Key Points
Question
Is lovastatin 2% plus cholesterol 2% cream safe and effective compared with lovastatin 2% cream alone for treating adults diagnosed with disseminated superficial actinic porokeratosis (DSAP)?
Findings
In this randomized clinical trial of 31 participants with DSAP, the disease severity decreased by 50% in the lovastatin-cholesterol treatment group and 51% in the lovastatin group. There were no significant differences in outcomes or adverse events between the treatment groups at the end of 12 weeks.
Meaning
The findings of this randomized clinical trial indicate that because the formulations were equally safe and effective, the addition of cholesterol may not be necessary and that the use of topical lovastatin 2% cream may be a new primary treatment option for patients diagnosed with DSAP.
Abstract
Importance
Disseminated superficial actinic porokeratosis (DSAP) is an inherited or sporadic disorder of keratinization associated with germline variations. There is no effective standard of care therapy for DSAP, but treatment with topical lovastatin combined with cholesterol cream has shown promise.
Objectives
To evaluate and compare the safety and efficacy of topical lovastatin 2% plus cholesterol 2% cream (lovastatin-cholesterol) and topical lovastatin 2% cream (lovastatin) alone in adults diagnosed with DSAP.
Design, Setting, and Participants
This patient- and assessor-blinded, randomized clinical trial was conducted at the Medical University of South Carolina between August 3, 2020, and April 28, 2021. Nonpregnant adults with a previous clinical or histological diagnosis of DSAP were eligible. Data were blindly analyzed after study completion.
Interventions
Participants were randomized to once- or twice-daily application of either lovastatin-cholesterol cream (n = 17) or lovastatin cream (n = 14) to symptomatic regions for 12 weeks.
Main Outcomes and Measures
The primary efficacy measure was the effect of the treatment on DSAP at the end of treatment (12 weeks) as measured by the DSAP General Assessment Severity Index (DSAP-GASI; scored from 0-4, with 0 indicating clear and 4 indicating severe). Treatment efficacy was based on investigator-standardized photographs provided by the participants because of the need for evaluation via telehealth during the COVID-19 pandemic. Secondary efficacy measures included patient-reported outcomes, application frequency, and adverse events (AEs).
Results
Of the 87 participants screened, 32 were enrolled. One participant randomized to receive lovastatin-cholesterol did not receive the intervention, leaving 17 participants (mean [range] age, 59.2 [40-83] years; 13 females [76.5%]; all White) allocated to receive lovastatin-cholesterol treatment and 14 participants (13 female [92.9%]; mean (range) age, 53.7 [33-71] years; all White) to receive lovastatin treatment. Twelve participants in each treatment group qualified for the analysis. Disease severity decreased from week 1 to week 12 by 50.0% (from 3.08 [95% CI, 2.57-3.60] to 1.54 (95% CI, 1.04-2.05] points on the DSAP-GASI; P < .001) in the lovastatin-cholesterol group and 51.4% (from 2.92 [95% CI, 2.40-3.43] to 1.50 [95% CI, 0.99-2.01] points; P < .001) in the lovastatin group. There was no significant difference between the treatment groups according to application frequency at the end of 12 weeks. Adverse events reported included myalgia (n = 2), elevation in the creatine kinase level (n = 1), application discomfort (n = 4), and rash (n = 1). No serious AEs occurred, and all participants with an AE were able to complete the study.
Conclusions and Relevance
This randomized clinical trial found improvements in DSAP severity in both treatment groups, without serious AEs, indicating a limited benefit with the addition of cholesterol. These results suggest that lovastatin cream may be a new primary treatment option for patients diagnosed with DSAP.
Trial Registration
ClinicalTrials.gov Identifier: NCT04359823
This randomized clinical trial of 31 adult patients in the US evaluates and compares the safety and efficacy of topical lovastatin plus cholesterol cream vs topical lovastatin cream for the treatment of disseminated superficial actinic porokeratosis.
