Abstract
Arterial spin labeling (ASL) is a noninvasive neuroimaging technique that allows for quantifying cerebral blood flow without intravenous contrast. Various neurovascular disorders and tumors have cerebral blood flow alterations. Identifying these perfusion changes through ASL can aid in the diagnosis, especially in entities with normal structural imaging. In addition, complications of tumor treatment and tumor progression can also be monitored using ASL. In this case-based review, we demonstrate the clinical applications of ASL in diagnosing and monitoring brain tumors and treatment complications.
Keywords: Arterial spin labeling, arterial spin labeling, neuroimaging, glioblastoma, meningioma, stroke-like migraines after radiation therapy, metastasis, radionecrosis, lymphocytic hypophysitis, primary CNS lymphoma
Introduction
Arterial spin labeling (ASL) is a noninvasive magnetic resonance imaging (MRI) technique that measures cerebral blood flow (CBF) at the capillary level. In this technique, a radiofrequency pulse inverts the magnetization of spinning protons in the blood prior to their movement to a region of interest (ROI). 1 After a post-labeling delay to allow the arrival of these labeled arterial spins, an image of the ROI is captured. 1 Control images of the ROI without labeling are also captured and subtracted from the ASL images, which results in a perfusion map that allows quantification of relative CBF (rCBF). 1 Raw CBF values may be normalized against CBF (normalized CBF, nCBF) of the whole brain or a reference region. Normalization helps factor out global modulation effects, thereby increasing the sensitivity of ASL in detecting regional CBF differences. 1 ASL has two major techniques, continuous ASL (CASL) and pulsed ASL (PASL). 2 The CASL technique utilizes continuous adiabatic inversion, whereas PASL is based on a single inversion pulse. 2 More recently introduced, the pseudo-continuous ASL (pCASL) is an intermediate technique between CASL and PASL, where a series of discrete RF pulses mimic the CASL method for spin labeling.2,3 Alterations in normal ASL perfusion can aid in the differentiation and diagnosis of various brain lesions and neurological disorders seen on imaging. 4
This case-based review explores the application of ASL in the diagnosis of intra-axial tumors, extra-axial tumors, and treatment-related complications.
Tumors
Intra-axial tumors
Glioblastoma
De novo and recurrent glioblastoma (GBM) typically demonstrates markedly increased CBF, consistent with the higher metabolism of the tumor tissue (Figures 1 and 2). Thus, ASL can play a role in initial tumor diagnosis and follow-up after treatment, aiding differentiation of treatment-induced changes such as radionecrosis or pseudoprogression from recurrence or early tumor progression (Figure 3). ASL was found to predict treatment response as early as 4–6 weeks following completion of concurrent chemoradiotherapy for glioblastoma,5-9 with improved accuracy over tumor volume measurements. Pseudoprogression frequently follows radiotherapy with concomitant and adjuvant temozolomide and typically occurs within the first 3 months after completing treatment. 10 Identification of pseudoprogression is important as it may interfere with treatment monitoring and result in temozolomide being withheld prematurely. 10 Choi et al. demonstrated utility of ASL grade as an independent predictor in differentiating between pseudoprogression and true early tumor progression in glioblastoma. 11
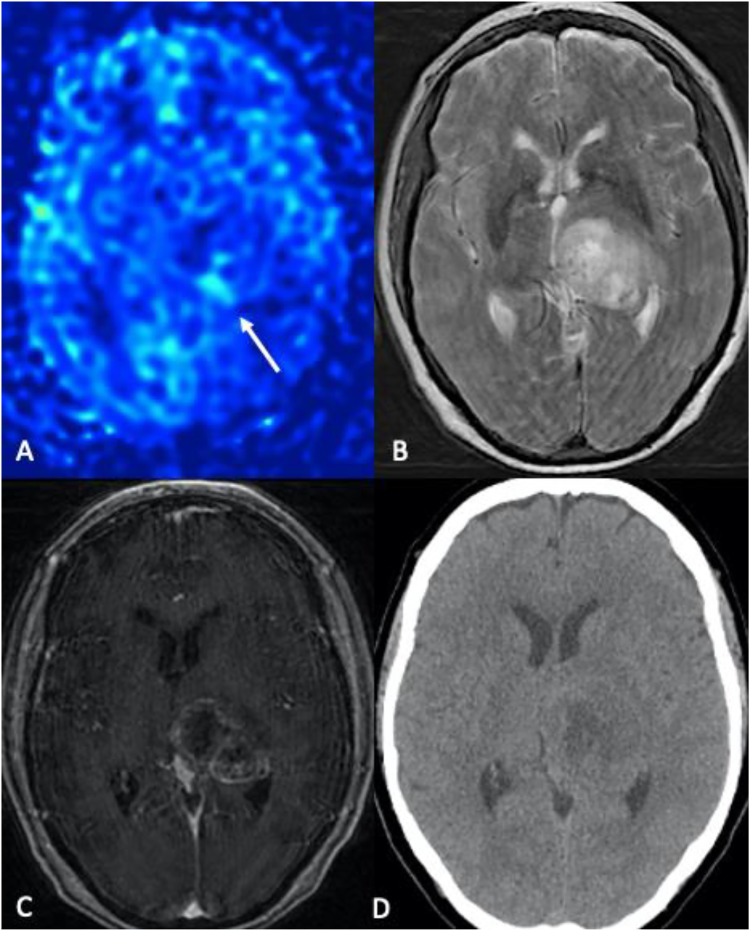
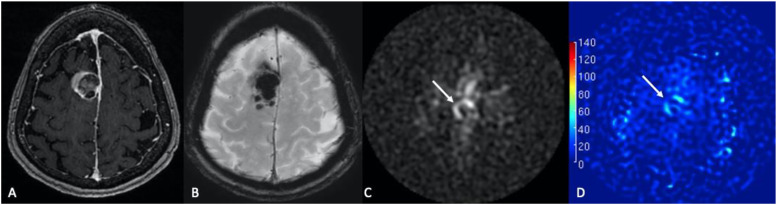
Figure 1.
Glioblastoma. (a) 79-year-old female with altered mental status and headache. (a) Color 3D pCASL, (b) axial T2-W, (c) axial T1-W post-contrast, (d) noncontrast CT. A heterogeneously enhancing expansile mass centered in the left thalamic region with central areas of necrosis is noted. Glioblastoma typically demonstrates markedly increased cerebral blood flow (CBF), consistent with the higher metabolism of the tumor tissue (arrow in A).
Figure 2.
Glioblastoma. A 46-year-old female presenting with acute onset right facial twitching and difficulty speaking. (a) Axial FLAIR demonstrates an expansile lesion involving the left precentral gyrus, with irregular rim-enhancement on post-contrast T1-WI. (b) Markedly increased relative cerebral blood flow (rCBF) and cerebral blood flow (CBF) corresponding to the enhancing areas are shown on dynamic susceptibility contrast (DSC, arrows in C) and color 3D pASL (arrows in D) perfusion sequences, respectively.
