Abstract
Exposure to traumatic stress is a major risk factor for the development of neuropsychiatric disorders in a subpopulation of individuals, whereas others remain resilient. The determinants of resilience and susceptibility remain unclear. Here, we aimed to characterize the microbial, immunological, and molecular differences between stress-susceptible and stress-resilient female rats before and after exposure to a traumatic experience. Animals were randomly divided into unstressed controls (n = 10) and experimental groups (n = 16) exposed to Single Prolonged Stress (SPS), an animal model of PTSD. Fourteen days later, all rats underwent a battery of behavioral tests and were sacrificed the following day to collect different organs. Stool samples were collected before and after SPS. Behavioral analyses revealed divergent responses to SPS. The SPS treated animals were further subdivided into SPS-resilient (SPS-R) and SPS-susceptible (SPS–S) subgroups. Comparative analysis of fecal 16S sequencing before and after SPS exposure indicated significant differences in the gut microbial composition, functionality, and metabolites of the SPS-R and SPS-S subgroups. In line with the observed distinct behavioral phenotypes, the SPS-S subgroup displayed higher blood-brain barrier permeability and neuroinflammation relative to the SPS-R and/or controls. These results indicate, for the first time, pre-existing and trauma-induced differences in the gut microbial composition and functionality of female rats that relate to their ability to cope with traumatic stress. Further characterization of these factors will be crucial for understanding susceptibility and fostering resilience, especially in females, who are more likely than males to develop mood disorders.
Keywords: Microbiome, SPS, Anxiety, Resilience, PTSD, Metabolites
Highlights
-
•
Animals in the SPS group revealed divergent responses to the traumatic stressors.
-
•
Host's stress response correlated with preexisting microbial composition.
-
•
The gut microbiota after SPS shifted differently in SPS-R and SPS-S subgroups.
-
•
Cecal levels of BCFAs were higher SPS-S subgroup and correlated with anxiety index.
-
•
SPS-S subgroup had higher neuroinflammation and BBB permeability.
1. Introduction
Susceptibility to developing mood disorders such as anxiety, depression, and post-traumatic stress disorder (PTSD) depends on a set of different genetic, biological, and environmental factors (Elwenspoek et al., 2017; Toomey et al., 2015). Currently, about 12 million adults in the U.S. suffer from PTSD, and their yearly productivity loss is estimated to be around $3 billion (Affairs, 2022; Gray et al., 2004). With the scarcity of preventive interventions or effective treatments, PTSD poses an immense burden on society. Epidemiological studies have indicated that while one third of the population will be exposed to traumatic experience (s), only 10–20% of these people will go on to develop PTSD, with women twice as likely to develop the disease than men (Breslau, 2009; Mendoza et al., 2016). Thus, elucidating the mechanism(s) that predispose to resilience (ability to cope with stress) or susceptibility (development of neurological and behavioral deficits after adverse experience) is fundamental for understanding mood disorders, fostering resilience, and developing targeted and effective treatments, especially in females.
In the past decade, the ability to target the brain via microbiota has emerged as a paradigm shift in neuroscience and psychiatry and has opened the possibility of non-invasive modulation of brain function (Cryan and Dinan, 2012; Mayer et al., 2014). Several animal and human studies have reported a strong correlation between gut microbial composition and behavior (Jiang et al., 2015, 2018; Naseribafrouei et al., 2014; Sonali et al., 2022; Tanelian et al., 2022); and probiotic and prebiotic interventions have been shown to alleviate some stress-induced behavioral alterations. For instance, in pre-clinical studies, oral supplementation with Bifidobacterium increased resilience to social defeat stress in mice, and Lactobacillus reuteri 23272 decreased despair-like behavior under chronic mild stress (Marin et al., 2017; Yang et al., 2017). Similarly, in clinical studies, patients administered L. helveticus R0052 and Bifidobacterium R0175 for 30 days showed decreased urinary cortisol levels from baseline and reduced depression and anxiety scores (Ait-Belgnaoui et al., 2018; Arseneault-Bréard et al., 2012).
Thus, if microbiota supplementation can ameliorate stress-induced alterations in behavior, it is plausible that pre-existing differences in the gut microbial composition of individuals can predispose them to stress resilience or vulnerability and/or assist them in overcoming the severity of trauma. In this context, a study conducted using chronic social defeat stress, reported an increased abundance of Bifidobacterium spp. in stress-resilient mice relative to that in control and stress-vulnerable mice (Yang et al., 2017). Similarly, using the single prolonged stress (SPS), animal model for PTSD, we observed significant correlation between pre-existing and trauma-induced differences in the gut microbial composition, functionality and bacterial metabolites of adult male rats, which subsequently became SPS-resilient or SPS-susceptible (Tanelian et al., 2022).
Despite women being more prone to developing mood disorders, most of the neurobiological studies examining mechanisms of resilience or susceptibility to stress-elicited neuropsychiatric disorders have been performed in males (Beery and Zucker, 2011). Thus, there is a clear need for preclinical studies of stress resilience and susceptibility in females. To address some of the gaps in knowledge, in this study, we aimed to: (1) establish whether exposure to SPS triggers segregated behavioral responses in females; (2) determine if pre-existing and SPS-induced differences in the gut microbial composition and functionality are associated with resilient or susceptible phenotypes; (3) evaluate the components of the gut-brain-axis which may foster the resilient or susceptible phenotype in females. The results of this study will aid in the discovery of new and more effective therapeutic measures for females.
2. Materials and methods
2.1. Animals
All animal experiments were performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the New York Medical College Institutional Animal Care and Use Committee (IACUC). Twenty-six 7-week-old outbred female Sprague-Dawley (SD) rats (150–170 g) from Charles River Laboratories (Wilmington, MA, USA) were housed for 14 days to acclimate to the animal facility under a 12-h light/dark cycle at 23 ± 1 °C. Food and water were provided ad libitum throughout the experiment.
2.2. Experimental design
The experimental timeline is shown in Fig. 1. Animals were housed 2 per cage throughout the experiment. After 14 days of acclimatization, the animals were randomly assigned to a control (n = 10) or SPS experimental group (n = 16). The SPS experimental group was exposed to SPS (day 15), and was left undisturbed for seven days to consolidate the experience of traumatic stress, whereas the control group was briefly handled on the day of SPS (Tanelian et al., 2022). After 2 weeks (day 31), all animals were tested for social behavior using the social interaction (SI) test, and on day 37, for anxiety-like behavior using the elevated plus maze (EPM) test. All animals were weighed after SPS and after each behavioral test (Supp. Fig. 1A) and euthanized by decapitation on day 38. Stool samples were collected 12 days after arrival and 3 days before the first behavioral test. Vaginal smears were collected before SPS (day 15) and after each behavioral test (days 31, 37). On the day of sacrifice, blood, cecum, and hippocampal tissues were collected and stored at −80 °C for further analyses.
Fig. 1.
Experimental Design.
The animals were allowed to accommodate to the animal facility for 14 days upon arrival. On day 15, they were randomly assigned into unstressed controls or SPS groups. The SPS group was left undisturbed for 7 days, to consolidate the experience of traumatic stress, while the unstressed controls were briefly handled on the day of SPS. On day 31, all animals were tested for social behavior on Social Interaction (SI) test, and on day 37 for anxiety-like behavior on Elevated Plus Maze (EPM) test. On day 38, all animals were sacrificed by decapitation and different tissues were collected. Stool samples were collected 12 days after arrival, and 3 days before the first behavioral test. Vaginal smears were collected before SPS, and after each behavioral test.
2.3. Single prolonged stress (SPS)
SPS was performed as described previously (Liberzon and Young, 1997; Serova et al., 2019). Briefly, the animals were restrained to a custom-made metal board for 2 h, after which they were immediately subjected to a 20 min forced swim in a plexiglass cylinder (50 cm height, 24 cm diameter; Stoelting, Wood Dale, IL, USA) filled two-thirds with fresh water at 24 °C. After 15 min of recuperation, the animals were exposed to diethyl ether in a glass desiccator chamber until loss of consciousness.
2.4. Vaginal smears
Vaginal smears were collected from each animal, as previously described (McLean et al., 2012; Nahvi et al., 2021) to determine the stage of ovulation. Briefly, the smears were collected using sterile swabs and distilled water and were left to dry on glass slides. The slides were then stained with 0.1% crystal violet and observed under a microscope at 10 × and 40 × magnifications. Views from distinct sections of the smear were analyzed by investigators blinded to the groups. Analysis of vaginal smears revealed that the vast majority of the animals were on Estrus, while two were on Proestrus during exposure to SPS (Supp. Fig. 2). As estrogen has a stress-protective effect (Jaric et al., 2019; Marcondes et al., 2001), these two animals were excluded from all subsequent analyses, bringing the total number of animals in this study to 24 rats.
2.5. Behavioral tests
All behavioral tests were performed in a room with dim light and administered between 8 a.m. and 12:30 p.m. to avoid the proestrus phase of the estrous cycle. Behavioral tests were videotaped with a ceiling camera and analyzed by trained individuals blinded to the groups.