Introduction
Disseminated superficial actinic porokeratosis (DSAP) is a porokeratosis variant appearing clinically as multiple pink or brown erythematous macules or plaques in photo-exposed areas.1 The lesions predominantly arise in White women during the third and fourth decades of life and can greatly alter the quality of life.2 The DSAP lesions are typically benign; however, malignant transformation is not uncommon and may occur at a higher rate than in the general population and other familial porokeratoses.3
The pathogenesis of DSAP is thought to be attributed to familial or sporadic heterozygous germline variations in the mevalonate pathway genes MVK, PMVK, MVD, and FDPS.4 The mechanism of disease is unknown, but it is hypothesized that cumulative cellular stress and UV radiation induce a second-hit variation at a critical genetic locus inducing a mutated metabolic sequela. This loss-of-function variation leads to dysregulated keratinocyte differentiation, reduction of end products including cholesterol, and accumulation of toxic mevalonate metabolites, presenting clinically as focal porokeratosis in areas of dyshomeostasis.5
Current off-label treatments are ineffective and focus on lesion destruction or inflammation mitigation (eg, topical fluorouracil, cryotherapy, laser photodestruction, photodynamic therapy, topical diclofenac, vitamin D, imiquimod, retinoids, and corticosteroids).5 None have targeted the molecular pathway underlying DSAP pathogenesis until recently, when Atzmony et al6 successfully pioneered topical therapy with lovastatin and cholesterol in 5 patients with porokeratosis, 1 of whom was diagnosed with DSAP. This has since prompted the use of topical therapy with lovastatin and cholesterol in other patients with DSAP, as described in other case series.6,7
As highlighted by multiple studies,5,6,8 lovastatin may exert its therapeutic effect in patients with DSAP by inhibiting β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) reductase in the mevalonate pathway and blocking the accumulation of toxic intermediate metabolites, thereby obviating the hyperactive local immune response thought to be responsible for the classic phenotypic appearance of DSAP. Additionally, it blocks the production of cholesterol, an essential compound for maintaining skin barrier function and prohibiting keratinocyte apoptosis. While the previous research9,10 showed success for both statin and cholesterol and statin monotherapy, the results were reported in individual case reports. Current literature is devoid of longitudinal, prospective clinical trials outlining risk-benefit profiles and comparative treatment efficacy. Moreover, the information on demographic characteristics and comorbidities associated with DSAP is very scarce or outdated.
In this study, we sought to elucidate basic demographic characteristics, current treatment regimens, and medical comorbidities associated with DSAP, the most common porokeratosis variant. The primary objective of this study was to measure, characterize, and compare the overall effectiveness of treatment with topical lovastatin 2% plus cholesterol 2% (hereinafter lovastatin-cholesterol) cream with topical lovastatin 2% (hereinafter lovastatin) cream alone. Secondary objectives were to measure whether cholesterol contributed to lesion clearance and to detail basic demographic characteristics and barriers to care regarding DSAP patient populations. We enrolled 31 participants with a history of DSAP to look at the efficacy of treatment of lovastatin-cholesterol vs lovastatin alone in the mitigation of these lesions over a 12-week period.
Methods
This was a phase 1, single-center, patient- and assessor-blinded, randomized clinical trial approved by the Institutional Review Board at the Medical University of South Carolina. The analysis was conducted between August 3, 2020, and April 28, 2021. The study followed the Consolidated Standards of Reporting Trials (CONSORT) guideline on reporting randomized clinical trials. Written informed consent was provided by all participants before randomization was performed. The study protocol and statistical analysis plan are provided in Supplement 1.
Study Participants
Participants were eligible for inclusion if they were aged 18 years or older; resided in South Carolina, North Carolina, or Pennsylvania; and had been clinically or histologically diagnosed with DSAP. Participants were excluded if they had allergies or a contraindication to lovastatin or cholesterol use; were outside the inclusion jurisdiction; used study drugs within 4 weeks of study initiation; or were pregnant, breastfeeding, or planning to become pregnant during the study period. All participants screened and enrolled were White, which was not surprising, given the proportion of patients with DSAP who are White. Use of other therapeutics for treating DSAP was not allowed 1 month prior to study initiation or anytime during the study. During the COVID-19 pandemic, access for the study team to the original funding was withdrawn. Equipment and other indirect costs were made either voluntary or waived for the study team. These funding limitations required participants to pay wholesale discounted drug costs, and the remaining costs were covered by the research team.