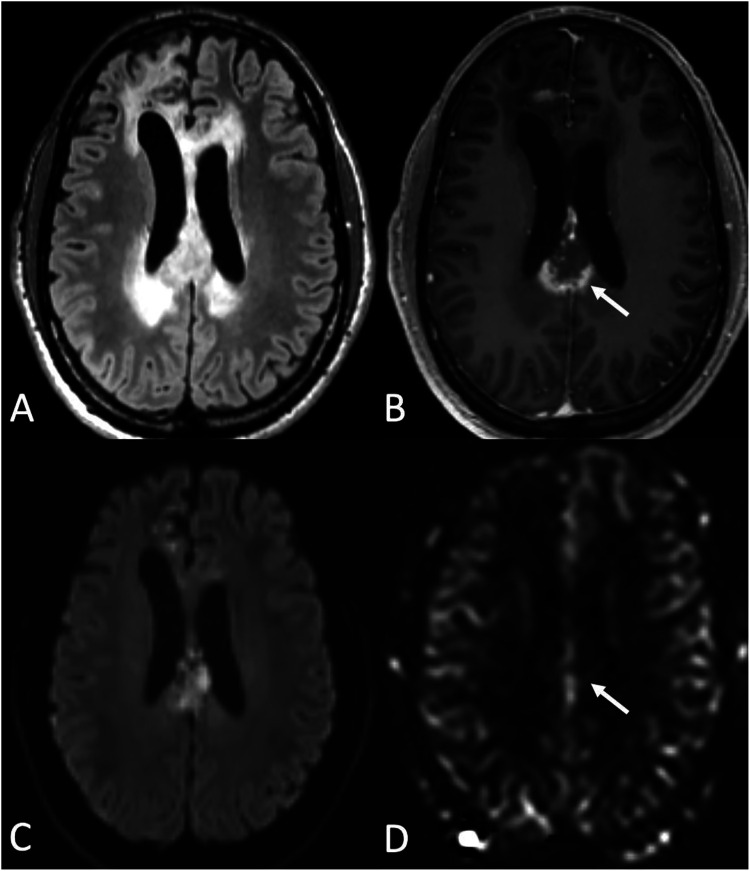
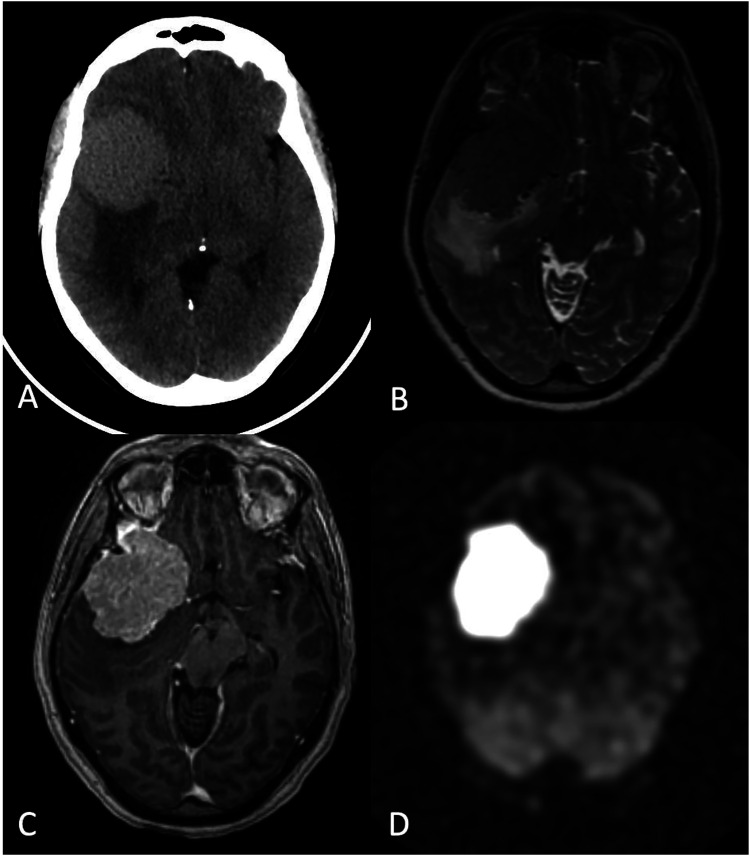
Figure 3.
High-grade glioma and posttreatment changes (radiation necrosis). A 41-year-old male with astrocytoma, IDH-mutant, grade IV, status post chemoradiation and adjuvant temozolomide. (a) axial FLAIR, (b) Contrast-enhanced T1-WI, (c) DWI, and (d) CBF map demonstrate, 10 months after treatment completion, progressive enlargement of a peripherally enhancing lesion with central necrosis and restricted diffusion in the posterior body/isthmus of corpus callosum (arrow in C) concerning for posttreatment change versus tumor progression. ASL showed no clear elevation of CBF in the corpus callosum (arrow in D). Biopsy confirmed treatment-related changes.
One distinct advantage of ASL in the posttreatment setting is that the readout can use spin-echo imaging, which helps in the detection of small regions of recurrence near the operative bed where blood products can cause distortion and signal loss on traditional dynamic susceptibility contrast perfusion-weighted images (DSC-PWI). 4 ASL imaging is less affected by postsurgical tissue distortion and has an advantage in diagnosing small foci of recurrence around the surgical bed.
Other gliomas
Gliomas are a pathologically varied set of primary brain tumors in which MRI plays a vital role in grading for treatment planning purposes. Tissue is obtainable in most of these tumors. However, failure to sample the highest grade portion of the tumor may result in under grading despite multiple biopsies.
Tumor angiogenesis is a critical factor in histologic tumor grading, and tumor blood flow serves as an in vivo marker that can be measured with ASL.4,12,13 Utilizing bolus PWI to differentiate high versus low-grade gliomas has been well established. As demonstrated by a meta-analysis of 20 studies by Luan et al., rCBF in ASL showed excellent diagnostic performances for differentiating high-grade gliomas (HGGs) from low-grade gliomas (LGGs). 14 In addition, it is important to note that antiangiogenic drugs used for the treatment of glioblastoma and some metastatic tumors, such as bevacizumab (Avastin), alter the process of angiogenesis, resulting in different perfusion patterns, including mixed perfusion, hypoperfusion, and normal perfusion, that must be known to avoid erroneous interpretation 15 (Figure 4).
