Abstract
Huangkui capsule (HKC), a traditional Chinese medicine, has been used for medication of kidney diseases, including diabetic nephropathy (DN). The current study aimed to evaluate the effects of HKC in the modulation of gut microbiota and the amelioration of metabolite levels by using non‐obese diabetes (NOD) mice with DN. The microbiota from three parts of intestines (duodenum, ileum and colon) in NOD mice with and without HKC treatment were analysed using 16S rDNA sequencing techniques. Untargeted metabolomics in plasma of NOD mice were analysed with liquid mass spectrometry. Results showed that HKC administration ameliorated DN in NOD mice and the flora in duodenum were more sensitive to HKC intervention, while the flora in colon had more effects on metabolism. The bacterial genera such as Faecalitalea and Muribaculum significantly increased and negatively correlated with most of the altered metabolites after HKC treatment, while Phyllobacterium, Weissella and Akkermansia showed an opposite trend. The plasma metabolites, mainly including amino acids and fatty acids such as methionine sulfoxide, BCAAs and cis‐7‐Hexadecenoic acid, exhibited a distinct return to normal after HKC treatment. The current study thereby provides experimental evidence suggesting that HKC may modulate gut microbiota and subsequently ameliorate the metabolite levels in DN.
The plasma metabolites, mainly including amino acids and fatty acids such as methionine sulfoxide, BCAAs and cis‐7‐Hexadecenoic acid, exhibited a distinct return to normal after HKC treatment. The flora in the duodenum of NOD mice were more sensitive to HKC intervention, while that in the colon have more effect on the metabolism. HKC may modulate gut microbiota and subsequently ameliorate the metabolite levels in DN.
INTRODUCTION
Diabetic nephropathy (DN) is one of the most common complications in patients with diabetes. Clinically, the most characteristic marker of DN for most of diabetes patients is albuminuria. DN occurs in 20–50% of patients with type 1 and type 2 diabetes (T1D, T2D), and has become the leading cause of end‐stage renal disease (ESRD) that requires treatment with dialysis or renal transplantation (Ahmad, 2015; Thomas et al., 2015). Therefore, elucidating the characteristic metabolic changes in the progression of DN is essential to better understand its pathogenesis and to identify potential biomarkers and drug targets (Wu et al., 2018).
Accumulating evidence has demonstrated that dysbiosis of the gut microbiota is associated with the development of diabetes and DN. Dysbiosis may trigger inflammatory responses by disrupting the gut epithelial barrier leading to increased gut permeability, translocation of pathogenic bacteria and accumulation of endotoxins, and may also accelerate the process of DN by affecting lipid metabolism and short chain fatty acid (SCFA) metabolism (Chen et al., 2015; Yacoub & Wyatt, 2017). Metabolic abnormalities in diabetes patients are exacerbated by microbial abnormalities, accompanied by increased leaky gut. The gut microbiota of patients with advanced renal disease is likewise significantly altered (Stadlbauer et al., 2017).
Abelmoschus Manihot (A. Manihot) is a member of the Malvaceae family of okra plants, its total flavonoids (TFA) are the main known active chemical constituents. Currently, Huangkui capsule (HKC), an A. Manihot extract preparation, has been widely used for the treatment of various types of kidney diseases (Li et al., 2021). In recent years, five multicentre randomized controlled clinical trials have been conducted and reported that HKC has the efficacy and safety for treatment of primary glomerular disease, IgA nephropathy and DN in reduction of proteinuria (Darshi et al., 2016; Li et al., 2017, 2020; Zhang et al., 2014; Zhao et al., 2022). The effects of HKC on gut microbiota, however, is still unclear.
In the current study, we have systematically analysed the changes in gut microbiota at three parts of the gut tract and at different times after the onset of disease in non‐obese diabetes (NOD) mice, which is a polygenic model for study T1D and diabetic complication, including DN (Mullen, 2017; Ridgway, 2006), and further evaluated the effects of HKC on modulation of gut microbiota and amelioration of metabolite levels in DN.
EXPERIMENTAL PROCEDURES
Animals and housing conditions
Thirteen‐week‐old female NOD/LtJ (usually called NOD) mice were purchased from Beijing Huafukang Bioscience Co., Ltd (Beijing, China). All mice were housed on a standard 12 h light/dark cycle in a temperature‐controlled room (22 ± 2°C) under specific pathogen‐free conditions in Animal Laboratory Center of China Pharmaceutical University (CPU, Nanjing, China). All animal experiments were approved by the Animal Ethics Committee of CPU.
Grouping and treatment
NOD mice were tested for random blood glucose once a week, and the mice with blood glucose level below 11.1 mmoL/L were considered as non‐diabetic controls (ND, n = 6). The mice, after two consecutive blood glucose measurements ≥11.1 mmol/L, were considered as hyperglycaemia. Twenty‐six mice with hyperglycaemia were used for collecting the physiological data. Six hyperglycaemia mice in each group were oral gavage treated with HKC (0.45 g/kg) (Suzhong Pharmaceutical Group Co., Ltd., Taizhou, China) or vehicle (purified water), respectively, once a day for 4 weeks.
The physiological data of mice such as dietary intake (g), water intake (ml) and urine production (ml) were recorded. During the administration period, the 24‐h urine of mice was collected once a week by metabolic cage. Urine samples were centrifuged (600 g, 10 min), and the supernatants were stored at −80°C. Concentrations of MAU and Cr were measured by ELISA quantitative kits (Elabscience Biotechnology Co, Ltd, Wuhan, China). After the animals were sacrificed, the blood samples were collected and centrifuged (1200 g, 5 min) to obtain plasma. Tissue samples were snap frozen in liquid nitrogen, and then stored at −80°C.
16S ribosomal DNA sequence analysis
The three parts of the intestine (duodenum, ileum and colon) were taken by surgical double ligation to measure about two centimetres for gut microbiota. Total g.DNA was extracted using the CTAB/SDS method and then amplified by PCR using primers 341F (5′‐CCTAYGGGRBGCASCAG‐3′) and 806R (5′‐GGACTACNNGGGTATCTAAT‐3′) belonging to the V3–V4 variable region of 16S rDNA. Each sample was repeated three times, and the mixed PCR products were detected by 2% agarose gel electrophoresis, and samples with main band brightness between 400 and 450 bp were selected for further experiments. The amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's instructions for 16S rDNA sequencing (Illumina, San Diego, CA, USA). Low‐quality and mice g.DNA reads were removed before analysis. Finally, the 16S RNA sequences were aligned using Silva database.
Bioinformatics analysis
Illumina PE250 was used to obtain high‐quality sequences after preliminary screening and optimization. High‐quality sequences were analysed using bioinformatics methods. (1) OTU (Operational Taxonomic Units, operable taxonomic units) cluster analysis: use Usearch software to press 97% similarity classifies and divide the resulting sequences, select the most abundant sequence in each OTU as the representative sequence, and then compare it with the database template sequence to obtain the taxonomic information corresponding to each OTU. (2) Alpha Diversity analysis: use Chao1 index (The Chao1 estimator) to analyse the community richness of the obtained OTU, and Shannon index (Shannon diversity index) to analyse the diversity of the community. (3) Analysis of taxonomic composition: according to the results of OTU division and classification status identification, the composition and abundance distribution of each sample at different classification levels of phylum, class, order, family, genus and species are obtained and clustered. (4) Lefse difference analysis: find the species with significant differences between the groups.
