Abstract
Pathogenic autoreactive antibodies that may be associated with life-threatening Coronavirus disease 2019 (COVID-19) remain to be identified. Here we show that self-assembled genome-scale libraries of full-length proteins covalently coupled to unique DNA barcodes for analysis by sequencing can be used for the unbiased identification of autoreactive antibodies in plasma samples. By screening 11,076 DNA-barcoded proteins expressed from a sequence-verified human ORFeome library, the method, which we named MIPSA (for molecular indexing of proteins by self-assembly), allowed us to detect circulating neutralizing type-I and type-III interferon (IFN) autoantibodies in plasma samples from 55 patients with life-threatening COVID-19. In addition to identifying neutralizing type-I IFN-α and IFN-ω autoantibodies and other previously known autoreactive antibodies in the patient samples, MIPSA enabled the detection of as yet unidentified neutralizing type-III anti-IFN-λ3 autoantibodies that were not seen in ten healthy plasma samples or in convalescent plasma from ten non-hospitalized individuals with COVID-19. The low cost and simple workflow of MIPSA will facilitate unbiased high-throughput analyses of protein–antibody, protein–protein and protein-small-molecule interactions.
Pathogenic autoreactive antibodies associated with severe COVID-19 can be identified via self-assembled genome-scale libraries of full-length proteins covalently coupled to uniquely identifying DNA barcodes for analysis by sequencing.
An unbiased analysis of antibody-binding specificities can provide insights into states of health and disease. We and others have used programmable phage-display libraries to identify novel autoantibodies, to characterize antiviral immunity and to profile allergen-specific IgE antibodies1–4. Although phage display has been useful for these and many other applications, most protein–protein, protein–antibody and protein–small-molecule interactions require a degree of conformational structure that is not captured by bacteriophage-displayed peptide libraries. Profiling conformational protein interactions at proteome scale has traditionally relied on protein microarray technologies. Protein microarrays, however, tend to suffer from high per-assay cost, and from a myriad of technical artifacts, including those associated with the high-throughput expression and purification of proteins, the spotting of proteins onto a solid support, the drying and rehydration of arrayed proteins, and the readout of slides imaged via scanning fluorescence imaging5,6. Alternative approaches to protein-microarray production and storage have been developed (such as Nucleic Acid-Programmable Protein Array, NAPPA7; or single-molecule PCR-linked in vitro expression; SIMPLEX8). However, a robust, scalable and cost-effective alternative is lacking.
To overcome the limitations associated with the array-based profiling of full-length proteins, we previously established a methodology, which we named ParalleL Analysis of Translated Open reading frames (PLATO), that uses ribosome display of open reading frame (ORF) libraries9. Ribosome display relies on the in vitro translation of mRNAs that lack stop codons, stalling ribosomes at the ends of mRNA molecules in a complex with the nascent proteins that they encode. PLATO suffers from several key limitations that have hindered its adoption. An ideal alternative is the covalent conjugation of proteins to short amplifiable DNA barcodes. Indeed, individually prepared DNA-barcoded antibodies and proteins have been employed successfully in a variety of applications10. One particularly attractive protein–DNA-conjugation method involves the HaloTag system, which adapts a bacterial enzyme that forms an irreversible covalent bond with halogen-terminated alkane moieties11. Individual DNA-barcoded HaloTag fusion proteins have been shown to greatly enhance the sensitivity and dynamic range of autoantibody detection, compared with traditional ELISA12. Scaling individual protein barcoding to entire ORFeome libraries would be immensely valuable yet formidable, owing to high costs and low throughput. A self-assembly approach could provide a much more efficient path to library production.
Here we describe a molecular-display technology, which we named molecular indexing of proteins by self-assembly (MIPSA), that overcomes key disadvantages of PLATO and other full-length protein-array technologies. MIPSA produces libraries of soluble full-length proteins, each uniquely identifiable via covalent conjugation to an amplifiable DNA barcode. Barcodes are introduced upstream of the ribosome-binding site (RBS). Partial reverse transcription (RT) of the in vitro transcribed RNA (IVT-RNA) creates a cDNA barcode, which is linked to a haloalkane-labelled RT primer. An N-terminal HaloTag fusion protein is encoded downstream of the RBS, such that in vitro translation results in the intra-complex (‘cis’) covalent coupling of the cDNA barcode to the HaloTag and its downstream ORF-encoded protein product. The resulting library of uniquely indexed full-length proteins can be used for inexpensive proteome-wide interaction studies, such as unbiased autoantibody profiling.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection ranges from an asymptomatic course to life-threatening pneumonia and death. A causal link between autoimmunity and severe COVID-19 has been supported by multiple studies13,14. Although a diverse array of autoantibodies have been documented15, neutralizing type-I interferon autoantibodies seem to play a particularly prominent role16,17. Here we investigate the utility of MIPSA by searching for novel autoantibodies in the plasma of patients with severe COVID-19.
Results
Development of the MIPSA system.
The MIPSA Gateway Destination vector for E. coli cell free translation contains the following key elements: a T7 RNA polymerase transcriptional start site, an isothermal unique clonal identifier (“UCI”) barcode sequence, an E. coli ribosome binding site (RBS), an N-terminal HaloTag fusion protein (891 nt), recombination sequences for ORF insertion, and a homing endonuclease (I-SceI) site for plasmid linearization. A recombined ORF-containing pDEST-MIPSA plasmid is shown in Fig. 1a.
Fig. 1 |. The MIPSA method.
