Abstract
Background
Viral acute respiratory tract infections (ARTIs) are a leading cause of hospitalization in infants and young children.
Methods
During the winter seasons of 2014–2018, hospitalized children (<18 years) with symptoms of ARTI were prospectively included at the University Hospital Heidelberg, Germany. Nasopharyngeal swabs were obtained for multiplex molecular analysis of 10 groups of respiratory viruses, and clinical data were obtained using a standardized questionnaire.
Results
Of 1353 children included in this study, 1142 (84.4%) were positive for ≥1 viral pathogen. Virus monoinfection was detected in 797 (69.8%) children, whereas 345 (30.2%) children had coinfections with 2–4 viral pathogens. Respiratory syncytial virus (RSV), rhinovirus, and influenza virus were the main pathogens detected. RSV-positive children had significantly more often lower ARTIs, including symptoms of severe cough, wheezing, chest indrawing, tachypnea, and pulmonary rales. Hospitalized children aged <6 months represented the largest age group with detection of ≥1 viral pathogen (455/528 [86.2%] children). Coinfection was more frequent in younger children and, particularly for RSV with rhinovirus, significantly associated with more severe respiratory symptoms (P = .01).
Conclusions
A better understanding of the etiology of viral ARTIs among hospitalized children plays a key role for future strategies in prevention, control, and treatment of respiratory viral infections.
Keywords: children, coinfection, multiplex PCR, respiratory tract infections, viral pathogens
Acute respiratory tract infections (ARTIs) are a common reason for hospital admissions in infants and young children and are mostly caused by viral pathogens [1, 2]. Clinical presentation of viral ARTI in children varies from asymptomatic viral replication over mild and self-limiting upper respiratory tract infection (URTI) to a more severe course of disease presenting as nondiphtheric croup, bronchitis, bronchiolitis, and pneumonia [3]. In 2010, approximately 15 million severe and very severe ARTIs led to hospitalization, particularly in infants [4]. Moreover, development of viral bronchiolitis in infancy predestines for wheezing/asthma later in childhood [5, 6]. A variety of respiratory viruses are well known to cause significant morbidity and mortality in children, including influenza, respiratory syncytial virus (RSV), and parainfluenza virus (PIV) [4]. Most respiratory viruses do not cause virus-type specific clinical syndromes [7]. However, the number of detectable viral pathogens associated with pediatric ARTI has grown since the introduction of multiplex polymerase chain reaction (PCR) assays in most hospitals, which detect a variety of respiratory viruses with high sensitivity and have become very popular in the diagnostic workup [3]. The increasing use of multiplex PCR methods has led to the more frequent detection of a wider range of respiratory viruses such as rhinovirus (RV), human metapneumovirus (HMPV), bocavirus (BV), parechovirus (PV), adenovirus (AV), and newly recognized variants of enterovirus (EV) or coronavirus (CoV) [4, 8]. However, the significance of detected viral pathogens, coinfections, and pathogenicity in children remains unclear [9, 10].
The purpose of this study was to investigate the prevalence of viral pathogens, their spread during winter seasons, possible coinfections, and clinical characteristics of ARTIs in hospitalized children in Heidelberg, Germany, to gain a better understanding of the epidemiology and etiology of viral ARTIs in children and their impact on disease severity.
METHODS
Patients and Clinical Data
We performed a single-center prospective study with children admitted with ARTI to the Pediatric Emergency Department at the Heidelberg University Hospital, Germany, during November–April of the 2014–2015 through 2017–2018 winter seasons. Study inclusion criteria were age <18 years, clinical symptoms of ARTI as part of the admission diagnosis or as concomitant symptoms, and hospitalization based on the clinical judgment of the attending physician. As part of a routine hospital screening for viral pathogens in hospitalized children with ARTI for the prevention of nosocomial infection, a nasopharyngeal swab as well as demographic and clinical data were collected, and a complete physical examination was performed. Medical information was prospectively collected by pediatricians on admission using a standardized data sheet. URTI was defined as the presence of respiratory symptoms (e.g. coughing, sneezing) without signs of lower respiratory tract infection (LRTI). LRTI was defined as the diagnosis of pneumonia, bronchiolitis, or bronchitis. Patient records and information were anonymized and de-identified prior to analysis. The Ethical Committee of the University of Heidelberg approved this study (S-166/2014) and waived individual written patient consent due to the observational character of the study.