Plasma metabolomics
For metabolomic analysis, 100 μl plasma was added to 400 μl pure methanol for the precipitation of protein. After vortex oscillation and ice incubation standing for 5 min, the supernatant was collected after centrifugation at 15,000 g for 5 min. The collection was centrifuged again at 15,000 g for 20 min after adding 1/2 volume of mass spectrometry‐grade water. The supernatant was collected for LC–MS analysis, and equal volume of each experimental sample was mixed as QC samples. Based on Novogene database (NovoDB), the multiple reaction monitoring mode (MRM) was used to test the experimental samples.
Statistical analysis
All analyses and graphics were performed using Graphpad Prism software (version 7.0), SPSS software (version 23.0), QIIME (version 1.8.0) and R software (version 3.6.0). Wilcoxon rank‐sum test was applied to assess the significant differences in α diversity between two compared groups, and β diversity was visualized by principal coordinates analysis (PCOA) using weighted Unirac distance matrix data. LEfSe differences between two groups were tested for significance using the Kruskal–Wallis sum‐rank test, and biological significance was subsequently investigated using a set of pairwise tests among groups with Wilcoxon rank‐sum test. Finally, linear discriminant analysis (LDA) was used to obtain the final differential species, followed by the Wilcoxon rank‐sum test. Prediction of gut microbiota function was imputed from 16S rDNA sequences using PICRUSt, which stores KO information corresponding to Greengene id (Hu et al., 2022). Metabolic pathway analyses (integrating pathway enrichment and pathway topology analyses) were visual using MetaboAnalyst (Xia et al., 2009). The correlations concerning the degree of association among different genera, different metabolites and disease indicators were analysed using Spearman correlation tests. The significance comparison between the groups was calculated by Student's t‐test. All values are presented as the means ± SEM and p < 0.05 was used to indicate a statistically significant difference.
RESULTS
Regression of DN in NOD mice after HKC treatment
In NOD mice, the diabetes onset time is different and overt diabetes develops in 60–80% of females (Ridgway, 2006). In the current study, we examined the changes in urinary albumin/creatinine ratio (UACR, MAU/UCr) of hyperglycaemia NOD mice and MAU/24 h (UAER) at different ages, and found that UACR was increased significantly 3 weeks after the onset of diabetes (Figure 1A, p < 0.05), while UAER was increased significantly by the week (Figure 1A, p < 0.05 between 0 and 1 or 2 weeks, p < 0.01 between 0 and 3 weeks). Furthermore, we found that UAER of mice three weeks after diabetes onset in late‐onset (in 30 weeks) mice was remarkable higher than that of early‐onset (p < 0.05 in 22 weeks) mice (Figure 1B). After HKC administration, UACR and UAER were decreased to some extend compared with those in the control group. Moreover, the physiological indexes of NOD mice such as food intake (p < 0.05) and urine volume (p < 0.05) but not water intake (p = 0.055) were lower after 3 weeks treatment with HKC (Figure 1D), indicating that HKC administration had certain improvement effects on the physiological indexes of DN mice.
FIGURE 1.
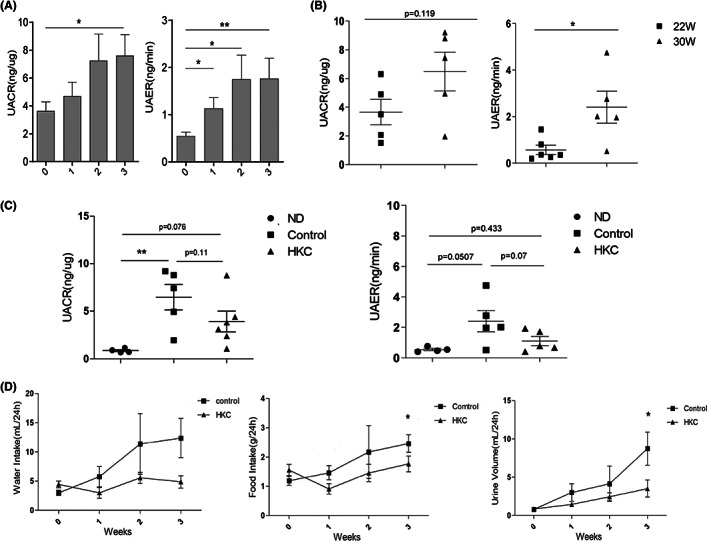
Effect of HKC on the physiological indexes of DN mice. (A) UACR and UAER changes of different ages in NOD mice, n = 25–26; (B) UACR and UAER changes between early‐onset and late‐onset mice, n = 5–6; (C) UACR and UAER changes before and after HKC administration, n = 4–6; (D) Water intake, food intake and urine volume of NOD mice before and after HKC administration, n = 4–6, *p < 0.05, **p < 0.01.
Comparison of microbiota in different parts of gut and at different disease onset time in NOD mice
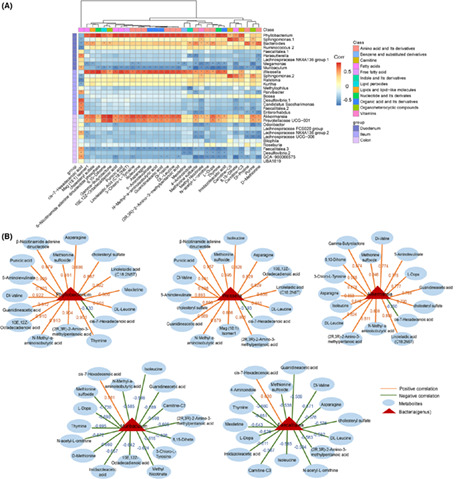
We used the surgical double ligation for NOD mice to intercept the duodenum, ileum and colon, and collect the faeces specimen for sequence analysis of gut microbiota (Figure S1). Based on Weighted UniFrac, PCoA analysis was performed on the bacterial community in three parts of intestines. We found that the flora in colon differed significantly from the duodenum and ileum samples, and the composition of the flora between the duodenum and ileum was somewhat similar (Figure 2A). From the chao1 and shannon indices, the flora richness and community diversity in colon were observed to be significantly higher than that in duodenum and ileum, and the community diversity in duodenum was significantly higher than that in ileum of early‐onset diabetic mice (22 weeks, Figure 2B, p < 0.01). In addition, the community diversity in the duodenum of late‐onset (30 weeks) diabetic mice was significantly decreased comparing with early‐onset diabetic mice (22 weeks, p < 0.001), while there were no significant changes in the ileum and colon (Figure 2B). The representative sequences of the samples OTU were compared with the database for taxonomic analysis, and at the phylum level, the species histogram was plotted, showing that the types of flora in colon differ significantly from the duodenum and ileum samples, while the flora in the duodenum and ileum were somewhat similar. Proteobacteria and Firmicutes were the main phyla in the duodenum and ileum, while in the colon, Bacteroidetes and Firmicutes were the dominant phyla (Figure 2C,D). The relative abundance of Bacteroidetes was decreased in the duodenum of late‐onset diabetic mice compared to early‐onset diabetic mice (p < 0.05), while the relative abundance of Proteobacteria was significantly increased in the ileum of late‐onset diabetic mice (Figure 2D, p < 0.05). Lefse analysis showed that more dominant flora in different levels including phylum, class, order, family and genus, in early‐onset than that in late‐onset diabetic mice. The dominant flora in duodenum of early‐onset mice were Bacteroidetes, Saccharimonadales, Lachnospiraceae and Megamonas, etc., while the dominant flora in duodenum of late‐onset mice were Phyllobacterium, Rhizobiaceae and Alphaproteobacteria. The dominant flora in ileum of early‐onset mice were Erysipelotrichaceae, Parabacteroides, Actinobacteria and Megamonas, etc., while the dominant flora in ileum of late‐onset mice were Proteobacteria and Fusobacteria, etc. The dominant flora in colon of early‐onset mice were Erysipelotrichaceae, Desulfovibrionales, Lachnospiraceae and Faecalitalea, etc., while the dominant flora in colon of late‐onset mice were Cyanobacteria and Staphylococcus, etc. (Figure 2E).
