Abstract
Oral drug delivery of peptides and proteins is limited by the degradative environment of the gastrointestinal tract and poor absorption, requiring parenteral administration of these drugs. Luminal mucus represents the initial steric and dynamic barrier to absorption. To overcome this barrier, we report the development of a robotic capsule (RoboCap), an orally ingestible drug delivery device that locally clears the mucus layer, enhances luminal mixing, and topically deposits the drug payload in the small intestine to enhance drug absorption. RoboCap’s mucus-clearing and churning movements are facilitated by an internal motor and surface features that interact with small intestinal plicae circulares, villi, and mucus. Vancomycin (1.4kiloDalton (kDa) glycopeptide) and insulin (5.8kDa peptide) delivery mediated by RoboCap resulted in enhanced bioavailability 20–40 fold in ex vivo and in vivo swine models when compared to standard oral delivery (p < 0.05). Insulin delivery via the RoboCap resulted in therapeutic hypoglycemia, supporting its potential to facilitate oral delivery of drugs that are normally precluded by absorption limitations.
One-sentence Summary:
An ingestible capsule was designed to robotically clear intestinal mucus in order to heighten drug absorption.
Introduction
Though it is the most common, cost-effective, and practical method of drug administration, oral drug delivery for macromolecules including nucleic acids and proteins is limited by the degradative environment of the GI tract and poor absorption (1). Drugs must overcome the harsh acidic environment of the stomach, dissolve in gastrointestinal fluid, remain stable amongst dynamic intestinal microbiota and degradative enzymes, penetrate through the viscous mucus barrier, and evade efflux pumps to achieve therapeutic bioavailability (2, 3). Subtherapeutic oral bioavailability levels lead to many drugs to require alternate, and often, more burdensome routes of administration. For instance, insulin, required daily for millions of diabetic patients globally, is a peptide with an oral bioavailability of less than 1%, necessitating subcutaneous injections, which can lead to injection-related anxiety, pain, and non-adherence (4–6). Alternatively, in the case of vancomycin, a small molecule commonly used in serious Gram-positive bacterial infections, an oral bioavailability of 0.069 – 4% forces intravenous administration, requiring costly hospitalization (7–9). Technologies to overcome the hurdles of absorption, distribution, metabolism, and elimination (ADME), which are necessary to allow chemical candidates to mature into drugs, present a major opportunity to help patients receive necessary pharmacological therapy as well as the pharmaceutical industry in supporting the development of more broadly acceptable drugs (10).
Absorption, the first stage of entry, is predominantly hindered by the mucus barrier. Through its viscous, hydrophilic, frequent turnover, and shear-thinning gel properties, mucus serves as a dynamic, steric, and interactive barrier, preventing drugs in the lumen from reaching the epithelial surface (11). Previously, microstirrers have been developed to perform in situ stirring and demonstrated the ability to increase absorption rate and bioavailability (12). Nanobiotechnology approaches, including tubular micrometers coated with pH-responsive polymers are capable of targeted delivery and have demonstrated increased retention in gastric tissues and mucosa, but their application is restricted to certain types of drugs and has not been scaled to large animal models or humans (13, 14). Mucus-penetrating PEGylated liposomes have increased tissue permeability, although they require cumbersome drug-specific optimization (15). Ultrasound vibrations (16) and low-frequency micro-vibrations (17) have also shown efficacy in mechanically inducing higher transport rates, but require more convenient administration modes for clinical utility. Drug transport rates across viscous mucus can be accelerated by increasing drug dispersion, inducing mixing in the mucus layer, and by temporarily exposing the epithelial layer.
Here, we describe the development of the RoboCap, an orally ingestible robotic drug delivery device that locally clears the mucus layer, enhances mixing, and topically deposits the drug payload to enhance drug absorption (Movie 1). The RoboCap’s rotational and churning movements are generated by surface features designed to interact directly with small intestinal plicae, villi, and mucus. We hypothesize that drug bioavailability will be significantly greater when delivered with the RoboCap compared to standard oral delivery. We test the efficacy of the RoboCap in delivering two model peptide drugs, vancomycin and insulin, through Franz cell diffusion and in vivo testing in swine.
Movie 1.
Results
RoboCap design, function and interaction with the small intestine
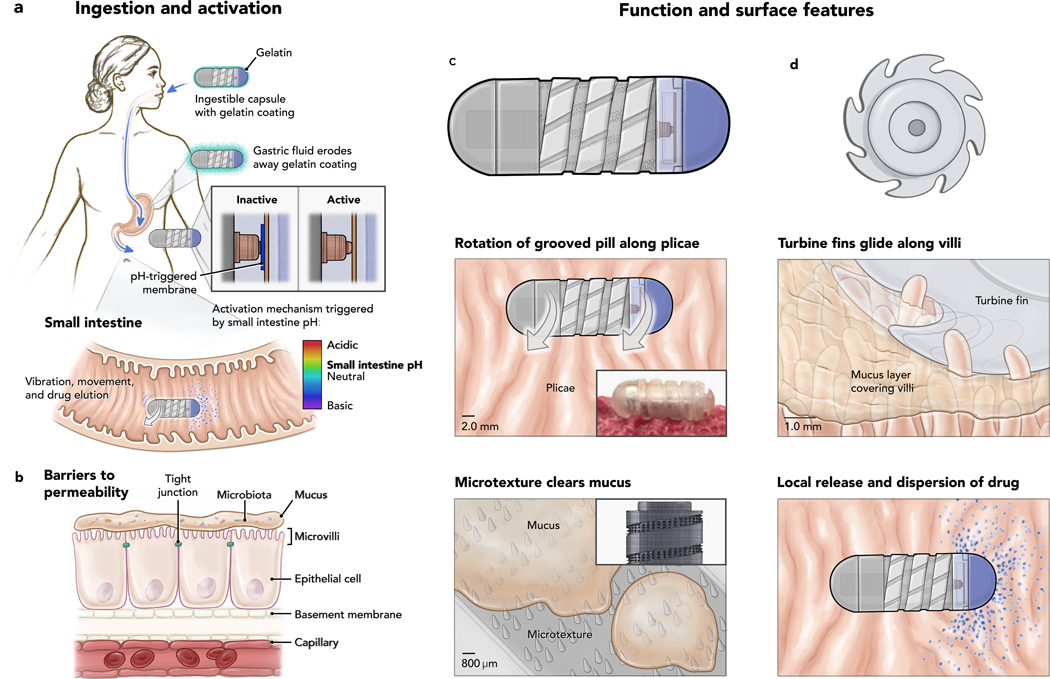
The RoboCap, sized as a triple-zero capsule, is orally ingestible and carries a drug payload volume of up to 342.6 mm3 in its cargo hold (purple component, Figure 1c, Figure 2a). A gelatinous coating hides the surface architecture to prevent abrasion or discomfort during swallowing. Then, during passage in the stomach, gastric fluid erodes this gelatinous coating to expose the RoboCap’s surface features (Figure 1a). Upon reaching the small intestine, the pH of the intestinal fluid triggers a dissolvable activation membrane, closing the onboard circuit to start the RoboCap (Figure 1a). Internal to the Robocap, an offset weight laterally mounted on a motor generates a centripetal force, , causing the RoboCap to vibrate and rotate against surface friction. , which pulls the RoboCap radially outward and changes its direction theta with the offset motor weight (Figure 2b, equation 1).