Study Design
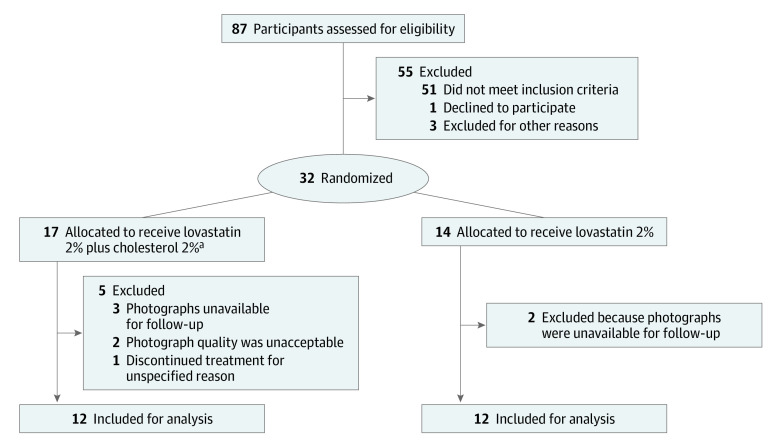
In this study, a cohort of 87 participants were assessed for eligibility, with 55 excluded for not meeting the stated inclusion criteria (Figure 1). Thirty-two participants were then allocated to treatment using a computer-generated block randomization with a double-blinded approach (ie, neither the patient nor researchers assessing the outcomes had access to the treatment group allocation). Allocation concealment was performed via coded order of recruitment. A conversion sheet was used separately to conceal the participant study number and identity for data analysis purposes. Thirty-one participants (1 of the 32 did not receive either therapy) with a clinical (n = 21) or histological (n = 10) diagnosis of DSAP received treatment with either lovastatin-cholesterol cream or lovastatin cream alone. A hydrophilic cream vehicle was chosen over ointment to facilitate comfort and compliance. Participants applied a thin coating of the specified cream to their preferred bilateral extremities once or twice daily (in some cases, participants changed from twice-daily to once-daily application due to an inability to pay for more medication). The study duration was 12 weeks. Lesion assessment was conducted either virtually or in person at days 28, 56, and 84. Most participants preferred telemedicine during the COVID-19 pandemic, with 93 out of 122 visits occurring virtually. The participants were blinded to the medication received. Medication was dispensed by 2 unblinded compounding pharmacies using vehicle-controlled, identical ingredients. Labels on the medications were randomly coded and logged by pharmacists and study team members. Pharmacists were instructed not to release the drug name until permitted to do so by the study team members. Lab monitoring was not required because of the inefficient transepidermal migration of topical lovastatin prohibiting systemic absorption. At the conclusion of the study, 2 physicians blinded to the treatment and participants graded the outcomes via photographic images.
Figure 1. Study Flow Diagram.
aThe number of participants in this group was 17 instead of 18 because 1 patient did not receive the intervention.
Assessments
The primary end point was the percentage of lesion clearance after 12 weeks of therapy using a clinical measurement tool modified from a validated psoriasis index called the Disseminated Superficial Actinic Porokeratosis General Assessment Severity Index (DSAP-GASI), previously named the DSAP Physician Global Assessment Scale. This scale included plaque or rim elevation, scaling, and color (0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, and 4 = severe) (Table 1). Secondary end points were self-reported evaluations (better, unchanged, or worse) on overall appearance, color, scale, pain, and itch. Application frequency and consistency along with safety and tolerability were also assessed at each visit. Finally, participants’ quality of life was analyzed using the survey instruments RAND-36 Measure of Health-Related Quality of Life11 and the Dermatology Life Quality Index.12
Table 1. Disseminated Superficial Actinic Porokeratosis General Assessment Severity Indexa.
| DSAP-GASI score | Category and Description |
|---|---|
| 0 (Clear) |
Plaque or rim elevation: no elevation over normal skin Scaling: no evidence of scaling Color: no discoloration except for residual hyper- or hypopigmentation |
| 1 (Almost clear) |
Plaque or rim elevation: possible but difficult to ascertain whether there is a slight elevation above normal skin Scaling: surface dryness with some desquamation Color: faint, diffuse pink, brown, or slightly red coloration |
| 2 (Mild) |
Plaque or rim elevation: slight but definite elevation; typically edges are indistinct or sloped Scaling: fine scale partially or mostly covering lesions Color: light red or brown coloration |
| 3 (Moderate) |
Plaque or rim elevation: marked definite elevation with rough or sloped edges in annular configuration Scaling: coarser scale covering most or all of the lesions Color: definite red or brown coloration |
| 4 (Severe) |
Plaque or rim elevation: marked elevation typically with definitive annular hard or sharp edges Scaling: coarse, nontenacious scale predominates covering most or all of the lesions Color: dark brownish to bright red coloration |
Abbreviation: DSAP-GASI, Disseminated Superficial Actinic Porokeratosis General Assessment Severity Index.
The DSAP-GASI is scored using the descriptors that best describe the overall appearance of the lesions at a given time. It is not necessary that all characteristics in the second column be present. Excoriations should not be considered when assessing disease severity.
Statistical Analysis
All enrolled participants were considered in the demographic data and safety analysis. Prior to the start of the study a power analysis based on a moderate effect size (ie, Cohen f = 0.25), power set to 0.80, and α = .05 was completed; and based on these parameters a total sample size of 24 participants was required. We sought to double that number during enrollment. Treatment efficacy was based on investigator-standardized photographs provided by the participants, which was necessary because of COVID-19 precautions. Repeat photographic documentation was obtained if image quality, lighting, angle, or location was inadequate or not standardized to prior photos. Thirty-one participants were enrolled in the study, of whom 24 (77.4%) provided clear pictures across all 4 visits and were considered in the efficacy analysis. Efficacy was calculated from the DSAP-GASI. The scores were graded by 2 blinded and trained physicians (including V.B.). Cronbach interrater reliability ranged from α = 0.810 to 0.875 among raters across visits, and the scores were averaged for each of the 4 visits.
