Abstract
In the summer of 2019, a cluster of cases were observed with users of battery-operated or superheating devices presenting with multiple symptoms, such as dyspnea, cough, fever, constitutional symptoms, gastrointestinal upset, and hemoptysis, that is now termed e-cigarette, or vaping, product use-associated lung injury (EVALI). The Centers for Disease Control and Prevention reported 2807 cases within the USA leading to at least 68 deaths as of February 18, 2020. The heterogeneous presentations of EVALI make diagnosis and treatment difficult; however, treatment focused on identifying and removal of the noxious substance and providing supportive care. Vitamin E acetate (VEA) is a likely cause of this lung injury, and others have reported other components to play a possible role, such as nicotine and vegetable glycerin/propylene glycol. EVALI is usually observed in adolescents, with a history of vaping product usage within 90 days typically containing tetrahydrocannabinol, and presenting on chest radiograph with pulmonary infiltrates or computed tomography scan with ground-glass opacities. Diagnosis requires a high degree of suspicion to diagnose and exclusion of other possible causes of lung disease. Here, we review the current literature to detail the major factors contributing to EVALI and primarily discuss the potential role of VEA in EVALI. We will also briefly discuss other constituents other than just VEA, as a small number of EVALI cases are reported without the detection of VEA, but with the same clinical diagnosis.
Keywords: E-cigarette, or vaping, product use-associated lung injury, lung injury, vaping, Vitamin E acetate
Electronic (e)-cigarettes and other vaping devices first arrived to world marketplace in 2007, and since then, there is a significant rise in the use of noncombustible tobacco products.[1] Approximately 7% of former smokers quit tobacco smoking by switching to electronic nicotine delivery systems.[2] However, the use of these vaping products can also introduce young adults to nicotine products and over 7000 flavors and other chemical constituents identified within these delivery systems.[3,4] The undoubted increase in demand for vaping products led to a surge of cluster cases in the summer of 2019 now identified as e-cigarette, or vaping, product use-associated lung injury (EVALI). EVALI became known as a constellation of symptoms including dyspnea, cough, fever, constitutional symptoms, gastrointestinal upset, and hemoptysis. Since February 18, 2020, the Centers for Disease Control and Prevention (CDC) reported a total of 2807 hospitalized EVALI cases or deaths within the US.[5] Analysis of the patient population in cases of EVALI found that 67% of patients were male while the median age was 24 and up to 86% of them had some association with vaping of tetrahydrocannabinol (THC)-containing products. Given the age distribution and frequency usage of e-cigarette by the younger population, it is apparent why most patients with EVALI are adolescents and young adults. Overall, there is a decrease of 1.73 million youths using e-cigarettes and vaping products, but there was a significant increase in disposable e-cigarette usage from 2019 to 2020 as these products gained popularity according to the National Youth Tobacco Survey.[6] Many unregulated THC-vaping products are known to include Vitamin E acetate (VEA), as VEA is used as a thickening agent for the THC oil. Following the first wave of EVALI cases, a study showed a correlation in EVALI diagnosis in patients with VEA detection. In December 2019, The Wadsworth center working in collaboration with the New York State Department of Health studied 206 e-cigarette fluids from 61 New York state EVALI cases.[7] Of the fluids obtained from these known cases, 147 (71%) contained THC, and 59 (29%) contained nicotine, and 101 (69%) contained VEA.[7]
In August 2019, the CDC collected 29 bronchoalveolar lavage fluid (BALF) samples of hospitalized EVALI patients and VEA was found in 100% of them.[8] In a 2020 study, 51 BALF samples were taken from EVALI patients across 16 states, and 49 (94%) of the participants had traces of VEA, but no VEA was found in the samples of the control group.[9] In general, EVALI appears to be primarily observed in the USA and suggests that this type of lung injury may be specific to vaping habits and products within a specific country. Of note, some cases were reported in Canada[10] and within South America.[11] In this review, we will discuss the effects of inhaled VEA on the cellular, inflammatory, immune, functional, physiological, histopathological, and radiographic changes noted in human studies, mouse models, and in vitro studies.
The Signs/Symptoms and Clinical Criteria/Definition of E-cigarette, or Vaping, Product Use-associated Lung Injury
EVALI patients, during the 2019 outbreak, presented with 1-week symptoms of dyspnea (81%), cough (74%), pleuritic chest pain (36%), and hemoptysis (10%).[12] Gastrointestinal symptoms such as nausea (66%), vomiting (64%), diarrhea (44%), and abdominal pain (34%) were also observed. Constitutional symptoms included headache (34%), malaise (47%), weight loss (26%), subjective fevers (84%), and chills (60%); however, only 33% had a temperature above 38°C upon presentation, tachycardia of over 100 beats per minute was seen in 63%, and an oxygen saturation of <88%–94% was seen in 58% of the patients (53).
The distinguishing factor that separates EVALI from other types of acute lung injury is the exposure to vaping/vaping devices. All patients endorsed a history of vaping within the last 90 days. The vast majority self reported vaping a product containing THC (89%) or nictine (73%). Only 9% reported using a vaping solution with cannabidiol (CBD). Given that symptoms such as cough, dyspnea, and fever and the radiographic findings of pulmonary infiltrates and ground-glass opacities (GGO) are seen in other forms of acute lung injury, the CDC's recommended workup centered around ruling out infectious causes.[13] The recommended workup on whether a concurrent pulmonary infection may be present is recommended, but if it does not explain the severity of illness, it can be presumed that EVALI is the primary illness. The CDC categorized EVALI patients into confirmed cases and probable cases. Confirmed cases are symptomatic patients with a history of vaping within 90 days, chest radiograph with pulmonary infiltrates, or CT scan with GGO who are proven to not have a pulmonary infection or any other likely explanation to symptoms such as cardiac, neoplastic, or rheumatologic disease.[14] A probable case is when a similar patient as described above has a pulmonary infection, but the infection is not thought to be the sole cause of lung injury.