| (1) |
| (2) |
where is the angular velocity of the motor weight and is the radial offset of the weight from the central axis of the RoboCap. The resulting vibrational frequency of the capsule is . The oscillatory movement of the capsule is caused by the offset of this force to one side of the capsule by from the center of mass, which causes the capsule to rock back and forth (teeter totter effect) as the weight moves with and against the force of gravity.
| (3) |
where angular momentum and
| (4) |
Figure 1. RoboCap Mechanism of Action.
a) Ingestion process and activation trigger serial dissolution of pH-sensitive gelatinous membranes to expose surface features and close the circuit to activate the RoboCap in the appropriate region of the GI tract. b) Barriers to drug absorption include the mucus, tight junctions, microbiota of the small intestine and other anatomical features of the tract. c) Side view and d) cross sectional view of the RoboCap. e) Helical surface grooves enable rotation against small intestinal plicae. f) Fin-shaped cuts enable the pill to glide and scrape mucus from villi. g) Microtexture comprising of an array of studs wick the mucus. h) Drug loaded into the capsule erodes away layer by layer during rotation of the Robocap onto the luminal surface.
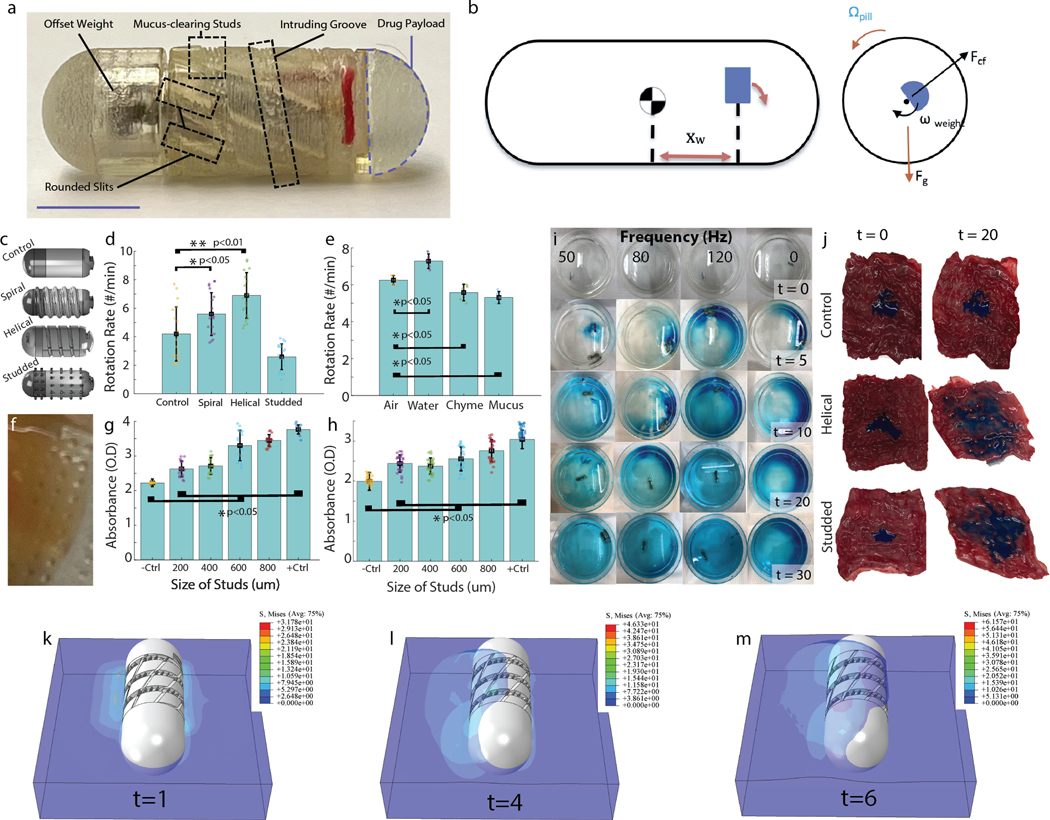
Figure 2. RoboCap Design and Optimization.
a) Robocap device with all major components labeled. Scale bar = 6.5mm b) (Left) A side view of the RoboCap with the center of gravity and offset weight (blue) market. (Right) Cross-sectional view of the RoboCap showing the main forces contributing to the rotational movement. c) Surface geometries to enhance rotation, movement and churning featured helical grooves, studs, and smooth surfaces. d) Rotation rate on swine small intestine ex vivo was significantly enhanced for helical and spiral surface geometries as compared to flat or studded controls. e) Rotation rate in various media demonstrates the influence of frictional constraints induced by fluid drag on movement. f) Close-up photograph of mucus adhered to the studs on the surface of the RoboCap. g) Optical absorbance of luminal fluid in a 4-cm segment of the intestine following 30 minutes of treatment with the RoboCap featuring studs with various heights. h) Optical absorbance quantification of mucus adhered to RoboCaps following 30 minutes of rotation in swine small intestine with various stud heights. i) Mixing of drug (blue) in reaction chamber with RoboCap at various frequencies. j) Dispersion of drug (blue dye) following delivery by a sham control, helically-grooved RoboCap or studded RoboCap at t = 0 and 20 minutes. (k,l,m) Numerical modeling of the stress field of the mucus adhered to RoboCaps following 6s of rotation, with a rotation speed of 0.5236 rad/s.