A factorial analysis of variance was used to examine the efficacy of the 2 drug groups, measured across the 4 time points of the study. Self-reported participant satisfaction on overall appearance, size, and color, were recorded across all 4 visits (n = 28) and assessed upon completion with χ2 analysis. Analyses were performed using IBM SPSS Statistics for Windows, version 28.0. IBM Corp), and all statistical analyses were 2-tailed with a significance threshold of α = .05.
Results
Participants
Of the 87 participants screened, 32 were enrolled (mean [range] age, 56.8 [33-83] years; 26 [83.9%] female) and randomized to receive 1 of the 2 treatments. Patient demographic and clinical characteristics are provided in Table 2. One of the 18 participants randomized to the lovastatin-cholesterol group did not receive the intervention, which left 17 participants (13 female [76.5%]; mean [range] age, 59.2 [40-83] years) in that group; 14 participants (13 female [92.9%]; mean [range] age, 53.7 [33-71] years) were randomized to the lovastatin group. After exclusions, 12 participants in each treatment group qualified for the analysis (Figure 1). All 17 participants from the lovastatin-cholesterol group and 12 participants from the lovastatin group completed the 12-week study (1 participant in the lovastatin group was unavailable for follow-up, and another voluntarily withdrew). Medication was applied once (n = 11) or twice (n = 20) a day. Assigned application was not followed by all participants; 2 participants had increased the application frequency from once to twice daily at week 4, and 3 participants had switched from twice to once daily at week 8. These participants were advised to continue the new treatment regimen. The treatment groups were well balanced for baseline demographic characteristics, baseline severity, application frequency, and application consistency (Table 2). The total numbers of missed doses in the lovastatin-cholesterol group and the lovastatin group were 65 and 113, respectively.
Table 2. Demographic and Clinical Characteristics of Patients With Disseminated Superficial Actinic Porokeratosis.
| Characteristica | Participants, No. (%) | |
|---|---|---|
| Lovastatin 2% plus cholesterol 2% (n = 17) | Lovastatin 2% (n = 14) | |
| Demographic variables | ||
| Age, mean (range), y | 59.2 (40-83) | 53.7 (33-71) |
| Sex | ||
| Male | 4 (23.5) | 1 (7.1) |
| Female | 13 (76.5) | 13 (92.9) |
| Disease history, mean (range), y | ||
| Duration of disease | 26.6 (0-35) | 20.1 (0-35) |
| Delay of diagnosis | 8.8 (0-33) | 7.2 (0-27) |
| DSAP diagnosis missed, mean (range), No. of practitioners | ||
| Dermatologistb | 3.13 (1-6) | 2.54 (1-6) |
| Other physicianc | 2.80 (1-6) | 2.92 (1-6) |
| Participant-reported history of DSAP disease | ||
| Stable | 3 (17.6) | 4 (28.6) |
| Progressive | 13 (76.5) | 10 (71.4) |
| Relapsing or remitting | 1 (5.9) | 0 |
| History of skin cancer, total No. | 9 (52.9) | 8 (57.1) |
| Basal cell carcinoma | 21 | 8 |
| Squamous cell carcinoma | 15 | 6 |
| Malignant melanoma | 1 | 0 |
| Other medical history | ||
| Autoimmune disorderd | 4 (23.5) | 1 (7.1) |
| Hyperlipidemia | 3 (17.6) | 5 (35.7) |
| Oral statin use | 0 | 3 (21.4) |
| Family history of DSAP | 12 (70.6) | 7 (50.0) |
| Application frequency | ||
| Once a day | 5 (29.4) | 6 (42.9) |
| Twice a day | 12 (70.6) | 8 (57.1) |
| Consistent application of medicine | ||
| Yes | 13 (76.5) | 8 (57.1) |
| No | 4 (23.5) | 6 (42.9) |
| Missed doses at study completion, total No. | 65 | 113 |
| Adverse events | ||
| Discomforte | 1 (5.9) | 3 (21.4) |
| Myalgiaf | 2 (11.8) | 0 |
| Rash | 1 (5.9) | 0 |
| Percentage reduction in disease severity per DSAP-GASI scoreg,h,i | ||
| Mild | 3 (60.0) | 3 (54.5) |
| Moderate | 2 (25.0) | 5 (51.7) |
| Severe | 7 (53.8) | 4 (43.3) |
| Overall DSAP-GASI score, mean (range)g | ||
| Baseline (1 wk) | 3.08 (0-4) | 2.92 (0-4) |
| End of study (12 wk) | 1.54 (0-4) | 1.50 (0-4) |
Abbreviations: DSAP, disseminated superficial actinic porokeratosis; DSAP-GASI, Disseminated Superficial Actinic Porokeratosis General Assessment Severity Index.