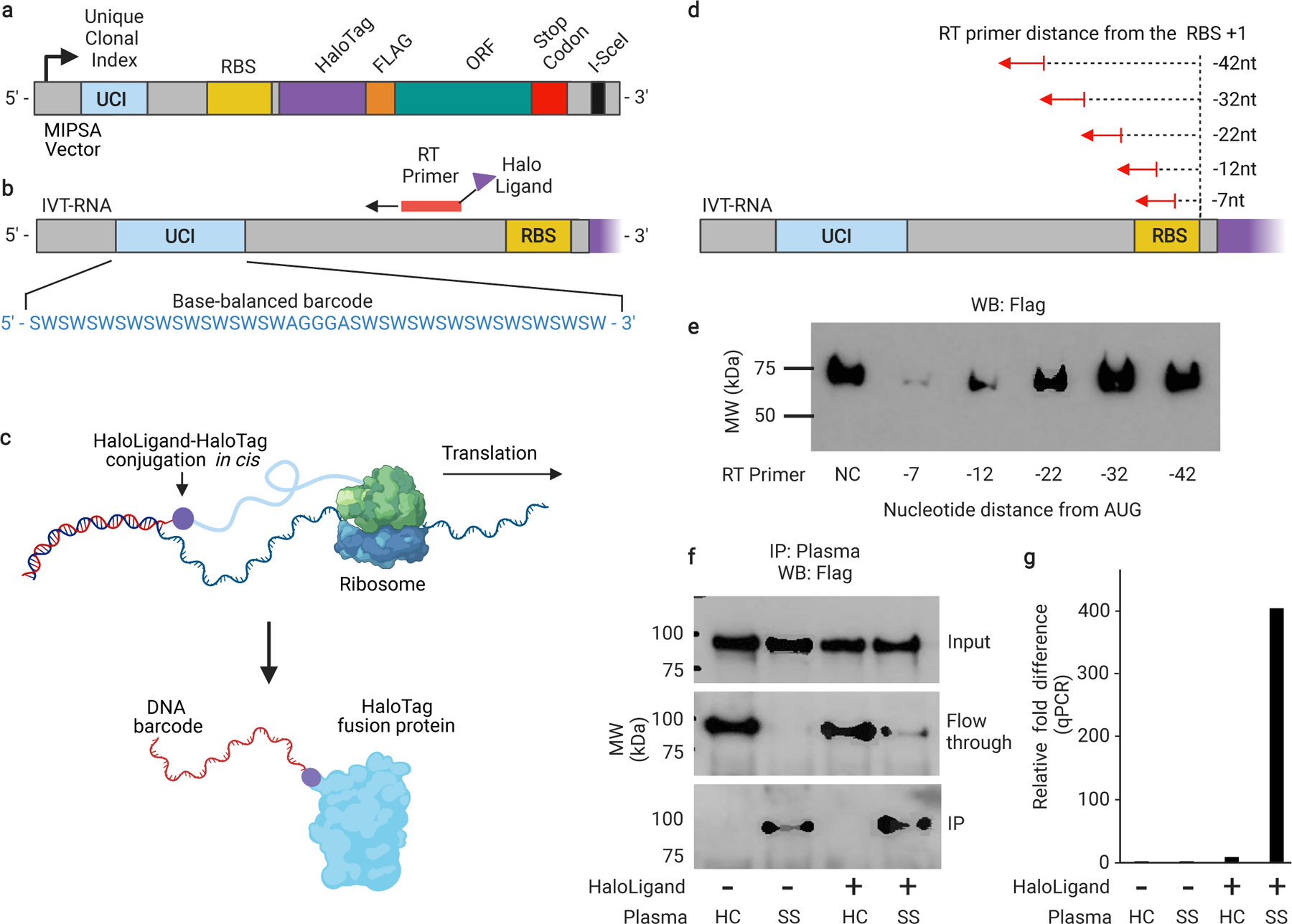
a, Schematic of the recombined pDEST-MIPSA vector with key components highlighted: unique clonal identifier (UCI, blue), ribosome binding site (RBS, yellow), N-terminal HaloTag (purple), FLAG epitope (orange), open reading frame (ORF, green), and the I-SceI restriction endonuclease site (black) for vector linearization. b, Schematic showing in vitro transcribed (IVT) RNA from the vector template shown in (a). Isothermal base-balanced UCI sequence: (SW)18-AGGGA-(SW)18. c, Cell-free translation of the RNA-cDNA shown in (b). HaloTag protein forms a covalent bond with the HaloLigand-conjugated UCI-containing cDNA in cis during translation. d, RT primer positions tested for impact on translation. e, α-FLAG western blot analysis of translation in presence of RT primers depicted in (d) (NC, negative control, no RT primer). f, Western blot analysis of TRIM21 protein translated from RNA carrying the UCI-cDNA primed from the −32 position, either conjugated (+) or not (−) with the HaloLigand. Sjogren’s Syndrome, SS; Healthy Control, HC. g, qPCR analysis of the IPed TRIM21 UCI. Fold-difference is by comparison with the HaloLigand (−) HC IP (technical replicates, n = 2).
We first sought to establish a library of pDEST-MIPSA plasmids containing stochastic, isothermal UCIs located between the transcriptional start site and the ribosome binding site. A degenerate oligonucleotide pool was synthesized, comprising melting temperature (Tm) balanced sequences: (SW)18-AGGGA-(SW)18, where S represents an equal mix of C and G, while W represents an equal mix of A and T (Fig. 1b). We reasoned that this inexpensive pool of sequences would (i) provide sufficient complexity (236 ~ 7 × 1010) for unique ORF labelling, (ii) amplify without distortion, and (iii) serve as ORF-specific forward and reverse qPCR primer binding sites for measurement of individual UCIs of interest. The degenerate oligonucleotide pool was amplified by PCR, restriction cloned into the MIPSA destination vector, and transformed into E. coli (Methods). About 800,000 transformants were scraped off selection plates to obtain the pDEST-MIPSA UCI plasmid library. ORFs encoding the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a known autoantigen, tripartite motif containing-21 (TRIM21, commonly known as Ro52), were separately recombined into the pDEST-MIPSA UCI plasmid library. Individually barcoded GAPDH and TRIM21 clones were isolated, sequenced, and used in the following experiments.