2.5.1. Social interaction (SI)
One day prior to the test, the animals were allowed to explore the open field arena for 5 min to reduce the anxiety component in a novel environment. On the testing day, the animals were acclimated to the room for 30 min and then allowed to explore the field for 2 min after which a juvenile rat (50–75 g) of the same sex was introduced into the center of the arena. The animals were allowed to interact for 5 min, and their behavior was recorded. The time spent interacting and the number of approaches initiated by the test rats were scored and analyzed by a trained individual blinded to the groups. The time spent in nose-to-nose sniffing, nose-to-anogenital sniffing, following, crawling over and under each other with physical contact, chasing, mounting, and wrestling initiated by the test rat was considered as the time spent engaged in social interaction (Tanelian et al., 2022; Varlinskaya and Spear, 2008).
2.5.2. Elevated plus maze (EPM)
Anxiety/avoidance-like behavior was tested on the EPM as previously described (Lapiz-Bluhm et al., 2008; Serova et al., 2019; Tanelian et al., 2022). The apparatus (Stoelting, Wood Dale, IL, USA), 50 cm above ground level, had four cross-shaped platforms; two platforms with a 2-cm-high plexiglass fence wall were open, while the other two platforms with 40-cm-high opaque walls on the sides were closed. Arms of the same type are located opposite to each other. The animals were acclimatized to the room for 30 min prior to the experiment. Each rat was placed on the central platform with its head towards an open arm and allowed 5 min to explore the maze. The maze was then cleaned with 70% ethanol. The following measurements were taken using tracking software (Viewer 3.0, Biobserve) or by video analysis from the overhead camera: open arm (OA) and closed arm (CA) entries, total entries into all arms, duration of exploration in open and closed arms, and number of head dips. Arm entry was defined as the entry of an arm with all four paws. The percentage of entries was calculated as the percentage of total open or closed arm entries to the total number of arm entries, and the time in the arms was calculated as a percentage of the total time of the test. The anxiety index (AI) was calculated as 1 − [(time spent in open arm/total time on the maze)/2 + (number of entries into the open arms/total exploration on the maze)/2] (Cohen et al., 2012). Head dips were defined as the frequency at which the animal lowered its head towards the floor over the sides of the open arms.
2.5.3. Grouping animals into SPS resilient or susceptible subgroups
The elevated plus maze test is extensively used to assess anxiety-like behavior in animals (Kraeuter et al., 2019; Lapiz-Bluhm et al., 2008). As the anxiety index calculation takes into consideration both the time spent and the number of entries into the open arms of the maze, we used it as a criterion to subdivide animals in the SPS group into resilient or susceptible subgroups.
The mean of the unstressed control was taken as a reference (Li et al., 2021; Nasca et al., 2015; Tanelian et al., 2022). Animals with anxiety index falling two standard deviations above the mean of the unstressed controls were considered susceptible (SPS–S).
2.6. Tissue collection
The brains were dissected using a brain matrix. Ventral hippocampus (vHipp) sections, −4.80 mm to −5.20 mm to bregma were dissected and flash frozen in liquid nitrogen and stored at −80 °C until further use.
2.7. Serum sampling for S100β quantification
Whole blood samples were collected from each animal by decapitation. The samples were stored at room temperature for 30 min, after which they were centrifuged at 4 °C for 10 min at 2000 g. Serum was collected and stored at −80 °C for later analysis. Serum S100β quantification was performed using a rat S100β ELISA kit (LSBio, cat # LS-F5632-1), according to the manufacturer's protocol.
2.8. Gene expression analysis
Total mRNA was isolated using RNA STAT-60 (Tel-test, cat # CS-502). RNA concentration was determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific) and cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, cat #K1622). Gene expression analysis was performed by quantitative real-time polymerase chain reaction (qRT-PCR) using FastStart Universal SYBR Green Master (ROX) kit (Roche Diagnostics cat #04913850001) and the following primers designed by QIAGEN: GAPDH (PPR06557B), IL-1α (PPR06403C), IL-1β (PPR06480B-200), and IL-6 (PPR06483B-200). Amplifications were performed in duplicates in 96 well plates on the QuantStudioTM 5 System. The data were normalized using GAPDH as an endogenous control and transformed using 2-ΔΔCT. All procedures were performed according to the manufacturer's instructions.
2.9. Cecum and cecal short chain fatty acids (SCFA) quantification
Ceca were isolated, weighed, snap-frozen in liquid nitrogen, and stored at −80 °C until further use. Cecal samples were sent for SCFA analysis to the Gnotobiotics, Microbiology, and Metagenomics Center (Boston, MA, USA). Chromatographic analysis was performed using an Agilent 7890 B system with a flame ionization detector and OpenLab ChemStation software (Agilent Technologies, Santa Clara, CA, USA). A volatile acid mix (10 mM acetic, propionic, isobutyric, butyric, isovaleric, valeric, isocaproic, caproic, and heptanoic acids) was used as the standard solution (Supelco CRM46975, Bellefonte, PA, USA). An internal standard control (1% 2-methyl pentanoic acid, Sigma-Aldrich, St Louis, MO, USA) was used for volatile acid extraction.
2.10. Fecal microbiota sequencing
To determine the microbiome profile of the cohorts, fecal samples were collected aseptically from individual rats at the indicated time points (Fig. 1) between 10 a.m. and 2 p.m. to limit circadian influences on the microbiome and were stored at − 80 °C until further use. Briefly, prior to SPS, each animal was placed in a sterile cage for up to 15 min to defecate voluntarily. Upon defecation, the pellets were collected immediately in sterile tubes using sterile forceps and placed on dry ice. Post SPS, stool pellets were collected using sterile forceps while weighing the animals. Total DNA was extracted from each stool sample using a DNeasy PowerSoil Pro Kit (Qiagen, cat # 47014) according to the manufacturer's protocol. The extracted DNA was subjected to 16S V3–V4 rDNA sequencing and analysis at Psomagen (Rockville, MD, USA). Briefly, the 16S metagenomic sequencing library protocol (Illumina) was used to amplify the designated variable regions using regions of interest-specific primers with attached overhang adapters:
Forward primer ’TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG Reverse primer 5′GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC. Each PCR reaction consisted of 2.5 μL of 5 ng/μL DNA template, 5 μL of 1 μM reverse primer, 5 μL of 1 μM forward primer and 12.5 μL of 2x KAPA HiFi HotStart ReadyMix. PCR was performed using the following program: initial denaturation at 95 °C for 3 min, followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min, and held at 4 °C. The size of the PCR product was verified using a Bioanalyzer DNA 1000 chip, followed by purifying the V3–V4 regions using AMPure XP beads. A second round of PCR was performed using the Nextera XT Index Kit, followed by purification and size verification to prepare samples for library quantification, normalization, and pooling using the Illumina guidelines. Samples were sequenced on the MiSeq sequencing platform using a 2 × 300 cycle kit following standard Illumina sequencing protocols. Bioinformatics analysis was performed by merging paired-end sequences using FLASH. The sequences were then filtered and trimmed, and error-free reads were selected for operational taxonomic unit (OUT) clustering using CD-HIT-OTU. Taxonomic composition for each sample from phylum to genus levels was assigned using QIIME-UCLAST Software and RDP Release 11 update 4 reference databases. The relative abundance of the bacterial taxa was expressed as the centered log-ratio (CLR)-transformed relative abundance of the identified sequences. A single value per group ≥2 SD from the mean was removed from the analyses. Samples sent for sequencing: before SPS, control n = 10, SPS-R n = 7, SPS-S n = 6; after SPS, control n = 8, SPS-R n = 8, SPS-S n = 6.
2.11. Statistical analysis
Statistical analysis was performed using the GraphPad Prism 9 software. Data were assessed for normality using the Shapiro-Wilk test and for equality of variances using Brown-Forsythe and Bartlett's tests. Comparisons of more than two groups were performed using one-way ANOVA followed by Tukey's multiple comparison test for Gaussian distributions, whereas the Kruskal-Wallis test followed by Dunn's multiple comparison test was used for non-Gaussian distributions. To compare group means at different time points, two-way ANOVA, repeated measures were used, with post-hoc Sídăk's and/or Tukey's multiple comparisons test. The Student's t-test was used to compare two groups. Pearson's correlation coefficient was used to assess correlations. For further analysis, R version 4.1.2 was used. Microbiome data were centered log-ratio (CLR) transformed using a composition library (Gloor et al., 2017; McLaren et al., 2019). Principal component analysis for beta diversity was performed in R using the Aitchison distance as a distance matrix. For metagenomic function prediction, phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) was used to infer KEGG pathways. Statistical significance was set at an α level of less than 0.05 (two-tailed). To correct for multiple testing, the Benjamini-Krieger-Yekutieli post-hoc test was used with a q-value of 0.05 as a cut-off to analyze microbiome data. Data are expressed as the mean ± SEM. The 16S sequencing data are deposited to NCBI SRA (BioProject ID PRJNA912323).
