Abstract
Purpose
In this pilot study, DKI measures of diffusivity and kurtosis were compared in active tumor regions and correlated to radiologic response to radiotherapy after completion of 2 weeks of treatment to derive potential early measures of tumor response.
Methods
MRI and Magnetic Resonance Spectroscopic Imaging (MRSI) data were acquired before the beginning of RT (pre-RT) and 2 weeks after the initiation of treatment (during-RT) in 14 glioblastoma patients. The active tumor region was outlined as the union of the residual contrast-enhancing region and metabolically active tumor region. Average and standard deviation of mean, axial, and radial diffusivity (MD, AD, RD) and mean, axial, and radial kurtosis (MK, AK, RK) values were calculated for the active tumor VOI from images acquired pre-RT and during-RT and paired t-tests were executed to estimate pairwise differences. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive capabilities of changes in diffusion metrics for progression-free survival (PFS).
Results
Analysis showed significant pairwise differences for AD (p = 0.035; Cohen’s d of 0.659) and AK (p = 0.019; Cohen’s d of 0.753) in diffusion measures after 2 weeks of RT. ROC curve analysis showed that percentage change differences in AD and AK between pre-RT and during-RT scans provided an Area Under the Curve (AUC) of 0.524 and 0.762, respectively, in discriminating responders (PFS>180 days) and non-responders (PFS<180 days).
Conclusion
This pilot study, although preliminary in nature, showed significant changes in AD and AK maps, with kurtosis derived AK maps showing an increased sensitivity in mapping early changes in the active tumor regions.
Keywords: diffusion kurtosis imaging (DKI), magnetic resonance spectroscopic imaging (MRSI), tumor response, progression-free survival (PFS), receiver operating characteristic (ROC)
Introduction
Diffusion Kurtosis Imaging (DKI) maps the non-Gaussian component of water diffusion within tissues, 1 which is sensitive to tissue heterogeneity on the microscopic scale of cellular compartments, organelles, and membranes. 2 DKI enables the estimation of standard diffusion weighted and diffusion tensor imaging parameters (DWI and DTI), such as fractional anisotropy (FA), and mean, axial, and radial diffusivity (MD, AD, and RD), as well as diffusion kurtosis parameters that include mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK).2–4 DKI measures have been shown to provide increased sensitivity and specificity relative to DWI and DTI in detecting several pathologies, including attention-deficit/hyperactivity disorder, 5 Huntington’s disease, 6 ischemia, 7 multiple sclerosis,8,9 and temporal lobe epilepsy, 10 in addition to normal aging.11,12 DKI has also been shown to improve tumor grading in gliomas providing better accuracy13–16 and has been shown to provide increased sensitivity in the evaluation of microstructural changes in low-grade gliomas as compared to DTI. 17
For malignant glioma, the Response Assessment in Neuro-Oncology (RANO) criteria are widely used radiologic response criteria that have been correlated with survival. 18 The three-dimensional measurements of tumor volume derived from structural MR measurements have also been suggested to have a strong association with survival; however, a disadvantage of these measures is the time for changes to occur with 8–10 weeks necessary to assess response as measures calculated earlier than that have found to be unreliable.19,20 Therefore, it would be of value to assess other early response criteria to monitor the treatment of gliomas.
DWI has been proposed as an early biomarker for tumor response. 20 Increased diffusion of water molecules shortly after successful treatment can be mapped to derive measures of radiologic response. Changes in diffusion correlate with the breakdown of cellular membranes and reduction in cell density that both precede changes in tumor size. Although the role of DWI has been widely studied for elucidating tumor response, the role of early DKI, which is based on and expands upon DWI, has not been studied.
In this pilot study, DTI measures of FA, MD, AD, and RD are compared in the active tumor regions to the kurtosis parameters of MK, AK, and RK. These are correlated with radiologic response to radiotherapy after completion of 2 weeks of treatment to derive potential early measures of tumor response.
Methods
Study population
Fourteen glioblastoma patients were included: nine men and five women, ages 24–69, at the University of Miami’s Miller School of Medicine Sylvester Comprehensive Cancer Center. Informed consent was acquired from each subject and the protocol was approved by the Review Board for Protection of Human Subjects in Research to analyze imaging findings and outcomes. Approximately 4 weeks after surgery, patients began a treatment regimen of temozolomide (TMZ) chemotherapy with concurrent intensity-modulated radiation therapy (RT) as part of a dose-escalation radiation clinical trial (NCT03137888). Patients received doses up to 75 Gy to regions identified as high risk for recurrence based on areas of metabolic abnormality identified using Magnetic Resonance Spectroscopic Imaging (MRSI). 21
Patients in this study received RT in 30 fractions using simultaneous integrated boost technique with three target volumes over 6 weeks (5 fractions per week), along with concurrent TMZ at standard-of-care dosing (75 mg/m2/day, 7 days/week) during RT. Following a 1-month rest period after RT, patients continued adjuvant cycles of TMZ (150–200 mg/m2/day days 1–5 every 28 days) per the standard of care as recommended by their treatment team.24 A minimum of six cycles of adjuvant TMZ were administered by each site unless there were signs of tumor progression or toxicity.