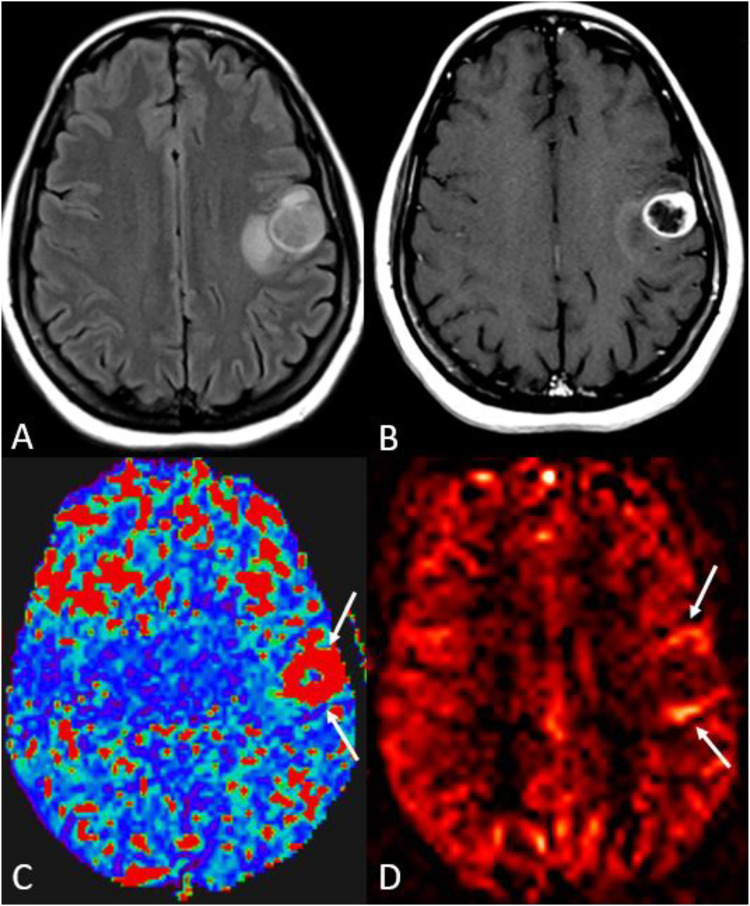
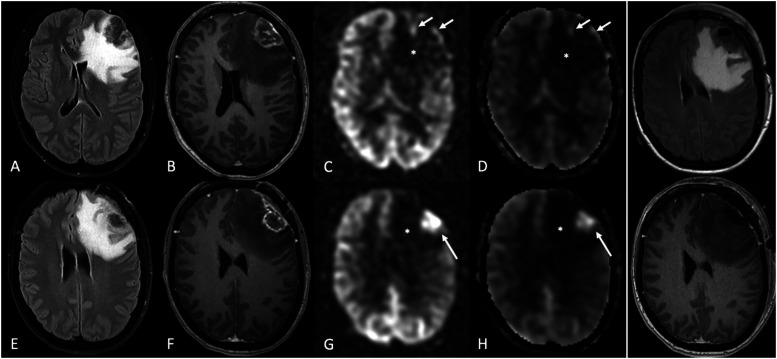
Figure 4.
Solitary intraparenchymal metastasis and posttreatment changes. A 41-year-old female with a history of rectal adenocarcinoma (Her2+, EGFR−) presented with several days of headache and was found to have a solitary brain metastasis in the left frontal lobe. A–D, initial presentation, and E–H, later presentation after subsequent treatment. (a) axial FLAIR and (b) axial post-contrast T1-WI demonstrate a left frontal mass with heterogenous enhancement, dural extension, and extensive surrounding vasogenic edema. (c–d) the lesion predominantly showed none or only mild hyperperfusion along its periphery (arrows) on 3D pCASL perfusion (c) and CBF map (d). The patient had been on a combination of chemotherapy drugs, including bevacizumab, for many months until the MRI (top row, panels a–d), which could account for the lesion’s predominant hypoperfusion. Subsequent imaging (after bevacizumab discontinuation, surgery, and radiotherapy) showed a high signal on ASL within an enhancing lesion at the treatment site (f), favoring tumor progression over radiation necrosis. Asterisks show hypoperfusion corresponding to the areas of FLAIR hyperintensity (a, e), consistent with vasogenic edema without tumor, which may not be the case with high-grade gliomas. In the far-right column, immediate postsurgical images (axial FLAIR [upper] and post-contrast T1-WI [bottom]) showed no abnormal enhancement at the surgical bed to suggest residual tumor.
Metastases
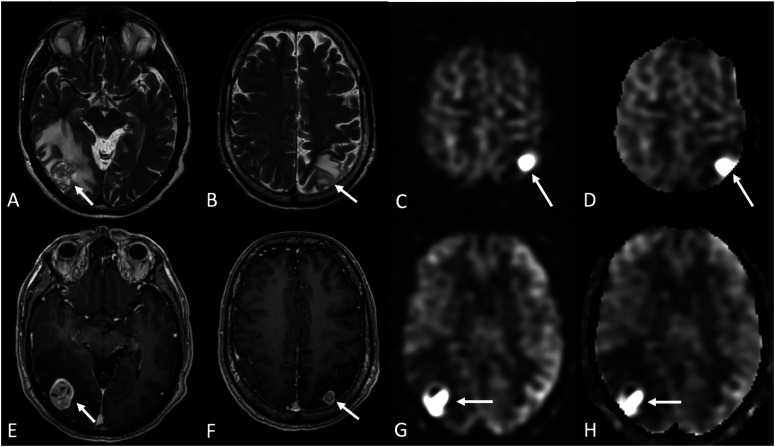
Although blood flow varies in brain metastases, it is typically slightly lower than that of gray matter. ASL nCBF values in metastases largely depend on the primary tumor histologic type. For example, with gastrointestinal adenocarcinoma primaries, since the metastasis tends to be cystic or necrotic, it is more common to see little ASL signal or only a peripheral rim of high ASL CBF values (Figure 4). The difference in perfusion may be very subtle; in such cases, differentiation between metastases and brain parenchyma may not be possible with ASL. 4 However, certain highly vascular tumor types, such as metastasis from renal cell carcinoma (RCC), thyroid malignancies, and melanoma, are easier to differentiate on ASL perfusion sequences 4 (Figure 5).
Figure 5.
Metastasis. A 62-year-old male with metastatic renal cell carcinoma (RCC) to the lung presented with 1 week of headaches, sudden onset of incoordination, and difficulty controlling his hands. MRI shows two solid heterogeneously enhancing lesions in the right temporo-occipital and left parietal regions surrounded by vasogenic edema (arrows in a, b [Axial T2-WI], e, and f [Axial T1 post-contrast]), consistent with metastases. In addition, markedly increased ASL perfusion signal (c, g) and relative cerebral blood flow (CBF; d, h) corresponding to the enhancing masses are shown on grayscale pCASL PWI (c, g) and CBF (d, h) maps.
Compared with HGG, metastasis shows only a slightly lower mean nCBF. Therefore, ASL may not be sufficient for discriminating between these two entities.16,17 However, this is being debated without a clear consensus. 18 On the other hand, perilesional edema ASL perfusion parameters are significantly lower in metastasis than HGG,16,19 which may be more helpful in distinguishing between these two entities (Figure 4). As with primary high-grade glial tumors, ASL can play a role in initial tumor diagnosis and follow-up after treatment, aiding differentiation of treatment-induced changes such as radionecrosis from tumor recurrence. The latter shows neovascularization and increased CBF on the surgical bed (Figure 4).
