Abstract
Rationale & Objective
Widespread delivery of high-quality care for acute kidney injury (AKI) survivors after hospital discharge requires a multidisciplinary team. We aimed to compare management approaches between nephrologists and primary care providers (PCPs) and explored strategies to optimize collaboration.
Study Design
Explanatory sequential mixed-methods study using a case-based survey followed by semi-structured interviews.
Setting & Participants
Nephrologists and PCPs providing AKI survivor care at 3 Mayo Clinic sites and the Mayo Clinic Health System were included.
Outcomes
Survey questions and interviews elucidated participants’ recommendations for post-AKI care.
Analytical Approach
Descriptive statistics were used to summarize survey responses. Qualitative data analysis used deductive and inductive strategies. A connecting and merging approach was used for mixed-methods data integration.
Results
148 of 774 (19%) providers submitted survey responses (24/72 nephrologists and 105/705 PCPs). Nephrologists and PCPs recommended laboratory monitoring and follow-up with a PCP shortly after hospital discharge. Both indicated that the need for nephrology referral, and its timing should be dictated by clinical and non-clinical patient-specific factors. There were opportunities for improvement in medication and comorbid condition management in both groups. Incorporation of multidisciplinary specialists (eg, pharmacists) was recommended to expand knowledge, optimize patient-centered care, and alleviate provider workload.
Limitations
Survey findings may have been affected by non-response bias and the unique challenges facing clinicians and health systems during the COVID-19 pandemic. Participants were from a single health system, and their views or experiences may differ from those in other health systems or serving different populations.
Conclusions
A multidisciplinary team-based model of post-AKI care may facilitate implementation of a patient-centered care plan, improve adherence to best practices, and reduce clinician and patient burden. Individualizing care for AKI survivors based on clinical and non-clinical patient-specific factors is needed to optimize outcomes for patients and health systems.
Plain-Language Summary.
We conducted this study to better understand how primary care providers and nephrologists approach delivering post-hospital care to survivors of acute kidney injury (AKI). Surveys and interviews probed recommendations of primary care providers and nephrologists on kidney function monitoring, comorbid condition management, and collaborating with experts from various medical specialties and disciplines. Results emphasized a need for patient-centered care, based on clinical and non-clinical factors, to optimize outcomes. A multidisciplinary team-based model of post-AKI care that includes other specialists (eg, pharmacists, nurses, dieticians) may facilitate implementation of a patient-centered care plan, improve adherence to best practices, and reduce clinician and patient burden.
Editorial, •••
Acute kidney injury (AKI) affects 20% of hospitalized patients and increases the risk for new or worsening chronic kidney disease (CKD), cardiovascular disease, rehospitalization, and death.1, 2, 3, 4, 5, 6, 7, 8 To mitigate these adverse health outcomes, focused post-hospital follow-up of AKI survivors is recommended. Though evidence-based guideline recommendations are lacking, expert-recommended best practices for follow-up include kidney function monitoring, education, medication review and reconciliation, and blood pressure optimization.9 The best way to deliver this care efficiently, effectively, and sustainably is unknown. Studies have tested nephrologist-directed AKI survivorship clinics, but the feasibility of recruitment and retention, acceptability to patients, and scalability of specialist-oriented models are concerns that have hindered dissemination of these models into routine care.10, 11, 12, 13 AKI survivors and nephrologists acknowledge that for widespread uptake of post-AKI care models to occur, there is a critical need to engage a multidisciplinary team in follow-up care.9
Primary care providers (PCPs) are ideal partners to collaboratively care for AKI survivors alongside nephrologists. PCPs have established relationships with patients and are familiar with the full spectrum of a patient’s conditions, of which AKI is typically one of many. PCPs also have insight into non-clinical factors that affect patients’ health and an understanding of local resource constraints, such as access to specialists. At most, outpatient nephrologists see one-third of AKI survivors in post-hospital follow-up,14, 15, 16 making the primary care setting the main source of post-AKI care for most patients. It is unknown how different provider specialties (PCPs and nephrologists) approach AKI survivor care practices following hospital dismissal and their preferred strategies for collaborative care delivery. This lack of knowledge hinders the ability to design and implement scalable health care delivery models that leverage coordinated care to meet the needs of the diverse population of AKI survivors.
To address these knowledge gaps, we conducted a mixed-methods study17 of PCPs and nephrologists who care for non–dialysis-dependent AKI survivors. The primary aim was to compare AKI survivor care management between nephrologists and PCPs using clinical cases. Key practices evaluated included the timing of post-dismissal follow-up, laboratory testing practices, medication and comorbid condition review, and provider and patient education. The secondary aim was to explore strategies to optimize collaborative care, specifically related to how and when to engage nephrologists and multidisciplinary team members. These data can inform how PCPs and other multidisciplinary team members can be integrated into a comprehensive care model to improve health care access and reduce the strain on nephrologists.