3. Results
3.1. Behavioral tests
3.1.1. Animals in SPS-S subgroup displayed anxiety-like behavior and impaired social interaction
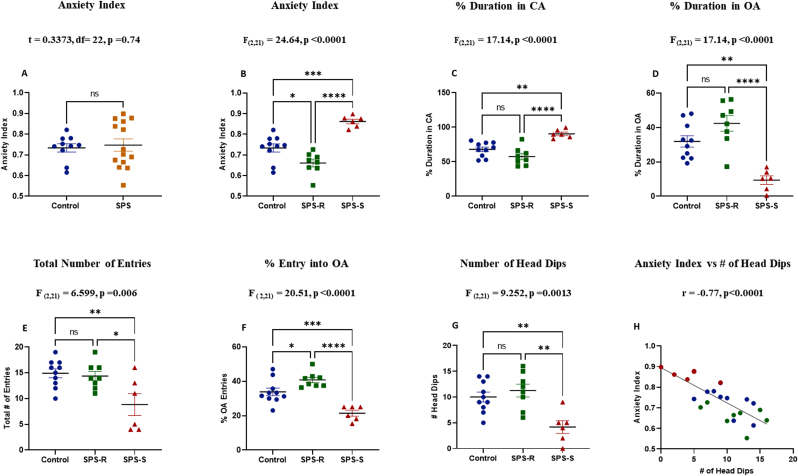
The SPS treated rats displayed a divergent response when tested on the EPM (Fig. 2A). Thus, the animals in the SPS group were further subdivided into two subgroups (Alves-Dos-Santos et al., 2020; Tanelian et al., 2022). Those with anxiety index (AI) two standard deviations (SD) above the mean of the controls were assigned to the SPS susceptible (SPS-S) subgroup, whereas the rest were grouped as SPS resilient (SPS-R) (Fig. 2B). The final animal groupings were as follows: controls (n = 10), SPS-R (n = 8), and SPS-S (n = 6).
Fig. 2.
Animals in the SPS-S Subgroup Displayed Increased Anxiety-like Behavior.
The effect of SPS on animals' anxiety-like behavior was assessed using Elevated Plus Maze (EPM) test. (A) anxiety Index, (B) anxiety Index after dividing the SPS animals into SPS-R and SPS-S subgroups, (C) Percent duration in closed arms (CA), (D) Percent duration in open arms (OA), (E) Total number of entries into all arms, (F) Percent entries into OA (G) Total number of head dips (H) Correlation between total number of head dips and anxiety Index (blue = Control, green = SPS-R, red = SPS-S). The behavioral data passed the normality test. Unpaired t-test was performed for comparison of the means of two groups, and one way-ANOVA, followed by Tukey's multiple comparisons test, for comparison of the means of three groups. Correlation analysis was performed using Pearson's correlation. Each dot represents value for an individual animal. All data are expressed as means ± SEM. ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The rats in the SPS-S subgroup spent more time in the closed arms (CA) (Fig. 2C) and significantly less time in the open arms (OA) (Fig. 2D) of the maze compared to the SPS-R and unstressed controls. The overall arm entries were also significantly lower in SPS-S subgroup (Fig. 2E), with SPS-S animals displaying fewer entries into the open arms relative to the SPS-R and unstressed controls (Fig. 2F). The SPS-S subgroup also differed significantly from SPS-R and the controls in the number of head dips (Fig. 2G), which correlated inversely with anxiety index (Fig. 2H).
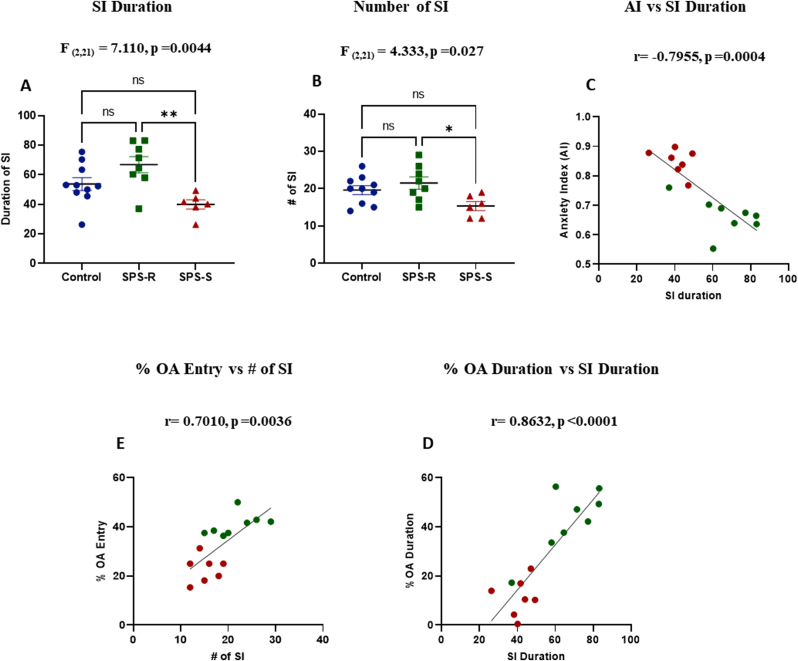
The animals’ social behavior was assessed by the SI test. The time spent interacting with a female juvenile rat was significantly shorter in the SPS-S subgroup than in the SPS-R subgroup (Fig. 3A). Likewise, the number of approaches towards the juvenile rat initiated by the SPS-S rats were significantly fewer than that of the SPS-R rats (Fig. 3B). The time spent engaged in SI was inversely correlated with AI (Fig. 3C), and positively with open arms duration (Fig. 3D). Similarly, the number of approaches initiated by the rats in SI correlated positively with the number of entries into the open arms of EPM (Fig. 3E).
Fig. 3.
Animals in the SPS-S Subgroup Displayed Social Impairments.
The effect of SPS on animals' social behavior was measured by Social Interaction (SI) test. (A) Duration engaged in social interaction, (B) Number of approaches/interactions initiated by the test rat towards the juvenile rat, (C) Correlation between social interaction duration and anxiety index, (D) Correlation between social interaction duration and % duration spent in OA, (E) Correlation between number of social interactions and % OA entries. The data passed the normality test and were analyzed using one way-ANOVA, followed by Tukey's multiple comparisons test. Correlations were performed using Pearson's correlation between the groups which showed significant differences (green = SPS-R, red = SPS-S). Each dot represents value for an individual animal. All data are expressed as means ± SEM. ns = not significant, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Gut microbial composition, functionality, and metabolites before and after SPS exposure
The 16S V3–V4 rDNA sequencing of fecal samples was used to determine the microbial composition of each group at the genus level before and after SPS.
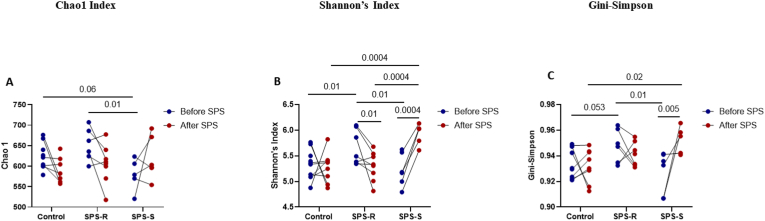
3.2.1. Alpha, beta diversity & volatility
The alpha diversity was analyzed using several indices (Fig. 4A–C, Supp. Table 1). Before exposure to SPS, the Chao1 index revealed higher community richness in the SPS-R rats (Fig. 4A) and Shannon (Fig. 4B), and Gini-Simpson (Fig. 4C) diversity indices revealed higher species evenness in the SPS-R than in the SPS-S subgroup. However, after SPS, both community richness and evenness became higher in SPS-S subgroup relative to the SPS-R subgroup and unstressed controls (Fig. 4A–C), due to the significant increases in these indices in the SPS-S subgroup. The beta diversity, which was calculated using the Aitchison distance matrix, showed no apparent separation among the groups either before or after SPS (Supp. Fig. 3A and B). Similarly, no group differences were observed in volatility, which quantifies the degree of compositional change of the gut microbial community before and after SPS (Bastiaanssen et al., 2021; Tanelian et al., 2022) (Supp. Fig. 3C).
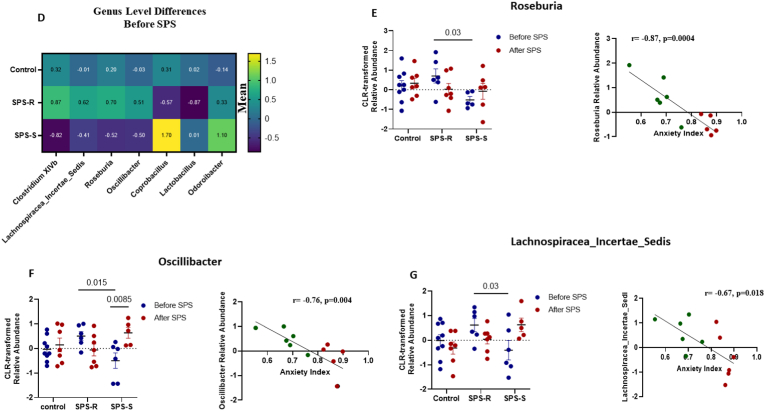
Fig. 4.