Primary central nervous system lymphoma
ASL signal and CBF measurement are typically lower in primary central nervous system lymphoma (PCNSL) than in GBM, which can aid in the differentiation of the two pathologies (Figure 6). You et al. compared nCBF in PCNSL and GBM. In their quantitative analysis, intratumoral nCBF and peritumoral nCBF in PCNSL were significantly lower than GBM. 20 This result may be explained by differences in neovascularization and proliferation patterns of these tumors. Neovascularization is a hallmark in GBM but an unusual feature in PCNSL (except when HIV-related). 21 Also, the PCNSL has higher intratumoral vascular permeability than GBM because of its characteristic vasocentric proliferation, wherein tumor cells proliferate concurrently with the destruction of the blood-brain barrier but without neovascularization. In contrast, the blood-brain barrier destruction in GBM proceeds with neovascularization.20,22 In addition, in a study by Yamashita et al., 23 the mean absolute tumor blood flow (aTBF) was measured in an ROI drawn over the enhancing area. Relative tumor blood flow (rTBF) was calculated by normalizing the aTBF by a blood flow measurement from the reference region based on a previous report. The lowest ADC value chosen from all ROIs was determined as the minimum ADC (ADCmin). They reported that aTBF, rTBF, and ADCmin were significantly higher in GBMs than in PCNSLs. 23
Figure 6.
Primary CNS lymphoma. A 70-year-old male with altered mental status. (a) Color 3D pCASL perfusion, (b) axial T1-WI, (c) axial FLAIR, (d) diffusion-weighted imaging (DWI) demonstrate enhancing foci centered in the basal ganglia regions, right greater than left (b), associated with restricted diffusion characterized by an increased signal on DWI (d), and low signal on ADC map (not shown). Although increased intratumoral normalized cerebral blood flow (nCBF, arrow in a) is seen in PCNSL, values are lower than those usually observed in the GBM.
Hemangioblastoma
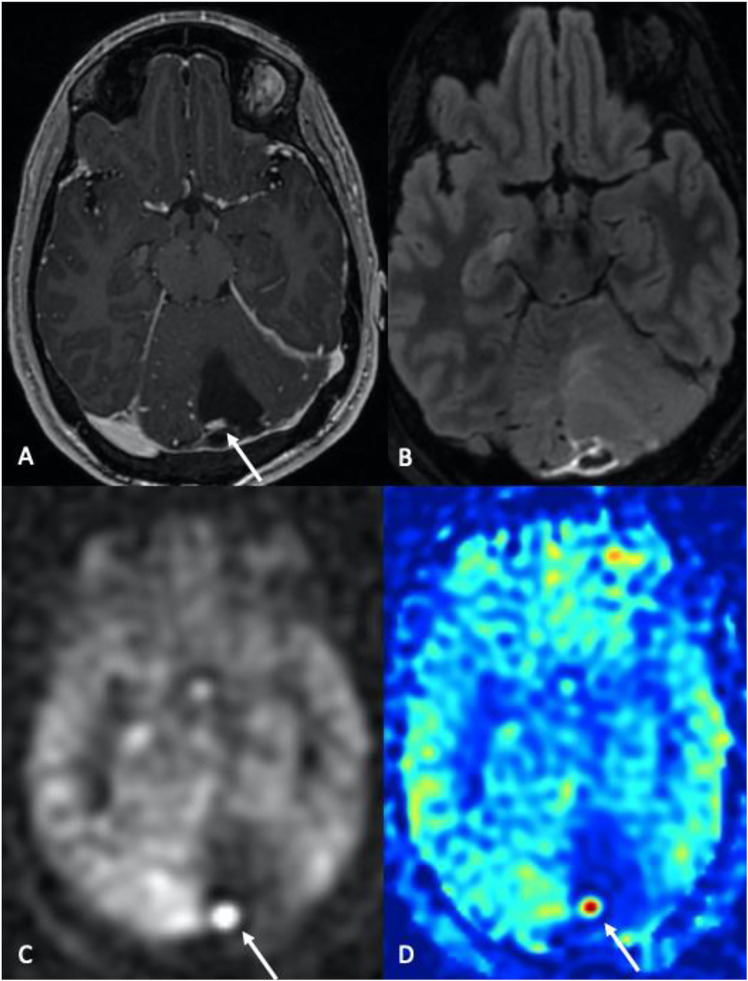
Differential diagnosis of hemangioblastoma from cystic metastatic lesions in the posterior fossa is challenging. Both may have cystic and enhancing nodular components. Enhancing mural nodules in hemangioblastoma demonstrate marked hyperperfusion on ASL and DSC-PWI compared to other hypervascular tumors (Figure 7). In addition, ASL CBF can aid in distinguishing hemangioblastoma from cystic metastasis in the posterior fossa,24,25 and in the identification and follow-up of small solid hemangioblastomas, particularly in the setting of von Hippel-Lindau (VHL) disease. 24
Figure 7.
Hemangioblastoma. 47-year-old male patient with a history of von Hippel-Lindau syndrome. Axial post-contrast T1-WI (a) and axial FLAIR (b) images demonstrate a left cerebellar predominantly cystic mass with an enhancing mural nodule (arrow in a). Note the markedly increased cerebral blood flow (CBF) associated with the mural nodule (c and d, grayscale and color 3D pCASL perfusion images) with an absence of ASL signal corresponding to the cystic components of the mass.
Extra-axial tumors
Meningioma
ASL can increase the detection of single or multifocal meningiomas, particularly in noncontrast studies.26,27 High ASL signal within meningiomas corresponds to increased vascularity, especially as seen in the angiomatous variant, which can have treatment implications 28 (Figure 8). Correlation with cancer history is advised to exclude metastases such as colon or thyroid for calcified lesions (Figure 9). ASL has also been used to identify residual or recurrent tumor in the postsurgical setting and to assess the WHO grade of meningiomas; 27 WHO grade I tumors demonstrate homogenous hyperperfusion signal with high mean CBF compared to WHO grade II and III tumors, which tend to show heterogeneous or no substantial hyperperfusion.1,29 Moreover, ASL can aid in differentiating meningioma from nerve sheath tumors, as meningiomas tend to have a “lightbulb bright” signal on ASL images (Figure 10), and nerve sheath tumors do not typically exhibit this finding. ASL also plays a role in identifying arteries that supply meningiomas, which is essential in surgical treatment planning. 30 Similar to meningioma, increased ASL signal within a solitary fibrous tumor (previous hemangiopericytoma) corresponds to increased vascularity within the tumor and can have treatment implications.31,32 These tumors also often show “lightbulb bright” ASL signal. 4
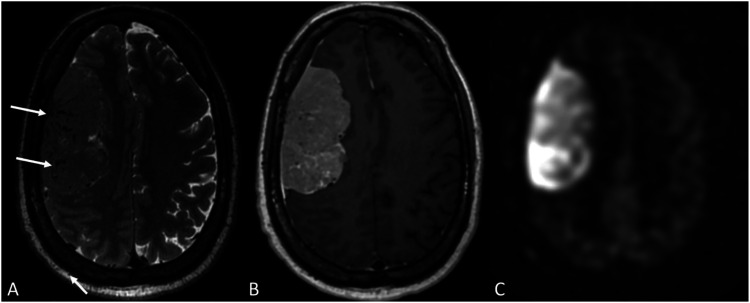
Figure 8.
