Abstract
Recently, SMARCA4-deficient undifferentiated tumor (SMARCA4-UT) has emerged as a distinct subset of lung cancer. Previous studies have suggested that SMARCA4-UT is often associated with smoking-related mutations, such as KRAS and STK11, rather than EGFR or ALK alterations. Nevertheless, no specific precision therapy has been identified for SMARCA4-UT. Here, we report the first case of concomitant ALK rearrangement and SMARCA4 (BRG1) deficiency in a nonsmoking female with thoracic cancer. Alectinib was given as the first-line therapy, and the patient achieved a remarkable complete response. Our case highlights the significance of ALK rearrangement identification for the precise therapeutic potential of SMARCA4-UT.
Keywords: Non–small cell lung cancer, Thoracic SMARCA4-deficient undifferentiated tumor, ALK-rearranged, Targeted therapy, Case report
Introduction
Recently, thoracic SMARCA4-deficient undifferentiated tumor (SMARCA4-UT) has emerged as a distinct subset of lung cancer.1 Available literature indicates that EGFR, ALK or ROS1 alterations are rarely associated with SMARCA4-UT.2,3 ALK gene rearrangements occur in approximately 3% to 7% of NSCLC.4 On the basis of an encouraging efficacy, alectinib has been approved for the treatment of patients with metastatic ALK-positive NSCLC.5 Nevertheless, little is known about the efficacy of ALK TKIs for patients synchronized with SMARCA4-UT. Here, we describe the first case of a nonsmoking female diagnosed with having SMARCA4-UT with concomitant EMLA4-ALK fusion. Alectinib was given as the first-line therapy, and the patient achieved remarkable tumor regression, which was sustained for more than 9 months without any adverse events.
Case Presentation
In February 2022, a 34-year-old woman who had never smoked presented to our emergency department with acute-onset chest pain and appearing pale and fatigued. Ultrasonography results indicated massive left pleural effusion, and emergency thoracentesis was performed. Nevertheless, her hemoglobin level dropped from 82 g/L to 57 g/L within 2 days. Chest computed tomography scans suggested left hemothorax and a slightly higher density shadow of adjacent diaphragmatic dough. The thoracic surgeon performed an emergency thoracoscopic thoracotomy for hemostasis. During the operation, the surgeon saw a bleeding fish-like mass with a diameter of approximately 4 cm without a clear boundary near the left posterior mediastinum. Multiple fish-like nodules with dark blood clots were also observed, with diameters ranging from 0.5 cm to 1.5 cm. Video-assisted thoracoscopic wedge resection of the left lower lobe was performed as a palliative surgery with informed consent.
The provisional diagnosis was poorly differentiated carcinoma with round cell and rhabdoid morphology. Immunohistochemistry presented positive pan-cytokeratin, but negative TTF-1 and BRG1, along with positive INI-1, partial positive BRM, and high Ki-67 (90%+). Subsequently, the diagnosis was corrected to SMARCA4-UT on the basis of the abovementioned results. The tumor proportion score of programmed death-ligand 1 was 35% with SP263 antibody. Fusion mutations in exon 13 of the EMLA4 gene and exon 20 of ALK were detected in the tumor sample with the ADx-amplification refractory mutation system and confirmed by target next-generation sequencing with the same sample.
From February 16, 2022, the patient received alectinib (600 mg twice daily) as first-line therapy and achieved partial response after 6 weeks. After 9 months, computed tomography scan confirmed the remarkable efficacy with complete remission (Fig. 1). During the treatment, she did not report any discomfort. In addition, no myelosuppression or abnormal liver function was identified during follow-up.
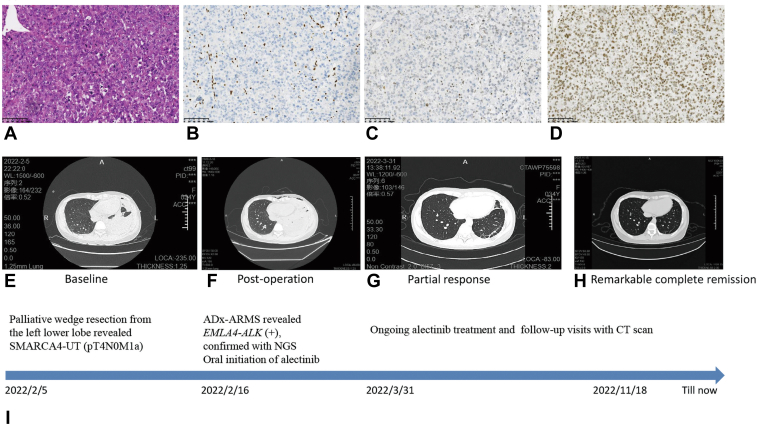
Figure 1.
Clinicopathological, radiologic information and timeline of the patient’s clinical course. Hematoxylin and eosin staining showed poorly differentiated carcinoma with round cell and rhabdoid morphology (A, 200×). Suggestive SMARCA4-UT by diffuse negative immunostaining BRG-1 (B, 200×). The tumor was partially positive for BRM (C, 200×) and positive for INI-1 (D, 200×). Computed tomography scans of the primary lung lesions at baseline before the initiation of alectinib (E), after palliative thoracoscopic wedge resection from the left lower lobe (F), partial response (PR) after 6 weeks (G), and remarkable complete remission after 9 months (H) with alectinib therapy. (I) Timeline of the patient’s clinical course (last follow-up on November 18, 2022). ARMS, amplification refractory mutation system; NGS, next generation sequencing; PR, partial response; SMARCA4-UT, thoracic SMARCA4-deficient undifferentiated tumor.