Pre-Existing Differences in Gut Microbial Composition of SPS-R and SPS-S Subgroups.
The fecal 16S sequencing was used to determine the microbial diversity and composition of each group before exposure to SPS. Alpha diversity measured by (A) Chao1, (B) Shannon's Index, (C) Gini-Simpson, (D) Heat map depicting the significantly different means of genera found among the groups, (E) Relative abundance of Roseburia and its correlation with anxiety index, (F) Relative abundance of Oscillibacter and its correlation with anxiety index, (G) Relative abundance of Lachnospiraceae_Incertae_Sedis, and its correlation with anxiety index. Data were CLR-transformed and were analyzed by Two-ways ANOVA, followed by FDR corrected Benjamini-Krieger-Yekutieli multiple comparisons test. Correlation analysis of the groups with significant differences was performed by Pearson's correlation (green = SPS-R, red = SPS-S). All data are expressed as means ± SEM. A single value, 2 SD away from the mean were excluded from analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2.2. Differences observed in the gut microbial composition at the genus level
A total of 27 genera were altered among the groups. Seven of these differences were present only before SPS, 15 were present only after SPS, and the remaining 5 were present both before and after SPS.
Among the 7 differences observed at the genus level before SPS (Fig. 4D, Supp. Table 2), four were unique to the SPS subgroups, with three of these genera correlating significantly with AI. For instance, the relative abundance of the genus Roseburia was significantly higher in the SPS-R subgroup and correlated inversely with AI (Fig. 4E). Similarly, the relative abundances of Oscillibacter (Fig. 4F), and Lachnospiracaea_incertae _sedis (Fig. 4G) were significantly higher in the SPS-R subgroup and correlated inversely with AI, suggesting that these genera might play a role in predisposing the animals to SPS susceptibility or resilience.
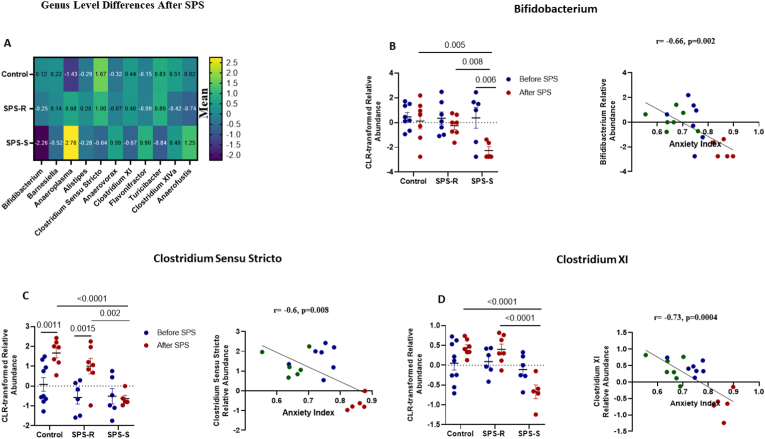
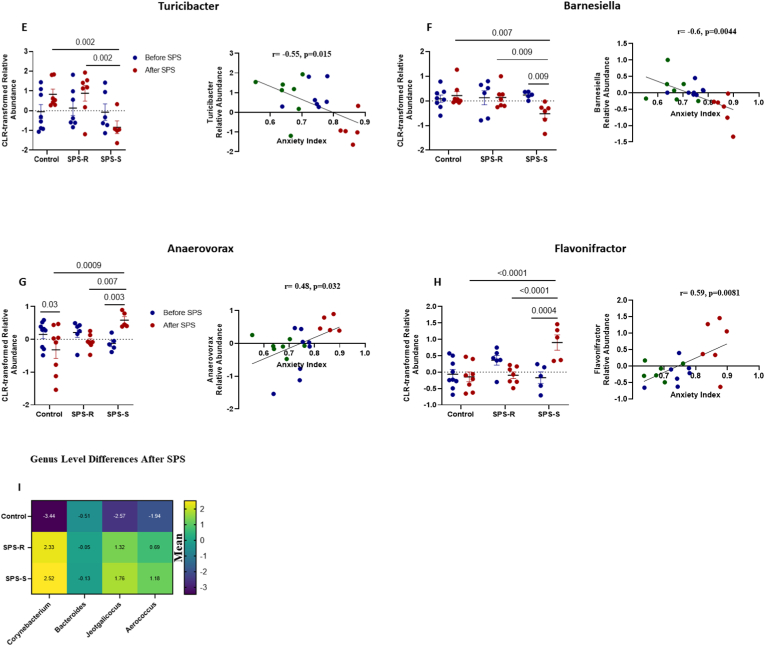
When analyzing the differences present only after exposure to SPS (Fig. 5A, Supp. Table 3), eight genera correlated significantly with AI. The abundances of Bifidobacterium (Fig. 5B), Clostridium Sensu Stricto (Fig. 5C), Clostridium XI (Fig. 5D), Turicibacter (Fig. 5E), and Barnesiella (Fig. 5F) correlated inversely with AI and were significantly lower in the SPS-S subgroup than in the SPS-R and unstressed controls. On the other hand, the relative abundances of Anaerovorax (Fig. 5G) and Flavonifractor (Fig. 5H) were significantly higher in the SPS-S subgroup than in the SPS-R subgroup and the controls and correlated positively with AI. Most of the shifts observed after SPS showed that the directionality of the microbial composition in SPS-R and unstressed controls were the same, and opposite to those in the SPS-S cohort. Interestingly, after SPS, the relative abundances of Corynebacterium, Bacteroides, Jeotgalicocus, and Aerococcus, were significantly higher in both SPS-subgroups than in the controls (Fig. 5I, Supp. Table 4), suggesting that these genera are unlikely to play a role in the observed distinct behavioral outcomes. Finally, five genera, namely Staphylococcus, Lactococcus, Marivinbryantia, Muscispirilium and Facklamia showed significant group differences both before and after SPS, yet none of these genera correlated with the anxiety index (Supp. Fig. 4, Supp Table 5).
Fig. 5.
Post-Trauma Differences in The Gut Microbial Communities of SPS-R and SPS-S Subgroups.
The 16S sequencing was used to determine the microbial composition of each group at genus level after SPS. (A) Heat map depicting the significantly different means of the genera found among the groups, (B) Relative abundance of the genus Bifidobacterium and its correlation with anxiety index, (C) Relative abundance of the genus Clostridium Sensu Stricto and its correlation with anxiety index, (D) Relative abundance of Clostridium XI and its correlation with anxiety index, (E) Relative abundance of the genus Turicibacter and its correlation with anxiety index, (F) Relative abundance of the genus Barnesiella and its correlation with anxiety index, (G) Relative abundance of the genus Anaerovorax and its correlation with anxiety index, (H) Relative abundance of the genus Flavonifractor and its correlation with anxiety index, (I) Heat map depicting the significantly different means of the genera found in SPS-subgroups relative to unstressed controls. All relative abundances are CLR-transformed. Data were analyzed by Two-way ANOVA followed by FDR corrected Benjamini-Krieger-Yekutieli multiple comparisons test. Correlation between relative abundances and anxiety Index of the groups with significant differences was performed by Pearson's correlation (blue = Control, green = SPS-R, red = SPS-S). All data are expressed as means ± SEM. A single value, 2 SD away from the mean were excluded from analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Predictive functionality of gut microbiota before and after SPS
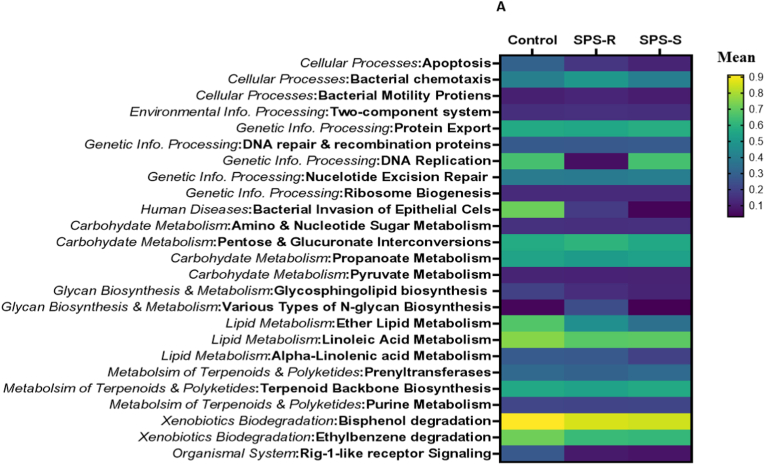
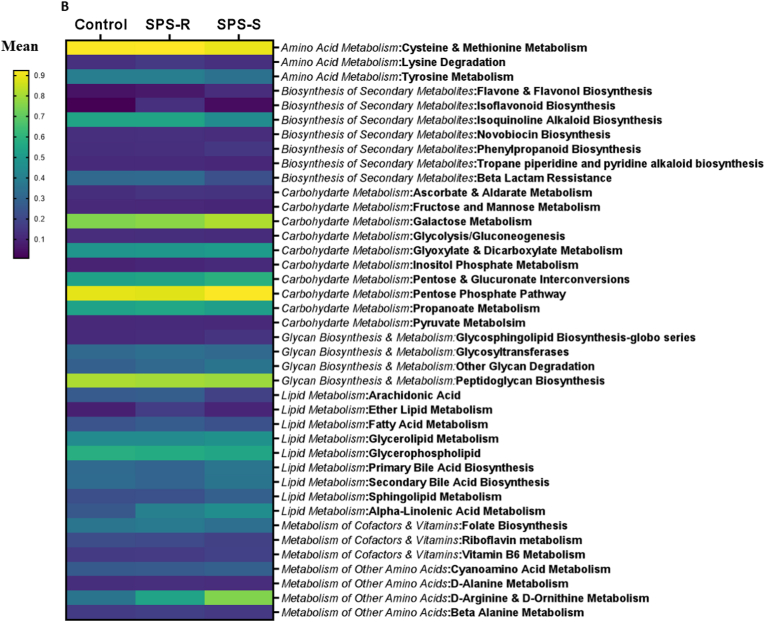
Alterations in gut microbial composition do not necessarily indicate changes in their functionality, as many of their functions are redundant. To evaluate this, we assessed the predictive functional profiles of the microbes residing in the gut of SPS-R and SPS-S subgroups before and after SPS exposure. Before SPS, 25 pathways were different among the groups with genetic information processing, carbohydrates, terpenoids, and polyketides metabolism being overall lower in the SPS-R subgroup, whereas pathways involved in cellular processes being higher relative to SPS-S and unstressed controls (Fig. 6A). After SPS, 83 pathways were different among the groups, with overall pathways in amino acid and secondary metabolites being higher in the SPS-R subgroup relative to SPS-S and controls, whereas pathways in xenobiotics, metabolism of cofactors, and vitamins being lower in the SPS-S subgroup than in the SPS-R and unstressed controls. The pathways in the other categories revealed mixed results (Fig. 6B, Supp. Fig. 5).