The rotational velocity of the capsule is governed by conservation of momentum within the system. In a frictionless environment, as the motor secured within the capsule spins at , the capsule will counter that spin with an angular velocity , proportional to the rotational rate of the motor and scaled by a ratio of the moment of inertia of the weight, , to that of the capsule, . Detailed analysis of the contribution of gravity and the motor to the RoboCap’s motion are provided in Supplementary Note 2.
During its rotation, the RoboCap’s surface features mechanically interact with the intestinal plicae, villi, and mucus (Figure 1e–h) to enhance drug delivery through various mechanisms. The external helix (1.0mm) enables optimal contact with plicae (1–10mm) and rounded slits (0.5mm) interface with villi (0.2 – 8mm), together facilitating rotation. The contoured surface also maximizes mucosal surface contact wherein microtextured (200–300 μm) studs seated on the recessed surfaces churn and clear the 500–800-μm-thick mucus layer coating the epithelium (18). With each rotation, the drug load erodes away, layer by layer, depositing drug particles. The RoboCap is active for ~35 minutes and is moved along the tract by peristalsis whereby it is passed by defecation. The RoboCap’s design incorporated practical considerations to enable versatile use. For example, the drug payload is positioned at one end of the capsule, allowing it to be easily manipulated by pharmacists who can load any drug of choice. Additionally, the Robocap’s pH sensitivity can be tuned to target other segments of the GI tract by modifying the properties of the dissolvable membrane. Fully dimensioned designs of the RoboCap can be found in Supplementary Figure 8.
Surface property optimization
To optimize rotation, surface geometries incorporating spiral, helical and studded features were compared to a smooth exterior. Rotation rate was measured as the RoboCap rotated on freshly excised small intestinal tissue. Rotation rate was found to be significantly increased with a helical groove (6.9 ± 1.6 rotations per minute (rpm), p<0.01, 2-tailed heteroscedastic t-test), likely due to alignment with plicae and accentuation of the oscillatory effect as compared to the smooth exterior (4.2 ± 1.9 rpm).
Spiral extrusions (5.6 ± 1.5 rpm, p<0.05, 2-tailed heteroscedastic t-test) also significantly enhanced the rotation rate, although studded exteriors did not (2.6 ± 0.9 rpm, p>0.05, 2-tailed heteroscedastic t-test, Figure 2c,d, n = 20 trials for each). Thus, an outer body comprising a helical groove was selected for the RoboCap. Rotation rates in air, water, chyme, and mucus were also tested to provide insight on the expected range of rotation rates as the RoboCap encounters diverse media in the small intestine. Rates were significantly different in chyme, water, and mucus as compared to air (p < 0.05, 2-tailed heteroscedastic t-test, Figure 2e, n = 5 trials each). However, less than 30% variability was observed between media, indicating that the RoboCap would function as desired even in the most viscous conditions. Rotation and mixing of viscous mucus (stained red) in luminal fluid (green) can be seen in Supplementary Movie 1.
In the recesses of the helical outer body, studs were fabricated to interrupt beds of mucus as the RoboCap strokes the surface. Studs with heights ranging from 200 μm to 800 μm were assessed for their capability to wick and remove mucus (Figure 2f). We compared these against a positive control in which we manually removed mucus using a comb-like device brushed against the tissue 10 times with a constant downward force. A negative control in which no mucus was removed and no RoboCap was placed was also utilized.
Following 20 minutes of treatment in freshly excised small intestinal tissue, the surface contents of the capsule and luminal fluid were collected using a standardized washing technique (Methods). The collected sample was then assessed with absorbance spectroscopy at 330nm, where higher absorbance indicated greater concentration of mucus displaced from the small intestinal lining. Studs of all lengths significantly increased mucus removal and presence in the luminal fluid (p < 0.05, 2-tailed heteroscedastic t-test, n = 9 trials/condition, Figure 2g,h). Moreover, the 800um studs enabled the greatest clearing and wicking of mucus. The effect of stud length was also validated on a Franz-cell experiment testing FITC-Dextran permeabilities. Studs significantly increased permeability as their length increased (Supplementary Figure 4, p< 0.05, two-tailed heteroscedastic t-test).
Inspired by torpedo blades, rounded slits serving as turbine fins (Figure 1f) were incorporated in the helical outer body to generate propulsion of dislodged mucus into the luminal cavity and enhance mixing of luminal fluids. Videography of this feature evinces greater mixing of viscous mucus (red) in luminal fluid (green) (Supplementary Movie 2).
To quantify the ability of the Robocap to wick mucus, we performed finite element analysis and assessed the displacement and stress fields of the mucus interacting with the rotating Robocap (Figure 2k,l,m, Supplementary Movie 2). Numerical results show that the studded features and grooves wick the mucus more effectively to cause it to turn with the pill as compared to a smooth exterior.
Heightened dispersion and mixing
The RoboCap’s mixing capabilities were characterized by imaging a reaction chamber at 0, 5, 10, 20 and 30 minutes with the drug (blue powder) and RoboCap operating at motor frequencies of 0 (control), 50Hz, 80Hz, and 120 Hz (Figure 2g). Absorbance measurements of liquid samples from the top, middle, and bottom of the chamber quantitively indicated that the RoboCap enabled faster dissolution of the drug and greater spatial dispersion when compared to the control (Supplementary Figure 1a). Motor frequencies of 80 and 120 Hz performed better than 50 Hz. Given power considerations, 80 Hz was chosen as the operational frequency. Following the removal or reduction in mucus at the surface of the intestinal epithelium, this increased dispersion enables a greater number of cells to achieve contact with the drug, thus, increasing the probability of uptake through mechanisms of mass transport and potential saturation of mucin fibers (19–22).
Drug erosion and dispersion on small intestinal (SI) tissue surfaces were also assessed utilizing swine SI tissue following 30 minutes of RoboCap activity. The drug was dispersed over a greater surface area when delivered with RoboCap as compared to the control (p < 0.05) (Figure 2h). With surface features and motor frequency optimized for mixing, dispersion, and clearing of mucus, we performed a range of ex vivo and in vivo studies to quantify the efficacy of the RoboCap in enhancing drug absorption. Using a Franz cell apparatus, vancomycin was delivered to the donor well either by direct dilution in donor or with the RoboCap.
