Highlights
-
•
The use of contrast agents in RT can improve the workflow of radiotherapy planning, both in target delineation and in delivery.
-
•
The use of contrast agent in MRI-guided radiotherapy (MRgRT) using MR Linac is currently not standardised among the various centres equipped with this technology.
-
•
This survey, administered to centres equipped with MR Linac, was developed to understand the use of contrast agent in the MRgRT workflow and future perspectives.
Keywords: Magnetic resonance guided radiation therapy, MRI contrast agents, Radiotherapy planning, Target delineation
Abstract
Aims
The introduction of on-line magnetic resonance image-guided radiotherapy (MRIgRT) has led to an improvement in the therapeutic workflow of radiotherapy treatments thanks to the better visualization of therapy volumes assured by the higher soft tissue contrast. Magnetic Resonance contrast agents (MRCA) could improve the target delineation in on-line MRIgRT planning as well as reduce inter-observer variability and enable innovative treatment optimization protocols. The aim of this survey is to investigate the utilization of MRCA among centres that clinically implemented on-line MRIgRT technology.
Methods
In September 2021, we conducted an online survey consisting of a sixteen-question questionnaire that was distributed to the all the hospitals around the world equipped with MR Linacs. The questionnaire was developed by two Italian 0.35 T and 1.5 T MR-Linac centres and was validated by four other collaborating centres, using a Delphi consensus methodology.
Results
The survey was distributed to 52 centres and 43 centres completed it (82.7%). Among these centres, 23 institutions (53.5%) used the 0.35T MR-Linac system, while the remaining 20 (46.5%) used the 1.5T MR-Linac system.
According to results obtained, 25 (58%) of the centres implemented the use of MRCA for on-line MRIgRT. Gadoxetate (Eovist®; Primovist®) was reported to be the most used MRCA (80%) and liver the most common site of application (58%). Over 70% of responders agreed/strongly agreed to the need for international guidelines.
Conclusions
The use of MRCA in clinical practice presents several pitfalls and future research will be necessary to understand the actual advantage derived from the use of MRCA in clinical practice, their toxicity profiles and better define the need of formulating guidelines for standardising the use of MRCA in MRIgRT workflow.
1. Background
Radiotherapy (RT) represents a crucial component in the management of cancer patients, with nearly 50% of patients undergoing RT during their oncological care path [1].
In the last decades, various technological developments have made RT less invasive and more precise and efficient. The recent introduction of magnetic resonance hybrid Magnetic Resonance linear accelerators (MR Linacs) into clinical practice represents the latest step in this sequence, opening new frontiers in radiation treatment delivery thanks to the advantages in imaging and the potential to influence patient outcomes [2], [3]. The technological advantages offered by MR guided RT (MRIgRT) may therefore improve the likelihood of tumour control and survival, while reducing the risk of treatment-related toxicities [4].
Nevertheless, despite the wide acceptance of online MRIgRT by the radiation oncology community, the most advantageous clinical applications are still far from being fully exploited and practice-changing evidence is still lacking. Two commercial solutions were available at the time of the survey, which have the same functioning principles but differ significantly in their design, both in terms of the magnetic resonance imaging (MRI) scanner and the way of handling the interaction between radiation and the magnetic field [5], [6].
The MRI scanners of these two hybrid devices (0.35T on the MRIdian, ViewRay Inc., USA and 1.5T on the Unity, Elekta SA, Sweden) allow daily acquisition of MR images to support treatment verification and daily plan adaptation (i.e. dose distribution re-optimisation on the patient's daily anatomy), with a more precise and reliable visualisation of the target volumes due to improved soft tissues contrast. Although MRI in principle has excellent soft-tissue contrast, compared to cone-beam CT, visualisation of the target volume can be challenging in some situations. For this reason, despite some limitations mainly related to potential toxicity and increased logistical burden, there has been growing interest for the introduction of MRI contrast agents (MRCA) in the framework of in-room MRIgRT [7], [8].
To date, as only a few studies have described the applications of contrast agents in this innovative setting [9], [10], their use is still not established in clinical practice. In addition, the scientific community is still exploring the actual need for their use, without fully understanding the relative potential benefits and pitfalls.
We conducted this survey to describe the different experiences with the use of contrast agents on MR Linacs around the world and to present the different practices and perspectives on the integration of contrast agents in clinical practice.