The MIPSA procedure involves RT of the UCI using a succinimidyl ester (O2)-haloalkane (HaloLigand)-conjugated RT primer (Supplementary Fig. 1). The bound RT primer should not interfere with the assembly of the E. coli ribosome and initiation of translation, but should be sufficiently proximal such that coupling of the HaloLigand-HaloTag-protein complex might hinder additional rounds of translation. We tested a series of RT primers that anneal at distances ranging from −42 nucleotides to −7 nucleotides relative to the 3’ end of the ribosome binding site (Fig. 1d). Based on the yield of protein product from mRNA saturated with primers at these differing locations, we selected the −32 position as it did not interfere with translation efficiency (Fig. 1e). In contrast, RT from primers located within 20 nucleotides of the RBS diminished or abolished protein translation, in agreement with the estimated footprint of assembled 70S E. coli ribosomes, which have been shown to protect a minimum of 15 nucleotides of mRNA.18
We next assessed the ability of SuperScript IV to perform RT from a primer labelled with the HaloLigand at its 5’ end, and the ability of the HaloTag-TRIM21 protein to form a covalent bond with the HaloLigand-conjugated primer during the translation reaction. HaloLigand conjugation and purification followed previously established methods. (Methods, Supplementary Fig. 1).19 Either an unconjugated RT primer or a HaloLigand-conjugated RT primer was used for RT of the barcoded HaloTag-TRIM21 mRNA. The translation product was then immuno-captured (i.e., immunoprecipitated, “IPed”) with plasma from a healthy donor or plasma from a TRIM21 autoantibody-positive patient with Sjogren’s Syndrome (SS), using protein A and protein G coated magnetic beads. The SS plasma efficiently IPed the TRIM21 protein, regardless of RT primer conjugation, but only pulled down the TRIM21 UCI when the HaloLigand-conjugated primer was used in the RT reaction (Fig. 1f-g).
Assessing levels of cis versus trans UCI barcoding.
While the previous experiment indicated that indeed the HaloLigand does not impede RT priming, and that the HaloTag can form a covalent bond with the HaloLigand during the translation reaction, it did not elucidate the amount of cis (intra-complex, desirable) versus trans (inter-complex, undesirable) HaloTag-UCI conjugation (Supplementary Fig. 2). Here, “intra-complex” is defined as conjugation to the UCI that is associated with the same RNA molecule encoding the protein. To measure the amount of cis and trans HaloTag-UCI conjugation, GAPDH and TRIM21 mRNAs were separately reverse transcribed (using HaloLigand-conjugated primer) and then either mixed 1:1 or kept separate for in vitro translation. As expected, translation of the mixture produced roughly equivalent amounts of each protein compared to the individual translations (Supplementary Fig. 3). SS plasma specifically IPed TRIM21 protein regardless of translation condition (Supplementary Fig. 3, IPed fraction). However, we noted that while the SS IPs contained high levels of the TRIM21 UCI, as intended, more of the GAPDH UCI was pulled down by the SS plasma compared to that by the HC plasma when the mRNA was mixed prior to translation. This indicates that indeed some amount of trans barcoding occurs (Fig. 2a). We estimate that ~50% of the protein is cis-barcoded, with the remaining 50% trans-barcoded protein equally conjugated to both UCIs. Thus, in this two-component system, 25% of the TRIM21 protein is conjugated to the GAPDH UCI (Supplementary Fig. 2).
Fig. 2 |. Cis- versus trans-UCI conjugation.
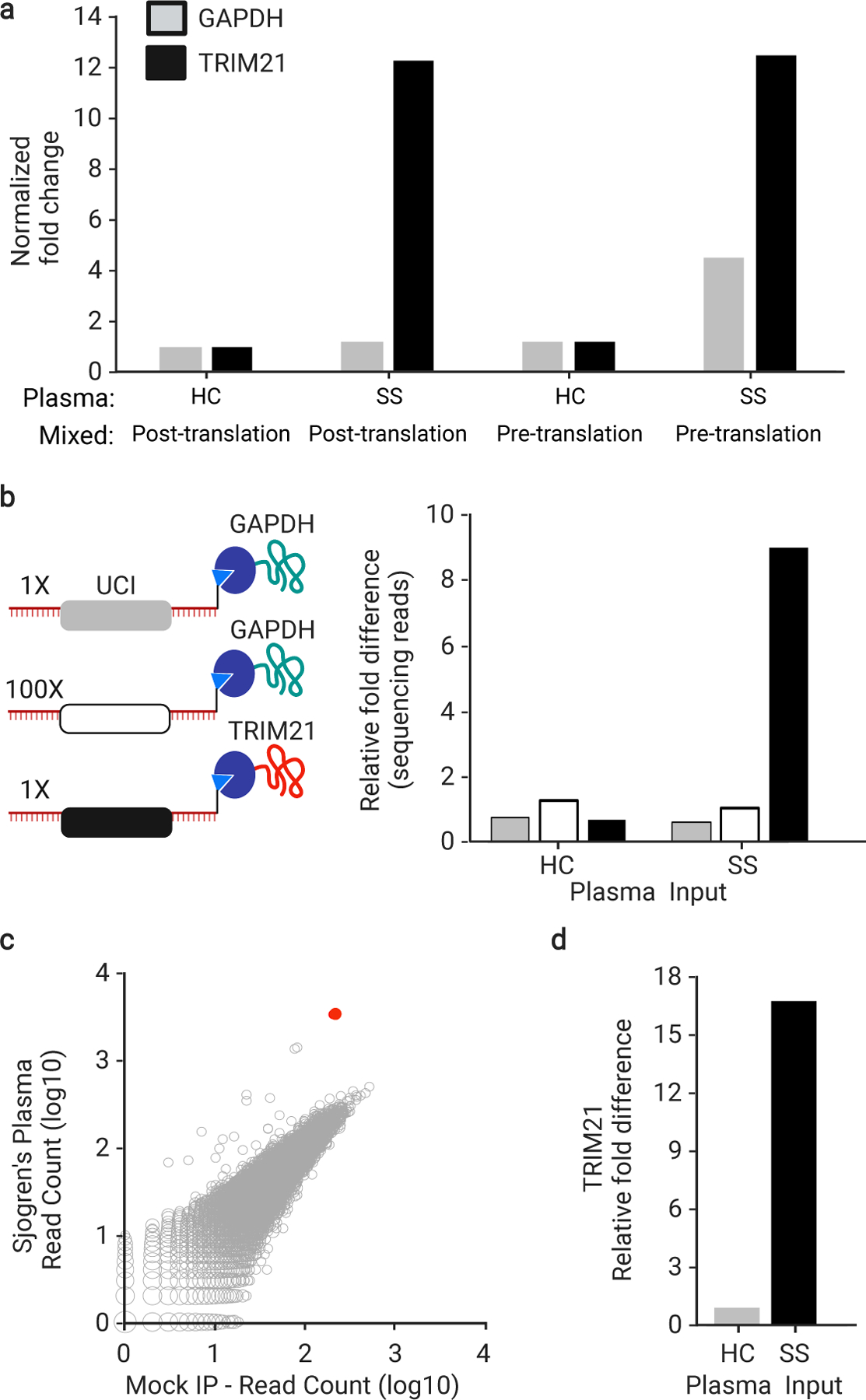