Methods
This was a 2-phase, explanatory sequential mixed-methods study17 conducted from October 2020 to January 2022. The Institutional Review Board at Mayo Clinic reviewed and approved this study (#20-008793) and participants provided informed consent. A detailed description of the methods was published previously.18
Phase 1—Quantitative Strand
Three sample cases of AKI survivors were developed. Following each case, closed-ended questions prompted respondents to select their preferred recommendations (Table S1). As no comprehensive evidence-based guideline exists for post-AKI care, questions were based on the Kidney Awareness Monitoring and Prevention Society framework, a kidney health care bundle recommended to improve post-AKI care quality, developed by experts (Table S2).9 Select questions contained evidence-based “best answers” (eg, guideline-directed blood pressure goals, package insert guidance for drug dosing in kidney dysfunction).19, 20, 21, 22, 23, 24 The survey was pilot-tested to enhance validity, and the instrument was refined.18
The sampling frame included staff physicians and advanced practice providers (APPs; nurse practitioners and physician assistants) with at least 1 year of experience in Nephrology, Internal Medicine, and Family Medicine who provide post-hospital care for AKI survivors at Mayo Clinic in Rochester, Florida, Arizona, and the Mayo Clinic Health System. These sites included providers affiliated with academic and community practices in urban, suburban, and rural settings who treat patients from various sociodemographic backgrounds. The invitation to participate in the study was extended via email and the survey was administered using RedCap Survey technology.
Data Collection and Analysis
Descriptive statistics were used to report demographics and summarize survey responses. For the 6 ‘best answer’ responses, 1 point was assigned for each best answer selected and 0 points assigned for other answers (total possible 6 points). Mean best answer scores were compared between nephrologists and PCPs using the t test. With 18 participants per group, we had 80% power to detect a mean between-group difference of 2 points, assuming a standard deviation of 2, using an alpha of 0.05.
We used a connecting and merging approach for mixed-methods data integration, where results from the survey informed the sampling strategy and questions for the qualitative strand.
Phase 2—Qualitative Strand
Sample
Purposive sampling of survey responders was used to recruit providers for semi-structured interviews. Participants were sampled based on key factors that may influence post-AKI care recommendations (eg, academic vs rural practice setting). Recruitment of participants ceased when data analysis suggested thematic saturation.25
Data Collection and Analysis
An open-ended interview guide was developed to establish a deeper understanding of select post-AKI care recommendations observed in the quantitative strand, with a focus on how and when to engage nephrologists and multidisciplinary specialists for collaborative care. We explored emerging issues not measured by the survey, including educational needs and themes identified during inductive analysis. Interviews were conducted by an experienced qualitative researcher (D.M.F.), recorded, and transcribed verbatim with permission from participants.
Deductive (using a priori codes related to post-AKI care components and collaborative care strategies) and inductive (identification of emerging themes) strategies were used to analyze data. Content experts (H.P.M., E.F.B.) and a qualitative data analyst (D.M.F.) discussed themes and created a preliminary codebook, which was then applied to all data. Themes were compared between nephrologists and PCPs, and areas of complementarity, concordance, and discordance were identified.
Results
Participants
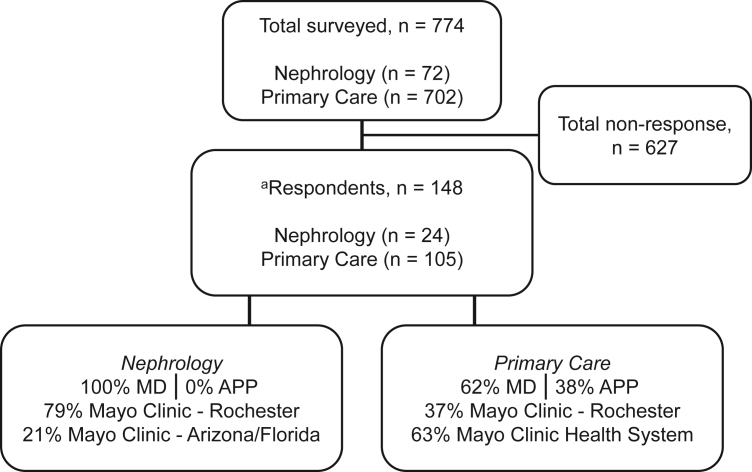
Of the 774 participants contacted, 148 providers submitted complete or partial responses to the study questionnaire (19% response rate). Of these, 24 were nephrologists and 105 were PCPs (Fig 1, Table 1). Nineteen responders did not provide demographic data and thus their responses could not be categorized according to specialty.
Figure 1.
Participant flowchart. Abbreviations: APP, advanced practice provider; MD, medical doctor. aIncomplete response, n=19.
Table 1.