The appropriate visualisation of the target volume and organs at risk (OARs) during in-room image guidance for safe RT delivery is essential for all RT treatments. Conventional RT planning based on CT imaging raises several issues related to poor visualisation of soft tissues and the presence and appropriate management of artefacts (e.g. artefacts induced by prostheses or by surgical procedures).
These issues can lead to uncertainties in treatment planning and during image guided radiotherapy (IGRT) (e.g. cone beam CT - CBCT), affecting the reliability of target alignment, and therefore the efficacy of treatment delivery. The use of intravenous contrast (IVC) is a possible way to overcome these limitations, as organ- and tumour-specific contrast agents that can discriminate between soft tissues with similar electronic density and CT imaging properties are available.
Several studies describe the implementation of contrast enhanced images in the target delineation process, resulting in an overall improved accuracy in target definition [5], [6].
The influence of IVC in planning CTs on the dose calculation was investigated for treatment planning in intensity modulated radiation therapy (IMRT) for prostate, rectal and head and neck tumours. The differences between the plans calculated with enhanced and non-enhanced CT were found not to be clinically significant. Therefore, contrast-enhanced CT can be used for both target delineation and treatment planning [7], [8].
The Royal College of Radiologists (RCR) published a report in 2004, suggesting nine tumour sites where contrast agents can be used and proposing its use for a further eleven entities for different imaging modalities [11]. These guidelines also included recommendations on the management of administration and any associated toxicities.
To date, the use of contrast agents in CT-based RT planning is widespread, although not yet standardised in terms of applicability and standardized procedures with variations strongly depending on the expertise, protocols of individual centres and existing national guidelines.
Williams et al in 2016, published a review of the use of CT IV contrast agents in the UK and concluded that there was no specific guideline was followed and overall compliance with RCR guidelines was limited. Furthermore, the use of specific iodine CT contrast agents requires accurate stratification of patients according to an estimated Glomerular Filtration Rate (eGFR) and specific clinical risk factors, as well as the establishment of appropriate protocols for the management of potential side effects (anaphylaxis and allergic reactions, extravasation, renal impairment) [12].
These findings were later confirmed in 2019 by Minogue et al, who conducted a similar survey on clinical practice in Ireland and highlighted the importance of introducing IVC into clinical practice and the training of radiation therapists (RTTs) to cannulate and administer IVC [13].
The recent introduction of MRI simulation and on-line MRIgRT has solved some of the problems associated with the use of CT and treatment delivery by taking advantage of the enhanced soft tissue contrast offered by on-board MR imaging [6], [5].
The use of MRCA for MR Linac based RT could potentially lead to improved characterisation of lesions for more accurate delineation of target volumes and OARs, both in simulation and on-line adaptive treatment steps in different anatomical sites [14], [15], [16], [17].
Furthermore, the application of MRCA may successfully reduce intra- and inter-observer variability [18] and pave the way to innovative dose escalation protocols, taking advantage of online and offline adaptive methods [19].
Magnetic resonance imaging offers numerous sequences that can describe different biological properties of the tissues under investigation. T1-weighted (T1w) and T2-weighted (T2w) imaging reflect the longitudinal (T1w) and transverse (T2w) relaxation time of protons in water and fat in the human body when exposed to an external radiofrequency pulsed magnetic field [20], [21].
Contrast agents in MR are divided according to their magnetic properties, chemical composition, presence or absence of metal atoms, type of administration (intravenous or oral), application, bio-distribution, and according to their characteristics in T1w and T2w sequences [22].
The most common MR contrast agent is a paramagnetic metal nanomaterial/complex originating from gadolinium (III) (Gd3+), which is characterised by stability and a high magnetic moment, causing bright contrast in T1w images, while reducing the signal in T2w images [21], [22], [23].
On the other hand, superparamagnetic nanoparticles (e.g. SPIONs) appear hypointense in T2w images [24]. Gadolinium-based contrast agents (GBCA) consist of a gadolinium ion and a chelating agent that allows them to be distributed throughout the body and prevent toxicity. GBCA are mostly excreted via the kidneys and some of them have the possibility to accumulate in tissues (such as liver, brain, bone and kidney itself), potentially leading to neurological, musculoskeletal and skin symptoms. However, there are currently few reliable data on the consequences of their repeated use, even if initial experiments did not show alterations of their chemical composition when irradiated [25], [26].