The pathophysiology of e-cigarette, or vaping, product use-associated lung injury
To date, the exact pathophysiology of EVALI is still unknown. While VEA was identified in most BALF of EVALI patients, other constituents within the vaping device could play a role. There may be a hyperactive immune response inciting sterile exogenous pneumonitis,[15] as all EVALI patients did not have a superimposed bacterial infection through testing and antibiotics did not help this disease.[16] One paper proposed two theories: the direct chemical theory and the two-hit phenomenon. The first theory states that an e-liquid constituent when inhaled within the aerosol product by vaping is cytotoxic to certain lung cells ultimately leading to cellular necrosis, neutrophilic inflammation, and an overall pro-inflammatory state.[17] The latter theory states that inhalation of the base liquid in e-cigarettes (such as vegetable glycerin/propylene glycol [VG/PG], medium-chain triglycerides oil, and VEA) can alter the homeostatic state of lung immune cells that results in extensive inflammation.[17] Pulmonary surfactant composed of phospholipids (90% being dipalmitoylphosphatidylcholine [DPPC]) is the foundation of preventing alveolar collapse by reducing surface tension during inspiration and expiration. Using neutron spin-echo and artificial DPPC with VEA in a model system suggested that it is capable of minimizing the elastic properties of surfactant and thereby could be playing a role in EVALI by modulating the critical function of surfactant.[18]
Radiological changes observed in e-cigarette, or vaping, product use-associated lung injury patients
As per the CDC prevention report, chest imaging and radiographic findings are a criterion for diagnosing EVALI.[13] The most common imaging findings observed in studies done on patients who fulfilled the CDC criteria for EVALI include: (a) GGO[19,20,21] with or without centrilobular nodules, consolidation, septal thickening,[19,20,21] subpleural sparing,[19,20,21] and peribronchovascular (PBV) sparing;[21] (b) diffuse alveolar damage/acute lung injury;[21] (c) acute eosinophilic-like pneumonia;[21,22] and (d) diffuse alveolar hemorrhage (DAH).[21,23] Subpleural sparing was observed in most patients with radiographic findings.[19,20,21]
The most common pattern of injury associated with EVALI is organizing pneumonia,[20,21,24] which is a pattern of lung injury that commonly manifests as diffuse or lower lobe predominant, bilateral, and mostly symmetrical GGO commonly seen with areas of subpleural and lobular sparing.[24] In patients with EVALI, organizing pneumonia can manifest in an airway-centered pattern with diffuse centrilobular nodules and little-to-no GGO on a CT scan.[21,25] A retrospective study of 14 pediatric patients who presented to a tertiary care hospital with EVALI had similar results to the adult population. Chest radiographic findings showed GGO in 14 of 14 (100%) and consolidation in 9 of 14 (64%). Findings in both chest radiographs were bilateral in 14 of 14 (100%) and symmetric in 13 of 14 (93%).[20]
A large multicenter study consisting of 160 patients comparing various imaging patterns of EVALI revealed that patients with mild disease severity on CT scan had less PBV sparing than those with moderate disease severity, but this difference was not determined to be significant (P = 0.11).[21] PBV sparing was also observed in younger patients (mean age, 25.1 ± 9.2 vs. 29.9 ± 12.4 years; P = 0.016).[21] Lymphadenopathy was less common in patients with milder disease albeit not statistically significant (P = 0.13). They also noted a negative correlation between vaping >6 months and diffuse alveolar damage patterns but noted no significant association between CT findings and vaping-related variables (THC, nicotine, and THC plus nicotine) nor with demographics.[21]
Diffuse alveolar damage is the histological finding of acute lung injury. It is observed during the 1st week of alveolar injury and manifests as GGO with or without consolidation on imaging.[25,26] It can progress to organizing pneumonia as a result of fibroblast proliferation in response to injury.[25,26] Hypersensitivity pneumonitis (HP) is another pattern commonly observed in patients with EVALI.[21,25,27] HP is a challenging diagnosis to make as it can be difficult to differentiate from interstitial lung disease.[27] There are many proposals in literature aiming to simplify this diagnosis by taking into consideration histological, clinical, and radiological findings. Radiological findings include diffuse GGO seen on imaging. Fibrosis, seen in chronic HP. has not been linked to vaping with statistical significance in the current literature.[21,27]
Lipoid pneumonia is a pattern also associated with vaping.[28] It presents as GGO on imaging and can be observed as early as 30 min after inhalation.[29] Acute eosinophilic pneumonia, characterized by diffuse GGO on imaging,[30] is rarely reported as an adverse effect from using e-cigarettes.[22,31,32] Pleural effusions and septal thickening are often present and could be mistaken for pulmonary edema.[30] DAH, a rare pattern seen on CT,[21,23] is characterized by centrilobular GGO seen on CT.[21,23] Unlike the previously described patterns where GGO is symmetrical, DAH can be asymmetric.[21]
Histopathological changes seen in patients with e-cigarette, or vaping, product use-associated lung injury
The pathology of EVALI based on imaging and clinical findings is poorly understood.[33] Nonspecific histological patterns consistent with acute to subacute lung injury were noted throughout the literature.[22,34,35] Studies exploring histopathological patterns seen in patients with EVALI or probable diagnosis of EVALI demonstrated patterns of acute lung injury, including acute fibrinous pneumonitis, interstitial edema, diffuse alveolar damage, or organizing pneumonia among patients.[22,34,36] Airway-centered accumulation of foamy macrophages and pneumocyte vacuolization was commonly observed.[34] However, there is no evidence of lipoid pneumonia associated with EVALI.[34,36,37] There are a few reported cases of lipoid pneumonia associated with vaping, with BALF cells staining positive for oil red O.[28,38,39] The significance of this finding remains unclear, and it is suggested to interpret this finding with caution, as it may simply be a marker of exposure[34] or a finding associated with aspiration, obstruction, or infection[34,37,40,41] and not necessarily associated with toxicity.[34]
Vitamin E Acetate and Pulmonary Toxicity
VEA is a viscous lipid oil that is added to several vaping mixtures, including THC and cannabidiol oil mixtures.[42] Unlike VEA, the synthetic O-acetylated analog of Vitamin E has been thoroughly studied for its antioxidant and anti-aging properties following oral ingested or dermal topical use.[43] However, we know little about inhaled VEA and also the effects of high-temperature pyrolysis conditions on its chemical stability and reactivity.
