Abstract
Background.
Draft DSM-5 criteria for a mixed major depressive episode have been proposed, but their predictive validity has not yet been established. We hypothesized that such symptoms would be associated with poorer antidepressant treatment outcomes.
Method.
We examined outcomes among individuals with major depressive disorder participating in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, an effectiveness study conducted at primary and specialty care centers in the USA. Mixed features were derived from the six self-report items of the mania subscale of the Psychiatric Diagnosis Screening Questionnaire. Primary analyses examined the association between the presence of at least two of these in the 6 months before study entry, and remission across up to four sequential treatment trials, as well as adverse outcomes.
Results.
Of the 2397 subjects with a major depressive episode of at least 6 months’ duration, 449 (18.7%) reported at least two mixed symptoms. The presence of such symptoms was associated with a greater likelihood of remission across up to four sequential treatments, which persisted after adjustment for potential confounding clinical and demographic variables (adjusted hazard ratio 1.16, 95% confidence interval 1.03–1.28). Two individual items, expansive mood and cheerfulness, were strongly associated with a greater likelihood of remission.
Conclusions.
Proposed DSM-5 mixed state features were associated with a greater rather than a lesser likelihood of remission. While unexpected, this result suggests the potential utility of further investigation of depressive mixed states in major depression.
Keywords: Bipolar disorder, bipolar spectrum, citalopram, DSM-5, major depressive disorder, mixed state, selective serotonin reuptake inhibitors, treatment-resistant depression
Introduction
The potential for coexistence of depressive and manic symptoms during a mood episode has long been recognized (Salvatore et al. 2002). The implications of such mixed symptoms in bipolar disorder have been well characterized (Swann et al. 2007; Goldberg et al. 2009). However, their significance in major depression has received relatively less attention; two cross-sectional studies suggested that individuals with such symptoms are more apt to have other indicators of bipolar disorder (Sato et al. 2003; Angst et al. 2011).
The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) included criteria for a mixed state, in which individuals met criteria for both a major depressive and a manic episode during the same interval. Draft DSM-5 criteria (Anon, 2011) introduce the broader concept of a mixed features specifier for a depressive episode, in which some manic/hypomanic symptoms are present, but irritability and agitation are excluded. As this modifier is intended to be applied in major depressive disorder (MDD) as well as bipolar disorder, its potential clinical significance in individuals diagnosed with MDD merits investigation.
The multi-center Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (Rush et al. 2004) provides an opportunity to examine the predictive validity of the proposed mixed criteria in a broad community-based cohort of individuals diagnosed with MDD. Previously, we have described the modest predictive validity of the bipolar spectrum construct in this cohort (Perlis et al. 2011). In the present analysis, we applied similar methodology to examine putative mixed features proposed for DSM-5. We hypothesized that, consistent with generally poorer outcomes in bipolar patients treated with antidepressant mono-therapy (Koukopoulos et al. 2007), the presence of mixed features would be associated with poorer anti-depressant treatment outcomes in major depression as well.
Method
The STAR*D study was a multi-center study designed to examine which of several treatments are most effective in out-patients with non-psychotic MDD who did not reach remission with up to 14 weeks of the selective serotonin reuptake inhibitor citalopram. The methods of the STAR*D study are detailed elsewhere (Fava et al. 2003); briefly, the study was conducted at 41 clinical sites across the USA, including 18 primary and 23 psychiatric care settings. Outcomes were assessed by a team of trained research outcome assessors, blinded to treatment type. The STAR*D study enrolled male and female out-patients, age 18–75 years, with a DSM-IV checklist-based diagnosis of non-psychotic MDD and a baseline score ⩾14 on the 17-item Hamilton Rating Scale for Depression (HAMD; Hamilton, 1960). No structured interview was included or required. Exclusion criteria included lifetime diagnosis of MDD with psychotic features, schizophrenia, schizo-affective disorder, or bipolar disorder I, II, or not otherwise specified, based on clinical assessment and self-report. Other exclusion criteria included a well-documented history of non-response or intolerability in the current major depressive episode to adequate doses (Fava, 2003) of one or more medications utilized in the first two protocol treatment steps; a current primary diagnosis of eating disorder or obsessive–compulsive disorder; presence of severe, unstable concurrent psychiatric conditions likely to require hospitalization within 6 months (for example, severe alcohol dependence with recent detoxification admissions); presence of concurrent medical or psychiatric conditions or concomitant medications that contraindicated a protocol treatment; and pregnancy or intent to conceive within the 9 months subsequent to study entry. The study recruited only individuals who sought treatment at the clinical sites, drawn from both primary and specialty care settings.