GBCA currently approved by FDA and EMA are gadobutrol (Gadavist®), gadopentate (Magnevist®), gadodiamide (Omniscan®), gadoterate (Dotarem®), gadoteridol (Prohance®), gadofosveset (Ablavar®), gadoversetamide (OptiMARK®) and gadotexate (Eovist®) [25].
2. Materials and methods
To better understand the current applications of MRCA in MR Linac clinical practice, we conducted an online survey and invited institutions equipped with hybrid 0.35 or 1.5T MR linear accelerators from different countries worldwide in September 2021. The questionnaire was developed by LB and LN, from two Italian 0.35T and 1.5T MR-Linac centres, respectively.
Prior to distribution, the questionnaire was validated and refined by four collaborating centres (two low T and two high T MR-Linac centres), using a Delphi consensus methodology before being distributed to all other centres [27].
The MRIgRT team leaders of each centre were asked to complete the questionnaire sent by e-mail from the main data supervisors for the two commercial systems, LB and LN.
Overall, one month was planned for the collection of responses and a reminder sent after two weeks for the non-responding centers.
Questionnaire collection closed on October 2021 as planned and all the data collected were analysed in January 2022. The Office suite was used for survey distribution and results analysis.
The survey was distributed to 52 centres across 14 different countries (USA, UK, Germany, South Korea, Israel, Netherlands, Denmark, Japan, Italy, Turkey, Australia, Sweden, France and Canada) worldwide. Of these 52 centres, 43 completed the survey (82.7%).
The questionnaire consisted of 17 questions (see supplementary material), divided into three sections.
The first section involved screening of the centres, covering the type of technology available (e.g. field strength of the MR Linac), the number of years of experience in the field of MRIgRT, and whether the use of MRCA was implemented in clinical practice or not.
The second section assessed the type of MRCA used, the clinical application, the anatomical sites treated, and the procedures used to administer the MRCA.
Finally, respondents were asked for their opinion on the inclusion of MRCA guidelines dedicated to its use within in-room MRIgRT and/or on the need to elaborate recommendations by the major scientific organisations.
3. Results
3.1. First section – General information
Among these centres, 23 hospitals (53.5%) use the 0.35T MR-Linac, while the remaining 20 centres (46.5%) use the 1.5T one.
Of all 43 responding centres, 37 (86%) of these hospitals reported using iodine contrast agents for standard CT-based RT treatments to facilitate contouring or improve image guidance, while the other 6 (14%) do not routinely use iodine contrast agents.
According to reports obtained from this survey, 25 (58%) of the centres implemented the use of MRCA for MRIgRT imaging, while the remaining 18 (42%) avoided its use for several reasons, such as lack of resources, national regulations, hospital policies, lack of trained personnel and concerns regarding the patient’s safety. Among the 25 centres that implemented the use of MRCA, 14 (56%) were centres with the 0.35T Linac and 11 (44%) with the 1.5T Linac, where the use of MRCA is less common.
3.2. Second section – MRCA usage
3.2.1. Type of MRCA
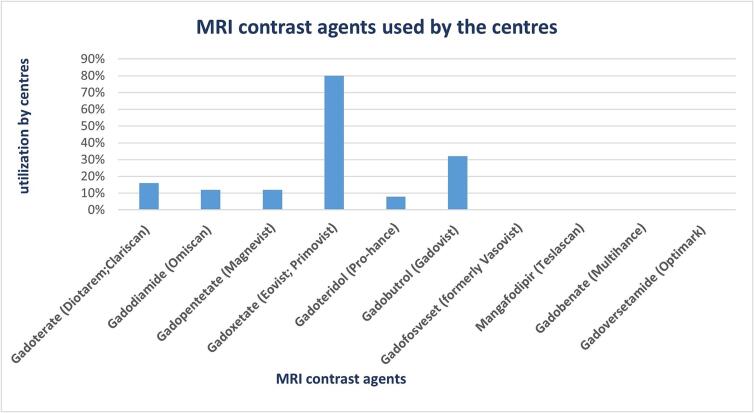
Among the different centres that implemented the use of one or more contrast agents, 80% used Gadoxetate (Eovist;Primovist), making it the most commonly used one. Other agents used included Gadobutrol (Gadovist) (32%), Gadoterate (Diotarem;Clariscan) (16%), Gadodiamide (Omiscan) (12%), Gadopentetate (Magnevist) (12%), Gadoteridol (Pro-hance) (8%).