Participant Characteristics
| Survey Respondents | |||
|---|---|---|---|
| Overall n=148 |
Neph n=24 |
PCP n=105 |
|
| Years in practice | |||
| 1-5 | 44 (30%) | 7 (29%) | 37 (35%) |
| 6-10 | 19 (13%) | 2 (8%) | 17 (16%) |
| >11 | 66 (44%) | 15 (63%) | 51 (49%) |
| No response | 19 (13%) | ||
| Degree | |||
| Physician | 89 (60%) | 24 (100%) | 65 (62%) |
| NP/PA | 40 (27%) | 0 | 40 (38%) |
| No response | 19 (13%) | ||
| Trained elsewhere | |||
| Yes | 57 (39%) | 15 (63%) | 42 (40%) |
| No | 72 (48%) | 9 (37%) | 63 (60%) |
| No response | 19 (13%) | ||
| Practice site | |||
| Mayo Clinic Rochester | 58 (39%) | 19 (79%) | 39 (37%) |
| Mayo Clinic Health System | 66 (45%) | 0 | 66 (63%) |
| Mayo Clinic Arizona | 3 (2%) | 3 (13%) | 0 |
| Mayo Clinic Florida | 2 (1%) | 2 (8%) | 0 |
| No response | 19 (13%) | ||
| Interview Participants | |||
|---|---|---|---|
|
Overall n=17 |
Neph n=8 |
PCP n=9 |
|
| Years in practice | |||
| 1-5 | 5 (29%) | 2 (25%) | 3 (33%) |
| 6-10 | 8 (47%) | 4 (50%) | 4 (45%) |
| >10 | 4 (24%) | 2 (25%) | 2 (22%) |
| Degree | |||
| Physician | 11 (65%) | 7 (86%) | 4 (44%) |
| NP/PA | 6 (35%) | 1 (14%) | 5 (56%) |
| Practice site | |||
| Mayo Clinic Rochester | 7 (41%) | 5 (63%) | 2 (22%) |
| Mayo Clinic Health System | 8 (47%) | 1 (13%) | 7 (78%) |
| Mayo Clinic Arizona | 1 (6%) | 1 (13%) | 0 |
| Mayo Clinic Florida | 1 (6%) | 1 (13%) | 0 |
Note: Data reported as n (% of the column total).
Abbreviations: Neph, nephrologist; NP, nurse practitioner; PA, physician assistant; PCP, primary care provider.
Seventeen of the 40 survey respondents contacted participated in qualitative interviews (Table 1). Eight interview participants were from a community or rural practice site. Of the 23 providers who did not respond to the invitation to interview, all but 2 were PCPs and 14 were affiliated with a community or rural practice site.
Overview of Findings
Quantitative and qualitative data were organized by the domains of (1) post-AKI care components and (2) collaborative care strategies. An emerging theme from qualitative interviews was the importance of delivering patient-centered post-AKI care, where providers and patients partner to co-design personalized care. To facilitate thematic analysis, we organized these findings using an established patient-centered care framework (Table 2).26
Table 2.
Implementing a Patient-Centered Care Model for Acute Kidney Injury Survivors
| Recommendation | Representative Quotes |
|---|---|
| Structure: Organizational-level components of health care delivery | |
| Increasing integration of, and access to, input from diverse health care professionals (eg, pharmacists, dieticians) |
|
| Development of patient-centered kidney health education |
|
| Process: Components of the patient-health care provider interaction | |
| Shared decision-making, patient goals of care |
|
| Patient access to resources necessary to complete follow-up (eg, transportation, technology) |
|
| Existing care team complexity |
|
| Outcomes: outcomes experienced by patients resulting from the care model | |
| Risk of future kidney-related complications |
|
| Timing of specialist availability |
|
| Care provider and laboratory accessibility (eg, physical location, hours of operation) |
|
| Timely availability of test results in rural areas |
|
Qualitative analysis of emerging themes revealed nephrologists and PCPs favored patient-centered care when recommending post-AKI care components and collaborative care. These can be organized according to factors related to organizational structure and processes and patient outcomes experienced as a result of the health care delivery model.26
Abbreviations: AKI, acute kidney injury; APP, advanced practice provider; CKD, chronic kidney disease; PCP, primary care provider.
Post-AKI Care Components
Timing of Post-Dismissal Follow-up
Eighty-five percent of nephrologists and 63% of PCPs recommended post-hospital laboratory follow-up within 2 weeks of discharge.
Interview participants elaborated that follow-up with a PCP should occur within 1-2 weeks of hospital discharge and variably thereafter with nephrologists. Nephrologists indicated preference for nephrology follow-up within 1-3 months of hospital dismissal. Some interviewees from both disciplines preferred an earlier evaluation by nephrologists (eg, within 1-3 weeks) for patients with multiple comorbid conditions, more severe AKI, or incomplete kidney function recovery by dismissal.
“Follow-up with a [PCP] within a week of discharge. Follow-up with a Nephrologist, that time period is a bit more nuanced.” (ID 5; Nephrologist, physician)
PCPs emphasized patient-centered care when scheduling follow-up, including the need to consider non-clinical factors (ie, transportation, access to technology, insurance and costs). They also expressed concerns about organizational barriers, such as limited access to nephrologists and long wait times for appointments (Table 2).
Laboratory Monitoring
Urine studies (eg, urine protein, urinalysis) were recommended more often by nephrologists than by PCPs (Table 3, questions 1, 5). The proportions of respondents recommending urine studies in both groups were highest when a patient had evidence of proteinuria on admission.
Table 3.
