Abstract
Background:
Many patients with bipolar disorder receive multi-drug treatment regimens, but the distinguishing profiles of patients who receive complex pharmacologies have not been established.
Method:
Prescribing patterns of lithium, anticonvulsants, antidepressants, and antipsychotics were examined for 4035 subjects with bipolar disorder (DSM-IV) immediately prior to entering the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Subjects were recruited for participation across 22 centers in the United States between November 1999 and July 2005. The quality receiver operating characteristic (ROC) method was used to develop composite profiles of patients receiving complex regimens (p < .01 for all iterations).
Results:
Use of 3 or more medications occurred in 40% of subjects, while 18% received 4 or more agents. Quality ROC analyses revealed that subjects had a 64% risk for receiving a complex regimen (≥ 4 medications) if they had (1) ever taken an atypical antipsychotic, (2) ≥ 6 lifetime depressive episodes, (3) attempted suicide, and (4) an annual income ≥ $75,000. Complex polypharmacy was least often associated with lithium, divalproex, or carbamazepine and most often associated with atypical antipsychotics or antidepressants. Contrary to expectations, a history of psychosis, age at onset, bipolar I versus II subtype, history of rapid cycling, prior hospitalizations, current illness state, and history of alcohol or substance use disorders did not significantly alter the risk profiles for receiving complex regimens.
Conclusion:
Complex polypharmacy involving at least 4 medications occurs in approximately 1 in 5 individuals with bipolar disorder. Use of traditional mood stabilizers is associated with fewer cotherapies. Complex regimens are especially common in patients with substantial depressive illness burden and suicidality, for whom simpler drug regimens may fail to produce acceptable levels of response.
Trial Registration:
clinicaltrials.gov Identifier: NCT00012558
Rapid growth in the pharmacopeia for bipolar disorder has generated more medication alternatives and, perhaps more importantly, a greater potential for patients to receive multiple medications simultaneously. However, little is known about the factors most likely to influence clinicians’ decisions to prescribe complex multi-drug regimens for individuals with bipolar disorder.
Intramural studies of patients treated at the National Institute of Mental Health (NIMH) identified a substantial rise in the number of medications being prescribed for patients from 1974 to 1996, but without a greater benefit in outcome.1 This observation has been interpreted to suggest that either contemporary patients with bipolar disorder require more elaborate drug regimens to attain symptomatic recovery as compared to patients from a generation ago, or that clinicians often add an increasing number of psychiatric medications as more options become available, even if there is not discernible additional benefit.
The empirical literature on polypharmacy, composed largely of commercially-sponsored, controlled, 2-drug combination trials in acute mania, lags behind clinical practice, in that it has focused mainly on the advantages or disadvantages of combining either a conventional antipsychotic2 or atypical antipsychotic (such as olanzapine,3 risperidone,4,5 or quetiapine6,7) with a traditional mood stabilizer (such as lithium, divalproex, or carbamazepine) during acute mania or continuation treatment (e.g., using 2 agents vs. 1 following an acute mood episode8,9). One recent study found no acute antimanic advantage, but more weight gain and dyslipidemia with olanzapine plus carbamazepine as compared to carbamazepine alone.10 Randomized controlled studies involving combinations of traditional mood stabilizers are few in number and limited to relatively small sample sizes (i.e., N ≤ 30)11-13 or active comparator combinations (e.g., 2 mood stabilizers vs. mood stabilizer plus antipsychotic14) that may have been underpowered to detect efficacy and/or tolerability differences between simpler versus more complex regimens. The combination of a traditional mood stabilizer and an antidepressant for acute bipolar depression showed no advantage over mood stabilizer monotherapy in 2 recent randomized placebo-controlled trials,15,16 although, for acute bipolar depression, the combination of lithium plus lamotrigine appears to yield greater efficacy than lithium plus placebo,17 as does the combination of olanzapine plus fluoxetine versus olanzapine monotherapy.18 Most studies have focused on monotherapy versus 2 antimanic drugs, yet in routine practice, a substantial number of patients with bipolar disorder receive 3 or more medications, regardless of their current illness phase.19
The present naturalistic study examined the prevalence of complex multi-drug prescriptions (i.e., 4 or more psychotropics) among entrants to the NIMH Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and developed composite profiles (i.e., combinations of variables) to identify patients who receive such regimens. Efforts to better understand psychiatrists’ prescribing behavior, and possible pharmacotherapy objectives, may help identify which aspects of illness complexity (e.g., symptom severity domains, episode recurrence, comorbidities) contribute most to the use of complex pharmacotherapy.