Fig. 6.
Predictive Functionality of Gut Microbiota Before and After SPS.
Heat map showing the significantly different KEGG pathways among the cohorts. (A) Before SPS. (B) After SPS. The comparison between the groups is presented as mean differences. Data were analyzed by Two-way ANOVA followed by FDR corrected Benjamini-Krieger-Yekutieli multiple comparisons test. A single value/group, 2 SD away from the mean was excluded from the analysis.
3.4. Cecal short chain fatty acids (SCFA)
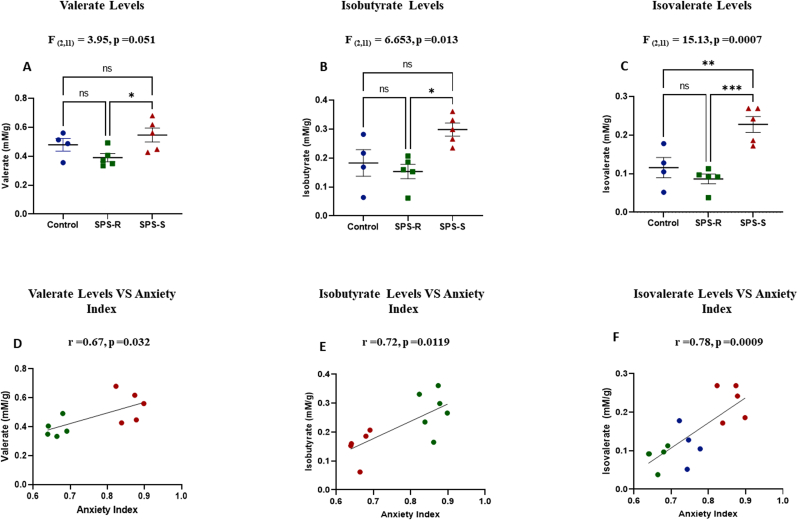
Short-chain fatty acids (SCFAs) are among the main metabolites produced by gut bacteria and play a key role in microbiota-gut-brain axis crosstalk (Verbeke et al., 2015). The levels of the major SCFA, acetate, propionate, and butyrate, were similar among the groups (Supp. Fig. 1B-E). However, the levels of the minor SCFA valerate (Fig. 7A), and branch-chain fatty acids (BCFA), isobutyrate (Fig. 7B) and isovalerate (Fig. 7C), were significantly higher in the SPS-S subgroup than in the SPS-R subgroup or unstressed controls and correlated positively with anxiety index (Fig. 7D, E and Fig 7F, respectively).
Fig. 7.
SPS Altered the Levels of Cecal BranchedChain Fatty Acids and Minor Forms of SCFA
Five cecal samples from SPS subgroups and four from the unstressed controls were sent for SCFA analysis. (A) Levels of Valerate, (B) Levels of Isobutyrate, (C) Levels of Isovalerate, (D) Correlation between levels of valerate and anxiety index, (E) Correlation between levels of isobutyrate and anxiety index, (F) Correlation between levels of isovalerate and anxiety index. Data from SCFA levels passed the normality test and were analyzed using one way-ANOVA, followed by Tukey's multiple comparisons test. Correlational analysis of the groups with significant differences were performed using Pearson's correlation (blue = Control, green = SPS-R, red = SPS-S). All data are expressed as means ± SEM. ns = not significant, *p < 0.05, **p < 0.01, ***P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Blood brain barrier permeability and neuroinflammation after SPS
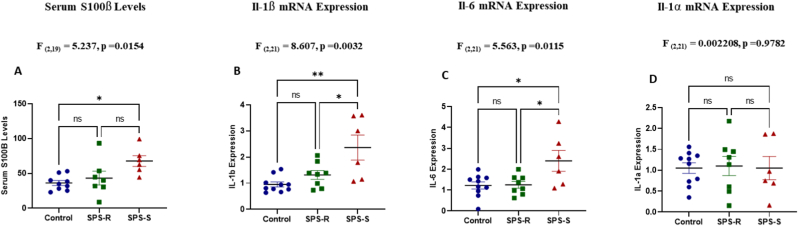
To further understand the underlying causes of the distinct behavioral phenotypes observed in the SPS subgroups, we assessed blood brain barrier (BBB) permeability. The levels of the astrocyte protein S100β in serum were significantly higher in the SPS-S subgroup compared to unstressed controls (Fig. 8A). Since neuroinflammation is commonly reported in mood disorders, we also assessed the inflammatory state of the ventral hippocampus in the subgroups. The mRNA levels of Il-1β (Fig. 8B), Il-6 (Fig. 8C), but not Il-1α (Fig. 8D) were significantly higher in the SPS-S subgroup compared to SPS-R and unstressed controls indicating neuroinflammation.
Fig. 8.
Exposure to SPS Increased Blood Brain Barrier (BBB) Permeability and Neuroinflammation in SPS-S Subgroup.
Serum samples and ventral hippocampal tissues were collected from each animal on the dissection day and used to assess BBB permeability and neuroinflammation respectively. (A) Serum levels of S100β analyzed by ELISA, (B) mRNA expression levels of Il-1β (C) mRNA expression levels of Il-6, (D) mRNA expression levels of Il-1α. All data passed the normality test and were analyzed using one way-ANOVA, followed by Tukey's multiple comparisons test. Each dot represents value for an individual animal. A single value/group, 2 SD away from the mean were excluded from the analysis. All data are expressed as means ± SEM. ns = not significant, *p < 0.05, **p < 0.01.
4. Discussion
This study demonstrated for the first time the ability of SPS model to segregate female rats with resilient and susceptible phenotypes. Significant pre-existing differences in gut microbial composition and predictive functionality of the SPS-resilient and susceptible rats were found, which correlated with their ability to cope with the traumatic stress-induced behavioral impairments. Following exposure to SPS, the gut microbial composition shifted in both subgroups, yet the microbial communities in SPS-R and SPS-S subgroups were shaped differently. This was reflected by the increased alpha diversity, altered abundance of several genera and the bacterial metabolites in SPS-S subgroup compared to SPS-R and unstressed controls, which displayed similar variations. The behavioral and microbial alterations in SPS-S rats were further accompanied by molecular changes in the brain as evident by the increased BBB permeability and neuroinflammation in the ventral hippocampus. The observed differences in the gut-brain axis in susceptible animals might be key to the development of new and more effective treatments to enhance resilience.
The SPS model, a translationally relevant and widely used animal model for PTSD (Lisieski et al., 2018), along with other stress models, has been shown to induce divergent responses to traumatic stress in male rodents, with only a subset of animals displaying anxiety-like behavior, whereas the remaining animals behaving like unstressed controls (Le Dorze and Gisquet-Verrier, 2016; Serova et al., 2019; Tanelian et al., 2022). This model has also been shown to induce some of the PTSD-associated behavioral deficits in females (Nahvi et al., 2021). Moreover, differences in the behavioral outcomes to SPS-triggered impairments between males and females have been observed (Keller et al., 2015; Mancini et al., 2021). Yet, none of the studies so far have assessed if the SPS model can differentiate distinct inter-individual coping responses in females. Here we demonstrated for the first time, that two weeks following the traumatic stress, a subgroup of animals in the SPS group displayed increased anxiety-like behavior on the EPM and impaired social interaction on SI than their SPS counterparts. Moreover, these animals maintained a consistent susceptible phenotype throughout the behavioral tests.