a, IVT-RNA encoding TRIM21 or GAPDH with their distinct UCI barcodes were translated before or after mixing at a 1:1 ratio. qPCR analysis of the IPs using UCI-specific primers, reported as fold-change versus IP with HC plasma, which is set to 1, when the IVT-RNA was mixed post-translation (technical replicates, n = 3). b, IVT-RNA encoding TRIM21 (black UCI) and GAPDH (gray UCI) were mixed 1:1 into a background of 100-fold excess GAPDH (white UCI) and then translated as a mock library. Sequencing analysis of the IPs, reported as fold-change versus the HC IP of the 100x GAPDH (technical replicates, n = 3). c, hORFeome MIPSA library containing spiked-in TRIM21, IPed with SS plasma and compared to average of 8 mock IPs (no plasma input). The TRIM21 UCI is shown in red. d, Relative fold difference of TRIM21 UCI in SS versus HC IPs, determined by sequencing.
In the setting of a complex library, even if ~50% of each protein is trans barcoded, this side product should be associated with a low level of randomly sampled UCIs. We tested this using a mock MIPSA library, composed of 100-fold excess of a second GAPDH clone, which was combined with a 1:1 mixture of the first GAPDH and TRIM21 clones (Fig. 2b).
Establishing and deconvoluting a stochastically barcoded human ORFeome MIPSA library.
The sequence-verified human ORFeome (hORFeome) v8.1 is composed of 12,680 clonal ORFs mapping to 11,437 genes in the Gateway Entry plasmid (pDONR223).20 Five subpools of the library were created, each composed of ~2,500 similarly sized ORFs. Each of the five subpools was separately recombined into the pDEST-MIPSA UCI plasmid library and transformed to obtain ~10-fold ORF coverage (~25,000 clones per subpool). Each subpool was assessed via Bioanalyzer electrophoresis, sequencing of ~20 colonies, and Illumina sequencing of the combined superpool. The TRIM21 plasmid was spiked into the superpooled hORFeome library at 1:10,000 – comparable to a typical library member. The SS IP experiment was then performed on the hORFeome MIPSA library, using sequencing as a readout. The read counts from all UCIs in the library, including the spiked-in TRIM21, are shown for the SS IP versus the average of 8 mock IPs in Fig. 2c. Reassuringly, the SS autoantibody-dependent enrichment of TRIM21 (17-fold) was similar to the model system (Fig. 2d). See Informatic analysis of MIPSA sequencing data in the Methods section for a description of the analytical pipeline for sequencing data.
Next, we established a system for creating a UCI-ORF lookup dictionary, using tagmentation and sequencing (Fig. 3a). Sequencing the 5’ 50 nt of the ORF inserts detected 11,076 of the 11,887 unique 5’ 50 nt library sequences. Of the 153,161 UCIs detected, 82.9% (126,975) were found to be associated with a single ORF (termed a “monospecific UCI”). Each ORF was uniquely associated with a median of 9 (ranging from 0 to 123) monospecific UCIs (Fig. 3b). Importantly, an ensemble of monospecific UCIs with consistent behaviour can provide additional, strong support for the reactivity of their associated ORF. We noted a weak, inverse correlation between UCI number and ORF size, which most likely reflects the less efficient recombination of larger ORF-containing plasmids in the pooled recombination reaction. After aggregation of the read counts corresponding to each ORF, over 99% of the represented ORFs were present within a 10-fold difference of the median ORF abundance (Fig. 3c). Taken together, these data indicate that we established a uniform library of 11,076 stochastically indexed human ORFs, and defined a lookup dictionary for downstream analyses. Fig. 3d shows UCI read counts of an SS IP versus the average of 8 mock IPs, and the 47 dictionary-decoded GAPDH monospecific UCIs (corresponding to two GAPDH isoforms present in the hORFeome library) appearing along the y = x diagonal as expected. To avoid ambiguity, any UCI associated with more than a single ORF was excluded from further analyses.
Fig. 3 |. Constructing the UCI-ORF dictionary.