Survey Responses
| Question/Responses | Neph n=24 |
PCP n=105 |
|---|---|---|
| Case 1 Summary: 40-year-old, AKI-Dialysis, no dialysis but incomplete recovery at dismissal | ||
| Question 1. What kidney function follow-up tests, if any, would you recommend for kidney function monitoring after discharge? (Select all that apply) | ||
| a. Serum creatinine | 22 (92%) | 105 (100%) |
| b. Cystatin C | 6 (25%) | 9 (9%) |
| c. Urine albumin-creatinine ratio | 13 (54%) | 35 (33%) |
| d. None/other | 8 (33%) | 10 (10%) |
| Question 2. When would you recommend follow-up laboratory monitoring of kidney function? | ||
| a. Within 14 days | 15 (63%) | 87 (83%) |
| b. Within 1-2 months | 9 (37%) | 17 (16%) |
| c. Within 6 months | 0 | 1 (1%) |
| d. Defer timing as indicated for other health care needs | 0 | 0 |
| Question 3. How would you optimize this patient’s medication regimen at discharge?19 | ||
| a. Switch atorvastatin to rosuvastatin | 0 | 2 (2%) |
| b. Monitor lithium levels and consider dose reduction (best answer) | 22 (92%) | 82 (78%) |
| c. Discontinue apixaban and initiate warfarin therapy | 1 (4%) | 4 (4%) |
| d. None of the above/unsure | 1 (4%) | 17 (16%) |
| Question 4. To what extent do you agree that this patient should be referred to a nephrologist for follow-up at the time of hospital discharge? | ||
| a. Agree | 21 (88%) | 64 (61%) |
| b. Disagree | 3 (12%) | 41 (39%) |
| Case 2 Summary: 65-year-old, AKI stage 2 with proteinuria, incomplete recovery at dismissal | ||
| Question 5. What kidney function follow-up tests, if any, would you recommend for kidney function monitoring after discharge? (Select all that apply) | ||
| a. Serum creatinine | 22 (92%) | 100 (100%) |
| b. Cystatin C | 5 (21%) | 4 (4%) |
| c. Urine albumin-creatinine ratio | 19 (79%) | 53 (51%) |
| d. None/other | 5 (21%) | 13 (12%) |
| Question 6. Based on the 2017 ACC/AHA guideline recommendations for the management of hypertension, what is an appropriate blood pressure goal for this patient?20 | ||
| a. <140/90 mm Hg | 2 (8%) | 22 (21%) |
| b. <130/80 mm Hg (best answer) | 21 (88%) | 80 (76%) |
| c. <120/90 mm Hg | 1 (4%) | 2 (2%) |
| d. <150/90 mm Hg | 0 | 0 |
| e. Unsure | 0 | 1 (1%) |
| Question 7. What additional therapy, if any, would you consider adding at outpatient follow-up, based on her kidney function at the time of discharge and past medical history? (Select all that apply)20,21,22 | ||
| a. Lisinopril (best answer) | 22 (92%) | 93 (89%) |
| b. Ezetimibe | 0 | 0 |
| c. Atorvastatin (best answer) | 8 (33%) | 28 (27%) |
| d. Furosemide | 3 (13%) | 3 (3%) |
| e. None | 1 (4%) | 6 (6%) |
| Question 8. To what extent do you agree that this patient should be referred to a nephrologist for follow-up at the time of hospital discharge? | ||
| a. Agree | 15 (63%) | 31 (30%) |
| b. Disagree | 9 (37%) | 74 (70%) |
| Case 3 Summary: 70-year-old, AKI stage 2 with proteinuria, multimorbidity, incomplete recovery at dismissal | ||
| Question 9. What action should be taken with regard to his metformin prescription at this time?23 | ||
| a. Discontinue (best answer) | 15 (63%) | 76 (72%) |
| b. Reduce the dose to metformin 500 mg twice daily | 6 (25%) | 23 (22%) |
| c. Continue at the current dose | 3 (13%) | 2 (2%) |
| d. Unsure | 0 | 4 (4%) |
| Question 10. What other work-up, if any, would you recommend in the outpatient setting given his baseline comorbidities? (Select all that apply) 24 | ||
| a. None | 2 (8%) | 9 (9%) |
| b. Rheumatologic work-up for recurrent gouty arthritis | 6 (25%) | 13 (12%) |
| c. Anemia and metabolic bone disease work-up in the setting of chronic kidney disease (best answer) | 19 (80%) | 73 (70%) |
| d. Hematologic work-up given his history of thromboembolism and bleeding | 10 (42%) | 41 (39%) |
| Question 11. To what extent do you agree that this patient should be referred to a nephrologist for follow-up at the time of hospital discharge? | ||
| a. Agree | 24 (100%) | 95 (90%) |
| b. Disagree | 0 | 10 (10%) |
Note: Data reported as n (% of the column total).
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; AKI, acute kidney injury; Neph, nephrologist; PCP, primary care provider.
Medication and Comorbid Condition Care
The mean (standard deviation) sum of “best answers” selected (maximum possible = 6) was 4.5 (1.2) for nephrologists and 4.1 (1.1) for PCPs (P = 0.20). Eight percent of nephrologists and 22% of PCPs did not recommend therapeutic drug monitoring of a high-risk medication (ie, lithium). Thirty-seven percent of nephrologists and 29% of PCPs did not recommend appropriate discontinuation of a glucose-lowering medication (ie, metformin) in the setting of advanced CKD. Sixty-seven percent of nephrologists and 73% of PCPs did not recommend guideline-directed statin therapy.