Discussion
The SMARCA4 gene encodes the BRG1 protein, which, as one of the subunits of the switch/sucrose nonfermentable complex, functions as a tumor suppressor.6 Mutations in SMARCA4 result in the loss of BRG1 expression and contribute to the development of malignant tumors.7 SMARCA4-UT is a rare tumor typically presenting as a mediastinal mass with poor prognosis. Consistent loss of BRG1 protein expression in cancer cells allows pathologic diagnosis. Meanwhile, BRG1 loss may occur in a subset of poorly differentiated NSCLCs that are TTF-1 and p40 negative.8 Previous studies have suggested that SMARCA4-UT is often associated with smoking-related mutations, such as KRAS, STK11, and KEAP1 mutations, rather than EGFR, ALK or ROS1 alterations.2,3 The efficacy of immunotherapy in SMARCA4-UT is controversial.3,9 Recent studies have found that most patients with SMARCA4-UT exhibit an immune desert tumor microenvironment, limited efficacy for immune checkpoint inhibitors, and poor prognosis.9,10 Currently, most patients with SMARCA4-UT are not suitable for molecularly targeted therapies because few targetable alterations have been identified. The young woman in our case was a nonsmoker who may have a higher incidence of ALK rearrangement. Although ALK genotyping has become a standard practice for NSCLC, the identification of concomitant co-mutations may affect the efficacy of ALK TKI and the prognosis of patients with ALK rearrangement. On the basis of our clinical and radiographic follow-up in 9 months, concomitant BRG1 loss did not affect the outstanding alectinib efficacy. Nevertheless, the mechanism of simultaneous BRG1 deletion is unknown.
Conclusions
Here, we present the first case of thoracic SMARCA4-UT harboring concomitant EML4-ALK rearrangement. The patient received alectinib as the first-line therapy and achieved a complete response lasting more than 9 months. Our case highlights the significance of ALK rearrangement identification in the quest for the precise therapeutic potential of SMARCA4-UT. Given the rarity of this condition and the lack of relevant literature, further studies are still needed.
CRediT Authorship Contribution Statement
Jin Sheng: Conceptualization, Methodology, Writing—original draft preparation.
Weidong Han: Writing—review and editing.
Hongming Pan: Supervision, Writing—review and editing.
Acknowledgments
The patient involved in this case report gave her informed consent authorizing the use and disclosure of her health information. This study was funded by the National Natural Science Foundation of China (number 81702809). All authors had full access to the data in the study and agreed to be held accountable for the integrity of this report. All the authors cooperated in the preparation, review, and approval of the manuscript, and agreed to the decision to submit this manuscript for publication. The authors thank Dr. Xiaotong Hu and Hui Li from the Department of Pathology, Dr. Ziyi Zhu from the Department of Thoracic Surgery, and all colleagues in the Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University, for their excellent work in technology support and clinical work.
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Sheng J, Han W, Pan H. Thoracic SMARCA4-deficient undifferentiated tumor with ALK fusion treated with alectinib achieved remarkable tumor regression: case report. JTO Clin Res Rep. 2023;4:100476.
Contributor Information
Jin Sheng, Email: shengjin@zju.edu.cn.
Hongming Pan, Email: panhongming@zju.edu.cn.
References
- 1.Nicholson A.G., Tsao M.S., Beasley M.B., et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Rekhtman N., Montecalvo J., Chang J.C., et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020;15:231–247. doi: 10.1016/j.jtho.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenfeld A.J., Bandlamudi C., Lavery J.A., et al. The genomic landscape of SMARCA4 alterations and associations with outcomes in patients with lung cancer. Clin Cancer Res. 2020;26:5701–5708. doi: 10.1158/1078-0432.CCR-20-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama R., Lovly C.M., Shaw A.T. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21:2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schokrpur S., Hilburn V., Giustini N., Bazhenova L. An overview of alectinib hydrochloride as a treatment option for ALK positive non-small cell lung cancer. Expert Opin Pharmacother. 2021;22:1815–1824. doi: 10.1080/14656566.2021.1948014. [DOI] [PubMed] [Google Scholar]
- 6.Mardinian K., Adashek J.J., Botta G.P., Kato S., Kurzrock R. SMARCA4: implications of an altered chromatin-remodeling gene for cancer development and therapy. Mol Cancer Ther. 2021;20:2341–2351. doi: 10.1158/1535-7163.MCT-21-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nambirajan A., Jain D. Recent updates in thoracic SMARCA4-deficient undifferentiated tumor. Semin Diagn Pathol. 2021;38:83–89. doi: 10.1053/j.semdp.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Nambirajan A., Singh V., Bhardwaj N., Mittal S., Kumar S., Jain D. SMARCA4/BRG1-deficient non-small cell lung carcinomas: a case series and review of the literature. Arch Pathol Lab Med. 2021;145:90–98. doi: 10.5858/arpa.2019-0633-OA. [DOI] [PubMed] [Google Scholar]
- 9.Dagogo-Jack I., Schrock A.B., Kem M., et al. Clinicopathologic characteristics of BRG1-deficient NSCLC. J Thorac Oncol. 2020;15:766–776. doi: 10.1016/j.jtho.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Gantzer J., Davidson G., Vokshi B., et al. Immune-desert tumor microenvironment in thoracic SMARCA4-deficient undifferentiated tumors with limited efficacy of immune checkpoint inhibitors. Oncologist. 2022;27:501–511. doi: 10.1093/oncolo/oyac040. [DOI] [PMC free article] [PubMed] [Google Scholar]