Clinical Samples and Multiplex PCR for Respiratory Viruses
Nasopharyngeal swabs were collected in viral transport media (MSwab; Copan, Brescia, Italy) and transferred to the virology diagnostic laboratory for further real-time multiplex PCR analysis. For detection of respiratory pathogens, RNA was extracted from respiratory specimens using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany). Amplification and detection of viral RNA was performed with FTD respiratory pathogens-21 PCR (Fast-Track Diagnostics; Abbott, Wiesbaden, Germany) on a LightCycler 480 instrument II (Roche, Mannheim, Germany) detecting the following pathogens: influenza virus A, influenza virus A/H1N1, influenza virus B, RV, RSV, BV, AV, PIV types 1–4, CoVs (NL63, 229E, OC43, and HKU1), PV, EV other than RV, HMPV, and Mycoplasma pneumoniae. For further data analysis, influenza A, influenza A/H1N1, and influenza B were clustered as influenza virus; CoVs NL63, 229E, OC43, and HKU1 were clustered as CoV; and PIV 1–4 were clustered as PIV. RV and EV infection can be distinguished by this assay. However, positive signals for RV and EV indicate that the sample is EV positive according to the manufacturer. As a result, conclusions about RV and EV coinfection are limited and were therefore excluded from the analysis.
Statistical Analysis of Epidemiological Factors
Demographic and clinical data were summarized. Group comparisons were performed using χ2 or Fisher exact test for categorical variables and Student t test or analysis of variance for continuous variables, as appropriate. P values <.05 were considered statistically significant. Stata/IC15.0 software (StataCorp, College Station, Texas) was used for all statistical analyses.
RESULTS
Characteristics of the Study Cohort
A total of 1353 hospitalized children with a median age of 10.5 months (range 1 week–17 years) were included in 4 consecutive winter seasons (2014–2015, 2015–2016, 2016–2017, and 2017–2018). Age and sex distribution in all 4 seasons was similar, with most children hospitalized being aged <6 months (n = 528 [39.0%]), followed by 6 months to 1 year (n = 493 [36.4%]), and few patients in the age groups of 2 to 4 years (n = 250 [18.5%]) and ≥5 years (n = 82 [6.1%]). Of all hospitalized children (54.6% male) with ARTI, 581 (42.9%) presented with symptoms of URTI, and of those, 61.4% were admitted primarily with a nonrespiratory diagnosis and concomitant symptoms of URTI. Among nonrespiratory admission diagnosis, the most common diagnosis was febrile convulsions (n = 119/357 [33.3%]), followed by fever of unknown origin and/or general bad condition including suspected sepsis or meningitis (n = 63/357 [17.6%]). Other diagnoses included gastroenteritis (n = 23/357 [6.4%]), convulsions/epilepsy (n = 14/357 [3.9%]), concussion (n = 13/357 [3.6%]), metabolic disorders (n = 10/357 [2.8%]), pyelonephritis (n = 9/357 [2.5%]), apparent life-threatening event (n = 9/357 [2.5%]), apnea (n = 7/357 [2.0%]), asthma (n = 4/357 [1.1%]), congenital heart disease (n = 4/357 [1.1%]), and allergic reaction (n = 4/357 [1.1%]). In total, 772 patients (57.1%) were admitted with LRTI; 24.5%, 18.5%, and 14.0% were diagnosed with bronchitis, bronchiolitis, and pneumonia, respectively. The median duration of hospitalization was 3 days for all hospitalized children.
Detection of Viral Pathogens
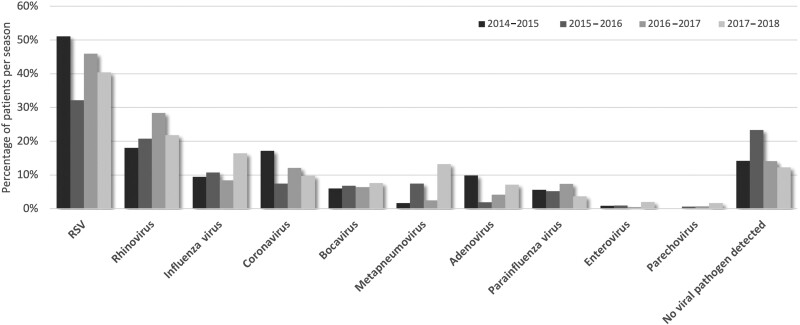
RSV was detected in 42.1% of all patients, followed by RV (22.9%), influenza (11.5%), and CoV (11.2%). No respiratory virus was detected in 15.6% of patients. Detection of HMPV varied with alternating lower and higher numbers, with up to 13.2% of infected children in 2017–2018. BV, AD, and PIV were less common (6.8%, 5.5%, and 5.5%, respectively). PV and EV infections were detected in a few cases only. The distribution of detected viral pathogens per winter season is shown in Figure 1.
Figure 1.
Viral pathogens detected in monoinfected and coinfected children (n = 1353) with acute respiratory tract infection per season. Abbreviation: RSV, respiratory syncytial virus.
