Abstract
Introduction and importance
Heterotopic Pancreas (HP) is defined by the presence of pancreatic tissue in an anatomically distinct location from the main pancreas. While often clinically silent, it may present symptomatically. If located in the gastric antrum, HP may cause gastric outlet obstruction (GOO). The objective of this paper is to present a rare case of HP in the gastric antrum causing GOO.
Case presentation
Herein, we report a 43-year-old man who presented with abdominal pain and non-bilious emesis in the setting of COVID-19 infection and alcohol consumption. During the initial workup, computed-tomography (CT) was non-specific but demonstrated GOO, concerning for cancer. Cold forceps biopsies taken during esophagogastroduodenoscopy (EGD) confirmed benign HP. Since the patient was symptomatic from gastric outlet compression, he underwent resection via laparoscopic distal gastrectomy and Billroth II gastrojejunostomy. At 1-month postoperative follow-up, the patient recovered uneventfully. We hypothesized that GOO by HP in this case may have been associated with cumulative effects of alcohol consumption and COVID-19 infection on the ectopic tissue.
Clinical discussion
HP is rare and difficult to diagnose preoperatively. When located in gastric antrum, HP can cause GOO, mimicking gastric malignancy. Combination of EGD/EUS, biopsy/FNA, and surgical resection are necessary to definitively make the diagnosis. Finally, it is important to consider that heterotopic pancreatitis or structural changes in HP may occur due to classic pancreatic stressors like alcohol and viral infections.
Conclusion
HP may cause GOO presenting with non-bilious emesis and abdominal pain, mistaken for malignancy on CT imaging.
Keywords: Heterotopic pancreas, Gastric outlet obstruction, COVID-19, Case report
Highlights
-
•
If located in gastric antrum, heterotopic pancreas can become enlarged and cause gastric outlet obstruction.
-
•
Gastric outlet obstruction caused by heterotopic pancreas can mimic a malignancy, in clinical presentation and radiologically.
-
•
Heterotopic pancreatitis may be related to excessive alcohol use.
1. Introduction
Heterotopic pancreas (HP), also known as ectopic pancreas, is a rare congenital condition wherein pancreatic tissue exists in an anatomically distinct location from the true pancreas [1]. The incidence rate on autopsy ranges from 0.5 % to 13.7 % [1]. HP can occur in various gastrointestinal organs including the stomach and small intestine [1]. Pancreatic pathologies can occur in HP, independently from the organ proper [1]. While this condition is often clinically silent, rarely, it may become symptomatic. If located in the gastric antrum, it can present with non-bilious emesis due to gastric outlet obstruction (GOO) [2]. Similar to the main pancreas, HP may be subject to inflammatory and neoplastic conditions alike and may even progress to pancreatic adenocarcinoma with attendant signs and symptoms of malignancy [3]. Due to anatomic similarities on imaging, HP may be mistaken for malignancies, such as gastrointestinal stromal tumors (GIST) [4].
Heavy alcohol consumption may cause heterotopic pancreatitis [5]. Moreover, recent studies suggest a potential association between COVID-19 and pancreatitis [6]. We report a rare case of HP causing GOO in the setting of alcohol use disorder and concomitant COVID-19 infection that was initially mistaken for GIST [7].
2. Presentation of case
A 43-year-old man presented to the emergency department (ED) in July 2022 with 8 days of persistent vomiting with occasional abdominal pain following heavy alcohol use. Prior to this presentation, he had been seen in an urgent care clinic and the ED for epigastric abdominal pain and emesis. He reported that he was at his normal state of health until he developed a non-radiating, sharp burning epigastric pain, nausea, and non-bloody, non-bilious emesis. He had a history of gastritis, peptic ulcer disease, and alcohol use disorder and had been binge drinking immediately prior to onset. Previously, he had never experienced abdominal pain after alcohol consumption or had pancreatitis. He denied fever, night sweats, and unintentional weight loss prior to the onset of symptoms, however, since onset, he lost 10 kg.