METHOD
The overall design and scope of the multi-site STEP-BD study have been described previously.20 Briefly, subjects were at least 15 years old, met DSM-IV criteria for any type of bipolar disorder (I, II, not otherwise specified [NOS], cyclothymic disorder, or schizoaffective bipolar disorder), and were recruited for participation across 22 centers in the United States between November 1999 and July 2005. Diagnoses were made by research psychiatrists using the STEP-BD Affective Disorders Evaluation (ADE)21 and independently confirmed with the Mini-International Neuropsychiatric Interview (MINI, version 5.0),22 administered by a trained master’s- or doctoral-level research clinician (psychiatrist, psychologist, social worker, or psychiatric nurse). The current study focused on all STEP-BD entrants who met DSM-IV criteria for bipolar I (N = 2666), II (N = 1084), or NOS (N = 285) disorders at the time of their baseline assessment. Study exclusion criteria were kept to a minimum to enhance the generalizability of findings to patients seen with bipolar disorder under ordinary clinical conditions. Analyses for the current study focused on medications that patients were taking at study entry as well as their past history of medication usage.
Clinical features were rated using the ADE, as this intake assessment tool gathers information regarding present and past psychiatric illness, family histories of psychiatric illness, medical illnesses, and current mental status. Affective or psychotic symptoms and current medications were assessed using the Clinical Monitoring Form (CMF),23 a 1-page instrument that contains a modified version of the Structured Clinical Interview for DSM-IV current mood modules as well as the Clinical Global Impressions-Severity of Illness (CGI-S) scale. The ADE and CMF were used to determine 1 of 8 clinical states for each subject at the time of enrollment, corresponding to 4 DSM-IV syndromal categories (depression, hypomania, mania, or mixed) and 4 subsyndromal categories (continued symptoms, recovering, recovered, and roughening, the latter term describing subjects with emerging signs of affective relapse after having previously been recovered).
Seven core medications and medication groups were defined: lithium, divalproex, carbamazepine, lamotrigine, other anticonvulsants without definitively established thymoleptic properties (e.g., topiramate, gabapentin, oxcarbazepine), any atypical antipsychotic, or any antidepressant. Data on the use of other medications that are not generally considered to be core treatments for syndromal symptoms in bipolar disorder, including benzodiazepines and other sedative-hypnotics (e.g., zolpidem), were recorded but not used in the current analyses.
All subjects provided written informed consent to participate in the study protocol, which was approved by the respective institutional review board at each of the STEP-BD study sites as well as the Data Safety Monitoring Board of STEP-BD.
Statistical Analyses
Between-group (i.e., complex polypharmacy present or absent) comparisons on dichotomous and continuous variables are presented using χ2 tests or t tests for the dichotomous and continuous independent variables, respectively, summarized in Table 1. Accompanying effect sizes (Cohen d) were calculated to provide estimates of the clinical significance of univariate analyses for the entire study group. A recursive receiver operating characteristic (ROC) analysis was performed using software available in the public domain, developed at the Sierra Pacific Mental Illness Research, Education, and Clinical Core (http://mirecc.stanford.edu; Stanford, Calif.). The event to be predicted, or “gold standard,” was whether a patient was on a complex regimen of 4 or more psychotropic medications. The gold standard was coded as a binary variable.
Table 1.
Characteristics of Subjects Taking Fewer Versus 4 or More Core Psychotropicsa at STEP-BD Entry
| Variable | Subjects Taking < 4 Medicationsb,c |
Subjects Taking ≥ 4 Medicationsb,c |
χ2 | T | df | p | Cohen d |
|---|---|---|---|---|---|---|---|
| Female, N/N (%) | 1713/3061 (56) | 446/676 (66) | 22.353 | … | 1 | < .0001 | 0.09 |
| White, N/N (%) | 2737/3324 (82) | 637/711 (90) | 21.959 | … | 1 | < .0001 | 0.09 |
| Clinical state at study entry, N/N (%) | |||||||
| Recovered | 580/3324 (17) | 116/711 (16) | 0.451 | … | 1 | .502 | 0.00 |
| Manic | 90/3323 (3) | 12/711 (2) | 2.476 | … | 1 | .116 | 0.07 |
| Hypomanic | 129/3323 (4) | 24/711 (3) | 0.412 | … | 1 | .521 | 0.03 |
| Mixed | 243/3323 (7) | 62/711 (9) | 1.660 | … | 1 | .198 | 0.05 |
| Depressed | 994/3323 (30) | 259/711 (36) | 11.609 | … | 1 | .001 | 0.18 |
| Income ≥ $75,000/y, N/N (%) | 747/3324 (22) | 158/711 (22) | 0.009 | … | 1 | .925 | 0.00 |