a, (i) Tagmentation randomly inserts adapters into the MIPSA vector library. (ii) Using a PCR1 forward primer and the reverse primer of the tagmentation-inserted adapter, DNA fragments are amplified and size selected to be ~1.5 kb, which captures the 5’ terminus of the ORF. (iii) These fragments are amplified with a P5-containing PCR2 forward primer and a P7 reverse primer. (iv) Illumina sequencing is used to read the UCI and the ORF from the same fragment, thus enabling their association in the dictionary. b, The number of monospecific UCIs is shown for each member of pDEST-MIPSA hORFeome library, superimposed on the length of the ORFs. c, Histogram of ORF representations in the library according to their aggregated UCI-associated read counts. Vertical red lines show +/−10x the median UCI-associated read count. d, IP of hORFeome MIPSA library using Sjogren’s Syndrome (SS) plasma is compared to the average of 8 mock IPs. Sequencing read count for each UCI are plotted. UCIs associated with the two GAPDH isoforms (filled black) and spiked-in TRIM21 (red) are indicated
Unbiased MIPSA analysis of autoantibodies associated with severe COVID-19.
Several recent reports have described elevated autoantibody reactivities in patients with severe COVID-19.21–25 We therefore used MIPSA with the human ORFeome library for unbiased identification of autoreactivities in the plasma of 55 severe COVID-19 patients, defined here based only on hospital admission, since the availability of clinical meta-data was incomplete. For comparison, we used MIPSA to detect autoreactivities in plasma from 10 healthy donors and 10 COVID-19 convalescent plasma donors who had not been hospitalized (Supplementary Table 1). As we have done previously for Phage ImmunoPrecipitation Sequencing (PhIP-Seq) analyses, each sample was compared to a set of 8 “mock IPs”, which contained all reaction components except for plasma, and were run on the same plate. Comparison to mock IPs accounts for bias in the library and background binding. The informatic pipeline used to detect antibody-dependent reactivity (Methods) yielded a median of 5 (ranging from 2 to 9) false positive UCI hits per mock IP. IPs using plasma from severe COVID-19 patients, however, yielded a mean of 83 reactive proteins among severe COVID-19 patients, which was significantly more than the mean of 64 reactive proteins among healthy pre-pandemic controls and significantly more than the mean of 62 reactive proteins among recovered individuals after mild to moderate COVID-19 (p = 0.02 and p = 0.05, respectively, one tailed t-test; Fig. 4a).
Fig. 4 |. MIPSA analysis of autoantibodies in severe COVID-19.

a, Box plots showing total numbers of autoreactive proteins in plasma from healthy controls (median 69.0 +/− s.d. of 36.3), mild-moderate COVID-19 patients (median 60.5 +/− s.d. of 44.7), or severe COVID-19 patients (median 106.0 +/− s.d. of 67.6). Boxes indicate quartiles and * indicates p = 0.02 and p = 0.05, respectively from a one-tailed t-test to compare means. b, Hierarchal cluster map of all proteins represented by at least 2 reactive UCIs in at least 1 severe COVID-19 plasma, but not more than 1 control (healthy or mild-moderate COVID-19 plasma). c, MIPSA analysis of autoantibodies in 10 inclusion body myositis (IBM) patients and 10 healthy controls (HCs), using the hORFeome library. Fold change of IPed 5’-nucleotidase, cytosolic 1A (NT5C1A), measured both as UCI-qPCR fold change (relative to average of 10 HCs) and as sequencing fold change (relative to mock IPs).
We next examined proteins in the severe COVID-19 IPs that had at least two reactive UCIs (in the same IP), which were reactive in at least one severe patient, and that were not reactive in more than one control (healthy or mild/moderate convalescent plasma). Proteins were excluded if they were reactive in a single severe patient and a single control. The 103 proteins that met these criteria are shown in the cluster map of Fig. 4b. Fifty one of the 55 severe COVID-19 patients exhibited reactivity to at least one of these proteins. We noted co-occurring protein reactivities in multiple individuals, the vast majority of which lack homology by protein sequence alignment. Supplementary Table 2 provides summary statistics about these reactive proteins, including whether they are previously defined autoantigens according to the human autoantigen database AAgAtlas 1.0.26 The Supplementary dataset provides the patient versus UCI fold change data used to construct the cluster map.
One notable autoreactivity cluster (Supplementary Table 2, cluster #5) includes 5’-nucleotidase, cytosolic 1A (NT5C1A), which is highly expressed in skeletal muscle and is the most well-characterized autoantibody target in inclusion body myositis (IBM). Multiple UCIs linked to NT5C1A were significantly increased in 3 of the 55 severe COVID-19 patients (5.5%). NT5C1A autoantibodies have been reported in up to 70% of IBM patients 1, in ~20% of Sjogren’s Syndrome (SS) patients, and in up to ~5% of healthy donors.27 The prevalence of NT5C1A reactivity in the severe COVID-19 cohort is therefore not necessarily elevated. However, we wondered whether MIPSA would be able to reliably distinguish between healthy donor and IBM plasma based on NT5C1A reactivity. We tested plasma from 10 healthy donors and 10 IBM patients, the latter of whom were selected based on NT5C1A seropositivity determined by PhIP-Seq.1 The clear separation of patients from controls in this independent cohort suggests that MIPSA may indeed have utility in clinical diagnostic testing using either UCI-specific qPCR or library sequencing, which were tightly correlated readouts (Fig. 4c).
Type I and type III interferon-neutralizing autoantibodies in severe COVID-19 patients.