Imaging protocol
MRI and MRSI data were acquired at 3T (Siemens Medical Solutions, Erlangen, Germany) before the beginning of RT (pre-RT) and 2 weeks after initiation of the 6-week course of treatment (during-RT). T1-weighted imaging was carried out using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo sequence with 0.9 x 0.9 x 0.7 mm3 resolution; TR/TE/TI = 2300/2.41/930 ms; flip angle 9°; and image matrix 320 x 216 x 192. The protocol also included fluid-attenuated inversion recovery (FLAIR) (TR/TE = 9000/106 ms, resolution = 0.36x0.36x3 mm3, flip angle = 120°), and T2-weighted (TR/TE = 4810/76 ms, resolution = 0.45x0.45x3 mm3, flip angle = 160°) images and DTI/DKI acquired with TR/TE of 6300/99 ms and a resolution 1.5x1.5x3 mm3. Diffusion-sensitizing gradient encoding with diffusion weighting factor of b =1000 and 2000 s/mm2 was applied in 30 directions along with nine averages for b = 0 s/mm2 (B0).
The radiation treatment plan incorporated metabolic information derived using whole-brain MR spectroscopic imaging (MRSI). This data was acquired using a spatial-spectral echo-planar readout with spin-echo excitation; TR/TE = 1551/50.0 ms; non-selective lipid inversion-nulling with TI = 198 ms; a field-of-view 280 x 280 x 180 mm3; matrix size 50x50x18 slices with elliptical k-space encoding; echo train length of 1000 points; bandwidth of 2500 Hz. The acquisition time was 15 min. Data was acquired using spatial oversampling with a nominal voxel volume of 0.313 cc.22,23 A water reference MRSI dataset was also obtained using an acquisition interleaved with the metabolite signal acquisition.
Imaging processing
DKI data were processed using Diffusional Kurtosis Estimator (www.nitrc.org/projects/dke) 24 following skull-stripping using the Brain Extraction Toolbox (FSL 4.0, www.fmrib.ox.ac.uk/fsl). 25 The conventional DTI measures were calculated by using the data obtained for b = 1000 s/mm2. Diffusion maps were corrected for geometric distortion using the Advanced Normalization Tools diffeomorphic registration tool 26 by registration of averaged B0 image to T2-weighted images. All DTI and DKI maps were registered to the pre-contrast T1 image using a rigid registration 27 and maps for MD, AD, RD, FA, MK, AK, and RK were evaluated.
MRSI data were processed using the MIDAS package (http://mrir.med.miami.edu).22,28 This included B0 and phase correction using the water reference data before any further processing in the frequency domain. Processing included generating masks for brain and lipid regions, k-space extrapolation to reduce the contribution of extracranial lipid into the brain, 29 tissue segmentation map obtained using FSL/FAST algorithm, 30 linear registration between the T1-weighted MR and MRSI, 27 and signal intensity normalization following the creation of individual metabolite maps. The spectral datasets were interpolated to 64x64x32 points and spatial smoothing was applied after B0 correction, resulting in an effective voxel volume of 1.55 cc. Automated spectral analysis was carried out for N-acetylaspartate (NAA), creatine, and phosphocreatine (Cr), choline, glycerophosphocholine, and phosphocholine (Cho). 31 Additional maps were generated for the fitted spectral linewidth and the Cramer-Rao lower bounds (CRLB) of fitting for each metabolite.