| Past year rapid cycling, N/N (%) | 1046/2152 (49) | 264/460 (57) | 6.165 | … | 1 | .013 | 0.13 |
| Alcohol abuse/dependence, N/N (%) | 325/3324 (10) | 48/711 (7) | 6.038 | … | 1 | .014 | 0.08 |
| Drug abuse/dependence, N/N (%) | 461/3324 (14) | 84/711 (12) | 1.944 | … | 1 | .163 | 0.05 |
| History of suicide attempt, N/N (%) | 1105/3201 (35) | 338/686 (49) | 52.030 | … | 1 | < .0001 | 0.05 |
| First episode polarity depressed, N/N (%) | 1602/3324 (48) | 369/711 (52) | 3.069 | … | 1 | .080 | 0.05 |
| History of psychosis, N/N (%) | 309/3324 (9) | 96/711 (13) | 11.014 | … | 1 | .001 | 0.02 |
| Age, mean ± SD, y | 39.4 ± 13.1 | 41.3 ± 12.1 | … | 3.468 | 3738 | < .001 | 0.11 |
| Age at illness onset, mean ± SD, y | 16.2 ± 3.6 | 16.4 ± 3.3 | … | 0.907 | 3913 | .182 | 0.03 |
| No. of lifetime manias, mean ± SD | 4.3 ± 1.8 | 4.5 ± 1.6 | … | 2.680 | 3518 | .007 | 0.09 |
| No. of lifetime depressions, mean ± SD | 4.5 ± 1.7 | 4.9 ± 1.4 | … | 5.820 | 3544 | < .0001 | 0.20 |
| CGI-S score at entry, mean ± SD | 3.0 ± 1.3 | 3.3 ± 1.3 | … | 6.230 | 3980 | < .0001 | 0.20 |
| BMI, mean ± SD | 28.2 ± 6.6 | 29.5 ± 7.1 | … | 4.078 | 2942 | < .0001 | 0.15 |
| Bipolar disorder diagnosis, N/N (%) | 16.6 | … | 2 | < .0001 | … | ||
| Bipolar I | 2156/3324 (65) | 510/711 (72) | |||||
| Bipolar II | 913/3324 (27) | 171/711 (24) | |||||
| Bipolar NOS | 255/3324 (8) | 30/711 (4) |
Core medications/medication groups are lithium, divalproex, carbamazepine, lamotrigine, other anticonvulsants, atypical antipsychotics, and antidepressants.
Percentages are column percentages.
Ns vary due to missing data for some analyses.
Abbreviations: BMI = body mass index, CGI-S = Clinical Global Impressions-Severity of Illness, STEP-BD = Systematic Treatment Enhancement Program for Bipolar Disorder.
Symbol: … = not applicable.
As described by Kraemer,24 the ROC analysis first identifies the best predictor and then splits patients into subgroups at a cut-point identified by the analysis. Within each subgroup, the remaining variables are searched for the next best predictor. Each time the “best” predictor is found, a χ2 test is performed and the variable is retained as a predictor if p < .01. The process of searching for predictors continues until a predictor is not significant or until there are fewer than 10 observations in a subgroup. Hence, the technique generates a collection of predictor variables that, taken together as a whole, yield a predictive result.
We chose to examine predictors of complex polypharmacy using ROC analyses rather than more traditional regression analyses in order to provide results that were more readily applicable in a clinical situation than those obtained through regression analyses. Importantly, unlike regression approaches, ROC analyses provide risk profiles that can be used as a focus of future study. While ROC analysis is commonly used to focus on balancing sensitivity and specificity in analyses of medical laboratory tests, this approach has more recently been used to predict composite risk profiles for dichotomous outcome states in clinical populations,25 including patients with bipolar disorder.26
RESULTS
Of the 7 core medications/medication categories studied, the mean ± SD number of medications being taken at study entry was 2.21 ± 1.41 (range, 0–7). Of the 4035 subjects, 472 (12%) were taking none of the core medications upon study entry, while 839 (21%) took 1 medication, 1130 (28%) took 2 medications, 883 (22%) took 3 medications, and 711 (18%) took 4 or more core medications. We operationally defined complex polypharmacy as being at least 1 standard deviation greater than the mean; hence, patients receiving 4 or more medications were classified as receiving complex polypharmacy.
Table 1 compares the characteristics of subjects taking fewer than 4 (N = 3324) versus 4 or more (N = 711) core medications/medication groups at STEP-BD entry, with accompanying effect sizes. As shown in the table, relatively modest effect sizes were associated with each of the univariate relationships examined with respect to polypharmacy prescriptions for the entire sample. Baseline severity at the time of study entry, as reflected by CGI-S scores, was modestly but significantly higher among subjects taking 4 or more core psychotropics (p < .001). In order to identify other factors related to the CGI-S, which could in turn potentially mediate the relationship between CGI-S scores and use of complex polypharmacy, we examined Pearson correlations between baseline CGI-S scores and several relevant clinical parameters, including current age, age at onset, gender, history of suicide attempts, income level, and lifetime use of an atypical antipsychotic, lithium, or carbamazepine. All such correlations were low (r < 0.18) and none was statistically significant.