Meningioma angiomatous features. A 58-year-old man presented with progressive left-hand weakness. Axial T2 (a) and post-contrast T1-WI (b) show a large right cerebral convexity dural-based enhancing mass, associated with markedly increased ASL perfusion signal (c). Flow voids suggest higher vascularity within the tumor (arrows in a).
Figure 9.
Calcified parasagittal meningioma. 85-year-old female with headache. (a), Axial post-contrast T1-WI shows a parasagittal frontal dural-based heterogeneously enhancing mass. (b), Susceptibility artifact from calcifications seen on susceptibility-weighted imaging (SWI). (c) and (d) Note the rim of circumferential increased ASL signal corresponding to the areas of enhancement on grayscale and color 3D pCASL images.
Figure 10.
Meningioma, “lightbulb bright” appearance. A 61-year-old woman presented with a few months of intermittent headaches and confusion. Noncontrast CT showed a sizeable hyperattenuating mass with perilesional edema (a). Axial T2-W and post-contrast T1-W MR images showed large right sphenoid wing extra-axial mass with marked enhancement (c) and “lightbulb bright” ASL perfusion signal (d).
Dural metastases
As discussed above, RCC classically exhibits the highest ASL signal as hypervascular intra-axial metastases. However, it is essential to consider the most common primaries, such as breast and lung, when assessing for hypervascular extra-axial metastases (Figure 11). If a solitary lesion is identified, meningioma is still a diagnostic consideration.
Figure 11.
Dural metastases. A 72-year-old female with a history of breast cancer. Metastases can have variable ASL signal depending on the vascularity. This patient had disseminated metastases, including calvarial lesions (arrowheads). (a), Axial FLAIR, (b), Axial post-contrast T1-WI, (c), grayscale 3D pCASL perfusion.
Treatment-related complications
Lymphocytic hypophysitis secondary to immunotherapy
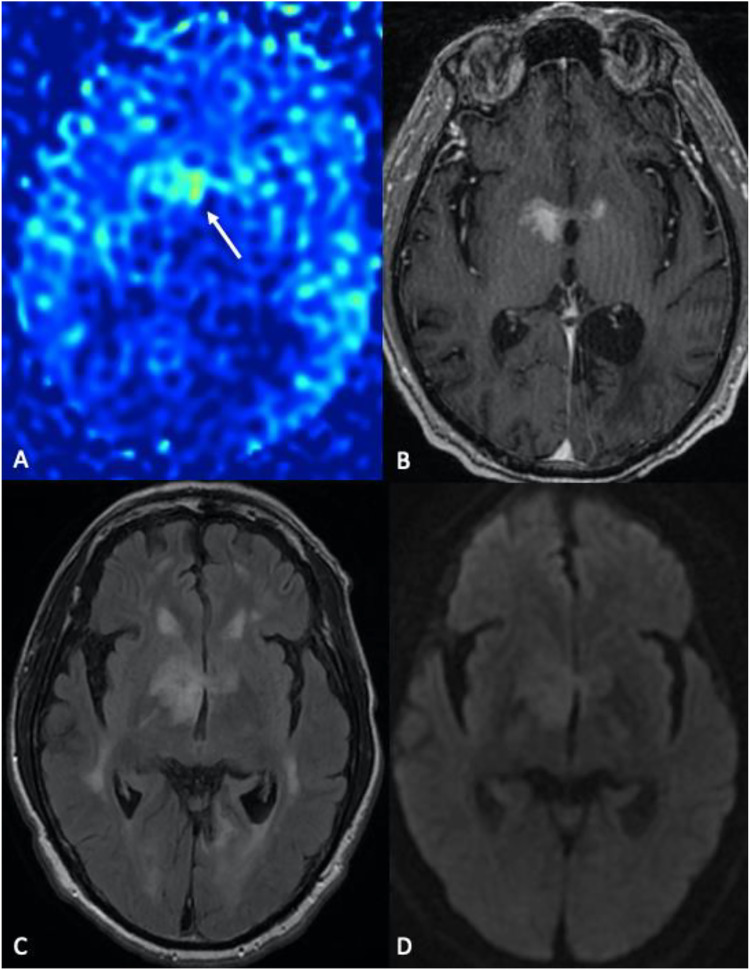
Immune checkpoint inhibitor therapy such as ipilimumab (Yervoy) and nivolumab (Opdivo) can result in acute lymphocytic (infundibulo-neuro) hypophysitis, which appears as thickening and infiltration of the pituitary gland and stalk (Figure 12). ASL typically shows increased signal in the pituitary gland; therefore, extent, intensity, correlation with anatomic imaging, and labs are advised to differentiate normal gland versus superimposed inflammatory process. 33 Correlation with the timing of therapy and prior imaging is also necessary. Lymphocytic hypophysitis is the leading differential consideration. Other differential diagnoses of increased ASL signal in the sellar/suprasellar region include Tolosa-Hunt syndrome, hypervascular metastases, infection (such as tuberculosis), meningioma, lymphoma, and cavernous sinus vascular entities (e.g., dural AVF or venous reflux).34–36
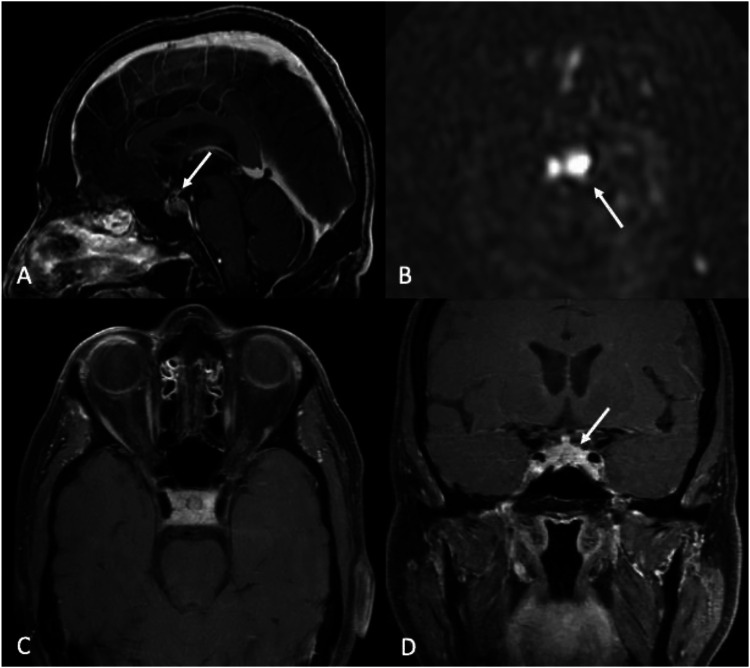
Figure 12.