Assessments collected at the initial study visit included sociodemographic features as well as the number of prior depressive episodes, current episode duration, age at first depressive episode, and family history of bipolar disorder among any first-degree relative. Study participants completed a modified version of the Psychiatric Diagnosis Screening Questionnaire (PDSQ; Zimmerman & Mattia, 2001a, b) to assess concurrent axis I disorders, which includes a mania symptom screen, assessing the prior 6 months. This screen includes six yes/no questions about elevated mood, extreme self-confidence, increased energy and decreased need for sleep, talkativeness, involvement in new projects, and impulsive or injudicious activities. The individual items correspond very closely to proposed DSM-5 criteria for mixed features (Anonymous, 2011).
As previously described, all eligible patients were treated with citalopram at level 1, with a goal of achieving symptomatic remission (Rush et al. 2004). Dosing was directed by a treatment manual (www.star-d.org) suggesting a starting dose of citalopram of 20 mg/day with increase to 40 mg/day by weeks 2–4 and 60 mg/day by weeks 4–6. Adjustments were permitted as needed to minimize side effects as well as to optimize the likelihood of therapeutic benefit for each patient. Treatment visits were advised at 0, 2, 4, 6, 9 and 12 weeks; an optional 14-week visit could be added if needed. Patients could also exit citalopram treatment before 12 weeks if they experienced intolerable side effects, could not reach an optimal anti-depressant dose because of side effects, or continued to have significant symptoms defined as a Quick Inventory of Depressive Symptomatology (QIDS-C16) score ⩾9 after at least 9 weeks at the maximal tolerable dose. After citalopram treatment, subjects could enter up to four sequential treatment levels until they reached remission or elected to discontinue participation. At level 2, next-step interventions included augmentation with buspirone, bupropion or cognitive therapy, or switch to venlafaxine, sertraline, bupropion or cognitive therapy.
Statistical analyses
Primary analyses dichotomized the mania score, comparing those with two or more symptoms to those with zero or one. This a priori division was intended to operationalize the DSM-5 criteria which require three or more symptoms, while accounting for the combination in the PDSQ of the sleep and energy items.
As the PDSQ mania score reflects the prior 6 months, only subjects with a current depressive episode duration of 6 months or more were analysed, in order to ensure that depressive and manic symptoms had co-occurred during the period assessed.
As in our previous analysis of STAR*D outcomes across levels (Perlis et al. 2011), we examined longitudinal outcomes to consider time to remission, utilizing survival analysis to account for censored observations (i.e. dropout), particularly for subjects who elected to discontinue treatment upon completion of a given treatment level even though they had not reached remission. Cox regression was used to examine association with manic symptoms, in crude models adjusted only for depression severity at study entry, as measured by the HAMD as well as models adjusted for potential confounding variables identified previously (Perlis et al. 2011) – these included age, marital status, race, ethnicity, presence of panic disorder, presence of substance-use disorder, history of three or more episodes, and illness onset at or before the age of 25 years.
We next examined two sets of adverse outcomes. First were those which might represent a switch into mania or other adverse effect – failure to return for a post-baseline visit, discontinuation with loss to follow-up, and psychiatric significant adverse event. Second were outcomes to be considered as reflecting antidepressant-induced effects postulated by Koukopoulos et al. (2007): these included worsening of suicidality (i.e. suicidal thoughts or behaviors), insomnia and psychomotor agitation. Emergence or worsening for each symptom was defined as a 1-point or greater increase in the QIDS-C16 item assessing that symptom compared with severity at baseline; subjects already maximally symptomatic on each item at baseline were excluded. For adverse effects, we utilized logistic regression, again in a crude model adjusted only for severity, and then in a model fully adjusted for potential confounding variables identified previously.
Survival and regression analyses utilized Stata 10.0 (StataCorp LP, USA).