Table 2 presents the proportion of subjects taking fewer than 4 medications versus 4 or more based on the presence of lithium, divalproex, carbamazepine, lamotrigine, other anticonvulsants, any atypical antipsychotic, and any antidepressant. Notably, the effect size associated with more extensive polypharmacy (i.e., ≥ 4 core medications/medication groups) was substantially smaller for subjects who were taking lithium (d = 0.03), divalproex (d = 0.11), or carbamazepine (d = 0.13) as compared to other core pharmacotherapies, suggesting the possibility that patients who receive these mood stabilizers may be less likely to receive more elaborate cotherapies. By contrast, antidepressants had the largest effect size (d = 0.78) associated with extensive polypharmacy, suggesting that individuals who took antidepressants were especially likely to receive more elaborate additional medications.
Table 2.
Prevalence of Subjects Receiving ≥ 4 Core Medications/Medication Groups, Stratified by Presence of an Individual Agent
| Current Agent | N | Subjects Taking < 4 Medications, N (%) |
Subjects Taking ≥ 4 Medications, N (%) |
Cohen d |
|---|---|---|---|---|
| Lithium | 266 | 225 (85) | 41 (15) | 0.03 |
| Divalproex | 1185 | 934 (79) | 251 (21) | 0.11 |
| Carbamazepine | 767 | 548 (16) | 219 (31) | 0.13 |
| Lamotrigine | 839 | 563 (17) | 276 (39) | 0.18 |
| Other anticonvulsant | 629 | 351 (56) | 278 (44) | 0.59 |
| Atypical antipsychotic | 1262 | 780 (62) | 482 (38) | 0.74 |
| Antidepressant | 1935 | 1251 (65) | 684 (35) | 0.78 |
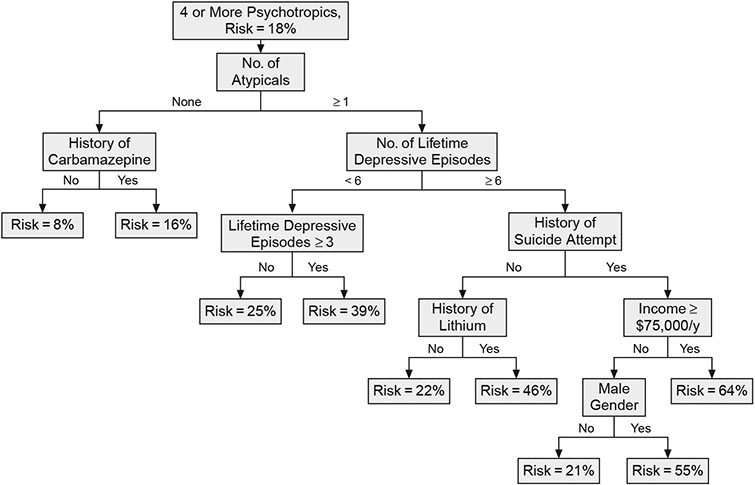
Figure 1 presents the ROC tree generated for identifying the profile of subjects who had been prescribed 4 or more core medications/medication groups. The tree can be read by following the path down to a particular risk. Thus, the profile associated with the greatest risk of receiving a complex polypharmacy regimen included patients who (1) received an atypical antipsychotic, (2) had 6 or more lifetime depressive episodes, (3) made a suicide attempt, and (4) had an annual income of at least $75,000. This model provided a 64% risk for receiving a complex medication regimen, compared to the overall 18% prevalence rate among all STEP-BD entrants for being prescribed 4 or more medications. By contrast, the risk profile for not receiving an extensive medication regimen was best accounted for by subjects who (1) did not receive an atypical antipsychotic and (2) did receive carbamazepine (risk for complex polypharmacy = 8%). No other clinical features contributed to the low-risk model. Factors that appear in the ROC tree may differ from what one might expect based on the effect sizes reported in Table 1 because the ROC creates subgroups according to risk, whereas the effect sizes were based on the full sample.
Figure 1. Receiver Operating Characteristic Tree Showing Risk of Receiving a Complex Polypharmacy Regimen for Bipolar Disorder Patients Prescribed 4 or More Core Medications/Medication Groupsa.
aCore medications/medication groups are lithium, divalproex, carbamazepine, lamotrigine, other anticonvulsants, atypical antipsychotics, and antidepressants.