In accordance with our hypothesis, we observed significant pre-existing differences in the gut microbial composition and predictive functionality between SPS-susceptible and resilient rats. For instance, before exposure to SPS, SPS-R subgroup harbored significantly higher abundances of Roseburia, Oscillibacter, and Lachnospiracea_Incertae_Sedis compared to the SPS-S subgroup. These three genera also correlated inversely and significantly with the anxiety index, indicating their beneficial contributions to the host's ability to withstand SPS-induced maladaptive behavioral alterations. Thus, our data suggest that pre-trauma low abundances of Roseburia, Oscilibacter and Lachnospiracea_Incertae_Sedis might represent a risk factor predicting the trauma susceptibility in females. In fact, the genus Roseburia plays a significant role in metabolic reprogramming, immune system maturation and modulation, and gut barrier sustainability (Tamanai-Shacoori et al., 2017). Several clinical and preclinical studies have reported lower abundance of Roseburia in psychiatric and inflammatory disorders (Gevers et al., 2014; Keshavarzian et al., 2015; Kumari et al., 2013; Mondot et al., 2022; Rajilić-Stojanović et al., 2013; Zheng et al., 2016), and accumulating evidence from animal and human studies supports its use as a probiotic (Kellermayer, 2019; Martínez et al., 2013; Seo et al., 2020; Shen et al., 2018; Tan et al., 2019; Xu et al., 2021). Similarly, the genera Oscillibacter and Lachnospiracea_Incertae_Sedis have been shown to promote health and play beneficial roles in the gut (Asnicar et al., 2021; Duncan et al., 2007; Liu et al., 2016; Rinninella et al., 2019; Shi et al., 2020; Wang et al., 2021).
Alterations in the gut microbial composition following exposure to stress have been reported in females (Bangsgaard Bendtsen et al., 2012; Gur et al., 2017; Liu et al., 2016); however, none of these studies included the aspect of discrimination between susceptible and resilient phenotypes. We uncovered significant shifts in the indices of alpha diversity following exposure to SPS, with the SPS-S subgroup displaying overall higher alpha diversity compared to the SPS-R subgroup. Similar increases were observed in stress-susceptible rats following social defeat stress (Pearson-Leary et al., 2020). It is plausible that this increase in community diversity could be attributed to the ability of SPS stressors to destabilize the gut microbial niche and introduce new pathobionts, especially in the SPS-S subgroup, which lacked a stable gut microbial niche, as was evident by the lower alpha diversity before SPS exposure. In turn, these newly introduced species may have exacerbated the effects of SPS. Indeed, after SPS, the abundance of the genera Anaerovorax and Flavonifractor increased significantly in the SPS-S subgroup and correlated positively with the anxiety index, indicating their possible involvement in the susceptibility of subjects to SPS. The genus Flavonifractor includes species whose metabolism is mainly focused on the consumption of γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the CNS, thus contributing to depression and anxiety (Strandwitz et al., 2019). A higher abundance of Flavonifractor and a positive correlation with disease severity were observed in patients with depression (Knuesel and Mohajeri, 2021). In contrast, the relative abundances of Bifidobacterium, Clostridium Sensu Stricto, Clostridium XI, and Turicibacter were significantly lower in the SPS-S subgroup compared to the SPS-R and unstressed controls and correlated negatively with anxiety index. In this regard, lower relative abundances of Bifidobacterium, Clostridium XI, and Turicibacter have been reported in patients with IBS and depression relative to healthy controls (Aizawa et al., 2016; Liu et al., 2016). Bifidobacterium can alleviate the symptoms of mood disorders and is well established as a potent probiotic in clinical and pre-clinical studies (Messaoudi et al., 2011; Savignac et al., 2014; Yang et al., 2017). Similarly, the genus Turicibacter has recently been suggested to alleviate depression (Fung et al., 2019). Ninety percent of the body's serotonin originate from the walls of the intestine, and it has been shown that T. Sanguinis, a member in the genus Turicibacter is responsible for the production of around half of the body's total serotonin (Fung et al., 2019).
SCFAs are among the major microbial metabolites, known to play a key role in influencing the subject's brain and behavior both directly and indirectly (Dalile et al., 2019). While there were no differences in the major forms of cecal SCFAs, the levels of minor and BCFAs were significantly different among the groups. BCFAs make up to 5–10% of the total SCFAs. They are derived from the fermentation of proteins and their production is suggested to be toxic to colonocytes and induce inflammation (Aguirre et al., 2016). In this study, SPS-S subgroup had significantly higher levels of isovalerate and isobutyrate relative to SPS-R and unstressed controls. BCFAs are not well studied, and their implications on host health and disease are still unclear. However, the levels of both BCFAs correlated positively and significantly with anxiety index in our study, similar to the correlation observed in patients with depression (Szczesniak et al., 2016). Thus, it is plausible that the local inflammation caused by BCFAs in the gut might be another pathway by which the microbiota contributed to development of the stress susceptible phenotype in SPS-S female rats.
One of the mechanisms by which the gut microbiota impacts brain function is through the regulation of BBB permeability (Braniste et al., 2014). The role of BBB in the pathogenesis of neurological disorders has gained attention, as alterations in its structure and permeability have been identified in a range of psychiatric disorders (Bell and Zlokovic, 2009; Nakagawa and Chiba, 2016; Salehi et al., 2017; Walker, 2018; Wang et al., 2022). The levels of the astrocyte protein S100β in serum is a widely used marker for increased BBB permeability (Abbott, 2000; Kawata et al., 2016; Xanthos and Sandkühler, 2014). In the current study, we observed higher serum levels of S100β in the SPS-S subgroup. In this regard, overexpression of S100β in female mice was shown to induce depressive-like behavior (Stroth and Svenningsson, 2015), and antidepressant treatments in MDD patients attenuated serum S100β levels (Schroeter et al., 2002, 2008). We analyzed BBB permeability only at the end of the experiment, and we cannot determine whether the observed differences between the cohorts pre-existed or resulted from SPS exposure. Yet, recent studies reported elevated levels of toxic microbial metabolites, such as p-crestol, capable of disrupting BBB integrity, which coincided with trauma-induced gut microbial alterations (Laudani et al., 2023; Shah et al., 2022). This further highlights the importance of gut microbial metabolites in the gut-brain communication axis. Finally, we also observed significantly higher levels of mRNA for proinflammatory cytokines IL-1β and IL-6 in the ventral hippocampus of the SPS-S subgroup compared to the SPS-R and unstressed controls. IL-1β is reported to lead to behavioral alterations by inhibiting hippocampal neurogenesis (Koo et al., 2010), and the levels of IL-6 are associated with decreased hippocampal volume in patients with MDD (Rothaug et al., 2016), and in SDS animal model (Cathomas et al., 2019; Hodes et al., 2016). Thus, our results are consistent with the likely involvement of increased BBB permeability and neuroinflammation in susceptibility to SPS in female rodents.
4.1. Limitations
In the present study, we used 16S rDNA sequencing, which limited our taxonomic profiling to the genus level. However, each genus contains different species and strains of bacteria that are involved in various metabolic, functional, and host regulatory roles. Thus, in future studies, we will use whole genome or shotgun metagenomics to obtain more comprehensive taxonomic, functional, and metabolomic analyses. Similarly, due to taxonomic limitations, our data enabled the identification of only correlative and not necessarily causative effects between the microbial composition and behavioral phenotypes in SPS-R and SPS-S animals. Further mechanistic studies are required to validate these findings. Additionally, bacterial metabolites (SCFA) were analyzed at the end of the experiment, and we could not determine whether the differences between the cohorts pre-existed or resulted from SPS exposure. Finally, estrogen plays an important role in stress regulation. Here, the experimental design and SPS time were adjusted to avoid peak estrogen levels as much as possible. Animals on Proestrus at the time of behavioral testing were excluded. Furthermore, only one day of the estrous cycle was sampled, as opposed to following the cycle for at least 4 consecutive days. The aim was to avoid the peak estrogen phase on the days of SPS and behavioral test and not determine whether the animals were cycling regularly. Further experiments at different times of the estrous cycle, might lead to varied results or change the proportion of susceptible to resilient animals.
To our knowledge, this is the first study to report pre-existing and post-trauma differences in the gut microbial composition, functionality, and metabolites of outbred female rats that correlate with their ability to cope with the traumatic stress experiences. This is of high relevance because if translatable to humans, microbial signatures can serve as non-invasive biomarkers to predict an individual's susceptibility to developing psychiatric disorders. Additionally, due to their ease of manipulation, they can serve as a promising target to foster resiliency, especially in females, through dietary and/or pro/prebiotic supplementations. Finally, the gut microbiota can also provide a new avenue for the development of more effective, predictive, and therapeutic interventions for mood disorders including PTSD, for which there is a major unmet need.
Funding
This study was supported by internal NYMC sources.