In qualitative interviews, nephrologists and PCPs alike endorsed the need to iteratively review medications because of dynamic kidney function among AKI survivors and strongly recommended engaging a clinical pharmacist for medication management. Pharmacist involvement was also recognized as a solution for overcoming barriers to medication-related guideline adherence identified in the survey.
“If I could have the ideal setup, my patients would all have easy access to a clinical pharmacist to review potential medication interactions, ensure medications are dosed correctly for the current kidney staging, help them with timing of medications, which ones can be taken together… We don’t have the time during our post-hospital clinic visits.” (ID 17; Nephrologist, APP)
Provider and Patient Education
Interview participants offered potential solutions for inconsistencies in follow-up care and guideline adherence. PCPs desired educational resources and practice guidance (eg, algorithms, online best-practice documents) endorsed by nephrologists addressing various clinical scenarios in AKI survivor care. Core components of interest included the ideal type and timing of kidney function tests, nephrology referral criteria, and the appropriateness of telehealth.
“Having an algorithm or guidelines of what to do for follow-up appointments wouldn’t be a bad idea…we try to provide [recommendations for follow-up] but I’m not sure we’re successful.” (ID 8; PCP, physician)
All providers emphasized the need for patient-centered education on AKI survivorship, tailored to the etiology of kidney injury, degree of kidney function recovery, presence or absence of underlying CKD, and the patient’s ability to understand and participate with recommendations (Table 2). Core components recommended by both specialties included explanation of kidney injury and disease and the importance of avoiding re-injury, self-monitoring and management (eg, blood pressure monitoring, holding antihypertensives and diuretics in dehydration), nephrotoxin avoidance, and essential dietary modifications (eg, avoidance of potassium in hyperkalemia, protein consumption in CKD). Although most nephrologists and PCPs encouraged education delivery before hospital dismissal, PCPs suggested continuation throughout the transition of care, given concerns about retention of in-hospital information.
“Patients can’t take [education] in because it’s stressful and confusing. Even patients without cognitive impairment cannot remember what’s told to them in the hospital because they’re dealing with the stress going on.” (ID 4, PCP, physician)
Collaborative Care Delivery for AKI Survivors
Perceptions of the nephrologist’s role varied by specialty. Survey responses indicated 63% of nephrologists and 30% of PCPs (42% among tertiary care center PCPs, 30% among community practice PCPs) recommended outpatient nephrology referral in a case with evidence of proteinuria and incomplete kidney function recovery at dismissal (Table 3, question 8). When proteinuria and incomplete recovery were combined with multiple comorbid conditions, 100% of nephrologists and 90% of PCPs recommended nephrology referral (Table 3, question 11). In the case of a younger patient with AKI requiring temporary dialysis, liberated by dismissal but with incomplete recovery, 88% of nephrologists and 61% of PCPs (73% among tertiary care center PCPs, 61% among community practice PCPs) recommended nephrology referral (Table 3, question 4).
Both quantitative and qualitative data showed outpatient nephrology referrals were universally desired in patients with higher severity or persistent AKI, pre-existing CKD (stage 3 or higher), and high comorbid condition burden. In interviews, nephrologists added the additional criteria of unresolved insults/risk factors for AKI or future CKD.
“AKI is a spectrum, right? If you have a severe AKI that would require renal replacement therapy, I think that needs to be [managed by] nephrology to make sure there is no chronic kidney disease after AKI. But if we’re talking about mild AKI that did not require a significant amount of specific nephrologic care, somebody in primary care trained for AKI follow-up… could review that. It’s a very heterogenous group …it’s challenging to group them in one big umbrella of AKI.” (ID 11; Nephrologist, physician)
Nephrologists and PCPs emphasized the key role of PCPs in AKI survivor care in qualitative interviews. Initial kidney follow-up performed by the PCP was deemed critical to maintaining care continuity, especially when access to timely nephrology follow-up was limited. This was particularly prominent in community and rural settings. In addition, both specialties preferred PCP follow-up to precede nephrology follow-up to facilitate non–kidney-related, transitional care and ensure the necessary laboratory and imaging studies were performed before a nephrology appointment. Several participants volunteered that provider-to-provider communication through a shared electronic health record would be ideal for care coordination, though this was not universal, as one provider preferred direct (ie, phone) conversation.
“I feel pretty strongly that if my patient has been admitted to the hospital, I would like to see them in the outpatient setting because I’m the one that knows them and should be coordinating their care…. Continuity is really important.” (ID 8; PCP, physician)
“We rely on our primary care colleagues to do the initial touch-base…and at least have lab work repeated within 5-10 days of discharge. … then follow-up with Nephrology.” (ID 17; Nephrologist, APP)
Qualitative interviews emphasized the importance of factoring in patient-specific considerations when recommending nephrology referral (Table 2). PCPs stressed shared decision-making when it comes to nephrology referral (eg, alignment with the patients’ care goals).