Two volumes of interest (VOIs) were created for each patient using the post-contrast T1 weighted imaging, FLAIR, and Cho/NAA map derived using whole-brain spectroscopy acquired before the beginning of RT. These included the residual contrast-enhancing region (ceVOI) derived from the post-contrast T1-weighted imaging, which was outlined semi-automatically, and a metabolically active tumor region (mVOI) that was obtained using the Cho/NAA and FLAIR images, in the following manner. The FLAIR images were first segmented using a mixture of three Gaussian distributions and the component with the largest mean value selected as the initial tumor region. Non-tissue regions and CSF were excluded using CSF labeled segmentation maps derived from T1w using FSL FAST. 32 Regions of interest were finally edited manually to limit the region of interest to the gross tumor region, which is inclusive of the solid tumor and any surrounding edema. The mVOI was then identified as regions within the gross tumor region that had a Cho/NAA ratio greater than 2.0 relative to the mean of the Cho/NAA value in contralateral white matter (relCho/NAA >2.0).33,34 Contralateral normal-appearing white matter regions were delineated using a semi-automatic segmentation approach using T1w and FLAIR images. 17 Generally, the ceVOI was found to be a subset of the mVOI; however, due to poor spectral quality (spectral linewidth >12 Hz) in some subjects the entire tumor was not mapped using MRSI. As a result, the final active tumor VOI was derived as a union of ceVOI and mVOI. For the delineation of volumes of interest, in-house programs were written in MatLab (MathWorks, Inc.) and IDL (L3Harris Geospatial Solutions, Inc) and all VOI visualization and editing was done in MIM Software (MIM Software Inc, Cleveland, OH) and MIDAS.22,28
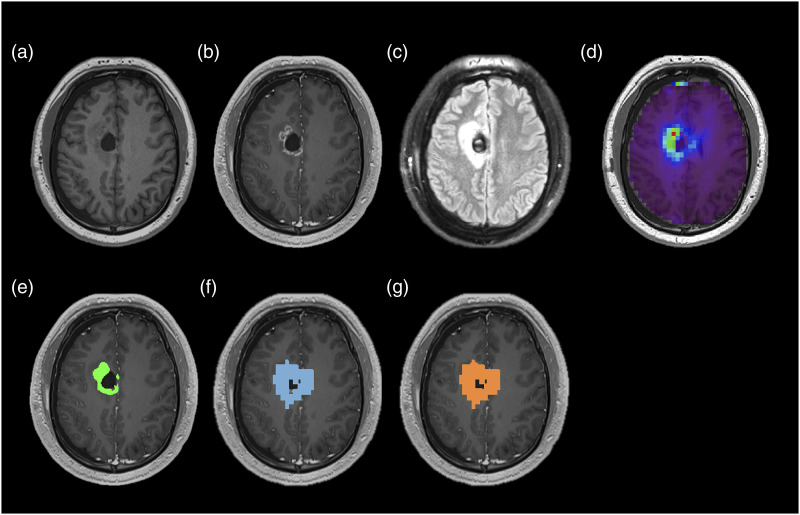
The VOI selection is illustrated in Figure 1, which shows an example images and illustrative volumes of interest of the ceVOI, mVOI, and active tumor acquired before the start of RT in a 24 year-old male subject demonstrating postsurgical changes from resection of a right frontal lobe lesion.
Figure 1.
T1-weighted image (a), T1 post-contrast image (b), FLAIR image (c), Cho/NAA map normalized to contralateral normal-appearing white matter (relCho/NAA) (d), and illustrative volumes of interest of the ceVOI (E), mVOI (F), and active tumor (g) acquired before the start of RT in a 24 year-old male subject.
All active tumor VOIs constructed on images acquired pre-RT were transferred to images acquired during-RT to study changes in diffusion maps in the same regions-of-interest employing a two-step approach using the MIDAS program. Diffusion and spectroscopy maps derived at the two-time points were registered to the pre-contrast T1 image acquired at the corresponding time point. T1-weighted images acquired during-RT were then registered rigidly to T1-weighted images acquired pre-RT to derive registration parameters. 27 Diffusion and spectroscopy maps acquired during-RT were applied the derived registration parameters to bring all images in the same spatial frame of reference, here chosen as the T1-weighted image acquired before the beginning of RT.
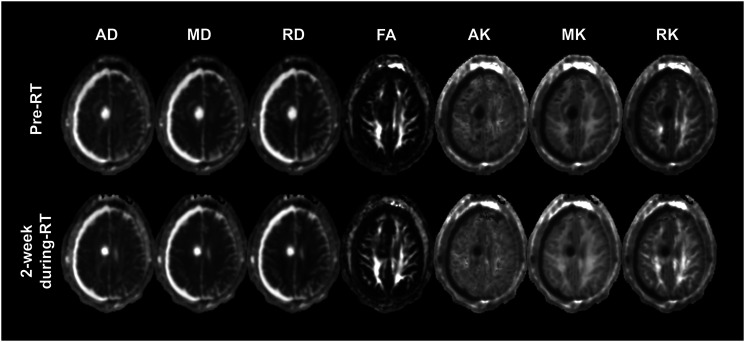
In Figure 2 are shown the co-registered diffusion maps acquired at pre-RT and during-RT for the same subject illustrated in Figure 1.
Figure 2.
Co-registered diffusion maps of axial (AD), mean (MD), and radial (RD) diffusivity, Fractional Anisotropy (FA), and kurtosis maps of Axial (AK), mean (MK), and Radial (RK) kurtosis acquired at pre-RT and during-RT for the same subject illustrated in Figure 1.