FIGURE 2.
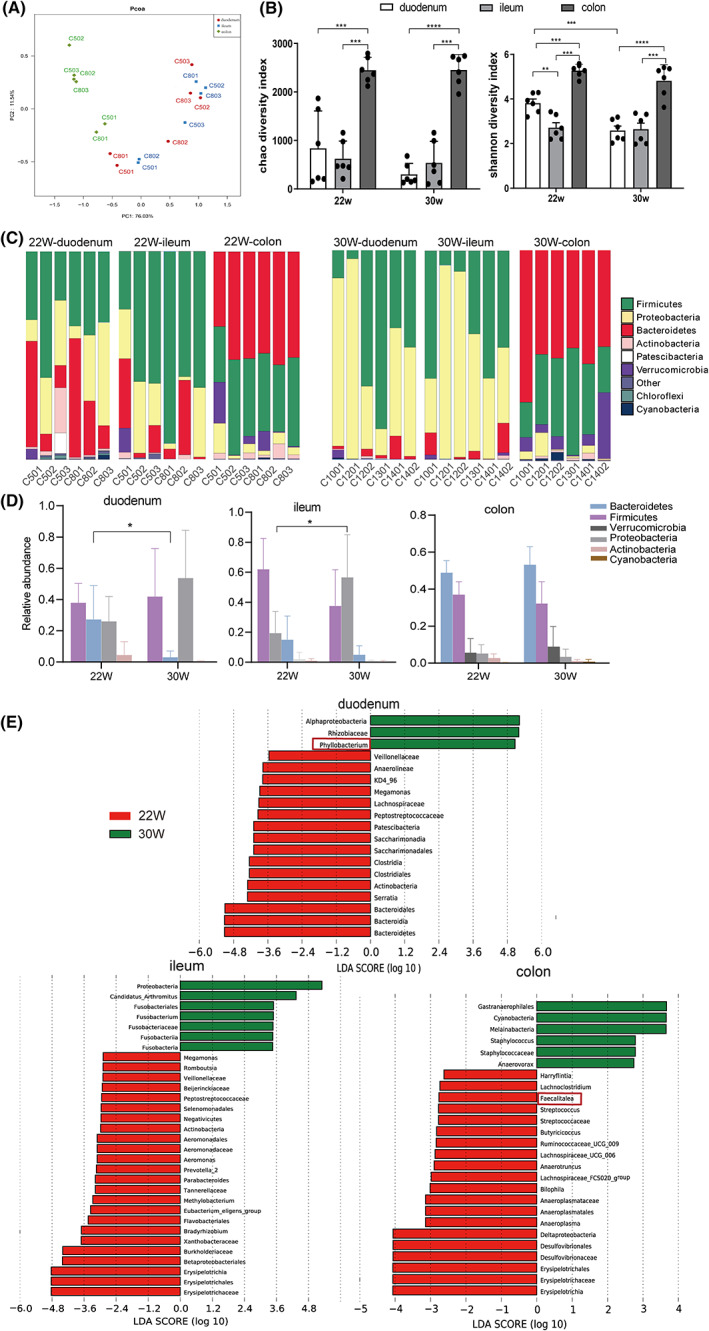
Comparison of microbiota in different parts of gut and at different disease onset time in NOD mice. (A) PCoA analysis of the three groups of intestines based on Bray‐Curtis distance; (B) Chao1 and Shannon indices between early‐onset and late‐onset mice; (C, D) Difference analysis of duodenum, ileum and colon at phylum level between early‐onset and late‐onset mice; (E) Lefse analysis of the duodenum, ileum and colon at genus level between early‐onset and late‐onset mice, n = 6 each group, *p < 0.05, ***p < 0.001, ****p < 0.0001.
Effects of HKC on gut microbiota composition in NOD mice
We further compared intestinal flora of three parts of intestines in mice between HKC and Control groups. The chao 1 index showed that HKC administration significantly increased the community richness in duodenum (p < 0.01) but had less effect on ileum and colon in NOD mice. Meanwhile, the community diversity was slightly increased in all three fractions (Figure 3A,B). The community structure of each fraction showed that the relative abundance of Bacteroidetes in duodenum was significantly increased after HKC administration (p < 0.05), but there was no significant difference in ileum and colon (Figure 3C,D). Boxplot of microbiota composition in two groups of mice revealed that the intestinal flora of three parts also differed significantly at the genus levels (Figure 3E). In the HKC group of mice, 4 genera were decreased, and 5 genera were increased in duodenum, 5 genera decreased and 6 genera increased in ileum, while 2 genera decreased and 10 genera increased in colon. The decreased genera, such as Phyllobacterium, Sphingomonas, Ralstonia, Kurthia and Akkermansia, have been reported to be associated with disease pathogenesis and promoted inflammation or alter intestinal leakage (Bolen et al., 2015; Huang et al., 2019; Zheng, Wang, et al., 2020). In contrast, most of the species that increased after HKC administration were beneficial bacteria to promote insulin secretion and produce short‐chain fatty acids (SCFA), such as Lachnospiraceae_ NK4A136_group, Muribaculum, Faecalitalea, Parasutterella, Roseburia, Lachnospiraceae_FCS020_ group, and Odoribacter (Bajaj et al., 2015; Lin et al., 2018; Ma et al., 2020; Yang et al., 2020; Yuan et al., 2020). The data also showed that the richness of Faecalitalea significantly increased in all three parts of intestines in NOD mice after HKC administration (Figure 3E, p < 0.05, framed in red). We then used PICRUSt to predict the metagenomes using the 16S‐based OUT tables, and found that membrane transport, carbohydrate metabolism, amino acid metabolism and energy metabolism are the main pathways in the intestinal flora in the two groups of mice (Figure 4A). The data also showed that the metabolic pathways, such as carbohydrate metabolism, amino acid metabolism, nucleotide metabolism and lipid metabolism were less activated in the colon microbiota of NOD mice after HKC treatment, while there were fewer changes in duodenum and ileum. This finding suggested that microbiota in the colon may have a more important effect on the metabolism of NOD mice with DN.
FIGURE 3.