“I’d refer to nephrology if the patient is not improving as we would expect or if there’s a significant, like one stage, change in CKD. But again that is dependent upon the patient’s situation. An 88-year old who has many other comorbidities and isn’t interested in a whole bunch of interventions and wouldn’t consider dialysis, I’m more likely to manage on my own…taking care of that patient in terms of goals of care.” (ID 8, PCP, APP)
Where patients received their local medical care (near the place of hospitalization vs a significant distance) and the transportation considerations influenced both specialties’ recommendations for patient-centered care (Table 2). Nephrologists considered whether the specialty care available in the patients’ home health system fit their needs (eg, dialysis support, transplantation assessment) and what other types of medical specialists were already part of the care team. When other specialists were involved in managing kidney disease risk factors (eg, cardiologists managing heart failure), several nephrologists were reluctant to increase the complexity of the care team by adding a nephrologist.
During qualitative interviews, both provider types recognized multidisciplinary care providers as team members with the capacity to offload PCPs’ and nephrologists’ burden. Leveraging their specialty knowledge was considered key to providing patient-centered care and optimizing the quality and efficiency of health care delivery. Several providers from both disciplines indicated nurse follow-up for patients at ‘low risk’ for post-hospital complications would be appropriate. They indicated this would require established guidance regarding issues to triage to a physician or APP but may expand capacity for post-AKI care and better allocate resources. Nurses were seen as preferred team members to deliver core education on kidney health. Pharmacists and dieticians were endorsed as key partners for discipline-specific consultation. PCPs from community and rural health systems cited a lack of awareness about multidisciplinary resources and limited access as reasons these team members were not more regularly consulted.
“Could we do a better job? Maybe. Do I think clinical pharmacy and/or [dieticians] being involved would be good? Yeah, probably, especially clinical pharmacy. They’re pretty stinking good.” (ID 14; PCP, APP)
Discussion
There were many commonalities in the approach to post-hospital AKI survivor care among PCPs and nephrologists, including recommendations for laboratory monitoring and follow-up with a PCP shortly after hospital discharge, using clinical (eg, severity of AKI) and non-clinical (eg, access to transportation) patient-specific factors to dictate the need and timing of nephrology referral, and more frequent incorporation of multidisciplinary specialists (eg, pharmacists, nurses, dieticians). Results complement existing expert recommendations for key care components by illuminating barriers and facilitators affecting ‘where’ and ‘how’ they might be delivered. This information is of particular importance in the absence of guidelines to direct post-AKI care.
Both disciplines supported early laboratory monitoring and assessment by a PCP shortly after hospital discharge, with nephrology referral based on indication and patient preferences. A team-based approach with follow-up in primary care, followed by specialty referral, could successfully contain health care costs and limited deleterious outcomes in patients needing complex care.25,27, 28, 29 These models can often accommodate a shorter time to follow-up than nephrologist-centric post-AKI care, where the median time to follow-up is 15-48 days, if accessible at all.10,11 Initial follow-up with primary care is also attentive to patient concerns about post-AKI care, including long travel time for appointments at referral centers, hospitalization fatigue, care fragmentation, and a reluctance to add additional specialists to their health care team.10,30 Guided by nephrologist recommendations for post-AKI care and referral criteria, PCPs’ clinical assessment and consideration of patient-specific socioeconomic and demographic factors may enhance the appropriate selection of patients for specialist referral and efficient use of health care resources. This is particularly important in the setting of a declining number of nephrology specialists, especially in low-income and rural areas, and increasing demand due to the burden of AKI.31 Longitudinal PCP follow-up also lends itself to continual evaluation of patient-specific risk factors for CKD (eg, kidney function recovery, comorbid conditions), which is recommended for AKI survivors at 90 days.9
Our results highlighted unique contributions from multidisciplinary specialists in a team-based care model. Whereas nephrologists demonstrated greater mastery of best practices for post-AKI care (eg, urine protein monitoring and individualized blood pressure goals), PCPs showed familiarity with a breadth of comorbid conditions (eg, diabetes) vital to providing comprehensive care following AKI. Pharmacists were widely acknowledged as critical to delivering expert-level medication management and monitoring throughout AKI survivors’ evolving kidney disease. Their involvement in peri-discharge care has been shown to reduce the risk of 30-day readmission and polypharmacy.32,33 Despite these findings and growing consensus of their importance in collaborative care models,9,34 pharmacists have not been a universal component of existing post-AKI care clinics. Other opportunities for multidisciplinary specialist contribution included nurse involvement in triage for patients with a low risk of complications and kidney health education, and dieticians to provide kidney-oriented nutrition counseling. Patient-centered education focused on knowledge about kidney injury and self-management may significantly improve kidney health outcomes.35
Heterogeneity in the AKI survivor population was widely acknowledged, and patient-specific factors were important determinants of the need and urgency for nephrology follow-up. In qualitative interviews, PCPs suggested creating algorithms and electronic health record-based clinical decision support tools to standardize post-AKI care and nephrology referral. Similarly, risk stratification for individuals with early-stage CKD has been recognized as a method for improved health equity, care quality, and resource utilization.36 This investigation and others have emphasized the importance of a patient-centered model for post-AKI care. A collaborative care model inclusive of diverse experts can augment care quality for patients with even the most complex needs.37 Attentiveness to transparency and shared decision-making can improve patient-provider communication. Recognition of the health care delivery outcomes patients experience can highlight areas of need and inform iterative process improvements.26 These results underscore factors in organizations’ structure, processes, and outcomes that must be attended to achieve patient-centered care.