No centre used Gadofosveset (formerly Vasovist), Gadobenate (Multihance), Gadoversetamide (Optimark) and Mangafodipir (Teslascan) (Fig. 1).
Fig. 1.
A graphical representation of MRI contrast agents used by the different centres.
3.2.2. Anatomical site
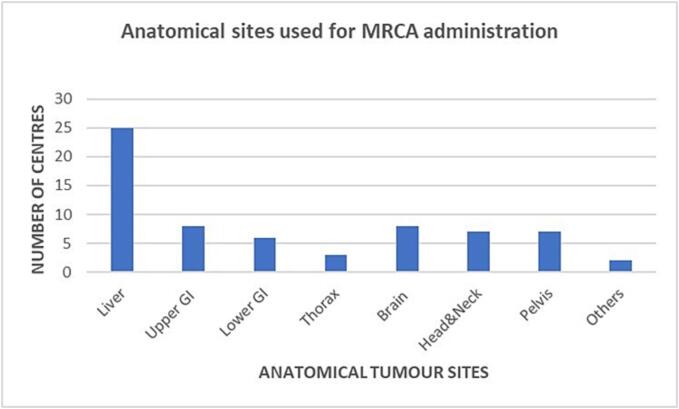
Regarding anatomical sites where contrast agents were used, eight different anatomical sites (liver primary and secondary lesions, upper gastrointestinal tract including pancreas and lower gastrointestinal tract, thorax, brain, head-and-neck, pelvis and other extremities) were mentioned in the survey. Out of all the institutions that used MRCA, only 2/25 (8%) used contrast agents for all indicated anatomical sites; 4/25 (16%) used MRCA for <8 but ≥5 different anatomical sites; 6/25 (24%) departments used MRCA for ≤4 anatomical sites; while most of the departments (12/25; 48%) used MRCA for only one anatomical site, generally represented by the liver (both primary and secondary lesions).
Fig. 2 illustrates the range of anatomical sites where MRCA are administered.
Fig. 2.
The range of tumour sites where MRCA are administered during MRgRT treatment by the different centres. GI: gastrointestinal.
3.2.3. Administration allowance
Regarding the administration of MRCA, this was mostly carried out by either radiation oncologists, RTTs, nurses or other medical doctors (i.e. radiologists). Most centres (n = 13/25) stated that they involved nurses only, as responsible trained personnel for MRCA administration; 4/25 centres appointed radiation oncologists only; 5/25 centres RTTs; and 3/25 centres radiologists only. The survey showed that 8/25 centres involved at least 2 different trained staff to administer MRCA.
3.2.4. MRCA for planning and treatment
All hospitals using MRCA in-room MRIgRT were of the opinion that contrast agents should be administered during in-room MRIgRT simulation, as it could be useful for segmentation and evaluation of tumour visibility, also reducing the burden of diagnostic MRI scanners which are generally used to this end.
Although, 52% of centres agreed that MRCA should only be administered if the target volume was not clearly visible on simulation imaging; 44% suggested that this should be done with a case by case approach; while the remaining 4% stated that it should always be administered.
Furthermore, when asked if MRCA should be administered throughout the in-room MRIgRT treatment, all centres agreed except for one centre, which gave the reason that contrast enhanced simulation imaging was sufficient, without the need of MRCA administration during the treatment.
3.2.5. Safety and surveillance
With regards to safety, it was asked how many centres planned any specific tests (e.g. blood test with renal function) for patients undergoing contrast enhanced MR simulation and whether other surveillance tests (i.e. serial blood tests) were planned for patients receiving MRCA throughout the in room MRIgRT treatment. Overall, 15 centres (60%) reportedly require blood tests with renal function for their patients undergoing contrast enhanced MR simulation, while eight centres (32%) perform blood tests only for high-risk patients and two (8%) centres have no tests scheduled.
In comparison, nine (36%) centres arrange further surveillance renal function tests to monitor patients undergoing MRCA throughout their MRIgRT treatment; four (16%) centres organize this only for high-risk patients; three (12%) centres were uncertain of their practice, as it was dependent on factors such as the length of treatment course and baseline creatinine values. The remaining nine (36%) did not perform any form of surveillance.