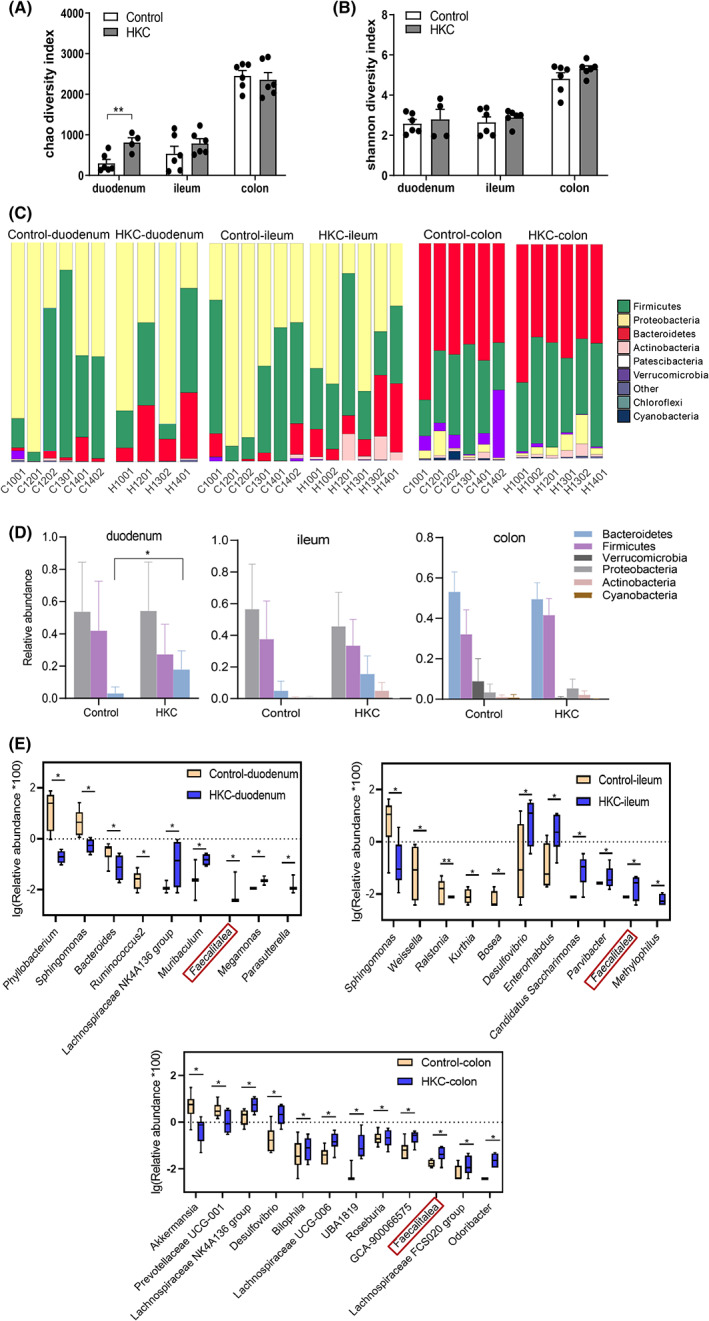
Effect of HKC on gut microbiota composition in NOD mice. (A, B) Comparison of the abundance and diversity of gut microbiota in HKC and Control groups of mice; (C, D) Difference analysis of duodenum, ileum and colon at phylum level in two groups of mice; (E) Boxplot of the duodenum, ileum and colon at genus level in two groups of mice, duodenum: n = 6 in Control and ND groups, n = 4 in HKC group, ileum and colon: n = 6 each group, *p < 0.05, **p < 0.01.
FIGURE 4.
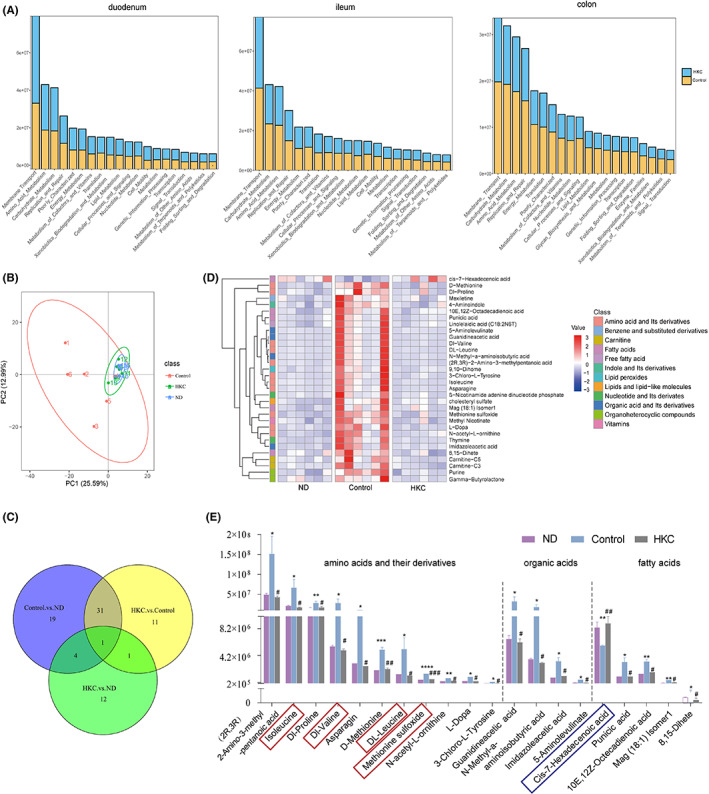
Changes of plasma metabolites in NOD mice after HKC treatment. (A) Prediction of gut microbiota function in two groups of mice; (B) PCA plot representing total plasma metabolites of three groups; (C) Venn diagramme showing the number of overlapping metabolites of three groups; (D) Heat map showing plasma metabolites with significant differences of three groups; (E) Metabolites with significant differences of three groups: amino acids and its derivatives, fatty acids, organic acids and its derivatives, plasma samples: n = 6 each group,*p < 0.05,**p < 0.01,***p < 0.001, ****p < 0.0001 vs ND; #p < 0.05, ##p < 0.01, ###p < 0.001 versus Control.
Difference of plasma metabolites in NOD mice caused by the intervention of HKC
To study the changes of metabolites in NOD mice after treatment with HKC, we analysed the differences in plasma metabolite profiles among HKC, Control and ND groups. From the PCA chart, we could see the significant differences in plasma metabolites in NOD mice among three groups, but the metabolites in HKC and ND groups were concentrated, suggesting that the plasma metabolites might return to normal after the administration of HKC (Figure 4B). We compared 44 differential metabolites between Control and HKC groups, and 55 differential metabolites between Control and ND groups, and identified 32 identical metabolites (Figure 4C). The cluster heat map analysis clearly reflected the changes of metabolites in each group. We found that the change of the metabolite content in Control group was completely opposite to what was in the other two groups. 31 metabolites were increased and 1 metabolite was decreased, suggesting that the administration of HKC on DN mice might produce the significant changes of plasma metabolites (Figure 4D). We further carried out a statistical analysis of these 32 metabolites by the category (Table S1) and found that most of them belong to amino acids and their derivatives (11 types), fatty acids (5 types) and organic acids (4 types), respectively (Figure 4E). Methionine sulfoxide was extremely increased after the onset of diabetes (p < 0.0001), and decreased after HKC administration (Figure 4E, p < 0.001, framed in red). Branched‐chain amino acids (BCAAs), including valine, leucine and isoleucine, were found significantly increased (p < 0.05) after the onset of diabetes, and decreased significantly after HKC treatment (Figure 4E, p < 0.01, framed in red). In addition, the changes of cis‐7‐Hexadecenoic acid were completely opposite to other differential metabolites (Figure 4E, framed in blue), which decreased after the onset of diabetes (p < 0.01) and increased after HKC administration (p < 0.01).