This study is not without limitations. Survey findings may have been affected by non-response bias. Though a response rate of 19% (33% and 15% among surveyed nephrologists and PCPs, respectively) is in line with previous clinician surveys,38, 39, 40 non-responders may have systematically differed from participants. This study was conducted during the COVID-19 pandemic and results may have been influenced by the unique challenges facing clinicians and health systems, including limited resources, high patient acuity and inpatient census, sweeping changes related to telehealth, and increasing demands on provider time. Qualitative interviews probed for information relevant to these factors, where able. Participants were from a single health system and their views or experiences may differ from those in other health systems or serving different populations. To account for this, we engaged a diverse array of providers from various training backgrounds, geographic locations, and practice settings to enhance generalizability of findings. Explicit guideline recommendations for the ‘who’ and ‘how’ to follow-up AKI survivors do not exist. Our questionnaire therefore probed provider perspectives, and only where clear recommendations are available in the published literature (ie, drug dosing thresholds) did we deem an answer preferred.
In conclusion, a multidisciplinary team-based model of post-AKI care may facilitate implementation of a patient-centered care plan than can improve adherence to best practices and promote collaborative care, while reducing clinician and patient burden. Individualizing care based on clinical and non-clinical patient-specific factors is needed to optimize outcomes for patients and health systems.
Article Information
ACT Study Group
Joe Herges, PharmD, Andrea Kattah, MD, MSc, Brenda Anderson, RN, Angeliki Tinaglia, RRT, LRT, Lauri Meade, RN, and the authors listed below.
Authors’ Full Names and Academic Degrees
Heather P. May, PharmD, MSc, Abby K. Krauter, PharmD, Dawn M. Finnie, MPH, Rozalina G. McCoy, MD, MS, Kianoush B. Kashani, MD, MSc, Joan M. Griffin, PhD, and Erin F. Barreto, PharmD, MSc; on behalf of the ACT Study Group.
Authors’ Contributions
Research idea and study design: HPM, AKK, DMF, JMG, RGM, KBK, EFB; data acquisition: HPM, DMF; data analysis/interpretation: HPM, AKK, DMF, RGM, KBK, EFB, JMG; statistical analysis: HPM; supervision or mentorship: EFB, JMG, RGM, KBK. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported in part by the Mayo Midwest Pharmacy Research Committee, Mayo Midwest Clinical Practice Committee Innovation Award, American College of Clinical Pharmacy, the National Institute of Allergy and Infectious Diseases of under award number K23AI143882 (PI; EFB), and the Agency for Healthcare Research and Quality HS028060-01 (PI; EFB). The funding sources had no role in study design; data collection, analysis, or interpretation; writing the report; or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received August 1, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form October 26, 2022.
Footnotes
Complete author and article information provided before references.
Supplementary File (PDF)
Table S1: Survey Instrument
Table S2: KAMPS Framework for Kidney Follow-up Care
Contributor Information
Heather P. May, Email: may.heather@mayo.edu.
ACT Study Group:
Joe Herges, Andrea Kattah, Brenda Anderson, Angeliki Tinaglia, and Lauri Meade
Supplementary Material
Table S1-S2.