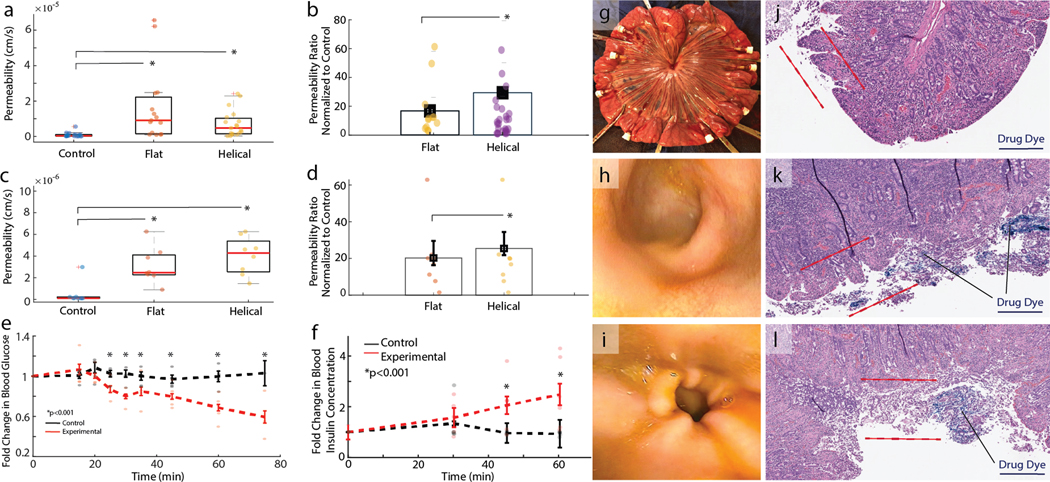
Various surface geometries were tested across 25 independent tissue samples derived from n = 5 animals. Given inter-animal variability of tissue properties, a ratio of permeability induced by the RoboCap to the control condition within the same animal was calculated. Vancomycin drug permeability was observed to increase over 10-fold with RoboCap delivery (either flat or helical surfaces) as compared to controls (p < 0.05, Figure 3a). Further, helical surface geometries significantly outperformed those with flat surface geometries (p < 0.05) (Figure 3b).
Figure 3. In Vivo RoboCap Function.
a) Drug permeabilities for vancomycin when delivered with a sham pill (control) or RoboCap with flat or helical surface geometries in a Franz cell apparatus on small intestinal swine tissue. B) Permeabilities are normalized to the control group and demonstrate over a 10-fold increase in efficacy when drug is delivered with a RoboCap. c) Drug permeability in swine small intestine in vivo for vancomycin delivery by sham (control) pills or a helical or flat RoboCap. d) Permeabilities are normalized to their matched pair in the control group, demonstrating over 20-fold improvements when drug is delivered with a RoboCap. e) Plasma glucose and f) blood insulin concentrations in swine following insulin delivery by endoscopic luminal spray (control, black) or via RoboCap (experimental, red). g) Isolation of independent small intestinal sections for permeability testing. Endoscopic appearance of the small intestine h) prior to and i) after treatment with a RoboCap. Hemotoxylin and eosin staining of cross sections of small intestine following treatment with a blue dyed drug to assess permeation in j) control and k,l) RoboCap-treated cases. Red lines indicate the region of microvilli, brush border and mucus residence. Indicator lines point to representative areas of dye deposition. Scale bars are 4mm. Box plots represent quartiles and dots represent individual measurements.
A chemical resistance test was performed to evaluate the chemical stability of the RoboCap. RoboCaps (n=5) with disabled activation mechanisms were immersed in simulated gastric fluid or simulated intestinal fluid for 72 hours at 37°C. Upon removal, 100% of the capsules were able to be activated and functioned normally (Supplementary Figure 6). Additionally, 10 capsules were placed in the small intestine of swine for at least 60 minutes. Following removal, 10 out of 10 capsules functioned normally when tested on the benchtop. No significant difference was observed in the rotation rate of RoboCaps between those that were exposed to the small intestine and air (control) (p > 0.05, 2-tailed heteroscedastic t-test, Supplementary Figure 6). Further, to evaluate potential thermal risks, the RoboCap was continuously operated in a volume of 10mL of simulated intestinal fluid. The temperature over the course of a 30-minute period shifted less than 1°C, posing no thermal risk for small intestinal tissue.
In Vivo Study
To assess the efficacy of the RoboCap in facilitating peptide drug delivery, we used the model peptide drugs of vancomycin and insulin.
In anesthetized swine, sections of the small intestine were first isolated to serve as independent testing sites while controlling for animal-specific properties such as hydration status, peristaltic rate, blood pressure, and perfusion (Figure 3g). RoboCaps or sham pills carrying 100 mg of vancomycin were placed into each section. Then, vancomycin permeability was assessed through venous blood collection from the mesenteric plexus stemming from each section. Consistent with the Franz cell studies, RoboCaps resulted in significantly higher tissue permeabilities, greater than 20 times the control (p < 0.001, Figure 3c). The helical surface additionally demonstrated a significant advantage over the smooth exterior when normalized to control samples (Figure 3d, p < 0.001,). Furthermore, vancomycin concentration significantly increased in the venous mesenteric blood over a 60-minute period when delivered with a RoboCap (p < 0.01), whereas control samples saw no trend (Supplementary Figure 2a).
Further, we delivered insulin (100 units) via the RoboCap (n= 7 animals, experimental) and compared it with an endoscopic spray in the small intestine (control, n = 5). Blood glucose and insulin concentrations were monitored for a 75-minute period with the drug delivery starting at 15 minutes. The RoboCap significantly increased the bioavailability of insulin, causing a sharp decrease in plasma glucose levels (p < 0.001, Figure 3e) and an increase in blood insulin levels (p < 0.001, n= 5 animals, Figure 3f) when compared to controls (n=5). Animals treated with the RoboCap demonstrated an average blood glucose reduction of 55.54 ± 16.1 mg/dL, while controls demonstrated a variance of 16.6 ± 17.3 mg/dL from baseline. Observed tissue permeabilities were over two-fold greater than previously reported permeabilities of insulin(23). These results are in a similar range as other strategies involving microneedles that circumvent the mucus barrier. When treated with the RoboCap, changes in plasma glucose levels were seen within 15 minutes and continued through the end of the monitoring period. In three animals, hypoglycemia (blood glucose < 20 mg/dL) ensued at 60 minutes, necessitating dextrose infusion. These indicated a steady and significantly enhanced drug absorption rate that makes oral insulin delivery viable for therapeutic applications.