Author contribution
Arax Tanelian: planning, experimentation, analysis, writing the manuscript: Bistra Nankova: planning, experimentation, analysis, writing the manuscript; Anish Cheriyan, Christopher Arens, Furung Hu: experimentation; Esther Sabban: planning, supervision of experimentation, analysis and writing. All authors read and approved the submitted manuscript.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We thank Dr. Edmund LaGamma for his insightful comments and support, and Mariam Miari for her assistance with Bioinformatic analysis.
Handling Editor: Dr. John Cryan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2023.100533.
Contributor Information
Arax Tanelian, Email: atanelia@student.touro.edu.
Bistra Nankova, Email: Bistra_Nankova@nymc.edu.
Anish Cheriyan, Email: acheriya@student.touro.edu.
Christopher Arens, Email: carens@student.touro.edu.
Furong Hu, Email: Furong_Hu@nymc.edu.
Esther L. Sabban, Email: sabban@nymc.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abbott N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000;20(2):131–147. doi: 10.1023/a:1007074420772. [DOI] [PubMed] [Google Scholar]
- Affairs U.S. D.o.V. National Center for PTSD; 2022. PTSD. [Google Scholar]
- Aguirre M., Eck A., Koenen M.E., Savelkoul P.H., Budding A.E., Venema K. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res. Microbiol. 2016;167(2):114–125. doi: 10.1016/j.resmic.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A., Payard I., Rolland C., Harkat C., Braniste V., Théodorou V., Tompkins T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal Axis modulation. J Neurogastroenterol Motil. 2018;24(1):138–146. doi: 10.5056/jnm16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa E., Tsuji H., Asahara T., Takahashi T., Teraishi T., Yoshida S., Ota M., Koga N., Hattori K., Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016;202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Alves-Dos-Santos L., Resende L.S., Chiavegatto S. Susceptibility and resilience to chronic social defeat stress in adolescent male mice: No correlation between social avoidance and sucrose preference. Neurobiol Stress. 2020;12 doi: 10.1016/j.ynstr.2020.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault-Bréard J., Rondeau I., Gilbert K., Girard S.A., Tompkins T.A., Godbout R., Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012;107(12):1793–1799. doi: 10.1017/s0007114511005137. [DOI] [PubMed] [Google Scholar]
- Asnicar F., Berry S.E., Valdes A.M., Nguyen L.H., Piccinno G., Drew D.A., Leeming E., Gibson R., Le Roy C., Khatib H.A., Francis L., Mazidi M., Mompeo O., Valles-Colomer M., Tett A., Beghini F., Dubois L., Bazzani D., Thomas A.M.…Segata N. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsgaard Bendtsen K.M., Krych L., Sørensen D.B., Pang W., Nielsen D.S., Josefsen K., Hansen L.H., Sørensen S.J., Hansen A.K. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanssen T.F.S., Gururajan A., van de Wouw M., Moloney G.M., Ritz N.L., Long-Smith C.M., Wiley N.C., Murphy A.B., Lyte J.M., Fouhy F., Stanton C., Claesson M.J., Dinan T.G., Cryan J.F. Volatility as a concept to understand the impact of stress on the microbiome. Psychoneuroendocrinology. 2021;124 doi: 10.1016/j.psyneuen.2020.105047. [DOI] [PubMed] [Google Scholar]
- Beery A.K., Zucker I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011;35(3):565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.D., Zlokovic B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118(1):103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., Gulyás B., Halldin C., Hultenby K., Nilsson H., Hebert H., Volpe B.T., Diamond B., Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263) doi: 10.1126/scitranslmed.3009759. 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10(3):198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Cathomas F., Murrough J.W., Nestler E.J., Han M.H., Russo S.J. Neurobiology of resilience: interface between mind and body. Biol. Psychiatr. 2019;86(6):410–420. doi: 10.1016/j.biopsych.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H., Liu T., Kozlovsky N., Kaplan Z., Zohar J., Mathé A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Duncan S.H., Louis P., Flint H.J. Cultivable bacterial diversity from the human colon. Lett. Appl. Microbiol. 2007;44(4):343–350. doi: 10.1111/j.1472-765X.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- Elwenspoek M.M.C., Kuehn A., Muller C.P., Turner J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology. 2017;82:140–154. doi: 10.1016/j.psyneuen.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Fung T.C., Vuong H.E., Luna C.D.G., Pronovost G.N., Aleksandrova A.A., Riley N.G., Vavilina A., McGinn J., Rendon T., Forrest L.R., Hsiao E.Y. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 2019;4(12):2064–2073. doi: 10.1038/s41564-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L.A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S.J., Yassour M., Morgan X.C., Kostic A.D., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J.…Xavier R.J. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor G.B., Macklaim J.M., Pawlowsky-Glahn V., Egozcue J.J. Microbiome datasets are compositional: and this is not optional. Front. Microbiol. 2017;8:2224. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.J., Elhai J.D., Frueh B.C. Enhancing patient satisfaction and increasing treatment compliance: patient education as a fundamental component of PTSD treatment. Psychiatr. Q. 2004;75(4):321–332. doi: 10.1023/b:psaq.0000043508.52428.6e. [DOI] [PubMed] [Google Scholar]
- Gur T.L., Shay L., Palkar A.V., Fisher S., Varaljay V.A., Dowd S., Bailey M.T. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 2017;64:50–58. doi: 10.1016/j.bbi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Ménard C., Russo S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol Stress. 2016;4:15–22. doi: 10.1016/j.ynstr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaric I., Rocks D., Cham H., Herchek A., Kundakovic M. Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model. Front. Mol. Neurosci. 2019;12:74. doi: 10.3389/fnmol.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., Li L., Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jiang H.Y., Zhang X., Yu Z.H., Zhang Z., Deng M., Zhao J.H., Ruan B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Kawata K., Liu C.Y., Merkel S.F., Ramirez S.H., Tierney R.T., Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci. Biobehav. Rev. 2016;68:460–473. doi: 10.1016/j.neubiorev.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.M., Schreiber W.B., Staib J.M., Knox D. Sex differences in the single prolonged stress model. Behav. Brain Res. 2015;286:29–32. doi: 10.1016/j.bbr.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer R. Roseburia species: prime candidates for microbial therapeutics in inflammatory bowel disease. Gastroenterology. 2019;157(4):1164–1165. doi: 10.1053/j.gastro.2019.05.073. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson's disease. Mov. Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- Knuesel T., Mohajeri M.H. The role of the gut microbiota in the development and progression of major depressive and bipolar disorder. Nutrients. 2021;14(1) doi: 10.3390/nu14010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J.W., Russo S.J., Ferguson D., Nestler E.J., Duman R.S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. U. S. A. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.K., Guest P.C., Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol. Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- Kumari R., Ahuja V., Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013;19(22):3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz-Bluhm M.D., Bondi C.O., Doyen J., Rodriguez G.A., Bédard-Arana T., Morilak D.A. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J. Neuroendocrinol. 2008;20(10):1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudani S., Torrisi S.A., Alboni S., Bastiaanssen T.F.S., Benatti C., Rivi V., Moloney R.D., Fuochi V., Furneri P.M., Drago F., Salomone S., Tascedda F., Cryan J.F., Leggio G.M. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav. Immun. 2023;107:385–396. doi: 10.1016/j.bbi.2022.11.004. [DOI] [PubMed] [Google Scholar]
- Le Dorze C., Gisquet-Verrier P. Sensitivity to trauma-associated cues is restricted to vulnerable traumatized rats and reinstated after extinction by yohimbine. Behav. Brain Res. 2016;313:120–134. doi: 10.1016/j.bbr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Li Z.L., Wang Y., Zou H.W., Jing X.Y., Liu Y.J., Li L.F. GABA(B) receptors within the lateral habenula modulate stress resilience and vulnerability in mice. Physiol. Behav. 2021;230 doi: 10.1016/j.physbeh.2021.113311. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Young E.A. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology. 1997;22(6):411–422. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Lisieski M.J., Eagle A.L., Conti A.C., Liberzon I., Perrine S.A. Single-prolonged stress: a review of two decades of progress in a rodent model of post-traumatic stress disorder. Front. Psychiatr. 2018;9:196. doi: 10.3389/fpsyt.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang L., Wang X., Wang Z., Zhang J., Jiang R., Wang X., Wang K., Liu Z., Xia Z., Xu Z., Nie Y., Lv X., Wu X., Zhu H., Duan L. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016;14(11):1602–1611.e1605. doi: 10.1016/j.cgh.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Mancini G.F., Marchetta E., Riccardi E., Trezza V., Morena M., Campolongo P. Sex-divergent long-term effects of single prolonged stress in adult rats. Behav. Brain Res. 2021;401 doi: 10.1016/j.bbr.2020.113096. [DOI] [PubMed] [Google Scholar]