All participants recruited and enrolled were White.
Any board-certified dermatologist who did not diagnose DSAP despite lesion of concern.
Predominantly primary care physicians.
Autoimmune disorders included ocular rosacea, myasthenia gravis, antiphospholipid syndrome, rosacea (2 patients), psoriasis, and psoriatic arthritis.
Discomfort at the time of application was defined as discomfort to the skin, such as itching, redness, or drying.
One of the 2 experiencing a transient elevation in creatine kinase levels. Participants reported recent strenuous activity correlating with the development of myalgia.
The DSAP-GASI assesses plaque or rim elevation, scaling, and color, ranking severity on a scale of 0 to 4: 0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, and 4 = severe.
Percentage reduction is the change of DSAP-GASI mean differences from visit 1 to visit 4.
Sample size was too small to determine significance.
Treatment Efficacy
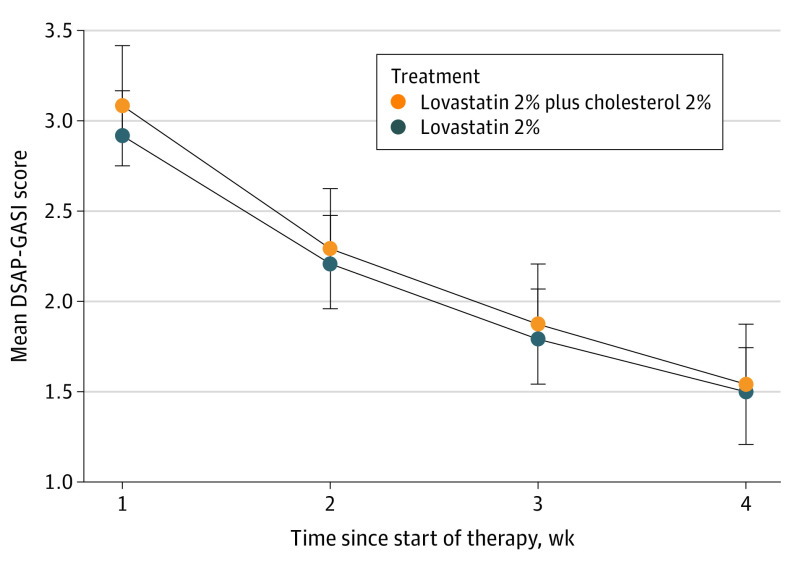
Starting at week 1 and until study completion (week 12), both treatment groups (n = 12 each) showed a statistically significant improvement in mean DSAP-GASI scores (Figure 2), without any significant differences in outcomes between the treatment groups. The disease severity decreased by 50% (from 3.08 [95% CI, 2.57-3.60] to 1.54 [95% CI, 1.04-2.05)] points on the DSAP-GASI; P < .001) in the lovastatin cholesterol group and 51.4% (from 2.92 [95% CI, 2.40-3.43] to 1.50 [95% CI, 0.99-2.01] points; P < .001) in the lovastatin group.
Figure 2. Disseminated Superficial Actinic Porokeratosis (DSAP) Severity Over Time.
The DSAP General Assessment Severity Index (DSAP-GASI) is scored from 0 to 4: 0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, and 4 = severe. Whiskers indicate 95% CIs.
The outcomes among participants applying the cream twice a day did not differ from those in participants applying it once a day, with comparable mean differences in DSAP-GASI scores from week 1 to week 12 (twice a day: change in score, 1.54 [95% CI, 0.90-2.19] points; once a day: change in score, 1.42 [95% CI, 0.77-2.06] points; P = .78). These nonsignificant findings are potentially attributable to the small sample size and application inconsistency. Participants with mild to moderate disease had greater improvements in overall appearance, size, and color than those with severe disease (Figure 3). No participants with severe disease experienced clearance of lesions within the study period. The assessment scores on overall appearance, color, size, itch, and pain did not differ significantly between the treatment groups. At the end of the study, 14 of 15 participants (93.3%) in the lovastatin-cholesterol group and 12 of 13 participants (92.3%) in the lovastatin group reported an improved overall appearance. The color of the lesions was reported to be lighter in 13 of 15 participants (86.7%) in the lovastatin-cholesterol group and in 12 of 13 participants (92.3%) in the lovastatin group. The size of the lesions was reported to be smaller in 12 of 15 participants (80.0%) in the lovastatin-cholesterol group and in 11 of 13 participants (84.6%) in the lovastatin group. In each group, participants first reported improvements in texture followed by a decrease in color and size. Few participants in the combination group noted a decrease in itch (4 participants [33.3%] at week 4, 1 participant [8.3%] at week 8, and no participants at week 16) or in pain (2 participants [16.7%] at week 4 and none at weeks 8 and 16); at week 8, 1 participant (8.3%) noted an increase in itch. One patient (0.8%) in the lovastatin group reported a decrease in itch at weeks 8 and 16 with unremarkable pain differences.