Lymphocytic hypophysitis. A 66-year-old male with a history of renal cell carcinoma (RCC) on immunotherapy (Opdivo/nivolumab) presented with worsening left ptosis, blurry vision, and retro-orbital eye pain over 5 days. Asymmetric thickening, enhancement, and infiltration of the pituitary gland and stalk are associated with increased CBF on ASL perfusion images (arrows). The differential included lymphocytic hypophysitis (leading consideration), RCC metastasis, lymphoma, and infection (such as tuberculosis). (a), (b), and (d) sagittal, axial, and coronal post-contrast T1-WI, respectively). D, 3D pCASL perfusion.
Stroke-like migraines after radiation therapy (SMART) syndrome
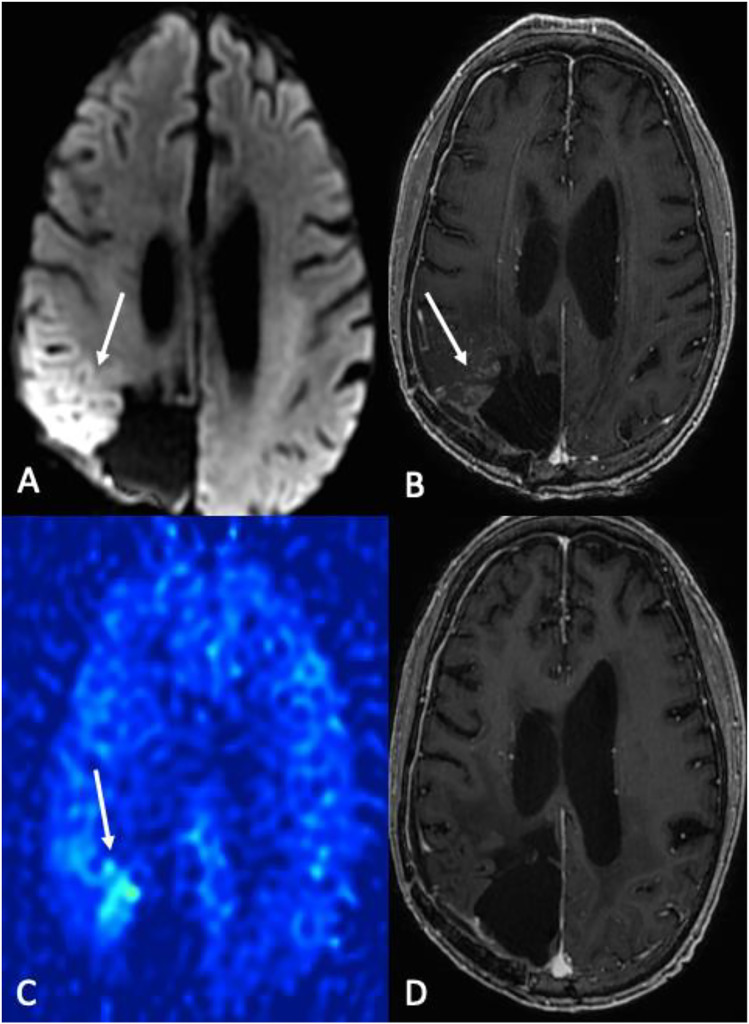
SMART syndrome is a delayed complication of radiation therapy leading to acute neurological abnormalities, most often characterized by headaches and seizures, and can occur 6–30 years after treatment. It is clinically associated with seizures in up to 72% of patients.37,38 Classically, the condition is self-limiting and gradually resolves over several weeks. There is controversy about whether this is a separate entity or a delayed seizure phenomenon. The imaging hallmark of SMART syndrome on MR images is prominent unilateral gyriform enhancement (cortical and leptomeningeal) with mild mass effect, usually in an area included in the radiation ports. Cortical thickening (hyperintense in T2 and FLAIR) with or without diffusion restriction cortical laminar necrosis is also reported. SMART usually responds to antiepileptics or steroids and resolves in most cases.37,38 SMART is associated with a high signal on ASL imaging (Figure 13) with essential differential considerations, including tumor recurrence and meningoencephalitis. Short interval follow-up after therapy may be necessary to distinguish tumor recurrence. Laboratory findings such as elevated WBC and symptoms such as fever can distinguish it from meningoencephalitis. The significantly lengthened time course can help differentiate subacute infarct, and other factors such as lack of volume loss and presence of gliosis further reduce its likelihood. Status epilepticus demonstrates variable enhancement patterns and marked hyperemia on the side of the epileptic focus, elevated ASL (depending on stage), DSC-PWI rCBF, and DSC-PWI rCBV maps. It is important to note that SMART can also present overlapping features such as seizures.
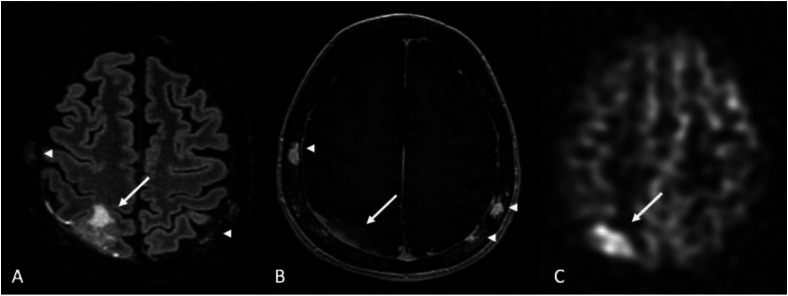
Figure 13.
SMART syndrome. 58-year-old male status post GBM resection and chemoradiation presenting with gait instability and weakness 13 years after therapy. (a), diffusion-weighted imaging, and (b), contrast-enhanced T1-WI showing demonstrated cortical restricted diffusion (ADC map not shown) and enhancement in the right parietal lobe, associated with hyperperfusion on 3D pCASL imaging (arrow in (c). (d) 6-month follow-up post-contrast T1-WI after anti-seizure medication and steroids showing resolution of findings.
Discussion
We have highlighted the utility of ASL perfusion in brain tumor imaging, including its role in the detection and differentiation of primary and secondary tumors, assessment of tumor grade, and as a noninvasive indicator of malignant progression or treatment response.