The effects of pyrolysis on VEA are important as temperatures in vaping devices are equivalent to a laboratory pyrolysis apparatus, ranging between 110°C and 1000°C. Thereby, the original contents within a vaping device may significantly change chemistry through this heating. The pyrolysis of VEA produces toxic ketene gas, carcinogen alkenes, and benzene,[44] similar to phenyl acetate[45] that shares a similar structural-functional group to VEA. Ketenes are highly pulmonary toxic at high concentrations but only induce minor irritation and central nervous system impairment when exposed to animals at low concentrations.[46] Severe damage to alveolar cells is observed 24 h after exposure and the minimum lethal in-air concentration of ketene was reported to be 200 ppm, and caused death after a single 10-min exposure in primates.[47] More recent guidelines suggest that the lethal 10-min exposure value for ketene is 0.24 ppm.[48] The formation of ketene from VEA is believed to be feasible at temperatures above 500°C or “dry puff“ conditions, where ketene lung concentrations could become severe (30-ppm).[49] The term “dry puff“ is used when the volume of the vaping liquid within the device are low and concentrated; thereby, not allowing sufficient heating and cooling of the coil.[50] These components can then become overheated and produce exaggerated heating temperatures that could generate ketenes from VEA. Interestingly, THC is highly viscous and requires more heat to aerosolize compared to other solvents and ingredients used in vaping products.[51] Thereby, VEA in THC would be undergoing greater heating than other vaping practices, especially when using customized counterfeit vaping devices that could be heating vaping components to high temperatures.[52] A recent rodent model of vaping utilizing a combination of nickel and chromium heating element at high power without THC, Vitamin E oil, or nicotine demonstrated lung lesions, including alveolar wall thickening with inflammation, red blood cell congestion, obliteration of alveolar spaces, pneumonitis, accumulation of bronchial fibrin, inflammatory cells, and mucus plugs.[53]
The impact of Vitamin E acetate on surfactant
Vitamin E is a linactant and a potent modulator of lateral phase separation that reduces the line tension at the two-dimensional phase boundaries, leading to increased surface viscosity of pulmonary surfactant. Unlike Vitamin E, inhaled VEA does not readily undergo esterase-mediated hydrolysis[54,55] but remains unhydrolyzed in the lung of vaping users. In theory, VEA may induce liquid crystalline phase in pulmonary surfactants, and influence respiratory compression-expansion cycling.[56]
VEA is also known to get incorporated into lipid drops and intra-alveolar lipid-laden macrophages (LLMs) are observed in EVALI patients with diagnosed acute lipoid pneumonia.[38] A similar macrophage phenotype was observed in a mouse model of VEA inhalation, where animals were exposed to 77.3–167.5 μg/g VEA daily for 2 weeks.[57] A recent study looking at LLM in tobacco smokers, e-cigarette users, and nonsmokers/vapers, found LLM in half of the healthy e-cigarette users and almost all healthy smokers.[58] Another animal study found that one inhalation dose of VEA to lipopolysaccharide-treated rats greatly attenuated the inflammation.[55] Since VEA has multiple functions, VEA is suggested to influence similar signaling such as the diacylglycerol kinase and protein kinase C signaling pathway,[59] xenobiotic-sensing pregnane X receptor signaling,[60] and modulate lateral phase separation.[61] A recent study did observe sex as a confounding factor in e-cigarette users, with females having lower levels of plasmalogens that are glycerophospholipids secreted by alveoli cells and required for normal surfactant formation and function.[62]
Comparison of exogenous lipoid pneumonia to participants who inhaled Vitamin E acetate
Exogenous lipoid pneumonia is an uncommon condition which is known to affect patients who ingest mineral oils largely present in laxatives or various aerosolized industrial products. It usually presents with nonspecific respiratory tract symptoms. When ingested, these oils cause a foreign body reaction which can ultimately cause fibrosis of the lung.[63] This diagnosis is usually one of the exclusions and can be confirmed by presenting with LLMs when stained in oil red O in various respiratory samples but not limited to sputum or BALF.[64] Radiographic analysis observes airspace consolidations, ground-glass attenuation, airspace nodules, and a “crazy-paving“ pattern.[64] This is unfortunately nonspecific and can include differential diagnosis such as carcinoma.