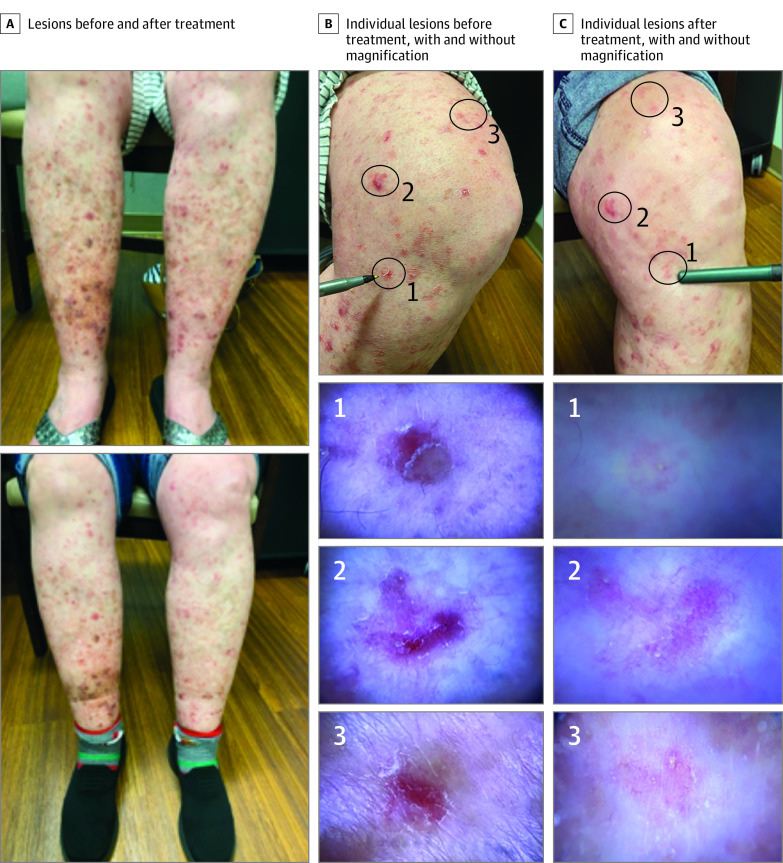
Figure 3. Clinical and Dermatoscopic Images of a Participant With Severe Disseminated Superficial Actinic Porokeratosis Treated With Topical Lovastatin 2% Cream Plus Cholesterol 2% Cream.
Safety and Tolerability
Both treatments were well tolerated. Seven adverse events were possibly related to the study drug (Table 2). One participant reported a rash that resolved with moisturization, and 4 participants reported brief application discomfort. Muscle soreness (myalgia) was briefly reported in 2 participants, with 1 experiencing a transient elevation in creatine kinase. Both individuals reported recent strenuous activity correlating with the development of myalgia. Participants were monitored, and the study drug was continued without reoccurrence of events. No serious adverse events were reported, and all participants with adverse events were able to complete the study.
Discussion
In this randomized clinical trial, we compared the efficacy and safety of topical lovastatin-cholesterol cream vs topical lovastatin cream in participants with DSAP. The addition of topical cholesterol is speculated to offset lovastatin’s influence on cholesterol downregulation.13 Although prior studies6,7 have suggested more favorable effects with the combination drug, results were nonsignificant, and our results showed no significant difference between the treatment groups, suggesting that the addition of cholesterol during the first 3 months of treatment was ineffective. This phase 1, single-center, randomized, patient- and assessor-blinded study is, to our knowledge, the first study to show an efficacious treatment response with a cream vehicle. Application frequency did not alter the outcomes, a result that may be attributable to a small sample size.
Lovastatin, an HMG-CoA receptor inhibitor, has been found to stop progression and promote regression of porokeratotic lesions.14 This receptor blocker alone, without supplemental cholesterol, may lead to a new standard of care for an underappreciated and underresearched disease.