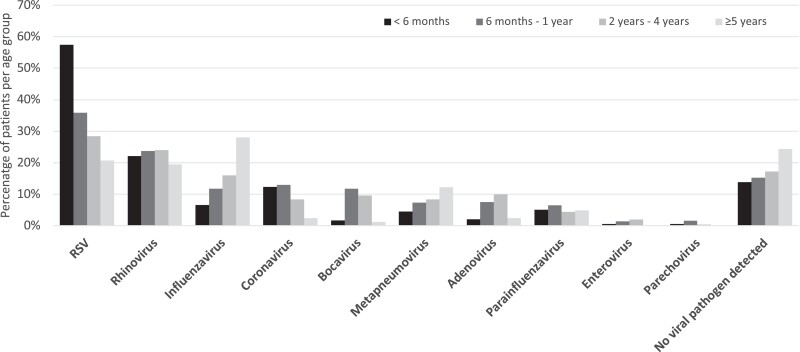
Patterns of age groups differed widely between viral pathogens (Figure 2). Children aged <6 months represented the largest age group being hospitalized with ARTI with a high positivity rate of 86.2% (n = 455/528), of whom 66.8% were RSV positive. The median age of children with RSV infection was 5 months, and they were significantly younger than children with influenza infection with a median age of 19 months (P < .001). Whereas the frequency of RSV-positive samples decreased to 20.7% in children aged ≥5 years, the proportion of influenza-infected children rose from 6.6% in patients aged <6 months to 28.0% in children aged ≥5 years. A similar effect could be observed for BV, HMPV, and AV, although percentages for BV and AV decreased again after 5 years. The mean age of children with RV and PIV infection was 18 and 19 months, respectively, and both pathogens were evenly distributed over all age groups.
Figure 2.
Viral pathogens detected in monoinfected and coinfected children (n = 1353) with acute respiratory tract infection per age group. Abbreviation: RSV, respiratory syncytial virus.
Information about subtypes was available for influenza, CoV, and PIV (Supplementary Table 1). Influenza A (69.2%; A/H1N1, 38.5%) was found more often than influenza B; 1 child had a coinfection with both types. CoV subtype OC43 was found in 44.1%, NL63 in 29.6%, HKU1 in 21.1%, and 229E in only 5.3% of patients, respectively. For PIV, mostly type 3 (68.9%) was detected.
Seasonality
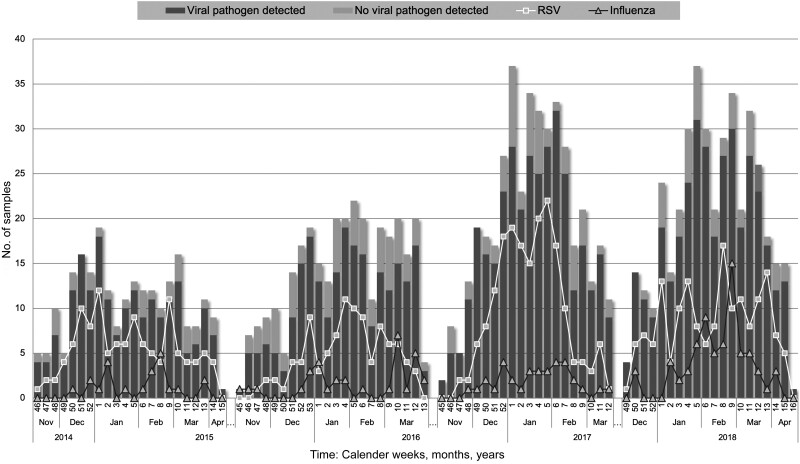
Increasing numbers of children with ARTI presented at the emergency department over the 2014–2015, 2015–2016, 2016–2017, and 2017–2018 seasons (n = 232, n = 308, n = 405, and n = 408, respectively). Starting with RSV, cases of ARTI increased in November, peaked in January, and decreased again in the month of April of each season. In 84.4% of patients, 1 or more of the complete set of viruses in the panel was detected, but their distribution during the winter seasons varied greatly. RSV reached epidemic peaks in late December and again in January/February, whereas influenza appeared late during winter seasons and peaked in February/March (Figure 3). The RSV and influenza seasons of 2016–2017 and 2017–2018 were particularly strong, with triple the number of cases for influenza compared with the 2014–2015 season. Subtype analysis revealed mainly influenza A in 2016–2017 and influenza A/H1N1 as well as influenza B in 2017–2018 (Supplementary Table 1). Other viral pathogens such as RV were more evenly distributed over the length of the season. CoV infection was also usually evenly distributed over the season, but in 2014–2015 a peak in late December to early January was observed; subtype analysis identified mainly CoV OC43.
Figure 3.
Annual peaks of respiratory syncytial virus (RSV) and influenza virus based on the overall detection of viral pathogens. Number of positive samples is shown in dark gray bars and negative samples are shown as light gray bars. The incidence of RSV is indicated by the white line with rectangles and influenza virus by the dark line with triangles per calendar week for all 4 seasons.
Clinical Presentation of Viral Monoinfections
A single viral pathogen was detected in 797 children. There was no monoinfection with PV. Like the overall study cohort, most monoinfected patients were RSV positive (n = 349 [44.3%]), followed by RV (n = 153 [19.4%]) and influenza (n = 100 [12.7%]). Most patients were admitted with a primary respiratory diagnosis (n = 611 [77.5%]), with four-fifths of children having LRTI. Rhinitis and cough comprised the most frequent clinical symptoms in patients with viral monoinfections (Table 1).