To visualize drug permeation in the tissue, a blue dye was encapsulated in the RoboCap and administered to small intestinal tissues ex vivo. Following 30 minutes of treatment, tissues were fixed, paraffin processed, and stained with hemotoxylin and eosin. Blue particulates can be visualized permeating more deeply into the microvilli and epithelial layers in tissues treated with RoboCaps (Figure 3k,l, Supplementary Figure 2) as compared to luminally administered controls (Figure 3j, Supplementary Figure 2). These visualizations reinforce the mechanism of the RoboCap clearing mucus and locally depositing drug for epithelial uptake.
Following oral or endoscopic delivery to the small intestine, RoboCaps safely transited through the GI tract of the animal without complications, perforation, or obstruction in 10 out of 10 trials. No erosion of the mucosa, inflammation, infection or hematological complications were sustained, as observed by endoscopy (Figures 3h,i) performed before and after RoboCap activity. Using radiography, the RoboCap was monitored passing through the animal alongside radiopaque (barium sulfate) beads, which serve as a proxy for motility rate (Supplementary Figure 3). Controls treated with a sham pill and those treated with RoboCap passed the beads in 7.6 ± 2.7 days and 6.3 ± 1.9 days. No significant difference in the rates of passage were observed at an alpha of 0.05 (Supplementary Figure 3).
Histological analysis was performed on cross-sectional samples from control (n = 9) and RoboCap-treated (n=16) samples (Supplementary Table 1) by a blinded pathologist. The epithelium, surface brush border, inflammation of the epithelium, and surface lamina propia were assessed on hemotoxylin and eosin and trichrome stained cross-sections to assay for damages to the epithelium caused by the RoboCap’s erosion of mucus. No significant differences in any category were assessed, as per two-tailed heteroscedastic t-tests. Further, there was no significant difference in the levels of edema (control = 1 ± 0.707, stimulated = .93 ± 0.25) and inflammation (control = 1.33 ± 0.866, experimental = 1.31 ± 0.47) between groups (p > 0.1). Macromolecular uptake is mediated by absorptive villous epithelial cells including previously described vacuolization (20–22), which can be regarded as a morphological indicator of absorptive activity. No control samples demonstrated a remarkable degree of vacuolization; however, 6 out of 16 experimental samples exhibited pronounced vacuolization, indicative of enhanced uptake behavior related to RoboCap activity (20–22). These data evince the safety of the RoboCap and its easy passage through the GI tract.
To assay the capability of the RoboCap to assist in the delivery of larger molecules, fluorescein isothiocyanate(FITC)-dextran of various molecular weights were delivered using the RoboCap, at various motor frequencies, and compared to direct application (controls). The RoboCap was able to significantly increase uptake even with molecular weights as high as 150kDa, although the greatest increases were seen at 40kDa and 70kDa (Supplementary Figure 4). The frequency of the internal motor did not have a measurable effect on the rate of uptake.
Discussion
This study demonstrates the utility of the RoboCap in enhancing oral drug absorption through localized drug delivery, increased drug dispersion and mucus-clearing mechanisms. Both ex vivo and in vivo testing consistently demonstrated a greater than 10-fold increase in drug permeability for small molecule and peptide drug models. Notably, insulin delivery using the RoboCap resulted in a more gradual uptake as compared to the pharmacodynamics of subcutaneous or intravascular injection – which may be a useful feature for various drugs requiring gradual or sustained release. RoboCap insulin delivery further resulted in a decrease of blood glucose levels in all animals and even caused unanticipated and supratherapeutic hypoglycemia in 3 out of 7 animals. This substantiates its significant potential to enable oral delivery of molecules that have previously seen little success by oral administration. Future studies in swine and humans should optimize dosage for such drugs to identify the therapeutic ranges via small intestinal delivery. Increasing the efficacy of orally administered drugs with poor availability can in turn limit dosages and thereby increase safety, compliance, convenience, and reduce cost.
Unlike other drug carrier systems, such as lipid-based formulations or nanoparticles, the RoboCap yields no biocompatibility concerns, because the electromechanical components remain sealed off and pass through the body after the drug is delivered (24). The mucus barrier serves to protect against pathogens; as such, excessive mucus clearing could pose an infectious risk. However, frequent turnover and constant production of mucus prevents the RoboCap from excessively depleting mucus. This can be benchmarked against more severe interventions that deplete the mucus barrier, such as polypectomies, where side effects of infection are extremely rare (< 0.2%)(25).
Capsule design can be further enhanced to improve RoboCap function and expand its utility to other applications. For instance, geometries that reduce the contact surface area or surface friction on the capsule-fluid boundary would result in an increased rotation rate of the RoboCap. Additionally, varying the material or geometry of the offset weight could increase the capsule’s inertia, resulting in an increased rotation rate. A relative rotation mechanism, where the two sides of the RoboCap rotate in opposite directions, could enable increased mixing of the surrounding fluid. Given RoboCap’s ability to rotate and create mixing, it can be adapted for the in situ generation of topical/intraluminal foams, which currently require endoscopic application (26). RoboCaps may also assist in topical administration of therapeutics including mesalamine and corticosteroids.
Clinical translation will be facilitated by design iterations to miniaturize components and safety and efficacy validations. Based on considerations including the chemical stability and safety of the drug, formulation media, and costs associated with manufacturing and scaling drug-specific capsules, RoboCap may be loaded with the desired drug during manufacturing or pharmaceutical preparation for personalized dosing. The presence of metal within the pill could make it difficult to use the RoboCap in patients that require imaging techniques involving magnets, such as magnetic resonance imaging. However, other ingestible devices such as the PillCam have overcome this limitation, and such techniques could be adapted for this capsule. Further, the current design incorporates rigid batteries to provide adequate motor power (~250mW). As wireless, battery-free, and energy-harvesting systems advance, they could potentially be incorporated to eliminate rigid electronics inside the RoboCap. The material components of the RoboCap are similar to those of FDA-approved ingestible devices such as the osmotic-controlled release oral delivery (OROS) capsules, ingestible temperature sensors, and capsule endoscopy systems, yielding comparable environmental considerations (27, 28). Systems to retrieve the RoboCaps from excreted waste must be considered to minimize the potential environmental complications of disposing such components into the common sewage systems.
In conclusion, as an ingestible robotic capsule, the RoboCap effectively clears mucus, enhances mixing, and topically deposits a drug payload leading to significantly improved drug absorption. As we demonstrated in the case of insulin delivery, the RoboCap makes it possible to achieve therapeutic absorption levels through oral ingestion for drugs that usually require more cumbersome and expensive methods like subcutaneous injections, inhalers, and intravenous administration, requiring hospitalization.