At the onset of symptoms, the patient tested positive for COVID-19 using a home test kit. On admission, he tested positive for COVID-19 again, in the absence of respiratory symptoms. Two days after admission, he had a negative COVID-19 test. On physical exam, he appeared well-nourished but had dry mucus membranes. He had epigastric tenderness with deep palpation and no palpable mass. Labs indicated hypokalemia (potassium 2.6 mEq/L), metabolic alkalosis (bicarbonate 50 mEq/L), and evidence of acute kidney injury (creatinine 3.13 mg/dL, baseline creatinine of 0.7 mg/dL). Lipase was 76 U/L. All other lab values were within normal limits. Blood carcinoembryonic antigen (CEA) was 1.1 ng/ml, and CA19-9 was less than 3 U/ml.
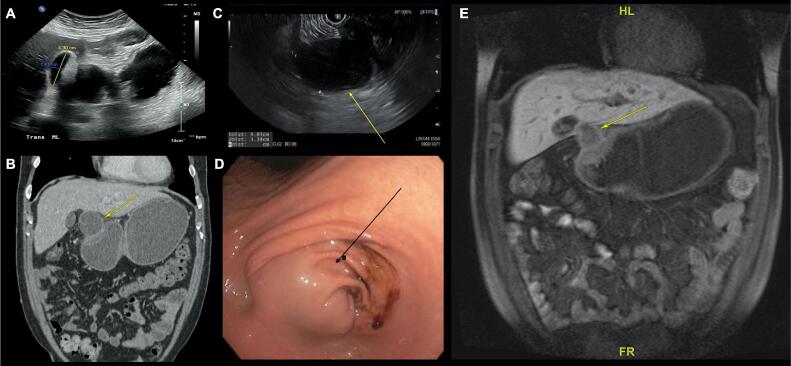
Abdominal X-ray on admission was unremarkable (Fig. 1). Ultrasound of the abdomen showed a 4.3 cm cystic structure near the pylorus, which was seen on a previous computed tomography (CT) of the abdomen, taken in the ED during this admission, which showed moderately distended stomach with a cystic structure at gastric antrum (Fig. 2A). Abdominal CT with intravenous (IV) contrast on this admission demonstrated a distended stomach with a cystic structure near the porta hepatis and gastric antrum (Fig. 2B). These findings were concerning for a possible malignancy. Esophagogastroduodenoscopy (EGD) revealed gastric antral distention due to a mass that was obstructing the pylorus (Fig. 2D). The mass appeared to be extrinsic or submucosal in origin. On endoscopic ultrasound (EUS), a 4.8 cm × 3.5 cm walled-off, extramural, cystic lesion was noted to be compressing the gastric antrum (Fig. 2C). The pancreatic parenchyma had normal architecture and the pancreatic duct and common bile duct were not dilated. Fine needle aspiration (FNA) of the cystic mass was performed, and aspirated fluid showed CEA levels of 1247 ng/ml, amylase levels of 35,850 U/L, and bacterial cultures grew Candida Glabrata. Magnetic resonance cholangiopancreatography (MRCP) demonstrated a partially cystic mass with hemorrhagic/proteinaceous components at the levels of pylorus and antrum (Fig. 2E). No evidence of distant disease was appreciated.
Fig. 1.
Abdominal X-ray (XR) on presentation. Abdominal XR showed a non-obstructed bowel gas pattern with mild gastric distension.
Fig. 2.
A) Abdominal ultrasound (US). Abdominal US showed a 4.3 cm × 1.4 cm cystic structure near the pylorus and the porta hepatis. B) Computed tomography (CT) of the abdomen with intravenous (IV) contrast. Abdominal CT with IV contrast demonstrated a moderately distended stomach consistent with gastric outlet obstruction due to a cystic structure (arrow) at the level of the pylorus and near the porta hepatis. No evidence of distant metastatic disease was noted in the abdomen. C) Esophagogastroduodenoscopy (EGD) with endoscopic ultrasound (EUS). EGD showed that the gastric antrum was distended by a mass lesion (arrow), likely extrinsic or submucosal in origin, which was obstructing the pylorus. D) EUS showed a 4.8 cm × 3.5 cm extramural, walled off cystic lesion (arrow) without any solid components in the mass. E) Magnetic resonance cholangiopancreatography (MRCP) with and without oral contrast. MRCP demonstrated gastric outlet obstruction which was likely secondary to a nodular and partially cystic mass (arrow) at the pylorus and antrum.