In tumors with high vascularity and metabolism, such as GBM, the role of ASL is more critical in the early treatment period as post-operative changes limit the evaluation of anatomical sequences as well as DSC-PWI. ASL imaging, when employed with a spin-echo readout method, is less affected by postsurgical local susceptibility effects and has an advantage in diagnosing small foci of recurrence around the surgical bed. In addition, ASL imaging can predict tumor response to therapy as early as four to 6 weeks after treatment.5–9
In some posttreatment changes, such as radiation necrosis in tumors and SMART syndrome, ASL findings are significant. Therefore, they should be differentiated from more severe pathologies such as recurrence or residual tumors that can mimic those ASL findings.
Disadvantages of ASL include lower signal intensity-to-noise ratio and longer acquisition time compared to DSC-PWI. 5 The low signal intensity-to-noise ratio in ASL is essential to consider in evaluating tumors in the posterior fossa, where susceptibility effect by the skull base leads to signal intensity loss. 39 A longer acquisition time can also make ASL more prone to motion artifacts that further degrade image quality. Another disadvantage is the image quality. Mora Alvarez et al. added a separate neck coil to the coil used during RF excitation over 6 minutes on 4.7-T MRI, which resulted in higher resolution and improved visibility of perfusion in small deep brain structures. 40 This may expand the clinical utility of ASL in identifying perfusion deficits in small areas.
Advantages of ASL include application in patients with renal insufficiency as it allows for quantification of CBF without exogenous contrast and does not require ionizing radiation. Thus, ASL is the MR perfusion technique of choice in pregnant women, children, and patients with gadolinium allergy.5,41,42 Repetition of ASL acquisitions during a single study is also advantageous. 5 As technological advances occur, improved signal detection and ease of use will continue to increase interest in clinical applications of ASL imaging.
Conclusion
Different neurological conditions, malignancies, and treatment complications present with specific changes in cerebral blood flow that can be quantified using ASL. ASL is a noninvasive, noncontrast-dependent technique and can be utilized as a safe approach to evaluating these perfusion patterns. ASL provides additional information that may not be found on structural imaging for many conditions. As technological improvements improve utility and reliability, ASL will become more commonly used in the clinical setting.
Abbreviations
- AV
arteriovenous
- aTBF
absolute tumor blood flow
- ADCmin
minimum apparent diffusion coefficient
- GBM
glioblastoma
- HGG
high-grade gliomas
- LGG
low-grade glioma
- nCBF
normalized cerebral blood flow
- PCNSL
Primary CNS Lymphoma
- PWI
perfusion-weighted imaging
- rCBF
regional cerebral blood flow
- rCBV
relative cerebral blood volume
- rTBF
relative tumor blood flow
- ROI
region of interest
- RCC
renal cell carcinoma
- SWI
susceptibility-weighted imaging
- SMART
stroke-like migraines after radiation therapy
- WI
weighted imaging
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Licia P Luna https://orcid.org/0000-0003-3539-4831
Laleh Daftaribesheli https://orcid.org/0000-0003-2640-8154
References
- 1.Soldozy S, Galindo J, Snyder H, et al. Clinical utility of arterial spin labeling imaging in disorders of the nervous system. Neurosurg Focus 2019; 47: E5. [DOI] [PubMed] [Google Scholar]
- 2.Aslan S, Xu F, Wang PL, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med 2010; 63: 765–771. DOI: 10.1002/mrm.22245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med 2007; 58: 1086–1091. DOI: 10.1002/mrm.21293 [DOI] [PubMed] [Google Scholar]
- 4.Telischak NA, Detre JA, Zaharchuk G, et al. Arterial spin labeling MRI: clinical applications in the brain. J Magn Reson Imaging 2015; 41: 1165–1180, DOI: 10.1002/jmri.24751 10.1002/jmri.24751 [DOI] [PubMed] [Google Scholar]
- 5.Huang RY, Neagu MR, Reardon DA, et al. Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy – detecting illusive disease, defining response. Front Neurol 2015; 6: 33. DOI: 10.3389/fneur.2015.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YJ, Kim HS, Jahng G-H, et al. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiologica 2013; 54: 448–454. DOI: 10.1177/0284185112474916 [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Wang Y, Pinho MC, et al. Intrasession reliability of arterial spin-labeled MRI–measured noncontrast perfusion in glioblastoma at 3 T. Tomography 2020; 6: 139–147. DOI: 10.18383/j.tom.2020.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grande FD, Subhawong T, Weber K, et al. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology 2014; 271: 499–511. DOI: 10.1148/radiol.13130844 [DOI] [PubMed] [Google Scholar]
- 9.Weber MA, Thilmann C, Lichy MP, et al. Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Invest Radiol 2004; 39: 277–287. DOI: 10.1097/01.rli.0000119195.50515.04 [DOI] [PubMed] [Google Scholar]
- 10.Balaña C, Capellades J, Pineda E, et al. Pseudoprogression as an adverse event of glioblastoma therapy. Cancer Med 2017; 6: 2858–2866. DOI: 10.1002/cam4.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YJ, Kim HS, Jahng G-H, et al. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiologica 2013; 54: 448–454. DOI: 10.1177/0284185112474916 [DOI] [PubMed] [Google Scholar]
- 12.Wolf RL, Wang J, Wang S, et al. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging 2005; 22: 475–482. DOI: 10.1002/jmri.20415 [DOI] [PubMed] [Google Scholar]
- 13.Silva AC, Kim SG, Garwood M, et al. Imaging blood flow in brain tumors using arterial spin labeling. Magn Reson Med 2000; 44: 169–173. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Luan J, Wu M, Wang X, et al. The diagnostic value of quantitative analysis of ASL, DSC-MRI and DKI in the grading of cerebral gliomas: a meta-analysis. Radiat Oncol 2020; 15: 204. DOI: 10.1186/s13014-020-01643-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gariani J, Hottinger A, Ben Aissa A, et al. New patterns of magnetic resonance images in high-grade glioma patients treated with bevacizumab (Avastin®). Clin Translational Neurosci 2018; 2: 2514183X17752903. DOI: 10.1177/2514183x17752903 [DOI] [Google Scholar]
- 16.Soni N, Srindharan K, Kumar S, et al. Arterial spin labeling perfusion: prospective MR imaging in differentiating neoplastic from non-neoplastic intra-axial brain lesions. Neuroradiol J 2018; 31: 544–553. DOI: 10.1177/1971400918783058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganbold M, Harada M, Khashbat D, et al. Differences in high-intensity signal volume between arterial spin labeling and contrast-enhanced T1-weighted imaging may be useful for differentiating glioblastoma from brain metastasis. J Med Invest 2017; 64: 58–63. DOI: 10.2152/jmi.64.58 [DOI] [PubMed] [Google Scholar]