Based on the changes in these metabolites, we used MetaboAnalyst (Xia et al., 2009) to perform an enrichment analysis of the metabolic pathways, and generated the perturbation of 20 networks. Among these networks, several metabolism or biosynthesis pathways, such as glycine, serine and threonine metabolism, phenylalanine metabolism, tyrosine metabolism, cysteine and methionine metabolism, valine, leucine and isoleucine biosynthesis etc., had higher pathway impact (Figure 5A). As shown in Figure 5B, 16 important altered metabolites after HKC administration were involved in six metabolic pathways. The organic acids and their derivatives such as guanidine acetic acid and 5‐aminolevulinate were downregulated, which affects the metabolism of glycine, serine and threonine, as well as proline and arginine biosynthesis. Meanwhile, N‐acetyl‐L‐ornithine and L‐Dopa were downregulated, and subsequently affected arginine biosynthesis. Thymine and asparagine were downregulated, which might affect the metabolism of tyrosine and aspartic acid. In the BCAA metabolism pathway, DL‐leucine, isoleucine and DL‐valine were downregulated. In fatty acid metabolism pathway, 10E, 12Z‐octadecadienoic acid, Mag (18:1) Isomer 1 and 8,15‐Dihete were downregulated, while cis‐7‐hexadecenoic acid was upregulated. Taken together, the data revealed that HKC treatment improved several metabolism pathways mainly involved amino acids and their derivatives, fatty acids and organic acids.
FIGURE 5.
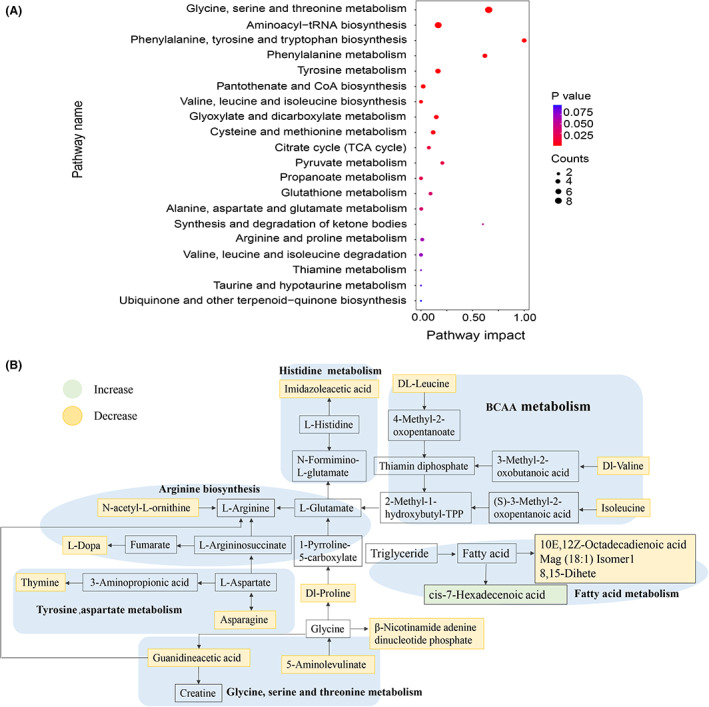
Difference of metabolic pathways in NOD mice caused by the intervention of HKC. (A) An enrichment analysis of the metabolic pathways using MetaboAnalyst; (B) Changes of different metabolites in the metabolic pathways between HKC and Control groups.
Correlation analysis of metabolites, diseases indicators and gut microbiota
We further performed correlation analyses to investigate the mechanism of action of HKC in treatment of DN. Firstly, the correlation analysis between differential flora and disease indicators showed that Phyllobacterium in duodenum, Weissella in ileum and Akkermansia in colon were positively correlated with disease indicators such as blood glucose, UACR, urinary albumin excretion rate (UAER), left/right kidney to body weight ratio (LKW/RKW) and volume of urine, while negatively correlated with MAU and UCr (Figure S2). On the contrary, Muribaculum in duodenum, Desulfovibrio, Faecalitalea and GCA‐900066575 in colon were positively correlated with UCr, but negatively correlated with blood glucose, LKW/RKW, water consumption and urine volume. The correlation analysis between differential metabolites and disease indicators suggested that changes in metabolites may more directly correlate with the development of DN (Figure S3). The results showed that most differential metabolites were negatively correlated with UCr and MAU, significantly positively correlated with disease indicators such as urine volume, LKW/RKW, blood glucose and water consumption, and relatively weakly correlated with food intake, and UACR. However, cis‐7‐Hexadecenoic acid has significantly negative correlations with blood glucose and UACR, while significantly positive correlations with UCr.
We further conducted a correlation analysis between 32 differential plasma metabolites and total of 32 differential intestinal bacteria (at genus level) of the three parts in NOD mice after HKC administration. As the heatmap shown in Figure 6A, the genera such as Phyllobacterium in duodenum, Weissella in ileum and Akkermansia and Prevotellaceae UCG‐001 in colon were positively correlated, while Muribaculum in duodenum and Faecalitalea in the colon were negatively correlated with most of the differential metabolites. Similarly, the correlation analysis between the differential flora and cis‐7‐Hexadecenoic acid showed an opposite trend (Figure 6A). Furthermore, we focused on the correlation between five differential bacterial genera, including Phyllobacterium, Weissella, Akkermansia, Muribaculum and Faecalitalea, which were simultaneously significantly correlated with most of disease indicators, and 16 important altered metabolites after HKC administration. The correlation coefficients were showed in Figure 6B. The bacterial genera, including Phyllobacterium, Weissella and Akkermansia were significantly decreased after HKC treatment and positively correlated with most of the altered metabolites except cis‐7‐Hexadecenoic acid. On the contrary, the bacterial genera such as Faecalitalea and Muribaculum were significantly increased after HKC treatment and negatively correlated with most of the altered metabolites except cis‐7‐Hexadecenoic acid. The results indicate that changes in intestinal flora are related to the changes of plasma metabolites. We thus speculate that HKC may modulate gut microbiota, and subsequently ameliorate plasma metabolite levels.
FIGURE 6.