Table 1.
Clinical Presentation of Viral Monoinfections (N = 797) on Admissiona
| Characteristic | Total (N = 797) |
RSV (n = 349) | Influenza Virus (n = 100) | HMPV (n = 56) |
PIV (n = 28) |
Rhinovirus (n = 153) |
Bocavirus (n = 30) | Adenovirus (n = 23) | Coronavirus (n = 49) | Enterovirus (n = 9) | P Valueb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission diagnosis | |||||||||||
| Nonrespiratory | 181/797 (22.7) | 31 (8.9) | 40 (40.0) | 11 (19.6) | 8 (28.7) | 51 (33.3) | 7 (23.3) | 9 (39.1) | 20 (40.8) | 4 (44.4) | <.001 |
| Respiratory | 616/797 (77.3) | 318 (91.1) | 60 (60.0) | 45 (80.4) | 20 (71.4) | 102 (66.7) | 23 (76.7) | 14 (60.9) | 29 (59.2) | 5 (55.6) | <.001 |
| URTI | 311/797 (39.0) | 76 (21.8) | 63 (63.0) | 23 (41.1) | 12 (42.9) | 73 (47.7) | 12 (40.0) | 13 (56.5) | 32 (65.3) | 7 (77.8) | <.001 |
| LRTI | 486/797 (61.0) | 273 (78.2) | 37 (37.0) | 33 (58.9) | 16 (57.1) | 80 (52.3) | 18 (60.0) | 10 (43.5) | 17 (34.7) | 2 (22.2) | <.001 |
| Symptoms on admissionc | |||||||||||
| Rhinitis | 697/772 (90.3) | 313 (90.0) | 85 (89.5) | 49 (90.7) | 22 (88.0) | 135 (90.0) | 22 (75.9) | 21 (91.3) | 42 (85.7) | 8 (88.9) | .06 |
| Hoarseness | 121/649 (18.6) | 74 (26.6) | 7 (8.6) | 8 (17.8) | 6 (26.1) | 15 (11.5) | 2 (8.3) | 2 (11.1) | 7 (16.7) | 0 (0.0) | <.001 |
| Stridor | 90/749 (12.1) | 40 (12.4) | 6 (6.3) | 7 (13.0) | 0 (0.0) | 22 (15.2) | 3 (11.5) | 1 (4.35) | 8 (18.2) | 3 (33.3) | .66 |
| Mild cough | 266/682 (39.0) | 103 (30.9) | 37 (48.7) | 22 (43.1) | 8 (36.4) | 59 (49.6) | 8 (33.3) | 9 (52.9) | 16 (50.0) | 4 (50.0) | <.001 |
| Moderate/severe cough | 416/682 (61.0) | 230 (69.1) | 39 (51.3) | 29 (56.9) | 14 (63.6) | 60 (50.4) | 16 (66.7) | 8 (47.1) | 16 (50.0) | 4 (50.0) | <.001 |
| Wheezing | 284/765 (37.1) | 153 (45.5) | 13 (13.4) | 23 (43.4) | 9 (34.6) | 63 (42.3) | 11 (39.3) | 3 (13.0) | 7 (15.6) | 2 (25.0) | <.001 |
| Rale | 338/768 (44.0) | 202 (59.4) | 25 (26.3) | 26 (48.2) | 8 (30.8) | 47 (31.3) | 8 (29.6) | 6 (26.1) | 12 (27.3) | 4 (44.4) | <.001 |
| Indrawings | 292/763 (38.3) | 160 (47.3) | 17 (18.3) | 18 (33.3) | 7 (28.0) | 64 (42.7) | 9 (32.1) | 5 (21.7) | 8 (18.6) | 4 (44.4) | <.001 |
| Tachypnea | 358/764 (46.9) | 194 (57.4) | 24 (25.3) | 23 (43.4) | 14 (51.9) | 68 (46.0) | 13 (48.2) | 6 (26.1) | 14 (31.8) | 2 (22.2) | <.001 |
| Flaring | 97/748 (13.0) | 53 (16.2) | 3 (3.26) | 9 (16.7) | 1 (3.9) | 19 (13.0) | 4 (14.8) | 2 (8.7) | 5 (11.6) | 1 (11.1) | .02 |
| Cyanosis | 61/750 (8.1) | 31 (9.31) | 6 (6.5) | 6 (11.1) | 0 (0.0) | 12 (8.1) | 1 (4.0) | 2 (9.1) | 3 (7.1) | 0 (0.0) | .34 |
| Apnea | 38/739 (5.1) | 17 (5.3) | 2 (2.2) | 6 (11.1) | 1 (3.9) | 10 (6.9) | 0 (0.0) | 0 (0.0) | 2 (4.7) | 0 (0.0) | .94 |
Data are presented as No. (%). Infections with relative frequency ≥60% are shown in bold.