References
- 1.Siew E.D., Parr S.K., Abdel-Kader K., et al. Predictors of recurrent AKI. J Am Soc Nephrol. 2016;27(4):1190–1200. doi: 10.1681/ASN.2014121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawhney S., Marks A., Fluck N., et al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92(2):440–452. doi: 10.1016/j.kint.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heung M., Steffick D.E., Zivin K., et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis. 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannu N., James M., Hemmelgarn B., Klarenbach S. Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odutayo A., Wong C.X., Farkouh M., et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377–387. doi: 10.1681/ASN.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rewa O., Bagshaw S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 8.Kashani K., Shao M., Li G., et al. No increase in the incidence of acute kidney injury in a population-based annual temporal trends epidemiology study. Kidney Int. 2017;92(3):721–728. doi: 10.1016/j.kint.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashani K., Rosner M.H.M.H., Haase M., et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14(6):941–953. doi: 10.2215/CJN.01250119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver S.A., Adhikari N.K., Bell C.M., et al. Nephrologist follow-up versus usual care after an acute kidney injury hospitalization (FUSION): a randomized controlled trial. Clin J Am Soc Nephrol. 2021;16(7):1005–1014. doi: 10.2215/cjn.17331120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh G., Hu Y., Jacobs S., et al. Post-discharge mortality and rehospitalization among participants in a comprehensive acute kidney injury rehabilitation program. Kidney360. 2021;2(9):1424–1433. doi: 10.34067/KID.0003672021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver S.A., Goldstein S.L., Harel Z., et al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2(1):36. doi: 10.1186/s40697-015-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver S.A., Harel Z., Harvey A., et al. Improving care after acute kidney injury: a prospective time series study. Nephron. 2015;131(1):43–50. doi: 10.1159/000438871. [DOI] [PubMed] [Google Scholar]
- 14.Karsanji D.J., Pannu N., Manns B.J., et al. Disparity between nephrologists’ opinions and contemporary practices for community follow-up after AKI hospitalization. Clin J Am Soc Nephrol. 2017;12(11):1753–1761. doi: 10.2215/CJN.01450217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harel Z., Wald R., Bargman J.M., et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83(5):901–908. doi: 10.1038/ki.2012.451. [DOI] [PubMed] [Google Scholar]
- 16.Siew E.D., Peterson J.F., Eden S.K., et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fetters M.D., Curry L.A., Creswell J.W. Achieving integration in mixed methods designs-principles and practices. Health Serv Res. 2013;48(6 Pt 2):2134–2156. doi: 10.1111/1475-6773.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May H.P., Krauter A.K., Finnie D.M., et al. Optimising transitions of care for acute kidney injury survivors: protocol for a mixed-methods study of nephrologist and primary care provider recommendations. BMJ Open. 2022;12(6) doi: 10.1136/bmjopen-2021-058613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin A., Stevens P.E., Bilous R.W., et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 20.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi P.Y., Naessens J.M., Peterson S.M., et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthcare. 2016;4(1):30–35. doi: 10.1016/j.hjdsi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Santana M.J., Manalili K., Jolley R.J., Zelinsky S., Quan H., Lu M. How to practice person-centred care: a conceptual framework. Health Expect. 2018;21(2):429–440. doi: 10.1111/hex.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner R., Li Q., Baier R.R., Butterfield K., Coleman E.A., Gravenstein S. Is implementation of the care transitions intervention associated with cost avoidance after hospital discharge? J Gen Intern Med. 2014;29(6):878–884. doi: 10.1007/s11606-014-2814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman E.A., Parry C., Chalmers S., Min S.J. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 29.Wee S.L., Loke C.K., Liang C., Ganesan G., Wong L.M., Cheah J. Effectiveness of a national transitional care program in reducing acute care use. J Am Geriatr Soc. 2014;62(4):747–753. doi: 10.1111/jgs.12750. [DOI] [PubMed] [Google Scholar]
- 30.Silver S.A., Saragosa M., Adhikari N.K., et al. What insights do patients and caregivers have on acute kidney injury and posthospitalisation care? A single-centre qualitative study from Toronto, Canada. BMJ Open. 2018;8(6) doi: 10.1136/bmjopen-2017-021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker M.G., Pivert K.A., Ibrahim T., Molitoris B.A. Recruiting the next generation of nephrologists. Adv Chronic Kidney Dis. 2013;20(4):326–335. doi: 10.1053/j.ackd.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Herges J.R., Herges L.B., Dierkhising R.A., Mara K.C., Davis A.Z., Angstman K.B. Effect of postdismissal pharmacist visits for patients using high-risk medications. Mayo Clin Proc Innov Qual Outcomes. 2018;2(1):4–9. doi: 10.1016/j.mayocpiqo.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal A., Babbott S., Wilkinson S.T. Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm. 2013;48(5):380–388. doi: 10.1310/hpj4805-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell J.D., Haag J.D., Klavetter E., et al. Development and implementation of a team-based, primary care delivery model: challenges and opportunities. Mayo Clin Proc. 2019;94(7):1298–1303. doi: 10.1016/j.mayocp.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 35.Diamantidis C.J., Burks E., Mohottige D., et al. What do acute kidney injury survivors want to know about their condition: a qualitative study. Kidney Med. 2022;4(4) doi: 10.1016/j.xkme.2022.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlipak M.G., Tummalapalli S.L., Boulware L.E., et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34–47. doi: 10.1016/j.kint.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Phipps D.L., Morris R.L., Blakeman T., Ashcroft D.M. What is involved in medicines management across care boundaries? A qualitative study of healthcare practitioners’ experiences in the case of acute kidney injury. BMJ Open. 2017;7(1) doi: 10.1136/bmjopen-2016-011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frazee E.N., Personett H.A., Bauer S.R., et al. Intensive care nurses’ knowledge about use of neuromuscular blocking agents in patients with respiratory failure. Am J Crit Care. 2015;24(5):431–439. doi: 10.4037/ajcc2015397. [DOI] [PubMed] [Google Scholar]
- 39.Torbic H., Bauer S.R., Personett H.A., et al. Perceived safety and efficacy of neuromuscular blockers for acute respiratory distress syndrome among medical intensive care unit practitioners: a multicenter survey. J Crit Care. 2017;38:278–283. doi: 10.1016/j.jcrc.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Weaver L., Beebe T.J., Rockwood T. The impact of survey mode on the response rate in a survey of the factors that influence Minnesota physicians’ disclosure practices. BMC Med Res Methodol. 2019;19(1):73. doi: 10.1186/s12874-019-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1-S2.