Materials and Methods
Design of the RoboCap
The RoboCap was designed using Solidworks. Its framework was based on a triple zero capsule’s dimensions to aid oral administration. A central compartment houses the battery, resistor, motor (1.5V 3V 6mm–by–10mm miniature micro vibrating coreless motor, A00000308), and offset weight. The circuitry in this compartment is closed upon dissolution of a polymer membrane that degrades at the pH of small intestinal fluid. This allows the pogo pin attached to the battery to contact the motor lead, thus closing the circuit. A secondary compartment houses the drug load and can be press fit onto the main compartment. A 1.55 volt 80 mAh silver oxide battery (DigiKey) was used due to its biocompatibility and its high capacity-to-size ratio. Prototypes were 3D printed (Stratasys) using the VeroClear photopolymer, which was selected for its biocompatibility, transparency, chemical resistance, and transparency. Capsules were thoroughly cleaned prior to administration. In preparation for assembly, the 3D-printed parts were submerged in 2% sodium hydroxide solution and stirred for 15 minutes. The parts were then rinsed in deionized (DI) water four times before being left to dry. Detailed assembly information is provided in Supplementary Note 1.
Motor frequency was modulated through the use of resistors (Digikey) ranging from 0 to 120 ohms placed between the battery and the motor (Supplementary Figure 7). The frequency of vibration was verified using a tachometer to measure the rotation rate of the offset weight over the period of 10 seconds. An inverse relationship was observed between the resistance in the circuit and the output operating frequency of the RoboCap.
Various surface geometries were designed and tested on the RoboCap in order to optimize rotation and mucosal disruption. The baseline geometry utilized a smooth exterior shell similar to standard triple-zero drug capsules. Grooved and protruding spiral geometries were then added to enhance the rotation rate of the RoboCap, taking inspiration from rotating screw mechanisms. Studded arrays along the spirals were incorporated to increase the churning effect on the SI mucosal layer and to further stimulate the villi for drug absorption. Due to the modular nature of the RoboCap, these features were easily incorporated and combined for fast prototyping of various geometries.
To coat RoboCap capsules with gelatin to prevent activation prior to reaching the small intestine, a 35% w/v solution of gelatin (Sigma Aldrich) was placed at room temperature in a large petri dish. Capsules were then be submerged in the solution for 1.2 hours until the desired thickness of the layer was achieved. Following removal, the capsule was gently rotated several times to ensure even distribution around the surface of the pill, and then left to set and dry at room temperature in a vented but covered dish. To load a drug into the capsule, a powdered formulation of the desired drug at the appropriate dosage was measured using a balance. A thin spatula was then used to scoop and pack the powder into the RoboCap’s drug compartment. This was then sealed and coated with Eudragit-L, which dissolves at pH 6, in the small intestine.
Tissue and In Vivo Experiments
All animal experiments were conducted in accordance with protocols approved by the Committee on Animal Care at the Massachusetts Institute of Technology (MIT) in a swine model [0 to 80kg Yorkshire pigs (Sus scrofa domesticus) ranging between 4 and 6 months of age]. The swine model was chosen because its gastric anatomy is similar to that of humans and has been widely used in the evaluation of biomedical GI devices (29). For bench tests assessing mixing and dispersion, mucus was collected from the swine small intestine through an endoscope. For all ex vivo studies, intestinal dissection was performed within 10 minutes of euthanasia and tissues were maintained in Krebs buffer during transport. 100 cm of the small intestine starting at the duodenum was used for all experiments, prioritizing proximal tissue whenever possible to maintain consistency across experiments.
Characterization of rotation rate
To characterize the rotation rate amongst various capsule shapes, RoboCaps were marked axially and rotations were counted for ten minutes in a 500-mL reaction chamber. For one set of trials, the reaction chamber was filled with distilled water. For a second set of trials, freshly harvested small intestinal tissue was transected longitudinally and laid flat in the chamber. The RoboCap was placed on top of a thick layer of mucus and observed.
Optimization of Surface Features
To optimize surface features, the RoboCap was observed operating on freshly harvested tissue. Studs ranging from 200–800 μm were evaluated for their ability to clear mucus. Following 20 rotations of the capsule in a single location, mucus that adhered to the tissue and to the RoboCap was collected using 3 washes of 5 mL in distilled water. The absorbance of the solution at 330nm was recorded at 9 points in each of the samples with mixing just prior to recording. This was performed in triplicates for each sample. A higher absorbance reading indicates a greater concentration of mucus in the sample, representing lesser adherent mucus on the small intestine.
Characterization of Dispersion and Mixing
Gelatin capsules filled with methylene blue were placed in a reaction chamber filled with distilled water along with the RoboCap operating at various frequencies. At each time point, 2 mL of fluid was sampled from the bottom, middle and top of the 500-mL chamber. Absorbance spectroscopy at 435 nm was performed to assess the concentration of the drug in the sample. Each sample was measured in triplicate.
The drug chamber of the RoboCap or sham pill was filled with a blue tissue-marking dye to simulate a drug. A helical RoboCap, studded RoboCap, or sham pill was placed in 3-cm-long isolated segments of the small intestine. Following 20 minutes of operation, the tissue was filleted open and photographed to measure dispersion.
Simulation of Rotation and Wicking of Mucus
Finite element analysis was conducted to characterize the interaction between the mucus and the Robocap, via the commercial finite element software Abaqus 2021 (SIMULIA). The Robocap was treated as a polypropylene rigid body, with a density of 900 kg/m3, Young’s modulus of 1,340 MPa and Poisson ratio of 0.39. Mucus was modeled as a non-Newtonian fluid under laminar flow, with a density of 1500 kg/m3. We used the Carreau-Yasuda model to describe the non-Newtonian shear-thinning behavior of the model, where the viscosity μ follows the formula
| (5) |
Here, is the shear rate, and the model properties are defined in Table 1.
Table 1.