- 18.Fu M, Han F, Feng C, et al. Based on arterial spin labeling helps to differentiate high-grade gliomas from brain solitary metastasis: a systematic review and meta-analysis. Medicine 2019; 98: e15580. DOI: 10.1097/md.0000000000015580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aparici-Robles F, Davidhi A, Carot-Sierra JM, et al. Glioblastoma versus solitary brain metastasis: MRI differentiation using the edema perfusion gradient. J Neuroimaging 2022; 32: 127–133, DOI: 10.1111/jon.12920 10.1111/jon.12920 [DOI] [PubMed] [Google Scholar]
- 20.You S-H, Yun TJ, Choi HJ, et al. Differentiation between primary CNS lymphoma and glioblastoma: qualitative and quantitative analysis using arterial spin labeling MR imaging. Eur Radiol 2018; 28: 3801–3810. DOI: 10.1007/s00330-018-5359-5 [DOI] [PubMed] [Google Scholar]
- 21.Roser F, Saini M, Meliss R, et al. Apoptosis, vascularity, and proliferation in primary central nervous system lymphomas (PCNSL): a histopathological study. Surg Neurol 2004; 62: 393–399. DOI: 10.1016/j.surneu.2003.11.038 [DOI] [PubMed] [Google Scholar]
- 22.Kickingereder P, Sahm F, Wiestler B, et al. Evaluation of microvascular permeability with dynamic contrast-enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic-pathologic correlation. Am J Neuroradiology 2014; 35: 1503–1508. DOI: 10.3174/ajnr.A3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita K, Yoshiura T, Hiwatashi A, et al. Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and 18F-fluorodeoxyglucose positron emission tomography. Neuroradiology 2013; 55: 135–143. DOI: 10.1007/s00234-012-1089-6 [DOI] [PubMed] [Google Scholar]
- 24.Kang KM, Sohn CH, You SH, et al. Added value of arterial spin-labeling MR imaging for the differentiation of cerebellar hemangioblastoma from metastasis. Am J Neuroradiology 2017; 38: 2052–2058. DOI: 10.3174/ajnr.A5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khashbat D, Abe T, Ganbold M, et al. Correlation of 3D arterial spin labeling and multi-parametric dynamic susceptibility contrast perfusion MRI in brain tumors. The J Med Invest 2016; 63: 175–181. DOI: 10.2152/jmi.63.175 [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi K, Hiwatashi A, Togao O, et al. Arterial spin-labeling is useful for the diagnosis of residual or recurrent meningiomas. Eur Radiol 2018; 28: 4334–4342. DOI: 10.1007/s00330-018-5404-4 [DOI] [PubMed] [Google Scholar]
- 27.Mayercik V, Ma M, Holdsworth S, et al. Arterial spin-labeling MRI identifies hypervascular meningiomas. Am J Roentgenology 2019; 213: 1124–1128. DOI: 10.2214/AJR.18.21026 [DOI] [PubMed] [Google Scholar]
- 28.Tamrazi B, Shiroishi MS, Liu C-SJ, et al. Advanced imaging of intracranial meningiomas. Neurosurg Clin N Am 2016; 27: 137–143. DOI: 10.1016/j.nec.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao XJ, Kim HG, Wang DJJ, et al. Application of arterial spin labeling perfusion MRI to differentiate benign from malignant intracranial meningiomas. Eur J Radiol 2017; 97: 31–36. DOI: 10.1016/j.ejrad.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Luan S, Liu L, et al. Evaluation of the applicability of territorial arterial spin labeling in meningiomas for presurgical assessments compared with 3-dimensional time-of-flight magnetic resonance angiography. Eur Radiol 2017; 27: 4072–4081. DOI: 10.1007/s00330-017-4760-9 [DOI] [PubMed] [Google Scholar]
- 31.Okamoto N, Itokawa H, Moriya M, et al. Efficacy of preoperative radiation therapy in hyper-vascular solitary fibrous tumor. Neurol Surg 2009; 37: 189–194. [PubMed] [Google Scholar]
- 32.Ma W, Yin H, Huan Y, et al. An unusual malignant solitary fibrous tumor of the pineal region: A case report and review of the literature. Eur J Radiol Extra 2010; 76: e39–e42, DOI: 10.1016/j.ejrex.2010.07.005 10.1016/j.ejrex.2010.07.005 [DOI] [Google Scholar]
- 33.Valecha G, Pant M, Ibrahim U, et al. Immunotherapy-induced autoimmune hypophysitis. J Oncol Pharm Pract 2019; 25: 217–220. DOI: 10.1177/1078155217727142 [DOI] [PubMed] [Google Scholar]
- 34.Deng F, Yoon BC. Brachiocephalic vein compression with jugular venous reflux may mimic cavernous dural arteriovenous fistula on arterial spin labeling. Neuroradiology 2021; 63: 291–294. DOI: 10.1007/s00234-021-02637-7 [DOI] [PubMed] [Google Scholar]
- 35.Imai M, Sunaga A, Aoki R, et al. Possibility of arterial spin labeling perfusion magnetic resonance imaging sequences with steroid therapy for Tolosa-Hunt syndrome: a case report and review of literature. Surg Neurol Int 2022; 13: 27–2022. DOI: 10.25259/sni_969_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noguchi T, Yakushiji Y, Nishihara M, et al. Arterial spin-labeling in central nervous system infection. Magn Reson Med Sci 2016; 15: 386–394. DOI: 10.2463/mrms.mp.2015-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan EP, Heiber G, Gerard EE, et al. Stroke-like migraine attacks after radiation therapy: a misnomer? Epilepsia 2018; 59: 259–268. DOI: 10.1111/epi.13963 [DOI] [PubMed] [Google Scholar]
- 38.Black DF, Morris JM, Lindell EP, et al. Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: a case series. Am J Neuroradiology 2013; 34: 2298–2303. DOI: 10.3174/ajnr.A3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noguchi T, Yoshiura T, Hiwatashi A, et al. Perfusion imaging of brain tumors using arterial spin-labeling: correlation with histopathologic vascular density. Am J Neuroradiology 2008; 29: 688–693. DOI: 10.3174/ajnr.A0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mora Álvarez MG, Stobbe RW, Beaulieu C, et al. High resolution continuous arterial spin labeling of human cerebral perfusion using a separate neck tagging RF coil. PLoS One 2019; 14: e0215998. DOI: 10.1371/journal.pone.0215998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falk Delgado A, Van Westen D, Nilsson M, et al. Diagnostic value of alternative techniques to gadolinium-based contrast agents in MR neuroimaging-a comprehensive overview. Insights Imaging 2019; 10. DOI: 10.1186/s13244-019-0771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morello A, Casseri T, Acampa M, et al. Stroke in pregnancy and review of current literature: arterial spin-labeling MRI can identify the presence and intensity of collateral circle. J Stroke Cerebrovasc Dis 2018; 27: 3575–3577, DOI: 10.1016/j.jstrokecerebrovasdis.2018.08.027 10.1016/j.jstrokecerebrovasdis.2018.08.027 [DOI] [PubMed] [Google Scholar]