Statistical analysis
Average and standard deviation of MD, MK, AD, RD, AK, RK, and FA values were calculated for the active tumor VOI from images acquired pre-RT and during-RT. Paired t-tests were executed to estimate pairwise differences between mean diffusion values obtained from the two imaging time-points. Cohen’s d was calculated to report effect sizes as a measure of the sensitivity of a diffusion map in mapping changes in the active tumor VOI following RT. 35
The clinical endpoint analyzed in this study was the progression-free survival (PFS) time, which was measured in days from surgical intervention to first tumor progression, death, or last follow-up. Based on the North American Brain Tumor Consortium (NABTC) criteria of 6 month progression-free survival which is used as an efficacy endpoint of therapy trials for adult patients with recurrent high-grade gliomas, 36 patients were divided into responders (PFS >180 days) and non-responders (PFS <180 days). A receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive capabilities of changes in diffusion metrics for PFS. Optimal cutoff values for each variable were determined using Youden’s index.37,38
Results
The extracted ceVOI, mVOI, and active lesion had an average volume of 9.4 ± 6.3 cc (Range: 2.0 cc–22.8 cc), 31.5 ± 26.2 cc (Range: 1.9 cc–89.4 cc), and 37.1 ± 26.0 cc (Range: 8.1 cc–93.1 cc), respectively (Volumes are reported as mean ± standard deviation). Of the 14 subjects enrolled in the study, eight were classified as non-responders with an average PFS of 102.5 ± 67.8 days (Range: 0–168 days), and six were classified as responders with an average PFS of 390.2 ± 198.4 days (Range: 217–632 days).
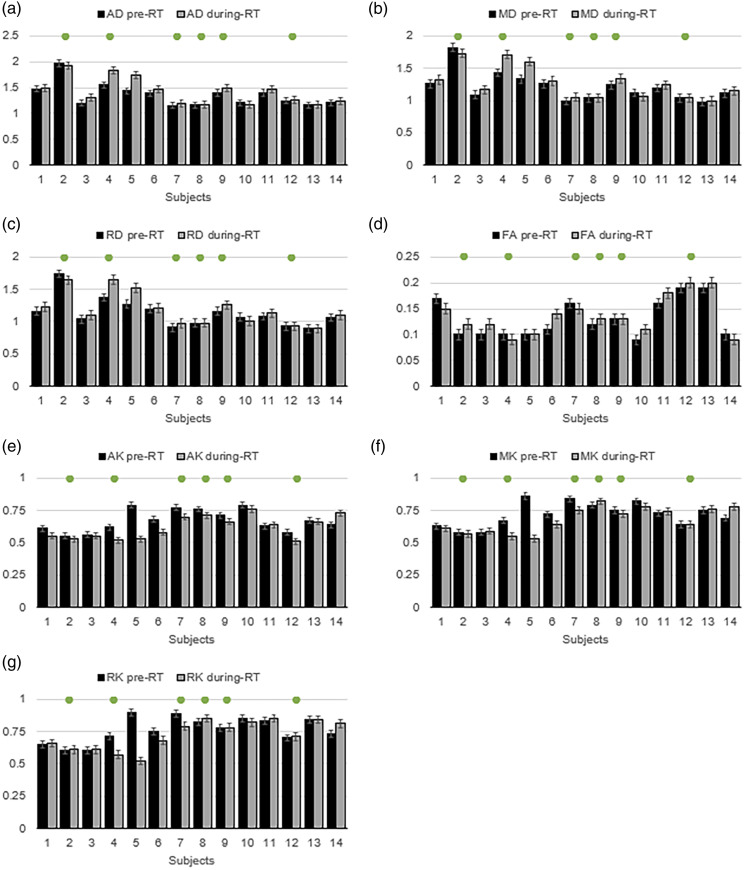
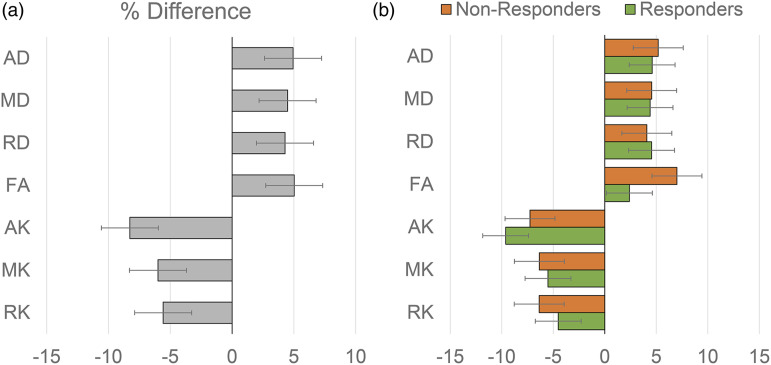
Figure 3 shows the mean value of the diffusion metrics of AD (A), MD (B), RD (C), FA (D), AK (E), MK (F), and RK (G) in the active tumor region for the 14 subjects at the two imaging time points. The subjects that show a clinical PFS >180 days are marked by the green dots in each graph. Figure 4 (a) shows the mean percentage difference of the diffusion metrics in the active tumors between the pre-RT and during-RT scans. The percentage difference is calculated as , with positive percent changes indicating an increase in the diffusion metric in the during-RT scan as compared to the pre-RT scan and vice-versa for negative percent changes. Figure 4 (b) shows the percentage changes in the diffusion metrics in responders and non-responders separately.