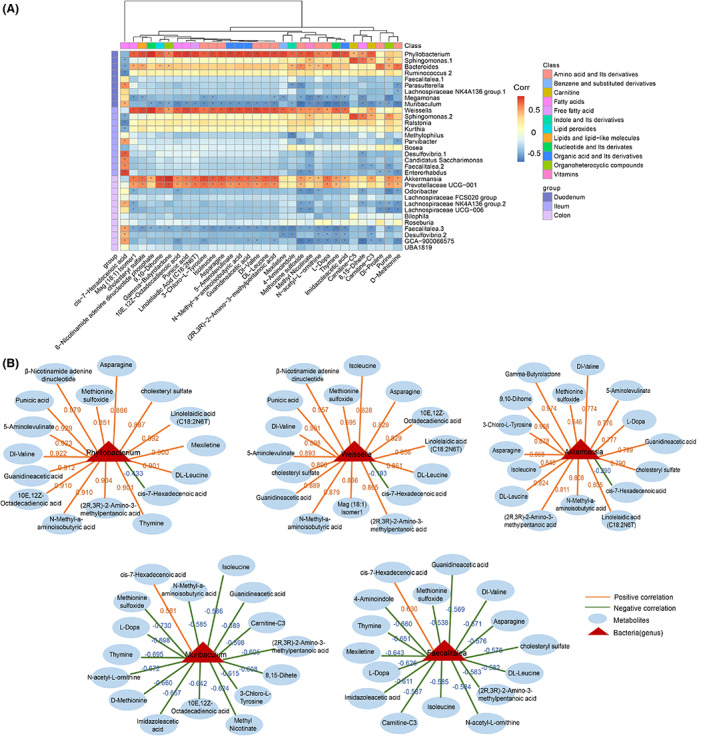
Fusion data is used to analyse the correlation between microbiota and metabolites in HKC and Control groups of mice. (A) Heatmap of the correlation analysis. (Wilcoxon rank‐sum test, p < 0.05, | Spearman correlation analysis, correlation coefficient| > 0.5); (B) Differential bacterial genera that are positively or negatively related to differential metabolites.
DISCUSSION
In the current study, both UACR and UAER in NOD mice were found to be significantly increased about 1 to 3 weeks after the onset of diabetes. After HKC administration, the aberrant UACR, UAER, water intake and urine output in NOD mice were remarkably improved. When comparing the flora in three parts of intestine, there was high community diversity of gut microbiota in colon of NOD mice with hyperglycaemia, while the least diversity was found in ileum. Furthermore, three parts of gut in NOD mice showed different dominant flora. Comparing either the gut microbiota of different onsets of diabetes or the gut microbiota with and without HKC administration, the data demonstrated that the community composition in duodenum was changed more. This may implicate that the flora species in duodenum are more sensitive to changes of intestinal microenvironment or drug intervention. When using PICRUSt to predict the metagenomes, however, the metabolic pathways in the microbiota were less activated mainly in colon, suggesting that it may be due to the highest flora richness in colon.
Accumulating evidence has demonstrated that DN is linked with the dysbiosis of intestinal microbiota (Fang et al., 2021). In the current study, specific bacterial genera altered significantly after HKC administration, and showed a significant correlation with changes in metabolite levels and disease indicators, suggesting that they may be associated with the mechanism of effects of HKC in treatment of DN. Among the differential intestinal bacteria, Phyllobacterium has been reported to be associated with the diseases in humans, such as liver cirrhosis and non‐liver cirrhosis‐induced HCC, and significantly enriched in the autism spectrum disorders patients and the cholestatic infants (Guo et al., 2018; Huang et al., 2021; Zheng, Wang, et al., 2020). Similarly, a recent study has demonstrated that Weissella confusa was higher in KK‐Ay mice with T2D (Liu et al., 2021) while other studies have shown that Weissella decreased after treatment with some effective medications in an animal model of T2D (Hu et al., 2019; Zheng, Chen, et al., 2020). According to the recent studies, strains of Akkermansia like A. muciniphila play a significant role in glucose metabolism and insulin resistance (Gurung et al., 2020; Hanninen et al., 2018; Hasani et al., 2021; Plovier et al., 2017). Another report, however, has suggested that A. muciniphila may contribute to creating an overall pro‐inflammatory environment in multiple sclerosis (Cekanaviciute et al., 2017). In the current study, all these microbiotas described above were decreased in colon of NOD mice after HKC administration.
On the contrary, Faecalitalea and Muribaculum significantly increased after HKC administration, especially Faecalitalea increased in three parts of intestine, and they were also negatively correlated with most of the differential metabolites and most disease indicators, suggesting that these two genera may be beneficial to treatment of DN. Muribaculum, a kind of probiotics, was reported to be correlated with a healthy phenotype and low‐fat diet consumption (Leigh et al., 2020). Moreover, a significant reduction in Muribaculum may lead to inflammation, dyslipidaemia and poor glucose tolerance (Yuan et al., 2020). Faecalitalea, belonging to Firmicutes, which can produce SCFAs, is reported to be beneficial for insulin secretion and insulin response to diabetes (De Maesschalck et al., 2014; Ma et al., 2020; Sanna et al., 2019). Overall, our results have indicated that HKC administration is effective in regulating the disorder of gut microbiota in NOD mice. Further investigation concerning the correlation between the intestinal flora disorder and the development of DN, as well as the impact of two potential probiotics in DN has been taken into our consideration.
Plasma metabolites might act as specific biomarkers for early diagnosis of DN (Colhoun & Marcovecchio, 2018). In the current study, a total of 32 differential metabolites were found to be related to DN in NOD mice and return to normal after HKC treatment. Moreover, the amino acids metabolism showed higher pathway impact among the perturbation of 20 networks. Among the altered amino acids, BCAAs and methionine sulfoxide, have been reported to be associated with metabolic abnormality. The serum metabolome revealed that BCAAs may not only be biomarkers, but also causal agents of insulin resistance and T2D (Fang et al., 2021). In T2D patients with stages 1 or 2 chronic kidney disease, high serum BCAA levels are independently associated with a decline in the estimated glomerular filtration rate (Lim et al., 2019). Methionine sulfoxide, as an oxidation product of methionine and also one of the markers of oxidative damage to proteins, was found to be higher in T1D patients and diabetic animal models compared with healthy controls (Karachalias et al., 2010; Monnier et al., 2022), but not in T1D with microalbuminuria (Perkins et al., 2012). Here, plasma BCAA and methionine sulfoxide was found to be increased in control group compared with ND group and decreased in HKC group. Furthermore, they were significantly positively correlated with disease indicators such as urine volume, LKW/RKW, blood glucose and water consumption, suggesting that they may directly correlate with the occurrence and development of DN.As a lipid hormone, cis‐7‐Hexadecenoic acid (also known as palmitoleic acid), can improve systemic insulin sensitivity (Astudillo et al., 2018), promote proliferation of pancreatic beta‐cells (Maedler et al., 2001) and have a strong anti‐inflammatory effect on monocytes and macrophages (Astudillo et al., 2020). In our study, the changes of cis‐7‐Hexadecenoic acid showed opposite trends comparing with other differential metabolites and were negatively correlated with blood glucose and UACR, suggesting that it may have beneficial effects against DN. Therefore, our data revealed that HKC treatment could ameliorate metabolite levels in DN. In addition, these differential metabolites, such as methionine sulfoxide, BCAAs and cis‐7‐Hexadecenoic acid may act as potential biomarkers for early diagnosis and therapeutic intervention of DN.
Previous studies have reported that total flavones of A. Manihot is effective in remodelling gut microbiota in Chronic Renal Failure Progression (Tu et al., 2020) and A. Manihot can attenuate DSS‐induced ulcerative colitis by regulating gut microbiota (Zhang et al., 2019). Our study showed that the gut microbiome in DN mice after HKC treatment had a large scale of alteration. Moreover, the metagenomes analysis revealed a lower number of sequences belonging to several metabolic pathway such as carbohydrate, amino acid, lipid and other metabolic pathway in the microbiota in colon of HKC‐treated group compared with the control group, which is consistent with significantly decreased metabolites like amino acids, fatty acids, and organic acids, etc. Indeed, the accumulating evidence has confirmed that dysbiosis of the gut microbiota is associated with metabolic abnormalities and the development of DN (Wang et al., 2022).