The lungs struggle to extract long-chain hydrocarbons from the airspaces. Most commonly, exogenous lipid pneumonia is a product of the aspiration of oils described by phagocytosis of oils by macrophages and subacute inflammation, ultimately leading to fibrotic lung lesions and gas exchange abnormalities as stated above.[64,65] In one study, paraffin oil was shown to cause alveolar type II (ATII) cell injury consisting of destruction of microvilli and cytoplasmic vacuolization.[66] The type of damage induced by oils is largely based on its chemical component and droplet size.[64,65,66] In typical exogenous lipoid pneumonia, aspiration is commonly by large oil globules into a focal lung region, while aerosolized VEA and other oils produce small droplets allowing them to reach distal airways into alveoli evenly throughout the lung. This unique exposure differs significantly from previous reports of lipoid pneumonia and would expect different patterns of toxicity.[26]
A retrospective study of 17 cases of EVALI showed the presence of LLMs in peribronchiolar airspace along with vacuolization of the cytoplasm of hyperplastic type 2 pneumocytes in every sample.[34] Although some did show cholesterol clefts, no sample showed the accumulation of large fat droplets seen in exogenous lipoid pneumonia. In true lipoid pneumonia, this would be predominant as water-insoluble oil droplets have exorbitant interfacial tension that can make fat droplets coalescence and form larger oil droplets.[67] At the same time, CT findings did show bilateral GGO in which six showed a distinct bronchocentric distribution.[34] In a retrospective study of 3 EVALI cases, a radiologic characteristic of both lipid pneumonia and EVALI appears to have areas of low attenuation (−30 to − 50 Hounsfield units) on computed tomography, proposing fatty infiltration within the airways and parenchyma.[68] Currently, the role of lipids in the pathogenesis of EVALI is unclear and this may represent a form of airway-centered chemical pneumonitis due to multiple inhaled toxic substances rather than exogenous lipoid pneumonia.[34]
The cellular and molecular changes in inhaled Vitamin E acetate
In one animal study, twice-daily exposure to 1 h of aerosolized VEA for 6 or 15 days resulted in increased extravascular lung water (EVLW), protein in BALF (a marker of lung injury), and increased plasma surfactant protein (SP)-D when inhaling aerosolized VEA compared to aerosolized JUUL product (Juul Labs, Inc., an American electronic cigarette company) or control.[69] SP-D is an indicator for alveolar epithelial injury and prognostic marker in ARDS.[70,71] An increase in EVLW is a readout of hydrostatic pulmonary edema and acute respiratory distress syndrome, which was seen in many EVALI patients.[72] A concentration-dependent surface pressure was also observed in serum albumin, which can surpass the respreading pressures of collapsed monolayer in vitro and ultimately play into the pathophysiology of EVALI.[73] The increase in protein can lead to inactivation of lung surfactant and cause alveolar collapse from overwhelming surface tension.
When comparing aerosolized VEA to VG/PG in mice, increased levels of airspace neutrophils, large vacuolated macrophages, and neutrophil chemoattractants MCP-3 and interleukin 8 (IL-8) were observed.[69] Histologically these animals exposed to aerosolized VEA versus JUUL showed monocytic and neutrophilic alveolar and interstitial inflammation in a bronchiolocentric pattern with increased LLMs in the airspaces.[69] Another mouse study observed an overall increase in BALF albumin, increased number of leukocytes, and LLMs in aerosolized VEA compared to PG/VG.[57] Tissue pathology demonstrated LLMs in the cytoplasm of the cells lining the alveoli. Aerosolized VEA induced cellular toxicity to human ATII cells but not in ATII cells exposed to JUUL aerosol.[69] A recent publication analyzed the byproducts of aerosolized VEA condensate by mass spectrometry and observed that VEA did not pyrolyze to Vitamin E plus ketene during vaping and that intact VEA vapor is toxic.[74] They did observe that airspace cells actively hydrolyzed the VEA to release α-tocopherol, increased plasma malonaldehyde, and detected the oxidized form of Vitamin E in lung cells. This study also observed not only lung inflammation in mice exposed to prolonged VEA but also systemic inflammation and greater lung injury upon influenza infection.[74]
Functional, physiological, and epigenetic changes observed in vaporized Vitamin E acetate
Patients with vaping-associated lung diseases can present with acute eosinophilic pneumonia,[31] DAH,[75] lipoid pneumonia,[28,76] and respiratory-bronchiolitis interstitial lung disease. Several respiratory and systemic symptoms are observed with EVALI that could be linked to VEA, including shortness of breath (85%), cough (85%), chest pain (52%), pleuritic chest pain (36%), hemoptysis (8%), fever (84%), and chills (60%).[77] All EVALI case patients had bilateral infiltrates on chest imaging, and 77% had gastrointestinal symptoms.[77] The majority of EVALI patients (83%) have a chest radiograph with diffuse hazy or consolidative opacities.[21,77,78] Bilateral opacities are observed frequently in EVALI patients with one study observing them in 100% of 98 patients, either on the chest radiograph or chest CT.[77] Immune cell profiling in EVALI patients gives variable findings, with increased neutrophil numbers frequently observed (58%, ranging from 10% to 91%), eosinophils are observed in isolated cases, and LLMs are a common feature.[31,77]
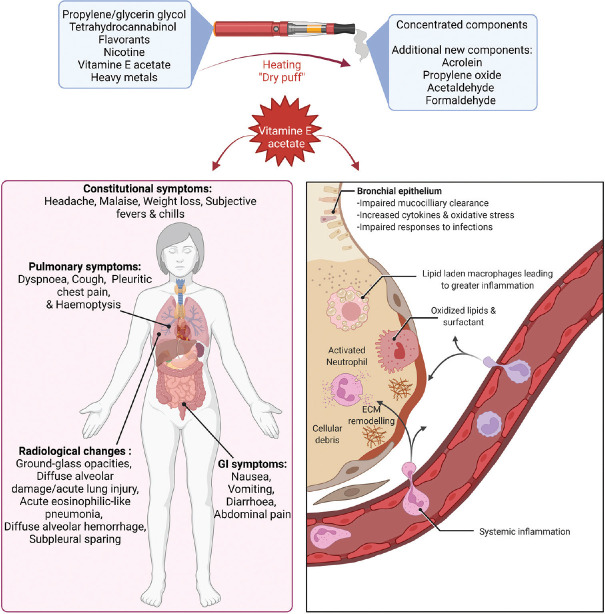
The question of whether VEA and other byproducts of it could cause epigenetic changes within ATII cells is of interest. There were noted to be 752 differential genes at a false discovery rate <0.1 between VEA exposed and control ATII cells. Pathway analysis divulged a significant increase in IL-17, MAPK, and tumor necrosis factor signaling pathways, as well at genes associated to osteoclast differentiation.[69] Figure 1 shows an overview of the major confounding factors for EVALI.