Abbreviations: HMPV, human metapneumovirus; LRTI, lower respiratory tract infection; PIV, parainfluenza virus; RSV, respiratory syncytial virus, URTI, upper respiratory tract infection.
No monoinfection detected for parechovirus.
P value for significant difference of RSV infection compared to other pathogens; tested with Pearson χ2 test.
Data were not available for all patients with monoinfections.
When compared to other single-pathogen infections, RSV-monoinfected children were significantly more often admitted with a primary respiratory diagnosis (91.1%) and LRTI (78.2%) including bronchitis/bronchiolitis (63.9%) and pneumonia (14.3%) (P < .001). They presented significantly more often with symptoms like hoarseness, moderate/severe cough, wheezing, pulmonary rales, chest indrawings, and tachypnea (P < .001).
Similarly, HMPV, PIV, RV, and BV also caused LRTI in particular, whereas influenza, AV, and CoV more often presented with URTI in monoinfected children.
Coinfections
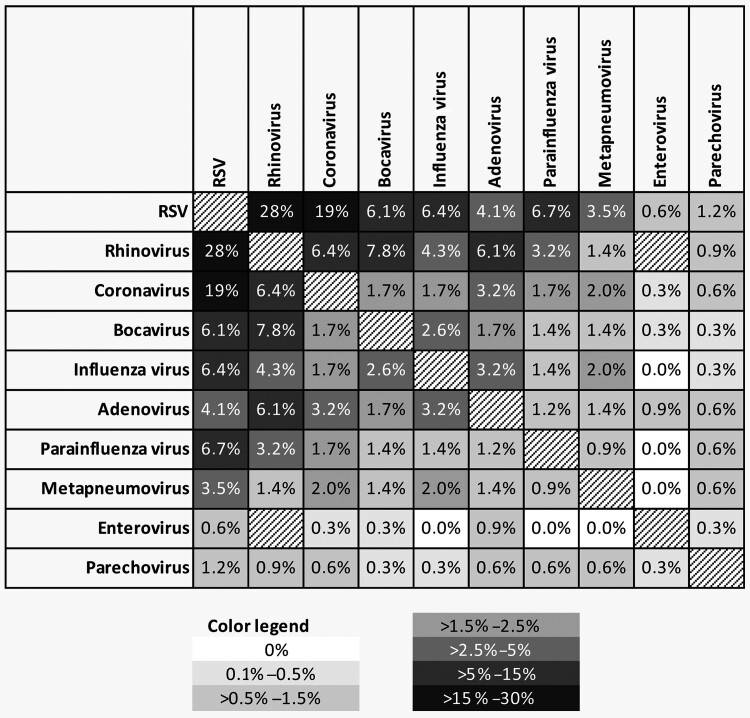
Coinfection with 2–4 viral pathogens was found in 345 cases, accounting for 25.5% of hospitalized children. Of all coinfected children, 292, 47, and 6 were infected with 2, 3, or 4 viral pathogens, respectively. RSV, RV, and CoV were the most common viruses in coinfected children. RSV monoinfection was significantly more often detected than RSV with coinfection (P < .001), whereas RV, CoV, BV, and AV were significantly more often detected as part of a coinfection (Supplementary Table 2). Furthermore, coinfection was significantly associated with younger age (P = .01) and mainly found in children aged <2 years (78.5%). A heat map of the frequency of different coinfections is shown in Figure 4. We found coinfections in 22.5% of samples from children with URTI and in significantly more samples (28.9%) from children with LRTI (P > .01). The most frequent coinfection was RSV/RV (n = 94/345 [27.2%]), followed by RSV/CoV (n = 64/345 [18.6%]) and BV/RV (n = 21/345 [6.1%]). RSV/RV coinfection was particularly often found in cases of LRTI (n = 69/221 [31.2%]), and this combination was also significantly associated with LRTI when compared to other coinfections (P = .01). However, when comparing RSV monoinfection with RSV coinfections, there was no evidence for a more severe course of disease.
Figure 4.
Schematic representation of the frequency of viruses detected in coinfections, expressed as percentage based on the number of patients with ≥2 respiratory pathogens (n = 345). The degree of shading corresponds to the frequency of each coinfection. Data are mirrored diagonally. Coinfections of rhinovirus and enterovirus were excluded from the analysis according to limitations of the multiplex assay (see Methods). Abbreviation: RSV, respiratory syncytial virus.
Mycoplasma pneumoniae Infection
Detection of M pneumoniae as a bacterial cause for respiratory infection was part of our multiplex panel. Only 13 children were M pneumoniae positive (age <6 months: n = 2 [15.4%]; age 6 months to 1 year: n = 4 [30.8%]; age 2 to 4 years: n = 6 [46.2%]; age ≥5 years: n = 1 [7.7%]). The majority of these children (n = 9/13) had a coinfection with 1–4 viral pathogens, particularly PIV and BV.