Figure 3.
Mean value of the diffusion metrics of AD (a), MD (b), RD (c), FA (d), AK (e), MK (f), and RK (g) in the active tumor region for the 14 subjects at the two imaging time points before and after 3-weeks of radiotherapy treatment. The subjects that show a clinical PFS >180 days are marked by the green dots in each graph.
Figure 4.
Mean percentage difference of the diffusion metrics in the active tumors between the pre-RT and during-RT scans with positive percent changes indicating an increase in the diffusion metric in the during-RT scan as compared to the pre-RT scan and vice-versa with all subjects pooled together (a) and with responders and non-responders shown separately (b).
Paired t-test analysis showed that significant pairwise differences were seen for AD (t = −2.3375, p = 0.035) and AK (t = 2.718, p = 0.019) between pre-RT and during-RT diffusion measures obtained from the active tumor region. Other diffusion measures showed no statistically significant (p < 0.05) change in diffusion measures after 2 weeks of RT. AD measures in the active tumor area showed an effect size (Cohen’s d) of 0.659, whereas AK showed a Cohen’s d of 0.753 in mapping changes in the active tumor VOI following RT.
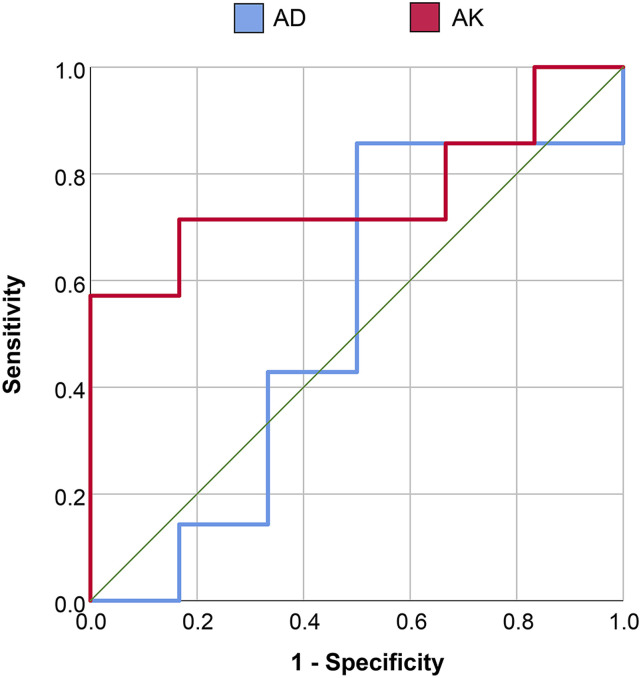
ROC curve analysis showed that percentage change differences in AD and AK between pre-RT and during-RT scans provided an Area Under the Curve (AUC) of 0.524 and 0.762, respectively, in discriminating responders and non-responders. Using the optimal cutoff values determined using Youden’s index changes in AD showed a sensitivity and specificity of 85.7 and 50%, respectively, whereas changes in AK showed a sensitivity of 71.4% and a specificity of 83.3% in discriminating responders and non-responders with a Youden’s cutoff of −5.33%, where a larger decrease in AK in during-RT images was a marker of response to RT (PFS >180 days). Figure 5 shows the ROC curves for AD and AK in discriminating responders and non-responders.
Figure 5.
ROC curves for axial diffusivity (AD) and axial kurtosis (AK) in discriminating responders and non-responders.
Discussion
This pilot study evaluated the relative value of diffusion kurtosis imaging derived maps in visualizing early changes in glioblastomas during radiotherapy treatment in 14 glioblastoma patients. This study aimed to evaluate the performance of DKI maps of AK, MK, and RK as compared to DWI maps of AD, MD, and RD in mapping changes in the active tumor areas defined using a combination of structural imaging and whole-brain spectroscopic imaging after 2 weeks of RT. The results indicate that significant changes are seen in AD and AK maps, with kurtosis derived AK maps showing an increased sensitivity in the mapping of during-RT changes.