3.3. Third section – General opinion and future perspectives
In the third section of the questionnaire, the last three questions were designed to get the general opinions of MR-Linac users concerning the introduction of international guidelines for patient safety. These questions were centered on standardising clinical indications for the use of contrast agents in MRIgRT, the need for the introduction of a minimal set of blood tests when using contrast agents in MRIgRT and the opportunity for international radiation oncology societies to set up working groups to foster the use of MRCA for MRIgRT purposes, whilst working closely with other diagnostic radiology societies. The results of these specific questions are described in Table 1.
Table 1.
The different responses by the 43 participating sites to the last 3 poll questions.
| Questions | Answers |
|||
|---|---|---|---|---|
| Strongly agree | Agree | Disagree | Strongly disagree | |
| Do you think that international guidelines should be introduced to standardize patient’s safety (e.g. minimal set of blood tests) for the use of contrast agents in-room MRIgRT | 12 (28%) | 19 (44%) |
10 (23%) |
2 (5%) |
| Do you think that international guidelines should be introduced to standardize clinical indications for the use of contrast agents in-room MRIgRT? | 12 (28%) |
21 (49%) |
8 (19%) |
2 (5%) |
| Do you think that working groups dedicated to foster the use of MRI contrast agents for in-room MRIgRT purposes should be set up? | 12 (28%) |
22 (51%) |
7 (16%) |
2 (5%) |
MRIgRT: Magnetic Resonance guided radiotherapy; MRI: Magnetic Resonance Imaging;
As reported in Table 1, over 70% of participants agreed or strongly agreed to the need for international guidelines and 28% of the centres strongly agreed to all three questions.
4. Discussion
Despite the advantages of the use of contrast agents in the setting of RT, reports and investigations into its application during in-room MRIgRT are very limited and it is still hard to determine if the use of contrast agents will represent an advantage in the evolving framework of modern MR Linac based radiotherapy or an unnecessary waste of resources [28], [29], [30].
The survey response rate of 83% allows to provide a reasonable representation of current MRCA practice patterns worldwide.
Considering the overall obtained results, it can be stated that MRCA are generally used in the MRIgRT workflow, only in the cases where the visualisation of the target provided by on board MRI is insufficient.
Patients safety and logistical burden are important considerations for the application of contrast agents in this clinical setting and may discourage the use of MRCA also in borderline decisional situations, where patients may be addressed to the implantation of fiducials. For this reason among others, some institutions (42%) that participated in this survey, prefer not to use MRCA in clinical MRIgRT practice, especially considering the lack of a clear advantage in using them.
Target volume delineation in the liver seems to benefit most from MRCA administration in current practice, probably due to the difficulty in delineating certain types of liver metastases and HCC lesions and the common use of on-line MRIgRT SBRT delivery technique, where very high doses with steep gradients are used [16], [30], [31].
In this context, Gadoxetate (Eovist/Primovist) resulted to be the most frequently used MRCA by the responding centres (80%).
Although the use of other GBCA such as Gadopentetate (Magnevist) and Gadodiamide (Omiscan) have been restricted by the EMA [32], these contrast agents were reportedly used by some centres outside the EU. This points out the varying regulations implemented by different countries or regions [33].
Regarding the involved staff members, different personnel were responsible for administering MRCA in the single centres, again indicating different practice policies in the different centres and countries.
The majority of participating institutions that implemented the use of MRCA agreed to their use during MR Linac simulation, although due to safety and possible toxicity concerns, only one of the centres opposed to its use throughout treatment.
Indeed, apart from individual centre practice, there are no validated protocols for the administration of contrast agents procedures and the management of related complications (i.e. extravasation policy, side effects, and anaphylaxis) in radiation oncology. In fact, 42% of centres avoid its use due to the lack of resources, lack of trained staff and general concerns regarding patient safety. Interestingly, significant inconsistencies were observed also between centres in patients safety screening: slightly more than half (60%) routinely perform dedicated blood tests for in-room MR simulation, whilst only one-third perform them for surveillance purposes during MRIgRT treatment (36%). This variability highlights the need to design shared recommendations or guidelines for the management of MRCA in clinical MRIgRT practice and, although the safety issues appear to be minimal, systematic research is still necessary.
Consequently, regarding the prospect of introducing international guidelines to standardise patient safety and clinical indications, the agreement of the respondents (strongly agree/agree) was 21/43 (72%) and 33/43 (77%), respectively. This result shows that about a quarter of the participants still have concerns about the feasibility/utility of such a project. This is further emphasized by 79% of responders agreeing to the need for working groups dedicated to developing the use of MRI contrast agents for MRIgRT purposes.