Figure 1.
Overview of primary confounding factors of EVALI. Created with BioRender.com. EVALI: e-cigarette, or vaping, product use-associated lung injury
Vaping and Pulmonary Toxicity: More Than One Constituent
Despite VEA being the major component in vaping products linked to EVALI, there are cases where no VEA was detected, but similar clinical manifestations were present. Essentially, the components of vaping equipment consist of a heating coil including varied metals including nickel, chromium, and lead,[27] an atomizer, a humectant such as polyethylene glycol, VG or glycerol, nicotine, tobacco-specific nitrosamines,[79] and flavoring such as vanillin, diacetyl, ethyl maltol, or menthol. It is important to note that the heating process of these liquids to aerosol can create volatile organic compounds such as acrylamide,[80] phenolic compounds such as catechol and hydroquinone,[81] and polyaromatic hydrocarbons that can further play a part in pulmonary toxicity. One study comparing e-cigarette vapor to a conventional cigarette in calf lungs suggested that nicotine did not affect surfactant interfacial properties and only marginally affected its lateral structure.[82] However, another study showed a decrease in SP-A in premature bovine lung epithelia after having the highest recorded mean of 49.8 μg/ml administered through nicotine patches to maternal sheep.[82] Furthermore, e-liquids contain nicotine salt rather than nicotine freebase (observed in conventional cigarettes) and its effects on alveoli, surfactant, and airways along with other organs are poorly understood.[83]
Vaping products also have a wide variety of chemicals added to enhance the flavor, but these additives could partake in pulmonary toxicity given that most safety studies are through oral ingestion. Notably, diacetyl (2,3-butanedione), a widely used volatile alpha-diketone flavoring agent with a butter-like taste, is notable for its toxicity in aerosolized form. In 2002 and 2006, diacetyl was found to be the culprit of bronchiolitis obliterans (nicknamed “popcorn lung“) linked to its direct effect on the bronchial epithelium leading to disorganized fibrotic repair in workers at a microwave-popcorn plant.[84] Diacetyl was detected in high concentrations in 39 of 51 electronic cigarettes sold by leading e-cigarette brands.[85] A more recent study was performed involving human donor respiratory epithelial cells exposed to 1100 ppm of diacetyl vapor. Proteomic analysis identified the presence of 11 novel proteins in both apical and basolateral supernatants collected including FBLN3, DDB1, ECM1, GDF15, and CXCL16.[86] E-cigarette flavorings prompt atypical activation of the lung epithelial cells and β-defensins, impaired macrophage activity, and increased levels of MUC5AC and NETosis.[87]
Conclusion
Even with the decline of EVALI after eliminating the use of VEA in e-cigarette products, there are still documented cases which did not contain VEA. Additional research is needed to determine the long-term outcomes of EVALI. Vaping products are a rapidly expanding industry, with increasing accessibility to younger populations. In 2020, 19.6% of high school students (3.02 million) and 4.7% of middle school students (550,000) reported current e-cigarette use within the USA. The use of vaping products, especially unregulated products, must continue to be of concern to clinicians. This undoubtedly remains an emerging field, though due to rising use, clinicians must remain up-to-date on this still new yet highly concerning topic having the potential to cause serious disease in the future.
Financial support and sponsorship
This work was supported by grants made available to P.G. (the Alpha-1 Foundation, 493373 and 614218).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sreedharan S, Mian M, Robertson RA, Rhodes A. Radiological findings of e-cigarette or vaping product use associated lung injury: A systematic review. Heart Lung. 2021;50:736–41. doi: 10.1016/j.hrtlng.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Mirbolouk M, Charkhchi P, Kianoush S, Uddin SM, Orimoloye OA, Jaber R, et al. Prevalence and distribution of E-cigarette use among U.S. Adults: Behavioral risk factor surveillance system, 2016. Ann Intern Med. 2018;169:429–38. doi: 10.7326/M17-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob Control. 2014;23(Suppl 3):i3–9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. cdc.gov, Atlanta, Georgia, USA: Centers for Disease Control and Preventation; 2020. [Lastupdated on 2021 Aug 03]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#latest-information . [Google Scholar]
- 6.National Youth Tobacco Survey (NYTS). Center for Disease Control and Protection; 2020. [Last updated on 2020 Dec 21]. https://www.cdc.gov/tobacco/data_statistics/surveys/nyts/index.htm.