DISCUSSION
The present findings indicate that a substantial proportion of individuals with bipolar disorder are prescribed complex polypharmacy regimens, particularly when they present with depression. In particular, bipolar disorder patients appear especially likely to receive more extensive medication combinations if they had been prescribed an atypical antipsychotic, had multiple depressive episodes, and made at least 1 suicide attempt. Inclusion of antidepressants as part of a treatment regimen was associated with the largest effect size leading to extensive polypharmacy with core medications. These observations underscore the extent to which depression in bipolar disorder continues to pose a formidable psychopharmacologic challenge, as also suggested by prior observations that antidepressants remain among the most widely prescribed psychotropic agents for patients with bipolar disorder.27
The previously demonstrated lack of efficacy of adjunctive antidepressants relative to mood stabilizers alone from randomized controlled trials for bipolar depression15,16,28 are consistent with prescribing habits observed in the present study, which suggest that clinicians often resort to numerous and more elaborate drug combinations when confronted by serious depression in patients with bipolar disorder. Recent prospective data from the STEP-BD program29 and other studies (e.g., Wehr and Goodwin30) also indicate that antidepressant use is associated with multiple depressive episodes. In the present study group, it is possible that clinicians added other medications if patients had become unstable as the result of antidepressant use in an effort to regain remission. However, the cross-sectional design of the present study did not permit the chronological assessment of when antidepressants were introduced relative to other treatments, leaving open the alternative explanation that antidepressants may have been added to pre-existing complex medication regimens.
Complex polypharmacy was somewhat less prevalent when medication regimens included lithium, divalproex, or carbamazepine rather than other agents, such as atypical antipsychotics, antidepressants, or anticonvulsants with less definitive data, to treat any phase of bipolar disorder. Whereas causal relationships cannot be inferred from these cross-sectional naturalistic data, one possible interpretation is that lithium, divalproex, or carbamazepine confer more robust overall efficacy than other medications for bipolar disorder, prompting a lesser need for additional pharmacotherapies. Alternatively, it is possible that psychiatrists refrain from including lithium, divalproex, or carbamazepine in the regimens of bipolar patients who ultimately receive multiple agents. This is particularly likely for carbamazepine, due to its complex drug interaction profile. In either case, practitioners may underestimate the value and parsimony that might be derived from incorporating lithium, divalproex, or carbamazepine in routine treatment regimens.
Strikingly, many clinical parameters related to illness severity that had initially been hypothesized to predict receipt of complex polypharmacy regimens did not emerge in the ROC analysis as being related to extensive polypharmacy. These variables included a history of psychosis, rapid cycling, number of prior hospitalizations, age at onset, comorbid alcohol or substance use disorders, current illness state (i.e., euthymic versus not euthymic), and bipolar I versus II subtype. The presence of multiple depressive episodes and a history of suicide attempts may especially compel prescribers to intervene with more numerous medications in people with bipolar disorder because clinicians often recognize these features as both difficult to treat and more proximal to morbidity and risk for suicide mortality.31 Patients with bipolar II disorder also were as likely as those with bipolar I disorder to receive complex polypharmacy, perhaps because depression in the former group is often frequent and unremitting,32 and suicide risk from depression may be higher in bipolar II than in bipolar I disorder.33 The observed association between higher income and more complex polypharmacy raises the possibility that prescribers may undertake more extensive, aggressive, and costly treatments (while relatively underutilizing lithium, divalproex, and carbamazepine) for patients with the fewest financial obstacles to them. Because a higher income increased the risk for complex polypharmacy by 18%, one cannot discount this hypothesis.
Methodologically, the present study illustrates the advantages of an ROC approach for establishing clinical profiles to define distinct patient subgroups. For example, many of the dependent variables distinguished the groups with simple or complex regimens but with small effect sizes. This could lead to the erroneous conclusion that particular variables were not relevant to the risk of complex polypharmacy. However, the ROC analyses revealed that the discriminatory power of variables can be much stronger for particular subgroups of patients. To cite one such instance, a history of suicide attempts alone showed little difference between the simple and complex regimen groups, but for patients who had taken an atypical antipsychotic and had more than 6 depressive episodes, it was important in predicting pharmacologic complexity. It should be noted that a logistic regression approach could also be applied to our data, but its outcome would not be as readily interpretable on a clinical basis. Specifically, logistic regression identifies statistically significant independent contributions of predictor variables for an entire sample, but does not allow for the creation of specific risk profiles as does ROC analysis.24
It remains to be demonstrated whether, and when, more complex regimens are associated with better outcomes than might occur with fewer medications for the average patient with bipolar disorder—or, more precisely, for bipolar disorder patients with histories of multiple depressive episodes and suicide attempts. Denicoff and colleagues12 previously found that the combination of lithium plus carbamazepine was associated with greater prophylactic efficacy than either monotherapy among bipolar disorder patients with rapid cycling, but not in those without rapid cycling. Although rapid cycling was not a predictor of complex polypharmacy prescriptions in the current study, it is often associated with extensive periods of depression,34 antidepressant use,29 and risk for suicidal behavior.35 Hence, such patients would seem to be a critical group for future studies of combination drug therapy.