Results
Clinical features of the 4041 subjects who entered citalopram treatment have been reported elsewhere (Rush et al. 2006); these included 3999 who completed the PDSQ at entry. Of the 3999, 2397 (59.9%) reported current depressive episode duration of at least 6 months and were included in the primary analyses. Proportions of subjects endorsing each symptom, and total symptom counts, are listed in Table 1; the most commonly endorsed were racing thoughts and talkativeness. Among the 2397, 439 (18.3%) reported two or more mixed symptoms (the ‘mixed’ group) in the previous 6 months and were subsequently compared with those endorsing zero or one in the primary analysis.
Table 1.
Prevalence of individual mixed features and association with likelihood of remission across treatment levels
| n | (%) | Crude HR (95% Cl) | Adjusted HR (95% Cl) | |
|---|---|---|---|---|
| Individual features | ||||
| Cheerfulness | 279 | (11.6) | 1.40 (1.19–1.65)* | 1.43 (1.20–1.69)* |
| Confidence | 249 | (10.4) | 1.45 (1.22–1.73)* | 1.49 (1.24–1.78)* |
| Increased energy/decreased sleep | 171 | (7.1) | 1.14 (0.91–1.42) | 1.15 (0.92–1.44) |
| Talkativeness | 356 | (14.9) | 1.06 (0.90–1.24) | 1.09 (0.92–1.28) |
| Involvement | 360 | (15.0) | 1.00 (0.86–1.18) | 1.00 (0.85–1.18) |
| Impulsivity | 342 | (14.3) | 1.02 (0.86–1.20) | 1.04 (0.88–1.24) |
| Total symptom count | ||||
| One or more | 884 | (36.9) | 1.08 (0.96–1.21) | 1.11 (0.98–1.25) |
| Two or more | 449 | (18.7) | 1.16 (1–1.34)* | 1.19 (1.03–1.38)* |
| Three or more | 231 | (9.6) | 1.29 (1.06–1.56)* | 1.33 (1.09–1.63)* |
| Four or more | 121 | (5.0) | 1.29 (1.02–1.64)* | 1.44 (1.12–1.86)* |
| Five or more | 51 | (2.1) | 1.14 (0.82–1.59) | 1.36 (0.93–1.99) |
HR, Hazard ratio; CI, confidence interval.
p<0.05.
A comparison of these two groups (Table 2) indicates that the mixed group was on average younger and more likely to meet criteria for panic disorder and drug abuse or dependence. More modest but nominally significant differences also indicated that the mixed group was more likely to be non-white, to be unmarried, Hispanic, and to have at least three prior depressive episodes. Other clinical features including severity and episode duration were similar between mixed and non-mixed subjects.
Table 2.
Clinical and sociodemographic features of patients endorsing or not endorsing two or more mixed features
| Feature | Zero or one mixed symptoms | Two or more mixed symptoms | χ2 | t | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | Mean (s.d..) | n | (%) | Mean (s.d.) | ||||
| Sex, male | 743 | (38) | 162 | (36) | 0.66 | 0.42 | |||
| Care setting, primary | 841 | (43) | 206 | (46) | 1.09 | 0.30 | |||
| Race, white | 1539 | (79) | 328 | (73) | 7.51 | 0.01* | |||
| Ethnicity, Hispanic | 267 | (14) | 80 | (18) | 4.98 | 0.03* | |||
| Marital status, married | 676 | (35) | 129 | (29) | 5.83 | 0.02* | |||
| Insurance, public payer | 404 | (21) | 96 | (21) | 0.09 | 0.76 | |||
| Education, not high school graduate | 282 | (14) | 68 | (15) | 0.13 | 0.72 | |||
| Episodes, 3+ previousa | 510 | (30) | 141 | (37) | 5.92 | 0.01* | |||
| Episode duration, 24+months | 823 | (42) | 198 | (44) | 0.50 | 0.48 | |||
| Panic disorder, PDSQ | 608 | (31) | 207 | (46) | 36.06 | 1.92×10−9* | |||
| Drug misuse, PDSQ | 179 | (9) | 75 | (17) | 21.75 | 3.1×10−6* | |||
| Alcohol misuse, PDSQ | 444 | (23) | 121 | (27) | 3.50 | 0.06 | |||
| Family history of bipolar disorder | 176 | (9) | 52 | (12) | 2.79 | 0.09 | |||
| Total | 1948 | (100) | 449 | (100) | |||||
| Age, decades | 1947 | 4.28 (1.33) | 449 | 3.93 (1.35) | 4.93 | 8.85 ×10−7* | |||
| HAMD, baseline | 1946 | 22.89 (5.27) | 449 | 22.98 (5.04) | −0.34 | 0.74 | |||
| Episode duration, months | 1947 | 40.38 (66.07) | 449 | 38.05 (53.69) | 0.70 | 0.49 | |||
s.d., Standard deviation; PDSQ, Psychiatric Diagnosis Screening Questionnaire; HAMD, Hamilton Rating Scale for Depression.
Excludes 320 subjects in whom the number of episodes is indeterminate.
p<0.05.
Time to remission across treatment levels for the mixed group was then compared with the non-mixed group using survival analysis (Table 1). In a Cox regression model, the crude hazard ratio for remission among the mixed group was 1.16 [95% confidence interval (CI) 1.00–1.34], and 1.20 (95% CI 1.04–1.39) in a fully adjusted model with terms for baseline depression severity and all other variables nominally significant in Table 2. Cox regression results with mixed state defined by number of symptoms ranging from one to five are shown in Table 1 and illustrated in Fig. 1.
Fig. 1.
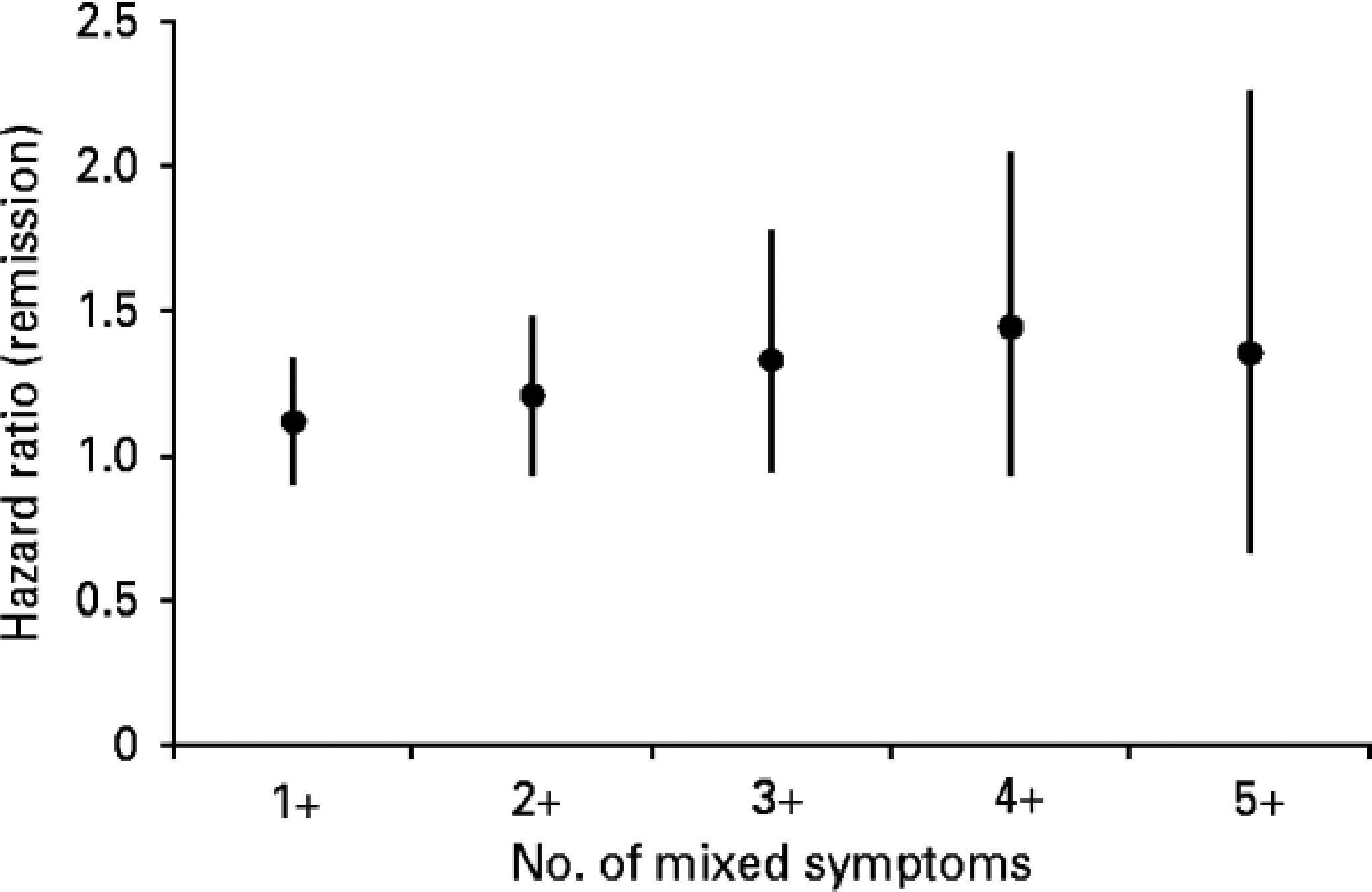
Association between the number of proposed DSM-5 mixed symptoms during a major depressive episode, and likelihood of remission in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Data are hazard ratios, with 95% confidence intervals represented by vertical bars.