DISCUSSION
In this epidemiological study, incidence, seasonality, coinfection patterns, and clinical features of 10 different respiratory viruses were investigated based on the prospective analysis of 1353 hospitalized children with ARTIs in Southern Germany over 4 consecutive winter seasons (2014–2018). RSV, RV, and influenza were the main viral pathogens detected in the study cohort. RSV stood out as being the major pathogen in severe ARTI, affecting very young children being hospitalized aged <6 months, presented mostly with LRTI in monoinfection, and was the most frequent part of coinfections, particularly with RV.
Of all hospitalized children with ARTI, the majority were aged <2 years (75.4%), and the largest age group was <6 months (39.0%). Other authors have found high rates of hospitalization in infants and young children with respiratory illnesses [11–14]. The proportion of hospitalized children presenting with symptoms of LRTI in our cohort was high (57.1% of all patients and 77.5% of those with primary respiratory diagnosis) and within the range of 48%–84% of LRTI in hospitalized children as described by others [15–17].
We were able to detect at least 1 viral pathogen in most of the hospitalized children (84.4%). Previously reported viral detection rates of multiplex PCR used in children with ARTI vary between 48% and 92% depending on the clinical inclusion criteria and sensitivity of the used assays, as well as number and selection of tested viruses [7, 15, 16, 18–20]. Like previous reports, we found that most children were positive for RSV (42.1%), followed by RV (23.9%) and influenza (11.5%) [7–9, 21, 22]. However, incidence of viral pathogens differed between age groups, and our data supported an opposite age trend for RSV compared to influenza-infected children [18]. Most RSV-positive children were <6 months of age (57.5%) with a decreasing trend toward older children, whereas influenza-positive children were significantly older, with increasing numbers with age. A similar increasing age trend was also observed for BV, HMPV, and AV.
RSV reached epidemic peaks in late December and again in January/February, whereas influenza appeared late during winter seasons and peaked in February/March. These seasonal patterns are comparable with other temperate climate regions [9]. Biennial patterns of higher RSV incidences every other year, as described by others, could not be clearly implied [23]. The 2017–2018 influenza season was particularly strong with triple numbers compared to 2014–2015, which is in line with the national surveillance data reporting the highest incidence of influenza cases since 2001 [24].
A single viral pathogen was detected in 58.2% of children, with most monoinfected patients being RSV positive (44.3%). Interestingly, most children with a single infection were primarily admitted with a respiratory diagnosis (77.5%), with four-fifths of children having LRTI. Thereby, RSV-monoinfected children presented significantly more often with severe symptoms with signs of respiratory failure (pulmonary rales, chest indrawings, tachypnea) compared to other viral pathogens. This finding provides supporting evidence for RSV as the major viral pathogen causing LRTI with bronchiolitis and pneumonia in young children [8, 25]. Similarly, HMPV, PIV, RV, and BV also caused LRTI in particular, whereas influenza, AV, and CoV more often presented with URTI in children infected with a single virus. Adams et al. did not observe clear differences between the clinical presentation of RSV, RV, and HMPV infection [26]; however, HMPV is closely related to RSV, and other authors describe the clinical picture of HMPV-related bronchiolitis in infants [1, 27]. Furthermore, several groups have suggested LRTI caused by PIV, RV, and BV infection [25, 28, 29]. Other authors have found influenza in young children with less severe respiratory symptoms [8], which fits with our study cohort with most children aged <5 years, whereas a higher incidence of influenza and a more severe course of disease has been described for older children and adults [9, 30].
In this study, coinfection was found in 26.2% of hospitalized children, within the range of 16%–47% as described by others, and we confirmed that the most common viruses in coinfected children were RSV, RV, and CoV [15, 18, 19, 21, 31]. RSV was found significantly more often in monoinfection, whereas RV, CoV, BV, and AV were mostly found in coinfections. Mengelle et al. also found coinfections more frequently in young children [8]. Opposing conclusions about the association of viral coinfections with LRTI have been reported. Some studies of hospitalized children were in line with our findings of an increased risk for LRTI [21, 32–34], whereas others could not identify clinical differences [7, 10, 15, 35]. Like Papadopoulos et al, we found particularly an association between coinfection and LRTI for the most frequent combination of RSV/RV compared to other coinfections (P = .01) [34]. However, like others, we did not find a difference for a severe infection directly comparing RSV monoinfection with RSV coinfection [36].
Our study has several limitations. First, the clustering of different virus subtypes itself could potentially lead to underestimation of some more harmful subtypes. Furthermore, the referral to the hospital after initial assessment by a primary care physician, as common in the German healthcare system, might cause bias. We also focused on hospitalized children only. Therefore, patients with milder disease may be underrepresented.
CONCLUSIONS
A high incidence of viral ARTI was found among hospitalized children in Southern Germany. In this study, RSV, RV, and influenza were the most common respiratory viruses detected. Moreover, this study provides an insight into the incidence and clinical presentation of 10 viral pathogens in a large pediatric cohort over 4 consecutive seasons and can therefore guide clinicians in the emergency department setting to prevent the abuse of antibiotics as well as inform future targeted interventions. We confirmed the high burden of RSV infection in young infants aged <6 months leading to hospitalization and particularly severe course of disease. Several decades after the discovery of RSV, there is still no established active vaccine, and prophylaxis with monoclonal antibodies is only recommended for premature babies and children with certain preexisting conditions [37, 38]. However, that might change as there are currently several promising trials on new vaccines, extended half-life antibodies, and maternal immunization strategies for the prevention of RSV hospitalization in infants aged <6 months [39]. The most advanced ones have already been approved or are in the approval process, and one will have to weigh up between year-round and seasonal vaccination programs. Therefore, epidemiological studies on respiratory viruses are an important prerequisite for the targeted development and verification of the effects of future vaccination programs and therapeutic approaches.
Supplementary Material
Contributor Information
Julia Tabatabai, Virology, Center for Infectious Diseases, University of Heidelberg, Heidelberg, Germany; German Center for Infectious Diseases, Heidelberg, Germany; Center for Child and Adolescent Medicine, University Hospital Heidelberg, Heidelberg, Germany.
Clara M Ihling, German Center for Infectious Diseases, Heidelberg, Germany; Dr von Haunersches Kinderspital, University Hospital of the Ludwig-Maximilians-University Munich, Munich, Germany.
Britta Manuel, Virology, Center for Infectious Diseases, University of Heidelberg, Heidelberg, Germany; German Center for Infectious Diseases, Heidelberg, Germany.
Rebecca M Rehbein, Virology, Center for Infectious Diseases, University of Heidelberg, Heidelberg, Germany; German Center for Infectious Diseases, Heidelberg, Germany.
Sarah V Schnee, Virology, Center for Infectious Diseases, University of Heidelberg, Heidelberg, Germany; German Center for Infectious Diseases, Heidelberg, Germany.
Johannes Hoos, Virology, Center for Infectious Diseases, University of Heidelberg, Heidelberg, Germany; German Center for Infectious Diseases, Heidelberg, Germany; Center for Child and Adolescent Medicine, University Hospital Heidelberg, Heidelberg, Germany.
Johannes Pfeil, Kinder- und Hausarztpraxis, Schwaigern, Germany.
Juergen Grulich-Henn, Center for Child and Adolescent Medicine, University Hospital Heidelberg, Heidelberg, Germany.
Paul Schnitzler, Virology, Center for Infectious Diseases, University of Heidelberg, Heidelberg, Germany.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the nurses in the Outpatient Department of the University of Heidelberg Children's Hospital for support in collecting respiratory samples and the technicians in the Center for Infectious Diseases, Virology, for excellent technical assistance.
Disclaimer. The funding agencies had no influence on concept, conduction, analysis, or the decision to publish the study.
Financial support. During the study period, J. T., C. M. I., B. M., R. M. R., J. H., and S. V. S. were supported by a research stipend of the German Centre for Infection Research. J. T. is also grateful for support from a research stipend from the Medical Faculty Heidelberg, Germany, in 2022, during the time the draft of this manuscript was finalized.
References
- 1. Sloots TP, Whiley DM, Lambert SB, Nissen MD. Emerging respiratory agents: new viruses for old diseases? J Clin Virol 2008; 42:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010; 23:74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krause JC, Panning M, Hengel H, Henneke P. The role of multiplex PCR in respiratory tract infections in children. Dtsch Arztebl Int 2014; 111:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Debiaggi M, Canducci F, Ceresola ER, Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J 2012; 9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piippo-Savolainen E, Korppi M. Wheezy babies—wheezy adults? Review on long-term outcome until adulthood after early childhood wheezing. Acta Paediatr 2008; 97:5–11. [DOI] [PubMed] [Google Scholar]
- 6. Lu S, Hartert TV, Everard ML, et al. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr Pulm 2016; 51:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wishaupt JO, van der Ploeg T, de Groot R, Versteegh FGA, Hartwig NG. Single- and multiple viral respiratory infections in children: disease and management cannot be related to a specific pathogen. BMC Infect Dis 2017; 17:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mengelle C, Mansuy JM, Pierre A, et al. The use of a multiplex real-time PCR assay for diagnosing acute respiratory viral infections in children attending an emergency unit. J Clin Virol 2014; 61:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramaekers K, Keyaerts E, Rector A, et al. Prevalence and seasonality of six respiratory viruses during five consecutive epidemic seasons in Belgium. J Clin Virol 2017; 94:72–8. [DOI] [PubMed] [Google Scholar]