This survey shows that there is considerable inconsistency in practice patterns and no standard practice among hospitals that implement the use of MRCA in hybrid MR-Linac, with a slight predominance of 0.35T adopters (14 centers versus 11).
However, there are indeed different limitations in this study, such as the exploratory nature of this survey; the lack of details about which anatomical site could benefit most from the administration of MRCA; billing issues; and how to overcome the logistical burden of this procedure.
5. Conclusions
Despite the growing number of MR-Linac units active worldwide and the accumulated clinical experience, the role of MRCA appears to still be unclear in the radiation oncology community. The issues that still need to be addressed mainly concern patient’s safety, the risk/benefit ratio (basic examinations to be performed routinely; assessment of risk factors to minimise complications; involvement of staff trained in the management of procedures and complications) and the definition of tumour sites where the use of MRCAs is beneficial for treatment planning.
CRediT authorship contribution statement
Luca Boldrini: Conceptualizaztion, Investigation, Resources, Supervision, Writing – review & editing. Filippo Alongi: Writing – review & editing. Angela Romano: Visualizazion, Writing – original draft. Diepriye Charles Davies: Data curation, Formal analysis, Project administration, Visualizazion, Writing – original draft. Michael Bassetti: Validation. Giuditta Chiloiro: Methodology. Stefanie Corradini: Supervision, Writing – review & editing. Lorenzo Placidi: Formal analysis, Software. Alison C. Tree: Validation. Rosalyne Westley: Validation. Luca Nicosia: Conceptualizaztion, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
LB received research grants and personal fees from Viewray Inc and Varian Medical Systems. SC received research grants and personal fees from Elekta and Viewray. AT declares research funding from Elekta, Varian and Accuray. RW declares institutional funding from Elekta. AT declares honoraria or travel assistance from Elekta, Accuray and Janssen. The Royal Marsden and the Institute of Cancer Research are part of the Elekta MR Linac consortium.
The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
AT and RW acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care. AT acknowledges Cancer Research UK RadNet Grant C7224/A28724 and C33589/A28284.
Ethics Approval and Consent to Participate
This research study was conducted retrospectively in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100615.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Baskar R., Lee K.A., Yeo R., Yeoh K.-W. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corradini S., Alongi F., Andratschke N., Belka C., Boldrini L., Cellini F., et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol. 2019;14(1) doi: 10.1186/s13014-019-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Mol van Otterloo S.R., Christodouleas J.P., Blezer E.L.A., Akhiat H., Brown K., Choudhury A., et al. The MOMENTUM study: an international registry for the evidence-based introduction of MR-guided adaptive therapy. Front Oncol. 2020;10:1328. doi: 10.3389/fonc.2020.01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keall P.J., Brighi C., Glide-Hurst C., Liney G., Liu P.Z.Y., Lydiard S., et al. Integrated MRI-guided radiotherapy — opportunities and challenges. Nat Rev Clin Oncol. 2022;19(7):458–470. doi: 10.1038/s41571-022-00631-3. [DOI] [PubMed] [Google Scholar]
- 5.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M., et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petronek M.S., Steinbach E.J., Kalen A.L., Builta Z.J., Callaghan C.M., Hyer D.E., et al. Assessment of Gadobutrol safety in combination with ionizing radiation using a preclinical MRI-guided radiotherapy model. Radiat Res. 2021;195(3) doi: 10.1667/RADE-20-00199.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Salzillo T., Jiang Y., Mackeyev Y., David Fuller C., Chung C., et al. Stability of MRI contrast agents in high-energy radiation of a 1.5T MR-Linac. Radiother Oncol. 2021;161:55–64. doi: 10.1016/j.radonc.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood F., Nielsen U.G., Jørgensen C.B., Brink C., Thomsen H.S., Hansen R.H. Safety of gadolinium based contrast agents in magnetic resonance imaging-guided radiotherapy – An investigation of chelate stability using relaxometry. Phys Imaging Radiat Oncol. 2022;21:96–100. doi: 10.1016/j.phro.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin B., Mustafayev T.Z., Gungor G., Aydin G., Yapici B., Atalar B., et al. First 500 fractions delivered with a magnetic resonance-guided radiotherapy system: initial experience. Cureus. 2019;11 doi: 10.7759/cureus.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London: The Royal College of, Radiologists. Imaging for Oncology. Oncology FoC, 2004.