Smaller case studies already support the use of topical statins and cholesterol for treating DSAP,13,14,15 although 2 studies6,8 so far have reported lesion clearance specifically with lovastatin, the first6 a trial of 4 weeks of twice-daily topical lovastatin 2% plus cholesterol 2% therapy, and the second8 with lovastatin 2% alone. Other statin derivatives have also shown success. An open-labeled, unblinded, split-body study16 compared simvastatin 2% plus cholesterol 2% with bland emollients in 8 participants for 6 weeks. The treated limbs had improvements in lesion number, erythema, and scale compared with the limbs receiving emollients. Adverse events included transient nausea (n = 1) and irritant dermatitis (n = 3), which led 1 patient to cease treatment.
Lesion clearance greatly varied between participants with mild to moderate disease (DSAP-GASI score of 2 or 3) and severe disease (DSAP-GASI score of 4) in both study drugs. Participants with mild to moderate disease had overall shorter disease durations and the greatest improvements at the end of 12 weeks. In contrast, participants with severe disease and long disease duration lacked noteworthy improvements, with skin texture being the greatest perceived difference. The time-to-effect differences suggest extending treatment length before withdrawing medication for participants with moderate to severe disease. Future comparative studies with longer treatment durations are warranted to assess long-term outcomes in patients with severe disease.
The demographic and clinical characteristics and care gaps highlighted in Table 2 justify the need for future trials on lovastatin-cholesterol and/or lovastatin alone. This treatment could potentially offset the increased risk of developing nonmelanoma skin cancer; 50.0% of the participants in our study reported a history of skin cancer compared with the national average of 20.0%.17 Of note, 8 participants had hyperlipidemia, 3 of whom were receiving oral statin therapy. None of these 3 participants reported myalgia or elevations in creatine kinase levels. Given the pathogenesis of DSAP, future research should explore comorbid lipid disease in patients with DSAP. Of note, 5 participants reported a history of an autoimmune disorder, which has been hypothesized to be a precipitant of DSAP.18 However, the most common associated variable was genetic inheritance, with 61.3% of the trial participants noted having a family history. Finally, DSAP’s association with quality of life, as depicted in our research group’s sister study,19 must not be forgotten. Disseminated superficial actinic porokeratosis has a negative association with mental health and body image, and in our study, DSAP was often repeatedly misdiagnosed by dermatologists, taking a mean of 3.13 and 2.54 dermatologists to correctly diagnose the disease in the lovastatin-cholesterol and lovastatin groups, respectively. The treatments with both lovastatin-cholesterol and lovastatin alone led to prompt, significant improvements in participants’ quality of life outcomes.19 This evidence underscores the need for a greater medical community awareness of DSAP to facilitate earlier diagnosis and intervention with evidence-based therapies such as topical lovastatin and cholesterol.
Limitations
This study has several limitations. Lack of study funding compounded by the coincidence of the COVID-19 pandemic required the necessity of study execution in a largely virtual setting. Disease prevalence, geographic scope, and the COVID-19 pandemic prevented our ability to meet the anticipated recruitment goal. The effect size was moderately powered; thus, if a small effect occurred, the study was not powered to detect it. Histopathologic confirmation of diagnosis was not performed in 21 participants and genetic confirmation of mevalonate pathway variations was not performed in any participant. An ointment vehicle was not studied as in prior research. There was no placebo group to assess assay sensitivity due to ethical considerations arising from participants purchasing treatment. Costs incurred by participants to procure the study medication may have exacerbated the Hawthorne effect and other biases. Our treatment period was limited to 12 weeks; however, follow-up for months to years is desirable because the time to effect is unknown. This study included the use of telemedicine, which prohibited the ability to calculate the clearance of cornoid lamella on dermoscopy. Patient-captured photographs led to minor variabilities in photograph quality, lighting, and positioning, potentially altering the DSAP-GASI grading. These errors were mitigated as much as possible by the researchers through requests for new photographs when quality did not match the standard. Additionally, the lack of literature on DSAP necessitated grading outcomes using a novel, nonvalidated assessment scale (Table 1) adapted from a validated psoriasis assessment scale. This scale matched the physical appearance of DSAP better than the Actinic Keratosis Field Assessment Scale,20 which is limited to the face and scalp only. Despite these limitations, this small-scale prospective randomized clinical trial provides a rationale for using either lovastatin-cholesterol or lovastatin alone for the treatment of this historically undertreated disease. Favoring the lovastatin-alone treatment could lead to a decrease in compounding costs and environmental waste and provide a therapy alternative for strict vegans.
Conclusions
In this randomized clinical trial, lovastatin 2% plus cholesterol 2% and lovastatin 2% alone equally improved DSAP lesions over the study period, suggesting that cholesterol may not be needed in formulations and that lovastatin cream may be a new primary treatment option for patients diagnosed with DSAP. This preliminary study provides a signal of effect to power future subsequent studies. A larger, longer-term, in-person clinical trial with a validated assessment scale may benefit this patient population.