Non-Newtonian mucus properties for the Carreau-Yasuda model
| Shear viscosity at low shear rates | 0.03 |
| Shear viscosity at high shear rates | 0.01 |
| Time constant | 25 |
| Flow behavior index | 0.25 |
The simulation space was set at 40 mm (L) by 40 mm (W) by 20 mm (H), where the Robocap rotates in the center of the cube space. The volume of the mucus was constrained to be half of the space, and initially the bottom half of the Robocap was immersed in the mucus. Gravity of 9.81 m/s2 was applied to the mucus and the Robocap. The interaction type between the Robocap and the mucus was set to be ‘hard’ for the normal behavior, with a friction coefficient of 0.02 for the tangential behavior. The dynamic explicit solver was implemented to simulate the first 12 s of interaction once the Robocap started rotating, with an angular velocity of 0.5236 rad/s.
Chemical Resistance Test
The robustness of the mechanical design and encased electronics were measured by immersing the RoboCaps in simulated gastric or intestinal fluid at 37°C. A small wax plug sealed the inlet to protect the pH-sensitive activation membrane. After 72 hours, the RoboCaps were visually inspected for any mechanical damage. The wax plug was removed and the capsules were placed in a 50-mL beaker of simulated intestinal fluid and observed for activation.
Thermal testing
To evaluate the thermal safety of the pill, we placed the RoboCap in a 10-mL vial of simulated intestinal fluid and monitored the temperature of the fluid over a 30-minute period using a digital thermometer.
In Vitro Tissue Permeability
To test RoboCap function and quantify tissue permeability, a Franz cell apparatus was used as previously described (23) with full thickness intestinal tissue surgically harvested from Yorkshire pigs. Tissue was filleted into rectangular strips, washed with 30 mL of saline to remove food contents, and placed between two magnetic compression plates to create an array of donor and receiver wells. Clear plastic film (Thermo Fischer) was used to seal the bottom plate, creating a receiver well. The receiver wells were filled with phosphate buffered saline (PBS, Thermo Fischer) and 2% bovine serum albumin (BSA, Sigma Aldrich). The donor well was filled with PBS (1X) along with the drug. Vancomycin hydrochloride (Sigma) was dosed at 1000–3000 μg/mL. Insulin was dosed at 500–2000 μg/mL. In the stimulation group, RoboCaps were placed in the wells of the plate for thirty minutes. Following treatment, internal standards were added to the receiver wells. For vancomycin and insulin experiments, we treated with bleomycin at 1 μg/mL and liraglutide at 1 μg/mL as internal standards, respectively. Then, the contents of the receiver wells were extracted with a 20-gauge needle. Tissues were maintained at 37°C and in Krebs buffer during processing to maximize viability. All tissues were used within 1 hour from the time of harvest. An internal standard was used to determine the losses due to processing or remove wells in which leaks occurred, and correct for inter-tissue variance.. The vancomycin or insulin signals in HPLC or LCMS are normalized to the signals of the respective internal standards to get a relative response and improve precision.
In Vivo Drug Delivery Study
To measure the efficacy of RoboCap as compared to traditional pill dissolution and drug uptake, 4-inch sections of the small intestine were isolated following laparotomy in an anesthetized pig (Figure 3g). Veins in the myenteric plexus were catheterized. Blood was sampled every 15 minutes during a 45-minute treatment period. In each intestinal section, which were separated from the adjacent section by a clamped-off section of small intestine at least 6 inches long, the RoboCaps or a drug in liquid form was administered. Every other section was kept blank and untreated as a buffer segment to prevent cross contamination and/or leaks. Tissue was kept at physiologic temperatures using warm towels with saline. Blood was centrifuged at 4°C for 15 minutes. Protease inhibitor (Sigma) was added to the plasma and stored at 4°C for further analysis.
In anesthetized pigs, RoboCaps loaded with insulin were endoscopically delivered to the small intestine. Blood glucose levels were assessed every 5–10 minutes. Blood was collected using an ear vein catheter and processed as described above. The RoboCap’s rotation and vibration rates were measured.
All samples were combined with acetonitrile in a 1:3 ratio (v/v), and then centrifuged at 1200 rpm at 4°C for fifteen minutes for protein precipitation and extraction. The supernatant of each tube was then loaded into microtubes and processed using high performance liquid chromatography (HPLC) to quantify the drug concentrations. Vancomycin concentration was analyzed using an Agilent 1260 Infinity II HPLC equipped with a quaternary pump, autosampler, thermostatted column compartment, and UV diode array detector (DAD). Output signal data processing was performed using ChemStation software. Chromatographic separation was performed using an Agilent Zorbax XDB C18, 4.6×150 mm analytical column with spherical particle size of 5 μm. Separations were performed at a temperature of 50°C. The optimized mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B). The gradient elution began at 100% A and 0% B, increasing to 5% A and 95% B over 3 minutes. The total run time was 5 minutes with a re-equilibration time of 2 minutes. The flow rate was 0.75 mL/min and the injection volume 10 μL. The DAD parameters were as follows: absorbance measured at a wavelength of 280 nm, bandwidth of 4.0 nm, and scan rate of 5 Hz. Standard curves were prepared using fresh vancomycin or insulin to calculate concentrations (Supplementary Figure 5).
Permeability was calculated with the following formula as per prior reports (23):
| (6) |
where V is the volume in the receiver chamber, A is the tissue surface area, is the initial concentration in the donor chamber and is the concentration increase in the receiver chamber in the incubation time .
Histology
Following euthanasia, small intestinal tissue sections were carefully harvested from animals in the control and experimental groups. Tissues were fixed in 4% paraformaldehyde for 24 hours, washed in phosphate buffered saline three times for 15 minutes each, and stored in 70% ethanol. They were then paraffin processed, embedded, and then sectioned (5 μm). Tissues were stained with hematoxylin and eosin to assess morphology and surveil for adverse side effects related to the intervention.
Tissue samples were evaluated by a blinded clinical pathologist for edema, basement membrane disruptions, inflammation, vacuolization and the presence of goblet cells as per the scales indicated in Supplementary Table 1.
Statistical analyses
Quantitative data are reported as mean (±standard deviation) or as a range when appropriate. The normality of the distributions was checked by the Shapiro-Wilk test. Comparative analyses were performed using student’s heteroscedastic two-tailed t-test, unless otherwise noted. P<0.05 was considered significant.
Supplementary Material
Acknowledgement:
We thank Virginia E. Fulford (Alar Illustration) for original artwork in Figure 1.
Funding:
This work was funded in part by a grant from the National Institutes of Health (R01EB000244), the Karl van Tassel (1925) Career Development Professorship and the Department of Mechanical Engineering at MIT.