Initially, alcoholic gastritis was considered in the differential diagnosis due to the patient's history of alcohol use disorder and recent binge drinking, in addition to initial imaging studies showing gastric distention. COVID-induced gastrointestinal inflammation was considered because the onset of symptoms coincided with a positive COVID test.
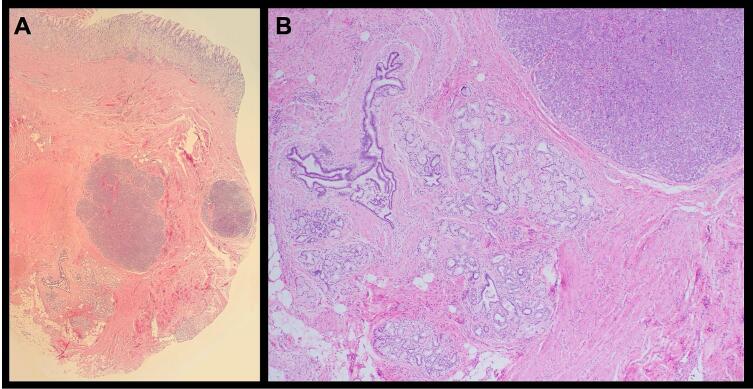
Given the EGD observations of GOO, and an apparent large submucosal antral mass with deformed duodenal bulb, malignancy was considered, and surgical oncology was consulted. Due to the mass at pylorus causing GOO, patient was counseled to undergo distal gastrectomy and resection of the mass for both diagnostic and therapeutic purposes. Laparoscopic-assisted distal gastrectomy with Billroth II gastrojejunostomy was performed, in addition to en bloc segment 4b liver resection since the lesion was inseparable to that portion of the liver. Upon entry of the abdomen, diagnostic laparoscopy showed no evidence of distant disease. The mass was easily identified to be straddling the inferolateral aspect of the gastrohepatic ligament, where it was adherent to segment 4b of the liver and compressing the gastric antrum. A combination of blunt dissection and electrocautery was used to circumferentially resect the mass along with 4b liver segment, distal stomach and pylorus, and portions of the lesser omentum. A loop of the proximal jejunum was brought up for a stapled anastomosis to the stomach. There were no intraoperative complications. Histopathology of the specimen showed pancreatic heterotopia with accompanying metaplastic epithelial changes, necrotic abscess cavity in adjacent pyloric adipose tissue (consistent with pseudocyst), and prominent Brunner gland hyperplasia at duodenal margin (Fig. 3). There was no evidence of malignancy. The patient had an uneventful recovery and was discharged home on postoperative day (POD) 5.
Fig. 3.
A) Benign gastric mucosa (top) with underlying pyloric muscle containing heterotopic pancreatic tissue, including clusters of acini (purple clusters) and ducts. B) Higher magnification view of the heterotopic pancreas residing within thick bands of pyloric smooth muscle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
On discharge, patient was sent home with oral analgesics, metoclopramide, polyethylene glycol, and was asked to resume his prior home medications including dicyclomine, docusate, famotidine, omeprazole, ondansetron, and sucralfate. On follow-up visit POD 18, he was recovering well with no complications. He had not consumed any alcoholic drinks since surgery.
3. Discussion
HP is a congenital condition, with the most common developmental cause believed to be heteroplastic differentiation of endodermal tissue in sites where pancreatic tissue does not normally develop, particularly gastric submucosa [8]. In our case, the submucosal layer of the gastric antrum was partially involved.