Neutralizing autoantibodies targeting type I interferons alpha (IFN-α) and omega (IFN-ω) have been associated with severe COVID-19.15,22,28 All type I interferons except IFN-α16 are represented in the human MIPSA ORFeome library and annotated in the lookup dictionary. IFN-α4, IFN-α17, and IFN-α21 are indistinguishable by the first 50 nucleotides of their encoding ORF sequences, and thus analysed as a single ORF. Two of the severe COVID-19 patients (P1 and P2) in this cohort (3.6%) exhibited dramatic type I IFN autoreactivity (49 and 46 type I interferon UCIs, across 11 distinct ORFs corresponding to many IFN-α and IFN-ω; Fig. 5a,b). The extensive co-reactivity of these proteins is likely attributable to their sequence homology (Supplementary Fig. 4). By requiring at least 2 reactive IFN UCIs to be considered positive, we identified two additional severe COVID-19 plasma (P3 and P4) with detectable levels of IFN-α reactivity, each with only 2 reactive IFN-α UCIs. Fifty percent of these four IFN-α autoreactive patients died, versus about 30% of the remaining cohort. Interestingly, one additional plasma (P5) precipitated no UCIs from any type I or II interferons, but five UCIs from the type III interferon IFN-λ3 (Fig. 5c-d). This patient also died of COVID-19. No additional interferon autoreactivities were detected among the severe COVID-19 patients. None of the healthy or non-hospitalized COVID-19 controls were positive for 2 or more interferon UCIs.
Fig. 5 |. MIPSA detects known and novel neutralizing interferon autoantibodies.
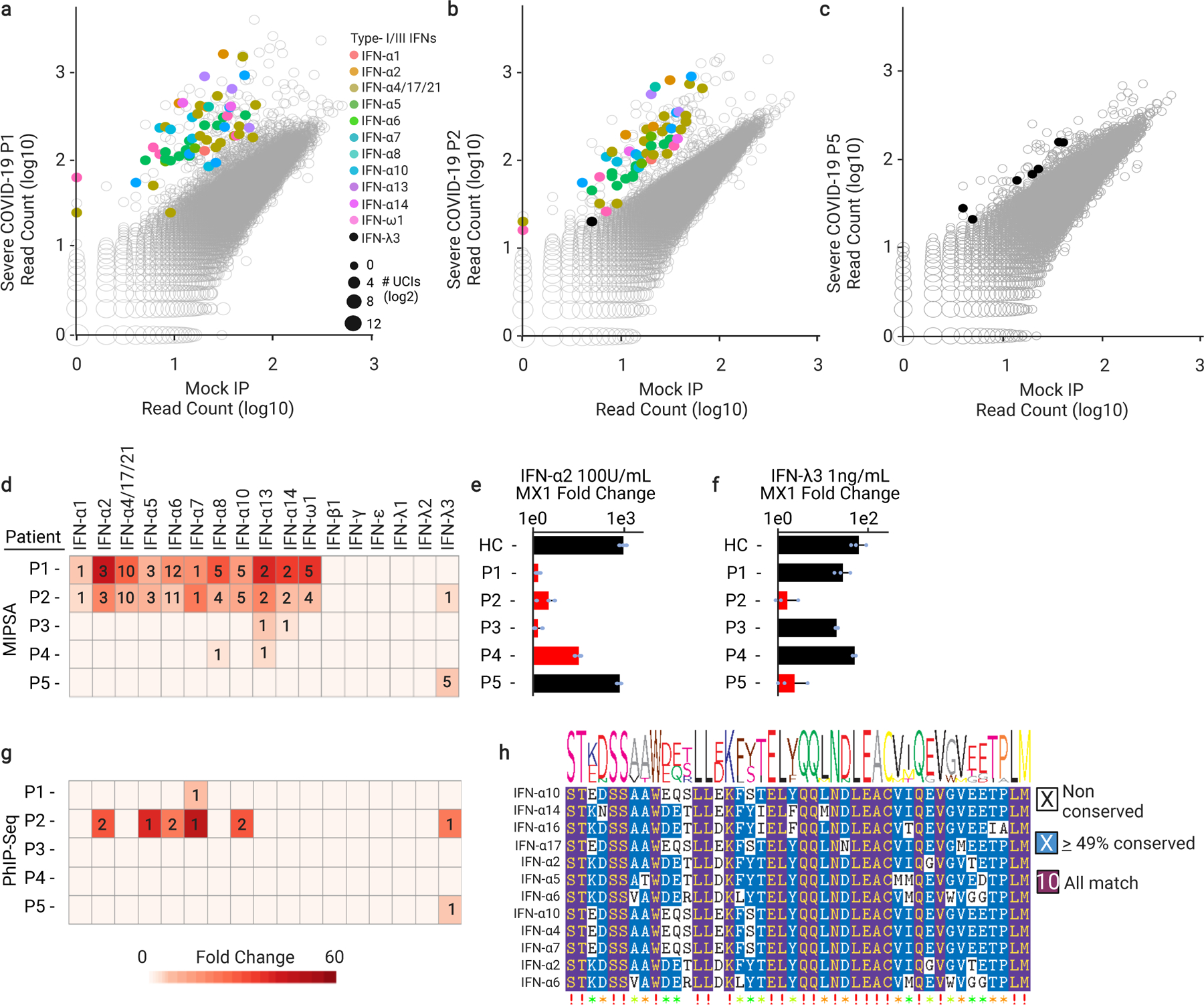
a-c, Scatterplots highlighting reactive interferon UCIs for three severe COVID-19 patients. d, Summary of interferon reactivity detected in 5 of 55 individuals with severe COVID-19. Hits fold-change values (colour of cell) and the number of reactive UCIs (number in cell) are provided. e, f, Recombinant interferon alpha 2 (IFN-α2) or interferon lambda 3 (IFN-λ3) neutralizing activity of the same patients shown in d. Plasma were pre-incubated with 100 U/ml of IFN-α2 or 1 ng/ml of IFN-λ3 prior to incubation with A549 cells. Fold changes of the interferon stimulated gene, MX1, were calculated by RT-qPCR relative to unstimulated cells (n = 3 for each of the 5 patient samples). For e, samples labelled HC, P1, P2, P3, P4, P5 have mean and standard deviation values of 977.7 +/−253.7, 1.39 +/−0.2, 3.1 +/−1.8, 1.4 +/−0.5, 32 +/−7.2, 741.1 +/−121, respectively. For f, samples labelled HC, P1, P2, P3, P4, P5 have mean and standard deviation values of 64.5 +/−26.8, 28.2 +/−12.1, 1.6 +/− 1.0, 20.3 +/−1.4, 52.4 +/− 5.1, 2.3 +/−2.0, respectively. GAPDH was used as a housekeeping control gene for normalization. Red bars indicate which samples were found to contain the corresponding anti-interferon antibodies using MIPSA. g, PhIP-Seq analysis of interferon autoantibodies in the 5 patients of d (row and column orders maintained). Hits fold-change values (colour of cell) and the number of reactive peptides (number in cell) are provided. h, Epitopefindr analysis of the PhIP-Seq reactive type I interferon 90-aa peptides.