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Chernosky ME. Disseminated superficial actinic porokeratosis (DSAP). Int J Dermatol. 1973;12(3):152-157. doi: 10.1111/j.1365-4362.1973.tb00026.x [DOI] [PubMed] [Google Scholar]
- 2.Shakhbazova A, Hinds B, Marsch AF. Lichenoid inflammation of DSAP lesions following treatment with durvalumab, olaparib and paclitaxel: a potential diagnostic pitfall mimicking lichenoid drug eruptions associated with PDL-1 inhibitors. Dermatol Online J. 2020;26(3):13030/qt7nf6c8hc. [PubMed] [Google Scholar]
- 3.Novice T, Nakamura M, Helfrich Y. The malignancy potential of porokeratosis: a single-center retrospective study. Cureus. 2021;13(2):e13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Liu Y, Liu F, et al. Loss-of-function mutation in PMVK causes autosomal dominant disseminated superficial porokeratosis. Sci Rep. 2016;6:24226. doi: 10.1038/srep24226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atzmony L, Choate KA. Second-hit somatic mutations in mevalonate pathway genes underlie porokeratosis. J Invest Dermatol. 2019;139(12):2409-2411. doi: 10.1016/j.jid.2019.07.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82(1):123-131. doi: 10.1016/j.jaad.2019.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomsitz D, Biedermann T. Successful treatment of disseminated superficial actinic porokeratosis with topical 2% cholesterol/2% lovastatin cream: a case series with 7 patients. J Eur Acad Dermatol Venereol. 2022;36(1)2:e52-e54. [DOI] [PubMed] [Google Scholar]
- 8.Ugwu N, Choate KA, Atzmony L. Two percent lovastatin ointment as a pathogenesis-directed monotherapy for porokeratosis. JAAD Case Rep. 2020;6(10):1110-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82(1):123-131. doi: 10.1016/j.jaad.2019.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ugwu N, Choate KA, Atzmony L. Two percent lovastatin ointment as a pathogenesis-directed monotherapy for porokeratosis. JAAD Case Rep. 2020;6(10):1110-1112. doi: 10.1016/j.jdcr.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hays RD, Morales LS. The RAND-36 Measure of Health-Related Quality of Life. Ann Med. 2001;33(5):350-357. doi: 10.3109/07853890109002089 [DOI] [PubMed] [Google Scholar]
- 12.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216. doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 13.Zhu T, Tian D, Zhang L, et al. Novel mutations in mevalonate kinase cause disseminated superficial actinic porokeratosis. Br J Dermatol. 2019;181(2):304-313. doi: 10.1111/bjd.17596 [DOI] [PubMed] [Google Scholar]
- 14.Maronese CA, Genovese G, Genovese C, Marzano AV. Refractory disseminated superficial actinic porokeratosis effectively treated with cholesterol/lovastatin cream: a case report. Dermatol Ther. 2021;34(1):e14583. doi: 10.1111/dth.14583 [DOI] [PubMed] [Google Scholar]
- 15.Jerjen R, Koh WL, Sinclair R. Effective treatment of disseminated superficial actinic porokeratosis using a novel topical cholesterol/simvastatin combination cream. Australas J Dermatol. 2021;62(1):93-94. doi: 10.1111/ajd.13473 [DOI] [PubMed] [Google Scholar]
- 16.Byth LA, Byth J. Topical simvastatin-cholesterol for disseminated superficial actinic porokeratosis: an open-label, split-body clinical trial. Australas J Dermatol. 2021;62(3):310-313. doi: 10.1111/ajd.13601 [DOI] [PubMed] [Google Scholar]
- 17.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-282. doi: 10.1001/archdermatol.2010.4 [DOI] [PubMed] [Google Scholar]
- 18.Sim CY, Shin JY, Lee SY, Park YL. Disseminated superficial actinic porokeratosis in a patient with psoriasis, after long-term narrowband ultraviolet B phototherapy. Ann Dermatol. 2018;30(2):211-213. doi: 10.5021/ad.2018.30.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucia GS, Snyder A, Lateef A, Devore A, Elston D. Disseminated superficial porokeratosis: impact on quality of life. J Am Acad Dermatol. 2022;87(5):1162-1163. doi: 10.1016/j.jaad.2022.02.032 [DOI] [PubMed] [Google Scholar]
- 20.Dréno B, Cerio R, Dirschka T, et al. A Novel Actinic Keratosis Field Assessment Scale for Grading Actinic Keratosis Disease Severity. Acta Derm Venereol. 2017;97(9):1108-1113. doi: 10.2340/00015555-2710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement