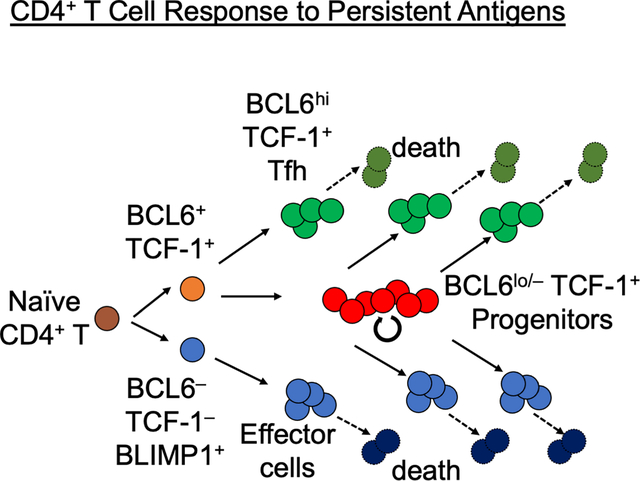
Summary
Soon after activation, CD4+ T cells are segregated into BCL6+ follicular helper (Tfh) and BCL6− effector (Teff) T cells. Here, we explored how these subsets are maintained during chronic antigen stimulation using the mouse chronic LCMV infection model. Using single cell-transcriptomic and epigenomic analyses, we identified a population of PD-1+ TCF-1+ CD4+ T cells with memory-like features. TCR clonal tracing and adoptive transfer experiments demonstrated that these cells have self-renewal capacity and continue to give rise to both Teff and Tfh cells, thus functioning as progenitor cells. Conditional deletion experiments showed Bcl6-dependent development of these progenitors, which were essential for sustaining antigen-specific CD4+ T cell responses to chronic infection. An analogous CD4+ T cell population developed in draining lymph nodes in response to tumors. Our study reveals the heterogeneity and plasticity of CD4+ T cells during persistent antigen exposure and highlights their population dynamics through a stable, bipotent intermediate state.
Keywords: CD4+ T cells, single-cell genomics, progenitor cells, chronic viral infection, follicular helper T cells, effector T cells, anti-tumor immunity
Graphical Abstract
eTOC blurbs
Understanding T cell responses in the presence of chronic antigen has largely focused on CD8+ T cells, while the persistance of CD4+ T cells during continued antigen exposure is underexplored. Xia, Sandor, and Pai et al. identify a self-renewing progenitor CD4+ T cell population required for sustaining both follicular helper and effector CD4+ T cells.
Introduction
CD4+ T cells play a central role in coordinating innate and adaptive immunity by differentiating into T effector (Teff) cells that produce context-specific cytokines (Crotty et al., 2010; Marshall et al., 2011; Pepper and Jenkins, 2011). Activated CD4+ T cells also differentiate into follicular helper T (Tfh) cells that provide help to B cells for their expansion and affinity maturation. In studies using acute pathogen infection or non-replicating antigen (Ag), CD4+ T cells diverge into BCL6− and BCL6+ subsets shortly after priming (Baumjohann et al., 2011; Choi et al., 2011; DiToro et al., 2018; Hondowicz et al., 2018). The early binary fate decision of CD4+ T cells is stable as early BCL6+ cells exhibit skewed developmental potential towards Tfh at the expense of Teff cells (Choi et al., 2013; DiToro et al., 2018).
CD4+ T cells also play essential roles in controlling chronic viral infection and tumors, in which their responses must be sustained for substantially longer periods, compared to responses to acute infection or vaccination. Similar to CD8+ T cells, activated CD4+ T cells acquire distinct phenotypes in the presence of chronic Ag compared to acute viral infection (Crawford et al., 2014; Hashimoto et al., 2018; Wherry et al., 2007). Whereas increased inhibitory receptor expression is the hallmark of exhausted CD8+ T cells, CD4+ T cells exposed to persistent Ag exhibit unique gene expression changes, including elevated expression of Bcl6 and additional similarities to Tfh cells (Fahey et al., 2011), while expression of multiple inhibitory receptors is less pronounced compared to CD8+ T cells (Crawford et al., 2014). In CD8+ T cells, recent studies identified a subset of stem-like or progenitor-like exhausted CD8+ T cells, referred to as T progenitor exhausted (TPEX) cells, that express the transcription factors (TF) TCF-1 and BCL6, and the canonical Tfh marker CXCR5 (He et al., 2016; Im et al., 2016; Leong et al., 2016; Utzschneider et al., 2016; Wu et al., 2016). TPEX cells are necessary to sustain CD8+ T cell effector responses and critical for response to immune checkpoint blockade (ICB). Given the terminally differentiated nature of CD4+ Teff and Tfh cells, the continued CD4+ T cell response may similarly be supported by continued differentiation from progenitor cells, which could be similar to the previously identified TCF-1+ cells generated by asymmetric divisions (Nish et al., 2017). However, it is unknown whether sustained CD4+ T cell response depends on such a progenitor population, or how they are distinct, if any, from pre-Tfh or memory CD4+ T cells.
To address this question, we performed an unbiased characterization of the heterogeneity of Ag-specific CD4+ T cells in response to infection with Lymphocytic choriomeningitis virus (LCMV) clone 13 (c13), which achieves prolonged Ag persistence. We identified a population of PD-1+ TCF-1+ BCL6lo/− progenitor CD4+ T (Tprog) cells distinct from TCF-1− BCL6− Teff and TCF-1+ BCL6hi Tfh cells. This population was detectable at the peak of T cell response and persisted into the chronic phase of infection in a B cell-independent manner. Trajectory analysis, TCR clonal tracing, and adoptive transfer experiments demonstrated that Tprog cells serve as common progenitors for Teff and Tfh cells. Epigenomic analysis suggested that the differentiation of Tprog cells is triggered by TCR signaling, followed by bifurcation to the two distinct terminal fates. Deletion of BCL6 in CD4 T cells not only results in the expected loss of Tfh, but also the ablation of Teff cells two weeks after infection despite their intact initial expansion. Finally, in tumor-bearing mice, an analogous heterogeneity of CD4+ T cells was observed in tumor-draining lymph nodes (tdLNs), while Ag-specific CD4+ T cells in the tumor were predominantly Teff cells, suggesting tumor-specific CD4+ T cells are maintained in secondary lymphoid organs rather than in the tumor microenvironment. These results collectively indicated that following an initial wave of naive T cell-derived Teff and Tfh cells, CD4+ Teff and Tfh cell responses to persistent Ag are maintained by a pool of TCF-1+ common Tprog cells, akin to how the CD8+ T cell response is maintained by CD8+ TPEX.
Results
Heterogeneity within Ag-specific CD4+ T cells responding to chronic viral infection.
To obtain an unbiased overview of the CD4+ T cell response to persisting Ag, we performed paired single-cell RNA-seq (scRNA-seq) and TCR sequencing of LCMV-gp66-specific (Tet+) and the remaining total CD4+ T cells (Tet−) in C57BL/6 (B6) mice infected either with LCMV-c13 or an acute strain of LCMV Armstrong strain (Arm) collected 8 or 21 days post infection (dpi) (Fig. S1A, Table S1, 2). In total, we obtained 80,785 scRNA-seq profiles after quality control filtering based on the number of genes detected per cell within 2 standard deviations of mean and percent mitochondrial reads per cell below 95th-percentile (see Methods). We obtained paired TCRαβ sequences in 89.0% of these cells (Fig. S1B). Dimensionality reduction and clustering of the cells based on their gene expression profiles identified 15 cell type clusters, most of which were grouped into 5 large clusters: Sell+ Ccr7+ Tcf7+ Slamf6− naive (Cluster 1 from RNA-seq as abbreviated as C1r), Tcf7+ Slamf6+ Bcl6− Cxcr5lo/− resting memory-like (C2r), Tcf7+ Slamf6+ activated memory-like (C3r/4r), Tcf7+ Slamf6+ Bcl6+ Cxcr5+ Tfh (C5r), and Prdm1+ Slamf1+ Cxcr6+ Teff cells (C6r/7r) (Fig. 1A, B; Fig. S1C). C3r and C4r were characterized by the expression of Tcf7, Slamf6, Pdcd1, Zbtb32, and Bcl2 at similar levels to C2r, and additionally expressed Cd69 and Mif, which are elevated in activated T cells (Choi et al., 2012; Ziegler et al., 1994). They expressed additional genes induced by TCR signaling, including Egr1, Egr2, Nr4a1, Tnfrsf4, Nfkbia, and Batf (Fig. 1A–C; Fig. S2A, B), suggesting that they represent activated, memory-like cells. We also identified 3 clusters of regulatory T (Treg) cells, including thymic Il2rahi and peripheral Il2ralo Treg cells (C8r and C9r, respectively), as well as a population of proliferating Treg cells (C10r). C11r comprised cells expressing high levels of IFN responsive genes, including Irf7 and IFN-stimulated genes Isg15, Ifit1, and Ifit3. Lastly, we found several additional small clusters, including an undefined population of non-Treg CD4+ T cells with elevated expression of the chemokine receptors, Ccr4 and Ccr6 (C12r), Myb (C13r), Gzmk (C14r), and proliferating markers including Hmgb2 and Stmn1 (C15r).
Figure 1. scRNA-seq and flow analysis reveals heterogeneity within antigen-specific CD4+ T cells in chronic LCMV infections. (See also Figures S1, S2, Table S1 and S2).
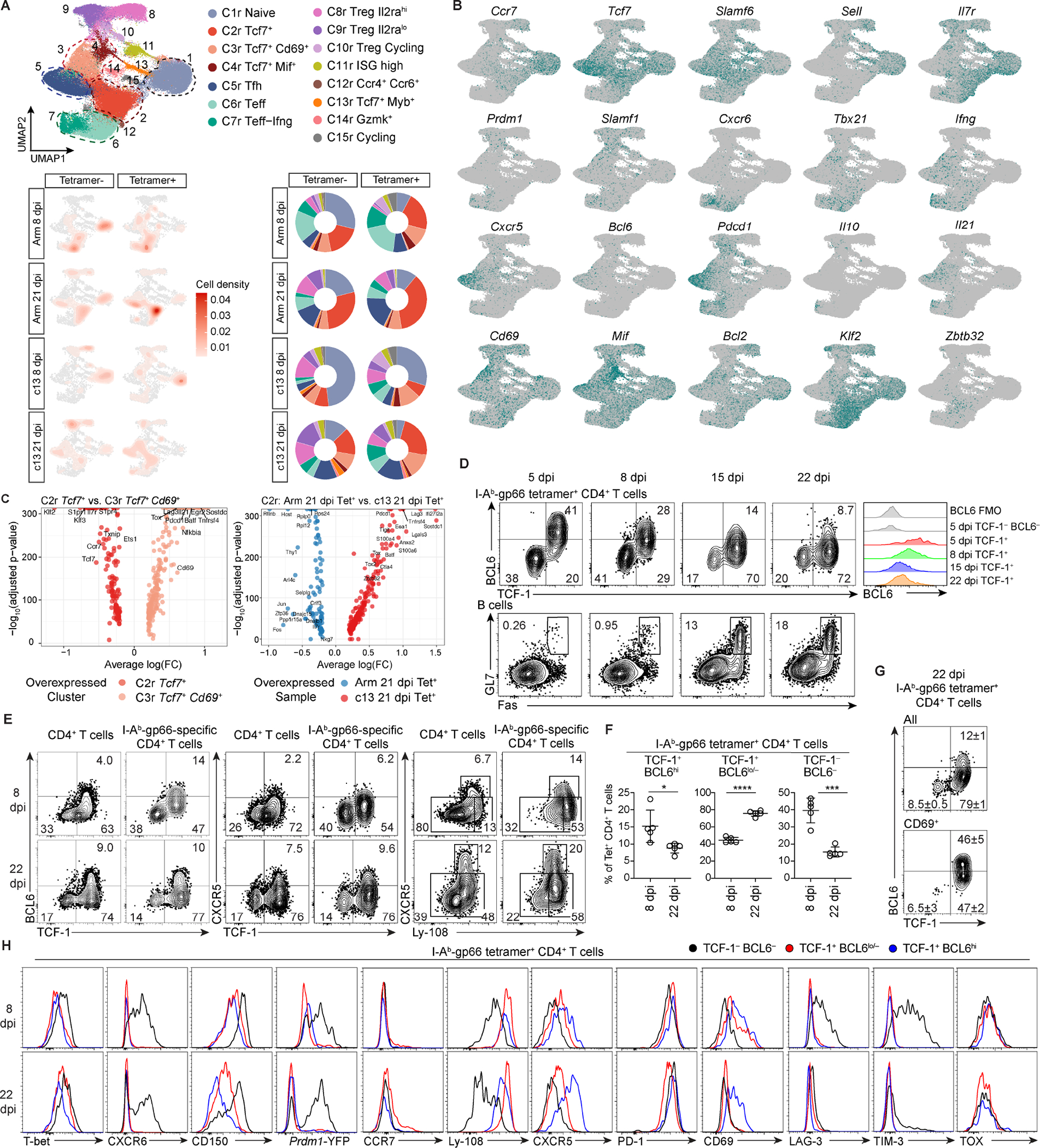
(A) UMAP of gp66 Tet+ and Tet− splenic CD4+ T cells in mice infected with LCMV-Arm or LCMV-c13 on 8 and 21 dpi. Cells were clustered by scRNA-seq gene expression profiles and colored by phenotypic clustering (top). Density UMAP of CD4+ T cells from each condition and tetramer-sorted population (bottom left). Proportion of cells from each condition in each phenotypic cluster (bottom right).
(B) Expression of cluster-defining markers.
(C) Differential gene expression of cells in the resting memory cluster C2r compared to the activated memory-like cluster C3r (left). Differential gene expression of gp66-Tet+ resting memory C2r cells from 21 dpi-LCMV-Arm compared to 21 dpi-LCMV-c13 samples (right).
(D-F) Expression of TCF-1, BCL6, CXCR5, and Ly-108 by gp66-specific splenic CD4+ T cells and expression of Fas and GL7 in splenic B cells in LCMV-c13-infected B6 mice at indicated time points. Representative flow plots (D) and pooled data for the frequencies of each PD-1+ CD4+ T cell population from 2 experiments (n = 2–3 / experiment) shown as mean±SD in (F). Unpaired t-test.
(G) Expression of indicated proteins or a Prdm1-YFP reporter in gp66-specific splenic CD4+ T cell subpopulations in LCMV-c13 infected B6 mice. Data are representative of 2 experiments with n = 2–3 / experiment.
Arm-8 dpi Tet− cells, containing a mixture of bystander naive cells, resting memory CD4+ T cells, and LCMV-reactive CD4+ T cells recognizing non-dominant epitopes, were present in all 5 major clusters, with a substantial proportion (~30%) in the C1r naive cluster (Fig. 1A). In contrast, Tet+ cells more frequently exhibited Teff and Tfh phenotypes, constituting approximately 40% of the whole population. A large proportion of the remaining Tet+ cells displayed the resting memory phenotype (Fig. 1A), similar to Tcmp (Ciucci et al., 2019; Pepper et al., 2011). In c13 infection at 8 and 21 dpi, the relative frequency of Teff cells in Tet− CD4+ T cells was reduced compared to CD4+ T cells responding to Arm infection, while that of Tfh cells was similar, indicating Tfh-skewing of expanding CD4+ T cells (Fig. 1A). As in Arm infection, Teff cells were enriched in Tet+ cells compared to Tet− cells in c13 infection. However, compared to Tet+ cells in Arm infection, the percentage of Teff cells within Tet+ cells in c13 infection was reduced on 8 dpi (Fig. 1A). Frequencies of Tet+ cells with the resting memory phenotype (C2r) were decreased in c13 infection compared to Arm infection on 8 and 21 dpi. However, c13-Tet+ cells in this cluster differed qualitatively from their Arm counterparts with increased expression of activation-induced genes (e.g., Pdcd1, Lag3, Tnfrsf4) and IFN-induced genes (e.g., Ifi27l2a), which are associated with Tfh cells (Ciucci et al., 2019), and downregulation of the Th1/Teff-associated genes, Ccl5, Nkg7, and Ly6c2 (Jenner et al., 2009; Lund et al., 2005; Marshall et al., 2011)(Fig. 1C, right and Fig. S2C, D). These changes in gene expression suggest that cells in the memory-like cell populations may be biased towards Tfh over Teff lineages. In addition, activated memory-like cells in C3r and C4r were significantly increased in c13-Tet+ samples compared to Arm infection samples on 21 dpi (Fig. 1A and Fig. S2E). Altogether, these results suggest the presence of a distinct Tcf7+ resting memory population (C2r) in c13 infection and mobilization to the activated memory-like cell pool (C3/4r).
To validate the scRNA-seq data, we used flow cytometry to subdivide splenic Tet+ CD4+ T cells in c13-infected B6 mice based on BCL6 and TCF-1 expression (Fig. 1D–F). On 5 dpi, TCF-1+ cells uniformly expressed a high level of BCL6, which was gradually and uniformly decreased on 8 and 15 dpi (Fig. 1D). On 22 dpi, TCF-1+ cells constituted a large proportion of Tet+ CD4+ T cells (Fig. 1E, F), and a subfraction of these cells expressed higher BCL6 as they underwent further differentiation into Tfh cells in germinal centers (GCs). A similar change was observed in expression of CXCR5 (Fig. 1E). Consistent with the scRNA-seq data, approximately 15% of Tet+ CD4+ T cells expressed CD69, and about half of them were TCF1+ BCL6lo/−. TCF-1− cells minimally expressed CD69. Both TCF-1+ populations expressed Ly-108 (encoded by Slamf6) but lacked expression of the Teff marker Prdm1 based on a Prdm1-YFP reporter (Fig. 1E, G). TCF-1+ BCL6lo/− cells expressed lower PD-1 and higher Ly-108 compared to TCF-1+ BCL6hi cells, and a minority (~5%) of them expressed CCR7, in contrast to resting Tcm. TCF-1− cells expressed elevated levels of T-bet, CXCR6, CD150 (encoded by Slamf1) and the Prdm1 reporter, indicating that they were highly enriched for differentiated Teff cells. TCF-1− cells also contained a small percentage (~5%) of Foxp3+ Treg cells (Fig. S2F). Consistent with previous reports, CXCR5 was a surrogate marker of BCL6, while Ly-108 was tightly associated with TCF-1 (Figs. 1E and S2G). In terms of tissue distribution, Tfh cells were barely detected in the bone marrow or liver, whereas Teff cells were detected at higher percentages (40–50%) in those organs compared to the spleen, and TCF-1+ BCL6lo/− cells were also present in those organs (Fig. S2H, I). While CD4+ T cells from all three populations produced cytokines following stimulation with gp61-77 peptide ex vivo, production of TNF and IL-2 was significantly reduced in TCF-1− cells (Fig. S2J), suggesting that they are relatively exhausted compared to two other populations of PD-1+ CD4+ T cells.
To investigate the similarities between CD4+ and CD8+ T cells in c13 infection, we compared the transcriptional profiles of each CD4+ T cell population to a single-cell CD8+ T cell dataset from LCMV-Arm and c13 infection (Daniel et al., 2021). We calculated gene signature scores of CD8+ T cell populations, including CD8+ memory (Tmem), TPEX (Texprog), KLR-expressing effector cells (TexKLR), intermediate/transitory cells (Texint), and terminally exhausted cells (Texterm) (see Methods). First, the CD4+ Tcf7+ C2r population exhibited the highest similarity to CD8+ Tmem in acute infection (mean CD8+ Tmem signature score: 0.231; Fig S2K), supporting their similarity to resting memory T cells as observed by our scRNA-seq analysis. Second, CD4+ Teff cells aligned closely to the exhausted CD8+ T cell clusters TexKLR, Texint, and Texterm in c13 infection, with the C7r Teff-Ifng cluster displaying the highest similarity to Texterm (mean CD8+ Texterm signature score: 0.295), in line with the elevated expression of exhaustion-related markers (e.g. Havcr2, Lag3) in this cluster (Fig S2B). Lastly, using the Texprog gene signature comprising 108 genes, including Tcf7, Slamf6, and Id3, CD8+ TPEX cells were most transcriptionally similar to Tcf7+ Cd69+ Pdcd1+ CD4+ T cells (mean CD8+ TPEX signature score: 0.354), and also showed similarity to Tfh cells (mean CD8+ TPEX signature score: 0.328; Fig S2K). In line with this similarity, the frequency of Tcf7+ Slamf6+ Bcl2+ Pdcd1+ memory-like cells (C2-4r) was increased in c13 infection, compared to Arm infection (Fig S2E), indicative of progenitor potential that actively supports the maintenance of Teff and Tfh cells in response to persistent Ag.
Collectively, our results indicate that with continued Ag persistence, a substantial fraction of Ag-specific CD4+ T cells acquire a TCF-1+ PD-1+ memory-like phenotype. These memory-like CD4+ T cells resemble CD8+ TPEX and contain a subpopulation of cells with a gene expression signature associated with TCR-mediated activation. They may thus act as progenitor cells to support the maintenance of Teff and Tfh cells in response to persistent LCMV infection.
Memory-like cells are enriched for common progenitors for both Tfh and Teff cells
To investigate the potential developmental relationships between CD4+ T cell clusters, we performed trajectory inference analysis (Monocle 3; see Methods) using the scRNA-seq data. To specifically analyze the transition of cells in response to chronic Ag stimulation, we re-clustered Tet+ cells from c13 infection on 8 and 21 dpi and labeled the cells according to the previously defined phenotypic clusters (Fig. 2A, S3A). Tfh (C5r) and Teff (C6/7r) clusters were mapped separately and most distantly in pseudotime from the naive cluster (C1r), supporting their terminally differentiated states. Naive cells were not directly projected to either terminally differentiated population, but were closest to the resting memory-like populations (C2r). Cells in C2r then transitioned into mature Tfh (C5r) or Teff (C6/7r) cells after passing through the activated memory-like state (C3r) and a subsequent bifurcation, suggesting that the memory-like state serves as a bifurcation point to Tfh and Teff fates. Along the trajectory from naive to mature Tfh cells (Fig. 2B, top), Tcf7 expression remained roughly constant across all differentiation time points (Fig. 2C). Bcl6 and Cxcr5 expression was increased as cells differentiated from C3/4r to the mature Tfh cluster (Fig. 2C). In contrast, Tcf7 expression decreased along the Teff trajectory (Fig. 2B, bottom), while Runx3, Prdm1, and Ifng expression increased (Fig. 2D).
Figure 2. Memory-like cells are enriched for common progenitors for both Tfh and Teff cells. (See also Figure S3, Tables S1 and S2).
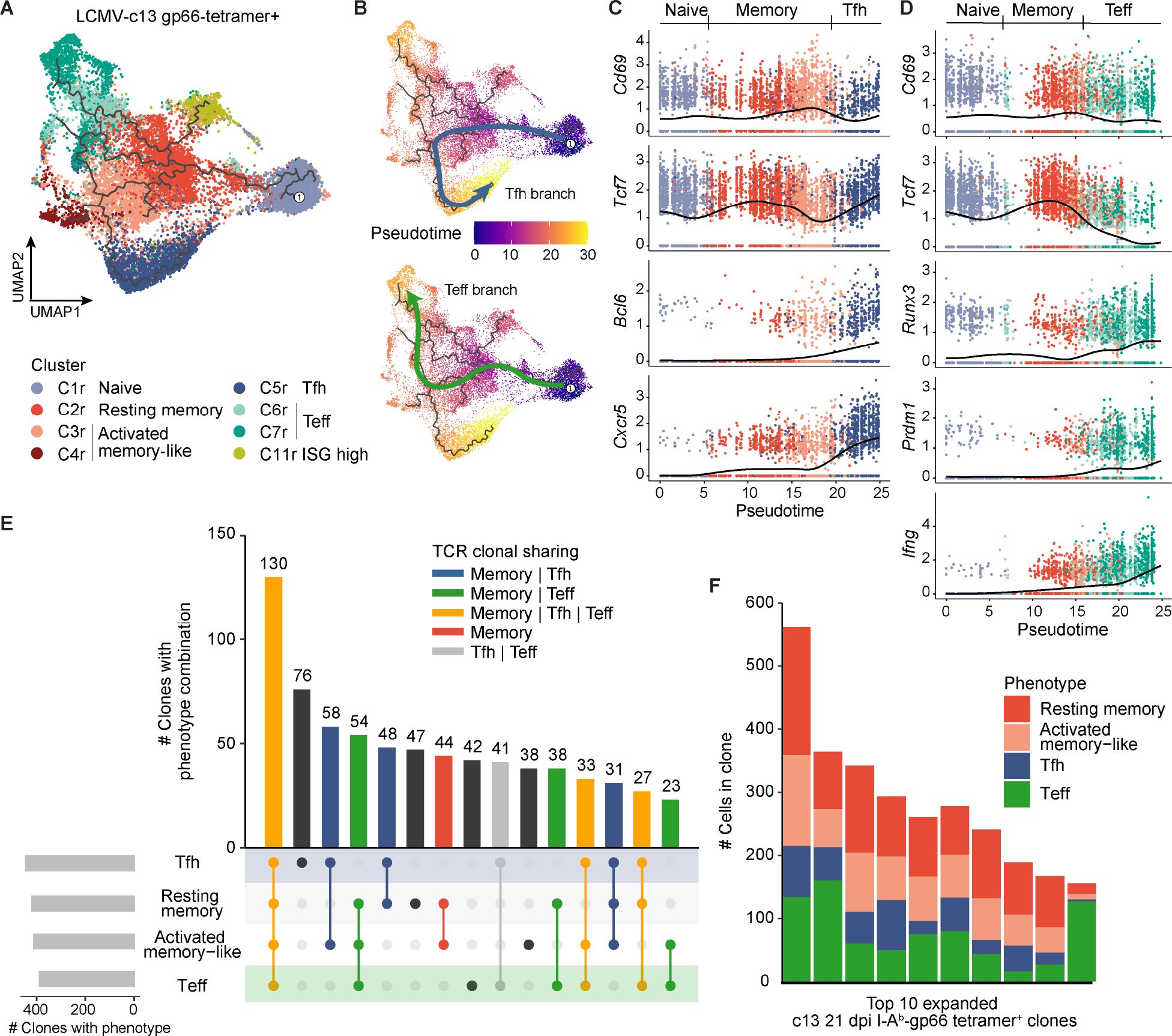
(A) UMAP trajectory graph of gp66-Tet+ CD4+ T cells from LCMV-c13 infected B6 mice on 8 and 21 dpi colored by phenotypic clusters as in Fig. 1A. The Treg clusters and other infrequent populations were excluded from the trajectory analysis for clarity.
(B) UMAP trajectory of gp66-Tet+ CD4+ T cells from LCMV-c13 infected B6 mice on 8 and 21 dpi along the Tfh branch (top) or Teff branch (bottom). Cells are colored by pseudotime.
(C) Gene expression dynamics of activation and Tfh lineage markers along the Tfh pseudotime trajectory.
(D) Gene expression dynamics of activation and Teff markers along the Teff pseudotime trajectory.
(E) Phenotypic overlap of expanded (>1 cell) T cell clones among the resting memory, activated memory-like, Tfh, and Teff clusters. Bars are colored by the category of clonal phenotypic overlap.
(F) Phenotypic composition of the top 10 most highly expanded gp66-Tet+ clones from LCMV-c13-infected B6 mice on 21 dpi.
To further support our hypothesis that cells in the memory-like clusters constitute a common progenitor pool for Tfh and Teff cells, we paired single-cell phenotyping with TCR sequence information to define clonal developmental trajectories of memory-like (C2r and C3/4r), Tfh (C5r), and Teff (C6/7r) cells. We defined TCR clones based on identical TCRαβ CDR3 sequences and identified 10,330 total clones from c13–21 dpi cells (Fig. S3B). Most of the expanded clones (>1 cell per clone) in all infection conditions and timepoints were observed in the Tet+ population, while the majority of Tet− clones were detected as unexpanded singletons (Fig. S3C–D). Among expanded Tet+ clones on c13–21 dpi (880 total expanded clones; 9,803 total cells), 483 clones were found in two or more of the three broad phenotypic clusters, and 442 of the 483 (83.9%) expanded clones were detected in the memory-like population, in addition to Tfh and/or Teff populations (Fig. 2E). Indeed, the most highly expanded clones from c13–21 dpi all comprised the memory-like, Teff, and Tfh cells (Fig. 2F). In contrast, only 41 expanded clones (7.8%) were detected in both Tfh and Teff populations, but not in the memory-like population (Fig. 2E). Taken together, these results suggest that during LCMV-c13 infection, the vast majority of the long-term clonal CD4+ T cell response is maintained by the memory-like cells that replace the initially expanded naive pool-derived clones and function as putative common Tprog cells.
Epigenetic differentiation trajectories of memory-like CD4+ T cells
To identify epigenetic pathways of TCF-1+ CD4+ Tprog cell differentiation, we performed single-cell assay for transposase accessible chromatin with sequencing (scATAC-seq) on c13–21 dpi splenic PD-1+ CD4+ T cells (Fig. S4A). In total, we obtained high quality scATAC-seq profiles from 11,611 cells (unique fragment count >1,000, transcription start site enrichment >11; Fig. S4B, C) and performed dimensionality reduction using iterative latent semantic indexing followed by UMAP (Satpathy et al., 2019), which identified 8 distinct chromatin state clusters after removing thymus-derived Treg cells (Fig. 3A). Next, we integrated scATAC-seq and scRNA-seq datasets to define the identity of each cell cluster, and linked pseudo-scRNA-seq expression values to each cell in the scATAC-seq data (Fig. 3B; Fig. S4A, D; scRNA-seq for integration analyses generated from the same experiments, see Methods). To perform integrative analysis, we first visualized the accessibility of cell cluster marker genes identified in scRNA-seq data shown in Fig. 1 by calculating gene scores, which is a measurement of the overall accessibility of the gene body and surrounding open chromatin regions (OCR) (Pliner et al., 2018). Second, we performed unconstrained integration of single-cell transcriptomic and chromatin accessibility profiles to calculate gene integration scores, yielding gene expression profiles at the single-cell level in the scATAC-seq defined UMAP space (see Methods). These analyses identified cells with Tfh (C4a and C5a), progenitor (C6a and C7a) and Teff (C2a and C3a) phenotypes; C6a and 7a were marked by intermediate accessibility of Tcf7 and Slamf6 and low to intermediate accessibility of Cxcr5 and Bcl6, corresponding to the putative Tprog cell clusters identified in Fig. 1 (Fig. 3C). Despite the overall epigenetic similarity between C6a and C7a, hierarchical clustering of differentially activated loci revealed increased accessibility in C6a at several loci related to T cell activation, including Cd69, Batf, Nr4a2, Irf4, and Tox (Fig. 3C; Fig. S4E, F). The result suggests that C7a and C6a correspond to the resting (C2r) and activated (C3r) Tprog cell clusters identified in Fig. 1, respectively. To further support these findings, we defined OCRs differentially accessible in each cluster and performed motif enrichment analysis, identifying several motifs associated with NFAT and AP-1 TF families in C6a compared to C7a (Fig. 3D). In contrast, Tfh cells showed high TF motif enrichment of TCF/LEF TF family members, while Teff cells showed high enrichment of TBX and RUNX motifs (Fig. 3D).
Figure 3. Chromatin accessibility analysis reveals heterogeneity and delineates the main CD4+ T cell differentiation pathways during chronic viral infection. (See also Figure S4 and Table S3).

(A) UMAP of scATAC-seq data of PD1+ CD4+ T cells on 21 dpi of LCMV-cl13 infection.
(B) UMAP of gene score (accessibility) and gene integration matrix (expression) for Cxcr5, Tcf7, Slamf6 and Tbx21.
(C) Heatmap visualization of marker gene accessibility scores across clusters represented by gene score values.
(D) Heatmap of marker peak scores of 5,227 cis-regulatory elements in scATAC-seq clusters (left). Heatmap of enriched motifs in marker peaks of the specific clusters (right).
(E) UMAP projection of Tfh and Teff differentiation trajectory, respectively. Cells that are not part of the trajectory are colored grey (top). Pseudotime heatmaps of motif deviation scores on the two differentiation trajectories (bottom).
(F) Volcano plot visualization of the differential peak analysis between the indicated clusters (FDR <= 0.1 and a Log2FC >= 0.5)
(G) Hockey plot representation of the enriched motifs under the cluster specific peak sets.
(H) Pseudotime analysis of the gene scores (accessibility) and gene integration scores (integrated expression values) of the indicated genes.
To further investigate the epigenetic dynamics between the Tprog, Teff, and Tfh populations, we constructed cellular trajectories for the transition to either terminally differentiated CD4+ subset, and analyzed changes in TF motif accessibility along the two paths (Fig. 3E, top). Tprog cells were clustered between the Tfh and Teff cells, both in scRNA-seq and scATAC-seq, and TCR-based analysis uncovered extensive clonal sharing of Teff and Tfh cells with this population (Figs. 1, 2). Therefore, we used Tprog cells as a starting point, and reconstructed two differentiation trajectories using a nearest-neighbor approach (sequential selection of similar chromatin states based on Euclidean distance, see Methods). As cells transitioned from C7a to C6a (representing the common path of the two trajectories), they gained accessibility at NFAT, BACH, and BATF/AP-1 motifs, representing the early chromatin remodeling events of Tprog commitment (Fig. 3E). After this common differentiation step, cells bifurcated into terminally differentiated Teff or Tfh phenotype. At these later stages of the trajectory, cells lost accessibility at common progenitor TF motifs and gained accessibility at Tfh-(TCF7, ASCL, CEBP, and MAF) (Liu et al., 2014; Shao et al., 2019; Tanaka et al., 2014; Wu et al., 2015), and Teff-related TF motifs (RUNX, TBX/EOMES) (Djuretic et al., 2007; Mullen et al., 2001; Naoe et al., 2007; Szabo et al., 2000)(Fig. 3E).
To quantify chromatin remodeling activities across the different phenotypic populations, we performed differential chromatin accessibility analysis between Tprog, Tfh and Th1 effector cells (Fig. 3F). Comparison of C7a and C6a Tprog cells identified 1,988 and 5,797 differentially accessible OCRs, while a comparison of C4a (Tfh) and C6a (Tprog) cells yielded 4,525 and 4,290 differential OCRs, respectively. Comparing C2a (Teff) to C6a (Tprog) cells identified 5,482 and 2,616 differentially accessible OCRs, respectively. Motif analyses at these specific OCRs confirmed the enrichment of NFAT and AP-1 binding motifs in C6a, compared to C7a (Fig. 3G), recapitulating the trajectory analysis. Enrichment of motifs for NFAT and AP-1 was lost during the early transition from Tprog to Teff in addition to loss of TCF/LEF-related motifs (Fig. 3G), which was consistent with loss of Tcf7 accessibility and expression (Fig. 3H). In contrast, the most significantly enriched motifs were associated with RUNX activity, which have been implicated in the commitment to the Th1 lineage (Djuretic et al., 2007; Naoe et al., 2007). During the transition from Tprog to Tfh cells, we detected a similar reduction of accessibility to NFAT and AP-1 motifs, while several motifs associated with the binding of basic helix-loop-helix (bHLH) proteins were enriched, which promote Tfh differentiation at the expense of Th1/Teff differentiation (Liu et al., 2014; Shaw et al., 2016) (Fig. S4G, H).
Altogether, these results suggest that clonally expanded CD4+ T cells adopt two distinct states of TCF-1+ phenotypes: resting (C2r/C7a) and activated (C3r/C6a) progenitor states. Resting memory-like cells are mobilized to terminal differentiation by transient TCR signaling, initially without polarization towards either lineage, and the specification of each terminal cell type is likely initiated after cells complete the transition to C6a through the activation of lineage-specific TF binding motifs. These two-step processes may be distinct from the initial differentiation towards either lineage as an immediate consequence of T cell activation during the initial priming of naive T cells.
TCF-1+ BCL6lo/− CD4+ T cells exhibit superior proliferative capacity and give rise to both TCF-1− Teff and TCF-1+ BCL6hi Tfh cells following adoptive transfer
To demonstrate the progenitor function of the TCF-1+ BCL6lo/− CD4+ T cells in vivo, we compared repopulation and differentiation capacities of the three major subsets of PD-1+ CD4+ T cells isolated from LCMV-c13 infected mice. We used Ly-108 and CXCR5 as surrogate surface markers for the expression of TCF-1 and BCL6 (Fig. S2G). Ly-108+ CXCR5− (enriched for TCF-1+ BCL6lo/− cells), Ly-108+ CXCR5+ (enriched for TCF-1+ BCL6hi cells), and Ly-108− CXCR5− (enriched for TCF-1− BCL6− cells) PD-1+ CD4+ T cells were harvested as donor cells from B6 mice that had been infected with LCMV-c13 for 21 days (Fig. S5A). They were adoptively transferred into infection-matched congenic B6-CD45.1 mice, followed by analyses for expansion and differentiation 15 days after transfer (36 dpi) (Fig. 4A–C). (Fig. 4; Fig. S5). Total and Tet+ Ly-108+ CXCR5− PD-1+ cells demonstrated the highest proliferation capacity with more than 10-fold expansion compared to Teff and Tfh donor cells (Fig. 4B–E). They gave rise to all three populations at a ratio similar to endogenous CD4+ T cells, further demonstrating their lineage plasticity and self-maintenance. Since the numbers of donor-derived cells 24 hour after transfer were comparable between Ly-108+ CXCR5− cells and Ly-108− Teff cells (Fig. S5B–D), we concluded that Teff cells are short-lived with limited expansion capacity. In contrast, the engraftment of Tfh cells was substantially lower compared to the other two populations, suggesting their impaired migration possibly due to impaired CXCR5 function caused by antibody staining during sort. While we detected small numbers of TCF-1+ BCL6lo/− cells in recipients of Teff or Tfh cells, it is likely that the observed phenotypic changes were caused by infrequent, contaminating memory-like cells during purification (Fig. 4E, F).
Figure 4. TCF-1+ BCL6lo/− PD-1+ CD4+ T cells are capable of superior expansion and differentiation into both Teff and Tfh cells following adoptive transfer. (See also Figure S5).
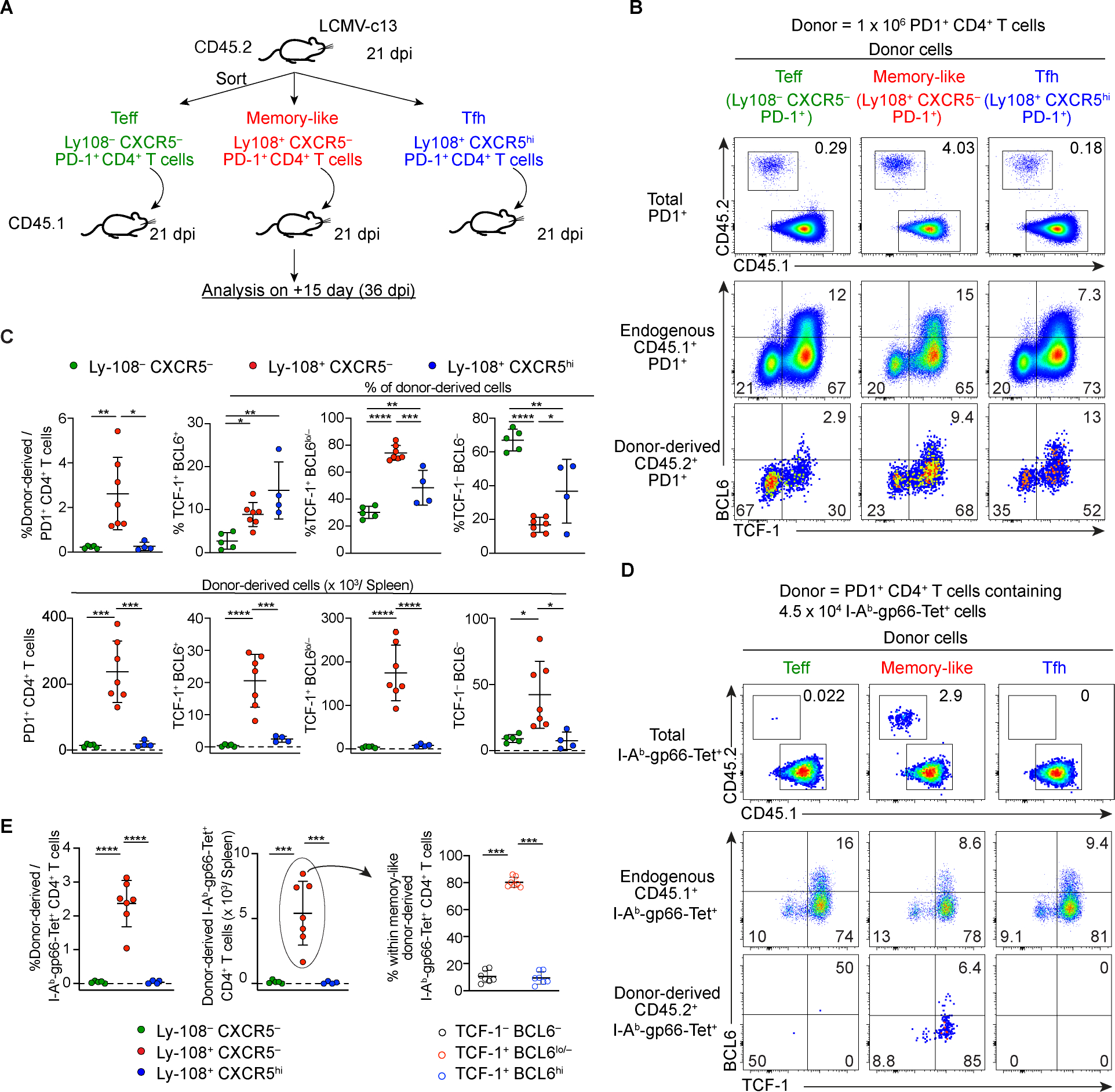
(A) Experimental design of adoptive transfer of distinct PD-1+ CD4+ T cell populations from LCMV-c13 infected mice to infection-matched recipient mice for the experiments shown in (B-E). For the experiments shown in (B) and (C), 1 × 106 PD-1+ CD4+ T cells were transferred. For the experiments shown in (D) and (E), 1–2 × 106 PD-1+ CD4+ T cells containing 4.5 × 104 Tet+ cells were transferred.
(B-E) Representative flow cytometry plots showing frequencies of total donor-derived cells (B) and Tet+ donor-derived cells (D) and their expression of TCF-1 and BCL6 15 days after transfer. Pooled data from 2 experiments with n = 2–4 / group / experiment are shown in (C, E) with mean±SD and statistical analysis by One-way ANOVA. Frequencies and total numbers of donor-derived cells in (B, C) were normalized to 1 × 106 transferred donor cells, and frequencies and total numbers of Tet+ donor-derived cells in (D, E) were normalized to 4.5 × 104 Tet+ transferred donor cells.
Together, these results demonstrated that the Ly-108+ CXCR5− PD-1+ CD4+ T cells retain high proliferative capacity and plasticity to differentiate into both TCF-1− Teff and TCF-1+ Tfh cells. These results suggest that they act as progenitor cells to maintain the pool of differentiated Teff and Tfh cells.
TCF-1+ BCL6lo/− PD-1+ CD4+ cells are progenitors that maintain TCF-1− effector cells
Our results thus far have shown that in the presence of chronic Ag, CD4+ T cells preferentially differentiate into TCF-1+ BCL6lo/− cells, which can give rise to TCF-1− Teff cells following adoptive transfer and thus function as progenitors for Ag-specific CD4+ T cells. To determine whether they are required for sustained Teff response, we conditionally deleted Bcl6 in a CD4+ T cell-specific manner. To achieve this, we generated a cre driver that facilitates deletion of a loxP-flanked gene in CD4+ T cells, with minimal impact on CD8+ T cells, by knocking-in cre into the Cd40lg locus on the X chromosome (Fig. S6A). This cre driver deleted a loxP-flanked transcriptional stop cassette in the Rosa26-stop-YFP allele in virtually all CD4+ T cells, whereas its activity in CD8+ T cells was substantially lower in naive, Arm- and c13-infected mice (Fig. S6B–G). YFP+ cells were barely detectable in B cells, myeloid cells, and NK cells (Fig. S6C, E). GC B cells were formed normally in Cd40lg-cre male mice (Fig. S6H). Comparable contribution of YFP+ and YFP− CD4+ T cells to Tfh cells in female Cd40lg-cre Rosa26-stop-YFP mice further indicates that the allele preserved the function of the Cd40lg gene (Fig. S6I).
Using this animal model, we investigated the role of Bcl6 in the maintenance of Ag-specific CD4+ T cell response. On 8 dpi with LCMV-c13, the number of Tet+ cells was comparable in Cd40lg-cre Bcl6F/F and Cd40lg-cre Bcl6+/+ mice (Fig. 5A, B). In Tet+ CD4+ T cells, TCF-1+ cells were absent in Cd40lg-cre Bcl6F/F mice while TCF-1− CXCR6+ Teff cells were unaffected (Fig. 5C, D), indicating that the development of both TCF-1+ BCL6hi Tfh and TCF-1+ BCL6lo/− cells requires Bcl6. In LCMV-Arm infected mice, Bcl6 deletion caused not only a loss of CXCR5+ PD1+ or Tet+ TCF-1+ BCL6hi Tfh cells but also a loss of TCF-1+ BCL6lo/− cells (Fig. S7A, B). The CD4+ Teff cells in c13-infected Cd40lg-cre Bcl6F/F and control mice expressed comparable levels of the inhibitory receptor TIM3 (Fig. S7C). Although Teff cell numbers were comparable between Cd40lg-cre Bcl6F/F and control mice on 8 dpi, Tet+ cells were reduced more than 10-fold by 15 dpi in Cd40lg-cre Bcl6F/F mice (Fig. 5C, D), indicating that the initially generated BCL6-independent Teff cells derived from naive CD4+ T cells are short-lived and that BCL6-dependent generation of TCF-1+ CD4+ T cells are essential for the maintenance of BCL6− TCF-1− Teff cells.
Figure 5. The maintenance of TCF-1− Teff cells requires BCL6-dependent, B cell-independent, TCF-1+ BCL6lo/− PD-1+ memory-like CD4+ T cells. (See also Figures S6 and S7).
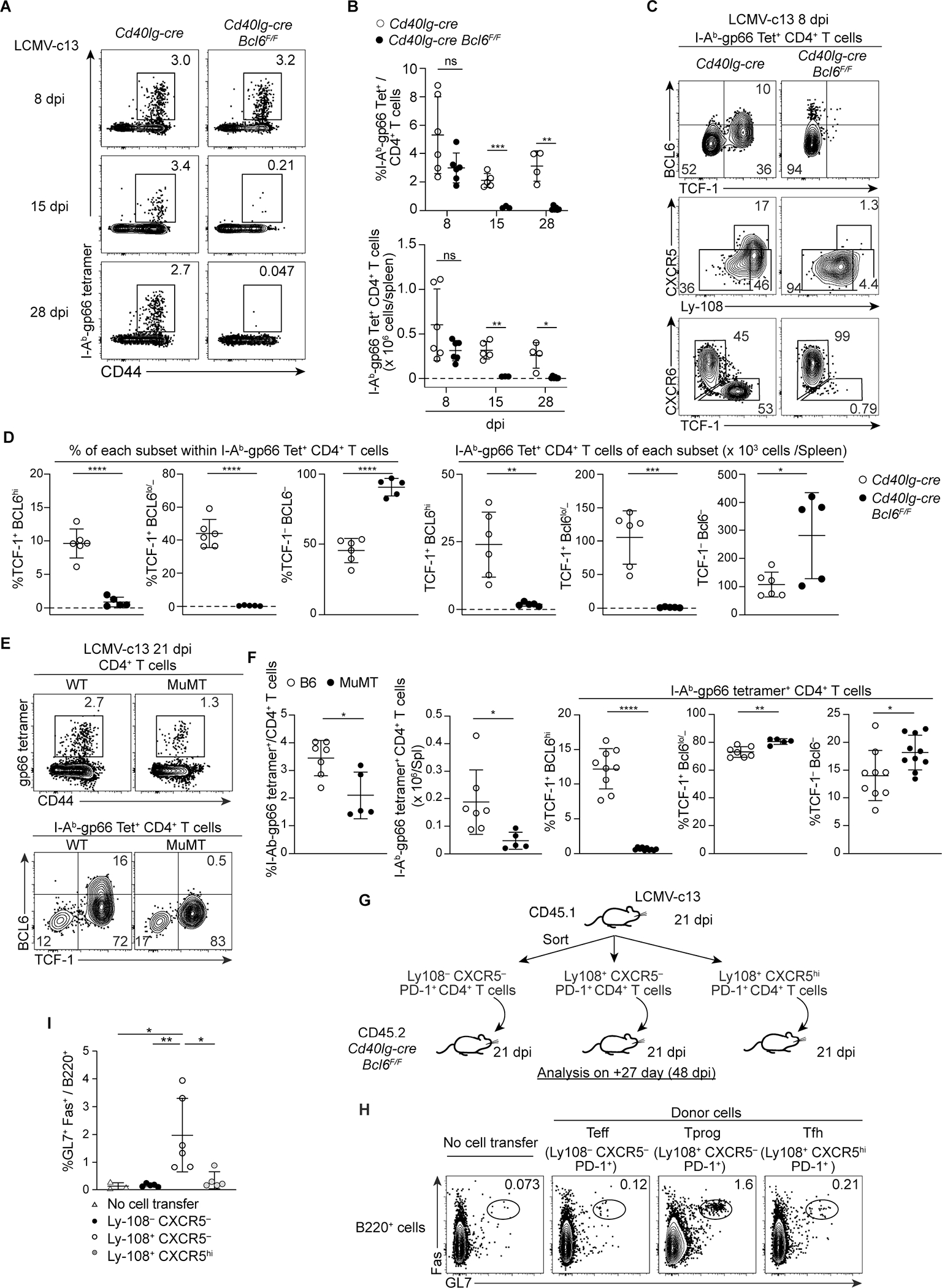
(A-B) Representative plots showing expression of CD44 and gp66-specific TCR in splenic CD4+ T cells in Cd40lg-cre Bcl6F/F and control Cd40lg-cre mice infected with LCMV-c13, analyzed on 8 dpi, 15 dpi or 28 dpi. Pooled data from 2 experiments with 2–3 mice/group/time point are shown with mean±SD. Unpaired t-test.
(C-D) Expression of TCF-1, BCL6, Ly-108, CXCR5 and CXCR6 in gp66-specific splenic CD4+ T cells from LCMV-c13-infected Cd40lg-cre Bcl6F/F and control Cd40lg-cre mice on 8 dpi. Data pooled from two experiments with n = 2–4 / genotype shown with mean±SD.
(E-F) Expression of TCF-1, BCL6 and CXCR6 in gp66-specific splenic CD4+ T cells from LCMV-c13-infected MuMT and age-matched B6 (WT) mice on 21 dpi. Data from two experiments with n = 2–5 / genotype / experiment shown with mean±SD.
(G-I) Adoptive transfer of distinct PD-1+ CD4+ T cell populations from LCMV-c13 infected B6-CD45.1 mice to infection-matched Cd40lg-cre Bcl6F/F recipients (CD45.2).
Experimental design (G): adoptive transfer of 0.7–1 × 106 cells of donor PD-1+ CD4+ T cells to infection-matched Cd40lg-cre Bcl6F/F recipients. 27 days after transfer, frequencies of splenic GL7+ Fas+ B cells in recipient mice were analyzed. Representative plots (H) and pooled data from 2 experiments (n = 2–4 / group / experiment) shown in (I) with mean±SD with statistical analysis by One-way ANOVA.
The TF Blimp1 plays antagonistic roles to BCL6 in the differentiation of Tfh versus Teff CD4+ T cells, memory versus effector CD8+ T cells, and memory B versus plasma cells (Kallies et al., 2009; Nutt et al., 2007; Rutishauser et al., 2009; Shaffer et al., 2002; Shao et al., 2019; Shapiro-Shelef et al., 2003; Welsh, 2009; Wu et al., 2015). In Cd40lg-cre Prdm1F/F mice, expansion of Tet+ CD4+ T cells was increased by 3-fold compared to control mice on 8 dpi with c13 (Fig. S7D, E). Aside from greater expansion, the vast majority of Tet+ CD4+ T cells in Cd40lg-cre Prdm1F/F mice were TCF-1+. Thus, Blimp1 not only regulates the expansion of Ag-specific CD4+ T cells, but also promotes the differentiation of TCF-1+ into TCF-1− CD4+ T cells.
To determine whether TCF-1+ BCL6hi or Ly-108+ CXCR5+ Tfh cells are necessary for the development of TCF-1+ BCL6lo/− cells or the maintenance of TCF-1− Teff cells, we analyzed MuMT mice lacking TCF-1+ BCL6hi Tfh cells. Although total Tet+ cells were mildly diminished on 21 dpi, the frequency of TCF-1+ BCL6lo/− cells was comparable to control mice (Fig. 5E, F), indicating that BCL6hi CXCR5hi cells are dispensable for the maintenance of Ag-specific CD4+ T cells.
Transferred Tprog cells are capable of supporting continued B cell responses
Furthermore, to determine whether Tprog cells are sufficient to support CD4+ T cell-dependent immune response, we adoptively transferred Ly-108+ CXCR5− PD-1+ CD4+ T cells to infection-matched Cd40lg-cre Bcl6F/F mice on 21 dpi, in which host-derived LCMV-specific CD4+ T cells were severely reduced (Fig. 5G–I). Four weeks after the transfer, the numbers of GL7+ Fas+ B cells, enriched for GC B cells, were increased by 10-fold in recipients of Ly-108+ CXCR5− CD4+ T cells compared to those of either of the two other subsets, indicating that transferred Tprog cells are sufficient to provide help to B cells. Taken together, our results establish Bcl6 as a critical regulator of the differentiation of TCF-1+ BCL6lo/− Tprog cells, which are indispensable for long-term, Ag-specific CD4+ T cell responses during chronic viral infection.
Finally, to gain insights into the contribution of Tprog or Tfh cells to the maintenance of IL-21-dependent CX3CR1+ exhausted CD8+ T cells (Raju et al., 2021; Zander et al., 2019), we examined LCMV-specific CD8+ T cells in LCMV-c13-infected MuMT mice lacking BCL6+ Tfh cells and Cd40lg-cre Bcl6F/F mice in which Ag-specific CD4+ T cells are severely reduced in the chronic phase of LCMV-c13 infection. Although CD4+ T cell depletion prior to infection almost completely depletes CX3CR1+ PD-1+ CD8+ T cells, frequencies of CX3CR1+ TCF-1− cells in gp33-specific CD8+ T cells were only mildly reduced in both MuMT and Cd40lg-cre Bcl6F/F mice (Fig. 6). Although the observed reduction confirms that Ag-specific CD4+ T cells, or more specifically BCL6+ Tfh cells, play roles in the maintenance of CX3CR1+ exhausted CD8+ T cells, these results suggest the presence of alternative sources of IL-21 that depend on CD4+ T cells specifically in early phases of infection.
Figure 6. IL-21-dependent CX3CR1+ exhausted CD8+ T cells in LCMV-c13-infected mice are maintained in the absence of Bcl6 in CD4+ T cells. (See also Figure S6).
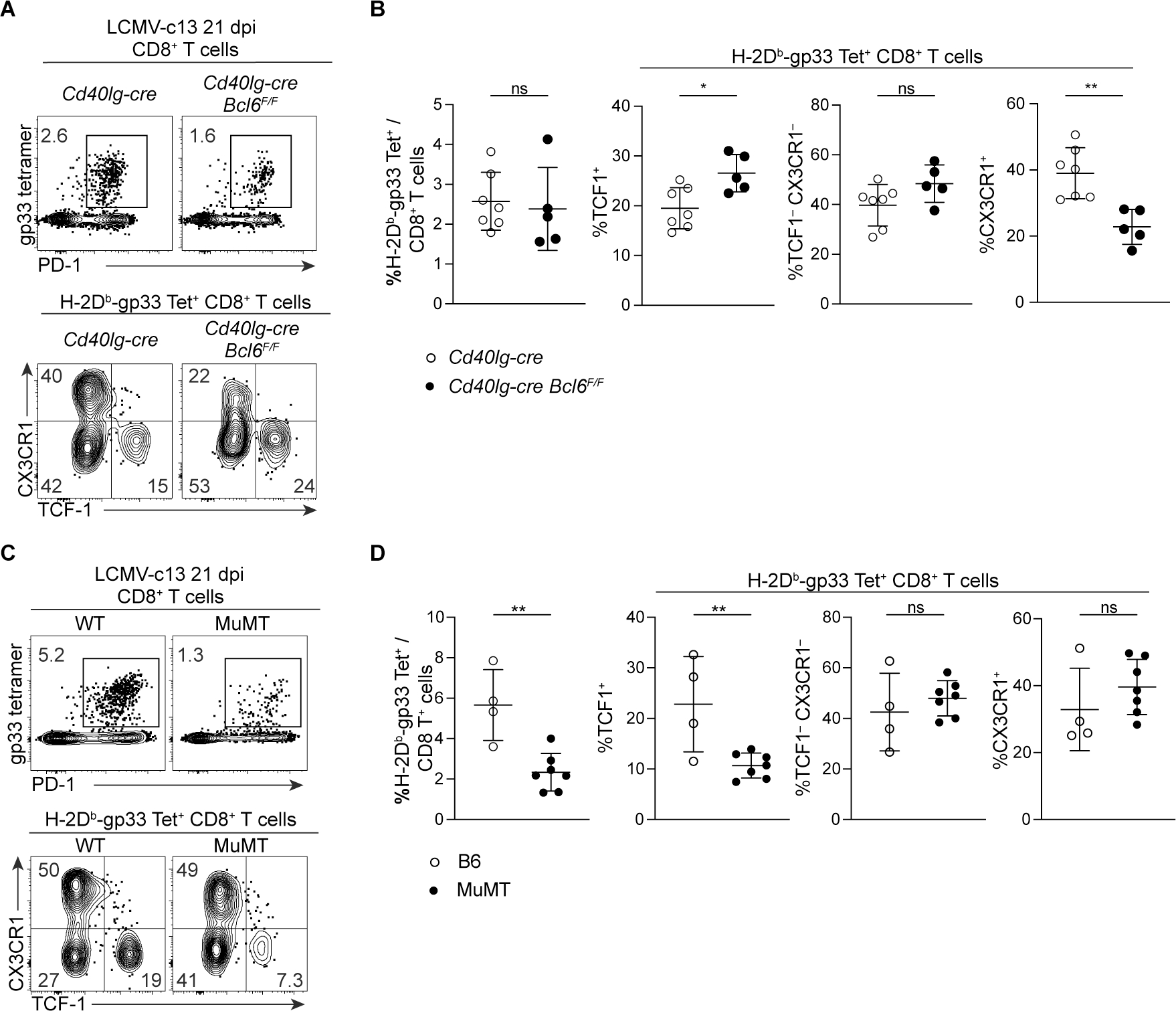
(A, B) Expression of PD-1, TCF-1 and CX3CR1 in splenic LCMV-gp33-specific CD8+ T cells from LCMV-c13-infected MuMT mice on 21 dpi. Representative plots (A) and pooled data from 2 experiments with n = 2–3 / genotype /experiment shown with mean±SD. Unpaired t-test.
(C, D) Expression of PD-1, TCF-1 and CX3CR1 in splenic LCMV-gp33-specific CD8+ T cells from LCMV-c13-infected Cd40lg-cre Bcl6F/F mice on 21 dpi. Representative plots (C) and pooled data from 2 experiments with n = 2–3 / genotype /experiment shown with mean±SD. Unpaired t-test.
Tumor Ag-specific CD4+ T cells differentiate predominantly into TCF-1+ BCL6lo/− cells in tumor-draining lymph nodes
To determine whether a similar progenitor population supports sustained Ag-specific CD4+ T cell responses during anti-tumor immune responses, we examined the differentiation and proliferation of OT-II CD4+ T cells in response to the subcutaneously transplanted OVA expressing 1956 sarcoma cell line (Ferris et al., 2020). We adoptively transferred CFSE-labeled, CD45.1/2 OT-II CD4+ T cells one day prior to tumor inoculation and tracked their proliferation by CFSE dilution and differentiation by staining for TCF-1 and BCL6 in the tumor and tdLNs (Fig. 7A). In tdLNs, OT-II T cells differentiated into all three populations defined by TCF-1 and BCL6 expression (Fig. 7B, C). As we observed in LCMV-c13 infected mice, the frequency of the TCF-1+ BCL6lo/− cells was the highest among expanded OT-II cells. Although TCF-1− Teff, BCL6+ Tfh and the majority of TCF-1+ BCL6lo/− cells fully diluted CFSE over several days, approximately 10% of TCF-1+ BCL6lo/− cells underwent limited rounds of division and expressed higher levels of BCL6 compared to fully divided TCF-1+ cells (Fig. 7D–F). Thus, the tumor-reacting TCF-1+ BCL6lo/− cells were heterogeneous, ranging from BCL6int/hi PD-1lo dormant cells to extensively divided BCL6lo/− PD-1+ cells, which resembled Tprog in LCMV-infected mice. In contrast to cells in tdLNs, OT-II cells isolated from the tumors were predominantly TCF-1− CXCR6+ Teff cells (Fig. 7B, C). Since a small fraction of extensively divided OT-II T cells in tdLN turned on CXCR6 expression before reduced the expression of TCF-1 (Fig. 7G), tumor-reactive CD4+ T cells likely initiate Teff differentiation prior to migration to the tumor microenvironment. These results suggest that in the setting of chronic Ag stimulation, rather than binary differentiation to TCF-1− BCL6− or TCF-1+ BCL6+ populations, CD4+ T cells preferentially differentiate into TCF-1+ BCL6lo/− Tprog cells, which may continuously generate fully differentiated Teff and Tfh cells as Ag persists. These results also suggest that such continued differentiation mainly takes place in tdLNs rather than in tumors, possibly because only the tdLNs contain the appropriate microenvironment to support TCF-1+ BCL6lo/− cells.
Figure 7. Antigen-specific CD4+ T cells differentiate into TCF-1+ PD-1+ cells following cell division in tumor-draining lymph nodes, but not in the tumor microenvironments.
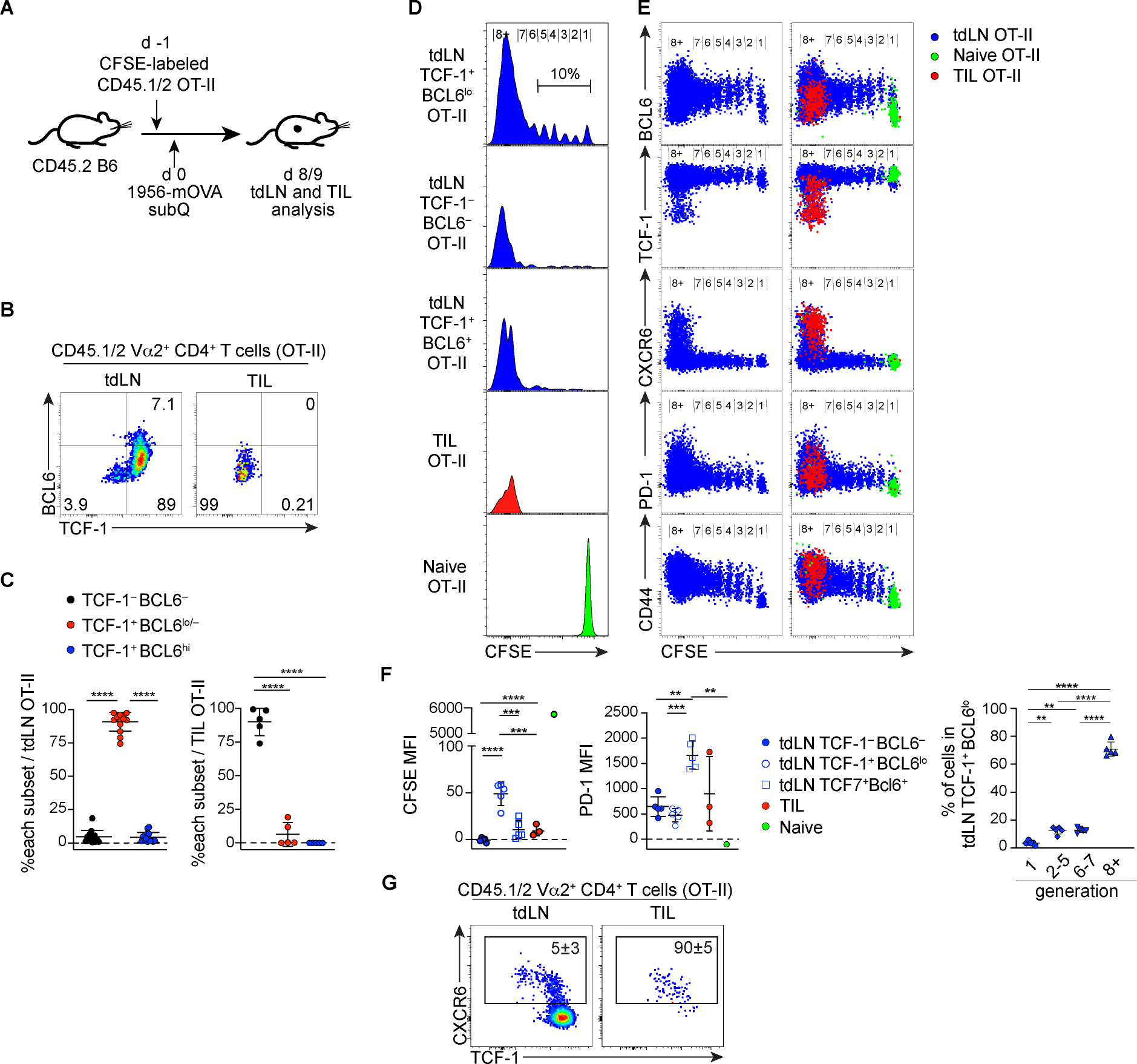
(A) Experiment design to analyze CD4+ T cell response to tumor antigen.
(B-C) Representative plots showing expression of TCF-1 and BCL6 by donor-derived OT-II CD4+ T cells harvested from tumor (TIL) and the tumor draining lymph node (tdLN). Data pooled from two experiments (n = 6 – 8 / experiment) and shown with mean±SD.
(D-F) CFSE dilution and expression of BCL6, TCF-1, CXCR6, PD-1 and CD44 by donor-derived OT-II cells. The CFSE level of naive OT-II cells was determined by recipient mice without tumor transplantation sacrificed at the same time points. Left panels in (E) show tdLN-derived OT-II cells without overlay of TIL or naive cells. Pooled data are shown with mean±SD in (F) with assessment of statistical differences by one-way ANOVA.
(G) Representative plots showing expression of CXCR6, TCF-1 and BCL6 by donor-derived OT-II CD4+ T cells harvested from tdLN and the tumor. Numbers show mean±SD.
Discussion
Using a combination of single-cell genomics and population-based experiments in the chronic LCMV-c13 model, we demonstrate that Ag-specific CD4+ T cells differentiated into TCF-1+ BCL6lo/− PD-1+ CD4+ Tprog cells that continued to give rise to Teff and Tfh cells in response to persistent Ag. During an acute infection or in response to vaccination, significant proportions of Teff and Tfh cells are derived directly from naive CD4+ T cells (Choi et al., 2011; DiToro et al., 2018). In contrast, CD4+ T cell response to chronic Ag was more complicated and required progenitor cells that can support long-term Teff responses as the initial Teff cells decay. This process was illustrated in our Cd40lg-cre Bcl6F/F model, where the initial Teff cell expansion from naive cells was intact, but Teff cells failed to persist due to a lack of Tprog cells. Thus, the developmental pathway of CD4+ Teff cells was shifted from BCL6-independent to -dependent as Ag persisted.
The CD4+ Tprog cells identified in this study exhibited similarities to CD4+ Tcm, Tcmp, and pre-Tfh cells that also express Tcf7, Slamf6, and Bcl2, which allows for their long-term survival, in contrast to differentiated Teff and Tfh cells (Choi et al., 2011; Ciucci et al., 2019; Pepper and Jenkins, 2011). Shortly after activation, ranging from several hours to a few days, CD4+ T cells diverge into IL2Rα+ Blimp1+ Teff cells and IL2Rα− BCL6+ cells in a B cell-independent manner (Baumjohann et al., 2011; Choi et al., 2011). These IL2Rα− BCL6+ cells contain Tcmp and pre-Tfh cells (Choi et al., 2013). This binary fate choice between Teff and Tfh lineages is stable, since differentiation of adoptively transferred IL2Rα− BCL6+cells is substantially biased towards the Tfh fate, and the depletion of these early IL2Rα− BCL6+ cells leads to a significant reduction of Tfh cells (Choi et al., 2013; DiToro et al., 2018). In the contexts of acute infection or immunization with non-replicating Ag, both Ag levels and inflammation wane by the time pre-Tfh or Tcmp cells develop. In contrast, following LCMV-c13 infection, Ag levels remain high for the first several days, rendering TCF-1+ PD-1+ CD4+ T cells that might otherwise become pre-Tfh or Tcmp cells are exposed to continued signals through TCR and other receptors. Such continued stimulation may result not only in changes in surface marker expression, such as increased PD-1 and reduced CCR7 and CXCR5 compared to Tcmp (Ciucci et al., 2019), but also epigenetic changes that preserve Teff differentiation, although the newly defined Tprog population was heterogeneous and may contain or overlap with the committed pre-Tfh populations with a similar surface phenotype.
Our single-cell analysis revealed two states of cells in the progenitor pool with distinct activation signatures. The transition between the two states was associated with NFAT- and AP-1-target gene activation; increased chromatin accessibility of these TF binding motifs was the dominant change between the two states. Trajectories between the resting progenitor state to Tfh or Teff lineages using both transcriptomic and epigenetic analyses suggested that the specification to either lineage occurs independently of the initial transition from the resting to activated states. Activation of bHLH-protein targets, which may be mediated by E2A and ASCL2 (Liu et al., 2014; Shaw et al., 2016), in the progenitor-Tfh trajectory and activation of RUNX targets for the Teff trajectory (Djuretic et al., 2007; Naoe et al., 2007) were detected after CD4+ Tprog cells acquired transient NFAT and AP-1 activation. Although a stringent validation requires fate tracing at the single cell level, this conclusion was also supported by the substantially overlapping TCR clonality among resting and activated Tprog, Tfh and Teff populations, while Teff and Tfh cells are derived from distinct clones in the context of acute infection (Khatun et al. 2021). The activated Tprog cells may overlap with previously described, BCL6-dependent PD-1+ CD4+ T cells that develop during M. tuberculosis infection (Moguche et al., 2015).
Finally, our study highlighted the similarities between CD4+ Tprog and CD8+ TPEX cells. Both cell types developed in the presence of persistent Ag, expressed a shared transcriptional program, including Tcf7, Slamf6, and Pdcd1, and continued generating differentiated effectors while they self-renew. Although these cell types resemble CD4+ Tcmp and CD8+ MPEC, respectively, they have unique gene expression and epigenetic signatures, such as those associated with activation. In addition, we observed the emergence of PD-1+ CD4+ T cells in tumor-bearing mice, which phenocopied progenitor CD4+ T cells in response to LCMV infection. Many studies have demonstrated the importance of CD8+ TPEX in enabling durable anti-tumor immunity and the immune response to ICB therapies. CD8+ TPEX cells are found in tumors and extratumoral tissues, such as lymph nodes. In mouse tumor transplantation models, intratumoral CD8+ TPEX cells are sufficient to promote anti-tumor immunity in response to vaccine or ICB (Siddiqui et al., 2019). In our tumor transplantation experiments, we found tumor-reactive TCF-1+ PD-1+ CD4+ T cells exclusively in the tdLNs while almost all Ag-specific CD4+ T cells in the tumor were differentiated TCF-1− Teff cells. In the LNs, a small fraction of tumor-reactive TCF-1+ CD4+ T cells initiated Teff differentiation as indicated by elevated CXCR6 expression. CD4+ T cell-dependent anti-tumor immunity requires expression of BCL6 in tumor-specific CD4+ T cells and is enhanced by the presence of cognate B cells (Cui et al., 2021). The abundance of non-Teff CD4+ T cells suggested that such interaction between CD4+ T and B cells may occur in tdLNs, and in turn promote anti-tumor immunity in multiple mechanisms, including promoting CD4+ Teff differentiation and establishing an IL-21-rich environment.
A recent study demonstrated the presence of a CXCR5− CXCR6− memory-like CD4+ T cell subset during chronic LCMV infection (Zander et al., 2022). This study showed that Tfh cells derived from the memory-like cells are an essential source of IL-21 to sustain CX3CR1+ exhausted CD8+ T cells. It is likely that the described memory-like CD4+ T cell population overlaps with Tprog cells defined in the current work. However, the extent to which CXCR5+ Tfh cells are the predominant source of IL-21, given that both MuMT and Cd40lg-cre Bcl6F/F mice largely maintain CX3CR1+ exhausted CD8+ T cells despite a specific lack of Tfh cells and a substantial loss of total Ag-specific CD4+ T cells, should be further investigated.
In summary, our analysis decoded the heterogeneity of CD4+ T cells that respond to persistent Ag in the context of antiviral and anti-tumor immunity and highlighted a population of TCF-1+ BCL6lo/− PD-1+ CD4+ T cells as progenitor cells that support the continued generation of differentiated effectors and helper CD4+ T cells. CD4+ Tprog cells went through a transitory state in which they retained an unbiased epigenetic signature towards either terminal fate, and subsequently resolved the bipotential states, which was distinct from the binary differentiation of naive CD4+ T cells into Teff and Tfh lineages early in an immune response. Our results revealed the population dynamics and differentiation hierarchy of CD4+ T cells for their sustained responses to persistent Ag.
LIMITATIONS OF THE STUDY
The mouse LCMV infection models have been extensively used to study immune responses to chronic antigen stimulation with many findings later confirmed to apply to anti-tumor immunity in both mice and humans. However, rigorous validation of the current findings is needed using multiple infection or disease models in both species to determine whether the proposed CD4+ T cell differentiation pattern serves as a general mechanism, since tumors in humans persist for substantially longer duration than that in mouse tumors and anti-tumor immunity could vary across different tissue types.
STAR METHODS
Resource availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Takeshi Egawa (egawat@wustl.edu).
Materials availability
Cd40lg-cre mice will be made available upon request and completion of a material transfer agreement.
Data and Code availability
All single-cell sequencing data generated in this paper have been deposited in the Gene Expression Omnibus (GEO) database under accession number GSE181474. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
In vivo animal models
Male C57BL/6N (B6) and B6-CD45.1 mice were purchased from Charles River Laboratories and JAX. Prdm1-EYFP, Bcl6-flox mice were obtained from the Jackson Laboratory. Cd40lg-cre mice were generated by knocking in a mammalian codon optimized cre coding sequence following an internal ribosomal entry sequence (IRES) into 3’ UTR of the Cd40lg locus by homologous recombination in JM8.N4 embryonic stem cells. After germline transmission, the FRT-flanked selection cassette was removed by crossing to Actb-Flpe transgenic mice (JAX). Because the Cd40lg locus is on X-chromosome, all female mice used in the Bcl6 deletion experiments were homozygous for cre knock-in. All mice were housed in a specific pathogen-free facility at Washington University in St. Louis and were used for infection at 8–12 wk of age, unless stated otherwise. LCMV infection was performed essentially as described (Chou et al., 2016). All experiments were performed according to a protocol approved by Washington University’s Institutional Animal Care and Use Committee.
Lymphocytic choriomeningitis virus (LCMV) infection
LCMV-Armstrong and clone 13 (LCMV-Arm and -c13) were propagated in BHK cells and viral titers were determined by a plaque forming assay using Vero cells. 2 × 105 plaque-forming units (PFU) of LCMV-Arm or 2 × 106 PFU of LCMV-c13 were inoculated into mice via intraperitoneal or intravenous route, respectively.
Tumor transplantation model
500,000 CFSE-labeled CD45.1/2 OT-II cells were intravenously transferred into B6 mice, which were subcutaneously inoculated with 1 million 1956-mOVA cells (Ferris et al., 2020) the next day. Tumor infiltrating lymphocytes (TILs) and cells in the tdLN were harvested 8 to 9 days after inoculation. LN cells were prepared by manual dissociation with frosted glass slides and TILs were prepared by digestion with Collagenase B, D1, and Dnase I, followed by staining with fluorescently labeled antibodies.
Method details
scRNA-seq and TCR-seq sample and library generation
Single-cell RNA-seq libraries were prepared using the 10X Chromium Next Gem Single Cell V(D)J Reagent Kit (v1.1 Chemistry), according to the manufacturer’s instructions. Briefly, FACS sorted cells were washed once with PBS + 0.04% BSA and resuspended in PBS containing 0.04% BSA. Following reverse transcription and cell barcoding in droplets, emulsions were broken, and cDNA purified using Dynabeads MyOne SILANE followed by PCR amplification (98°C for 45 sec; 14 cycles of 98°C for 20 sec, 67°C for 30 sec, 72°C for 1 min; 72°C for 1 min). Amplified cDNA was then used for both 5′ gene expression library construction and TCR enrichment. For gene expression library construction, 50 ng of amplified cDNA was used for fragmentation, following by and end-repair, double-sided size selection with SPRIselect beads, PCR amplification with sample indexing primers (98°C for 45 sec; 14 cycles of 98°C for 20 sec, 54°C for 30 sec, 72°C for 20 sec; 72°C for 1 min), and double-sided size selection with SPRIselect beads. For TCR library construction, TCR transcripts were enriched from 2 μl of amplified cDNA by PCR (primer sets 1 and 2: 98 °C for 45 s; 10 cycles of 98 °C for 20 s, 67 °C for 30 s, 72 °C for 1 min; 72 °C for 1 min). Following TCR enrichment, 5 – 50 ng of enriched PCR product was fragmented and end-repaired, size-selected with SPRIselect beads, PCR-amplified with sample-indexing primers (98 °C for 45 s; 9 cycles of 98 °C for 20 s, 54 °C for 30 s, 72 °C for 20 s; 72 °C for 1 min), and size-selected with SPRIselect beads. scRNA/TCR-seq libraries were quantified using a Qubit dsDNA HS Assay kit (Invitrogen) and a HighSensitivity DNA chip run on a Bioanalyzer 2100 system (Agilent). Sequencing was performed on NovaSeq S4 (Illumina) with paired-end reads (2 × 150 cycles).
scRNA-seq and TCR-seq library processing
Reads from 10x scRNA expression libraries were aligned to mouse genome assembly GRCm38 (mm10) and quantified using cellranger count (10x Genomics, version 3.1.0). The filtered feature-barcode matrices containing only cellular barcodes were used for further analysis. Single cell gene expression matrices were imported into R (version 3.6.1) and analyzed using Seurat (version 3.1.1)(Stuart et al., 2019). Cells for which the number of genes captured fell within two standard deviations of the mean of all cells in the library were kept. Additionally, cells with mitochondrial RNA content percentages above the 95th-percentile were excluded from subsequent analyses.
Single cell TCR reads were aligned to mouse genome assembly GRCm38 (mm10) and assembled into reconstructed TCR consensus sequences using cellranger vdj (10x Genomics, version 3.1.0). Only productive TCRα and TCRβ sequences were considered for further analysis. Overall, TCR sequences were annotated for 80,785 cells that passed RNA quality filtering, with paired TCR αβ sequences detected for 71,818 cells (89.0%). Only cells with conventional paired TCR chain combinations αβ or ααβ were kept for downstream clonotype analyses. Cells sharing the same CDR3αβ amino acid sequences were defined as belonging to the same TCR clone.
scRNA-seq data integration and clustering
scRNA-seq libraries of I-Ab-LCMV-gp66 tetramer-sorted CD4+ T cell populations from LCMV-Arm and LCMV-c13 at 8 dpi and 21 dpi were normalized individually while regressing out cell cycle score. Cells passing quality filtering (80,785 cells) from all 19 samples (Table S1, Fig. S1B–C) were then integrated by identifying anchors between datasets using 30 reciprocal PCA dimensions. TCR genes were excluded from the selection of integration anchors to prevent TCR chain driven biases. Dimensionality reduction of the integrated matrix was performed using Uniform Manifold Approximation and Projection (UMAP) with the first 15 principal components. Phenotypic clusters were defined by constructing a k-nearest neighbors graph and identifying groups of cells using the Louvain algorithm with resolution of 0.5.
CD8+ T cell gene signature scoring
To compare the CD4+ T cell populations in LCMV infection identified by scRNA-seq to previously described CD8+ T cell populations, we computed gene signature scores for each cell with Seurat’s AddModuleScore() function (Stuart et al., 2019) using gene signatures of CD8+ T cell subsets in LCMV-c13 infection from Daniel et al., 2021 (Daniel et al., 2021). Mitochondrial, TCR, and BCR genes were removed from all gene sets in order to prevent biases due to library quality or TCR clonal composition. Scores were then averaged per CD4+ T cell cluster to generate a mean composite signature score for each CD4+ T cell population.
Trajectory inference
To perform trajectory analysis of the CD4+ T cell response in LCMV-c13 infection, dimensionality reduction of I-Ab-LCMV-gp66 Tet+ cells on 8 dpi (2 samples) and 21 dpi (4 samples) was performed using UMAP as described above after excluding cells belonging to the Treg clusters C8-10r and other infrequent populations (C12-15r). Pseudotime analysis was then performed with Monocle 3 (Cao et al., 2019) by learning a principal graph for the data and ordering cells along the graph using the cells in the naive phenotype cluster to select a root node.
scATAC-seq sample and library generation
Single cell ATAC-seq dataset is obtained from two biological replicates. Experiments were performed on the 10x Chromium platform as described previously(Satpathy et al., 2019). Briefly, following sorting, cells were subjected to nuclei isolation according to the manufacturer’s recommendation. After tagmentation, nuclei were processed for generating scATAC-seq libraries and loaded to the 10x Chromium controller. For GEM incubation the standard thermocycler conditions were used and library construction was done as described by 10x Genomics for scATAC-seq. Libraries were quantified using a Qubit dsDNA HS Assay kit (Invitrogen) and a HighSensitivity DNA chip run on a Bioanalyzer 2100 system (Agilent). Sequencing was performed on NovaSeq S4 (Illumina) with paired-end reads (2 × 150 cycles), and demultiplexed using CellRanger-ATAC v1.2.
scATAC-seq analysis
scATAC-seq datasets were processed as described previously (Granja et al., 2021). Briefly, reads were filtered, trimmed, and aligned to the mm10 reference genome using the 10x cellranger atac-count pipeline. Fragment files were loaded into ArchR for additional processing and analysis. Doublets were identified and removed using ArchR’s default doublet simulation and calling procedures. Barcodes were removed that had an enrichment of Tn5 insertions in transcription start sites (TSS enrichment) less than 4 or less than 1000 fragments. Tiles and GeneScores matrices were computed by summing Tn5 insertions in predefined genomic windows. After clustering the cells, peaks were called by macs2 (Zhang et al., 2008) on pseudoreplicates sampled from each cluster to obtain a reproducible peak set retaining cell type specific peaks. scATAC-seq and scRNA-seq datasets were integrated using the ArchR addGeneIntegrationMatrix() function, which directly aligns cells from scATAC-seq with cells from scRNA-seq by comparing the scATAC-seq gene score matrix with the scRNA-seq gene expression matrix. For each cell in the scATAC-seq data, this integration process finds the cell in the scRNA-seq data that looks most similar and assigns the gene expression data from that scRNA-seq cell to the scATAC-seq cell. Dynamics of scRNA-seq expression data can then be computed using this paired cell matrix. TF motif deviations were computed using chromVar (Schep et al., 2017). Imputation was performed using Magic (van Dijk et al., 2018). scATAC-seq dataset was obtained from two biological replicates, integrated with scRNA-seq dataset (two biological replicates, 1456 and 1746 cells respectively) from the same animals. For these experiments, PD-1+ CD4+ splenocytes were sorted and used.
Cell preparation, cell staining, and flow cytometry
Single-cell suspensions of splenocytes were prepared by manual disruption with frosted glass slides. Bone marrow cells were dissociated from the femur with mortar and pestle. Liver cells were prepared by manual disruption with frosted glass slides followed by gradient centrifugation with 40% Percoll. Absolute live cell counts were determined by trypan blue exclusion using Vi-CELL (Beckman Coulter). PE- or APC-labeled I-Ab-LCMV-gp66 tetramer reagents were obtained from the NIH Tetramer Core at Emory and PE- or APC-labeled H2-Db-LCMV-gp33 tetramer reagents were obtained from MBL (TB-M512-1). Total splenocytes were stained with MHC-tetramers at room temperature for 60 min. For analysis of T cells in the liver and lung, circulating T cells were excluded by staining for biotinylated anti-CD3e that was intravenously injected (3 ug) 3 minutes before euthanasia. For intracellular cytokine staining, total splenocytes were cultured in RPMI-1640 supplemented with 10% FBS in the presence of 1 ug/ml of LCMV-gp61-80 peptide (GenScript Biotech) and 5 ug/ml of Brefeldin A (Biolegend) for 4 h before staining. Cells for intracellular staining were staining for surface proteins and then labeled with LIVE/DEAD Aqua (Thermo Fisher Scientific) before fixation and intracellular staining using the Foxp3 staining kit (eBioscience) according to the manufacturer’s instructions.
The following antibodies were purchased from Biolegend, unless otherwise indicated: Alexa Fluor (AF)-conjugated donkey polyclonal anti-rabbit IgG (Thermo Fisher Scientific, catalog no. R37118); AF647–conjugated goat polyclonal anti-rabbit IgG (Cell Signaling, catalog no. 4414S); AF700-conjugated anti-CD44 (IM7); APC-conjugated anti-PD-1 (29F.1A12); APC-Cy7-conjugated anti-CD8a (53-6.7, BD Biosciences); BV421- or BV711-conjugated anti-CXCR5 (L138D7); Biotinylated anti-CD3e (145-2C11); PE-conjugated anti-GL7 (GL7); PerCP-Cy5.5-conjugated anti-CD4 (GK1.5); BUV395-conjugated anti-CD4 (GK1.5, BD Biosciences); PerCP-Cy5.5-conjugated anti-CD45.1 (A20), A700-conjugated anti-CD45.2 (104); PerCP-Cy5.5-conjugated anti-Ki67 (B56); PE-Cy7-conjugated anti-PD-1 (29F.1A12), PE-Cy7-conjugated anti-CD95 (Fas)(Jo2); PE-Dazzle 594-conjugated anti-B220 (RA3-6B2), PE-Dazzle594-conjugated anti-CXCR6 (SA051D1); BV605-conjugated anti-Ly-108 (13G3, BD Biosciences); BV650-conjugated anti-CD150 (SLAM)(TC15-12F12.2); FITC-conjugated anti-CD69 (H1.2F3); BV605-conjugated anti-CX3CR1 (SA011F11); PE-Cy7-conjugated anti-LAG3 (C9B7W); BV421- or APC-conjugated anti-Tim-3 (RMT3-23); eFluor660-conjugated anti-TOX (TXRX10, Thermofisher); unconjugated anti-TCF-1 (Cell Signaling Technology, C63D9), BV421-conjugated anti-BCL6 (K112-91); PE-conjugated anti-IFN-𝛄 (XMG1.2); APC-conjugated anti-IL-2 (JES6-5H4, BD Pharmingen); and APC-conjugated TNF (MP6-XT22). Stained samples were analyzed with BD FACS LSR Fortessa, X20, or Symphony A3 or sorted on Aria II or III. Data were analyzed using FlowJo Software (FlowJo).
Adoptive Transfer
CD4+ T cells from the spleen and peripheral lymph nodes of B6 (CD45.2) mice on 21 dpi with LCMV-c13 were harvested and enriched for CD4+ T cells using a MojoSort Mouse CD4 T Cell Isolation Kit (Biolegend) prior to surface staining. 1–2 million Ly-108− CXCR5−, Ly-108+ CXCR5−, or Ly-108+ CXCR5+ PD-1+ CD4+ T cells were transferred into infection-matched CD45.1 congenic recipients. Splenocytes from the recipient mice were analyzed at time points described in Figure legends.
Quantification and Statistical analysis
The P values were calculated with an unpaired two-tailed Student’s t-test and by one-way ANOVA for multigroup comparisons with the Tukey post hoc test using Prism 9 software (GraphPad): *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplementary Material
Supplementary Table 1, Related to Figures 1, 2, S1, S2, and S3. Replicates and metrics of CD4+ T cell scRNA/TCR-seq libraries from LCMV infection.
Supplementary Table 2, Related to Figures 1, 2, S1, S2, and S3. Metadata for CD4+ T cell scRNA/TCR-seq samples deposited in GEO.
Supplementary Table 3, Related to Figures 3 and S4. Metadata for PD-1+ CD4+ T cell scRNA/scATAC-seq samples deposited in GEO.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alexa Fluor (AF)-conjugated donkey polyclonal anti-rabbit IgG | Thermo Fisher Scientific | catalog no. R37118 RRID:AB_2556546 |
| AF647–conjugated goat polyclonal anti-rabbit IgG | Cell Signaling | catalog no. 4414S RRID:AB_10693544 |
| AF700-conjugated anti-CD44 (IM7) | BD Pharmingen | catalog no. 560568 RRID:AB_1727481 |
| APC-conjugated anti-PD-1 (29F.1A12) | Biolegend | catalog no. 135209 RRID:AB_2251944 |
| APC-Cy7-conjugated anti-CD8a (53-6.7) | BD Biosciences | catalog no. 557654 RRID:AB_396769 |
| BV421 - conjugated anti-CXCR5/CD185 (L138D7) | Biolegend | catalog no. 145512 RRID:AB_2562128 |
| BV711- conjugated anti-CXCR5/CD185 (L138D7) | Biolegend | catalog no. 145529 RRID:AB_2734207 |
| Biotinylated anti-CD3e (145-2C11) | Biolegend | catalog no. 100304 RRID:AB_312669 |
| PE-conjugated anti-GL7 Antigen (GL7) | Biolegend | catalog no. 144608 RRID:AB_2562926 |
| PerCP-Cy5.5-conjugated anti-CD4 (GK1.5) | Biolegend | catalog no. 100434 RRID:AB_893324 |
| BUV395-conjugated anti-CD4 (GK1.5) | BD Biosciences | catalog no. 563790 RRID:AB_2738426 |
| PerCP-Cy5.5-conjugated anti-CD45.1 (A20) | Biolegend | catalog no. 110728 RRID:AB_893345 |
| AF700-conjugated anti-CD45.2 (104) | Biolegend | catalog no. 109822 RRID:AB_493731 |
| PerCP-Cy5.5-conjugated anti-Ki67 (B56) | BD Biosciences | catalog no. 561284 RRID:AB_10611574 |
| PE-Cy7-conjugated anti-PD-1 (29F.1A12) | Biolegend | catalog no. 135216 RRID:AB_10689635 |
| PE-Cy7-conjugated anti-Fas/CD95 (Jo2) | BD Biosciences | catalog no. 557653 RRID:AB_396768 |
| APC-Cy7-conjugated anti-B220 (RA3-6B2) | Biolegend | catalog no. 103224 RRID:AB_313007 |
| PE-Dazzle 594-conjugated anti-B220 (RA3-6B2) | Biolegend | catalog no. 103258 RRID:AB_2564053 |
| PE-Dazzle 594-conjugated anti-CXCR6 (SA051D1) | Biolegend | catalog no. 151117 RRID:AB_2721700 |
| BV605-conjugated anti-Ly-108 (13G3) | BD Biosciences | catalog no. 745250 RRID:AB_2742834 |
| BV650-conjugated anti-SLAM/CD150 (TC15-12F12.2) | Biolegend | catalog no. 115932 RRID:AB_2715765 |
| FITC-conjugated anti-CD69 (H1.2F3) | Biolegend | catalog no. 104506 RRID:AB_313109 |
| BV605-conjugated anti-CX3CR1 (SA011F11) | Biolegend | catalog no. 149027 RRID:AB_2565937 |
| PE-Cy7-conjugated anti-LAG3 (C9B7W) | Biolegend | catalog no. 125226 RRID:AB_2715764 |
| BV421-conjugated anti-Tim-3 (RMT3-23) | Biolegend | catalog no. 119723 RRID:AB_2616908 |
| APC-conjugated anti-Tim-3 (RMT3-23) | Biolegend | catalog no. 119706 RRID:AB_2561656 |
| eFluor660-conjugated anti-TOX (TXRX10) | Thermo Fisher Scientific | catalog no. 50-6502-82 RRID:AB_2574265 |
| unconjugated anti-TCF-1 | Cell Signaling Technology | catalog no. 2203 RRID:AB_2199302 |
| BV421-conjugated anti-BCL6 (K112-91) | BD Biosciences | catalog no. 563363 RRID:AB_2738159 |
| PE-conjugated anti-IFN-g (XMG1.2) | Biolegend | catalog no. 505808 RRID:AB_315402 |
| APC-conjugated anti-IL-2 (JES6-5H4, BD Pharmingen) | BD Biosciences | catalog no. 554429 RRID:AB_398555 |
| APC-conjugated TNF-a (MP6-XT22) | Biolegend | catalog no. 506308 RRID:AB_315429 |
| MojoSort Mouse CD4 T Cell Isolation Kit | Biolegend | catalog no. 480033 RRID: N/A |
| APC-Cy7-conjugated anti-CD4 | Biolegend | catalog no. 100414 RRID:AB_312699 |
| Bacterial and virus strains | ||
| LCMV-Armstrong | Prepared in Lab | N/A |
| LCMV-clone 13 | Prepared in Lab | N/A |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| RPMI-1640 | GenDEPOT | catalog no. CM059-050 |
| LCMV-gp61-80 peptide | GenScript Biotech | Custom order |
| Brefeldin A | Sigma | catalog no. B6542 |
| Foxp3 staining kit | eBioscience | catalog no. 00-5523-00 |
| Collagenase B | Sigma | catalog no. C9891 |
| Collagenase D1 | Roche | catalog no. 11088882001 |
| DNase I | Sigma | catalog no. 10104159001 |
| Percoll | Sigma | catalog no. P4937 |
| Critical commercial assays | ||
| Chromium Single Cell V(D)J Enrichment Kit, Mouse T Cell | 10× Genomics | 1000071 |
| Chromium Single Cell 5' Library Construction Kit | 10× Genomics | 1000020 |
| Chromium Next GEM Single Cell 5' Library and Gel Bead Kit v1.1 | 10× Genomics | 1000165 |
| Chromium Next GEM Single Cell ATAC Library & Gel Bead Kit, 16 rxns | 10× Genomics | PN-1000175 |
| Deposited data | ||
| Raw and processed data | This paper | GEO: GSE181474 |
| Experimental models: Cell lines | ||
| Fibrosarcoma cell line 1956-mOVA | Laboratory of Kenneth Murphy | Ferris et al., 2020 |
| Experimental models: Organisms/strains | ||
| C57BL/6N (B6) mice | Charles River Laboratories | Strain Code 556 PPID: N/A |
| B6-CD45.1 mice | Charles River Laboratories | RRID: IMSR_CRL:564 |
| C57BL/6N (B6) mice | Jackson Laboratory | RRID: IMSR_JAX:000664 |
| B6-CD45.1 mice | Jackson Laboratory | RRID: IMSR_JAX:002014 |
| Prdm1-EYFP mice | Jackson Laboratory | RRID: IMSR_JAX:008828 |
| Bcl6-flox mice | Jackson Laboratory | RRID: IMSR_JAX:023727 |
| Cd40lg-cre mice | This paper | N/A |
| Actb-Flpe mice | Jackson Laboratory | RRID: IMSR_JAX:005703 |
| OT-II mice | Jackson Laboratory | RRID: IMSR_JAX:004194 |
| B6.129X1-Gt (ROSA)26Sortm1(EYFP)Cos/J | Jackson Laboratory | RRID: IMSR_JAX:006148 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and algorithms | ||
| GraphPad Prism, version 9.3.1(350) | Graph Pad | RRID:SCR_002798 |
| Flowjo 10.8.0 | Flowjo, LLC | RRID:SCR_008520 |
| Cell Ranger, version 3.1.0 | 10× Genomics | https://support.10xgenomics.com/single-cell-vdj/software |
| Cell Ranger ATAC, version 1.2.0 | 10× Genomics | https://support.10xgenomics.com/single-cell-atac/software |
| Seurat, version 3.1.1 |
Stuart et al., 2019 PMID: 31178118 |
https://satijalab.org/seurat |
| Monocle 3, version 0.2.1 |
Cao et al., 2019 PMID: 30787437 |
https://cole-trapnell-lab.github.io/monocle3/ |
| R, version 3.6.1 | The R Project | https://www.r-project.org/ |
| ArchR, version 1.0.1 | Granja et al., 2019 PMID: 33633365 |
RRID:SCR_020982 https://www.nature.com/articles/s41587-019-0332-7 |
| Other | ||
| PE- or APC-labeled I-Ab-LCMV-gp66 tetramer | NIH Tetramer Core at Emory | N/A |
| PE- or APC-labeled H2-Db-LCMV-gp33 tetramer | MBL | TB-M512-1 RRID: N/A |
| LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit | Thermo Fisher Scientific |
L34965 RRID: N/A |
| CellTrace™ CFSE Cell Proliferation Kit, for flow cytometry | Life technologies |
C34554 RRID: N/A |
Highlights.
Activated memory-like PD-1+ CD4+ T cells develop in the presence of chronic Ag.
They contain progenitor CD4+ T (Tprog) cells that give rise to Tfh and Teff cells.
The CD4+ Tprog cells express TCF-1 and require BCL6 for their development.
Tprog cells are critical for sustained CD4+ T cell responses to chronic antigen.
Acknowledgments
We thank the NIH tetramer core at Emory for providing the I-Ab-LCMV-gp66 tetramers. This study was supported by NIH grants R01AI130152 (to T.E.), R21AI161040 (to T.E.), F30CA247262 (to R.W.), T32AI007290 (to J.A.P.), K08CA230188 (to A.T.S.), U01CA260852 (to A.T.S.), UMHG012076 (to A.T.S.), R01CA190700 (to R.D.S), P30AR073752 (Rheumatic Diseases Research Resource-Based Center at Washington University), the Leukemia and Lymphoma Society Scholar Award (to T.E.), Stanford Propel Scholarship (to KJHG), the Parker Institute for Cancer Immunotherapy (to A.T.S. and R.D.S), the Burroughs Wellcome Fund Career Award for Medical Scientists (to A.T.S.), a Technology Impact Award from the Cancer Research Institute (to A.T.S.), a Pew-Stewart Scholars for Cancer Research Award (to A.T.S.), and a Baxter Foundation Scholar Award (to A.T.S.).
Footnotes
Declaration of interests
A.T.S. is a scientific co-founder of Immunai and founder of Cartography Biosciences and receives research funding from Arsenal Biosciences, Merck Research Laboratories, and Allogene Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumjohann D, Okada T, and Ansel KM (2011). Cutting Edge: Distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 187, 2089–2092. [DOI] [PubMed] [Google Scholar]
- Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, Zhang F, Mundlos S, Christiansen L, Steemers FJ, et al. (2019). The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Kim H-R, Leng L, Kang I, Jorgensen WL, Cho C-S, Bucala R, and Kim W-U (2012). Role of macrophage migration inhibitory factor in the regulatory T cell response of tumor-bearing mice. J. Immunol. 189, 3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, and Crotty S (2011). ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, and Crotty S (2013). Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 190, 4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C, Verbaro DJ, Tonc E, Holmgren M, Cella M, Colonna M, Bhattacharya D, and Egawa T (2016). The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity 45, 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci T, Vacchio MS, Gao Y, Tomassoni Ardori F, Candia J, Mehta M, Zhao Y, Tran B, Pepper M, Tessarollo L, et al. (2019). The Emergence and Functional Fitness of Memory CD4+ T Cells Require the Transcription Factor Thpok. Immunity 50, 91–105.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, and Wherry EJ (2014). Molecular and Transcriptional Basis of CD4+ T Cell Dysfunction during Chronic Infection. Immunity 40, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S (2011). Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663. [DOI] [PubMed] [Google Scholar]
- Crotty S (2019). T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50, 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, and Schoenberger SP (2010). Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Wang J, Fagerberg E, Chen P-M, Connolly KA, Damo M, Cheung JF, Mao T, Askari AS, Chen S, et al. (2021). Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell 184, 6101–6118.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel B, Yost KE, Sandor K, Xia Y, Qi Y, Hiam-Galvez KJ, Meier SL, Belk JA, Giles JR, John Wherry E, et al. (2021). Divergent clonal differentiation trajectories of T cell exhaustion. Biorxiv, doi: 10.1101/2021.12.16.472900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk D, Sharma R, Nainys J, Yim K, Kathail P, Carr AJ, Burdziak C, Moon KR, Chaffer CL, Pattabiraman D, et al. (2018). Recovering Gene Interactions from Single-Cell Data Using Data Diffusion. Cell 174, 716–729.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiToro D, Winstead CJ, Pham D, Witte S, Andargachew R, Singer JR, Wilson CG, Zindl CL, Luther RJ, Silberger DJ, et al. (2018). Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, and Mark Ansel K (2007). Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nature Immunology 8, 145–153. [DOI] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, and Brooks DG (2011). Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 208, 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, Davidson JT, Bagadia P, Liu T, Briseño CG, et al. (2020). cDC1 prime and are licensed by CD4 T cells to induce anti-tumour immunity. Nature 584, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja JM, Corces MR, Pierce SE, Bagdatli ST, Choudhry H, Chang HY, and Greenleaf WJ (2021). ArchR is a scalable software package for integrative single-cell chromatin accessibility analysis. Nat. Genet. 53, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, and Ahmed R (2018). CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu. Rev. Med. 69, 301–318. [DOI] [PubMed] [Google Scholar]
- He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. (2016). Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–428. [DOI] [PubMed] [Google Scholar]
- Hondowicz BD, Kim KS, Ruterbusch MJ, Keitany GJ, and Pepper M (2018). IL-2 is required for the generation of viral-specific CD4+ Th1 tissue-resident memory cells and B cells are essential for maintenance in the lung. Eur. J. Immunol. 48, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. (2016). Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, and Lord GM (2009). The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc. Natl. Acad. Sci. U. S. A. 106, 17876–17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, and Crotty S (2009). Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, and Nutt SL (2009). Blimp-1 Transcription Factor Is Required for the Differentiation of Effector CD8+ T Cells and Memory Responses. Immunity 31, 283–295. [DOI] [PubMed] [Google Scholar]
- Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, et al. (2016). CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 17, 1187–1196. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, et al. (2014). Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature 507, 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund R, Ahlfors H, Kainonen E, Lahesmaa A-M, Dixon C, and Lahesmaa R (2005). Identification of genes involved in the initiation of human Th1 or Th2 cell commitment. Eur. J. Immunol. 35, 3307–3319. [DOI] [PubMed] [Google Scholar]
- Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, et al. (2011). Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity 35, 633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguche AO, Shafiani S, Clemons C, Larson RP, Dinh C, Higdon LE, Cambier CJ, Sissons JR, Gallegos AM, Fink PJ, et al. (2015). ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J. Exp. Med. 212, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, et al. (2001). Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292, 1907–1910. [DOI] [PubMed] [Google Scholar]
- Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, Littman DR, and Taniuchi I (2007). Repression of interleukin-4 in T helper type 1 cells by Runx/Cbfβ binding to the Il4 silencer. Journal of Experimental Medicine 204, 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nish SA, Zens KD, Kratchmarov R, Lin W-HW, Adams WC, Chen Y-H, Yen B, Rothman NJ, Bhandoola A, Xue H-H, et al. (2017). CD4+ T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J. Exp. Med. 214, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Fairfax KA, and Kallies A (2007). BLIMP1 guides the fate of effector B and T cells. Nat. Rev. Immunol. 7, 923–927. [DOI] [PubMed] [Google Scholar]
- Pepper M, and Jenkins MK (2011). Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 12, 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Pagán AJ, Igyártó BZ, Taylor JJ, and Jenkins MK (2011). Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner HA, Packer JS, McFaline-Figueroa JL, Cusanovich DA, Daza RM, Aghamirzaie D, Srivatsan S, Qiu X, Jackson D, Minkina A, et al. (2018). Cicero Predicts cis-Regulatory DNA Interactions from Single-Cell Chromatin Accessibility Data. Mol. Cell 71, 858–871.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju S, Xia Y, Daniel B, Yost KE, Bradshaw E, Tonc E, Verbaro DJ, Kometani K, Yokoyama WM, Kurosaki T, et al. (2021). Identification of a T-bethi Quiescent Exhausted CD8 T Cell Subpopulation That Can Differentiate into TIM3+ CX3CR1+ Effectors and Memory-like Cells. The Journal of Immunology 206, 2924–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruterbusch M, Pruner KB, Shehata L, and Pepper M (2020). In Vivo CD4 T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annual Review of Immunology 38, 705–725. [DOI] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, and Kaech SM (2009). Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Granja JM, Yost KE, Qi Y, Meschi F, McDermott GP, Olsen BN, Mumbach MR, Pierce SE, Corces MR, et al. (2019). Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol. 37, 925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schep AN, Wu B, Buenrostro JD, and Greenleaf WJ (2017). chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, et al. (2002). Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62. [DOI] [PubMed] [Google Scholar]
- Shao P, Li F, Wang J, Chen X, Liu C, and Xue H-H (2019). Cutting edge: Tcf1 instructs T follicular helper cell differentiation by repressing Blimp1 in response to acute viral infection. J. Immunol. 203, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin K-I, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, and Calame K (2003). Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19, 607–620. [DOI] [PubMed] [Google Scholar]
- Shaw LA, Bélanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, Goulding J, Lasorella A, Lu L-F, Crotty S, et al. (2016). Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat. Immunol. 17, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AA, and Groom JR (2021). Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment. Cell. Mol. Immunol. 18, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, et al. (2019). Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10. [DOI] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, and Satija R (2019). Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, and Glimcher LH (2000). A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Tanaka K, Magnusson F, Chung Y, Martinez GJ, Wang Y-H, Nurieva RI, Kurosaki T, and Dong C (2014). CCAAT/enhancer-binding protein α negatively regulates IFN-γ expression in T cells. J. Immunol. 193, 6152–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, et al. (2016). T Cell Factor 1-Expressing Memory-like CD8+ T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 45, 415–427. [DOI] [PubMed] [Google Scholar]
- Welsh RM (2009). Blimp hovers over T cell immunity. Immunity 31, 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, John Wherry E, Ha S-J, Kaech SM, Nicholas Haining W, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, et al. (2007). Molecular Signature of CD8 T Cell Exhaustion during Chronic Viral Infection. Immunity 27, 824. [DOI] [PubMed] [Google Scholar]
- Wu T, Shin HM, Moseman EA, Ji Y, Huang B, Harly C, Sen JM, Berg LJ, Gattinoni L, McGavern DB, et al. (2015). TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. CellReports 12, 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, et al. (2016). The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, and Cui W (2019). CD4+ T Cell Help Is Required for the Formation of a Cytolytic CD8+ T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 51, 1028–1042.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander R, Kasmani MY, Chen Y, Topchyan P, Shen J, Zheng S, Burns R, Ingram J, Cui C, Joshi N, et al. (2022). Tfh-cell-derived interleukin 21 sustains effector CD8 T cell responses during chronic viral infection. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Ramsdell F, and Alderson MR (1994). The activation antigen CD69. Stem Cells 12, 456–465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1, Related to Figures 1, 2, S1, S2, and S3. Replicates and metrics of CD4+ T cell scRNA/TCR-seq libraries from LCMV infection.
Supplementary Table 2, Related to Figures 1, 2, S1, S2, and S3. Metadata for CD4+ T cell scRNA/TCR-seq samples deposited in GEO.
Supplementary Table 3, Related to Figures 3 and S4. Metadata for PD-1+ CD4+ T cell scRNA/scATAC-seq samples deposited in GEO.
Data Availability Statement
All single-cell sequencing data generated in this paper have been deposited in the Gene Expression Omnibus (GEO) database under accession number GSE181474. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