We assessed the performance of MIPSA using P2 plasma, which neutralizes both type I and III interferons. MIPSA was run on P2 plasma in triplicate, yielding a high level of assay reproducibility (Supplementary Fig. 5a,b), both in consistent detection of hits and low coefficients of variation (mean CV = 22%). We assessed the linearity of the assay by diluting P2 plasma 10-fold into a healthy plasma and then performing MISPA again. The results demonstrate a consistent decrease in signal among the reactivities (mean of 5.4-fold for reactive interferons), and loss of detection of some hits, particularly of ORFs with single reactive UCIs.
Incubation of A549 human adenocarcinomatous lung epithelial cells with 100 U/ml IFN-α or 1 ng/ml of IFN-λ3 for 4 hours in serum-free medium results in a robust upregulation of the IFN-response gene MX1 by ~1,000-fold and ~100-fold, respectively. Pre-incubation of IFN-α2 with plasma P1, P2, or P3 completely abolished MX1 upregulation (Fig. 5e). The plasma with the weakest IFN-α reactivity by MIPSA, P4, only partially neutralized the cytokine. Neither HC nor P5 plasma had any effect on the response of A549 cells to IFN-α2 treatment. However, pre-incubation of the IFN-λ3 cytokine with the MIPSA-positive plasma, P2 and P5, ablated the interferon response (Fig. 5f). None of the other plasma (HC, P1, P3, or P4) had any effect on the response of A549 cells to IFN-λ3. By comparison against titration curves using IFN-α2 and IFN-λ3 monoclonal antibodies, a serial titration using patient P2 plasma in triplicate indicated circulating levels of these autoantibodies to be ~20 μg/ml and ~100 ng/ml, respectively (Supplementary Fig. 6). MIPSA analysis of the serially diluted IFN-α2 mAb revealed broad IFN-α cross-recognition, but mutually exclusive binding of the mAbs to the appropriate type I or type III interferon (Supplementary Fig. 7a,b). Importantly, we noted that loss of MIPSA detection sensitivity corresponded to the same or greater plasma dilutions at which IFN-α2 and IFN-λ3 neutralization activities were also lost. Finally, the titre of P2’s autoantibodies exhibited at least a 10-fold preference for IFN-λ3 neutralization over IFN-λ1 neutralization (Supplementary Fig. 8). In summary, MIPSA-based autoantibody profiling of this severe COVID-19 cohort identified strongly neutralizing IFN-α autoantibodies in 7.3% of patients and strongly neutralizing IFN-λ3 autoantibodies in 3.6% of patients, with a single patient (1.8%) harbouring both autoreactivities.
We then determined whether Phage ImmunoPrecipitation Sequencing (PhIP-Seq) with a 90-aa human peptidome library29 might also detect interferon antibodies in this cohort. PhIP-Seq detected IFN-α reactivity in plasma from P1 and P2, although to a much lesser extent (Fig. 5g). The two weaker IFN-α reactivities detected by MIPSA in the plasma of P3 and P4 were both missed by PhIP-Seq. PhIP-Seq identified a single additional weakly IFN-α reactive sample, which was negative by MIPSA (not shown). Both technologies detected type III interferon autoreactivity (directed exclusively at IFN-λ3). PhIP-Seq data was used to narrow the location of a dominant epitope in these type I and type III interferon autoantigens (Fig. 5h for IFN-α; amino acid position 45–135 for IFN-λ3).
We next wondered about the prevalence of the previously unreported IFN-λ3 autoreactivity in the general population, and whether it might be increased among patients with severe COVID-19. PhIP-Seq was previously used to profile the plasma of 423 healthy controls, none of whom were found to have detectable IFN-λ3 autoreactivity.30 These data suggest that IFN-λ3 autoreactivity is likely to be more frequent among individuals with severe COVID-19. Therefore, neutralizing IFN-λ3 autoantibodies may be involved in a pathogenic mechanism contributing to life-threatening COVID-19 in a subset of patients.
Discussion
We have described a molecular-display technology for full-length proteins that provides key advantages over protein microarrays (such as PLATO) and alternative techniques. MIPSA uses self-assembly to produce a library of proteins, linked to relatively short (158 nt) single-stranded DNA barcodes via the 25-kDa HaloTag domain. This compact barcoding approach will likely have many applications not accessible to alternative display formats with bulky linkage cargos (such as yeast, bacteria, viruses, phages, ribosomes, mRNAs and cDNAs). Indeed, individually conjugating minimal DNA barcodes to proteins, especially antibodies and antigens, has already proven useful in several settings, including CITE-Seq,31 LIBRA-seq32 and related methodologies33. At proteome scale, MIPSA will enable unbiased analyses of protein–antibody, protein–protein and protein–small-molecule interactions, as well as studies of post-translational modifications, such as hapten-modification studies34 or protease-activity profiling35. Key advantages of MIPSA include its high throughput, low cost, simple sequencing-library preparation, inherent compatibility with PhIP-Seq, and the stability of the protein–DNA complexes (important for the manipulation and storage of display libraries). Importantly, MIPSA can be adopted by standard molecular biology laboratories, since it does not require specialized training or instrumentation (yet it requires access to a high-throughput DNA-sequencing instrument or facility).