- 10. Peng D, Zhao D, Liu J, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J 2009; 6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourgeois FT, Valim C, Wei JC, McAdam AJ, Mandl KD. Influenza and other respiratory virus–related emergency department visits among young children. Pediatrics 2006; 118:e1–8. [DOI] [PubMed] [Google Scholar]
- 12. Christensen KLY, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis 2009; 49:1025–35. [DOI] [PubMed] [Google Scholar]
- 13. Reiche J, Schweiger B. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J Clin Microbiol 2009; 47:1800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fillatre A, François C, Segard C, et al. Epidemiology and seasonality of acute respiratory infections in hospitalized children over four consecutive years (2012–2016). J Clin Virol 2018; 102:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Y, Lin G-Y, Wang Q, et al. A 3-year prospective study of the epidemiology of acute respiratory viral infections in hospitalized children in Shenzhen, China. Influenza Other Resp 2014; 8:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshida LM, Suzuki M, Yamamoto T, et al. Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatric Infect Dis J 2010; 29:75–7. [DOI] [PubMed] [Google Scholar]
- 17. Yen C-Y, Wu W-T, Chang C-Y, et al. Viral etiologies of acute respiratory tract infections among hospitalized children—a comparison between single and multiple viral infections. J Microbiol Immunol Infect 2019; 52:902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai X, Wang Q, Lin G, et al. Respiratory virus infections among children in South China. J Med Virol 2014; 86:1249–55. [DOI] [PubMed] [Google Scholar]
- 19. Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis 2013; 207:982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nascimento-Carvalho AC, Vilas-Boas A-L, Fontoura M-SH, Vuorinen T, Nascimento-Carvalho CM; PNEUMOPAC-Efficacy Study Group . Respiratory viruses among children with non-severe community-acquired pneumonia: a prospective cohort study. J Clin Virol 2018; 105:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paranhos-Baccalà G, Komurian-Pradel F, Richard N, Vernet G, Lina B, Floret D. Mixed respiratory virus infections. J Clin Virol 2008; 43:407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogel M, Grund S, Pandey S, et al. What we have learned from the influenza A pH1N1 2009/10 pandemic: high clinical impact of human metapneumovirus and respiratory syncytial virus in hospitalized pediatric patients. Jpn J Infect Dis 2016; 69:6–11. [DOI] [PubMed] [Google Scholar]
- 23. Gunell M, Antikainen P, Porjo N, et al. Comprehensive real-time epidemiological data from respiratory infections in Finland between 2010 and 2014 obtained from an automated and multianalyte mariPOC respiratory pathogen test. Eur J Clin Microbiol 2016; 35:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robert Koch Institut . Epidemiology of influenza in Germany for the 2017/18 season [in German].2018; [cited 2023 Mar 13]. Available at: https://influenza.rki.de/Saisonberichte/2017.pdf.
- 25. Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta–analysis. J Glob Health 2015; 5:010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adams O, Weis J, Jasinska K, Vogel M, Tenenbaum T. Comparison of human metapneumovirus, respiratory syncytial virus and rhinovirus respiratory tract infections in young children admitted to hospital. J Med Virol 2015; 87:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev 2006; 19:546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis 2006; 43:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finianos M, Issa R, Curran MD, et al. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J Med Virol 2016; 88:1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piralla A, Mariani B, Rovida F, Baldanti F. Frequency of respiratory viruses among patients admitted to 26 intensive care units in seven consecutive winter-spring seasons (2009–2016) in northern Italy. J Clin Virol 2017; 92:48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wishaupt JO, Russcher A, Smeets LC, Versteegh FGA, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics 2011; 128:e1113-20. [DOI] [PubMed] [Google Scholar]
- 32. Richard N, Komurian-Pradel F, Javouhey E, et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatric Infect Dis J 2008; 27:213–7. [DOI] [PubMed] [Google Scholar]
- 33. Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 2005; 191:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papadopoulos NG, Moustaki M, Tsolia M, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Resp Crit Care 2002; 165:1285–9. [DOI] [PubMed] [Google Scholar]
- 35. Scotta MC, Chakr VC, de Moura A, et al. Respiratory viral coinfection and disease severity in children: a systematic review and meta-analysis. J Clin Virol 2016; 80:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gagliardi TB, Paula FE, Iwamoto MA, et al. Concurrent detection of other respiratory viruses in children shedding viable human respiratory syncytial virus. J Med Virol 2013; 85:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peeples ME. Next-generation RSV vaccines avoid flipping out. Sci Transl Med 2022; 14:eade9984. [DOI] [PubMed] [Google Scholar]
- 38. Bergeron HC, Tripp RA. RSV replication, transmission, and disease are influenced by the RSV G protein. Viruses 2022; 14:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng Z, Weinberger DM, Pitzer VE. Predicted effectiveness of vaccines and extended half-life monoclonal antibodies against RSV hospitalizations in children. NPJ Vaccines 2022; 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.