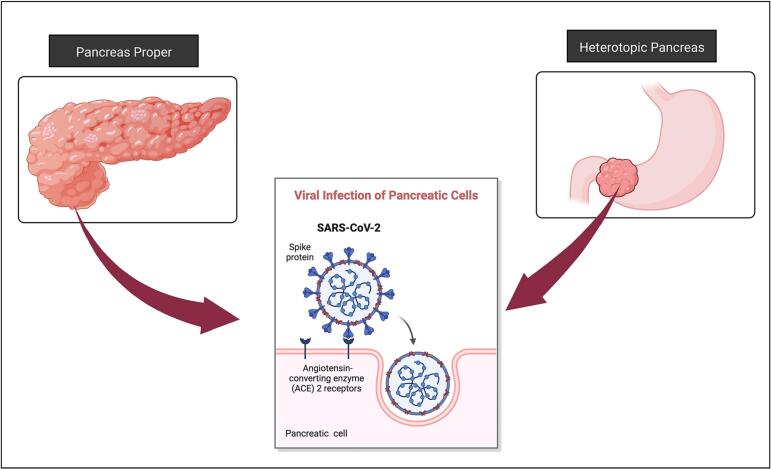
Since HP is histologically similar to the main organ, pathologies may present similarly. Irritation of the pancreatic tissue as a result of mechanical stimulation or inflammation can change the structure of HP. One of the most common causes of pancreatitis is alcohol consumption. Several recent studies suggest that COVID-19 infection may also be associated with pancreatitis [6]. The underlying molecular mechanism of COVID-19 pancreatitis is still unknown. However, the proposed hypothesis indicates that the virus can enter the cell through angiotensin-converting enzyme 2 (ACE2) surface protein (Fig. 4) [9]. Pancreatic cells express a significant amount of ACE2; therefore, they may be particularly prone to COVID-19 infection [9]. Additionally, recent cases were reported with heterotopic pancreatitis in the setting of COVID-19 infection [10]. In our case, the patient had a history of alcohol use disorder and a recent episode of binge drinking, in addition to 2 positive COVID-19 test results. We hypothesize that our patient had an episode of gastritis or heterotopic pancreatitis weeks prior to presentation, which stimulated the HP tissue and incited structural changes that led to GOO. Since our patient had no history of alcoholic pancreatitis prior to this episode, we postulate that heterotopic pancreatitis in this case was perhaps associated with the cumulative effects of both alcohol and COVID-19 infection. However, our current methods for histopathological analysis of COVID-19 are limited to lung tissue, therefore, viral infection could still have contributed to the pathology.
Fig. 4.
Hypothesized mechanism of pancreatitis secondary to SARS-CoV-2 infection.
HP is often clinically silent, however may rarely become symptomatic. If located in the gastric antrum, in the setting of inflammation or enlargement of HP, GOO may occur (Table 1) [11], [12], [13], [14], [15], [16], [17], [18]. HP can develop pancreatic adenocarcinoma as previously reported by other groups [11]. In the case of diagnostic uncertainty, or anatomical difficulties such as obstruction, it is recommended to resect the ectopic tissue.
Table 1.
Cases reported to present with gastric outlet obstruction due to heterotopic pancreas.
| First author, year | Age/gender | Presentation | Associated pathology | Treatment | Outcome |
|---|---|---|---|---|---|
| Xiong 2020 | 44 Female |
Abdominal distention and vomiting | Malignant transformation of heterotopic pancreas | Distal gastrectomy and chemotherapy | Asymptomatic during a 12-month follow-up |
| Iwahashi 2019 | 40 Male |
Abdominal pain and vomiting | Submucosal tumor | Open distal gastrectomy | Asymptomatic during a 12-month follow-up |
| Jin 2017 | 40 Male |
Abdominal pain, nausea, and vomiting | Gastric intramural pseudocyst formation | EUS-guided single pigtail stent insertion | Asymptomatic during a 4-year follow-up |
| Rodriguez 2014 | 16 Female |
Epigastric pain, nausea, and non-bilious emesis | Viral infection prior to presentation, H. pylori | Open distal gastrectomy | Residual mild epigastric pain and nausea |
| Bryan 2014 | 31 Female |
abdominal pain, nausea, and vomiting | Heterotopic pancreatitis and gastric intramural pseudocyst formation | Distal gastrectomy | Asymptomatic during a 12-month follow-up |
| Trifan 2012 | 31 Male |
Abdominal pain and vomiting | Submucosal tumor | Distal gastrectomy | Asymptomatic during a 6-month follow-up |
| Jung 2011 | 21 Female |
Intermittent abdominal pain, nausea, and vomiting | Pseudocystic degeneration of heterotopic pancreas | Subtotal gastrectomy | Asymptomatic during a 8-month follow-up |
| Jiang 2008 | 46 Female |