In conclusion, the current study provides experimental evidence that the microbiota in duodenum is more sensitive to HKC intervention, and the microbiota in colon may have greater impact on metabolic pathways after HKC treatment and suggests that by altering the composition of intestinal flora, HKC may ameliorate the levels of circulating metabolites and subsequently delay the development of DN.
AUTHOR CONTRIBUTIONS
Ruiya Shi: Formal analysis (equal); writing – review and editing (equal). Yingjun Tao: Investigation (equal); methodology (equal); writing – review and editing (equal). Haitao Tang: Data curation (equal); validation (equal). Chenhua Wu: Formal analysis (equal). Jingjin Fei: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Haitao Ge: Data curation (equal); validation (equal). Harvest F. Gu: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – review and editing (equal). Jie Wu: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – review and editing (equal).
FUNDING INFORMATION
National Natural Science Foundation of China (Grant/Award Number: No. 81973224), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the start grants from China Pharmaceutical University (CPU20180815 HFG) and the Cooperation Research Project (CPU20200228 HFG).
CONFLICT OF INTEREST
None declared.
Supporting information
Appendix S1.
Shi, R. , Tao, Y. , Tang, H. , Wu, C. , Fei, J. , Ge, H. et al. (2023) Abelmoschus Manihot ameliorates the levels of circulating metabolites in diabetic nephropathy by modulating gut microbiota in non‐obese diabetes mice. Microbial Biotechnology, 16, 813–826. Available from: 10.1111/1751-7915.14200
Ruiya Shi and Yingjun Tao are contributed equally to this work.
Contributor Information
Harvest F. Gu, Email: feng.gu@cpu.edu.cn.
Jie Wu, Email: wujie@cpu.edu.cn.
REFERENCES
- Ahmad, J. (2015) Management of diabetic nephropathy: recent progress and future perspective. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 9, 343–358. [DOI] [PubMed] [Google Scholar]
- Astudillo, A.M. , Meana, C. , Bermudez, M.A. , Perez‐Encabo, A. , Balboa, M.A. & Balsinde, J. (2020) Release of anti‐inflammatory palmitoleic acid and its positional isomers by mouse peritoneal macrophages. Biomedicine, 8(11), 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astudillo, A.M. , Meana, C. , Guijas, C. , Pereira, L. , Lebrero, P. , Balboa, M.A. et al. (2018) Occurrence and biological activity of palmitoleic acid isomers in phagocytic cells. Journal of Lipid Research, 59, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj, J.S. , Betrapally, N.S. , Hylemon, P.B. , Thacker, L.R. , Daita, K. , Kang, D.J. et al. (2015) Gut microbiota alterations can predict hospitalizations in cirrhosis independent of diabetes mellitus. Scientific Reports, 5, 18559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen, R.D. , Palavecino, E. , Gomadam, A. , Balakrishnan, N. & Datar, S. (2015) Sphingomonas paucimobilis meningitis and ventriculitis in an immunocompromised host. Journal of the Neurological Sciences, 359, 18–20. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute, E. , Yoo, B.B. , Runia, T.F. , Debelius, J.W. , Singh, S. , Nelson, C.A. et al. (2017) Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proceedings of the National Academy of Sciences of the United States of America, 114, 10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Zhu, J. , Liu, Y. , Dong, Z. , Liu, H. , Liu, Y. et al. (2015) Lipopolysaccharide induces chronic kidney injury and fibrosis through activation of mTOR signaling in macrophages. American Journal of Nephrology, 42, 305–317. [DOI] [PubMed] [Google Scholar]
- Colhoun, H.M. & Marcovecchio, M.L. (2018) Biomarkers of diabetic kidney disease. Diabetologia, 61, 996–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darshi, M. , Van Espen, B. & Sharma, K. (2016) Metabolomics in diabetic kidney disease: unraveling the biochemistry of a silent killer. American Journal of Nephrology, 44, 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck, C. , Van Immerseel, F. , Eeckhaut, V. , De Baere, S. , Cnockaert, M. , Croubels, S. et al. (2014) Faecalicoccus acidiformans gen. Nov., sp. nov., isolated from the chicken caecum, and reclassification of streptococcus pleomorphus (Barnes et al. 1977), Eubacterium biforme (Eggerth 1935) and Eubacterium cylindroides (Cato et al. 1974) as Faecalicoccus pleomorphus comb. nov., Holdemanella biformis gen. Nov., comb. nov. and Faecalitalea cylindroides gen. Nov., comb. nov., respectively, within the family Erysipelotrichaceae. International Journal of Systematic and Evolutionary Microbiology, 64, 3877–3884. [DOI] [PubMed] [Google Scholar]
- Fang, Q. , Liu, N. , Zheng, B. , Guo, F. , Zeng, X. , Huang, X. et al. (2021) Roles of gut microbial metabolites in diabetic kidney disease. Frontiers in Endocrinology, 12, 636175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C. , Li, Y. , Wang, P. , Li, Y. , Qiu, C. , Li, M. et al. (2018) Alterations of gut microbiota in cholestatic infants and their correlation with hepatic function. Frontiers in Microbiology, 9, 2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung, M. , Li, Z. , You, H. , Rodrigues, R. , Jump, D.B. , Morgun, A. et al. (2020) Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine, 51, 102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanninen, A. , Toivonen, R. , Poysti, S. , Belzer, C. , Plovier, H. , Ouwerkerk, J.P. et al. (2018) Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut, 67, 1445–1453. [DOI] [PubMed] [Google Scholar]
- Hasani, A. , Ebrahimzadeh, S. , Hemmati, F. , Khabbaz, A. , Hasani, A. & Gholizadeh, P. (2021) The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. Journal of Medical Microbiology, 70, 10. [DOI] [PubMed] [Google Scholar]
- Hu, T.G. , Wen, P. , Shen, W.Z. , Liu, F. , Li, Q. , Li, E.N. et al. (2019) Effect of 1‐Deoxynojirimycin isolated from mulberry leaves on glucose metabolism and gut microbiota in a streptozotocin‐induced diabetic mouse model. Journal of Natural Products, 82, 2189–2200. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Xu, J. , Sheng, Y. , Liu, J. , Li, H. , Guo, M. et al. (2022) Pleurotus Ostreatus ameliorates obesity by modulating the gut microbiota in obese mice induced by high‐fat diet. Nutrients, 14, 1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Liu, J. , Liu, K. , Chen, J. , Wei, Z. , Feng, Z. et al. (2021) Microbiome‐specific statistical modeling identifies interplay between gastrointestinal microbiome and neurobehavioral outcomes in patients with autism: a case control study. Frontiers in Psychiatry, 12, 682454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T. , Zhang, M. , Tong, X. , Chen, J. , Yan, G. , Fang, S. et al. (2019) Microbial communities in swine lungs and their association with lung lesions. Microbial Biotechnology, 12, 289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachalias, N. , Babaei‐Jadidi, R. , Rabbani, N. & Thornalley, P.J. (2010) Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia, 53, 1506–1516. [DOI] [PubMed] [Google Scholar]