We next examined individual mixed features for outcome with remission (Table 2). Presence of either cheerfulness or self-esteem, but not other items, was associated with a greater likelihood of remission in both crude and adjusted models. To examine the possibility that these items were serving as proxy measures for mood reactivity, further models were fit incorporating the IDS-C30 pleasure and reactivity items; results were essentially unchanged (results not shown), indicating that they are unlikely to be confounded by mood variability per se.
We also analysed the association between the presence of two or more mixed features and adverse outcomes. In simple logistic regression such features were associated with an increased risk for loss to follow-up [odds ratio (OR) 1.43, 95% CI 1.02–2.00] but not early dropout (OR 1.00, 95% CI 0.70–1.44) or psychiatric adverse event (OR 1.51, 95% CI 0.75–3.01). However, in adjusted models, these effects were diminished and no longer statistically significant: for loss to follow-up, OR 1.23, 95% CI 0.87–1.74; for early dropout, OR 0.88, 95% CI 0.61–1.28; and for psychiatric significant adverse event, OR 1.16, 95% CI 0.57–2.38.
Finally, we examined the possibility that mixed features might be associated with more subtle exacerbation of symptoms with citalopram treatment. Based in part on a previous description of mixed depression (Koukopoulos et al. 2007), we analysed worsening of suicidality, psychomotor agitation and insomnia, during citalopram treatment, all measured on the QIDS-C16, among subjects not reporting maximal symptoms already at study entry. For suicidality, 288/2363 (12.2%) experienced 1-point or greater worsening at any point. For psychomotor agitation, 673/2366 (28.4%) experienced 1-point or greater worsening. No association between the presence of two or more mixed symptoms and either of these was identified (OR 0.98, 95% CI 0.71–1.35 and OR 0.92, 95% CI 0.73–1.16, respectively). For insomnia, 877/2122 (41%) experienced a 1-point or greater worsening. Here the presence of two or more mixed symptoms was actually associated with a protective effect – that is, individuals were less likely to report a worsening of insomnia during citalopram treatment (OR 0.77, 95% CI 0.62–0.96).
Discussion
A major challenge in the study of depressive mixed states is the shifting definitions of the condition. Early work by Wilhelm Weygandt, a student of Emil Kraepelin, included a typology of mixed states which might be most akin to rapid cycling (Salvatore et al. 2002). The concept later became operationalized in DSM as simultaneously meeting criteria for both mania and depression, which was criticized as being overly stringent. Empirical study in bipolar disorder suggested that subthreshold states such as mixed hypomania are common (Suppes et al. 2005), and even subthreshold manic symptoms might be associated with differential course (Swann et al. 2007) or treatment response (Goldberg et al. 2009).
In MDD, the notion of a depressive mixed state has been more malleable. In part, this may be a result of the inclusion of agitated depression or melancholia agitate (Koukopoulos et al. 2007). A number of authors have suggested that the presence of such symptoms was a marker of occult bipolarity, or bipolar spectrum illness (Ghaemi et al. 2001; Sato et al. 2003; Benazzi et al. 2004; Moreno & Andrade, 2010; Angst et al. 2011). In this analysis of data from the largest effectiveness study in MDD to date, we find an association between proposed DSM-5 mixed features and a greater likelihood of remission. Although these results do suggest that mixed features possess some predictive validity, the association is in the opposite direction of what was hypothesized. This challenges the view that mixed features may be associated with a poorer outcome in MDD and may question the notion that these features are truly in the ‘bipolar spectrum’.
The proposed DSM-5 mixed state criteria simplify the definition of this type of episode by omitting those symptoms which may be seen in either pole of illness, including irritability, psychomotor agitation and distractibility. While this decision greatly facilitates empirical study, it does omit features that have previously-established predictive validity. For example, previous work in STAR*D suggests that irritability is associated with poorer treatment response (Perlis et al. 2011).
Several limitations should be underscored in interpreting these findings. First, while the PDSQ items correspond closely to proposed DSM-5 mixed symptoms, it is not optimal for assessment of such symptoms. In a validation study, the screen was not specific for bipolar disorder (Zimmerman & Mattia, 2001a). Moreover, its criteria do not fully correspond to those proposed for mixed states, combining the energy and sleep items and omitting the requirement that symptoms are observable by others.
A further limitation is that STAR*D did not include detailed assessment for manic symptoms, so it is possible that some hypomania or even frank mania could emerge during antidepressant treatment without being recognized. The finding that mixed symptoms were not significantly associated with other adverse outcomes which could be proxies for mania, such as study dropout and loss to follow-up, lends some confidence that widespread mood switch or exacerbation of mixed states was not missed. Still, measures that consider a broader range of mixed symptoms may be useful for future investigations in MDD (Zimmerman et al. 2010; Angst et al. 2011).
Systematic, prospective assessment of mixed symptoms in large population-based cohorts of individuals with MDD will ultimately be required to understand the utility of these symptoms. Ideally, those assessments should be done by individuals blinded to hypotheses about bipolar spectrum illness and mixed states. The present data nonetheless provide at least an initial estimate of effects based on proposed criteria. The cohort far more closely resembles clinical populations than most randomized, controlled trials, while retaining the benefits of structured longitudinal treatment and measurement.
Our analysis suggests that mixed symptoms, and specifically cheerfulness and elevated self-esteem at some point in the 6 months preceding treatment, predict better acute outcomes. While it is tempting to conclude that such symptoms are indicators of bipolarity, and perhaps shorter episode length or cycle acceleration with antidepressants, we would caution against assuming this interpretation. First, as we have noted, systematic consideration of adverse effects finds no evidence of other switch-like outcomes. Second, apart from a greater number of episodes, these symptoms do not appear to be associated with other putative markers of bipolar liability such as family history. Likewise, the presence of these symptoms does not appear to be a proxy for mood reactivity or hedonic capacity per se, as adjusting for these items from the IDS-C30 does not diminish the association with outcome.
The association observed with panic disorder also merits further investigation. A century ago, some descriptions of mixed states incorporated both psychic and physical anxiety (Salvatore et al. 2002). More recent nosologic investigations focus on the well-established co-occurrence of mood and anxiety disorders (Simon et al. 2003, 2004). Whether panic attacks or substance use, like mixed features, indicate a common underlying feature of depression requires additional study.
Taken together, this analysis suggests that mixed criteria as operationalized in DSM-5 possess some predictive validity. They are not necessarily markers of bipolar disorder or bipolar spectrum illness. However, their clinical significance in this data set suggests that further investigation from both a nosologic and biological perspective is warranted.
Acknowledgements
The STAR*D study is supported by federal funds from the National Institute of Mental Health (NIMH) under contract no. N01 MH-90003 to the University of Texas – SouthWestern Medical Center at Dallas (A. J. Rush, principal investigator). R.H.P. is supported by NIMH MH086026.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
We thank all the STAR*D investigators for all their help in making this large and complex multi-center study possible and for generating the data for this report, and acknowledge Stephen Wisniewski for creating the STAR*D database.
Declaration of Interest
R.H.P.: Research support: Proteus Biomedical. Advisory/consulting: Genomind; Proteus Biomedical; RID-Ventures. Equity holdings and patents: Concordant Rater Systems. Royalty/patents: Concordant Rater Systems. M.F.: Research support: Abbott Laboratories, Alkermes, Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmith-kline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., Pam-Lab, LLC, Pfizer Inc., Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., Wyeth-Ayerst Laboratories. Advisory/consulting: Aspect Medical Systems, AstraZeneca, Bayer AG, Biovail Pharmaceuticals, Inc., BrainCells, Inc., Bristol-Myers Squibb Company, Cephalon, Compellis, Cypress Pharmaceuticals, DOV Pharmaceuticals, Eli Lilly & Company, EPIX Pharmaceuticals, Fabre-Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., GlaxoSmithkline, Grunenthal GmbH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Lundbeck, MedAvante, Inc., Neuronetics, Novartis, Nutrition 21, Organon Inc., PamLab, LLC, Pfizer Inc., PharmaStar, Pharmavite, Roche, Sanofi/Synthelabo, Sepracor, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, Wyeth-Ayerst Laboratories. Speaking: AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmith-kline, Novartis, Organon Inc., Pfizer Inc., PharmaStar, Wyeth-Ayerst Laboratories.