Information about the relationship between complex polypharmacotherapy and medication adherence, as well as cumulative adverse effects, was unavailable in the current study, although both treatment complexity and number of medications have been cited in previous work as predictors of nonadherence.36 Relationships between the number of prescribed medications and treatment adherence may differ depending on which medications are combined. For example, among bipolar disorder patients taking lithium, divalproex, carbamazepine, or lamotrigine, long-term adherence appears greater with use of 2 rather than 1 of these agents,37 perhaps because combinations of these agents are more effective. In the current study, patients taking any of these agents were less likely to receive 4 or more core medications, in contrast to those taking atypical antipsychotics or antidepressants. With respect to potential adverse drug effects and complex polypharmacy, some clinicians may prescribe additional medications if adverse drug effects (such as low energy or poor concentration) are mistaken for signs of untreated psychopathology and perceived as targets for additional pharmacotherapy.
Historical exposure to a particular medication may influence subsequent decisions to prescribe that same medication, or other medications, and hence we included past history of psychotropic medications in the ROC model to profile current complex polypharmacy. Unavailability of information regarding response to past medications, or issues related to duration of use or adherence, represents limitations of the current study. Other limitations include the cross-sectional study design and retrospective assessment of prior treatments and historical illness characteristics (e.g., assessing the lifetime number of episodes). The observed lifetime prevalence of comorbid alcohol and drug abuse in the study group was somewhat lower than has been reported in other studies of multi-episode patients with bipolar disorder.38 Although enrollment of STEP-BD participants was intended to optimize generalizability about individuals with bipolar disorder by imposing few study exclusion criteria, subjects were voluntary patients who sought treatment at academically-affiliated specialty centers for the treatment of bipolar disorder and were willing to participate in research-based treatment. Hence, the results may have differed had other types of patients with bipolar disorder been studied.
In summary, the present study indicates that a substantial proportion of individuals with bipolar disorder receive 4 or more core psychotropic agents during routine treatment and that features related to recurrent depression and suicidality may represent driving forces behind clinician decisions to undertake more extensive drug regimens. Conversely, patients appear less likely to receive complex polypharmacy regimens if they do not require an atypical antipsychotic but do receive lithium or an anticonvulsant with demonstrated efficacy in bipolar disorder. These naturalistic observations underscore both the limitations of existing treatments for bipolar depression as well as the need for controlled studies to determine which multi-drug regimens offer greater utility in depressed bipolar patients with suicidal features.
Acknowledgments
Portions of this research were funded by the National Institute of Mental Health (NIMH) K-23 Career Development Award MH01936 (to Dr. Goldberg) and by federal funds from NIMH and the National Institutes of Health (NIH), under contract N01MH80001. The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was funded with Federal funds from NIMH and NIH under contract N01MH80001. No pharmaceutical company funding or study drug was received in support of this study.
Footnotes
Presented at the 46th annual meeting of the American College of Neuropsychopharmacology, December 9—13, 2007, Boca Raton, Fla.
Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the NIMH. The contents of this article were reviewed and approved by the STEP-BD Steering Committee. Additional details on STEP-BD are available at: http://www.nimh.nih.gov/health/trials/practical/step-bd/questions-and-answers-for-the-systematic-treatment-enhancement-program-for-bipolar-disorder-step-bd-study-background.shtml.
Drug names: carbamazepine (Carbatrol, Equetro, and others), divalproex (Depakote and others), fluoxetine (Prozac and others), gabapentin (Neurontin and others), lamotrigine (Lamictal and others), lithium (Lithobid, Eskalith, and others), olanzapine (Zyprexa), olanzapine-fluoxetine combination (Symbyax), oxcarbazepine (Trileptal and others), quetiapine (Seroquel), risperidone (Risperdal and others), topiramate (Topamax), zolpidem (Ambien and others).