- 7.Lu SJ, Li L, Duffy BC, Dittmar MA, Durocher LA, Panawennage D, et al. Investigation of vaping fluids recovered from New York State E-cigarette or vaping product use-associated lung injury patients. Front Chem. 2021;9:748935. doi: 10.3389/fchem.2021.748935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blount BC, Karwowski MP, Morel-Espinosa M, Rees J, Sosnoff C, Cowan E, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of E-cigarette, or vaping, product use-associated lung injury – 10 states, August-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1040–1. doi: 10.15585/mmwr.mm6845e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. Vitamin E acetate in Bronchoalveolar-Lavage fluid associated with EVALI. N Engl J Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canada Go. Vaping-Associated Lung Illness; 2019. [Last accessed on 2019 Dec 13, Last updated on 2020 Aug 20]. Available from: https://www.canada.ca/en/public-health/services/diseases/vaping-pulmonary-illness.html .
- 11.Boloña E, Felix M, Vanegas E, Vera Paz C, Cherrez-Ojeda I. A case of Vaping-Associated pulmonary illness in South America: Highlighting the need for awareness and surveillance programs in the region. Am J Respir Crit Care Med. 2020;201:733–5. doi: 10.1164/rccm.201910-2002LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonas AM, Raj R. Vaping-Related acute parenchymal lung injury: A systematic review. Chest. 2020;158:1555–65. doi: 10.1016/j.chest.2020.03.085. [DOI] [PubMed] [Google Scholar]
- 13.Siegel DA, Jatlaoui TC, Koumans EH, Kiernan EA, Layer M, Cates JE, et al. Update: Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury – United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:919–27. doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schier JG, Meiman JG, Layden J, Mikosz CA, VanFrank B, King BA, et al. Severe pulmonary disease associated with electronic-cigarette-product use – Interim guidance. MMWR Morb Mortal Wkly Rep. 2019;68:787–90. doi: 10.15585/mmwr.mm6836e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chand HS, Muthumalage T, Maziak W, Rahman I. Pulmonary toxicity and the pathophysiology of electronic cigarette, or vaping product, use associated lung injury. Front Pharmacol. 2019;10:1619. doi: 10.3389/fphar.2019.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belok SH, Parikh R, Bernardo J, Kathuria H. E-cigarette, or vaping, product use-associated lung injury: A review. Pneumonia (Nathan) 2020;12:12. doi: 10.1186/s41479-020-00075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander LE, Bellinghausen AL, Eakin MN. What are the mechanisms underlying vaping-induced lung injury? J Clin Invest. 2020;130:2754–6. doi: 10.1172/JCI138644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiPasquale M, Gbadamosi O, Nguyen MH, Castillo SR, Rickeard BW, Kelley EG, et al. A mechanical mechanism for vitamin e acetate in E-cigarette/vaping-associated lung injury. Chem Res Toxicol. 2020;33:2432–40. doi: 10.1021/acs.chemrestox.0c00212. [DOI] [PubMed] [Google Scholar]
- 19.Girvin F, Naidich D. CT features of electronic-cigarette or vaping-associated lung injury (EVALI); Our experience during the recent outbreak. BJR Case Rep. 2020;6:20200027. doi: 10.1259/bjrcr.20200027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artunduaga M, Rao D, Friedman J, Kwon JK, Pfeifer CM, Dettori A, et al. Pediatric chest radiographic and CT findings of Electronic Cigarette or Vaping Product Use-Associated Lung Injury (EVALI) Radiology. 2020;295:430–8. doi: 10.1148/radiol.2020192778. [DOI] [PubMed] [Google Scholar]
- 21.Kligerman SJ, Kay FU, Raptis CA, Henry TS, Sechrist JW, Walker CM, et al. CT findings and patterns of e-cigarette or vaping product use-associated lung injury: A multicenter cohort of 160 cases. Chest. 2021;160:1492–511. doi: 10.1016/j.chest.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arter ZL, Wiggins A, Hudspath C, Kisling A, Hostler DC, Hostler JM. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. doi: 10.1016/j.rmcr.2019.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmonds PJ, Copeland C, Conger A, Richmond BW. Vaping-induced diffuse alveolar hemorrhage. Respir Med Case Rep. 2020;29:100996. doi: 10.1016/j.rmcr.2020.100996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panse PM, Feller FF, Butt YM, Smith ML, Larsen BT, Tazelaar HD, et al. Radiologic and pathologic correlation in EVALI. AJR Am J Roentgenol. 2020;215:1057–64. doi: 10.2214/AJR.20.22836. [DOI] [PubMed] [Google Scholar]
- 25.Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: Organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33:1951–75. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 26.Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381:1486–7. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 27.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from E-Cigarette Use. Pediatrics. 2018;141:e20163927. doi: 10.1542/peds.2016-3927. [DOI] [PubMed] [Google Scholar]
- 28.Viswam D, Trotter S, Burge PS, Walters GI. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018;2018:r–224350. doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betancourt SL, Martinez-Jimenez S, Rossi SE, Truong MT, Carrillo J, Erasmus JJ. Lipoid pneumonia: Spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010;194:103–9. doi: 10.2214/AJR.09.3040. [DOI] [PubMed] [Google Scholar]
- 30.Jeong YJ, Kim KI, Seo IJ, Lee CH, Lee KN, Kim KN, et al. Eosinophilic lung diseases: A clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617–37. doi: 10.1148/rg.273065051. [DOI] [PubMed] [Google Scholar]
- 31.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47:15–7. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Wolf M, Richards J. Acute eosinophilic pneumonia due to vaping-associated lung injury. J Crit Care Med (Targu Mures) 2020;6:259–62. doi: 10.2478/jccm-2020-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wackowski OA, Gratale SK, Jeong M, Delnevo CD, Steinberg MB, O’Connor RJ. Over 1 year later: Smokers' EVALI awareness, knowledge and perceived impact on e-cigarette interest. Tob Control. 2022 doi: 10.1136/tobaccocontrol-2021-057190. doi: 10.1136/tobaccocontrol-2021-057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381:1780–1. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 35.Reagan-Steiner S, Gary J, Matkovic E, Ritter JM, Shieh WJ, Martines RB, et al. Pathological findings in suspected cases of e-cigarette, or vaping, product use-associated lung injury (EVALI): A case series. Lancet Respir Med. 2020;8:1219–32. doi: 10.1016/S2213-2600(20)30321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhopadhyay S, Mehrad M, Dammert P, Arrossi AV, Sarda R, Brenner DS, et al. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping) Am J Clin Pathol. 2020;153:30–9. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 37.Fathima S, Zhang H. Histologic patterns of lung injury in patients using e-cigarettes. Proc (Bayl Univ Med Cent) 2020;33:619–20. doi: 10.1080/08998280.2020.1775052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicpinigaitis PV, Trachuk P, Fakier F, Teka M, Suhrland MJ. Vaping-associated acute respiratory failure due to acute lipoid pneumonia. Lung. 2020;198:31–3. doi: 10.1007/s00408-019-00277-6. [DOI] [PubMed] [Google Scholar]
- 39.Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, Raman SM, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381:1488–9. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 40.Colby TV. Pathologic aspects of bronchiolitis obliterans organizing pneumonia. Chest. 1992;102:38S–43S. doi: 10.1378/chest.102.1_supplement.38s. [DOI] [PubMed] [Google Scholar]
- 41.Rossi G, Cavazza A, Spagnolo P, Bellafiore S, Kuhn E, Carassai P, et al. The role of macrophages in interstitial lung diseases: Number 3 in the series “pathology for the clinician“ edited by peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26:170009. doi: 10.1183/16000617.0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledford H. Scientists chase cause of mysterious vaping illness as death toll rises. Nature. 2019;574:303–4. doi: 10.1038/d41586-019-03033-1. [DOI] [PubMed] [Google Scholar]
- 43.Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311–5. doi: 10.4103/2229-5178.185494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu D, O’Shea DF. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci U S A. 2020;117:6349–55. doi: 10.1073/pnas.1920925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurd CD, Blunck FH. The pyrolysis of esters. J Am Chem Soc. 1938;60:2419–25. [Google Scholar]
- 46.Mendenhall RM, Stokinger HE. Tolerance and cross-tolerance development to atmospheric pollutants ketene and ozone. J Appl Physiol. 1959;14:923–6. [Google Scholar]
- 47.Treon JF, Sigmon HE. Physiologic response of animals exposed to air-borne ketene. J Ind Hyg Toxicol. 1949;31:209–19. [PubMed] [Google Scholar]
- 48.Committee on Acute Exposure Guideline Levels; Committee on Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals. Vol. 16. Washington, DC: National Academies Press; 2014. [PubMed] [Google Scholar]
- 49.Narimani M, da Silva G. Does 'Dry Hit' vaping of vitamin E acetate contribute to EVALI. Simulating toxic ketene formation during e-cigarette use? PLoS One. 2020;15:e0238140. doi: 10.1371/journal.pone.0238140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health. 2013;10:2500–14. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strongin RM. E-cigarette chemistry and analytical detection. Annu Rev Anal Chem (Palo Alto Calif) 2019;12:23–39. doi: 10.1146/annurev-anchem-061318-115329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthumalage T, Friedman MR, McGraw MD, Ginsberg G, Friedman AE, Rahman I. Chemical constituents involved in e-cigarette, or vaping product use-associated lung injury (EVALI) Toxics. 2020;8:25. doi: 10.3390/toxics8020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleinman MT, Arechavala RJ, Herman D, Shi J, Hasen I, Ting A, et al. E-cigarette or vaping product use-associated lung injury produced in an animal model from electronic cigarette vapor exposure without tetrahydrocannabinol or vitamin E Oil. J Am Heart Assoc. 2020;9:e017368. doi: 10.1161/JAHA.120.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hybertson BM, Kitlowski RP, Jepson EK, Repine JE. Supercritical fluid-aerosolized vitamin E pretreatment decreases leak in isolated oxidant-perfused rat lungs. J Appl Physiol (1985) 1998;84:263–8. doi: 10.1152/jappl.1998.84.1.263. [DOI] [PubMed] [Google Scholar]
- 55.Hybertson BM, Chung JH, Fini MA, Lee YM, Allard JD, Hansen BN, et al. Aerosol-administered alpha-tocopherol attenuates lung inflammation in rats given lipopolysaccharide intratracheally. Exp Lung Res. 2005;31:283–94. doi: 10.1080/01902140590918560. [DOI] [PubMed] [Google Scholar]
- 56.Lee H. Vitamin E acetate as linactant in the pathophysiology of EVALI. Med Hypotheses. 2020;144:110182. doi: 10.1016/j.mehy.2020.110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. An animal model of inhaled Vitamin E acetate and EVALI-like lung injury. N Engl J Med. 2020;382:1175–7. doi: 10.1056/NEJMc2000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shields PG, Song MA, Freudenheim JL, Brasky TM, McElroy JP, Reisinger SA, et al. Lipid laden macrophages and electronic cigarettes in healthy adults. EBioMedicine. 2020;60:102982. doi: 10.1016/j.ebiom.2020.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin E isoforms directly bind PKCα and differentially regulate activation of PKCα. Biochem J. 2012;441:189–98. doi: 10.1042/BJ20111318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galli F, Polidori MC, Stahl W, Mecocci P, Kelly FJ. Vitamin E biotransformation in humans. Vitam Horm. 2007;76:263–80. doi: 10.1016/S0083-6729(07)76009-0. [DOI] [PubMed] [Google Scholar]
