid/7398/Default.aspx. Accessed 16 November 2022.
- 32.Fenton S.E., Ducatman A., Boobis A., DeWitt J.C., Lau C., Ng C., et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2021;40:606–630. doi: 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong L.E., Guo G.L. Understanding environmental contaminants’ direct effects on non-alcoholic fatty liver disease progression. Curr Environ Health Rep. 2019;6:95–104. doi: 10.1007/s40572-019-00231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez F., Nadal M., Navarro-Ortega A., Fàbrega F., Domingo J.L., Barceló D., et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z., Yang T., Walker D.I., Thomas D.C., Qiu C., Chatzi L., et al. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ Int. 2020;145 doi: 10.1016/j.envint.2020.106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon E.D., Nardo A.D., Claudel T., Trauner M. The role of lipid sensing nuclear receptors (PPARs and LXR) and metabolic lipases in obesity, diabetes and NAFLD. Genes (Basel) 2021;12:645. doi: 10.3390/genes12050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen P., Qadri S., Luukkonen P.K., Ragnarsdottir O., McGlinchey A., Jäntti S., et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J Hepatol. 2022;76:283–293. doi: 10.1016/j.jhep.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Ho S.H., Soh S.X.H., Wang M.X., Ong J., Seah A., Wong Y., et al. Perfluoroalkyl substances and lipid concentrations in the blood: a systematic review of epidemiological studies. Sci Total Environ. 2022;850 doi: 10.1016/j.scitotenv.2022.158036. [DOI] [PubMed] [Google Scholar]
- 39.Fragki S., Dirven H., Fletcher T., Grasl-Kraupp B., Bjerve Gützkow K., Hoogenboom R., et al. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: what do we know and what not? Crit Rev Toxicol. 2021;51:141–164. doi: 10.1080/10408444.2021.1888073. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention, U.S. National Report on Human Exposure to Environmental Chemicals. Updated March 2022. Department of Health and Human Services 2022. https://www.cdc.gov/exposurereport. Accessed 16 November 2022.
- 41.Kirk A.B., Michelsen-Correa S., Rosen C., Martin C.F., Blumberg B. PFAS and potential adverse effects on bone and adipose tissue through interactions with PPARγ. Endocrinology. 2021;162:bqab194. doi: 10.1210/endocr/bqab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salihovic S., Lind L., Larsson A., Lind P.M. Plasma perfluoroalkyls are associated with decreased levels of proteomic inflammatory markers in a cross-sectional study of an elderly population. Environ Int. 2020;145 doi: 10.1016/j.envint.2020.106099. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R., Porwal Y.C., Dev N., Kumar P., Chakravarthy S., Kumawat A. Association of high-sensitivity C-reactive protein (hs-CRP) with non-alcoholic fatty liver disease (NAFLD) in Asian Indians: a cross-sectional study. J Fam Med Prim Care. 2020;9:390–394. doi: 10.4103/jfmpc.jfmpc_887_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vítek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol. 2012;3:55. doi: 10.3389/fphar.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwak M.S., Kim D., Chung G.E., Kang S.J., Park M.J., Kim Y.J., et al. Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2012;18:383–390. doi: 10.3350/cmh.2012.18.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian J., Zhong R., Liu C., Tang Y., Gong J., Chang J., et al. Association between bilirubin and risk of non-alcoholic fatty liver disease based on a prospective cohort study. Sci Rep. 2016;6 doi: 10.1038/srep31006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerra Ruiz A.R., Crespo J., López Martínez R.M., Iruzubieta P., Casals Mercadal G., Lalana Garcés M., et al. Measurement and clinical usefulness of bilirubin in liver disease. Adv Lab Med. 2021;2:352–361. doi: 10.1515/almed-2021-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahl T., Hofmann A., Cöllen M., Falk S., Brunn H. Analysis of selected perfluoroalkyl substances (PFASs) in beer to evaluate the effect of beer consumption on human PFAS exposure: a pilot study. Eur Food Res Technol. 2013;238:443–449. [Google Scholar]
- 49.Salihovic S., Stubleski J., Kärrman A., Larsson A., Fall T., Lind L., et al. Changes in markers of liver function in relation to changes in perfluoroalkyl substances – a longitudinal study. Environ Int. 2018;117:196–203. doi: 10.1016/j.envint.2018.04.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES data used in this study are publicly available (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm).