Abstract
Deep eutectic solvents (DESs) are multicomponent designer solvents that exist as stable liquids over a wide range of temperatures. Over the last two decades, research has been dedicated to developing noncytotoxic, biodegradable, and biocompatible DESs to replace commercially available toxic organic solvents. However, most of the DESs formulated until now are hydrophilic and disintegrate via dissolution on coming in contact with the aqueous phase. To expand the repertoire of DESs as green solvents, hydrophobic DESs (HDESs) were prepared as an alternative. The hydrophobicity is a consequence of the constituents and can be modified according to the nature of the application. Due to their immiscibility, HDESs induce phase segregation in an aqueous solution and thus can be utilized as an extracting medium for a multitude of compounds. Here, we review literature reporting the usage of HDESs for the extraction of various organic compounds and metal ions from aqueous solutions and absorption of gases like CO2. We also discuss the techniques currently employed in the extraction processes. We have delineated the limitations that might reduce the applicability of these solvents and also discussed examples of how DESs behave as reaction media. Our review presents the possibility of HDESs being used as substitutes for conventional organic solvents.
1. Introduction
Deep eutectic solvents (DESs) are a class of designer solvents that generally have low volatility and flammability and can exist as chemically inert liquids over a wide temperature range.1−4 Currently, research in the areas of Chemistry and Chemical Engineering is focused on promoting sustainable and green practices with an emphasis on the use of novel renewable sources, practices5 that minimize negative impacts on the environment and economy. Recent developments in DESs show immense potential as green substitutes for commercially available toxic organic solvents.6,7 However, the noncytotoxic, biodegradable, and biocompatible nature of these solvents are often overemphasized and needs to be assessed carefully. As opposed to ionic liquids8 that are salts of an organic cation and anion (having a melting point below 100 °C), DESs are mixtures of two or more components with melting points lower than that of the individual components. The depression in melting point is due to the nonideality of these solutions arising from favorable van der Waals interaction, extensive hydrogen bonding, and positive entropy of mixing among components.9,10 These factors work together to stabilize the liquid phase at room temperature. The “deepness” of a eutectic mixture depends on the chemical identity of the components involved and the strength and structure of the interactions among them.11 In addition to availability of the components (often from natural sources) and tunability of solvent properties, DESs are easy to prepare without any purification steps.12−14
Based on the nature of the constituents, DESs can be categorized into the following five types: (i) type I prepared from a mixture of a metal halide (MXn, M = metal, X = halide) and a quaternary ammonium salt (QS), (ii) type II prepared from a mixture of a hydrated metal halide (MXn·mH20) and a quaternary ammonium salt, (iii) type III prepared from a mixture of quaternary ammonium salt and a hydrogen bond donor (HBD), (iv) type IV obtained from a mixture of MXn and HBD,9 and (v) type V consisting of a mixture of a neutral hydrogen bond acceptor (HBA) and a neutral HBD.15 Further classification of DESs on the basis of their hydrophobic and hydrophilic character was proposed by Florindo et al.16 The majority of the DESs reported in the literature are hydrophilic in nature and belong to type I or II. However, the water solubility of hydrophilic DESs limits their application in an aqueous medium, as the components undergo dissolution. Alternatively, DESs from category V (and often III and IV) are hydrophobic in nature due to the inherent hydrophobicity of their constituents. For ionic DESs, the hydrophobic nature is manifested in the low water content and reduced salt leakage in contact with water.17 For nonionic DESs, the hydrophobicity is estimated from the total organic carbon (TOC) content and the pH of the aqueous phase in contact with the DES phase.18 Additionally, the water–octanol partition coefficient (log10Kow) also serves as a metric for assessing the hydrophobicity of hydrophobic DESs (HDESs).19
Though earlier reported instances of DESs were all hydrophilic in nature, recently, HDESs have received much attention. The very first example of HDES was reported by van Osch et al., where a mixture of quaternary ammonium salts with decanoic acid was successfully employed to separate volatile fatty acids from aqueous solutions.20 To reduce toxicity and increase environmental compatibility while maintaining sustainability, the authors later encouraged the use of natural ingredients in the preparation of DESs, leading to a new category termed “NADES”.18 HDESs exhibit low melting points (often below 25 °C) and are normally liquids at room temperature. HDESs with neutral components generally result in a shallow depression in melting points and, consequently, solutions of low viscosity. HDESs are characterized by positive log10Kow values and often have densities lower than that of water, which helps in the segregation of the aqueous phase and the extractant. Due to the inherent hydrophobicity, HDESs retain their integrity in the presence of water with extremely low water uptake capability and minimum leaching of the DES constituents into the aqueous phase. All these factors combined make HDESs ideal for use as extractant media. Added to these properties is the flexibility in designing and tuning the physicochemical behavior of DES, keeping in mind the nature of the target compound to be extracted.11,17 In this paper, we have carried out an extensive literature review of HDESs. We explain in detail how HDESs have been used as extraction media for various organic compounds and metal ions. We have also incorporated instances of HDESs being utilized for absorption of CO2. We then discuss techniques that are commonly employed in the extraction processes. We finally addressed the challenges of working with HDESs and also discussed possible areas where they can be useful. We have delineated the composition, density, viscosity and melting (or glass transition) temperatures of each of the DES considered in this review.
The rest of the review is divided into the following sections: in section 2, we review the usage of HDES in the extraction of organic compounds and metal ions from aqueous solution and absorption of carbon dioxide; in section 3, we explore the popular techniques employed in the extraction process; and in section 4, we discuss the challenges associated with HDES as extraction media and prospective areas where these solvents can be utilized.
Table 1. Physicochemical Properties of the HDESs Reported in This Review.
| DESs [HBD:HBA] | molar ratio | density (g·mL–1) | temperaturea (°C) | viscosity (mPa·s) | ref |
|---|---|---|---|---|---|
| acetic acid:dl-menthol | 1:1 | (21−23) | |||
| anise alcohol:dl-menthol | 1:1 | 0.99 | 4.17 | 64.1 | (24) |
| 1:2 | 0.95 | 6.47 | 66.2 | (24) | |
| 2:1 | 1.01 | 6.41 | 66.9 | (24) | |
| atropine:thymol | 1:2 | 1.06 | 9255.5 | (18) | |
| BisZ:[TOPO] | 1:2 | (25) | |||
| [BTEAC]:thymol | 1:4 | 0.91 | –18.0 (M) | 230.0 | (26) |
| 1-butanol:N8881Cl | 2:1 | (27) | |||
| 1-butanol:[TOPO] | 1:1 | (28) | |||
| n-butanol:N8881Cl | 3:1 | (29) | |||
| 1-butyric acid:[TOPO] | 1:1 | (28) | |||
| n-butyric acid:thymol | 1:1 | (30) | |||
| 1,2-butanediol:N8881Cl | 2:1 | (27) | |||
| 4-bromophenol:ChCl | 2:1 | 1.21 | 132.0 | (31) | |
| n-butyl alcohol:menthol | 1:1 | (21) | |||
| n-butyl alcohol:N8888Br | 2:1 | 0.92 | –99.0 (M) | 22.0 | (32) |
| butylparaben:[DEHP] | 1:3 | 0.93 | 18.0 | (33) | |
| p-chlorophenol:camphor | 2:1 | 1.15 | (34) | ||
| ChCl:m-cresol | 1:2 | 1.08 | –59.30 (M) | (35) | |
| ChCl:o-cresol | 1:2 | 1.10 | –30.20 (M) | (35) | |
| ChCl:p-cresol | 1:2 | 1.08 | –62.70 (M) | (35) | |
| ChCl:Ph-EtOH | 1:4 | (36),37 | |||
| cyclohexanol:N8881Cl | 2:1 | (27) | |||
| 4-cyclophenol:N8881Cl | 1:1 | 0.94 | –62.0 | (38) | |
| 1:2 | 0.91 | –63.0 | (38) | ||
| 2:1 | 0.97 | –63.0 | (38) | ||
| DecaA:Aliquat 336 | 1:2 | 783.41 | (39) | ||
| DecaA:atropine | 2:1 | 1.02 | 80.5 | (18) | |
| DecaA:[BTEAC] | 3:1 | 0.91 | 4.0 | (40) | |
| DecaA:DodecaA | 2:1 | 0.89 | 18.00 (M) | 10.7 | (41) |
| DecaA:[HDC] | 1:1 | 0.97 | 11.2 | (42) | |
| DecaA:Lid | 2:1 | 0.96 | 237.5 | (18,43−46) | |
| 3:1 | 0.95 | 208.5 | (44),46 | ||
| 4:1 | 0.94 | 142.0 | (44),46 | ||
| DecaA:dl-menthol | 1:1 | (47) | |||
| 1:2 | 0.91 | (48) | |||
| DecaA:menthol | 1:1 | 0.90 | 20.4 | (18),21,46 | |
| 1:2 | 0.90 | 26.2 | (18) | ||
| 1:3 | 8.50 (M) | (49) | |||
| 2:1 | 0.89 | (50) | |||
| DecaA:N4444Br | 1:1 | 5.0 (M) | (51) | ||
| 2:1 | 0.95 | 16–17 (M) | (52) | ||
| 2:1 | 5.3 (M) | (51) | |||
| 3:1 | 5.7 (M) | (51) | |||
| 4:1 | 6.1 (M) | (51) | |||
| DecaA:N4444Cl | 1:2 | (53) | |||
| 2:1 | 0.91 | –11.95 (M) | 265.3 | (20,54−56) | |
| DecaA:N7777Cl | 2:1 | 0.89 | –16.65 (M) | 172.8 | (20),55,57 |
| DecaA:N8881Br | 2:1 | 0.94 | 8.95 (M) | 576.5 | (20),55 |
| DecaA:N8881Cl | 1:1 | 0.89 | 13.0 (M) | 1214.0 | (58) |
| 1:2 | 0.89 | 1.0 (M) | 2515.0 | (53)(58), | |
| 2:1 | 0.89 | 2.0 (M) | 288.0 | (58) | |
| 2:1 | 0.89 | –0.05 (M) | 783.4 | (20),32 | |
| 3:1 | (59) | ||||
| 3:1 | (60) | ||||
| DecaA:N8888Br | 2:1 | 0.92 | 8.95 (M) | 636.3 | (20),46,55,61,62 |
| DecaA:N8888Cl | 1.5:1 | 0.88 | (55)(62), | ||
| 2:1 | 0.88 | 1.95 (M) | 472.5 | (20) | |
| DecaA:P14,666Cl | 2:1 | 0.81 | (61) | ||
| DecaA:N8881Cl | 1:1 | 0.89 | 13.0 (M) | 1214.0 | (58) |
| 1:2 | 0.89 | 1.0 (M) | 2515.0 | (53),58 | |
| 2:1 | 0.89 | 2.0 (M) | 288.0 | (58) | |
| 3:1 | (59) | ||||
| DecaA:Thy | 1:1 | 0.93 | (46) | ||
| DecaA:[TOPO] | 1:1 | 0.88 | 44.1 | (25),42 | |
| 1:2 | 0.88 | 39.0 | (25),63 | ||
| DecaA+oleic acid:N4444Br | 2:1 | 1.02 | –7.9 (M) | 628.5 | (64) |
| DecaA+DodecaA:N4444Br | 5:4 | 0.99 | –8.2 (M) | 211.3 | (64) |
| decyl alcohol:N8881Cl | 2:1 | (27) | |||
| DodecaA:dl-menthol | 1:2 | 0.89 | 15.0 (M) | 27.3 | (47),57 |
| DodecaA:menthol | 3:1 | 18.2 (M) | (49) | ||
| DodecaA:Lid | 2:1 | 0.94 | (46) | ||
| DodecaA:[TOPO] | 1:1 | (25) | |||
| 1:2 | 0.88 | 46.5 | (63) | ||
| dodecanol:N4444Br | 2:1 | 0.91 | 1.0 (M) | 366.5 | (32) |
| dodecanol:N8888Cl | 2.5:1 | (65) | |||
| dodecyl alcohol:N8881Cl | 2:1 | (27) | |||
| [DHTU]:[TOPO] | 1:2 | (25) | |||
| [DTBC]:[TOPO] | 1:1 | (25) | |||
| 1-dodecanol:menthol | 1:1 | (21) | |||
| 1,2-decanediol:thymol | 1:2 | 0.95 | 42.5 | (18) | |
| 1,2-decanediol:[TOPO] | 1:2 | (25) | |||
| 3,5-di-tert-butylcatechol:[TOPO] | 1:2 | (25) | |||
| dl-menthol:acetic acid | 1:1 | 0.93 | –7.8 | (66) | |
| dl-menthol:DodecaA | 2:1 | 0.89 | –7.1; 13.8 | (66) | |
| dl-menthol:lactic acid | 1:2 | 1.03 | –61.1 | (66) | |
| dl-menthol:Lid | 5:5 | 0.90 | –54.71 | 38.6 | (67) |
| dl-menthol:N8881Cl | 2:1 | (27) | |||
| dl-menthol:PS | 7:3 | 0.88 | 13.2 (M) | 24.9 | (67) |
| dl-menthol:pyruvic acid | 1:2 | 0.99 | –58.8; −6.7 | (66) | |
| ethylene glycol:N8888Br | 3:1 | 1.02 | (68) | ||
| ethylene glycol:N8881Cl | 2:1 | (27) | |||
| formic acid:menthol | 1:1 | (21) | |||
| Gemfibrozil:N8881Cl | 1:1 | 0.94 | –70.0 | 3040.0 | (58) |
| 1:2 | 0.92 | –57.0 | 3034.0 | (58) | |
| glycerol:ChCl | 1.5:1 | 1.22 | (68) | ||
| glycerol:N8881Cl | 2:1 | (27) | |||
| HeptaA:N4444Br | 2:1 | –17.2 (M) | (51) | ||
| HeptaA:thymol | 2:1 | (69) | |||
| Heptanol:N4444Br | 2:1 | –10.0 (M) | (70) | ||
| HexaA:menthol | 1:1 | (21) | |||
| 2:1 | 0.91 | (50) | |||
| 3:1 | 0.86 | (71) | |||
| HexaA:N4444Cl | 3:1 | (72) | |||
| HexaA:N8888Br | 2:1 | 0.73 | (61) | ||
| hexyl alcohol:N8881Cl | 2:1 | (27) | |||
| 1-hexanoic acid:[TOPO] | 1:1 | (28) | |||
| hexanol:dl-menthol | 2:1 | (73) | |||
| 1-hexanol:[TOPO] | 1:1 | (28) | |||
| n-hexyl alcohol:N8888Br | 2:1 | 0.90 | –85.0 (M) | 29.3 | (32) |
| [HFIP]:betaine | 2:1 | 1.48 | –39.4 (M) | 76.0 | (74) |
| [HFIP]:l-carnitine | 2:1 | 1.50 | –18.7 (M) | 698.0 | (74) |
| [HFIP]:Brij-35 | 5:1 | 1.22 | –10.68 (M) | 110.6 | (75) |
| 10:1 | 1.33 | –13.26 (M) | 76.7 | (75) | |
| 15:1 | 1.39 | –28.57 (M) | 55.7 | (75) | |
| 20:1 | 1.42 | –13.35 (M) | 23.5 | (75) | |
| [HFIP]:PONPE-7.5 | 5:1 | 1.27 | –23.86 (M) | 40.2 | (75) |
| [HFIP]:Triton X-100 | 5:1 | 1.26 | –18.79 (M) | 90.6 | (75) |
| [HFIP]:Triton X-114 | 5:1 | 1.29 | –19.28 (M) | 40.8 | (75) |
| Ibu:N7777Cl | 3:7 | 0.89 | 1029.0 | (57) | |
| isoamyl alcohol:N8881Cl | 4:1 | (76) | |||
| Ketoprofen:N8881Cl | 1:1 | 0.99 | –46.0 | 4717.0 | (58) |
| 1:2 | 0.99 | –57.0 | 4670.0 | (58) | |
| 2:1 | 1.02 | –40.0 | 4915.0 | (58) | |
| lactic acid:serine | 3:1 | 0.88 | 1.0 (M) | 209.41 | (77) |
| 4:1 | 0.91 | 5.0 (M) | 83.21 | (77) | |
| 5:1 | 0.94 | 11.0 (M) | 76.27 | (77) | |
| lactic acid:N8881Cl | 3:1 | (78) | |||
| levulinic acid:thymol | 3:1 | 1.07 | (71) | ||
| l-menthol:[TOPO] | 1:1 | (28) | |||
| [MAA]:Lid | 9:1 | 1.11 | –61.1 | 10.8 | (67) |
| [MAA]:PS | 9:1 | 1.12 | –63.2 | 9.4 | (67) |
| menthol:DecaA | 3:2 | 0.87 | 8.8 (M) | 4.5 | (79) |
| menthol:DodecaA | 3:1 | 0.86 | 21.2 (M) | 5.4 | (79),80 |
| menthol:HexadecaA | 17:3 | 0.86 | 33.1 (M) | 6.0 | (79) |
| menthol:OctaA | 3:2 | 0.87 | –1.8 (M) | 3.8 | (79) |
| menthol:OctadecaA | 9:1 | 0.86 | 37.8 (M) | 6.2 | (79) |
| menthol:TetradecaA | 4:1 | 0.86 | 26.6 (M) | 5.8 | (79) |
| menthol:Lid | 2:1 | 0.94 | 59.0 | (18) | |
| menthol:thymol | 3:1 | 0.91 | (71) | ||
| [MTC]:[MHB] | 1:1 | 0.96 | –77.7 | 1088 | (81) |
| 1:2 | 1.01 | –57.8 | 967 | (81) | |
| 2:1 | 0.93 | –77.9 | 2437 | (81) | |
| [MTC]:[BHB] | 1:1 | 0.95 | –24.2 | 1435 | (81) |
| 1:2 | 0.98 | –60.4 | 778 | (81) | |
| 1:3 | 1.04 | –65.1 | 910 | (81) | |
| 2:1 | 0.92 | –77.7 | 1547 | (81) | |
| [MTC]:[IHB] | 1:1 | 0.94 | –37.0 | 1525 | (81) |
| 1:2 | 0.97 | –62.3 | 1807 | (81) | |
| 1:3 | 1.05 | –58.6 | 2031 | (81) | |
| 2:1 | 0.92 | –36.7 | 1530 | (81) | |
| [MTC]:[OHB] | 1:1 | 0.93 | –77.9 | 1526 | (81) |
| 1:2 | 0.96 | –78.7 | 1045 | (81) | |
| 1:3 | 0.97 | –77.6 | 930 | (81) | |
| 2:1 | 0.92 | –77.6 | 1491 | (81) | |
| [MTC]:[EHB] | 1:1 | 0.94 | –76.4 | 1680 | (81) |
| 1:2 | 0.96 | –78.3 | 1730 | (81) | |
| 1:3 | 0.97 | –78.5 | 1327 | (81) | |
| 1:4 | 0.98 | –79.1 | 1490 | (81) | |
| 2:1 | 0.92 | –69.1 | 1436 | (81) | |
| 1-napthol:menthol | 1:2 | 0.98 | 74.4 | (18) | |
| 4-nitrobenzaldehyde:N4444Br | 1.5:2.2 | 1.02 | (68) | ||
| NonaA:DodecaA | 3:1 | 0.89 | 9.0 (M) | 8.6 | (41) |
| n-NonaA:menthol | 1:3 | 9.8 (M) | (49) | ||
| OctaA:menthol | 1:1 | (21) | |||
| 2:1 | 0.90 | (50) | |||
| 3:1 | 0.90 | (71) | |||
| OctaA:dl-menthol | 1:1 | (47) | |||
| OctaA:N4444Br | 2:1 | 0.97 | 0.58 (M) | (52),60 | |
| OctaA:N8881Cl | 1:2 | (53) | |||
| 2:1 | (60) | ||||
| OctaA:thymol | 1:2 | (82) | |||
| 1:3 | (82) | ||||
| 3:1 | (82) | ||||
| 4:1 | (82) | ||||
| 5:1 | (82) | ||||
| octanol:menthol | 1:1 | (83) | |||
| 1-octanol:N4444Br | 2:1 | (84) | |||
| 1-octanol:N8881Cl | 2:1 | (27) | |||
| 1-octanol:[TOPO] | 1:1 | (28) | |||
| 2-octanol:menthol | 1:3 | 10.7 (M) | (49) | ||
| 2-octanol:N8881Cl | 2:1 | (60) | |||
| n-octyl alcohol:menthol | 1:2 | 15.5 (M) | (49) | ||
| n-octyl alcohol:DecaA | 2:1 | 0.84 | –55.0 (M) | 18.8 | (32) |
| n-octyl alcohol:N4444Br | 2:1 | 0.94 | –26.0 (M) | 317.5 | (32) |
| n-octyl alcohol:N8888Br | 2:1 | 0.86 | –82.0 (M) | 494.7 | (32) |
| n-octyl alcohol:DecaA | 2:1 | 0.89 | –15.0 (M) | 573.8 | (32) |
| oleic acid:menthol | 1:3 | 6.2 (M) | (49) | ||
| oleic acid:N4444Br | 2:1 | 0.95 | 1.86 (M) | (52) | |
| oleic acid:N7777Cl | 2:1 | 0.87 | 244.7 | (57) | |
| oleic acid:N8881Cl | 2:1 | (60) | |||
| oleyl alcohol:menthol | 1:1 | (21) | |||
| perfluorodecanoic acid:N8888Cl | 2:1 | (62) | |||
| perfluorooctanol:N4444Cl | 1:1 | 1.15 | 7.0 (M) | 264.0 | (85) |
| 1:2 | 1.19 | 12.0 (M) | 319.0 | (85) | |
| 1:3 | 1.29 | 25.0 (M) | (85) | ||
| 2:1 | 1.09 | –1.0 (M) | 198.0 | (85) | |
| 3:1 | 1.02 | 4.0 (M) | 81.0 | (85) | |
| PropaA:menthol | 1:1 | (21) | |||
| 4-phenylbutyric acid:N4444Br | 2:1 | 5.10 (M) | (51) | ||
| pyruvic acid:dl-menthol | 2:1 | (86) | |||
| 1-propanol:N8881Cl | 2:1 | (27) | |||
| 1,3-propanediol:N8881Cl | 2:1 | (27) | |||
| 1,5-pentadiol:N8888Br | 3:1 | 1.00 | (68) | ||
| salicylic acid:l-menthol | 1:4 | 0.95 | 23.00 (M) | (87) | |
| 1-tetradecanol:menthol | 1:2 | 0.87 | 36.6 | (18) | |
| 1-tetradecanol:N8881Cl | 2:1 | (27) | |||
| 1-tetradecanol:thymol | 1:2 | (19) | |||
| thymol:[BTEAC] | 4:1 | (88) | |||
| thymol:camphor | 1:1 | 0.98 | –44.0 (M) | 25.8 | (89) |
| 3:2 | 0.97 | –37.0 (M) | 20.5 | (89) | |
| 7:3 | 0.96 | –33.0 (M) | 18.8 | (89) | |
| thymol:ChCl | 5:1 | (90) | |||
| thymol:coumarin | 1:1 | 1.09 | 25.8 | (18) | |
| 2:1 | 1.05 | 26.7 | (18) | ||
| thymol:DecaA | 1:1 | 0.94 | 17.0 (M) | 11.2 | (89) |
| 1:1 | 0.90 | 18.86 (M) | 3.7 | (79) | |
| 1:2 | 0.93 | 18.0 (M) | 10.8 | (89) | |
| 1:3 | 0.92 | 19.0 (M) | 10.4 | (89) | |
| 3:2 | 0.95 | 18.0 (M) | 13.0 | (89) | |
| thymol:DodecaA | 11:9 | 0.89 | 24.83 (M) | 4.4 | (79) |
| thymol:HexadecaA | 4:1 | 0.91 | 41.22 (M) | 4.5 | (79) |
| thymol:Lid | 1:1 | 0.99 | 149.8 | (18) | |
| 2:1 | 0.99 | 100.2 | (18),46 | ||
| thymol:menthol | 1:1 | 0.93 | 42.0 | (18),50 | |
| 1:2 | 0.92 | 52.1 | (18) | ||
| 2:1 | 0.94 | (50) | |||
| 3:1 | 0.95 | (50) | |||
| 4:1 | 0.95 | (50) | |||
| thymol:N8888Cl | 2:1 | (90) | |||
| thymol:OctaA | 21:29 | 0.90 | 6.68 (M) | 2.8 | (79) |
| thymol:OctadecaA | 9:1 | 0.92 | 46.22 (M) | 4.0 | (79) |
| thymol:TetradecaA | 3:1 | 0.91 | 38.16 (M) | 4.3 | (79) |
| thymol:[TEPA]Cl | 3:1 | 1.05 | 295.7 | (91) | |
| thymol:[TEPA]Cl | 5:1 | 1.04 | 198.8 | (91) | |
| thymol:[TETA]Cl | 3:1 | 1.03 | 205.1 | (91) | |
| thymol:[TETA]Cl | 5:1 | 1.04 | 69.1 | (91) | |
| thymol:[TMGH]Cl | 2:1 | (90) | |||
| thymol:[TOPO] | 1:1 | 0.89 | 69.9 | (28),42 | |
| thymol:10-undecylenic acid | 1:1 | 0.94 | 11.0 (M) | 13.2 | (89) |
| 1:2 | 0.93 | 10.0 (M) | 13.1 | (89) | |
| 1:3 | 0.93 | 9.0 (M) | 12.4 | (89) | |
| 1:4 | 0.93 | 7.5 (M) | 11.8 | (89) | |
| 3:2 | 0.95 | 16.5 (M) | 14.4 | (89) | |
| 7:3 | 0.95 | 19.0 (M) | 15.6 | (89) | |
| thymol:vanillin | 1:1 | (92) |
Glass transition temperature of the HDESs. “M: denotes melting temperature.
2. Applications of HDESs
2.1. Extraction of Organic Compounds from Aqueous Solutions
2.1.1. Volatile Fatty Acids
As industrialization and the human population have increased, so has waste generation, resulting in environmental degradation. Organic waste can be processed and converted into volatile fatty acids (VFAs), which can be considered an alternative pathway to the production of petroleum-based chemicals.93,94 VFAs are composed of short-chain monocarboxylic acids containing five or fewer carbon atoms.95 VFAs can be produced via fermentation,96,97 can be extracted from water with the help of the liquid–liquid extraction (LLE) method,94,98 and so on. Recently, HDESs have been successfully used as solvent media in the extraction of VFAs due to their advantages99 over their counterparts. In 2015, the first use of HDESs for removing VFAs from a diluted aqueous solution was tested.20 This work used six HDESs comprising decanoic acid as a hydrogen bond donor and quaternary ammonium salts as hydrogen bond acceptors. For examining their extraction properties, the VFAs, namely, acetic acid (CH3COOH), propionic acid (C2H5COOH), and butyric acid (C3H7COOH) were taken as water contaminants. Finally, the results obtained were compared with a conventional extracting agent, trioctylamine (TOA) (Table 2). It was observed that all six HDESs exceeded the TOA in terms of extraction performance, and this ability of extraction increases with the increment in chain length of the VFAs. More particularly, decanoic acid:methlytrioctylammonium chloride (DecaA:N8881Cl) in the ratio 2:1 extracted maximum VFAs, most presumably because the CH3 group leads to less steric hindrance. However, in the presence of turbidities in the aqueous medium after centrifugation, the extraction of butyric acid and propionic acid by the DES comprising of decanoic acid and tetraheptylammonium chloride (DecaA:N7777Cl) in the ratio 2:1 was found to be higher, implying that no proper phase separation had taken place. In addition to that, the extraction abilities of the HDESs decreased when chloride was replaced with bromide anion. This was because of the increase in steric hindrance in the chemical structures of the respective DESs in the presence of bromide anion.
Table 2. Extraction Efficiencies (in Terms of Percentage) of Acetic Acid (CH3COOH), Propionic Acid (C2H5COOH), and Butyric Acid (C3H7COOH) from Water with Hydrophobic DESs and Industrial Extracting Agent Trioctylamine20.
| extraction
efficiency (%) |
|||
|---|---|---|---|
| hydrophobic DESs | CH3COOH | C2H5COOH | C3H7COOH |
| DecaA:N8881Cl (2:1) | 38.0 | 70.5 | 89.8 |
| DecaA:N7777Cl (2:1) | 32.0 | 76.5a | 91.5a |
| DecaA:N8888Cl (2:1) | 25.0a | 52.7 | 81.3 |
| DecaA:N8881Br (2:1) | 29.7 | 63.4 | 83.1 |
| DecaA:N8888Br (2:1) | 30.6 | 65.9 | 87.4 |
| TOA | 18.6 | 45.9 | 73.5 |
The top phase (DES phase) was turbid. Here, DecaA = decanoic acid.
Another study related to VFA extractions by HDESs was reported in 2019,25 where numerous combinations of 16 hydrophobic substituents of DESs were analyzed. The hydrophobicity of the DESs in this study was determined by two criteria: (a) each of the DES components should have a water solubility of less than 1 g L–1, and (b) they should have a logarithmic water–octanol partition coefficient greater than 4. It was noticed that DES made of dihexylthiourea and trioctylphosphine (TOPO) had an efficient extraction ability because of its high stability over a wide pH range. The extraction ability of the DES TOPO:dihexylthiourea increased toward VFAs with the increment in the hydrophobicity of the VFAs, in the order of acetic acid < propionic acid < butyric acid. This result goes with the findings of other studies100,101 related to the use of nonprotic organic solvents (like kerosene, hexane, and methyl isobutyl ketone) for extracting propionic acid and butyric acid via TOPO.
Again, Riveiro and colleagues investigated the extraction of adipic, levulic, and succinic acids from water using two hydrophobic TOPO-based DESs as alternatives to organic solvents.63 However, the extraction efficiencies of the HDESs were found to be lower than those of TOPO.
It has been presumed that the interactions within components of the HDESs may skew the vital attractive forces between DES and VFAs, thereby reducing the effectiveness of elimination of VFAs.102−104 Therefore, a novel strategy is required to tackle this problem by modifying the initial DES constituents that lessen the interference with the intermolecular forces of attraction and repulsion required for the removal of volatile fatty acids.
2.1.2. Biomolecules
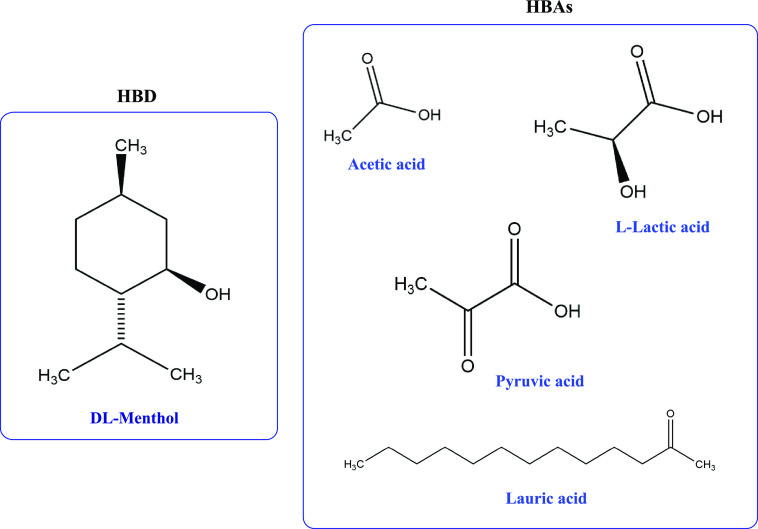
The application of HDESs was first studied by the team of Marrucho in 2015,66 where they used HDESs based on dl-menthol and naturally occurring acids for the removal of different biomolecules from water (Figure 1). Out of the four HDESs, namely, dl-menthol:lactic acid with molar ratio 1:2, dl-menthol:dodecanoic acid with molar ratio 2:1, dl-menthol:pyruvic acid with molar ratio 1:2, and dl-menthol:acetic with molar ratio 1:1, except the one with pyruvic acid, showed sufficiently high partition coefficients for the removal of tryptophan, tetracycline, caffeine, and vanillic acid. The presence of an absorption band in the UV–visible region at about 350 nm, caused by the π* ← n transition because pyruvic acid is a keto acid, made it impossible to evaluate partition data using dl -menthol:pyruvic acid. The results show that at a pH of 1.18, the partition coefficient values obtained were sufficiently high for the elimination of tryptophan and caffeine from an aqueous solution using the HDES comprising menthol and lactic acid with ratio of 2:1. All HDESs had modest partition coefficients for vanillin, while the highest partition coefficient for isopthalic acid was obtained with the same DES that consists of menthol and lactic acid with a ratio 2:1 at a pH of 1.47.
Figure 1.
Chemical structure of HBDs (hydrogen bond donors) and HBAs (hydrogen bond acceptors) used for the preparation of the menthol-based HDESs used in the study. Adapted from ref (66). Copyright 2015 American Chemical Society.
Another important application of HDESs was studied in the field of biorefineries for the production of 5-hydroxymethylfurfural (HMF) in an aqueous solution.46 A total of eight HDESs, namely, decaA:n-tetraoctylammonium bromide (2:1), decaA:lidocaine (2:1), decaA:lidocaine (3:1), decaA:lidocaine (4:1), decaA:thymol (1:1), decaA:menthol (1:1), thymol:lidocaine (2:1), and dodecaA:lidocaine(2:1) were prepared, and their solubility in HMF and water was examined. Here, decaA and dodecaA stand for decanoic acid and dodecanoic acid, respectively. The solubilities of HMF in three selected DESs (decanoic acid and thymol in ratio 1:1, decanoic acid, and n-tetraoctylammonium bromide in ratio 2:1, and decanoic acid and lidocaine in ratio 2:1) were found to be 80, 75, and 85 wt %, respectively. Furthermore, the HMF solubility values obtained from the experiment were validated using PC-SAFT (perturbed-chain statistical associating fluid theory) modeling.
2.1.3. Micropollutants
Water pollution is a hot topic in today’s world. In addition to traditional water pollutants, micropollutants are a new type of pollutant present in water at trace concentrations ranging from ng/L to mg/L.105,106 Despite their low concentration, these pollutants pose a serious threat to human and animal health due to their adverse effects on endocrine function, antibiotic resistance, and short- and long-term toxicity.107 Scientists all over the world are working tirelessly to find effective ways to remove these pollutants from wastewater. In this regard, HDESs have received a lot of attention because they are a biofriendly, cost-effective, and less-toxic solvent. Below are some studies where HDESs were used to extract or eliminate these toxic micropollutants from wastewater.
2.1.3.1. Industrial Wastes
A study related to the elimination of Bisphenol A, a microcontaminant, was reported using three fatty acid–based HDESs, namely, decanoic acid (C10):dodecanoic acid (C12) (2:1), nonanoic acid (C9):dodecanoic acid (C12) (3:1), and octanoic acid (C8):dodecanoic acid (C12) (3:1).41 In addition to these three binary HDESs, the extraction abilities of Bisphenol A using ternary HDESs were also calculated, the details of which are shown in Table 3. Out of the three binary HDESs, the extraction efficiency of Bisphenol A was found to be highest for DES nonanoic acid (C9):dodecanoic acid (C12) (3:1) i.e., 88.32% (Figure 2). On the other hand, for ternary HDESs, nonanoic acid (C9):decanoic acid (C10):dodecanoic acid (C12) with the molar ratio 2:2:1 had the highest extraction ability of 91.52%. In all cases, however, ternary HDESs had a higher extraction ability of Bisphenol A as compared to binary HDESs. Again in another study, the elimination of Bisphenol A from an aqueous environment was carried out with the help of menthol-based HDESs.21 Nine HDESs were studied in a ratio of 1:1. The HDESs menthol:propionic acid and menthol:formic acid were found to possess the highest extraction properties of 98.2 and 99.0%, respectively. They concluded that the extraction efficiencies of the HDESs were greatly affected by the nature of the hydrogen bond donors that were used.
Table 3. Extraction Efficiencies (%) of the Microcontaminant Bisphenol A with the Help of Binary and Ternary HDESsa That Were Based on Fatty Acids41.
| fatty-acid-based HDESs | molar ratio | extraction efficiency (%) | |
|---|---|---|---|
| binary HDESs | OctaA:DodecaA | 3:1 | 76.04 ± 1.13 |
| NonaA:DodecaA | 3:1 | 88.32 ± 0.23 | |
| DecaA:DodecaA | 2:1 | 81.81 ± 0.34 | |
| ternary HDESs | OctaA:NonaA:DodecaA | 1:1:1 | 85.49 ± 0.86 |
| OctaA:NonaA:DodecaA | 1:2:1 | 84.53 ± 0.43 | |
| OctaA:NonaA:DodecaA | 2:1:1 | 82.34 ± 1.10 | |
| OctaA:NonaA:DodecaA | 3:1:1 | 79.42 ± 0.54 | |
| OctaA:NonaA:DodecaA | 3:2:1 | 80.32 ± 0.78 | |
| NonaA:DecaA:DodecaA | 1:1:1 | 87.65 ± 1.06 | |
| NonaA:DecaA:DodecaA | 1:2:1 | 87.81 ± 0.67 | |
| NonaA:DecaA:DodecaA | 2:1:1 | 89.01 ± 0.72 | |
| NonaA:DecaA:DodecaA | 2:1:1 | 89.06 ± 0.34 | |
| NonaA:DecaA:DodecaA | 2:2:1 | 91.52 ± 0.41 | |
| NonaA:DecaA:DodecaA | 3:2:1 | 90.50 ± 0.57 | |
| OctaA:DecaA:DodecaA | 1:1:1 | 82.77 ± 1.03 | |
| OctaA:DecaA:DodecaA | 2:1:1 | 79.45 ± 0.46 | |
| OctaA:DecaA:DodecaA | 3:1:1 | 77.75 ± 0.72 | |
| OctaA:DecaA:DodecaA | 3:2:1 | 79.62 ± 0.58 | |
Stirring speed = 300 rpm, ratio DES/water = 1:1, T = 25 °C, mixing time = 15 min. Again, OctaA = octanoic acid, NonaA = nonanoic acid, DecaA = decanoic acid, and DodecaA = decanoic acid.
Figure 2.
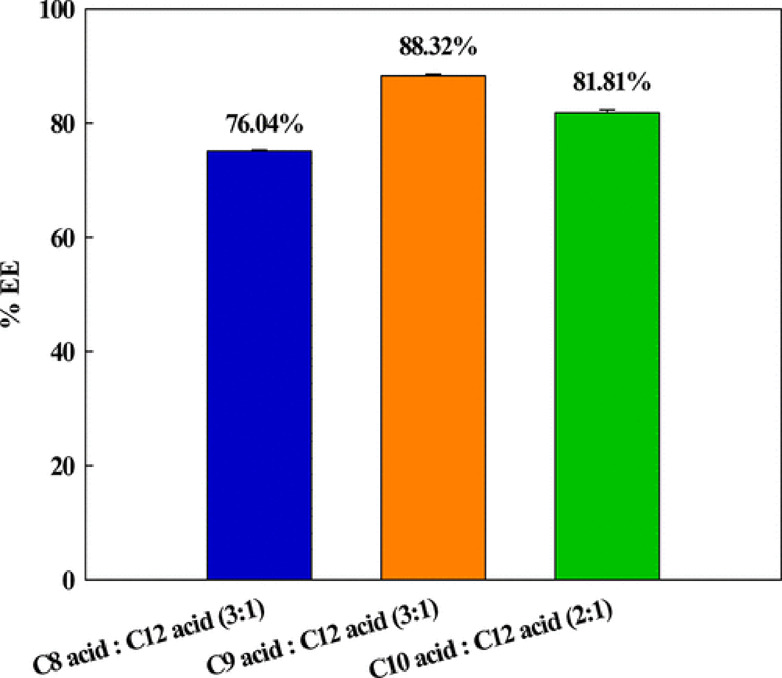
Extraction efficiencies (%) of Bisphenol A using the developed binary fatty-acid-based HDESs. Reprinted from ref (41). Copyright 2018 American Chemical Society.
Chlorophenol, a potential environmental hazard, is known to possess genotoxicity, carcinogenicity, mutagenicity, etc. Therefore, the extraction of 3-chlorophenol, 2-chlorophenol, and 2,4-dichlorophenols from wastewater was carried out experimentally using menthol-based HDESs50 in different molar ratios. The experimental results showed extraction ability higher than 94% for all the chlorophenol species. For the menthol–alkanoic acid HDESs, the extraction efficiencies of the chlorophenols proceeded in the following order: 2,4-dichlorophenol < 2-chlorophenol < 3-chlorophenol. Moreover, the extraction process was modeled with the help of the conductor-like screening model for real solvents (COSMO-RS). The extraction of chlorophenols by the HDESs was observed to be due to hydrogen bonding and hydrophobic interactions.
Apart from the already mentioned micropollutants, isopropanol compounds are another prevalent toxic component of wastewater that has been widely disposed of by various cosmetic, rubber, etc. industries into watersources.108,109 A study on the separation of isopropanol from aqueous solution was reported using liquid–liquid extraction with the help of two HDESs, namely, 1-decanol:methlytrioctylammonim chloride (2:1) and 1-hexanol:methyltrioctylammonium chloride (2:1).110 The distribution coefficient and separation factor for isopropyl alcohol extraction were found to be in the ranges of 1.38–4.13 and 2.50–23.02, respectively, which indicated that the two HDESs were effective at removing isopropyl alcohol from wastewater.
Another class of biopollutants present in wastewater is polycyclic aromatic hydrocarbons (PAHs). They have attracted much attention due to their toxic, mutagenic, and carcinogenic properties, and so the removal of these compounds from wastewater is of utmost necessity in today’s world.111−113 A number of natural and nonionic HDESs comprising camphor, decanoic acid, 10-undecylenic acid, and thymol were prepared and tested for the removal of PAHs from aqueous environments.89 A total of 16 different PAHs, such as biphenyl, fluorine, anthracene, pyrene, naphthalene, etc., in the range of 0.12–46.2 μg/L (in terms of concentration), were extracted by these HDESs. In another study, carboxylic acid-based HDESs with the composition of tetrabutylammonium bromide with decanoic acid, acrylic acid, octanoic acid, propionic acid, oleic acid, butyric acid, and acetic acid were used to extract PAHs from the water via solidification of the floating drop microextraction method.52 Out of all of the seven HDESs, decanoic acid:tetrabutylammonium bromide (2:1) was found to be the most desirable solvent for the elimination of PAHs. Moreover, analysis of the six different kinds of PAHs (fluoranthene, anthracene, pyrene, fluorene, naphthalene, and phenanthrene) in the samples from the aqueous solutions revealed a high extraction rate ranging from 83 to 117%. As a result, using HDESs instead of traditional organic solvents improved the efficiency, simplicity, speed, cost-effectiveness, and environmental friendliness of the extraction process, which was based on the solidification of the floating drop.
2.1.3.2. Pesticides and Dyes
Pesticides of various types are used in agriculture to control pest diseases and maintain high-quality products.114 However, some nonbiodegradable pesticides accumulate in plants, water, and animal bodies, endangering human health throughout the food chain.115−117 Florindo et al. used two different families of HDESs in order to remove four pesticides, namely, imidacloprid, acetamiprid, nitenpyram, and thiamethoxam, from water.47 With an extraction efficiency of 80%, the HDESs comprising dl-menthol with dodecanoic acid, decanoic acid, and octanoic acid in the molar ratios of 2:1, 1:1, and 1:1, respectively, displayed as the most stable HDESs in aqueous solution. The findings also indicated that tetrabutylammonium chloride consisting of HDESs was less effective than dl-menthol consisting of DESs at extracting pesticides.
In another study, a series of HDESs were synthesized using hexafluoroisopropanol and l-carnitine or betaine and tested as solvents for eliminating pyrethroid from tea beverages and fruit juices.74 Out of all, HDES l-carnitine:hexafluoroisopropanol showed the highest extraction capacity via dispersive liquid–liquid microextraction. In addition to that, this novel HDES had more advantages in comparison to other standard solvents, like chloroform, chlorobenzene, tetrachloromethane, etc.,118−121 because of their optimum efficiency rate of extraction (85–109%), short extraction time (5.3 min), less expensive, and high recovery (85–109%). Moreover, the HDES comprising l-carnitine and hexafluoroisopropanol was found to be nonvolatile as well as nonflammable, which also makes it less harmful to the environment and safer for human health than typical solvents.
Again, Liu and his team studied the extraction of pyrethroid from different environmental water samples using three HDESs, namely, trihexyl(tetradecyl)phosphonium tetrafluoroborate:decaA, trihexyl(tetradecyl)phosphonium tetrafluoroborate:dodecaA, and trihexyl(tetradecyl)phosphonium tetrafluoroborate:octaA.122 Here, decaA, dodecaA, and octaA stand for decanoic acid, dodecanoic acid, and octanoic acid, respectively. They separated pyrethroid from various water samples using the ultrasound-assisted dispersive liquid–liquid microextraction technique, and it was discovered that all three HDESs had high extraction abilities between 80.93 and 109.88%.
In a different study,51 HDESs comprising tetrabutylammonium bromide and fatty acids were used to extract five organophosphorus pesticides (OPPs) comprising azinphosemethyl (AZP), fenitrothion (FNT), diazinon (DIZ), parathionemethyl (PRT) and chlorpyrifos (CPF), and two dyes constituting malachite green (MG) and acid blue 29 (AB29) from wastewater, agricultural water, and soil samples. Deep eutectic solvent-embedded melamine sponge (DES-MS) was used in a quick and comparatively easy method that was both efficient and effective, with removal efficiencies for various pesticide samples exceeding 70%. Furthermore, under ideal conditions, the removal of different dyes was accomplished with an efficiency of greater than 65%.
2.1.3.3. Medical Components
Pharmaceutical products, mainly drugs and care products with a chemical base, have gained popularity as the world’s population continues to grow. Their importance in contemporary life cannot be overstated, but at the same time, their usage and disposal are raising serious concerns about environmental degradation. The drug manufacturing facilities frequently fail to filter out all of the chemicals employed in the process, which causes the chemicals to leak into nearby freshwater systems before finally reaching the sea, lakes, streams, and rivers, thus causing water pollution. Tang and his team carried out the extraction of two antibiotic drugs,123 ciprofloxacin and levofloxacin, using fatty acid/alcohol-based HDESs via the liquid–liquid microextraction method. Both ciprofloxacin and levofloxacin are commonly used as therapeutic and preventative antimicrobial medicines in aquaculture and animal husbandry.124,125 They found that 1-octanol:methyltriooctylammonium chloride (1:1) showed the highest extraction efficiency. In addition, the study demonstrated that when compared to ultrasound, heating, and microwave techniques, the vortex-assisted procedure was the most effective way for extracting ciprofloxacin and levofloxacin.
In another study, HDESs containing terpenes were tested for the extraction of riboflavin (vitamin B2) from water.18 Out of numerous combinations, finally, eight HDESs were selected for the extraction, namely, decanoic acid:menthol (1:1), 1-tetradecanol:menthol (1:2), decanoic:lidocaine (2:1), thymol:coumarin (1:1), thymol:coumarin (2:1), thymol:menthol (1:2), and thymol:menthol (1:1). With extraction efficiencies ranging from 20.5 to 81.1%, these DESs surpassed the efficiency of one of the first reported HDES tetraoctylammonium bromide:decanoic acid in the ratio 1:2.
Again, a HDES, namely, trioctylmethylammonium chloride:2-octanol (1:2),60 was reported to perform exceptionally well in the ultrasound assistance method for the extraction of synthetic antibiotic sulfonamides that are present in fruit juices. High yields of recovery (88.09 to 97.84%) were achieved using this ultrasonic-assisted microextraction methodology. It is consistent with a prior study84 on the removal of erythrosine pollutants from an aqueous solution, in which the HDES tetrabutylammonium bromide:1-octanol (1:2) emerged as a potent extracting agent based on ultrasonic-assisted methodology with high extraction rate (i.e., 90–100% yield) in contrast to prior published traditional procedures.126−129 Numerous chemical compounds have been extracted from different solid or liquid matrices using vortex- and ultrasonic-assisted methods.130−132 These results also imply that different types of antibiotics require different separation and removal techniques.
2.2. Extraction of Bioactive Compounds from Plant Sources
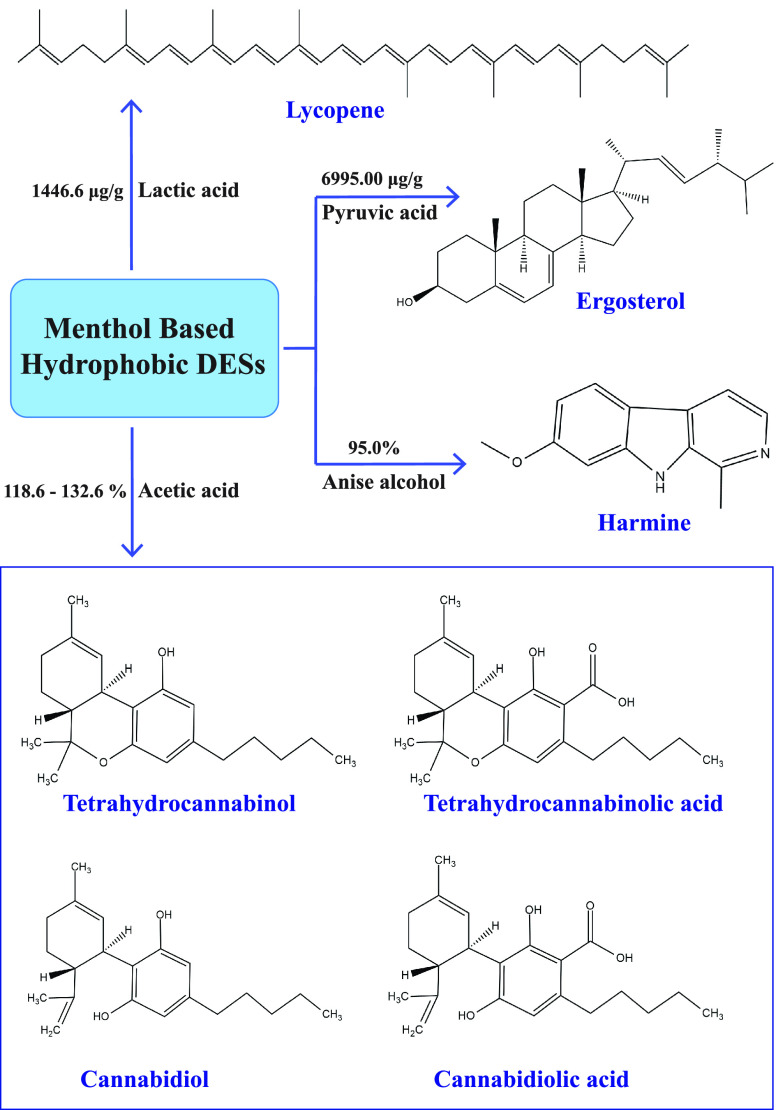
Apart from the extraction of VFAs and micropollutants from aqueous solutions, HDESs are also used for the extraction of several essential bioactive compounds from different plant sources. A study was reported recently where 39 HDESs were used for the extraction of ergosterol86 (precursor of vitamin D2) from a mushroom, Agaricus bisporus, via the response surface technique under optimized conditions. Out of all the HDESs, dl-menthol:pyruvic acid with a molar ratio of 1:2 exhibited the highest extraction capacity of 6995.00 μg/g. The DES was reused up to six times in a row with an extraction efficiency of 28%, and the extracted ergosterol was purified using a novel technique. The high stereoisomer similarity in the ergosterol compound can be attributed to the high removal rate demonstrated by menthol-based HDESs. Again, menthol-based HDESs were used for the extraction of harmine compounds under specific conditions.24 Studies indicate that harmine may exert neuroprotective, cognitively enhancing, and anti-inflammatory effects.133−135 The extraction ability of DES dl-menthol:anise alcohol (1:1) was found to be higher than the traditional organic solvents and ionic liquids at conditions such as pH 8.0, extraction time of 5 min, and 50:1 water to extractant ratio. Menthol-based HDESs were also used to extract lycopene (nonpolar antioxidant) from the pulpy residue of tomatoes.136 The maximum yield of lycopene was shown by HDES menthol:lactic acid, with a concentration of 1446.6 μg/g at conditions such as T = 70 °C, extraction time = 10 min, 120 mL of solvent over 1 g of sample. Additionally, HDESs composed of menthol and naturally occurring organic acids were tested for the removal of phytocannabinoids present in cannabis plants.22 In comparison to a traditional medium for phytocannabinoid elimination (for example, a mixture of methanol and chloroform in the ratio 9:1), the HDESs, notably menthol:acetic acid (1:1), performed exceptionally well with extraction efficiency ranging from 118.6 to 132.6%. Moreover, the extraction rate was better than that of methanol (97.7–112%) and ethanol (102.3–118.4%), two common organic solvents. All of the above work indicates that menthol-based HDESs are highly efficient in the extraction of bioactive compounds (Figure 3).
Figure 3.
Molecular structure of several bioactive compounds that were extracted using menthol-based HDESs. The HBD components along with respective extraction efficiencies are listed over the arrows.
Under ideal conditions, a HDES methyltrioctylammonium chloride:1-butanol with a molar ratio of 1:4 unveiled the highest yield (i.e., 7.9936 mg/g) in the extraction of artemisinin from the leaves of the Artemisia annua plant that is effective for treating malaria.27 The HDES had hydrophobicity nearly identical to that of hexane and petroleum ether, suggesting that it could be used to extract artemisinin.137 With extraction yields of 7.99 and 6.18 mg/g, respectively, methyltrioctylammonium chloride-based DESs, particularly methyltrioctylammonium chloride:1-butanol (1:4), performed better in comparison to petroleum ether.
In another study, the most effective DESs for the separation of carnosol and carnosic acid from the Rosemary plant were scanned using a thermodynamic model based on computational chemistry known as the COSMO-RS method.138 The interaction of carnosol and carnosic acid in terms of hypothetical thermodynamic properties in HDESs was discovered by using the COSMO-RS, and a total of 49 hydrogen bond donor and 28 hydrogen bond acceptor combinations were found to be the probable candidates of DESs for the extraction. With a yield of 18.9 mg/g of carnosol as well as 14.8 mg/g of carnosic acid, tetrapropylammonium chloride:1,2-propanediol:water (1:3.7:2) drew the maximum extraction rate. The experimental findings supported the findings of the COSMO-RS procedure and were in strong accord with earlier observations.50,139
Additionally, using a two-phase system made up of hydrophilic as well as hydrophobic DES, bioactive compounds with different polarities from Ginkgo biloba leaves were extracted in a study.140 The hydrophilic DESs were betaine and ethylene glycol at a ratio of 1:3 with a water content of 40% choline chloride, and malonic acid at a ratio of 1:2 with a water content of 55% and choline chloride and levulinic acid with a ratio of 2:1 with water content of 40%. The ternary DESs comprising methyltrioyctylammonium chloride, capryl alcohol, and octylic acid in the ratio of 1:2:3 was used as the HDES. The two-phase DES system was prepared by mixing the hydrophobic DES with hydrophilic DES at a volume ratio of 1:1. Procyanidine, flavonoids, polyprenyl acetates, and trilactones all had extraction yields of 94.63, 93.29, 86.07, and 77.72%, respectively, whereas polyprenyl acetates transitioned into the hydrophobic phase while flavonoids remained in the hydrophilic phase. Another two-phase system was studied,141 where hexafluoroisopropanol:choline chloride (1:1) was used as a hydrophilic DES, and menthol:tricaprylylmethylammonium chloride (2:1) was chosen as a HDES. From the leaves of Artemisia annua, this biphasic mixture successfully extracted four bioactive compounds, namely, anthocyanidins (8.9 mg/g), chlorogenic acid (7.86 mg/g), arteminism (6.21 mg/g), and quercetin (5.5 mg/g). Thus, it can be highly advantageous to use this two-phase approach for the elimination of various compounds that are bioactive and possess a wide range of polarities. Further, by adjusting the polarity of the DESs’ phases, we may enhance the utilization of this new DES system.
2.3. Extraction of Metal Ion from Aqueous Solutions
Population overgrowth has led to rapid industrialization and unplanned urbanization. As a result, industrial sectors such as, metallurgy, petroleum, and mining release substantial quantities of aqueous solutions polluted with a high level of toxic metals.142,143 Conventional solvent extraction procedures rely on volatile organic compounds (VOCs) and potentially harmful chemicals,144 necessitating the development of more effective and ecofriendly techniques for the recovery and removal of these metals and subsequent water purification. Extraction is typically quantified based on extraction efficiency (EE), given by the equation145
| 1 |
where C0 denotes the concentration of the analyzed metal ion in the aqueous medium prior to extraction while C1 is the analyte concentration in the aqueous medium following extraction.
Further, the distribution ratio (D) of the analyte is given by the equation43
| 2 |
Here, Maq,ini represents the initial metal concentration and Maq,eql represents the equilibrium metal concentration in the water phase.
Following the outset of research into the usage of HDESs in 2015, two articles were published that reported the removal of metallic species from aqueous solutions.44,57 Tereshatov et al. first reported the successful extraction of the near-critical strategic metal indium from hydrochloric and oxalic acid solutions into novel hydrophobic mixtures based on quaternary ammonium salts and menthol.57 Four distinct HDESs were employed in the study, viz. tetraheptylammonium chloride:decanoic acid (N7777Cl:DecaA)in 1:2 molar ratio, tetraheptylammonium chloride:oleic acid (N7777Cl:OleA) in 1:2 molar ratio, tetraheptylammonium chloride:ibuprofen (N7777Cl:Ibu) in 7:3 molar ratio, and dl-menthol:dodecanoic acid (menthol:DodecaA) in 2:1 molar ratio. Decanoic and dodecanoic acids were chosen based on literature data due to their low viscosity among the fatty acids.20,66 A kinetic analysis demonstrated the requirement of 5 min of shaking for the metal species to attain equilibrium in the water and DES phases. Additionally, the impact of concentration variations of hydrochloric and oxalic acids on the distribution coefficients was explored. The extraction process was shown to be aided by the addition of HCl and oxalic acid solutions to the water phase. The HCl concentrations ranged from 0.1 to 10 M HCl. The highest distribution coefficients were measured in 6 M HCl, with values of 20 (1:2 molar ratio N7777Cl:DecaA), 280 (1:2 molar ratio N7777Cl:OleA), and 600 (7:3 molar ration N7777Cl:Ibu), respectively. Menthol:DodecaA (2:1) solution, on the contrary, recorded the lowest distribution coefficient, less than 0.025 (v/v). Correspondingly, oxalic acid concentrations varied from 1 × 10–7 to 8 × 10–1 M. The highest distribution coefficient values of 3 × 103 and 1.5 × 103 were recorded for 7:3 molar ratio N7777Cl:Ibu and 1:2 molar ratio N7777Cl:DecaA or N7777Cl:OleA solutions, respectively. Successful reverse extraction of the metal ion to the water phase was also obtained by the formation of a stable indium complex with the addition of a 0.1 M solution of diethylene triamine pentaacetic acid (DTPA) to the aqueous phase. In a related study, the same group accomplished the removal and recovery of indium from HCl solutions employing low-viscosity, nonionic, hydrophobic binary mixtures based on active pharmaceutical and food-grade components.67 In 0.05 M HCl, the distribution coefficients of dl-menthol:lidocaine, MAA:lidocaine, dl-menthol:PS, and MAA:PS spanned from 2 to 800. Additionally, in certain systems containing PS and lidocaine as hydrogen bond donor (HBD) counterparts, an extraction efficiency (EE) exceeding 99% was observed for the HDESs.
The removal of metals from aqueous solutions is governed by several parameters. One such factor is the molar ratio of hydrogen bond acceptor to hydrogen bond donor in the binary mixtures, i.e., metal extraction from an aqueous medium depends significantly on HBA:HBD. The impact of DecaA:lidocaine molar ratio on the distribution coefficient was shown by van Osch et al.44 The group employed HDESs composed of a mixture of decanoic acid and lidocaine in 2:1, 3:1, and 4:1 molar ratios to extract a variety of metal chlorides, including LiCl, NaCl, KCl, MnCl2, FeCl2, CoCl2, NiCl2, CuCl2, and ZnCl2, without the addition of acids to the aqueous environment. The distribution coefficients obtained for the metal and chloride ions over the HDES and aqueous phases are presented in Table 4.
Table 4. Distribution Coefficients of the Metal and Chloride Ions Reported by van Osch et al. over the Aqueous and HDES Phases.44a.
| exp no. | ion | DecaA:Lid (2:1) | DecaA:Lid (3:1) | DecaA:Lid (4:1) |
|---|---|---|---|---|
| 1 | cobalt | >0.996 ± 0.001 | >0.996 ± 0.001 | 0.983 ± 0.002 |
| chloride | 0.113 ± 0.002 | 0.078 ± 0.008 | 0.101 ± 0.059 | |
| 2 | iron | >0.992 ± 0.001 | >0.991 ± 0.001 | >0.991 ± 0.001 |
| chloride | 0.197 ± 0.003 | 0.080 ± 0.001 | 0.113 ± 0.007 | |
| 3 | manganese | >0.992 ± 0.001 | >0.991 ± 0.001 | 0.983 ± 0.004 |
| chloride | 0.086 ± 0.002 | 0.081 ± 0.027 | 0.065 ± 0.011 | |
| 4 | potassium | 0.457 ± 0.001 | 0.397 ± 0.011 | 0.457 ± 0.001 |
| chloride | 0.141 ± 0.001 | 0.078 ± 0.031 | 0.072 ± 0.001 | |
| 5 | lithium | 0.266 ± 0.015 | 0.166 ± 0.001 | 0.128 ± 0.036 |
| sodium | 0.195 ± 0.001 | 0.140 ± 0.009 | 0.127 ± 0.040 | |
| potassium | 0.211 ± 0.028 | 0.161 ± 0.018 | 0.134 ± 0.005 | |
| cobalt | 0.990 ± 0.001 | 0.946 ± 0.012 | 0.777 ± 0.008 | |
| copper | >0.996 ± 0.001 | >0.996 ± 0.001 | >0.996 ± 0.001 | |
| nickel | >0.996 ± 0.001 | >0.983 ± 0.001 | 0.880 ± 0.004 | |
| zinc | >0.995 ± 0.001 | >0.995 ± 0.001 | >0.995 ± 0.001 |
D → 0 indicates low efficient ion extraction, i.e., the ion remains in the water phase, while D → 1 indicates highly efficient ion extraction into the DES phase.
The coefficient recorded lower values with an increase in the metal salt concentrations, specifically for CoCl2. This impact was negligible for the molar ratios 2:1 and 3:1 but it was fairly significant for the 4:1 HDES. A change in the DES:water mass ratios likewise exhibited a similar pattern. It was further demonstrated that the distribution coefficients obtained for the divalent metal ions were found to be higher, indicating more efficient extraction than the monovalent ions. An ion exchange mechanism induced by the interaction of the metal salts with partially positive-charged lidocaine was proposed to aid the extraction procedure. This mechanism proposed by van Osch et al. is supported by the following equations.
| 3 |
| 4 |
In accordance with this, a novel class of HDES was prepared by the binary combination of quaternary ammonium salt with parabens, and the impact of the HDESs’ molar ratio in the extraction of the hazardous metal Cr(VI) from water was evaluated.81 Methyltrioctylammonium chloride (MTC) acting as HBA was combined in a variety of molar ratios with the HBDs, viz., 2-ethylhexyl 4-hydroxybenzoate (EHB), methyl 4-hydroxybenzoate (MHB), isobutyl 4-hydroxybenzoate (IHB), butyl 4-hydroxybenzoate (BHB), and n-octyl 4-hydroxybenzoate (OHB). The extraction efficiency of Cr(VI) for each HDES was seen to depict a noticeable increase (∼50 to ∼95%) on altering the HBA:HBD ratios from 0.5:1 to 1:2, respectively, suggesting the increase in EE with an increase in paraben content in the HDESs.
The type of hydrogen bond donor and acceptor of the HDESs is another important aspect influencing metal ion extraction. Liu and co-workers investigated the influence of HBD of HDES on the recovery of Pt(IV) from secondary resources in HCl medium, using a 1:1 molar ratio of trioctylphosphine oxide (TOPO), and environmentally benign compounds such as 1-butyric acid, 1-hexanoic acid, 1-hexanol, 1-butanol, 1-octanol, l-menthol, and thymol.28 All of the HDESs prepared exhibited excellent Pt(IV) extraction selectivity, and the EE of Pt(IV) with the three HDESs surpassed 92%. TOPO:1-butanol, among the three, recorded the highest extraction ability for Pt(IV). For instance, in 5.6 mmol L–1 chloride solution, the EE of Pt(IV) followed the order: TOPO:l-menthol (94.4%) < TOPO:1-hexanol (98.3%) < TOPO/1-butanol (98.9%). This was attributed to the difference in hydrogen bonding energies between TOPO and the HBD reagents. Furthermore, the variation in the binding energies between the extractant and PtCl62– aided the process. Another article reported by Schaeffer et al. focused on the impact of varying lengths of the alkyl chain of carboxylic acids on the efficiency of copper(II) extraction from other transition metals, specifically cobalt(II) and nickel(II).79 The study employed biosourced sustainable HDESs based on menthol or its aromatic counterpart thymol mixed with long chain carboxylic acids bearing alkyl chains of 8, 10, 12, 14, 16, and 18 carbon groups. At pH 4.9 and 20 °C, the thymol:DecaA HDES displayed optimum selectivity in extracting Cu(II) from a concentrated aqueous solution (0.1 M) comprising other metal ions. Notably, the EE recorded a consistent decrease with an increase in the alkyl chain length. The possibility of recovering and recycling HDESs was also highlighted in the study. The same group of researchers further examined the influence of hydrogen bond acceptor counterparts of nonionic HDESs on the specific extraction of platinum group metals (PGMs) and transition metal ions in hydrochloric acid media.42 The HDESs employed comprised trioctylphosphine oxide (TOPO) and hydrocinnamic acid (HDC) as the HBA, while decanoic acid and thymol acted as the HBD in 1:1 molar ratios. These highly hydrophobic, low viscous binary solvents were capable of serving as both the extractant and the hydrophobic medium, establishing them as a viable option for solvent extraction procedures. At 2 M concentration of hydrochloric acid in aqueous solutions, TOPO-based HDESs demonstrated high selectivity toward PGMs over other transition metals. This higher extraction efficiency in the eutectic TOPO:DecaA solvent for most metals was attributed to the formation of their corresponding anionic halometalates in the aqueous media. On the contrary, the unfavorable electrostatic interactions arising between the anionic platinate and palladate chloro- complexes and the carboxylate ligands resulted in no significant metal removal/recovery on the application of the HDC:DecaA mixture to the aqueous phase. The volumetric ratio of DES to the aqueous medium was also proven to impart a substantial influence on the process of metal extraction. Phelps and co-workers reported the efficient recovery of tracer levels of radioactive pertechnetate (99mTcO4) ion from an aqueous solution consisting of excess competing anions, employing monocarboxylic acid-based HDESs.61 The deep eutectic mixtures employed comprised tetraoctylammonium (N8888+) or trihexyltetradecylphosphonium (P14666) with saturated fatty acids, viz. hexanoic acid or decanoic acid in 1:2 molar ratio. The group examined the impact of volumetric proportions of the extractant to an aqueous medium on the removal procedure of the pertechnate anion. The distribution ratio of the analyte ion between the DES and 0.15 M aqueous solution of ReO4– indicated a consistent decrease in its value, with a decrease in the volumetric ratio of the phase components. This was attributable to the surrogate perrhenate anion’s ReO4 effective interference with the extraction of the tracer-level pertechnate, which ultimately outcompeted the extractants’ ability to pick the tetra-oxo anions. Zante et al. reported another study that looked at the influence of the volume ratio of HDES to an aqueous medium on Ni ion removal from a leach liquor containing a combination of Li(I) and Ni(II).45 The extraction efficiency employing decanoic acid:lidocaine (DecaA:Lid) HDES in a 2:1 molar ratio was observed to increase with an increase in the volumetric ratio, resulting in a non-negligible recovery of the metal on the ratio exceeding a value of 1. However, to attain the requisite efficiency, the HDES-aqueous phase ratio of 1:1 was proven acceptable and economically viable.
The pH of the analyte solution has a substantial impact on the existing chemical state of the targeted analyte as well as the efficacy of their extraction procedures. To analyze the effect of pH on the extraction of two widely used metals, Fe(III) and Mn(II), the HDES comprised a 2:1 molar ratio of DecaA:Lid44 was further explored. Ola et al. employed 25 and 300 g/L of the HDES concentrations for the complete removal of Fe(III) and Mn(II) ions from the aqueous phase.43 The study revealed that the pH of the initial metal solutions had a significant impact on the extraction efficiency. In particular, a pH between 1.0 and 2.0 was ideal for the extraction of Fe(III). This was attributed to the interaction of the decanoic anion and Fe3+ ion pair. However, at pH >2.0, precipitation in the aqueous phase hindered the extraction procedure. Mn(II), on the other hand, was effectively separated at pH values of ≤2.2 and ≥3.5. In contrast, at pH levels ranging from 2.2 to 3.5, Mn(II) extraction was inefficient, presumably due to the predominance of the cation exchange reaction between lidocaine and Mn(II) ions in the extraction procedure. The extraction performance of HDESs is also measured with the help of extraction recovery (ER) and enrichment factor (EF) in metal removal processes. The ER(%) and EF are defined by the equations,81
| 5 |
| 6 |
where Vset and Cset designate the volume and concentration of the extractant medium while V0 and C0 are the initial volume and concentration of the aqueous medium, respectively.
Rad and co-workers investigated the impact of pH on nickel removal/recovery in water samples with pH ranging from 1 to 10.31 In the pH range of 7–9, the findings showed practically consistent recovery. Reduced recovery was seen at pH values less than 7, as H+ and Ni2+ compete in the complex formation process.146 The Cr(VI) and Cu(II) extraction studies stated in the preceding discussion, using TOMC-based and terpene-based HDESs, also explored the impact of pH on metal separation and recovery processes.79,81 Shi et al. recorded optimum extraction of Cr(VI) ion in the pH range of 2–5, attributable to the electrostatic interactions between the predominated form of chromium, HCrO4– and N+(R3R′) of TOMC, the HBA counterpart of the HDES, thereby resulting in enhanced Cr(VI) ion transfer from the aqueous to the DES phase. On pH exceeding 7, the extraction rate displayed a reduction due to a rise in the OH– ion concentration hindering the CrO4–DES interactions. Likewise, Cu(II) extraction also exhibited a decline with an increase in the pH of the solution.145 At pH less than 3, the extraction rate was minimal for both HDESs studied, while it increased and reached a maximum at pH 5.2. Beyond this threshold value, no increase in pH was recorded as a result of the hindrance offered by the hydrolysis of Cu(II) ions.
As demonstrated, metal extraction from buffered solutions offers a multitude of challenges, including hydroxide precipitation in alkaline solutions,44 the low solubility of metal ions such as Cu2+, Ni2, Cr3+ in phosphate buffer,54 and so on. To address these constraints, the use of HDESs in metal removal/recovery procedures in unbuffered aqueous solutions was investigated. The first report on the application of HDESs in unbuffered solutions includes the previously stated study by van Osch et al.,44 employing varied molar ratios of DecaA:Lid DESs. Analogous extraction experiments in unbuffered aqueous solutions were also conducted by Ruggeri et al. for the removal of Cr(VI) employing prototypical TBAC:DecaA HDES combined in a 1:2 molar ratio.54 According to the study, a 500 mM unbuffered Cr(VI) solution with a pH of 5.6 was able to selectively recover the Cr(VI) ion with 99% efficiency.
In addition to the numerous HDESs stated in the preceding discussion, an effort for the development of novel metal extraction methods facilitated the invention of highly selective, environmentally friendly supported liquid membrane (SLM) systems using HDES as the liquid phase.87 Shahrezaei et al. employed the SLM extraction method with a l-menthol:salicylic acid-based HDES as an optimal membrane carrier to selectively extract Ag+ ions to generate a highly selective metal–ligand complex in the absence of a carrier ligand. The process relied on the SA–Ag+ ion interaction, resulting in the formation of a hydrophobic complex. The formation of the strong anionic complex between Ag+ and thiosulfate anions present in the strip phase was found to be primarily responsible for the remarkable selectivity of the SLM system. These observations were consistent with previous findings that demonstrated the stable complexation of SA with silver ions through Ag-p interactions.147,148 Further, in comparison to previously reported SLM systems, the proposed HDES–SLM system demonstrated excellent permeability and improved selectivity for the extraction of silver ions from an aqueous medium consisting of a mixture of competing metal ions. The remarkable selectivity and effectiveness for removal and recovery of Ag+ ions with reduced transport times established the SA-based HDESs as suitable alternative solvents for the SLM systems over conventional supported liquid membranes.
2.4. Absorption of CO2
Another potentially significant use of HDESs is their CO2 absorption capacity. Anthropogenic emissions of greenhouse gases (GHGs), primarily CO2 have been steadily increasing since the preindustrial era, posing major environmental challenges to ecosystems and humanity. This almost certainly can be regarded as the primary cause of the recent unusual changes in the global climate system, necessitating a viable solution to the problem.149−151 This led to the development of CO2 capture and storage (CCS) as a feasible strategy to combat this global issue.152,153
Over the years, a number of studies have been conducted to assess the solubility of carbon dioxide,150,154−160 while a few also investigated the solubility of other gases such as hydrogen sulfide, methane, and sulfur dioxide.161−166 Aqueous amine solutions are one type of chemical solvent that has traditionally been used to absorb CO2 by chemical absorption from flue gases. Despite their low cost, strong absorption capacity, high selectivity, and high reactivity, the adverse effects of the solvents on the environment restricted their usage.150,167,168 To overcome these limitations, scientists conducted substantial research on greener solvents, until DESs gained attention as a novel choice.7,154,157,161 Hydrophilic DESs have been shown to be a potential solvent pertaining to gas solubility, with a high ability to dissolve hazardous gases as well as CO2.169 Yet, in addition to CO2 solubilization, these hygroscopic DESs can also absorb water in the process, reducing their mass absorption capacity and increasing the energy expenditure for CO2 desorption.91 This turned the quest for highly efficient solvents for CO2 capture to HDESs as they exhibited a capacity for CO2 solubility equivalent to that of ionic liquids (ILs).55 Several factors influenced the solubility of CO2 in HDESs. It was noted to increase with an increase in pressure and reported to be particularly sensitive in the low-pressure range.55 On the other hand, the solubility of CO2 in HDESs declined with increasing temperature across all pressure ranges.64,91
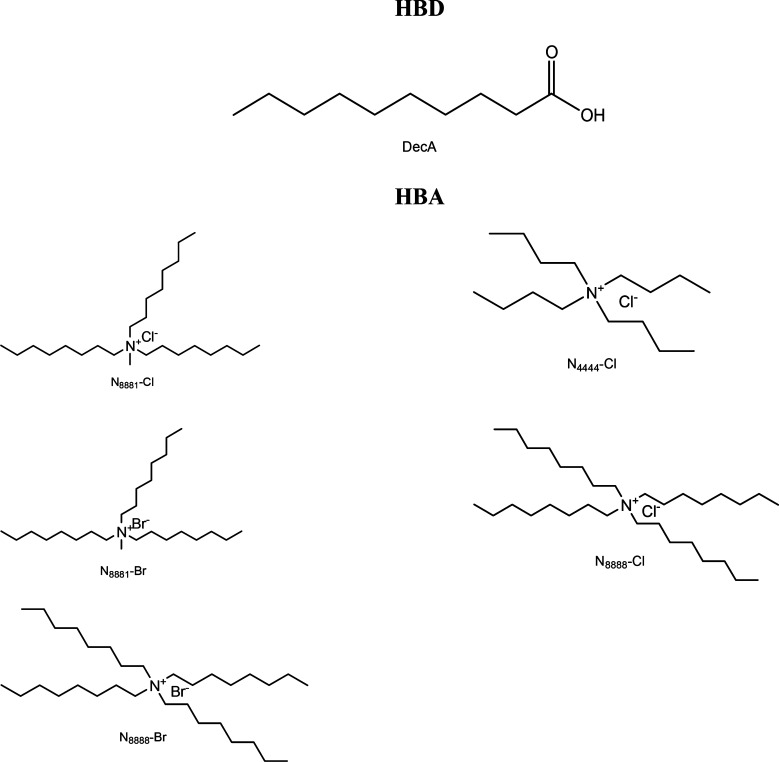
The first study concerning the use of HDESs in CO2 solubilization was reported recently in 2018. Using decanoic acid (DecA) as a hydrogen bond donor (HBD) combined with five different quaternary ammonium salts serving as hydrogen bond acceptors (HBAs), Zubeir’s research group examined the solubility of CO2 in six distinct HDESs.55Figure 4 displays the molecular structures of the HBD and HBAs employed in the study. The HDESs viz. DecaA:N4444Cl, DecaA:N8881Cl, DecaA:N8881Br, DecaA:N8888Cl, and DecaA:N8888Br were investigated at temperatures of 298.15, 308.15, and 323.15 K and CO2 pressures ranging from 0.1 to 2 MPa. The HBD was employed in various molar ratios with the HBAs, and the impacts of the halide ion, the length of the alkyl chain in the quaternary ammonium salts, and the hydrogen bond donor-to-acceptor ratio on CO2 solubilization were accessed. A comparative analysis based on Henry’s law and the heat of solution was also undertaken to evaluate the CO2 solubility of the HDESs with the currently established physical solvents. When compared to other DESs described in the existing literature, it was observed that the investigated HDESs displayed the maximum solubility of CO2 with values ranging between 0.239 and 0.284 mol CO2/mol of DES. Further, it was established that lengthening the alkyl chain and significantly reducing the HBD:HBA ratio enhanced the CO2 solubilities. Among the examined DESs, DecaA:N8888Cl demonstrated an efficiency higher than that of [C4mim][BF4] for the molar ratio 1.5:1, which provided a promising avenue for enhancing solvent performances without requiring complicated synthesis and subsequent purification processes.
Figure 4.
Molecular structures of the HBD and HBAs constituting the HDESs used in this study. Reprinted from ref (55). Copyright 2018 American Chemical Society.
Additionally, to streamline the testing procedure for optimal HDESs, the same research group modeled the CO2 solubility by applying the PC-SAFT methodology.62 Employing the “pseudopure” approach, segment number, temperature-independent segment diameter, and dispersion-energy parameters were estimated solely on liquid density data without any modification in the binary interaction parameter. Dietz and co-workers modeled the solubility of CO2 in four HDESs and one DES/IL mixture, viz. DecaA:N8888Cl in 2:1 ratio, DecaA:N8888Cl in 1.5:1 ratio, DecaA:N8888Br in 2:1 ratio, DecaA:N4444Cl in 2:1 ratio, and perfluorordecanoic acid (PerFDecaA):N8888Cl in 2:1 molar ratio. Of all the examined mixes, PerFDecaA:N8888Cl is partly ionized, making it an IL rather than a DES. The model demonstrated a reasonable correlation between the experimental and theoretically modeled densities, with Absolute Average Relative Deviation (AARD) (%) ranging from 2.27 to 12.01%. The promising results thus obtained from the PC-SAFT technique indicated its viability in screening HDESs for the CO2 solubilization.
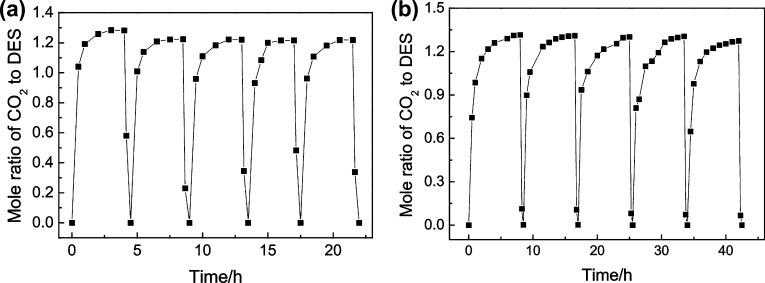
At 313.15 K and 1 bar, Gu et al. evaluated the solubility of CO2 in a novel class of hydrophobic functional DES composed of [TETA]Cl:thymol and [TEPA]Cl:thymol.91 The HDESs exhibited absorption capacities of 1.298 and 1.355 mol CO2/mol of DES and were shown to efficiently solubilize the gas even at low partial pressures. The values obtained were significantly greater than those reported in the literature for numerous ILs170,171 and DESs,55 (presented in Table 5). The authors highlighted that contrary to hydrophilic DESs, the amount of water in the HDESs remained unchanged during the absorption process from flue gases. Another intriguing result reported in the study was the formation of a new chemical bond between CO2 and the amino residue of the DESs, resulting in the formation of carboxylate. Despite the new chemical bond formed, both DESs remained hydrophobic during the CO2 absorption. The HDESs were further observed to retain their dissolving efficiency for a minimum of five absorption–desorption cycles (Figure 5).
Table 5. CO2 Absorption Capacity of Some Reported ILs and HDESs91.
| absorbent | extractant type | T (K) | PCO2 (kPa) | CO2 (mol abs) | CO2 (g abs) | ref |
|---|---|---|---|---|---|---|
| [BMIM][PF6] | ILs | 298 | 100 | 0.019 | 0.003 | (170) |
| [TMPDA][Tf2N] | ILs | 298 | 100 | 0.026 | 0.0028 | (171) |
| [BDMAEE][Tf2N] | ILs | 298 | 100 | 0.015 | 0.0015 | (171) |
| [TMHDA][Tf2N] | ILs | 298 | 100 | 0.023 | 0.0022 | (171) |
| DecA–[N8881Cl] | DESa | 298 | 90 | 0.013 | 0.0024 | (55) |
| DecA–[N8881Br] | DESa | 298 | 90 | 0.014 | 0.0024 | (55) |
| DecA–[N4444Cl] | DESa | 298 | 90 | 0.013 | 0.0027 | (55) |
| DecA–[N8888Cl] | DESa | 298 | 90 | 0.016 | 0.0024 | (55) |
| DecA–[N8888Br] | DESa | 298 | 90 | 0.016 | 0.0023 | (55) |
| [TETA]Cl–thymol | DESb | 313 | 101.3 | 1.298 | 0.09 | (91) |
| [TEPA]Cl–thymol | DESb | 313 | 101.3 | 1.355 | 0.088 | (91) |
nHBA/nHBD = 1:2.
nHBA/nHBD = 1:3.
Figure 5.
Absorption–desorption capacity of CO2 up to five consecutive cycles for HDESs reported by Gu et al. (a) [TETA]Cl–thymol DES in 1:3 molar ratio; (b) [TEPA]Cl–thymol DES in 1:3 molar ratio. Absorption parameters (CO2, 101.3 kPa, 323 K, 40 mL/min); desorption parameters (N2, 101.3 kPa, 373 K, 40 mL/min). Reprinted from ref (91). Copyright 2020 American Chemical Society.
In a recent experimental study, Haider et al. reported the synthesis of a class of ternary HDESs employing the binary combination of fatty acids, specifically capric, lauric, and oleic acids, with tetrabutylammonium bromide and their utilization in CO2 solubilization.64 The pressure drop method was used to measure the CO2 uptake of the HDESs, and the observed results were further correlated with the Peng–Robinson equation of state. The obtained CO2 solubility data were in good agreement with experimental results, which indicated a significant gaseous intake by the investigated HDESs.
Amidst the several experimental analyses conducted on carbon dioxide capture employing HDESs, Gutiérrez and co-workers very recently reported a theoretical exploration of the solubility of carbon dioxide in an archetypical type III HDES.172 The study focused on analyzing the nanoscopic characteristics of the HDES composed of tetraoctylammonium chloride:decanoic acid (N8888Cl:DecaA) in a 1:2 molar ratio, employing a multiscale molecular modeling approach. For this purpose, Density Functional Theory calculations and Classical Molecular Dynamics simulations were conducted for both the pure DES and DES-CO2 mixtures over a wide range of pressures and temperature. The DES–CO2 interactions were examined through DFT, taking into account five distinct sites in the DES cluster. Interatomic distances measured between the CO2 molecules and the OH sites of DecaA spanned from 2.9 to 3.5 Å, indicating fairly strong interactions. The modest disruption of interactions between the DES components, demonstrated by a comparison of the binding energies in the presence and absence of CO2 molecules, provided additional support for DES–CO2 cluster formation. Additionally, MD simulations were run for the DES-CO2 liquid mixtures as a function of DES concentration up to CO2 partial pressure (χCO2) of 0.1. Across the considered range of concentrations, CO2 adsorption by the HDES was supported by a linear rise in density, suggesting the proper fitting of CO2 molecules into the DES structure. The empty space in the low-density, pure, DESs offered adequate room for the gas molecules to occupy without any significant interference in the DES interactions. This was corroborated by the virtually negligible increase in the volume upon absorption of CO2. Consequently, DFT calculations and the obtained volumetric data of the DES systems implied low-density HDESs as promising solvents for CO2 capture.
In a nutshell, HDESs are established as effective absorbents with significantly high CO2 affinity and selectivity. The solvents offer a plethora of applications to be employed, either in the precombustion stage such as natural gas sweeteners173 or postcombustion, in the elimination of acidic gases from flue gases. Despite the fact that the observed capacity of DESs for CO2 solubilization is lower than that of certain ILs (e.g., fluorinated ILs),174 HDESs provide great control over their physicochemical characteristics as well as inexpensiveness and environmental viability.
3. Extraction Techniques
In contrast with the traditional liquid–liquid extraction method, liquid–liquid microextraction shows a superior edge in terms of a lower prerequisite for solvent and sample and a higher extraction efficiency. HDESs have been successfully implemented as solvents in such extraction techniques, substituting the typical organic solvents. This technique can be modified further by using a trinary structure: aqueous sample, extracting solvent (water-soluble), and disperser solvent (soluble in both phases). This is known as dispersive liquid–liquid microextraction, and in the presence of the disperser solvent, the extractant is dispersed throughout the medium as tiny droplets. An increased surface area of the extractant leads to a much faster recovery of the analyte. Moreover, the dispersion process can also be completed with the help of a vortex agitator instead of the disperser solvent, and this technique is termed vortex-assisted liquid–liquid microextraction.
3.1. Liquid–Liquid Microextraction
Liquid–liquid microextraction in tandem with HDES was used to extract synthetic pigments in commercial beverages.53 Eight synthetic pigments were isolated from samples, and optimal analysis was done through HPLC. The DESs were composed of quaternary ammonium salts (trioctylmethylammonium chloride and tetrabutylammonium chloride) as the hydrogen bond acceptor and fatty acids as the donor (decanoic acid and octanoic acid), which were mixed in 1:2 molar ratios. Variables, in particular, DES volume, salt effect, extraction time, and pH of the sample, were under investigation. Although the authors did not report any studies on the hydrophobicity of the prepared DES, the low solubility of the fatty acids may justify their assumption. The HDES formed with tetrabutylammonium chloride and octanoic acids exhibited the best results, with extraction recovery lying in the 74.5–94.5% range. Potent stimulants, amphetamine, and methamphetamine, were also extracted from human plasma and medicinal wastewater using HDES combined with an air-assisted emulsification microextraction technique.36 Choline chloride and phenethyl alcohol were mixed in a molar ratio of 1:4 to obtain the desired HDES. This methodology avoided any additional emulsifier, making it economically more viable. The stimulants were extracted in the 63–66% range having a reasonable relative standard deviation (RSD) below 8.4%. HDESs were further used for separating nonsteroidal anti-inflammatory drugs present in human urine samples via liquid–liquid microextraction.175 The drugs in focus were Ketoprofen and Diclofenac, and the analysis was done through HPLC-UV. Deep eutectic mixtures are formed in situ due to the hydrogen bonds formed among the OH group of menthol and the prevalent COOH moiety of the drug molecules. The effect of pH, extraction time, and amount of menthol used was some of the factors that were considered. Adequate extraction recovery of 93 to 97% was achieved using this methodology.
The decomposition of HDES in contact with an aqueous phase, leading to the formation of a dispersed organic phase in situ, was used to separate steroidal estrogen 17 β-estradiol (E2) from transdermal gel samples. Tetrabutylammonium bromide (TBABr) was mixed with heptanol (1:1, 1:2, 1:3), octanol (1:2), decanol (1:2), and dodecanol (1:2) to obtain the required HDESs.70 TBABr is water-soluble, but upon exposure to an aqueous medium, the long-chain alcohols lead to the creation of the dispersed organic phase. After the dissolution of DES, TBABr acts as a dispersive agent as well as a salting-out agent. E2 was extracted with a recovery range of 95% with a satisfactory repeatability of 6%. Removal of parabens from environmental water samples was achieved using a similar form of a liquid–liquid extraction method. In situ HDES formation was involved, and the analysis was done via high-performance liquid chromatography diode array detector.48dl-menthol and decanoic acid were mixed in a 2:1 molar ratio in an aqueous medium and heated, resulting in the formation of the DES. Short extraction time, absence of any emulsifier, and high relative recovery of 84.8–104.7% were some of the advantages of this methodology.
A three-phase hollow fiber liquid–liquid microextraction process was applied to withdraw antiarrhythmic agents from the water samples. Choline chloride and 1-phenyl ethyl alcohol were blended in a molar ratio of 1:4 to generate the HDES acting as the extracting solvent.37 Propranolol, carvedilol, verapamil, and amlodipine were the four target analytes, where the methodology yielded extraction recovery in the range of 44–54%. Quantification of cinnamic acid from medicine samples was done via the hollow fiber liquid phase microextraction technique, with the hollow fiber filled with a HDES.72 The plasma protein binding rates were also investigated. After several steps of optimization, the DES was synthesized by using tetrabutylammonium chloride and hexanoic acid in a 1:3 molar ratio. The recovery range of the analyte was stated to be 86.7–110.5%. A natural HDES centered on serine and lactic acid was designed to quantify caffeic acid from beverages.77 A similar hollow phase microextraction was utilized, which was followed by the analysis performed with HPLC-UV. The DES was prepared using serine and lactic acid in a 1:5 molar ratio. It is theorized that strong π-type hydrogen bonding occurs between the DES and caffeic acid, which leads to the high affinity of the DES toward the target analyte. A suitable recovery range of 95–99.3% was achieved after investigating various beverage samples with adequate repeatability of less than 5.2%.
Terpene-based HDESs were synthesized to examine DES derived from natural compounds. Seventeen HDES were prepared and were used for purging riboflavin from water samples.18 The compounds used for DES were decanoic acid, dodecanoic acid, menthol, thymol, 1-tetradecanol, 1,2-decanediol, 1-10-decanediol, cholesterol, trans-1,2-cyclohexanediol, 1-napthol, atropine, tryptamine, lidocaine, cyclohexanecarboxyaldehyde, caffeine, and coumarin with molar ratios of 2:1, 1:2, and 1:1 between the donor and acceptor of hydrogen bonds. The results of riboflavin extraction were juxtaposed with the HDES made of a 2:1 molar ratio of decanoic acid and tetraoctylammonium bromide (extraction efficiency of 19%). Maximum extraction efficiency of 81% was obtained for DecaA:lidocaine (2:1).
Four neonicotinoid pesticides, imidacloprid, acetamiprid, nitenpyram, and thiamethoxam, were extracted from water samples using a combination of HDES and liquid–liquid microextraction techniques.47 The DES were based on two hydrogen bond acceptors, dl-menthol and tetrabutylammonium chloride (N4444Cl), which were mixed with acids acting as the donors. dl-Menthol:acetic acid (1:1), dl-menthol:levulinic acid (1:1), dl-menthol:pyruvic acid (1:2), dl-menthol:butyric acid (1:1), dl-menthol: hexanoic acid (1:1), dl-menthol:octanoic acid (1:1), dl-menthol:decanoic acid (1:1), dl-menthol:dodecanoic acid (2:1), N4444Cl:acetic acid (1:1), N4444Cl:levulinic acid (1:2), N4444Cl:hexanoic acid (1:2), N4444Cl:octanoic acid (1:2), and N4444Cl:decanoic acid (1:2) were the HDES prepared as the extraction solvents. It was observed that the hydrophobicity of the pesticides impacts their extraction efficiencies, with imidacloprid having the highest extraction efficiency.
HDES centered on menthol was also used for extracting alcohol from water.139 Separation of lower alcohols from water can be tricky to an extent due to the formation of azeotropic mixtures. Liquid–liquid microextraction, along with a dl-menthol and lauric acid (molar ratio 2:1) mixture, as the extraction solvent, was used for this procedure. Among the alcohols extracted, butanol had the highest extraction efficiency of 90%, followed by propanol (80%) and ethanol (50%).
Selenium ions were separated from water and food samples by employing low viscous HDES via ultrasound-assisted liquid–liquid microextraction.49 Six HDESs were developed, menthol:DodecaA, menthol:n-octyl alcohol, menthol:n-NonaA, trioctylmethylammonium chloride:2-octanol, trioctylmethylammonium chloride:oleic acid, and trioctylmethylammonium chloride:DecaA, with molar ratios of 1:3, 2:1, 1:1, 1:2, 1:2, and 1:3, respectively. The analysis of the ion was performed by using hydride generation atomic absorption spectrometry. It was observed that the HDES menthol:lauric acid provides optimum recovery of the Se(IV) ions. The recovery of the analyte was found to be 91–104% with reasonable repeatability of 1.8–3.9%. HDESs hinged on tetrabutylammonium chloride, and decanoic acid were fabricated for the determination of nickel (Ni(II))and zinc(Zn(II)) in food samples.56 The conventional liquid–liquid microextraction method was applied, followed by flame atomic absorption spectroscopy for the analysis of the ions. Although six types were synthesized, the HDES fabricated from tetrabutylammonium chloride and decanoic acid in a molar ratio of 1:2 was the most efficient for extraction. From interference studies, it was determined that this method is highly selective for nickel and zinc ions. A recovery range of 95.37–103.5% having a relative standard deviation below 4.2% was observed after evaluating various food samples.
Extraction of three anesthetics, specifically eugenol, isoeugenol, and methyl isoeugenol, from marine consumable samples was accomplished by utilizing the ultrasound-assisted liquid–liquid microextraction method in tandem with gas chromatography for analysis. The HDESs used as the extraction solvent was based on menthol and thymol as the HBAs, while levulinic acid, lactic acid, hexanoic acid, and octanoic acid acted as the donors.71 After optimizing the DESs, with respect to their viscosity and density, thymol:levulinic acid with the molar ratio of 1:2 was preferred for this methodology. After extracting the three anesthetics from various samples, recovery of 86–101% was achieved, having a relative standard deviation under 5%.
3.2. Dispersive Liquid–Liquid Microextraction
HDES was employed in dispersive liquid–liquid microextraction for the analysis of sulfonamides in environmental water samples.35 Here the analytical parameters, such as extraction time, the volume of HDES used, and the pH of the water samples were focused upon. Choline chloride was the common hydrogen bond donor in all DES, which was mixed with o-cresol, m-cresol, and p-cresol in molar ratios of 1:2. Under the optimized conditions, the proposed methodology yielded sulfonamide extraction with a recovery range of 80.17–93.5% within extraction time of 0.5 to 12 min. Another study was performed for the detection of sulfonamides in fruit samples using HDES with an ultrasound-assisted dispersive liquid–liquid microextraction technique.60 Three sulfonamides were investigated, namely, sulfapyridine, sulfamethazine, and sulfamethoxine, from various fruit samples. Five HDESs were prepared with the combination of trioctylmethylammonium chloride and tetrabutylammonium bromide as the hydrogen bond acceptors with 2-octanol, capric acid, octanoic acid, and oleic acid as the acceptors. After optimization, the DES prepared from trioctylmethylammonium chloride and 2-octanol (molar ratio 1:2) was selected as the extracting solvent. A recovery range of 88.09–97.84% with a repeatability of 0.32–9.43% was observed for the samples.
Separation of UV filters from aqueous samples was also possible through the utilization of HDES in an ultrasound-assisted dispersive liquid–liquid microextraction technique.59 The HDESs were tailored from trioctylmethylammonium chloride (TAC) as the hydrogen bond acceptor and decanoic acid (DecaA) as the donor, with molar ratios of 1:1, 1:2, 1:3, 1:4, and 1:5. Several parameters, such as the effect of sample and DES volume, the impact of salt addition, ultrasound duration, etc., were under investigation. Among the HDES prepared, the DES with a molar ratio of 1:3 showed optimal extraction rates for the sample analytes. This methodology was implemented in commercial water samples with a 90.2–103.5% recovery range and suitable repeatability (relative standard deviation of 5.2%). Ultrasound-aided liquid–liquid microextraction further came into play to assess four nonsteroidal anti-inflammatory drugs (NSAID) in water and milk samples. The HDES used as the extraction solvent was designed with thymol as the hydrogen bond donor and 1,1,3,3-tetramethylguanidine ([TMGH]Cl), methyltrioctylammonium chloride (N8881Cl), and choline chloride at molar ratios of 2:1, 2:1, and 5:1, respectively.90 The hydrophobic nature of the DESs was vindicated using the contact angle of DES with water which lay in the range of 41.75–57.65° at 25 °C. Salicylic acid, oxaprozin, diclofenac, and ibuprofen were the NSAIDs investigated. The HDES generated with [TMGH]Cl and thymol produced comparatively higher efficiency with relative recoveries ranging between 79.42 and 107.52%.
HDESs were also used to evaluate the amount of dye in food samples with an effervescence-assisted dispersive liquid–liquid microextraction technique, where the analysis was done through UV–vis spectroscopy.39 Synthetic dyes such as Sunset Yellow and Brilliant Blue FCD were extracted, and carbon dioxide formed during the effervescent reaction was used as the disperser for DESs. For the synthesis of HDESs, Aliquat 336 was preferred as the hydrogen bond acceptor and was combined with decanoic acid, oleic acid, and ibuprofen in molar ratios of 1:2, 1:2, and 7:3, respectively. After the optimization of several parameters influencing extraction rates, this methodology was applied to food samples. The dyes were extracted in a suitable recovery range of 98.04–102.5% with a relative standard deviation below 5%.
Determination of the insecticide pyrethroid in environmental water samples was also possible via the use of HDES.122 Dispersive liquid–liquid microextraction in combination with high-performance liquid chromatography and an ultraviolet detector was utilized to increase the extraction efficiency. Trihexyl(tetradecyl)phosphonium tetrafluoroborate was mixed with octanoic acid, decanoic acid, and dodecanoic acid at varied molar ratios to generate the HDESs. Several factors were under investigation, including DES and sample volume, salt effect, and centrifugation rate. The water samples contained five pyrethroid types: deltamethrin, fenvalerate, permethrin, etofenprox, and bifenthrin. An adequate recovery range of 80.93 and 109.88% was observed, with good repeatability (RSD less than 6%). The analysis of pyrethroid was further investigated with a different type of HDES, precisely pairing thymol and octanoic acid to extract the insecticide from cereals.82 The dispersive liquid–liquid microextraction technique can be coupled with a step called solidification of a floating organic drop (SFOD) to ease the collection of the analyte, where the extraction solvent is at a solid phase at a low temperature. Thymol and octanoic acid were mixed at molar ratios of 1:5, 1:4, 1:3, 1:2, 1:1, 2:1, and 3:1 to synthesize the desired HDES. The extraction recovery was found to be in the range of 75.6 to 87.2% with reasonable repeatability (RSD less than 3.6%).
Another implementation of dispersive liquid–liquid microextraction in an amalgam with HDES was the determination of folic acid present in wheat samples.76 The DES used as the extracting solvent was prepared using trioctylmethylammonium chloride as the hydrogen bond acceptor with amyl alcohol as the donor. Five types of HDESs were synthesized with molar ratios: 1:1, 1:2, 1:3, 1:4, and 1:5, with the DES comprising the molar ratio 1:4 showing the highest recovery of folic acid. A relatively high recovery range of 91.6–99.7% with a relative standard deviation of less than 7% was observed in this investigation.
HDES was used as the extracting solvent to accumulate benzoylureas from water samples, with a ferric salt being used as the dispersive-demulsified solvent.65 Solidification of a floating organic drop (SFOD) technique was utilized, making collection of the sample analyte more manageable. Tricaprylmethylammonium chloride and 1-dodecanol were mixed at varied molar ratios, and the ratio of 1:2.5 was used after proper optimization. Triflumuron, hexaflumuron, flufenoxuron, and lufenuron were the benzoylureas analyzed by using this methodology. Extraction recovery of 82.36–93.82% was achieved with a relative standard deviation below 5%. β-lactam antibiotics were also extracted using the ultrasound-assisted dispersive liquid–liquid microextraction solidified floating organic drop method in the presence of HDES.40 Penicillin G, ampicillin, and amoxicillin were the β-lactams considered, with the analysis being performed through high-performance liquid chromatography with a photodiode array. Benzyl triethylammonium chloride and decanoic acid were mixed in a molar ratio of 1:3 to synthesize the HDES, and the analytes were extracted with relative recoveries of 97 to 99.6% (relative standard deviation <6.1%). The HDES had a melting temperature of 4 °C and the stabilization at such temperatures eases the process of solvent and analyte collection.
HDES composed of terpenoids and long-chain alcohols, along with dispersive liquid–liquid microextraction, were implemented to analyze mycotoxin in food samples.73 A mixture of dl-menthol and hexanol of molar ratio 2:1 was picked as the preferred DES, while acetonitrile was used as the disperser solvent. The mycotoxin zearalenone was under investigation, and a recovery range of 66–110% was observed for the varied food samples. The focus was on the stability of DES under different conditions as well as on the specific contribution of the two components of the HDES. Here it was articulated that after the addition of the disperser solvent, the aqueous phase is devoid of DES formation. The HDES components may contribute individually through intermolecular hydrogen bonding with the analyte to accelerate the extraction process. Thymol-based HDESs were fabricated to extract herbicides from rice samples via a dispersive liquid–liquid microextraction technique.30 Bentazone, pyrazosulfuronethyl, pyribenzoxim, fenoxaprop-P-ethyl, and anilofos were the herbicides studied, and HPLC was used for analysis. To synthesize the HDESs, thymol was combined with decanoic acid, hexanoic acid, n-butyric acid, and undecylenic acid in molar ratios of 3:2, 1:1, 1:1, 1:1, 1:2, and 1:3, respectively. Among them, the HDES designed with thymol and n-butyric acid was highly efficient, providing extraction recovery in the range of 70.38–122.91% with a relative standard deviation of 0.997–9.669%.
Trace amounts of cadmium and arsenic in wine samples were detected by using the ultrasound-assisted liquid–liquid microextraction technique.78 Eight types of HDES with a molar ratio of 1:3 were prepared, among which dl-lactic acid:trioctylmethylammonium chloride was preferred for this methodology. The effects of salt, sonication time, and the volume of HDES were a few of the parameters in focus. Adequate recoveries of 90.6–103.6% were achieved, with a relative standard deviation of 2.7–8.1%.
Of late, a green dispersive liquid–liquid microextraction technique was developed employing both hydrophilic and HDES for examining pesticides in water samples.19 Thymol and myristyl alcohol were mixed in a 2:1 molar ratio to generate HDES, used as the extractor solvent. The disperser solvent was hydrophilic DES comprising alanine, kojic acid, and water in a 1:2:5 ratio. The logKow value for thymol:myristyl alcohol and alanine:kojic acid:water were 4 and −0.11, respectively, indicating the hydrophobic and hydrophilic nature of the DES. Optimizable factors that can affect extraction, such as DES volume, salt effect, and pH of the sample, were regulated. Further minute changes to the pH of water were observed after the addition of the HDES. Sixteen types of pesticides were investigated, with recoveries in the range of 64–105% achieved.
3.3. Vortex-Assisted Liquid–Liquid Microextraction
HDESs have been employed in liquid–liquid microextraction in combination with a vortex-aided microextractor for the extraction of levofloxacin and ciprofloxacin in standard water samples.123 HPLC was used for the analysis of the antibiotics. Sixteen types of fatty acid and alcohol-based DES, having the pedigree to form two phases with aqueous solutions were assembled for this investigation, among which tetrabutylammonium bromide:HexaA (1:1), tetrabutylammonium bromide:OctaA (1:1), tetrabutylammonium bromide:DecaA (1:1), tetrabutylammonium bromide:n-butyl alcohol (1:1), tetrabutylammonium bromide:1-octanol (1:1), tetrabutylammonium bromide:1-dodecanol (1:1), tetrabutylammonium bromide:oleyl alcohol (1:1), tricaprylylmethylammonium chloride:HexaA (1:1), tricaprylylmethylammonium chloride:OctaA (1:1), tricaprylylmethylammonium chloride:DecaA (1:1), tricaprylylmethylammonium chloride:n-butyl alcohol (1:1), tricaprylylmethylammonium chloride:1-octanol (1:1), tricaprylylmethylammonium chloride:1-dodecanol (1:1), and tricaprylylmethylammonium chloride:oleyl alcohol (1:1) were stable at room temperature. The focus was emphasized on the DES volume and vortex-assisted time. Levofloxacin and ciprofloxacin were recovered in 94.4–98.3% and 95.1–98.4% range, respectively.
HDESs coupled with vortex-assisted microextraction were employed to extract formaldehyde from zoological and indoor air samples and further quantified with HPLC.38 Trioctylmethylammonium chloride (TAC) was preferred as the hydrogen bond acceptor, combining it with 4-cyanophenol in 1:2, 1:1, and 2:1 molar ratios and with hydroquinone and phenylphenol in 1:1 ratios to design the HDES. The DES prepared using TAC and 4-cyanophenol was considered, and it showed valid results with recovery in the range of 83.1–104.4%.
To analyze pesticides in olive oil samples, HDES prepared with thymol and vanillin coupled with vortex-assisted liquid–liquid microextraction was utilized, and the methodology was validated using GC-μ ECD determination.92 Thymol and vanillin were mixed in 1:2, 2:1, and 1:1 molar ratios, among which the composition with the 1:1 molar ratio remained as a clear fluid at room temperature. A positive logKow of 4.30 rationalizes the hydrophobicity of the DES. Sixteen pesticides were extracted using this procedure, where the extraction process was completed in 1 min, with recovery in the range of 63.1–119.4% having relative standard deviations of 2.5–7.4%.
Vortex-aided liquid–liquid microextraction involving HDES was used to detect dyes in food sauces in tandem with an HPLC-DAD system.75 For the preparation of DES, hexafluoroisopropanol (HFIP) was selected as the hydrogen bond donor, which was combined with nonionic surfactants like Brij-35 (1:5, 1:10, 1:15, 1:20 molar ratios), PONPE-7.5(1:5 molar ratio), Triton X-100 (1:5 molar ratio), and Triton X-114 (1:5 molar ratio), respectively. It was observed that all of the DES showed adequate stability at room temperature. Five Sudan dyes were evaluated, and the recovery of the dyes ranged in the region of 87–110.1%, with a relative standard deviation of less than 8.1%. Moreover, a similar procedure utilizing HDES benzyltriethylammonium chloride:thymol as an extracting solvent was used for the determination of five red dyes: amaranth, ponceau 4R, allura red, azorubine, and erythrosine from consumable items.26 Benzyltriethylammonium chloride (BTEAC) and thymol were combined in a molar ratio of 1:4 to generate the DES. Strong π–π interaction and hydrogen bonding with the analytes led to the ease of extraction of the dyes. Extraction recovery range of 94.2–100.8% was attained with a relative standard deviation below 6%.
Thymol-based HDESs were further used in the analysis of tetracycline antibiotics in infant formulas using the vortex-assisted liquid–liquid microextraction method, and the investigation was accomplished using HPLC.176 The ternary HDESs prepared in this investigation had their hydrogen bond donor in thymol (Thy) and composed as Thy:ethylene glycol:benzyl alcohol (2:2:1), Thy:ethylene glycol:octanol (1:1:1), Thy:DecaA:benzyl alcohol (1:1:1), and Thy:DecaA:octanol (1:1:1), and focus was emphasized on the greenness of this methodology. Among the assembled DES, thymol:ethylene glycol:benzyl alcohol showed maximum extraction efficiency, and the recovery of the antibiotics was in the range of 69–102% with RSD less than 9%. The hydophobicity of the DES was analyzed by evaluating octanol–water partition coefficient, where the logKow value was found to be 1.99.
In recent times, vortex-assisted dispersive liquid–liquid microextraction complemented by HDES was implemented for the extraction of vincristine alkaloids from the plasma of children suffering from leukemia in tandem with HPLC-UV.29 For the synthesis of the HDESs, methyltrioctylammonium chloride (MTOAC) was chosen as the hydrogen bond acceptor, with several alcohols as hydrogen bond donors, namely, n-nonanol, n-heptanol, n-butanol, glycerol, and ethylene glycol. After optimization, all the DESs were prepared in a 1:3 molar ratio, among which MTOAC:n-butanol showed satisfactory results for the extraction of vincristine. Analyses were done on various brands of drug and plasma samples as well as on live samples, with recovery ranging from 92.0 to 108.6%. Anilines were determined from food samples through a HDES-based dispersive liquid–liquid extraction with vortex assistance.33 Bis(2-ethylhexyl) phosphate was mixed in a 1:3 ratio with butylparaben to generate the desired HDES. The extracted anilines were further exposed to a diazotization process, and the final quantification was done via microvolume spectrophotometry. Strong ion pairing interaction, π–π interaction, and hydrogen bonding between the DES and the aromatic amine resulted in adequate extraction efficiency, with a relative recovery of 90.0–99.0%. Benzyltriethylammonium chloride (BTEAC) and thymol-based HDES were used for the removal of malachite green and crystal violet from aqueous samples.88 Vortex-assisted dispersive liquid–liquid extraction came into play with HPLC being used for the analysis of the dyes. Benzyltriethylammonium chloride was used as the hydrogen bond acceptor with thymol as the donor and was mixed in a 1:4 molar ratio. The BTEAC:thymol DES was compared with the choline chloride:thymol DES and due to the higher hydrophobicity of the ion pair formed of BTEAC as well as stronger π–π interaction, BTEAC-based DES showed better performance. Relative recoveries of 90.0–97.4% were achieved, with RSD of less than 9.4%.
Interestingly HDES can be used for the preparation of ferrofluids, which can then be implemented in vortex-assisted liquid–liquid microextraction coupled with HPLC for the determination of mefenamic acid in human urine samples.23 Ferrofluid volume, ionic strength, and vortex time were optimized initially for this study. The HDES was generated by combining acetic acid and dl-menthol with a molar ratio of 1:1. The ferrofluid was further prepared by dispersing oleic acid-coated Fe3O4 nanoparticles in 1 mL volume of the DES. The extraction process of mefenamic acid was achieved in 7 min with an extraction efficiency in the range of 80.25–97.45% and a relative standard deviation below 4.60%.
Bisphenol contaminants from water samples were extracted using HDES based on trioctylmethylammonium chloride.58 Vortex-assisted liquid–liquid microextraction along with high-performance liquid chromatography (HPLC) and fluorescence detection were used for the analysis of the sample. Trioctylmethylammonium chloride was mixed with decanoic acid, ketoprofen, and gemfibrozil in molar ratios of 2:1 1:1, 1:2, 2:1, 1:1, 1:2, 1:2, and 1:1, respectively, to synthesize 8 types of DES. Among them DecaA:trioctylmethylammonium chloride (2:1 molar ratio) DES was marked as the most suitable solvent for extraction. The hydrophobicity was validated using the water contact angle, which was shown to be 47°. The relative recoveries of the bisphenols were in the 82.0–109.4% range, with a relative standard deviation of 0.7–6.9%. Twelve quinolones from honey were extracted using the vortex-assisted liquid–liquid microextraction technique, with a thymol-based HDES as the extracting solvent.69 The quinolones were analyzed using ultraperformance liquid chromatography–mass spectrometry. The extraction was achieved inside a syringe without centrifugation, reducing extraction time and requiring less sample. The DES was fabricated by using heptanoic acid and thymol mixed in a 2:1 molar ratio. The quinolones were recovered in the range of 75.08–117.46%, with a relative standard deviation of below 10.11%.
Phenolic acids were extracted from the medicinal herb Taraxacum mongolicum (TM) using the rapid recycle HDES-assisted water extraction technique.34 Compared to conventional extraction methods, the target analyte is concentrated in the aqueous phase. The assistance of a vortex is required for dispersion of the DES components. Thirty-six types of HDESs based on camphor, dl-menthol, lidocaine, and thymol as the hydrogen bond acceptors were prepared, among which the camphor:p-chlorophenol (1:3 molar ratio) was found to be the most suitable. A high recovery range of 90.76–94.73% was achieved with a relative standard deviation below 2.97%.
Low viscous HDES were synthesized, and their properties were evaluated in extracting phthalate esters in plastic samples in contact with food.32 Vortex-assisted liquid–liquid microextraction in association with gas chromatography (GC) was used in this procedure. Eight HDESs were prepared such as, n-octyl alcohol:DecaA (2:1), n-octyl alcohol: N4444Br (2:1), 1-dodecanol: N4444Br (2:1), n-butyl alcohol: N4444Br (4:1), n-hexyl alcohol: N4444Br (4:1), n-octyl alcohol: N8881Cl (2:1), DecaA: N8881Cl (2:1), DodecaA: N8881Cl (2:1). Among them, n-hexyl alcohol: N4444Br was the least viscous, along with showing a high GC response value for the phthalate esters analytes. After various plastic samples were analyzed, recovery was observed in the 86.4–103.2% range with a relative standard deviation of 3.6–5.6%.
HDESs centered upon thymol and menthol were used for drawing out thiophanate–methyl and carbendazim from aqueous samples.83 Vortex-assisted liquid–liquid extraction in tandem with HPLC was the preferred methodology. Hexanoic acid, octanoic acid, decanoic acid, n-butyl alcohol, 1-octanol, and 1-dodecanol were used as the hydrogen bond donors and mixed with thymol and menthol in a 1:1 molar ratio to synthesize 12 HDESs. Among them, menthol:1-octanol DES was selected for the extraction procedure. After optimization for several parameters, thiophanate–methyl and carbendazim were extracted with the highest recovery of 94.0 and 95.6%, respectively.
Herbicides of triazine and phenylurea were segregated from milk samples using vortex-assisted dispersive liquid–liquid microextraction hinged on the solidification of sedimentary DES. Five HDESs were designed based on tetrabutylammonium chloride and perfluorooctanol as the hydrogen bond donor and acceptor, respectively, in molar ratios of 3:1, 2:1, 1:1, 1:2, and 1:3.85 The HDES with a 2:1 molar ratio was preferred. A significant advantage of this method over the conventional ones is the conversion into a solid–liquid separation, thus curtailing the waste of the target analyte. Six such herbicides were extracted with a recovery of 87.01–97.92% with an error of less than 6.8%.
Recently, quantification of antiprostate cancer triple therapy from river water and human plasma was carried out using natural HDES following the vortex-assisted dispersive liquid–liquid microextraction technique.177 Lutamide, resveratrol, and ethynylestradiol were the target analytes. The HDES was composed of α-terpineol as the hydrogen bond acceptor, with octanoic acid as the donor in a molar ratio of 1:1. Recoveries of the three drugs were in the 92.1–100.4% range with a relative standard deviation below 6.1%.
4. Future Scope and Challenges
4.1. HDESs as Enzymatic Reaction Media
Numerous hydrophilic DESs as improved biocompatible enzymatic reaction media have been presented with the introduction of nonaqueous enzymatic catalysis, displaying increased solubility and selectivity of the substrate, in addition to suppression of hydrolytic side reactions.178−180 However, the application range of hydrophilic DESs was constrained by the low solubility of the hydrophobic substrates in it. Additionally, water adsorption brought about by catalytic reactions reduced the reaction rates in hydrophilic DESs.
Hummer et al. reported an interesting study that employed HDESs with long-chain carboxylic acids combined with (−)-menthol in lipase catalyzed esterification.181 Interestingly, the DESs in use simultaneously served as the substrate and the reaction medium. Under solvent-free conditions, the components of the HDESs were esterified to generate menthyl fatty acid esters. To activate the lipase by generating a phase interface, 10% water was added. Notably, after 7 days of reaction, 50, 71, and 83% of octanoic acid, dodecanoic acid, and decanoic acid yielded their respective menthyl esters. To improve the esterification of menthol:DodecaA HDES, Pätzold and co-workers further examined the requisite enzyme quantity, thermodynamic water activity, and temperature of the esterification reaction, respectively.80 Under optimal reaction conditions, the group observed that 95% of the acid converted to ester within a day. Furthermore, a 1.36 mol/L concentration of the product was generated in 2.25 days. Following the complete conversion of the dodecanoic acid, a vacuum distillation process was employed to recover the residual (−)-menthol and, thereafter, reuse it for subsequent esterification cycles. 94% pure menthyl dodecanoic ester was obtained from the reaction. As a future perspective, to enhance the efficiency of the HDES-based enzymatic synthesis of (−)-menthyl esters, the authors proposed the reuse of the lipase component from the reaction system.
In a different study, porcine pancreatic lipase (PPL) was used to investigate the catalytic aldose activity on acetone and 4-nitrobenzaldehyde using four DESs viz. glycerol:choline chloride (Gly:ChCl) in 1:1.5 molar ratio, ethylene glycol:tetraoctylammonium bromide (EG:N8888Br) in 1:3 molar ratio, 1,5-pentadiol:tetraoctylammonium bromide PD:N8888Br in 1:3 molar ratio, and 4-nitrobenzaldehyde:tetrabutylbromide (4-NBA:N4444Br) in 2.2:1.5 molar ratio.68 The product composition of the PPL-catalyzed reactions was evidenced to be closely associated with the choice of DES, which differed in its hydrophobicity. Although the aldol addition exhibited a faster initial reaction velocity in the hydrophilic DES ChCl:Gly, the low solubility of the 4-NBA substrate limited the effectiveness of the DES. The 4-NBA containing DES described the highest 4-NBA solubility. Nevertheless, the fastest reaction was accomplished in the cosolvent acetone, resulting in a solvent-free reaction and increasing the productivity up to 40.5 mM h–1 in comparison to DES-mediated reactions.
4.2. Challenges and Limitations
Although several authors claimed the synthesized DESs to be nontoxic and biodegradable owing to the properties of the individual components, minimal studies were performed to justify their assumption. While synthesizing the ternary HDES thymol:ethylene glycol:benzyl alcohol, Sereshti et al. analyzed the greenness of the procedure using the Analytical Eco-Scale (AEC) method. Here an ideal green solvent scores an AEC value of 100, and any deviation results in penalty points (PPs) being deducted. The methodology attained a score of 68, which is considered acceptable (scores of 50–75 are in the accepted range). Cytotoxic behavior and nonbiodegradability were observed in some of the compounds, which may raise questions about the greenness of the DESs. Dodecanol, cresols, 4-cyanophenol, para-chlorophenol, Triton X-100, Triton X-, TOPO, and perfluorodecanoic acid, as well as quarternary amines, are associated with toxic traits.182−191 Constituents such as para-chlorophenol, perfluorodecanoic acid, and ibuprofen also show bioaccumulative tendencies.192−194 Moreover, the functionality of DESs depends upon the interactions among the components, and they can show properties that discrete components do not possess. There are reports on hydrophilic DESs where the synthesized DES shows higher toxicity than the individual components.195,196 Hayyan et al. accessed the toxicity effect of choline chloride-centered DESs on mice cells and, on the basis of the IC50 and LC50 values, concluded that the assembled DESs were more toxic than the individual components.197 To summarize, the toxicity property depends upon the interactions among the DES constituents, and further insight into this topic is needed to get a lucid picture on the utility of HDESs as green solvents. One of the significant concerns with HDESs is the recyclability of the solvent postextraction. Recyclability ensures sustainability in solvent usage, which is desired to promote green practices. Several recovery techniques have been employed, with the most popular being back extraction.141,198 However, further research needs to be carried out to improve the retrieval efficiency. Few HDESs show a tendency to salt out. The additional requirement of some toxic disperser solvents in a few extraction techniques73 can again be problematic as they reduce the greenness of the methodology. In HDESs, viscosity arises from a combination of hydrogen bonding and electrostatic interactions that reduce the mobility of the DES components. With the increase in viscosity, it becomes challenging to inject the medium into GC or HPLC. Thus, the solution needs to be adequately diluted before injection, which limits its applicability in the field of detection and sensing. Also, the extraction efficiency is negatively correlated to the solvent’s viscosity.17 Higher viscous drag slows the uptake of compounds into the extracting media. In such cases, viscosity is decreased by increasing the temperature of the medium and is thus energetically costly. A minimum density difference of 50 kg/m3 between HDES and water is required to ensure segregation into phases that helps in the extraction process. The definition of HDESs is often misused, as they are formulated with chemicals that are hydrophilic. Care must be taken not to confuse HDESs with “quasi-hydrophobic” DESs in which one of the components is hydrophilic. In numerous cases, HDESs have been reported to cause contamination of the aqueous phase due to leaching of the DES ingredients. This issue must be addressed, as it might alter the solution’s properties and even harm the environment. An area that needs to be explored is the long-term stability of the DESs—both shelf life and after prolonged usage. The components present in DESs might chemically react with each other, in which case the mixture is not considered a DES. The extremely slow kinetics associated with specific reactions makes it challenging to assess the reactivity of the components with each other. Notwithstanding their limitations, HDESs have proven helpful as extraction media and significantly reduced our dependence on conventional organic solvents. The tunable feature of DESs can be exploited in other fields, as well, which might pave the way for a greener future.
Acknowledgments
The authors are grateful to MHRD and IIT Guwahati for the financial assistance during their research tenure.
Author Contributions
M.D., R.M., and S.T. contributed equally to this work while preparing the initial draft and should be considered as first authors. A.M. furnished the roadmap, composed the introduction and limitation section, and compiled the final version of the manuscript. S.P. is the corresponding author.
The authors declare no competing financial interest.
References
- Abo-Hamad A.; Hayyan M.; AlSaadi M. A.; Hashim M. A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. 10.1016/j.cej.2015.03.091. [DOI] [Google Scholar]
- Dai Y.; van Spronsen J.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Mbous Y. P.; Hayyan M.; Hayyan A.; Wong W. F.; Hashim M. A.; Looi C. Y. Applications of deep eutectic solvents in biotechnology and bioengineering - Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. 10.1016/j.biotechadv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Yang R.; Cao Q.; Liang Y.; Hong S.; Xia C.; Wu Y.; Li J.; Cai L.; Sonne C.; Le Q. V.; Lam S. S. High capacity oil absorbent wood prepared through eco-friendly deep eutectic solvent delignification. Chem. Eng. J. 2020, 401, 126150. 10.1016/j.cej.2020.126150. [DOI] [Google Scholar]
- Anastas P. T.; Warner J. C.. Green Chemistry: Theory and Practice; Oxford University Press, 1998. [Google Scholar]
- Cabezas R.; Zurob E.; Gomez B.; Merlet G.; Plaza A.; Araya-Lopez C.; Romero J.; Olea F.; Quijada-Maldonado E.; Pino-Soto L.; Gonzalez T.; Castro-Muñoz R. Challenges and Possibilities of Deep Eutectic Solvent-Based Membranes. Ind. Eng. Chem. Res. 2022, 61, 17397. 10.1021/acs.iecr.2c02747. [DOI] [Google Scholar]
- Hansen B. B.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. 10.1021/acs.chemrev.0c00385. [DOI] [PubMed] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Francisco M.; van den Bruinhorst A.; Kroon M. C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem., Int. Ed. 2013, 52, 3074–3085. 10.1002/anie.201207548. [DOI] [PubMed] [Google Scholar]
- van Osch D. J. G. P.; Dietz C. H. J. T.; Warrag S. E. E.; Kroon M. C. The curious case of hydrophobic deep eutectic solvents: A story on the discovery, design, and applications. ACS Sustainable Chem. Eng. 2020, 8, 10591–10612. 10.1021/acssuschemeng.0c00559. [DOI] [Google Scholar]
- Hayyan M.; Hashim M. A.; Al-Saadi M. A.; Hayyan A.; AlNashef I. M.; Mirghani M. E. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. 10.1016/j.chemosphere.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Benlebna M.; Ruesgas-Ramón M.; Bonafos B.; Fouret G.; Casas F.; Coudray C.; Durand E.; Cruz Figueroa-Espinoza M.; Feillet-Coudray C. Toxicity of natural deep eutectic solvent betaine: glycerol in rats. J. Agric. Food Chem. 2018, 66, 6205–6212. 10.1021/acs.jafc.8b01746. [DOI] [PubMed] [Google Scholar]
- Wang T.; Wang Q.; Li P.; Yang H. High-speed countercurrent chromatography-based method for simultaneous recovery and separation of natural products from deep eutectic solvent extracts. ACS Sustainable Chem. Eng. 2020, 8, 2073–2080. 10.1021/acssuschemeng.9b06893. [DOI] [Google Scholar]
- Abranches D. O.; Martins M. A. R.; Silva L. P.; Schaeffer N.; Pinho S. P.; Coutinho J. A. P. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: the quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. 10.1039/C9CC04846D. [DOI] [PubMed] [Google Scholar]
- Florindo C.; Lima F.; Ribeiro B. D.; Marrucho I. M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustainable Chem. 2019, 18, 31–36. 10.1016/j.cogsc.2018.12.003. [DOI] [Google Scholar]
- Zainal-Abidin M. H.; Hayyan M.; Wong W. F. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. 10.1016/j.jiec.2021.03.011. [DOI] [Google Scholar]
- van Osch D. J. G. P.; Dietz C. H. J. T.; van Spronsen J.; Kroon M. C.; Gallucci F.; van Sint Annaland M.; Tuinier R. A search for natural hydrophobic deep eutectic solvents based on natural components. ACS Sustainable Chem. Eng. 2019, 7, 2933–2942. 10.1021/acssuschemeng.8b03520. [DOI] [Google Scholar]
- Sereshti H.; Seraj M.; Soltani S.; Rashidi Nodeh H.; Hossein Shojaee AliAbadi M.; Taghizadeh M. Development of a sustainable dispersive liquid–liquid microextraction based on novel hydrophobic and hydrophilic natural deep eutectic solvents for the analysis of multiclass pesticides in water. Microchem. J. 2022, 175, 107226. 10.1016/j.microc.2022.107226. [DOI] [Google Scholar]
- van Osch D. J. G. P.; Zubeir L. F.; van den Bruinhorst A.; Rocha M. A. A.; Kroon M. C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. 10.1039/C5GC01451D. [DOI] [Google Scholar]
- An Y.; Row K. H. Evaluation of menthol-based hydrophobic deep eutectic solvents for the extraction of Bisphenol A from environment water. Anal. Lett. 2021, 54, 1533–1545. 10.1080/00032719.2020.1811716. [DOI] [Google Scholar]
- Křížek T.; Bursová M.; Horsley R.; Kuchař M.; Tüma P.; Čabala R.; Hložek T. Menthol-based hydrophobic deep eutectic solvents: Towards greener and efficient extraction of phytocannabinoids. J. Clean. Prod. 2018, 193, 391–396. 10.1016/j.jclepro.2018.05.080. [DOI] [Google Scholar]
- Dil E. A.; Ghaedi M.; Asfaram A. Application of hydrophobic deep eutectic solvent as the carrier for ferrofluid: A novel strategy for pre-concentration and determination of mefenamic acid in human urine samples by high performance liquid chromatography under experimental design optimization. Talanta 2019, 202, 526–530. 10.1016/j.talanta.2019.05.027. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Wu H.; Cai D.; Yang T.; Yang L. Effective extraction of harmine by menthol/anise alcohol-based natural deep eutectic solvents. Sep. Purif. Technol. 2020, 250, 117211. 10.1016/j.seppur.2020.117211. [DOI] [Google Scholar]
- van den Bruinhorst A.; Raes S.; Maesara S. A.; Kroon M. C.; Esteves A. C. C.; Meuldijk J. Hydrophobic eutectic mixtures as volatile fatty acid extractants. Sep. Purif. Technol. 2019, 216, 147–157. 10.1016/j.seppur.2018.12.087. [DOI] [Google Scholar]
- Faraji M. Determination of some red dyes in food samples using a hydrophobic deep eutectic solvent-based vortex assisted dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. J. Chromatogr. A 2019, 1591, 15–23. 10.1016/j.chroma.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Cao J.; Yang M.; Cao F.; Wang J.; Su E. Well-designed hydrophobic deep eutectic solvents as green and efficient media for the extraction of artemisinin from Artemisia annua leaves. ACS Sustainable Chem. Eng. 2017, 5, 3270–3278. 10.1021/acssuschemeng.6b03092. [DOI] [Google Scholar]
- Liu R.; Geng Y.; Tian Z.; Wang N.; Wang M.; Zhang G.; Yang Y. Extraction of platinum (IV) by hydrophobic deep eutectic solvents based on trioctylphosphine oxide. Hydrometallurgy 2021, 199, 105521. 10.1016/j.hydromet.2020.105521. [DOI] [Google Scholar]
- Golpayegani M. R.; Akramipour R.; Gheini S.; Amini M. V.; Fattahi F.; Mohebbi A.; Fattahi N. Sensitive determination of vincristine in plasma of children with leukaemia using vortex-assisted dispersive liquid–liquid microextraction based on hydrophobic deep eutectic solvent. RSC Adv. 2022, 12, 3611–3617. 10.1039/D1RA07981F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Wang Y.; Chen M.; Qin Y.; Zhou Y. Hydrophobic deep eutectic solvent based dispersive liquid–liquid microextraction for the preconcentration and HPLC analysis of five rice paddy herbicides in water samples. Microchem. J. 2022, 181, 107790. 10.1016/j.microc.2022.107790. [DOI] [Google Scholar]
- Rad A. S.; Rahnama R.; Zakeri M.; Jamali M. R. Dispersive liquid-liquid microextraction based on green type solvents-”deep eutectic solvents”-for highly selective separation and efficient preconcentration of nickel in water samples. J. Iran. Chem. Soc. 2019, 16, 1715–1722. 10.1007/s13738-019-01643-0. [DOI] [Google Scholar]
- Li T.; Song Y.; Li J.; Zhang M.; Shi Y.; Fan J. New low viscous hydrophobic deep eutectic solvents in vortex-assisted liquid-liquid microextraction for the determination of phthalate esters from food-contacted plastics. Food Chem. 2020, 309, 125752. 10.1016/j.foodchem.2019.125752. [DOI] [PubMed] [Google Scholar]
- Faraji M.; Salehi N.; Shirani M.; Mahmoudi-Maymand M. Development of a deep eutectic solvent-based dispersive liquid–liquid microextraction method followed by back-extraction and diazotization coupled to spectrophotometry for determination of total primary aromatic amines from food simulants. J. Iran. Chem. Soc. 2022, 19, 3539–3548. 10.1007/s13738-022-02548-1. [DOI] [Google Scholar]
- Liao M.; Zhao Y.; Yang X.; Yang L.; Liu E.-H.; Lu B.; Wang J.; Liu X.; Chang Y.; Duan L. A greener and sustainable route for medicinal plant analysis: Recycle utilization of hydrophobic deep eutectic solvent. Microchem. J. 2022, 178, 107372. 10.1016/j.microc.2022.107372. [DOI] [Google Scholar]
- Qu Q.; Lv Y.; Liu L.; Row K. H.; Zhu T. Synthesis and characterization of deep eutectic solvents (five hydrophilic and three hydrophobic), and hydrophobic application for microextraction of environmental water samples. Anal. Bioanal. Chem. 2019, 411, 7489–7498. 10.1007/s00216-019-02143-z. [DOI] [PubMed] [Google Scholar]
- Rajabi M.; Ghassab N.; Hemmati M.; Asghari A. Emulsification microextraction of amphetamine and methamphetamine in complex matrices using an up-to-date generation of eco-friendly and relatively hydrophobic deep eutectic solvent. J. Chromatogr. A 2018, 1576, 1–9. 10.1016/j.chroma.2018.07.040. [DOI] [PubMed] [Google Scholar]
- Rajabi M.; Ghassab N.; Hemmati M.; Asghari A. Highly effective and safe intermediate based on deep eutectic medium for carrier less-three phase hollow fiber microextraction of antiarrhythmic agents in complex matrices. J. Chromatogr. B 2019, 1104, 196–204. 10.1016/j.jchromb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Liu C.; Li S.; Fan J. A hydrophobic deep eutectic solvent based vortex-assisted liquid-liquid microextraction for the determination of formaldehyde from biological and indoor air samples by high performance liquid chromatography. J. Chromatogr. A 2019, 1589, 39–46. 10.1016/j.chroma.2018.12.063. [DOI] [PubMed] [Google Scholar]
- Ghorbani Ravandi M.; Fat’hi M. R. Green effervescence assisted dispersive liquid–liquid microextraction based on a hydrophobic deep eutectic solvent for determination of Sunset Yellow and Brilliant Blue FCF in food samples. New J. Chem. 2018, 42, 14901–14908. 10.1039/C8NJ00782A. [DOI] [Google Scholar]
- Shirani M.; Akbari-adergani B.; Shahdadi F.; Faraji M.; Akbari A. A hydrophobic deep eutectic solvent-based ultrasound-assisted dispersive liquid–liquid microextraction for determination of β-lactam antibiotics residues in food samples. Food Anal. Methods 2022, 15, 391–400. 10.1007/s12161-021-02122-0. [DOI] [Google Scholar]
- Florindo C.; Romero L.; Rintoul I.; Branco L. C.; Marrucho I. M. From phase change materials to green solvents: Hydrophobic low viscous fatty acid-based deep eutectic solvents. ACS Sustainable Chem. Eng. 2018, 6, 3888–3895. 10.1021/acssuschemeng.7b04235. [DOI] [Google Scholar]
- Schaeffer N.; Conceição J. H.; Martins M. A.; Neves M. C.; Pérez-Sánchez G.; Gomes J. R.; Papaiconomou N.; Coutinho J. A. Non-ionic hydrophobic eutectics-versatile solvents for tailored metal separation and valorisation. Green Chem. 2020, 22, 2810–2820. 10.1039/D0GC00793E. [DOI] [Google Scholar]
- Ola P. D.; Matsumoto M. Use of deep eutectic solvent as extractant for separation of Fe (III) and Mn (II) from aqueous solution. Sep. Sci. Technol. 2019, 54, 759–765. 10.1080/01496395.2018.1517796. [DOI] [Google Scholar]
- van Osch D. J.; Parmentier D.; Dietz C. H.; van den Bruinhorst A.; Tuinier R.; Kroon M. C. Removal of alkali and transition metal ions from water with hydrophobic deep eutectic solvents. Chem. Commun. 2016, 52, 11987–11990. 10.1039/C6CC06105B. [DOI] [PubMed] [Google Scholar]
- Zante G.; Braun A.; Masmoudi A.; Barillon R.; Trebouet D.; Boltoeva M. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents. Miner. Eng. 2020, 156, 106512. 10.1016/j.mineng.2020.106512. [DOI] [Google Scholar]
- Dietz C. H. J. T.; Erve A.; Kroon M. C.; van Sint Annaland M.; Gallucci F.; Held C. Thermodynamic properties of hydrophobic deep eutectic solvents and solubility of water and HMF in them: Measurements and PC-SAFT modeling. Fluid Ph. Equilibria 2019, 489, 75–82. 10.1016/j.fluid.2019.02.010. [DOI] [Google Scholar]
- Florindo C.; Branco L. C.; Marrucho I. M. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Ph. Equilibria 2017, 448, 135–142. 10.1016/j.fluid.2017.04.002. [DOI] [Google Scholar]
- Ge D.; Wang Y.; Jiang Q.; Dai E. A deep eutectic solvent as an extraction solvent to separate and preconcentrate parabens in water samples using in situ liquid-liquid microextraction. J. Braz. Chem. Soc. 2019, 30, 1203–1210. 10.21577/0103-5053.20190014. [DOI] [Google Scholar]
- Altunay N.; Tuzen M. A simple and green ultrasound liquid–liquid microextraction method based on low viscous hydrophobic deep eutectic solvent for the preconcentration and separation of selenium in water and food samples prior to HG-AAS detection. Food Chem. 2021, 364, 130371. 10.1016/j.foodchem.2021.130371. [DOI] [PubMed] [Google Scholar]
- Adeyemi I.; Sulaiman R.; Almazroui M.; Al-Hammadi A.; AlNashef I. M. Removal of chlorophenols from aqueous media with hydrophobic deep eutectic solvents: Experimental study and COSMO RS evaluation. J. Mol. Liq. 2020, 311, 113180. 10.1016/j.molliq.2020.113180. [DOI] [Google Scholar]
- Gissawong N.; Mukdasai S.; Boonchiangma S.; Sansuk S.; Srijaranai S. A rapid and simple method for the removal of dyes and organophosphorus pesticides from water and soil samples using deep eutectic solvent embedded sponge. Chemosphere 2020, 260, 127590. 10.1016/j.chemosphere.2020.127590. [DOI] [PubMed] [Google Scholar]
- Yousefi S. M.; Shemirani F.; Ghorbanian S. A. Hydrophobic deep eutectic solvents in developing microextraction methods based on solidification of floating drop: Application to the trace HPLC/FLD determination of PAHs. Chromatographia 2018, 81, 1201–1211. 10.1007/s10337-018-3548-7. [DOI] [Google Scholar]
- Zhu S.; Zhou J.; Jia H.; Zhang H. Liquid–liquid microextraction of synthetic pigments in beverages using a hydrophobic deep eutectic solvent. Food Chem. 2018, 243, 351–356. 10.1016/j.foodchem.2017.09.141. [DOI] [PubMed] [Google Scholar]
- Ruggeri S.; Poletti F.; Zanardi C.; Pigani L.; Zanfrognini B.; Corsi E.; Dossi N.; Salomäki M.; Kivelä H.; Lukkari J.; Terzi F. Chemical and electrochemical properties of a hydrophobic deep eutectic solvent. Electrochim. Acta 2019, 295, 124–129. 10.1016/j.electacta.2018.10.086. [DOI] [Google Scholar]
- Zubeir L. F.; van Osch D. J.; Rocha M. A.; Banat F.; Kroon M. C. Carbon dioxide solubilities in decanoic acid-based hydrophobic deep eutectic solvents. J. Chem. Eng. Data 2018, 63, 913–919. 10.1021/acs.jced.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi F.; Arain M. B.; Ali Khan W.; Ul Haq H.; Khan A.; Jan F.; Castro-Munoz R.; Boczkaj G. Ultrasound-assisted deep eutectic solvent-based liquid–liquid microextraction for simultaneous determination of Ni (II) and Zn (II) in food samples. Food Chem. 2022, 393, 133384. 10.1016/j.foodchem.2022.133384. [DOI] [PubMed] [Google Scholar]
- Tereshatov E.; Boltoeva M. Y.; Folden C. First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: Indium extraction from hydrochloric and oxalic acids. Green Chem. 2016, 18, 4616–4622. 10.1039/C5GC03080C. [DOI] [Google Scholar]
- Li T.; Song Y.; Dong Z.; Shi Y.; Fan J. Hydrophobic deep eutectic solvents as extractants for the determination of bisphenols from food-contacted plastics by high performance liquid chromatography with fluorescence detection. J. Chromatogr. A 2020, 1621, 461087. 10.1016/j.chroma.2020.461087. [DOI] [PubMed] [Google Scholar]
- Wang H.; Hu L.; Liu X.; Yin S.; Lu R.; Zhang S.; Zhou W.; Gao H. Deep eutectic solvent-based ultrasound-assisted dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for the determination of ultraviolet filters in water samples. J. Chromatogr. A 2017, 1516, 1–8. 10.1016/j.chroma.2017.07.073. [DOI] [PubMed] [Google Scholar]
- Ji Y.; Meng Z.; Zhao J.; Zhao H.; Zhao L. Eco-friendly ultrasonic assisted liquid-liquid microextraction method based on hydrophobic deep eutectic solvent for the determination of sulfonamides in fruit juices. J. Chromatogr. A 2020, 1609, 460520. 10.1016/j.chroma.2019.460520. [DOI] [PubMed] [Google Scholar]
- Phelps T. E.; Bhawawet N.; Jurisson S. S.; Baker G. A. Efficient and selective extraction of 99m TcO4–from aqueous media using hydrophobic deep eutectic solvents. ACS Sustainable Chem. Eng. 2018, 6, 13656–13661. 10.1021/acssuschemeng.8b03950. [DOI] [Google Scholar]
- Dietz C. H.; van Osch D. J.; Kroon M. C.; Sadowski G.; van Sint Annaland M.; Gallucci F.; Zubeir L. F.; Held C. PC-SAFT modeling of CO2 solubilities in hydrophobic deep eutectic solvents. Fluid Phase Equilib. 2017, 448, 94–98. 10.1016/j.fluid.2017.03.028. [DOI] [Google Scholar]
- Riveiro E.; González B.; Domínguez Á. Extraction of adipic, levulinic and succinic acids from water using TOPO-based deep eutectic solvents. Sep. Purif. Technol. 2020, 241, 116692. 10.1016/j.seppur.2020.116692. [DOI] [Google Scholar]
- Haider M. B.; Jha D.; Kumar R.; Sivagnanam B. M. Ternary hydrophobic deep eutectic solvents for carbon dioxide absorption. Int. J. Greenhouse Gas Control 2020, 92, 102839. 10.1016/j.ijggc.2019.102839. [DOI] [Google Scholar]
- Yang M.; Hong K.; Li X.; Ge F.; Tang Y. Freezing temperature controlled deep eutectic solvent dispersive liquid–liquid microextraction based on solidification of floating organic droplets for rapid determination of benzoylureas residual in water samples with assistance of metallic salt. RSC Adv. 2017, 7, 56528–56536. 10.1039/C7RA11030H. [DOI] [Google Scholar]
- Ribeiro B. D.; Florindo C.; Iff L. C.; Coelho M. A. Z.; Marrucho I. M. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustainable Chem. Eng. 2015, 3, 2469–2477. 10.1021/acssuschemeng.5b00532. [DOI] [Google Scholar]
- Edgecomb J. M.; Tereshatov E. E.; Zante G.; Boltoeva M.; Folden C. M. III Hydrophobic amine-based binary mixtures of active pharmaceutical and food grade ingredients: Characterization and application in indium extraction from aqueous hydrochloric acid media. Green Chem. 2020, 22, 7047–7058. 10.1039/D0GC02452J. [DOI] [Google Scholar]
- Milker S.; Pätzold M.; Bloh J.; Holtmann D. Comparison of deep eutectic solvents and solvent-free reaction conditions for aldol production. Mol. Catal. 2019, 466, 70–74. 10.1016/j.mcat.2019.01.012. [DOI] [Google Scholar]
- Wang Y.; Zhao S.; Yang L.; Liu C.; Wang H.; Li D.; Zhang W.; Li L.; Song C.; Li C. Determination of 12 quinolones in honey by vortex-assisted dispersive liquid liquid microextraction performed in syringe based on deep eutectic solvent combine with ultra performance liquid chromatography-mass spectrometry. Eur. Food Res. Technol. 2022, 248, 263–272. 10.1007/s00217-021-03878-9. [DOI] [Google Scholar]
- Shishov A.; Chromá R.; Vakh C.; Kuchár J.; Simon A.; Andruch V.; Bulatov A. In situ decomposition of deep eutectic solvent as a novel approach in liquid-liquid microextraction. Anal. Chim. Acta 2019, 1065, 49–55. 10.1016/j.aca.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Yan J.; Ma S.; Feng M.; Zheng J.; Guo M. Hydrophobic deep eutectic solvent-based ultrasonic-assisted liquid-liquid microextraction combined with GC for eugenol, isoeugenol, and methyl isoeugenol determination in aquatic products. Food Addit Contam 2022, 39, 1718–1730. 10.1080/19440049.2022.2112764. [DOI] [PubMed] [Google Scholar]
- Zhang S.-m.; Zhang X.-x.; Chen X.; Hu S.; Bai X.-h. Deep eutectic solvent-based hollow fiber liquid-phase microextraction for quantification of Q-markers of cinnamic acid derivatives in traditional Chinese medicines and research of their plasma protein binding rates. Microchem. J. 2020, 155, 104696. 10.1016/j.microc.2020.104696. [DOI] [Google Scholar]
- Pochivalov A.; Pavlova K.; Garmonov S.; Bulatov A. Behaviour of deep eutectic solvent based on terpenoid and long-chain alcohol during dispersive liquid-liquid microextraction: Determination of zearalenone in cereal samples. J. Mol. Liq. 2022, 366, 120231. 10.1016/j.molliq.2022.120231. [DOI] [Google Scholar]
- Deng W.; Yu L.; Li X.; Chen J.; Wang X.; Deng Z.; Xiao Y. Hexafluoroisopropanol-based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of pyrethroids in tea beverages and fruit juices. Food Chem. 2019, 274, 891–899. 10.1016/j.foodchem.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Chen J.; Li X.; Huang A.; Deng W.; Xiao Y. Nonionic surfactants based hydrophobic deep eutectic solvents for liquid–liquid microextraction of Sudan dyes in tomato chili sauces. Food Chem. 2021, 364, 130373. 10.1016/j.foodchem.2021.130373. [DOI] [PubMed] [Google Scholar]
- Faraji M.; Mahmoodi-Maymand M.; Dastmalchi F. Green, fast and simple dispersive liquid-liquid microextraction method by using hydrophobic deep eutectic solvent for analysis of folic acid in fortified flour samples before liquid chromatography determination. Food Chem. 2020, 320, 126486. 10.1016/j.foodchem.2020.126486. [DOI] [PubMed] [Google Scholar]
- Nia N. N.; Hadjmohammadi M. R. Amino acids-based hydrophobic natural deep eutectic solvents as a green acceptor phase in two-phase hollow fiber-liquid microextraction for the determination of caffeic acid in coffee, green tea, and tomato samples. Microchem. J. 2021, 164, 106021. 10.1016/j.microc.2021.106021. [DOI] [Google Scholar]
- Ji Y.; Zhao M.; Li A.; Zhao L. Hydrophobic deep eutectic solvent-based ultrasonic-assisted dispersive liquid-liquid microextraction for preconcentration and determination of trace cadmium and arsenic in wine samples. Microchem. J. 2021, 164, 105974. 10.1016/j.microc.2021.105974. [DOI] [Google Scholar]
- Schaeffer N.; Martins M. A.; Neves C. M.; Pinho S. P.; Coutinho J. A. Sustainable hydrophobic terpene-based eutectic solvents for the extraction and separation of metals. Chem. Commun. 2018, 54, 8104–8107. 10.1039/C8CC04152K. [DOI] [PubMed] [Google Scholar]
- Pätzold M.; Burek B.; Liese A.; Bloh J.; Holtmann D. Product recovery of an enzymatically synthesized (−)-menthol ester in a deep eutectic solvent. Bioprocess Biosyst. Eng. 2019, 42, 1385–1389. 10.1007/s00449-019-02125-6. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Xiong D.; Zhao Y.; Li T.; Zhang K.; Fan J. Highly efficient extraction/separation of Cr (VI) by a new family of hydrophobic deep eutectic solvents. Chemosphere 2020, 241, 125082. 10.1016/j.chemosphere.2019.125082. [DOI] [PubMed] [Google Scholar]
- Wu B.; Guo Z.; Li X.; Huang X.; Teng C.; Chen Z.; Jing X.; Zhao W. Analysis of pyrethroids in cereals by HPLC with a deep eutectic solvent-based dispersive liquid–liquid microextraction with solidification of floating organic droplets. Anal. Methods. 2021, 13, 636–641. 10.1039/D0AY02121K. [DOI] [PubMed] [Google Scholar]
- He S.; Tang W.; Row K. H. Determination of thiophanate-methyl and carbendazim from environmental water by liquid-liquid microextraction (LLME) using a terpenoid-based hydrophobic deep eutectic solvent and high-performance liquid chromatography (HPLC). Anal. Lett. 2022, 55, 1235–1248. 10.1080/00032719.2021.1993237. [DOI] [Google Scholar]
- Yuvali D.; Seyhaneyildizi M.; Soylak M.; Narin İ.; Yilmaz E. An environment-friendly and rapid liquid-liquid microextraction based on new synthesized hydrophobic deep eutectic solvent for separation and preconcentration of erythrosine (E127) in biological and pharmaceutical samples. Spectrochim. Acta A Mol. 2021, 244, 118842. 10.1016/j.saa.2020.118842. [DOI] [PubMed] [Google Scholar]
- Ji F.; Zhe Z.; Jilong L.; Zhenhua S.; Yi Y.; Zhibing W.; Hanqi Z. Vortex-assisted dispersive liquid–liquid microextraction based on the solidification of sedimentary deep eutectic solvents for the determination of triazine and phenylurea herbicides in milk samples. Anal. Methods. 2022, 14, 460–468. 10.1039/D1AY01788H. [DOI] [PubMed] [Google Scholar]
- Khare L.; Karve T.; Jain R.; Dandekar P. Menthol based hydrophobic deep eutectic solvent for extraction and purification of ergosterol using response surface methodology. Food Chem. 2021, 340, 127979. 10.1016/j.foodchem.2020.127979. [DOI] [PubMed] [Google Scholar]
- Shahrezaei F.; Shamsipur M.; Gholivand M. B.; Zohrabi P.; Babajani N.; Abri A.; Zonouz A. M.; Shekaari H. A highly selective green supported liquid membrane by using a hydrophobic deep eutectic solvent for carrier-less transport of silver ions. Anal. Methods 2020, 12, 4682–4690. 10.1039/D0AY01266A. [DOI] [PubMed] [Google Scholar]
- Faraji M.; Adeli M.; Noormohammadi F. Deep eutectic solvent-based dispersive liquid-liquid micro-extraction for extraction of malachite green and crystal violet in water samples prior their determination using high performance liquid chromatography. J. Environ. Anal. Chem. 2022, 102, 681–689. 10.1080/03067319.2020.1724992. [DOI] [Google Scholar]
- Makoś P.; Przyjazny A.; Boczkaj G. Hydrophobic deep eutectic solvents as “green” extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. 10.1016/j.chroma.2018.07.070. [DOI] [PubMed] [Google Scholar]
- Qiao L.; Sun R.; Yu C.; Tao Y.; Yan Y. Novel hydrophobic deep eutectic solvents for ultrasound-assisted dispersive liquid-liquid microextraction of trace non-steroidal anti-inflammatory drugs in water and milk samples. Microchem. J. 2021, 170, 106686. 10.1016/j.microc.2021.106686. [DOI] [Google Scholar]
- Gu Y.; Hou Y.; Ren S.; Sun Y.; Wu W. Hydrophobic functional deep eutectic solvents used for efficient and reversible capture of CO2. ACS Omega 2020, 5, 6809–6816. 10.1021/acsomega.0c00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani S.; Sereshti H.; Nouri N. Deep eutectic solvent-based clean-up/vortex-assisted emulsification liquid-liquid microextraction: Application for multi-residue analysis of 16 pesticides in olive oils. Talanta 2021, 225, 121983. 10.1016/j.talanta.2020.121983. [DOI] [PubMed] [Google Scholar]
- Lee W. S.; Chua A. S. M.; Yeoh H. K.; Ngoh G. C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. 10.1016/j.cej.2013.09.002. [DOI] [Google Scholar]
- Agler M. T.; Wrenn B. A.; Zinder S. H.; Angenent L. T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol 2011, 29, 70–78. 10.1016/j.tibtech.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Kim N.-J.; Lim S.-J.; Chang H. N. Volatile fatty acid platform: Concept and application. Emerg. Area Bioeng. 2018, 1, 173–190. 10.1002/9783527803293.ch10. [DOI] [Google Scholar]
- Pratt S.; Liew D.; Batstone D. J.; Werker A. G.; Morgan-Sagastume F.; Lant P. A. Inhibition by fatty acids during fermentation of pre-treated waste activated sludge. J. Biotechnol. 2012, 159, 38–43. 10.1016/j.jbiotec.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Royce L. A.; Liu P.; Stebbins M. J.; Hanson B. C.; Jarboe L. R. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl. Microbiol. Biotechnol. 2013, 97, 8317–8327. 10.1007/s00253-013-5113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Garzón C. S.; Straathof A. J. J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. 10.1016/j.biotechadv.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Boateng I. D. A critical review of emerging hydrophobic deep eutectic solvents’ applications in food chemistry: Trends and opportunities. J. Agric. Food Chem. 2022, 70, 11860–11879. 10.1021/acs.jafc.2c05079. [DOI] [PubMed] [Google Scholar]
- Keshav A.; Wasewar K. L.; Chand S. Equilibrium and kinetics of the extraction of propionic acid using tri-n-octylphosphineoxide. Chem. Eng. Technol. 2008, 31, 1290–1295. 10.1002/ceat.200700490. [DOI] [Google Scholar]
- Bilgin M.; Arısoy Ç.; Kırbaşlar Ş. İ. Extraction equilibria of propionic and butyric acids with tri-n-octylphosphine oxide/diluent systems. J. Chem. Eng. Data 2009, 54, 3008–3013. 10.1021/je900063p. [DOI] [Google Scholar]
- Gilmore M.; McCourt E. N.; Connolly F.; Nockemann P.; Swadźba-Kwaśny M.; Holbrey J. D. Hydrophobic deep eutectic solvents incorporating trioctylphosphine oxide: Advanced liquid extractants. ACS Sustainable Chem. Eng. 2018, 6, 17323–17332. 10.1021/acssuschemeng.8b04843. [DOI] [Google Scholar]
- Marták J.; Schlosser Š. Phosphonium ionic liquids as new, reactive extractants of lactic acid. Chem. Pap. 2006, 60, 395–398. 10.2478/s11696-006-0072-2. [DOI] [Google Scholar]
- Schlosser Š.; Marták J.; Blahušiak M. Specific phenomena in carboxylic acids extraction by selected types of hydrophobic ionic liquids. Chem. Pap. 2018, 72, 567–584. 10.1007/s11696-017-0365-7. [DOI] [Google Scholar]
- Benner J.; Helbling D. E.; Kohler H.-P. E.; Wittebol J.; Kaiser E.; Prasse C.; Ternes T. A.; Albers C. N.; Aamand J.; Horemans B.; Springael D.; Walravens E.; Boon N. Is biological treatment a viable alternative for micropollutant removal in drinking water treatment processes?. Water Res. 2013, 47, 5955–5976. 10.1016/j.watres.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Guo W.; Ngo H. H.; Nghiem L. D.; Hai F. I.; Zhang J.; Liang S.; Wang X. C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Tijani J. O.; Fatoba O. O.; Petrik L. F. A review of pharmaceuticals and endocrine-disrupting compounds: sources, effects, removal, and detections. Water, Air, Soil Pollut 2013, 224, 1770. 10.1007/s11270-013-1770-3. [DOI] [Google Scholar]
- Tsai M.-L.; Zhang Y.-R.; Chien I.-L. Improved design of separation system for the recovery of benzene and isopropanol from wastewater. Sep. Purif. Technol. 2021, 260, 118227. 10.1016/j.seppur.2020.118227. [DOI] [Google Scholar]
- Geng Y.; Deng Y.; Chen F.; Jin H.; Hou T.; Tao K. Isopropanol biodegradation by immobilized Paracoccus denitrificans in a three-phase fluidized bed reactor. Prep. Biochem. Biotechnol. 2016, 46, 747–754. 10.1080/10826068.2015.1135446. [DOI] [PubMed] [Google Scholar]
- Xu D.; Meng X.; Liu Z.; Zhang L.; Wang Y.; Gao J. Separation of isopropanol from its aqueous solution with deep eutectic solvents: liquid–liquid equilibrium measurement and thermodynamic modeling. Braz. J. Chem. Eng. 2020, 37, 569–576. 10.1007/s43153-020-00041-x. [DOI] [Google Scholar]
- Fan C.; Cao X.; Han T.; Pei H.; Hu G.; Wang W.; Qian C. Selective microextraction of polycyclic aromatic hydrocarbons using a hydrophobic deep eutectic solvent composed with an iron oxide-based nanoferrofluid. Microchim. Acta 2019, 186, 560. 10.1007/s00604-019-3651-y. [DOI] [PubMed] [Google Scholar]
- Grimmer G.; Dettbarn G.; Brune H.; Deutsch-Wenzel R.; Misfeld J. Quantification of the carcinogenic effect of polycyclic aromatic hydrocarbons in used engine oil by topical application onto the skin of mice. Int. Arch. Occup. Environ. Health 1982, 50, 95–100. 10.1007/BF00432496. [DOI] [PubMed] [Google Scholar]
- Andersson J. T.; Achten C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. 10.1080/10406638.2014.991042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Su E. Hydrophobic deep eutectic solvents: The new generation of green solvents for diversified and colorful applications in green chemistry. J. Clean. Prod. 2021, 314, 127965. 10.1016/j.jclepro.2021.127965. [DOI] [Google Scholar]
- Munz N. A.; Burdon F. J.; de Zwart D.; Junghans M.; Melo L.; Reyes M.; Schönenberger U.; Singer H. P.; Spycher B.; Hollender J.; Stamm C. Pesticides drive risk of micropollutants in wastewater-impacted streams during low flow conditions. Water Res. 2017, 110, 366–377. 10.1016/j.watres.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Sereshti H.; Jamshidi F.; Nouri N.; Nodeh H. R. Hyphenated dispersive solid-and liquid-phase microextraction technique based on a hydrophobic deep eutectic solvent: application for trace analysis of pesticides in fruit juices. J. Sci. Food Agric. 2020, 100, 2534–2543. 10.1002/jsfa.10279. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kim H.; Kang S.; Baik N.; Hwang I.; Chung D. S. Applications of deep eutectic solvents to quantitative analyses of pharmaceuticals and pesticides in various matrices: a brief review. Arch. Pharm. Res. 2020, 43, 900–919. 10.1007/s12272-020-01266-7. [DOI] [PubMed] [Google Scholar]
- Wang K.; Xie X.; Zhang Y.; Huang Y.; Zhou S.; Zhang W.; Lin Y.; Fan H. Combination of microwave-assisted extraction and ultrasonic-assisted dispersive liquid-liquid microextraction for separation and enrichment of pyrethroids residues in Litchi fruit prior to HPLC determination. Food Chem. 2018, 240, 1233–1242. 10.1016/j.foodchem.2017.08.061. [DOI] [PubMed] [Google Scholar]
- Boonchiangma S.; Ngeontae W.; Srijaranai S. Determination of six pyrethroid insecticides in fruit juice samples using dispersive liquid–liquid microextraction combined with high performance liquid chromatography. Talanta 2012, 88, 209–215. 10.1016/j.talanta.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Hu L.; Wang H.; Qian H.; Liu C.; Lu R.; Zhang S.; Zhou W.; Gao H.; Xu D. Centrifuge-less dispersive liquid-liquid microextraction base on the solidification of switchable solvent for rapid on-site extraction of four pyrethroid insecticides in water samples. J. Chromatogr. A 2016, 1472, 1–9. 10.1016/j.chroma.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Mansour F. R.; Khairy M. A. Pharmaceutical and biomedical applications of dispersive liquid–liquid microextraction. J. Chromatogr. B 2017, 1061, 382–391. 10.1016/j.jchromb.2017.07.055. [DOI] [PubMed] [Google Scholar]
- Liu X.; Liu C.; Qian H.; Qu Y.; Zhang S.; Lu R.; Gao H.; Zhou W. Ultrasound-assisted dispersive liquid-liquid microextraction based on a hydrophobic deep eutectic solvent for the preconcentration of pyrethroid insecticides prior to determination by high-performance liquid chromatography. Microchem. J. 2019, 146, 614–621. 10.1016/j.microc.2019.01.048. [DOI] [Google Scholar]
- Tang W.; Dai Y.; Row K. H. Evaluation of fatty acid/alcohol-based hydrophobic deep eutectic solvents as media for extracting antibiotics from environmental water. Anal. Bioanal. Chem. 2018, 410, 7325–7336. 10.1007/s00216-018-1346-6. [DOI] [PubMed] [Google Scholar]
- Lamei N.; Ezoddin M.; Ardestani M. S.; Abdi K. Dispersion of magnetic graphene oxide nanoparticles coated with a deep eutectic solvent using ultrasound assistance for preconcentration of methadone in biological and water samples followed by GC–FID and GC–MS. Anal. Bioanal. Chem. 2017, 409, 6113–6121. 10.1007/s00216-017-0547-8. [DOI] [PubMed] [Google Scholar]
- Mȩczykowska H.; Kobylis P.; Stepnowski P.; Caban M. Ionic liquids for the passive sampling of sulfonamides from water-applicability and selectivity study. Anal. Bioanal. Chem. 2017, 409, 3951–3958. 10.1007/s00216-017-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvolová M.; Táborský P.; Vrábel P.; Krásenský P.; Preisler J. Sensitive determination of erythrosine and other red food colorants using capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 2007, 1141, 206–211. 10.1016/j.chroma.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Ozkantar N.; Yilmaz E.; Soylak M.; Tuzen M. Separation, enrichment and spectrophotometric determination of erythrosine (E127) in drug, cosmetic and food samples by heat-induced homogeneous liquid–liquid microextraction method. J. Environ. Anal. Chem. 2019, 99, 1135–1147. 10.1080/03067319.2019.1616718. [DOI] [Google Scholar]
- Mahajan P. G.; Bhopate D. P.; Kolekar G. B.; Patil S. R. FRET sensor for Erythrosine dye based on organic nanoparticles: Application to analysis of food stuff. J. Fluoresc. 2016, 26, 1467–1478. 10.1007/s10895-016-1839-7. [DOI] [PubMed] [Google Scholar]
- Shokrollahi A.; Pili H. B.; Doust K. H. Microspectrophotometric determination of erythrosine in beverage and water samples after ultrasonic assisted supramolecular-based dispersion solidification liquid–liquid microextraction. J. Anal. Chem. 2017, 72, 617–623. 10.1134/S1061934817060028. [DOI] [Google Scholar]
- Gładysz M.; Król M.; Własiuk P.; Piwowar M.; Zadora G.; Kościelniak P. Development and evaluation of semi-destructive, ultrasound assisted extraction method followed by gas chromatography coupled to mass spectrometry enabling discrimination of red lipstick samples. J. Chromatogr. A 2018, 1577, 92–100. 10.1016/j.chroma.2018.09.055. [DOI] [PubMed] [Google Scholar]
- Babaee S.; Daneshfar A. Magnetic deep eutectic solvent-based ultrasound-assisted liquid-liquid microextraction for determination of hexanal and heptanal in edible oils followed by gas chromatography-flame ionization detection. Anal. Methods 2018, 10, 4162–4169. 10.1039/C8AY01058G. [DOI] [Google Scholar]
- Altunay N.; Elik A.; Gürkan R. Monitoring of some trace metals in honeys by flame atomic absorption spectrometry after ultrasound assisted-dispersive liquid liquid microextraction using natural deep eutectic solvent. Microchem. J. 2019, 147, 49–59. 10.1016/j.microc.2019.03.003. [DOI] [Google Scholar]
- Moloudizargari M.; Mikaili P.; Aghajanshakeri S.; Asghari M. H.; Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199. 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos C. M. H.; Mason N. L.; Kuypers K. P. C. Psychedelics and neuroplasticity: A systematic review unraveling the biological underpinnings of psychedelics. Front. Psychiatry 2021, 1575. 10.3389/fpsyt.2021.724606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Li M.; Tan S.; Wang C.; Fan S.; Huang C. Harmine is an inflammatory inhibitor through the suppression of NF-κB signaling. Biochem. Biophys. Res. Commun. 2017, 489, 332–338. 10.1016/j.bbrc.2017.05.126. [DOI] [PubMed] [Google Scholar]
- Silva Y.; Ferreira T. A. P. C.; Jiao G.; Brooks M. S. Sustainable approach for lycopene extraction from tomato processing by-product using hydrophobic eutectic solvents. J. Food Sci. Technol. 2019, 56, 1649–1654. 10.1007/s13197-019-03618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briars R.; Paniwnyk L. Effect of ultrasound on the extraction of artemisinin from Artemisia annua. Ind. Crops Prod. 2013, 42, 595–600. 10.1016/j.indcrop.2012.06.043. [DOI] [Google Scholar]
- Wojeicchowski J. P.; Ferreira A. M.; Abranches D. O.; Mafra M. R.; Coutinho J. A. P. Using COSMO-RS in the design of deep eutectic solvents for the extraction of antioxidants from Rosemary. ACS Sustainable Chem. Eng. 2020, 8, 12132–12141. 10.1021/acssuschemeng.0c03553. [DOI] [Google Scholar]
- Verma R.; Banerjee T. Liquid-liquid extraction of lower alcohols using Menthol-based hydrophobic deep eutectic solvent: Experiments and COSMO-SAC predictions. Ind. Eng. Chem. Res. 2018, 57, 3371–3381. 10.1021/acs.iecr.7b05270. [DOI] [Google Scholar]
- Cao J.; Chen L.; Li M.; Cao F.; Zhao L.; Su E. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018, 20, 1879–1886. 10.1039/C7GC03820H. [DOI] [Google Scholar]
- Tang W.; Row K. H. Design and evaluation of polarity controlled and recyclable deep eutectic solvent based biphasic system for the polarity driven extraction and separation of compounds. J. Clean. Prod. 2020, 268, 122306. 10.1016/j.jclepro.2020.122306. [DOI] [Google Scholar]
- Vardhan K. H.; Kumar P. S.; Panda R. C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. 10.1016/j.molliq.2019.111197. [DOI] [Google Scholar]
- Polmear I.; St. John D.; Nie J.-F.; Qian M.. Light Alloys: Metallurgy of the Light Metals; Butterworth-Heinemann, 2017. [Google Scholar]
- Flett D. S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 2426–2438. 10.1016/j.jorganchem.2004.11.037. [DOI] [Google Scholar]
- Wazeer I.; Hizaddin H. F.; Hashim M. A.; Hadj-Kali M. K. An Overview about the extraction of heavy metals and other critical pollutants from contaminated water via hydrophobic deep eutectic solvents. J. Environ. Chem. Eng. 2022, 10, 108574. 10.1016/j.jece.2022.108574. [DOI] [Google Scholar]
- Rahnama R.; Najafi M. The use of rapidly synergistic cloud point extraction for the separation and preconcentration of trace amounts of Ni (II) ions from food and water samples coupling with flame atomic absorption spectrometry determination. Environ. Monit. Assess. 2016, 188, 150. 10.1007/s10661-016-5146-1. [DOI] [PubMed] [Google Scholar]
- Stathopoulou M.-E. K.; Banti C. N.; Kourkoumelis N.; Hatzidimitriou A. G.; Kalampounias A. G.; Hadjikakou S. K. Silver complex of salicylic acid and its hydrogel-cream in wound healing chemotherapy. J. Inorg. Biochem. 2018, 181, 41–55. 10.1016/j.jinorgbio.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Maier J. M.; Li P.; Hwang J.; Smith M. D.; Shimizu K. D. Measurement of silver-π interactions in solution using molecular torsion balances. J. Am. Chem. Soc. 2015, 137, 8014–8017. 10.1021/jacs.5b04554. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Ye B.; Liu J. Research on the peak of CO2 emissions in the developing world: Current progress and future prospect. Appl. Energy 2019, 235, 186–203. 10.1016/j.apenergy.2018.10.089. [DOI] [Google Scholar]
- Li G.; Deng D.; Chen Y.; Shan H.; Ai N. Solubilities and thermodynamic properties of CO2 in choline-chloride based deep eutectic solvents. J. Chem. Thermodyn. 2014, 75, 58–62. 10.1016/j.jct.2014.04.012. [DOI] [Google Scholar]
- Ghaedi H.; Zhao M.; Clough P. T.; Anthony E. J.; Fennell P. S. High CO2 absorption in new amine based-transition-temperature mixtures (deep eutectic analogues) and reporting thermal stability, viscosity and surface tension: Response surface methodology (RSM). J. Mol. Liq. 2020, 316, 113863. 10.1016/j.molliq.2020.113863. [DOI] [Google Scholar]
- Sarmad S.; Mikkola J.-P.; Ji X. Carbon dioxide capture with ionic liquids and deep eutectic solvents: A new generation of sorbents. ChemSusChem 2017, 10, 324–352. 10.1002/cssc.201600987. [DOI] [PubMed] [Google Scholar]
- Pires J.; Martins F.; Alvim-Ferraz M.; Simões M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. 10.1016/j.cherd.2011.01.028. [DOI] [Google Scholar]
- Liu X.; Gao B.; Jiang Y.; Ai N.; Deng D. Solubilities and thermodynamic properties of carbon dioxide in guaiacol-based deep eutectic solvents. J. Chem. Eng. Data 2017, 62, 1448–1455. 10.1021/acs.jced.6b01013. [DOI] [Google Scholar]
- Lu M.; Han G.; Jiang Y.; Zhang X.; Deng D.; Ai N. Solubilities of carbon dioxide in the eutectic mixture of levulinic acid (or furfuryl alcohol) and choline chloride. J. Chem. Thermodyn. 2015, 88, 72–77. 10.1016/j.jct.2015.04.021. [DOI] [Google Scholar]
- Deng D.; Jiang Y.; Liu X.; Zhang Z.; Ai N. Investigation of solubilities of carbon dioxide in five levulinic acid-based deep eutectic solvents and their thermodynamic properties. J. Chem. Thermodyn. 2016, 103, 212–217. 10.1016/j.jct.2016.08.015. [DOI] [Google Scholar]
- Chen Y.; Ai N.; Li G.; Shan H.; Cui Y.; Deng D. Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols. J. Chem. Eng. Data 2014, 59, 1247–1253. 10.1021/je400884v. [DOI] [Google Scholar]
- Leron R. B.; Caparanga A.; Li M.-H. Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T= 303.15–343.15 K and moderate pressures. J. Taiwan Inst. Chem. Eng. 2013, 44, 879–885. 10.1016/j.jtice.2013.02.005. [DOI] [Google Scholar]
- Leron R. B.; Li M.-H. Solubility of carbon dioxide in a choline chloride–ethylene glycol based deep eutectic solvent. Thermochim. Acta 2013, 551, 14–19. 10.1016/j.tca.2012.09.041. [DOI] [Google Scholar]
- Ali E.; Hadj-Kali M. K.; Mulyono S.; Alnashef I.; Fakeeha A.; Mjalli F.; Hayyan A. Solubility of CO2 in deep eutectic solvents: experiments and modelling using the Peng–Robinson equation of state. Chem. Eng. Res. Des. 2014, 92, 1898–1906. 10.1016/j.cherd.2014.02.004. [DOI] [Google Scholar]
- Deng D.; Han G.; Jiang Y. Investigation of a deep eutectic solvent formed by levulinic acid with quaternary ammonium salt as an efficient SO2 absorbent. New J. Chem. 2015, 39, 8158–8164. 10.1039/C5NJ01629K. [DOI] [Google Scholar]
- Altamash T.; Amhamed A. I.; Aparicio S.; Atilhan M. Combined experimental and theoretical study on high pressure methane solubility in natural deep eutectic solvents. Ind. Eng. Chem. Res. 2019, 58, 8097–8111. 10.1021/acs.iecr.9b00702. [DOI] [Google Scholar]
- Haider M. B.; Kumar R. Solubility of CO2 and CH4 in sterically hindered amine-based deep eutectic solvents. Sep. Purif. Technol. 2020, 248, 117055. 10.1016/j.seppur.2020.117055. [DOI] [Google Scholar]
- Yang D.; Hou M.; Ning H.; Zhang J.; Ma J.; Yang G.; Han B. Efficient SO2 absorption by renewable choline chloride–glycerol deep eutectic solvents. Green Chem. 2013, 15, 2261–2265. 10.1039/c3gc40815a. [DOI] [Google Scholar]
- Sun S.; Niu Y.; Xu Q.; Sun Z.; Wei X. Efficient SO2 absorptions by four kinds of deep eutectic solvents based on choline chloride. Ind. Eng. Chem. Res. 2015, 54, 8019–8024. 10.1021/acs.iecr.5b01789. [DOI] [Google Scholar]
- Chen Y.; Jiang B.; Dou H.; Zhang L.; Tantai X.; Sun Y.; Zhang H. Highly efficient and reversible capture of low partial pressure SO2 by functional deep eutectic solvents. Energy Fuels 2018, 32, 10737–10744. 10.1021/acs.energyfuels.8b01794. [DOI] [Google Scholar]
- Shunji K.; Xizhou S.; Wenze Y. Investigation of CO2 desorption kinetics in MDEA and MDEA+DEA rich amine solutions with thermo-gravimetric analysis method. Int. J. Greenhouse Gas Control 2020, 95, 102947. 10.1016/j.ijggc.2019.102947. [DOI] [Google Scholar]
- Nozaeim A. A.; Tavasoli A.; Mortaheb H. R.; Mafi M. CO2 absorption/desorption in aqueous DEEA/MDEA and their hybrid solutions with sulfolane. J. Nat. Gas Sci. Eng. 2020, 76, 103219. 10.1016/j.jngse.2020.103219. [DOI] [Google Scholar]
- Chen Y.; Han X.; Liu Z.; Yu D.; Guo W.; Mu T. Capture of toxic gases by deep eutectic solvents. ACS Sustainable Chem. Eng. 2020, 8, 5410–5430. 10.1021/acssuschemeng.0c01493. [DOI] [Google Scholar]
- Anthony J. L.; Maginn E. J.; Brennecke J. F. Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium Hexafluorophosphate. J. Phys. Chem. B 2002, 106, 7315–7320. 10.1021/jp020631a. [DOI] [Google Scholar]
- Huang K.; Zhang X.-M.; Hu X.-B.; Wu Y.-T. Hydrophobic protic ionic liquids tethered with tertiary amine group for highly efficient and selective absorption of H2S from CO2. AIChE J. 2016, 62, 4480–4490. 10.1002/aic.15363. [DOI] [Google Scholar]
- Gutiérrez A.; Rozas S.; Hernando P.; Alcalde R.; Atilhan M.; Aparicio S. A theoretical study of CO2 capture by highly hydrophobic type III deep eutectic solvents. J. Mol. Liq. 2022, 366, 120285. 10.1016/j.molliq.2022.120285. [DOI] [Google Scholar]
- Zubeir L. F.; Lacroix M. H.; Kroon M. C. Low transition temperature mixtures as innovative and sustainable CO2 capture solvents. J. Phys. Chem. B 2014, 118, 14429–14441. 10.1021/jp5089004. [DOI] [PubMed] [Google Scholar]
- Zubeir L. F.; Held C.; Sadowski G.; Kroon M. C. PC-SAFT modeling of CO2 solubilities in deep eutectic solvents. J. Phys. Chem. B 2016, 120, 2300–2310. 10.1021/acs.jpcb.5b07888. [DOI] [PubMed] [Google Scholar]
- Shishov A. Y.; Chislov M. V.; Nechaeva D. V.; Moskvin L. N.; Bulatov A. V. A new approach for microextraction of non-steroidal anti-inflammatory drugs from human urine samples based on in-situ deep eutectic mixture formation. J. Mol. Liq. 2018, 272, 738–745. 10.1016/j.molliq.2018.10.006. [DOI] [Google Scholar]
- Sereshti H.; Zarei-Hosseinabadi M.; Soltani S.; Taghizadeh M. Green vortex-assisted emulsification microextraction using a ternary deep eutectic solvent for extraction of tetracyclines in infant formulas. Food Chem. 2022, 396, 133743. 10.1016/j.foodchem.2022.133743. [DOI] [PubMed] [Google Scholar]
- El-Deen A. K.; Elmansi H.; Shimizu K. Natural hydrophobic deep eutectic solvent for vortex-assisted dispersive liquid-liquid microextraction of anti-prostate cancer triple therapy from water and human plasma. Microchem. J. 2022, 184, 108124. 10.1016/j.microc.2022.108124. [DOI] [Google Scholar]
- Guajardo N.; Müller C. R.; Schrebler R.; Carlesi C.; Dominguez de Maria P. Deep eutectic solvents for organocatalysis, biotransformations, and multistep organocatalyst/enzyme combinations. ChemCatChem. 2016, 8, 1020–1027. 10.1002/cctc.201501133. [DOI] [Google Scholar]
- Kourist R.; González-Sabín J. Non-conventional media as strategy to overcome the solvent dilemma in chemoenzymatic tandem catalysis. ChemCatChem. 2020, 12, 1903–1912. 10.1002/cctc.201902192. [DOI] [Google Scholar]
- Durand E.; Lecomte J.; Villeneuve P. Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. 10.1002/ejlt.201200416. [DOI] [Google Scholar]
- Hümmer M.; Kara S.; Liese A.; Huth I.; Schrader J.; Holtmann D. Synthesis of (−)-menthol fatty acid esters in and from (−)-menthol and fatty acids–novel concept for lipase catalyzed esterification based on eutectic solvents. Mol. Catal. 2018, 458, 67–72. 10.1016/j.mcat.2018.08.003. [DOI] [Google Scholar]
- Safety data for 1-dodecanol: http://msds.chem.ox.ac.uk/DO/1-dodecanol.html (accessed on April 2, 2007).
- Sanders J.; Bucher J.; Peckham J.; Kissling G.; Hejtmancik M.; Chhabra R. Carcinogenesis studies of cresols in rats and mice. Toxicology 2009, 257, 33–39. 10.1016/j.tox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romonchuk W.; Bunge A. Mechanism of enhanced dermal permeation of 4-cyanophenol and methyl paraben from saturated aqueous solutions containing both solutes. Skin Pharmacol Physiol 2010, 23, 152–163. 10.1159/000272121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbinosa E. O.; Odjadjare E. E.; Chigor V. N.; Igbinosa I. H.; Emoghene A. O.; Ekhaise F. O.; Igiehon N. O.; Idemudia O. G. Toxicological profile of chlorophenols and their derivatives in the environment: the public health perspective. Sci. World J. 2013, 2013, 460215. 10.1155/2013/460215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh V. R.; Chow S. L.; Schirmer K.; Lynn D. H.; Bols N. C. Evaluating the toxicity of Triton X-100 to protozoan, fish, and mammalian cells using fluorescent dyes as indicators of cell viability. Ecotoxicol. Environ. Saf. 2004, 57, 375–382. 10.1016/S0147-6513(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Safety Data Sheet: https://www.bio.vu.nl/~microb/Protocols/chemicals/MSDS/Triton20X-114.pdfhttps://www.bio.vu.nl/~microb/Protocols/chemicals/MSDS/Triton20X-114.pdf (accessed on September 23, 2014).
- Hu L.; Zhang C.; Zeng G.; Chen G.; Wan J.; Guo Z.; Wu H.; Yu Z.; Zhou Y.; Liu J. Metal-based quantum dots: synthesis, surface modification, transport and fate in aquatic environments and toxicity to microorganisms. RSC Adv. 2016, 6, 78595–78610. 10.1039/C6RA13016J. [DOI] [Google Scholar]
- Li K.; Zhao Q.; Fan Z.; Jia S.; Liu Q.; Liu F.; Liu S. The toxicity of perfluorodecanoic acid is mainly manifested as a deflected immune function. Mol. Biol. Rep. 2022, 49, 4365–4376. 10.1007/s11033-022-07272-w. [DOI] [PubMed] [Google Scholar]
- Dave G.; Blanck H.; Gustafsson K. Biological effects of solvent extraction chemicals on aquatic organisms. J. Chem. Technol. Biotechnol. 1979, 29, 249–257. 10.1002/jctb.503290406. [DOI] [Google Scholar]
- Compound Summary Benzyltriethylammonium chloride: https://pubchem.ncbi.nlm.nih.gov/compound/Benzyltriethylammonium-chloride (accessed on January 23, 2021).
- Hamdaoui O.; Naffrechoux E.; Suptil J.; Fachinger C. Ultrasonic desorption of p-chlorophenol from granular activated carbon. J. Chem. Eng. 2005, 106, 153–161. 10.1016/j.cej.2004.10.010. [DOI] [Google Scholar]
- Sáez M.; de Voogt P.; Parsons J. R. Persistence of perfluoroalkylated substances in closed bottle tests with municipal sewage sludge. Environ. Sci. Pollut. Res. 2008, 15, 472–477. 10.1007/s11356-008-0020-5. [DOI] [PubMed] [Google Scholar]
- Zeng J.; Yang B.; Wang X.; Li Z.; Zhang X.; Lei L. Degradation of pharmaceutical contaminant ibuprofen in aqueous solution by cylindrical wetted-wall corona discharge. J. Chem. Eng. 2015, 267, 282–288. 10.1016/j.cej.2015.01.030. [DOI] [Google Scholar]
- Halder A. K.; Cordeiro M. N. D. S. Probing the environmental toxicity of deep eutectic solvents and their components: An in silico modeling approach. ACS Sustainable Chem. Eng. 2019, 7, 10649–10660. 10.1021/acssuschemeng.9b01306. [DOI] [Google Scholar]
- Lomba L.; Ribate M.; Sangüesa E.; Concha J.; Garralaga M.; Errazquin D.; García C. B.; Giner B. Deep eutectic solvents: Are they safe?. Appl. Sci. 2021, 11, 10061. 10.3390/app112110061. [DOI] [Google Scholar]
- Hayyan M.; Looi C. Y.; Hayyan A.; Wong W. F.; Hashim M. A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PloS One 2015, 10, e0117934 10.1371/journal.pone.0117934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-T.; Yang Q.; Cui Q.; Fan X.-H.; Dong M.-Z.; Gao M.-Z.; Lv M.-J.; An J.-Y.; Meng D.; Zhao X.-H.; Fu Y.-J. Recyclable menthol-based deep eutectic solvent micellar system for extracting phytochemicals from Ginkgo biloba leaves. J. Clean. Prod. 2020, 244, 118648. 10.1016/j.jclepro.2019.118648. [DOI] [Google Scholar]