Chronic epigastric pain and recurrent vomiting | Submucosal tumor | Distal gastrectomy | Asymptomatic during a 6-month follow-up |
| Ikematsu 2003 | 26 Female |
Abdominal pain, nausea, and vomiting | Submucosal tumor | Distal gastrectomy | Asymptomatic during a 3-month follow-up |
Diagnosis of HP is often an incidental finding, as most imaging studies are non-specific. EGD/EUS has the capacity to make the diagnosis, however needle biopsy may be inconclusive if the sample is taken only from superficial layers [19]. FNA can also be helpful since fluid cytology has a diagnostic sensitivity of 80–100 % [13]. Another consideration is the tumor markers, while the serum CA19.9 was normal, the aspirated fluid analysis demonstrated elevated CEA levels. CEA is a non-specific tumor marker that can aid the diagnosis and monitoring of multiple carcinomas including pancreatic cancer. Previous literature has not reported on or explored the significance of elevation of CEA in the aspirated fluid in relation to HP. However, elevated CEA levels in the aspirated fluid analysis have been used in the diagnosis of mucinous pancreatic cysts and have not been associated with malignant disease or radiographic cyst progression [20].
Ultimately, due to its similarity to malignant tumors on imaging, surgical resection and pathologic examination of the mass remains the most definitive method of diagnosis. Cross-sectional imaging such as CT and MRCP can localize the mass but is unable to identify its histological nature.
4. Conclusions
Even though HP is rare, it is relevant to include in the differential diagnosis of a patient presenting with GOO. HP maybe visualized as a mass and mistaken for malignancy. It is pertinent to further study and report such cases to develop more specific guidelines for diagnosis and treatment.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval is exempt/waived at our institution.
Sources of funding
None.
Author contribution
Sarah Mirzaie: Study design, data collection, data interpretation, writing the paper.
Theodore X. Hu: Data collection, data interpretation, editing the paper.
Lu Yang: Data collection, data interpretation, editing the paper.
Katy L. Lawson: Data collection, data interpretation, editing the paper.
Mark D. Girgis: Study design, data collection, data interpretation, editing the paper.
Guarantor
Sarah Mirzaie.
Research registration number
Not applicable.
Declaration of competing interest
None.
Acknowledgements
Fig. 4 was created using Biorender.com.
References
- 1.Yang C.-W., Che F., Liu X.-J., Yin Y., Zhang B., Song B. Insight into gastrointestinal heterotopic pancreas: imaging evaluation and differential diagnosis. Insights Imaging. 2021;12(1):144. doi: 10.1186/s13244-021-01089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skertich N.J., Madonna M.B., Shah A.N. Gastric outlet obstruction due to gastric duplication cysts with heterotopic pancreatic tissue treated by marsupialization. J. Pediatr. Surg. Case Reports. 2019;50 doi: 10.1016/j.epsc.2019.101309. [DOI] [Google Scholar]
- 3.Hisanaga E., Sano T., Kubo N., et al. Adenocarcinoma with intraductal papillary mucinous neoplasm arising in a duodenal heterotopic pancreas: a case report. Clin. J. Gastroenterol. 2020;13(6):1373–1382. doi: 10.1007/s12328-020-01224-2. [DOI] [PubMed] [Google Scholar]
- 4.Štor Z., Hanžel J. Gastric ectopic pancreas mimicking a gastrointestinal stromal tumour: a case report. Int. J. Surg. Case Rep. 2018;53:348–350. doi: 10.1016/j.ijscr.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatelain D., Vibert E., Yzet T., et al. Groove pancreatitis and pancreatic heterotopia in the minor duodenal papilla. Pancreas. 2005;30(4) doi: 10.1097/01.mpa.0000161885.79373.1d. https://journals.lww.com/pancreasjournal/Fulltext/2005/05000/Groove_Pancreatitis_and_Pancreatic_Heterotopia_in.23.aspx [DOI] [PubMed] [Google Scholar]