Financial disclosure: Dr. Goldberg has received honoraria from Abbott and Eli Lilly and has participated in speakers/advisory boards for AstraZeneca, GlaxoSmithKline, Eli Lilly, and Pfizer. Dr. Brooks has participated in speakers/advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and Pfizer. Dr. Hoblyn has participated in speakers/advisory boards for Bristol-Myers Squibb and Eli Lilly. Dr. Ghaemi has received grant/research support from Pfizer and has participated in speakers/advisory boards for AstraZeneca and Pfizer. Dr. Perlis is a consultant for Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, and Pfizer. Dr. Miklowitz has received book royalties from Guilford Press and John Wiley and Sons. Dr. Ketter has received grant/research support from Abbott, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Pfizer, Repligen, and Wyeth; is a consultant to Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Jazz, Novartis, Organon, Solvay, Vanda, Wyeth, and XenoPort; and has received lecture honoraria from Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Otsuka, and Pfizer. Dr. Ketter’s spouse is an employee of Johnson & Johnson. Dr. Sachs is a consultant to or a member of advisory boards for AstraZeneca, Bristol-Myers Squibb, Cephalon, Concordant Rater Systems, Eli Lilly, GlaxoSmithKline, Janssen, Memory, Novartis, Organon, Otsuka, Pfizer, Repligen, Sanofi-Aventis, Schering-Plough, Sepracor, and Wyeth; is a member of speakers bureaus for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Memory, Novartis, Pfizer, Sanofi-Aventis, and Wyeth; and Dr. Sachs’ spouse is a stock share-holder of Concordant Rater Systems. Dr. Thase is a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, MedAvante, Neuronetics, Novartis, Organon, Sepracor, Shire, Supernus, and Wyeth; has received grant/research support from Eli Lilly and Sepracor; has participated in speakers bureaus for AstraZeneca, Bristol-Myers Squibb, Cyberonics, Eli Lilly, GlaxoSmithKline, Sanofi-Aventis, Schering Plough (formerly Organon), and Wyeth; has delivered expert testimony for Jones Day (Wyeth Litigation), Phillips Lytle (GlaxoSmithKline Litigation), and Pepper Hamilton LLP (Eli Lilly Litigation); has equity holdings in MedAvante; and has received royalties/patents from American Psychiatric Publishing, Guilford Publications, Herald House, and W.W. Norton & Company. Dr. Thase’s spouse is Senior Medical Director of Advogent (formerly Cardinal Health). Ms. Kurita reports no additional financial or other relationships relevant to the subject of this article.
Contributor Information
Joseph F. Goldberg, Department of Psychiatry, Mount Sinai School of Medicine, New York, N.Y., and the Affective Disorders Research Program, Silver Hill Hospital, New Canaan, Conn.
John O. Brooks, III, Palo Alto Veterans Affairs Health Care System, Calif.; Department of Psychiatry and Behavioral Sciences, Stanford University, Palo Alto, Calif.
Keiko Kurita, Palo Alto Veterans Affairs Health Care System, Calif.; Department of Psychiatry and Behavioral Sciences, Stanford University, Palo Alto, Calif.
Jennifer C. Hoblyn, Palo Alto Veterans Affairs Health Care System, Calif.; Department of Psychiatry and Behavioral Sciences, Stanford University, Palo Alto, Calif.
S. Nassir Ghaemi, Department of Psychiatry, Tufts New England Medical Center, Boston, Mass.
Roy H. Perlis, Department of Psychiatry, Harvard Medical School, and the Department of Psychiatry, Massachusetts General Hospital, Boston
David J. Miklowitz, Departments of Psychology and Psychiatry, University of Colorado, Boulder
Terence A. Ketter, Department of Psychiatry and Behavioral Sciences, Stanford University, Palo Alto, Calif
Gary S. Sachs, Department of Psychiatry, Harvard Medical School, and the Department of Psychiatry, Massachusetts General Hospital, Boston
Michael E. Thase, Department of Psychiatry, University of Pennsylvania, Philadelphia, and the Department of Psychiatry, the University of Pittsburgh, Pa.
REFERENCES
- 1.Frye MA, Ketter TA, Leverich GS, et al. The increasing use of polypharmacotherapy for refractory mood disorders: 22 years of study. J Clin Psychiatry 2000;61(1):9–15 [DOI] [PubMed] [Google Scholar]
- 2.Müller-Oerlinghausen B, Retzow A, Henn FA, et al. Valproate as an adjunct to neuroleptic medication for the treatment of acute episodes of mania: a prospective, randomized, double-blind, placebo-controlled multicenter study. European Valproate Mania Study Group. J Clin Psychopharmacol 2000;20:195–203 [DOI] [PubMed] [Google Scholar]
- 3.Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62–69 [DOI] [PubMed] [Google Scholar]
- 4.Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159:1146–1154 [DOI] [PubMed] [Google Scholar]
- 5.Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania: international, double-blind, randomised controlled trial. Br J Psychiatry 2003;182:141–147 [DOI] [PubMed] [Google Scholar]
- 6.Sachs G, Chengappa KN, Suppes T, et al. Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord 2004;6:213–223 [DOI] [PubMed] [Google Scholar]
- 7.DelBello MP, Kowatch RA, Adler CM, et al. A double-blind, randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry 2006;45:305–313 [DOI] [PubMed] [Google Scholar]
- 8.Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser versus mood stabiliser alone. Br J Psychiatry 2004;184:337–345 [DOI] [PubMed] [Google Scholar]
- 9.Vieta E, Suppes T, Eggens I, et al. Efficacy and safety of quetiapine in combination with lithium or divalproex for maintenance of patients with bipolar I disorder (international trial 126). J Affect Disord 2008;109:251–263 [DOI] [PubMed] [Google Scholar]
- 10.Tohen M, Bowden CL, Smulevich AB, et al. Olanzapine plus carbamazepine versus carbamazepine alone in treating manic episodes. Br J Psychiatry 2008;192:135–143 [DOI] [PubMed] [Google Scholar]
- 11.Denicof KD, Smith-Jackson EE, Bryan AL, et al. Valproate prophylaxis in a prospective clinical trial of refractory bipolar disorder. Am J Psychiatry 1997;154:1456–1458 [DOI] [PubMed] [Google Scholar]
- 12.Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997;58(11):470–478 [DOI] [PubMed] [Google Scholar]
- 13.Solomon DA, Ryan CE, Keitner GI, et al. A pilot study of lithium carbonate plus divalproex sodium for the continuation and maintenance treatment of patients with bipolar I disorder. J Clin Psychiatry 1997;58(3):95–99 [DOI] [PubMed] [Google Scholar]
- 14.Small JG, Klapper MH, Marhenke JD, et al. Lithium combined with carbamazepine or haloperidol in the treatment of mania. Psychopharmacol Bull 1995;31:265–272 [PubMed] [Google Scholar]
- 15.Nemerof CB, Evans DL, Gyulai L, et al. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001;158:906–912 [DOI] [PubMed] [Google Scholar]
- 16.Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007;356:1711–1722 [DOI] [PubMed] [Google Scholar]
- 17.van der Loos M, Nolen WA, Vieta E, et al. Lamotrigine as add-on to lithium in bipolar depression. Presented at the 5th European Stanley Conference on Bipolar Disorder; Oct 5–7, 2006; Barcelona, Spain [Google Scholar]
- 18.Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079–1088 [DOI] [PubMed] [Google Scholar]
- 19.Levine J, Chengappa KN, Brar JS, et al. Psychotropic drug prescription patterns among patients with bipolar I disorder. Bipolar Disord 2000;2:120–130 [DOI] [PubMed] [Google Scholar]
- 20.Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028–1042 [DOI] [PubMed] [Google Scholar]
- 21.Sachs GS. Use of clonazepam for bipolar affective disorder. J Clin Psychiatry 1990;51(suppl):31–34, discussion 50–53 [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(suppl 20):22–33 [PubMed] [Google Scholar]
- 23.Sachs GS, Guille C, McMurrich S. A clinical monitoring form for mood disorders. Bipolar Disord 2002;4:323–327 [DOI] [PubMed] [Google Scholar]
- 24.Kraemer HC. Evaluating Medical Tests: Objective and Quantitative Guidelines. Newbury Park, Calif: Sage Publications, Inc; 1992 [Google Scholar]
- 25.Brooks JO, Hoblyn JC, Kraemer HC, et al. Factors associated with psychiatric hospitalization of individuals diagnosed with dementia and comorbid bipolar disorder. J Geriatr Psychiatry Neurol 2006;19:72–77 [DOI] [PubMed] [Google Scholar]
- 26.Hoblyn JC, Balt SL, Woodard SA, et al. Substance use disorders as risk factors for psychiatric hospitalization in bipolar disorder. Psychiatr Serv 2009;60:50–55 [DOI] [PubMed] [Google Scholar]
- 27.Baldessarini RJ, Leahy L, Arcona S, et al. Patterns of psychotropic drug prescription for US patients with diagnoses of bipolar disorders. Psychiatr Serv 2007;58:85–91 [DOI] [PubMed] [Google Scholar]
- 28.Young LT, Jofe RT, Robb JC, et al. Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 2000;157:124–126 [DOI] [PubMed] [Google Scholar]
- 29.Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry 2008;165:370–377 [DOI] [PubMed] [Google Scholar]
- 30.Wehr TA, Goodwin FK. Can antidepressants cause mania and worsen the course of afective illness? Am J Psychiatry 1987;144:1403–1411 [DOI] [PubMed] [Google Scholar]
- 31.Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. J Clin Psychiatry 2003;64:506–515 [DOI] [PubMed] [Google Scholar]
- 32.Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003;60:261–269 [DOI] [PubMed] [Google Scholar]
- 33.Rihmer Z, Pestality P. Bipolar II disorder and suicidal behavior. Psychiatr Clin North Am 1999;22:667–673 [DOI] [PubMed] [Google Scholar]
- 34.Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: focus on depression as its hallmark. J Clin Psychiatry 2001;62(suppl 14):34–41 [PubMed] [Google Scholar]
- 35.Hawton K, Sutton L, Haw C, et al. Suicide and attempted suicide in bipolar disorder: a systematic review of risk factors. J Clin Psychiatry 2005;66(6):693–704 [DOI] [PubMed] [Google Scholar]
- 36.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 37.Sajatovic M, Valenstein M, Blow F, et al. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv 2007;58:855–863 [DOI] [PubMed] [Google Scholar]
- 38.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2004;61:807–816 [DOI] [PubMed] [Google Scholar]