Significant differences between pre-RT scans and during-RT scans were seen in the axial component of both diffusion (AD) and kurtosis (AK) images. Overall, subjects showed an increase in diffusivity with around 5% increase in all measures of diffusivity (AD, MD, RD, and FA) but showed a decrease in kurtosis measures following RT. AK maps showed the largest decrease of 8.2% after 2 weeks of RT in patients with poorer response. This finding of reduced kurtosis can be attributed to the greater cellularity with large tumor cells in non-responding tumors resulting in a reduced extracellular space.14,16,39 In the current study, during-RT changes were predominantly in the axial component of both diffusion and kurtosis. This change in AD and AK as compared to a non-significant change in radial components suggests that primary early changes in the active tumor can be attributed to axonal degeneration rather than to microstructural disruptions to the cellular membrane or demyelination, which can be visualized as reductions in RK.1,40 Although axial components were found to be the most sensitive in mapping early changes in this study, it should be noted that an apparent change in AD or AK be the result of a sorting bias 41 or due structural differences between subjects not eliminated by the registration step. 42 As a result, instead of assessing individually, the combination of diffusion and kurtosis parameters, especially directional parameters, may improve our understanding of early pathological changes in the active tumor areas during RT.
Several studies have shown that diffusion MRI is a sensitive and early indicator of both treatment response and overall survival in brain tumors,43–45 however, few studies have carried out a direct comparison between DWI and DKI for estimating early response following treatment. Liu et al. demonstrated the advantage of MD over MK in monitoring tumor response to preoperative chemotherapy in patients with osteosarcoma, 46 whereas Yuan et al. showed that kurtosis parameters showed early and larger changes in detecting response to docetaxel chemotherapy in rat epithelial ovarian cancer. 47 The current study showed the axial kurtosis metric to be more sensitive to studying early RT related changes in the tumor offering a 13.3% larger effect sizes than corresponding diffusion metrics in the active tumor regions. This increased sensitivity is also seen in the evaluation of microstructural changes in low-grade gliomas as compared to DTI imaging, where DKI offers significant increases in the effect sizes for delineating different regions of the gross tumor. 17
The role of DKI imaging towards predicting survival has been studied using measures derived from pre-treatment gliomas. Hempel et al. 48 have shown that MK from DKI is a relevant factor for preoperatively predicting overall and progression-free survival. Zhang et al. 49 using ROC analysis showed that MK was better associated with overall survival than MD, leading to a more accurate evaluation of high-grade glioma patient survival. However, changes in diffusion and kurtosis following treatment have been not studied widely to assess response to treatment. In this study, changes in active tumor diffusion and kurtosis metrics showed that axial kurtosis offers significantly higher sensitivity and specificity in discriminating responders and non-responders. Moreover, this pilot study, establishes a threshold of change between pre-RT and during-RT AK images, as a marker of response to RT (PFS >180 days). It is seen in this study, that generally, active tumor regions show a decrease in AK following RT and a decrease of greater than 5.33% was attributed to positive tumor response and a PFS of greater than 180 days. Although AD images also showed significant difference between pre-RT and during-RT scans, an ROC AUC of 0.52 for AD in evaluating its predictive capabilities towards PFS is not statistically significant.
Finally, to the best of our knowledge, this is the first study where whole-brain spectroscopic imaging has been used to create regions of interest for evaluation of DKI measures. The use of spectroscopic imaging to outline active tumor region ascertains that the entire active part of the glioma is imaged rather than a subset which may be the case using only structural imaging. 33 In this study, we see that metabolically active tumor regions (mVOI) were found to be about 3 times larger than those estimated from contrast-enhanced T1-weighted images. It should be noted that the larger mVOI volumes seen in the study may be impacted to an extent by the broad spatial response function (SRF) of the imaging system in case of MRSI.50–52 The broad SRF results in signal from tumors bleeding in to surrounding voxels resulting in larger tumor volumes.
Limitations of this study include that the measurements were based on a VOI definition for the metabolically active tumor region using a threshold of Cho/NAA ratio of 2.0 times that of the contralateral side. This may not include the whole tumor environment, thereby possibly introducing a selection bias, although this threshold has been shown to be highly correlated to Sox2 density. 34 Furthermore, the diffusion and kurtosis imaging measures that report average values for a region of interest may not account for possible heterogeneity within the tumor. The small sample size of this pilot study does present a drawback but the results show a significant trend that could be ascertained with larger studies in the future. Progression in this study was marked by the identification of first tumor progression as ascertained from clinical charts as per recommendations of neurologists, however, the presence of pseudoprogression cannot be ruled out. The dose-escalation radiation clinical trial has shown that few subjects showed significant pseudoprogression, lasting longer than clinically expected. Finally, mutations that affect tumor proliferation such as in isocitrate dehydrogenase (IDH), Epidermal growth factor receptors (EGFR), and Phosphatase and tensin homolog (PTEN) were not accounted for due to sample size limitations.
Footnotes
Authors’ contributions: Conceptualization: [Mohammed Goryawala, Hyunsuk Shim]; Methodology: [Mohammed Goryawala, Andrew Maudsley]; Formal analysis and investigation: [Mohammed Goryawala]; Writing - original draft preparation: [Mohammed Goryawala]; Writing - review and editing: [Eric Mellon, Hyunsuk Shim, Andrew Maudsley]; Funding acquisition: [Hyunsuk Shim, Andrew Maudsley]; Resources: [Andrew Maudsley]; Supervision: [Andrew Maudsley, Hyunsuk Shim]
Availability of data and material: Anonymized imaging data will be made available on request.