- 61.Muddana HS, Chiang HH, Butler PJ. Tuning membrane phase separation using nonlipid amphiphiles. Biophys J. 2012;102:489–97. doi: 10.1016/j.bpj.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middlekauff HR, William KJ, Su B, Haptonstall K, Araujo JA, Wu X, et al. Changes in lipid composition associated with electronic cigarette use. J Transl Med. 2020;18:379. doi: 10.1186/s12967-020-02557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spickard A, 3rd, Hirschmann JV. Exogenous lipoid pneumonia. Arch Intern Med. 1994;154:686–92. [PubMed] [Google Scholar]
- 64.Hadda V, Khilnani GC. Lipoid pneumonia: An overview. Expert Rev Respir Med. 2010;4:799–807. doi: 10.1586/ers.10.74. [DOI] [PubMed] [Google Scholar]
- 65.Marchiori E, Zanetti G, Mano CM, Hochhegger B. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med. 2011;105:659–66. doi: 10.1016/j.rmed.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Baskerville A. Ultrastructural changes in experimental lipid pneumonia. Res Vet Sci. 1969;10:4–6. [PubMed] [Google Scholar]
- 67.Boyson TK, Pashley RM. A study of oil droplet coalescence. J Colloid Interface Sci. 2007;316:59–65. doi: 10.1016/j.jcis.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, English S, Ogilvie JA, Siu MK, Tammara A, Haas CJ. All up in smoke: Vaping-associated lung injury. J Community Hosp Intern Med Perspect. 2020;10:571–8. doi: 10.1080/20009666.2020.1800978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumoto S, Fang X, Traber MG, Jones KD, Langelier C, Hayakawa Serpa P, et al. Dose-dependent pulmonary toxicity of aerosolized Vitamin E acetate. Am J Respir Cell Mol Biol. 2020;63:748–57. doi: 10.1165/rcmb.2020-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–96. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K Acute Respiratory Distress Syndrome Network. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–8. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kushimoto S, Taira Y, Kitazawa Y, Okuchi K, Sakamoto T, Ishikura H, et al. The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: A prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care. 2012;16:R232. doi: 10.1186/cc11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warriner HE, Ding J, Waring AJ, Zasadzinski JA. A concentration-dependent mechanism by which serum albumin inactivates replacement lung surfactants. Biophys J. 2002;82:835–42. doi: 10.1016/S0006-3495(02)75445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsumoto S, Traber MG, Leonard SW, Choi J, Fang X, Maishan M, et al. Aerosolized Vitamin E acetate causes oxidative injury in mice and in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2022;322:L771–83. doi: 10.1152/ajplung.00482.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018:2018. doi: 10.1155/2018/9724530. doi: 10.1155/2018/9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davidson K, Brancato A, Heetderks P, Mansour W, Matheis E, Nario M, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia – North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68:784–6. doi: 10.15585/mmwr.mm6836e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary Illness related to e-cigarette use in Illinois and Wisconsin – Final report. N Engl J Med. 2020;382:903–16. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 78.Aberegg SK, Cirulis MM, Maddock SD, Freeman A, Keenan LM, Pirozzi CS, et al. Clinical, bronchoscopic, and imaging findings of e-cigarette, or vaping, product use-associated lung injury among patients treated at an academic medical center. JAMA Netw Open. 2020;3:e2019176. doi: 10.1001/jamanetworkopen.2020.19176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farsalinos KE, Gillman G, Poulas K, Voudris V. Tobacco-specific nitrosamines in electronic cigarettes: Comparison between liquid and aerosol levels. Int J Environ Res Public Health. 2015;12:9046–53. doi: 10.3390/ijerph120809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.St Helen G, Liakoni E, Nardone N, Addo N, Jacob P, 3rd, Benowitz NL. Comparison of systemic exposure to toxic and/or carcinogenic Volatile Organic Compounds (VOC) during vaping, smoking, and abstention. Cancer Prev Res (Phila) 2020;13:153–62. doi: 10.1158/1940-6207.CAPR-19-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Hage R, El-Hellani A, Salman R, Talih S, Shihadeh A, Saliba NA. Vaped humectants in e-cigarettes are a source of phenols. Chem Res Toxicol. 2020;33:2374–80. doi: 10.1021/acs.chemrestox.0c00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Przybyla RJ, Wright J, Parthiban R, Nazemidashtarjandi S, Kaya S, Farnoud AM. Electronic cigarette vapor alters the lateral structure but not tensiometric properties of calf lung surfactant. Respir Res. 2017;18:193. doi: 10.1186/s12931-017-0676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herman M, Tarran R. E-cigarettes, nicotine, the lung and the brain: Multi-level cascading pathophysiology. J Physiol. 2020;598:5063–71. doi: 10.1113/JP278388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–8. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 85.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in e-cigarettes: Diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit, candy, and cocktail-flavored E-cigarettes. Environ Health Perspect. 2016;124:733–9. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brass DM, Gwinn WM, Valente AM, Kelly FL, Brinkley CD, Nagler AE, et al. The diacetyl-exposed human airway epithelial secretome: New insights into flavoring-induced airways Disease. Am J Respir Cell Mol Biol. 2017;56:784–95. doi: 10.1165/rcmb.2016-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quinones Tavarez Z, Li D, Croft DP, Gill SR, Ossip DJ, Rahman I. The interplay between respiratory microbiota and innate immunity in flavor e-cigarette vaping induced lung dysfunction. Front Microbiol. 2020;11:589501. doi: 10.3389/fmicb.2020.589501. [DOI] [PMC free article] [PubMed] [Google Scholar]