Competing Interests:
Shriya Srinivasan, Amro Alshareef, Robert Langer and Giovanni Traverso are co-inventors on provisional patent applications describing the developments presented here. R.L. and G.T. Report receiving consulting fees from Novo Nordisk. Complete details of all relationships for profit and not for profit for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0. Complete details for R.L. can be found at the following link: https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0.
Data and materials availability:
All data associated with this study are presented in the manuscript or the Supplementary Materials.
References:
- 1.Billat P-A, Roger E, Faure S, Lagarce F, Models for drug absorption from the small intestine: where are we and where are we going? Drug Discov. Today 22, 761–775 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Paul A, in Introduction to Basics of Pharmacology and Toxicology: Volume 1: General and Molecular Pharmacology: Principles of Drug Action, Raj GM, Raveendran R, Eds. (Springer, Singapore, 2019), pp. 81–88. [Google Scholar]
- 3.Vertzoni M, Augustijns P, Grimm M, Koziolek M, Lemmens G, Parrott N, Pentafragka C, Reppas C, Rubbens J, Van Den Αbeele J, Vanuytsel T, Weitschies W, Wilson CG, Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci 134, 153–175 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Aronson R, The Role of Comfort and Discomfort in Insulin Therapy. Diabetes Technol. Ther 14, 741–747 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaklotar D, Agrawal P, Abdulla A, Singh RP, Sonali AK Mehata, Singh S, Mishra B, Pandey BL, Trigunayat A, Muthu MS, Transition from passive to active targeting of oral insulin nanomedicines: enhancement in bioavailability and glycemic control in diabetes. Nanomed. 11, 1465–1486 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Banerjee A, Ibsen K, Brown T, Chen R, Agatemor C, Mitragotri S, Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci 115, 7296–7301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauter M, Uhl P, Meid AD, Mikus G, Burhenne J, Haefeli WE, New Insights Into the Pharmacokinetics of Vancomycin After Oral and Intravenous Administration: An Investigation in Beagle Dogs. J. Pharm. Sci 109, 2090–2094 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Shibata N, Ishida M, Venkata Rama Prasad Y, Gao W, Yoshikawa Y, Takada K, Highly sensitive quantification of vancomycin in plasma samples using liquid chromatography–tandem mass spectrometry and oral bioavailability in rats. J. Chromatogr. B 789, 211–218 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Geary RS, Wade Schlameus H, Vancomycin and insulin used as models for oral delivery of peptides. J. Controlled Release 23, 65–74 (1993). [Google Scholar]
- 10.Hodgson J, ADMET—turning chemicals into drugs. Nat. Biotechnol 19, 722–726 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Boegh M, Nielsen HM, Mucus as a Barrier to Drug Delivery – Understanding and Mimicking the Barrier Properties. Basic Clin. Pharmacol. Toxicol 116, 179–186 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Mundaca-Uribe R, Karshalev E, Esteban-Fernández de Ávila B, Wei X, Nguyen B, Litvan I, Fang RH, Zhang L, Wang J, A Microstirring Pill Enhances Bioavailability of Orally Administered Drugs. Adv. Sci 8, 2100389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Thamphiwatana S, Liu W, de Ávila BE-F, Angsantikul P, Sandraz E, Wang J, Xu T, Soto F, Ramez V, Wang X, Gao W, Zhang L, Wang J, An Enteric Micromotor Can Selectively Position and Spontaneously Propel in the Gastrointestinal Tract. ACS Nano 10, 9536–9542 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maric T, Atladóttir S, Thamdrup LHE, Ilchenko O, Ghavami M, Boisen A, Self-propelled Janus micromotors for pH-responsive release of small molecule drug. Appl. Mater. Today 27, 101418 (2022). [Google Scholar]
- 15.Yamazoe E, Fang J-Y, Tahara K, Oral mucus-penetrating PEGylated liposomes to improve drug absorption: Differences in the interaction mechanisms of a mucoadhesive liposome. Int. J. Pharm 593, 120148 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Swiston A, Zervas M, Barman R, DiCiccio AM, Brugge WR, Anderson DG, Blankschtein D, Langer R, Traverso G, Ultrasound-mediated gastrointestinal drug delivery. Sci. Transl. Med 7, 310ra168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty SM, Russell IJ, Lukashkin AN, Drug distribution along the cochlea is strongly enhanced by low-frequency round window micro vibrations. Drug Deliv. 28, 1312–1320 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dörffel Y, Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56, 343–350 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schittny A, Huwyler J, Puchkov M, Mechanisms of increased bioavailability through amorphous solid dispersions: a review. Drug Deliv. 27, 110–127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Z, Paunović N, Leroux J-C, Physical methods for enhancing drug absorption from the gastrointestinal tract. Adv. Drug Deliv. Rev 175, 113814 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Kesarla R, Omri A, Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN Pharm. 2013, e848043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai SK, Wang Y-Y, Hanes J, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev 61, 158–171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Erlach T, Saxton S, Shi Y, Minahan D, Reker D, Javid F, Lee Y-AL, Schoellhammer C, Esfandiary T, Cleveland C, Booth L, Lin J, Levy H, Blackburn S, Hayward A, Langer R, Traverso G, Robotically handled whole-tissue culture system for the screening of oral drug formulations. Nat. Biomed. Eng 4, 544–559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ, Advances in Oral Drug Delivery. Front. Pharmacol 12, 62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S-H, Kim K-J, Yang D-H, Jeong KW, Ye BD, Byeon J-S, Myung S-J, Yang S-K, Kim J-H, Postpolypectomy Fever, a Rare Adverse Event of Polypectomy: Nested Case-Control Study. Clin. Endosc 47, 236–241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christophi GP, Rengarajan A, Ciorba MA, Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach. Clin. Exp. Gastroenterol 9, 125–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass DM, Prevo M, Waxman DS, Gastrointestinal safety of an extended-release, nondeformable, oral dosage form (OROS: a retrospective study. Drug Saf. 25, 1021–1033 (2002). [DOI] [PubMed] [Google Scholar]
- 28.McKenzie JE, Osgood DW, Validation of a new telemetric core temperature monitor. J. Therm. Biol 29, 605–611 (2004). [Google Scholar]
- 29.Stamatopoulos K, O’Farrell C, Simmons M, Batchelor H, In vivo models to evaluate ingestible devices: Present status and current trends. Adv. Drug Deliv. Rev 177, 113915 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are presented in the manuscript or the Supplementary Materials.