- 6.Tomasi I., Scott L., Cullen J., di Maggio F., Ebied H., Wheatstone S. A rare case of heterotopic pancreatitis and intestinal malrotation in a COVID-19 positive patient. COVID-19, causative or coincidence? Int. J. Surg. Case Rep. 2021;82 doi: 10.1016/j.ijscr.2021.105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agha R.A., Franchi T., Sohrabi C., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Gore R.M., MSBT-H-YIG Levine, editors. High Yield in Radiology. W.B. Saunders; Philadelphia: 2010. Heterotopic pancreatic tissue; pp. 695–696. [DOI] [Google Scholar]
- 9.Capurso G., de-Madaria E. COVID-19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol. 2021;18(1):3–4. doi: 10.1038/s41575-020-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang W., Nemeth K., White A., Bonomo L. Combination of ectopic pancreas and intestinal malrotation presenting as non-specific right iliac fossa pain in a SARS-CoV-2 positive patient. BMJ Case Rep. 2021;14(5) doi: 10.1136/bcr-2021-241926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong Y., Xie Y., Jin D.-D., Wang X.-Y. Heterotopic pancreas adenocarcinoma in the stomach: a case report and literature review. World J. Clin. Cases. 2020;8(10):1979–1987. doi: 10.12998/wjcc.v8.i10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwahashi S., Nishi M., Yoshimoto T., et al. A case of gastric heterotopic pancreas with gastroduodenal invagination. Surg. Case Rep. 2019;5(1):110. doi: 10.1186/s40792-019-0669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H.-B., Lu L., Yang J.-F., et al. Interventional endoscopic ultrasound for a symptomatic pseudocyst secondary to gastric heterotopic pancreas. World J. Gastroenterol. 2017;23(34):6365–6370. doi: 10.3748/wjg.v23.i34.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez A.A., Berquist W., Bingham D. Gastric outlet obstruction caused by heterotopic pancreas in an adolescent. Dig. Dis. Sci. 2015;60(4):835–837. doi: 10.1007/s10620-014-3314-0. [DOI] [PubMed] [Google Scholar]
- 15.Bryan D.S., Waxman I., Matthews J.B. Gastric obstruction due to intramural pseudocyst associated with heterotopic pancreas. J. Gastrointest. Surg. 2014;18(6):1225–1226. doi: 10.1007/s11605-014-2511-7. [DOI] [PubMed] [Google Scholar]
- 16.Trifan A., Târcoveanu E., Danciu M., Huţanaşu C., Cojocariu C., Stanciu C. Gastric heterotopic pancreas: an unusual case and review of the literature. J Gastrointest Liver Dis. 2012;21 https://www.jgld.ro/jgld/index.php/jgld/article/view/2012.2.18 (2 SE-Case Reports) [PubMed] [Google Scholar]
- 17.Jung G.O., Park D.E., Yun K.J., Chae K.M. Partial gastric outlet obstruction caused by a huge submucosal tumor originating in the heterotopic pancreas. Korean J. Hepato-Biliary-Pancreatic Surg. 2011;15(3):194–197. doi: 10.14701/kjhbps.2011.15.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikematsu Y., Nishiwaki Y., Kida H., et al. Gastric outlet obstruction caused by a heterotopic pancreas in a pregnant woman: report of a case. Surg. Today. 2003;33(12):952–955. doi: 10.1007/s00595-003-2614-3. [DOI] [PubMed] [Google Scholar]
- 19.Kung J.W., Brown A., Kruskal J.B., Goldsmith J.D., Pedrosa I. Heterotopic pancreas: typical and atypical imaging findings. Clin. Radiol. 2010;65(5):403–407. doi: 10.1016/j.crad.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Nagula S., Kennedy T., Schattner M.A., et al. Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous cysts of the pancreas. J. Gastrointest. Surg. 2010;14(12):1997–2003. doi: 10.1007/s11605-010-1281-0. [DOI] [PubMed] [Google Scholar]