Code availability: Metabolite Imaging and Data Analysis System (MIDAS) software was used for processing and analysis of data. It can be downloaded at http://mrir.med.miami.edu:8000/midas
Ethics approval: The protocol was approved by the Review Board for Protection of Human Subjects in Research at participating institutions (University of Miami, Emory University) to analyze imaging findings and outcomes.
Consent to participate: Informed consent was acquired from each subject and approved by the human subjects’ research review boards at the University of Miami and Emory University.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by National Institute of Health (NIH) grants [R01CA172210], [R01EB016064], and [U01EB028145].
Consent for publication: Informed consent for publication of anonymized data was acquired from each subject.
ORCID iD
Mohammed Goryawala https://orcid.org/0000-0002-6875-2191
References
- 1.Steven AJ, Zhuo JC, Melhem ER. Diffusion Kurtosis Imaging: An Emerging Technique for Evaluating the Microstructural Environment of the Brain. Am J Roentgenol 2014; 202: W26–W33. [DOI] [PubMed] [Google Scholar]
- 2.Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005; 53: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 3.Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010; 23: 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu HZ, Jensen JH, Ramani A, et al. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed 2006; 19: 236–247. [DOI] [PubMed] [Google Scholar]
- 5.Adisetiyo V, Tabesh A, Di Martino A, et al. Attention-deficit/hyperactivity disorder without comorbidity is associated with distinct atypical patterns of cerebral microstructural development. Hum Brain Mapp 2014; 35: 2148–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blockx I, Verhoye M, Van Audekerke J, et al. Identification and characterization of Huntington related pathology: An in vivo DKI imaging study. Neuroimage 2012; 63: 653–662. [DOI] [PubMed] [Google Scholar]
- 7.Cheung JS, Wang EF, Lo EH, et al. Stratification of Heterogeneous Diffusion MRI Ischemic Lesion With Kurtosis Imaging Evaluation of Mean Diffusion and Kurtosis MRI Mismatch in an Animal Model of Transient Focal Ischemia. Stroke 2012; 43: 2252–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kouchkovsky I, Fieremans E, Fleysher L, et al. Quantification of normal-appearing white matter tract integrity in multiple sclerosis: a diffusion kurtosis imaging study. J Neurol 2016; 263: 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida M, Hori M, Yokoyama K, et al. Diffusional kurtosis imaging of normal-appearing white matter in multiple sclerosis: preliminary clinical experience. Jpn J Radiol 2013; 31: 50–55. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Zhang YZ, Wong CS, et al. Diffusion abnormalities in temporal lobes of children with temporal lobe epilepsy: a preliminary diffusional kurtosis imaging study and comparison with diffusion tensor imaging. NMR Biomed 2012; 25: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 11.Das SK, Wang JL, Bing L, et al. Regional Values of Diffusional Kurtosis Estimates in the Healthy Brain during Normal Aging. Clin Neuroradiol 2017; 27: 283–298. [DOI] [PubMed] [Google Scholar]
- 12.Falangola MF, Jensen JH, Babb JS, et al. Age-Related Non-Gaussian Diffusion Patterns in the Prefrontal Brain. J Magn Reson Imaging 2008; 28: 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado AF, Nilsson M, van Westen D, et al. Glioma Grade Discrimination with MR Diffusion Kurtosis Imaging: A Meta-Analysis of Diagnostic Accuracy. Radiology 2018; 287: 119–127. [DOI] [PubMed] [Google Scholar]
- 14.Jiang RF, Jiang JJ, Zhao LY, et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 2015; 6: 42380–42393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raab P, Hattingen E, Franz K, et al. Cerebral Gliomas: Diffusional Kurtosis Imaging Analysis of Microstructural Differences. Radiology 2010; 254: 876–881. [DOI] [PubMed] [Google Scholar]
- 16.Van Cauter S, Veraart J, Sijbers J, et al. Gliomas: Diffusion Kurtosis MR Imaging in Grading. Radiology 2012; 263: 492–501. [DOI] [PubMed] [Google Scholar]
- 17.Goryawala MZ, Heros DO, Komotar RJ, et al. Value of diffusion kurtosis imaging in assessing low-grade gliomas. J Magn Reson Imaging 2018; 48: 1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010; 28: 1963–1972. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey MF, Condon BR, Hadley DM. Measurement of tumor "size" in recurrent malignant glioma: 1D, 2D, or 3D? Am J Neuroradiol 2005; 26: 770–776. [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang EJ, Cha Y, Lee AL, et al. Early response evaluation for recurrent high grade gliomas treated with bevacizumab: a volumetric analysis using diffusion-weighted imaging. J Neurooncol 2013; 112: 427–435. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh K, Mellon EA, Gurbani SS, et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neuro-Oncology Adv 2022; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maudsley AA, Domenig C, Govind V, et al. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med 2009; 61: 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebel A, Maudsley AA. Improved spectral quality for 3D MR spectroscopic imaging using a high spatial resolution acquisition strategy. Magn Reson Imaging 2003; 21: 113–120. [DOI] [PubMed] [Google Scholar]