- Marcondes F.K., Miguel K.J., Melo L.L., Spadari-Bratfisch R.C. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol. Behav. 2001;74(4–5):435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Marin I.A., Goertz J.E., Ren T., Rich S.S., Onengut-Gumuscu S., Farber E., Wu M., Overall C.C., Kipnis J., Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017;7 doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I., Lattimer J.M., Hubach K.L., Case J.A., Yang J., Weber C.G., Louk J.A., Rose D.J., Kyureghian G., Peterson D.A., Haub M.D., Walter J. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7(2):269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014;34(46):15490–15496. doi: 10.1523/jneurosci.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren M.R., Willis A.D., Callahan B.J. Consistent and correctable bias in metagenomic sequencing experiments. Elife. 2019;8 doi: 10.7554/eLife.46923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean A.C., Valenzuela N., Fai S., Bennett S.A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012;67:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza C., Barreto G.E., Ávila-Rodriguez M., Echeverria V. Role of neuroinflammation and sex hormones in war-related PTSD. Mol. Cell. Endocrinol. 2016;434:266–277. doi: 10.1016/j.mce.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.F., Rougeot C., Pichelin M., Cazaubiel M., Cazaubiel J.M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105(5):755–764. doi: 10.1017/s0007114510004319. [DOI] [PubMed] [Google Scholar]
- Mondot S., Lachkar L., Doré J., Blottière H.M., Hanachi M. Roseburia, a decreased bacterial taxon in the gut microbiota of patients suffering from anorexia nervosa. Eur. J. Clin. Nutr. 2022;76(10):1486–1489. doi: 10.1038/s41430-022-01116-3. [DOI] [PubMed] [Google Scholar]
- Nahvi R.J., Tanelian A., Nwokafor C., Hollander C.M., Peacock L., Sabban E.L. Intranasal neuropeptide Y as a potential therapeutic for depressive behavior in the rodent single prolonged stress model in females. Front. Behav. Neurosci. 2021;15 doi: 10.3389/fnbeh.2021.705579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Chiba K. Involvement of neuroinflammation during brain development in social cognitive deficits in autism spectrum disorder and schizophrenia. J. Pharmacol. Exp. Therapeut. 2016;358(3):504–515. doi: 10.1124/jpet.116.234476. [DOI] [PubMed] [Google Scholar]
- Nasca C., Bigio B., Zelli D., Nicoletti F., McEwen B.S. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol. Psychiatr. 2015;20(6):755–763. doi: 10.1038/mp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R., Rudi K. Correlation between the human fecal microbiota and depression. Neuro Gastroenterol. Motil. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Pearson-Leary J., Zhao C., Bittinger K., Eacret D., Luz S., Vigderman A.S., Dayanim G., Bhatnagar S. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol. Psychiatr. 2020;25(5):1068–1079. doi: 10.1038/s41380-019-0380-x. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M., Shanahan F., Guarner F., de Vos W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel Dis. 2013;19(3):481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1) doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaug M., Becker-Pauly C., Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta. 2016;1863(6 Pt A):1218–1227. doi: 10.1016/j.bbamcr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Salehi A., Zhang J.H., Obenaus A. Response of the cerebral vasculature following traumatic brain injury. J. Cerebr. Blood Flow Metabol. 2017;37(7):2320–2339. doi: 10.1177/0271678x17701460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neuro Gastroenterol. Motil. 2014;26(11):1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Abdul-Khaliq H., Diefenbacher A., Blasig I.E. S100B is increased in mood disorders and may be reduced by antidepressive treatment. Neuroreport. 2002;13(13):1675–1678. doi: 10.1097/00001756-200209160-00021. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Abdul-Khaliq H., Krebs M., Diefenbacher A., Blasig I.E. Serum markers support disease-specific glial pathology in major depression. J. Affect. Disord. 2008;111(2–3):271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Seo B., Jeon K., Moon S., Lee K., Kim W.K., Jeong H., Cha K.H., Lim M.Y., Kang W., Kweon M.N., Sung J., Kim W., Park J.H., Ko G. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice. Cell Host Microbe. 2020;27(1):25–40.e26. doi: 10.1016/j.chom.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Serova L.I., Nwokafor C., Van Bockstaele E.J., Reyes B.A.S., Lin X., Sabban E.L. Single prolonged stress PTSD model triggers progressive severity of anxiety, altered gene expression in locus coeruleus and hypothalamus and effected sensitivity to NPY. Eur. Neuropsychopharmacol. 2019;29(4):482–492. doi: 10.1016/j.euroneuro.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Shah S.N., Knausenberger T.B.-A., Connell E., Gall G.L., Hardy T.A.J., Randall D.W., McCafferty K., Yaqoob M.M., Solito E., Müller M., Stachulski A.V., Glen R.C., Vauzour D., Hoyles L., McArthur S. Cerebrovascular damage caused by the gut microbe-derived uraemic toxin <em>p</em>-cresol sulfate is prevented by blockade of the epidermal growth factor receptor. bioRxiv. 2022;2022 doi: 10.1101/2022.11.12.516113. 2011.2012. [DOI] [Google Scholar]
- Shen Z., Zhu C., Quan Y., Yang J., Yuan W., Yang Z., Wu S., Luo W., Tan B., Wang X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018;33(10):1751–1760. doi: 10.1111/jgh.14144. [DOI] [PubMed] [Google Scholar]
- Shi Z., Qiu Y., Wang J., Fang Y., Zhang Y., Chen H., Du Q., Zhao Z., Yan C., Yang M., Zhou H. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: a cross sectional study. J. Neuroimmunol. 2020;339 doi: 10.1016/j.jneuroim.2019.577126. [DOI] [PubMed] [Google Scholar]
- Sonali S., Ray B., Ahmed Tousif H., Rathipriya A.G., Sunanda T., Mahalakshmi A.M., Rungratanawanich W., Essa M.M., Qoronfleh M.W., Chidambaram S.B., Song B.J. Mechanistic insights into the link between gut dysbiosis and major depression: an extensive review. Cells. 2022;11(8) doi: 10.3390/cells11081362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz P., Kim K.H., Terekhova D., Liu J.K., Sharma A., Levering J., McDonald D., Dietrich D., Ramadhar T.R., Lekbua A., Mroue N., Liston C., Stewart E.J., Dubin M.J., Zengler K., Knight R., Gilbert J.A., Clardy J., Lewis K. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N., Svenningsson P. S100B interacts with the serotonin 5-HT7 receptor to regulate a depressive-like behavior. Eur. Neuropsychopharmacol. 2015;25(12):2372–2380. doi: 10.1016/j.euroneuro.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Szczesniak O., Hestad K.A., Hanssen J.F., Rudi K. Isovaleric acid in stool correlates with human depression. Nutr. Neurosci. 2016;19(7):279–283. doi: 10.1179/1476830515y.0000000007. [DOI] [PubMed] [Google Scholar]
- Tamanai-Shacoori Z., Smida I., Bousarghin L., Loreal O., Meuric V., Fong S.B., Bonnaure-Mallet M., Jolivet-Gougeon A. Roseburia spp.: a marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- Tan B., Luo W., Shen Z., Xiao M., Wu S., Meng X., Wu X., Yang Z., Tian L., Wang X. Roseburia intestinalis inhibits oncostatin M and maintains tight junction integrity in a murine model of acute experimental colitis. Scand. J. Gastroenterol. 2019;54(4):432–440. doi: 10.1080/00365521.2019.1595708. [DOI] [PubMed] [Google Scholar]
- Tanelian A., Nankova B., Miari M., Nahvi R.J., Sabban E.L. Resilience or susceptibility to traumatic stress: potential influence of the microbiome. Neurobiol Stress. 2022;19 doi: 10.1016/j.ynstr.2022.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey R., Panizzon M.S., Kremen W.S., Franz C.E., Lyons M.J. A twin-study of genetic contributions to morningness-eveningness and depression. Chronobiol. Int. 2015;32(3):303–309. doi: 10.3109/07420528.2014.971366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya E.I., Spear L.P. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav. Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke K.A., Boobis A.R., Chiodini A., Edwards C.A., Franck A., Kleerebezem M., Nauta A., Raes J., van Tol E.A., Tuohy K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015;28(1):42–66. doi: 10.1017/s0954422415000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.C. Pathophysiology of status epilepticus. Neurosci. Lett. 2018;667:84–91. doi: 10.1016/j.neulet.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Wang S., Wei Y., Liu L., Li Z. Association between breastmilk microbiota and food allergy in infants. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.770913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ma L., Luo Y., Yang Y., Upreti B., Cheng Y., Cui R., Liu S., Xu J. Increasing of blood brain barrier permeability and the association with depression and anxiety in systemic lupus erythematosus patients. Front. Med. 2022;9 doi: 10.3389/fmed.2022.852835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthos D.N., Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014;15(1):43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- Xu F., Cheng Y., Ruan G., Fan L., Tian Y., Xiao Z., Chen D., Wei Y. New pathway ameliorating ulcerative colitis: focus on Roseburia intestinalis and the gut-brain axis. Therap. Adv. Gastroenterol. 2021;14 doi: 10.1177/17562848211004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Fujita Y., Ren Q., Ma M., Dong C., Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017;7 doi: 10.1038/srep45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., Zeng L., Chen J., Fan S., Du X., Zhang X., Yang D., Yang Y., Meng H., Li W., Melgiri N.D., Licinio J., Wei H., Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatr. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.