- 12.Williams K., Probst H. Use of IV contrast media in radiotherapy planning CT scans: a UK audit. Radiography. 2016;22:S28–S32. doi: 10.1016/j.radi.2016.06.006. [DOI] [Google Scholar]
- 13.Minogue S., Gillham C., Kearney M., Mullaney L. Intravenous contrast media in radiation therapy planning computed tomography scans - Current practice in Ireland. Tech Innov Patient Support Radiat Oncol. 2019;12:3–15. doi: 10.1016/j.tipsro.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hales R.B., Chuter R., McWilliam A., Salah A., Dubec M., Freear L., et al. The impact of gadolinium-based MR contrast on radiotherapy planning for oropharyngeal treatment on the MR Linac. Med Phys. 2022;49(1):510–520. doi: 10.1002/mp.15325. [DOI] [PubMed] [Google Scholar]
- 15.Tummala P., Junaidi O., Agarwal B. Imaging of pancreatic cancer: an overview. J Gastrointest Oncol. 2011;2:168–174. doi: 10.3978/j.issn.2078-6891.2011.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencioni R., Cioni D., Della Pina C., Crocetti L., Bartolozzi C. Imaging diagnosis. Semin Liver Dis. 2005;25:162–170. doi: 10.1055/s-2005-871196. [DOI] [PubMed] [Google Scholar]
- 17.Neri E., Bali M.A., Ba-Ssalamah A., Boraschi P., Brancatelli G., Alves F.C., et al. ESGAR consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur Radiol. 2016;26(4):921–931. doi: 10.1007/s00330-015-3900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill B.D.P., Salerno G., Thomas K., Tait D.M., Brown G. MR vs CT imaging: low rectal cancer tumour delineation for three-dimensional conformal radiotherapy. Br J Radiol. 2009;82:509–513. doi: 10.1259/bjr/60198873. [DOI] [PubMed] [Google Scholar]
- 19.Chiloiro G., Cusumano D., Boldrini L., Romano A., Placidi L., Nardini M., et al. THUNDER 2: THeragnostic utilities for neoplastic DisEases of the rectum by MRI guided radiotherapy. BMC Cancer. 2022;22(1) doi: 10.1186/s12885-021-09158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Sun Y., Ma L., Liu G., Wang Z. The renal clearable magnetic resonance imaging contrast agents: state of the art and recent advances. Molecules. 2020;25 doi: 10.3390/molecules25215072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Y.-D., Paudel R., Liu J., Ma C., Zhang Z.-S., Zhou S.-K. MRI contrast agents: classification and application (Review) Int J Mol Med. 2016;38:1319–1326. doi: 10.3892/ijmm.2016.2744. [DOI] [PubMed] [Google Scholar]
- 23.Caravan P., Ellison J.J., McMurry T.J., Lauffer R.B. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A.K., Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Rogosnitzky M., Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29:365–376. doi: 10.1007/s10534-016-9931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Salzillo T., Jiang Y., Mackeyev Y., David Fuller C., Chung C., et al. Radiother Oncol. 2021;161:55–64. doi: 10.1016/j.radonc.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centre (UK) NCG. Delphi consensus methods. National Institute for Health and Care Excellence (NICE); 2014.
- 28.Koudrina A., DeRosa M.C. Advances in medical imaging: aptamer- and peptide-targeted MRI and CT contrast agents. ACS Omega. 2020;5:22691–22701. doi: 10.1021/acsomega.0c02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahsner J., Gale E.M., Rodríguez-Rodríguez A., Caravan P. Chemistry of MRI contrast agents: current challenges and new frontiers. Chem Rev. 2019;119:957–1057. doi: 10.1021/acs.chemrev.8b00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corradini S., Alongi F., Andratschke N., Azria D., Bohoudi O., Boldrini L., et al. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol. 2021;159:146–154. doi: 10.1016/j.radonc.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Kanematsu M., Kondo H., Goshima S., Kato H., Tsuge U., Hirose Y., et al. Imaging liver metastases: review and update. Eur J Radiol. 2006;58(2):217–228. doi: 10.1016/j.ejrad.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Kanal E., Maravilla K., Rowley H.A. Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol. 2014;35:2215–2226. doi: 10.3174/ajnr.A3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marasini R., Thanh Nguyen T.D., Aryal S. Integration of gadolinium in nanostructure for contrast enhanced-magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1580. doi: 10.1002/wnan.1580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.