References
- Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, Gamma A, Young AH (2011). Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode: the BRIDGE study. Archives of General Psychiatry 68, 791–798. [DOI] [PubMed] [Google Scholar]
- Anon. (2011). DSM-V Proposed Revision: Mixed Features Modifier (http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=483).
- Benazzi F, Koukopoulos A, Akiskal HS (2004). Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression). European Psychiatry 19, 85–90. [DOI] [PubMed] [Google Scholar]
- Fava M (2003). Diagnosis and definition of treatment-resistant depression. Biological Psychiatry 53, 649–659. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ (2003). Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatric Clinics of North America 26, 457–494, x. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Ko JY, Goodwin FK (2001). The bipolar spectrum and the antidepressant view of the world. Journal of Psychiatric Practice 7, 287–297. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Perlis RH, Bowden CL, Thase ME, Miklowitz DJ, Marangell LB, Calabrese JR, Nierenberg AA, Sachs GS (2009). Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. American Journal of Psychiatry 166, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukopoulos A, Sani G, Koukopoulos AE, Manfredi G, Pacchiarotti I, Girardi P (2007). Melancholia agitata and mixed depression. Acta Psychiatrica Scandinavica Supplementum 433, 50–57. [DOI] [PubMed] [Google Scholar]
- Moreno DH, Andrade LH (2010). Latent class analysis of manic and depressive symptoms in a population-based sample in Sao Paulo, Brazil. Journal of Affective Disorders 123, 208–215. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Uher R, Ostacher M, Goldberg JF, Trivedi MH, Rush AJ, Fava M (2011). Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Archives of General Psychiatry 68, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz B, Ritz L, Niederehe G (2004). Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Controlled Clinical Trials 25, 119–142. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Salvatore P, Baldessarini RJ, Centorrino F, Egli S, Albert M, Gerhard A, Maggini C (2002). Weygandt’s On the Mixed States of Manic-Depressive Insanity: a translation and commentary on its significance in the evolution of the concept of bipolar disorder. Harvard Review of Psychiatry 10, 255–275. [DOI] [PubMed] [Google Scholar]
- Sato T, Bottlender R, Schroter A, Moller HJ (2003). Frequency of manic symptoms during a depressive episode and unipolar ‘depressive mixed state’ as bipolar spectrum. Acta Psychiatrica Scandinavica Supplementum 107, 268–274. [DOI] [PubMed] [Google Scholar]
- Simon NM, Otto MW, Wisniewski SR, Fossey M, Sagduyu K, Frank E, Sachs GS, Nierenberg AA, Thase ME, Pollack MH (2004). Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). American Journal of Psychiatry 161, 2222–2229. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, Fava M, Sachs G, Racette SR, Perlis R, Sonawalla S, Rosenbaum JF (2003). Comparing anxiety disorders and anxiety-related traits in bipolar disorder and unipolar depression. Journal of Psychiatric Research 37, 187–192. [DOI] [PubMed] [Google Scholar]
- Suppes T, Mintz J, McElroy SL, Altshuler LL, Kupka RW, Frye MA, Keck PE Jr, Nolen WA, Leverich GS, Grunze H, Rush AJ, Post RM (2005). Mixed hypomania in 908 patients with bipolar disorder evaluated prospectively in the Stanley Foundation Bipolar Treatment Network: a sex-specific phenomenon. Archives of General Psychiatry 62, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Swann AC, Moeller FG, Steinberg JL, Schneider L, Barratt ES, Dougherty DM (2007). Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disorders 9, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Galione JN, Chelminski I, Young D, Ruggero CJ (2010). Performance of the Bipolar Spectrum Diagnostic Scale in psychiatric outpatients. Bipolar Disorders 12, 528–538. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI (2001a). The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Comprehensive Psychiatry 42, 175–189. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI (2001b). A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Archives of General Psychiatry 58, 787–794. [DOI] [PubMed] [Google Scholar]