- 24.Tabesh A, Jensen JH, Ardekani BA, et al. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 2011; 65: 1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- 26.Avants BB, Epstein CL, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008; 12: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studholme C, Hill DLG, Hawkes DJ. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recogn 1999; 32: 71–86. [Google Scholar]
- 28.Maudsley AA, Darkazanli A, Alger JR, et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed 2006; 19: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haupt CI, Schuff N, Weiner MW, et al. Removal of lipid artifacts in 1H spectroscopic imaging by data extrapolation. Magn Reson Med 1996; 35: 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Taub E, Salibi N, et al. Comparison of reproducibility of single voxel spectroscopy and whole-brain magnetic resonance spectroscopy imaging at 3T. NMR Biomed 2018; 31: e3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soher BJ, Young K, Govindaraju V, et al. Automated spectral analysis - III: Application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med 1998; 40: 822–831. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE T Med Imaging 2001; 20: 45–57. [DOI] [PubMed] [Google Scholar]
- 33.Cordova JS, Kandula S, Gurbani S, et al. Simulating the Effect of Spectroscopic MRI as a Metric for Radiation Therapy Planning in Patients with Glioblastoma. Tomography 2016; 2: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordova JS, Shu HK, Liang Z, et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro-oncology 2016; 18: 1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falangola MF, Guilfoyle DN, Tabesh A, et al. Histological correlation of diffusional kurtosis and white matter modeling metrics in cuprizone-induced corpus callosum demyelination. NMR Biomed 2014; 27: 948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-oncology 2008; 10: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruopp MD, Perkins NJ, Whitcomb BW, et al. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biometrical J 2008; 50: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–35. [DOI] [PubMed] [Google Scholar]
- 39.Delgado Anna F, Fahlström M, Nilsson M, et al. Diffusion kurtosis imaging of gliomas grades II and III - a study of perilesional tumor infiltration, tumor grades and subtypes at clinical presentation. Radiol Oncol 2017; 51: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrale M, Collura G, Brai M, et al. Physics, Techniques and Review of Neuroradiological Applications of Diffusion Kurtosis Imaging (DKI). Clin Neuroradiol 2016; 26: 391–403. [DOI] [PubMed] [Google Scholar]
- 41.Martin KM, Papadakis NG, Huang CL, et al. The reduction of the sorting bias in the eigenvalues of the diffusion tensor. Magn Reson Imaging 1999; 17: 893–901. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med 2009; 61: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 43.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 2000; 92: 2029–2036. [DOI] [PubMed] [Google Scholar]
- 44.Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 2008; 26: 3387–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross BD, Moffat BA, Lawrence TS, et al. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther 2003; 2: 581–587. [PubMed] [Google Scholar]
- 46.Liu C, Xi Y, Li M, et al. Monitoring Response to Neoadjuvant Chemotherapy of Primary Osteosarcoma Using Diffusion Kurtosis Magnetic Resonance Imaging: Initial Findings. Korean J Radiol 2019; 20: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan SJ, Qiao TK, Qiang JW. Diffusion-weighted imaging and diffusion kurtosis imaging for early evaluation of the response to docetaxel in rat epithelial ovarian cancer. J Transl Med 2018; 16: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hempel JM, Brendle C, Bender B, et al. Diffusion kurtosis imaging histogram parameter metrics predicting survival in integrated molecular subtypes of diffuse glioma: An observational cohort study. Eur J Radiol 2019; 112: 144–152. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Jiang J, Zhao L, et al. Survival prediction of high-grade glioma patients with diffusion kurtosis imaging. Am J Transl Res 2019; 11: 3680–3688. [PMC free article] [PubMed] [Google Scholar]
- 50.Ford EC, Kinahan PE, Hanlon L, et al. Tumor delineation using PET in head and neck cancers: threshold contouring and lesion volumes. Med Physics 2006; 33: 4280–4288. [DOI] [PubMed] [Google Scholar]
- 51.Maudsley AA. Lesion segmentation for MR spectroscopic imaging using the convolution difference method. Magn Reson Med 2019; 81: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaidi H, El Naqa I. PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur Journal Nuclear Medicine Molecular Imaging 2010; 37: 2165–2187. [DOI] [PubMed] [Google Scholar]