- Leigh, S.J. , Kaakoush, N.O. , Bertoldo, M.J. , Westbrook, R.F. & Morris, M.J. (2020) Intermittent cafeteria diet identifies fecal microbiome changes as a predictor of spatial recognition memory impairment in female rats. Translational Psychiatry, 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Tang, H. , Wu, L. , Ge, H. , Wang, Y. , Yu, H. et al. (2021) Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytotherapy Research, 35, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. , Chen, Y.Z. , Lin, H.L. , Ni, Z.H. , Zhan, Y.L. , Wang, R. et al. (2017) Abelmoschus manihot – a traditional Chinese medicine versus losartan potassium for treating IgA nephropathy: study protocol for a randomized controlled trial. Trials, 18, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. , Lin, H. , Ni, Z. , Zhan, Y. , He, Y. , Yang, H. et al. (2020) Efficacy and safety of Abelmoschus manihot for IgA nephropathy: a multicenter randomized clinical trial. Phytomedicine, 76, 153231. [DOI] [PubMed] [Google Scholar]
- Lim, L.‐L. , Lau, E.S. , Lee, H.M. , Tam, C.H. , Lim, C.K.P. , Luk, A. et al. (2019) 533‐P: Association of Serum Branched‐Chain Amino Acids with kidney function decline in type 2 diabetes: the Hong Kong diabetes register. Diabetes, 68, 533‐P. [Google Scholar]
- Lin, R. , He, X. , Chen, H. , He, Q. , Yao, Z. , Li, Y. et al. (2018) Oil tea improves glucose and lipid levels and alters gut microbiota in type 2 diabetic mice. Nutrition Research, 57, 67–77. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Shu, A. , Zhu, Y. & Chen, Y. (2021) Cornuside alleviates diabetes mellitus‐induced testicular damage by modulating the gut microbiota. Evidence‐based Complementary and Alternative Medicine, 2021, 5301942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Q. , Li, Y. , Wang, J. , Li, P. , Duan, Y. , Dai, H. et al. (2020) Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high‐throughput sequencing. Biomedicine & Pharmacotherapy, 124, 109873. [DOI] [PubMed] [Google Scholar]
- Maedler, K. , Spinas, G.A. , Dyntar, D. , Moritz, W. , Kaiser, N. & Donath, M.Y. (2001) Distinct effects of saturated and monounsaturated fatty acids on beta‐cell turnover and function. Diabetes, 50, 69–76. [DOI] [PubMed] [Google Scholar]
- Monnier, V.M. , Sell, D.R. , Gao, X. , Genuth, S.M. , Lachin, J.M. , Bebu, I. et al. (2022) Plasma advanced glycation end products and the subsequent risk of microvascular complications in type 1 diabetes in the DCCT/EDIC. BMJ Open Diabetes Research & Care, 10, e002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, Y. (2017) Development of the nonobese diabetic mouse and contribution of animal models for understanding type 1 diabetes. Pancreas, 46, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, B.A. , Rabbani, N. , Weston, A. , Ficociello, L.H. , Adaikalakoteswari, A. , Niewczas, M. et al. (2012) Serum levels of advanced glycation endproducts and other markers of protein damage in early diabetic nephropathy in type 1 diabetes. PLoS One, 7, e35655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovier, H. , Everard, A. , Druart, C. , Depommier, C. , Van Hul, M. , Geurts, L. et al. (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nature Medicine, 23, 107–113. [DOI] [PubMed] [Google Scholar]
- Ridgway, W.M. (2006) Dissecting genetic control of autoimmunity in NOD congenic mice. Immunologic Research, 36, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna, S. , van Zuydam, N.R. , Mahajan, A. , Kurilshikov, A. , Vich Vila, A. , Vosa, U. et al. (2019) Causal relationships among the gut microbiome, short‐chain fatty acids and metabolic diseases. Nature Genetics, 51, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer, V. , Horvath, A. , Ribitsch, W. , Schmerbock, B. , Schilcher, G. , Lemesch, S. et al. (2017) Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Scientific Reports, 7, 15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, M.C. , Brownlee, M. , Susztak, K. , Sharma, K. , Jandeleit‐Dahm, K.A. , Zoungas, S. et al. (2015) Diabetic kidney disease. Nature Reviews. Disease Primers, 1, 15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Y. , Fang, Q.J. , Sun, W. , Liu, B.H. , Liu, Y.L. , Wu, W. et al. (2020) Total flavones of Abelmoschus manihot remodels gut microbiota and inhibits microinflammation in chronic renal failure progression by targeting autophagy‐mediated macrophage polarization. Frontiers in Pharmacology, 11, 566611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhao, J. , Qin, Y. , Yu, Z. , Zhang, Y. , Ning, X. et al. (2022) The specific alteration of gut microbiota in diabetic kidney diseases‐a systematic review and meta‐analysis. Frontiers in Immunology, 13, 908219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T. , Qiao, S. , Shi, C. , Wang, S. & Ji, G. (2018) Metabolomics window into diabetic complications. Journal of Diabetes Investigation, 9, 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, J. , Psychogios, N. , Young, N. & Wishart, D.S. (2009) MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Research, 37, W652–W660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub, R. & Wyatt, C.M. (2017) Manipulating the gut microbiome to decrease uremic toxins. Kidney International, 91, 521–523. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Chen, W. , Xia, P. & Zhang, W. (2020) Dynamic comparison of gut microbiota of mice infected with shigella flexneri via two different infective routes. Experimental and Therapeutic Medicine, 19, 2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Y. , Zhou, J. , Zheng, Y. , Xu, Z. , Li, Y. , Zhou, S. et al. (2020) Beneficial effects of polysaccharide‐rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomedicine & Pharmacotherapy, 127, 110182. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Li, P. , Xing, C.Y. , Zhao, J.Y. , He, Y.N. , Wang, J.Q. et al. (2014) Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. American Journal of Kidney Diseases, 64, 57–65. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Cheng, C. , Han, Q. , Chen, Y. , Guo, J. , Wu, Q. et al. (2019) Flos Abelmoschus manihot extract attenuates DSS‐induced colitis by regulating gut microbiota and Th17/Treg balance. Biomedicine and Pharmacotherapy, 117, 109162. [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Tostivint, I. , Xu, L. , Huang, J. , Gambotti, L. , Boffa, J.J. et al. (2022) Efficacy of combined Abelmoschus manihot and irbesartan for reduction of albuminuria in patients with type 2 diabetes and diabetic kidney disease: a multicenter randomized double‐blind parallel controlled clinical trial. Diabetes Care, 45, e113–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Chen, M. , Ye, C. , Sun, X. , Jiang, N. , Zou, X. et al. (2020) BuZangTongLuo decoction improved hindlimb ischemia by activating angiogenesis and regulating gut microbiota in diabetic mice. Journal of Ethnopharmacology, 248, 112330. [DOI] [PubMed] [Google Scholar]
- Zheng, R. , Wang, G. , Pang, Z. , Ran, N. , Gu, Y. , Guan, X. et al. (2020) Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Medicine, 9, 4232–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