Autoantibodies detected in severe COVID-19 patients using MIPSA.
Neutralizing IFN-α/ω autoantibodies have been described in patients with severe COVID-19 disease and are presumed to be pathogenic.22 These likely pre-existing autoantibodies, which occur very rarely in the general population, block restriction of viral replication in cell culture, and are thus likely to interfere with disease resolution. This discovery paved the way to identifying a subset of individuals at risk for life-threatening COVID-19 and proposed therapeutic use of interferon beta in this population of patients. In our study, MIPSA identified two individuals with extensive reactivity to the entire family of IFN-α cytokines. Indeed, plasma from both individuals, plus two individuals with weaker IFN-α reactivity detected by MIPSA, robustly neutralized recombinant IFN-α2 in a lung adenocarcinomatous cell culture model.
Type III IFNs (IFN-λ, also known as IL-28/29) are cytokines with potent anti-viral activities that act primarily at barrier sites. The IFN-λR1/IL-10RB heterodimeric receptor for IFN-λ is expressed on lung epithelial cells and is important for the innate response to viral infection. Previous studies in mice determined that IFN-λ diminished pathogenicity and suppressed replication of influenza viruses, respiratory syncytial virus, human metapneumovirus, and severe acute respiratory syndrome coronavirus (SARS-CoV-1).36 It has been proposed that IFN-λ exerts much of its antiviral activity in vivo via stimulatory interactions with immune cells, rather than through induction of the antiviral cell state.37 However, IFN-λ has been found to robustly restrict SARS-CoV-2 replication in primary human bronchial epithelial cells38, primary human airway epithelial cultures39, and primary human intestinal epithelial cells40. Collectively, these studies suggest multifaceted mechanisms by which neutralizing IFN-λ autoantibodies may exacerbate SARS-CoV-2 infections.
Among 55 severe COVID-19 patients, MIPSA detected two individuals with IFN-λ3 reactive autoantibodies. The same autoreactivities were also detected using PhIP-Seq. We tested the IFN-λ3 neutralizing capacity of these patients’ plasma, observing near complete ablation of the cellular response to the recombinant cytokine (Fig. 5f). These data suggest that IFN-λ3 autoreactivity is a potentially pathogenic mechanism contributing to severe COVID-19 disease.
In one study, type III IFN neutralizing antibodies were not detected among a cohort of 101 individuals with type I IFN autoantibodies tested.22 In our study, one of the four IFN-α autoreactive individuals (P2, a 22-year-old male) also harboured autoantibodies that neutralized IFN-λ3. It is possible that this co-reactivity is extremely rare and thus not represented in the aforementioned 101 patient study. Alternatively, it is possible that the differing assay conditions exhibit different detection sensitivity. Also, whereas in the previous study, cultured A549 cells were incubated with IFN-λ3 at 50 ng/ml without plasma preincubation, we cultured A549 cells with IFN-λ3 at 1 ng/ml after pre-incubation with plasma for one hour. Their readout of STAT3 phosphorylation may also provide different detection sensitivity compared to the upregulation of MX1 expression. A larger study is needed to determine the true frequency of these reactivities in severe COVID-19 patients and matched controls. Here, we report detection of strongly neutralizing IFN-α and IFN-λ3 autoantibodies in 4 (7.3%) and 2 (3.6%) individuals, respectively, in a cohort of 55 patients with severe COVID-19. IFN-λ3 autoantibodies were not detected via PhIP-Seq in a larger cohort of 423 healthy controls collected prior to the pandemic.
Exogenously administered Type III interferons have been proposed as a therapeutic for SARS-CoV-2 infection,39,41–45 and there are currently three ongoing clinical trials to test pegylated IFN-λ1 for efficacy in reducing morbidity and mortality associated with COVID-19 (ClinicalTrials.gov Identifiers: NCT04343976, NCT04534673, NCT04344600). One recently completed double-blind, placebo-controlled trial, NCT04354259, reported a significant reduction by 2.42 log copies per ml of SARS-CoV-2 at day 7 among mild to moderate COVID-19 patients in the outpatient setting (p = 0.0041).46 Future studies will determine whether anti-IFN-λ3 autoantibodies are pre-existing or arise in response to SARS-CoV-2 infection, and how often they also cross-neutralize IFN-λ1. Based on neutralization data from P2 (Supplementary Fig. 8) and sequence alignment of IFN-λ1 and IFN-λ3 (~29% homology, Fig. S4), cross-neutralization is expected to be rare, raising the possibility that patients with neutralizing IFN-λ3 autoantibodies may derive benefit from pegylated IFN-λ1 treatment.
While clusters of uncharacterized autoreactivities were observed in multiple individuals, it is not clear what role, if any, they may play in severe COVID-19. In larger scale studies, we expect that patterns of co-occurring reactivity, or reactivities towards proteins with related biological functions, may ultimately define new autoimmune syndromes associated with severe COVID-19.
Complementarity of MIPSA and PhIP-Seq.
Display technologies frequently complement one another but may not be amenable to routine simultaneous use. MIPSA is more likely than PhIP-Seq to detect antibodies directed at conformational epitopes on proteins expressed well in vitro. This was exemplified by the robust detection of interferon alpha autoantibodies via MIPSA, which were less sensitively detected via PhIP-Seq. PhIP-Seq, on the other hand, is more likely to detect antibodies directed at less conformational epitopes contained within proteins that are either absent from an ORFeome library or cannot be expressed well in cell-free lysate. Because MIPSA and PhIP-Seq naturally complement one another in these ways, we designed the MIPSA UCI amplification primers to be the same as those we have used for PhIP-Seq. Since the UCI-protein complex is stable – even in phage preparations – MIPSA and PhIP-Seq can readily be performed together in a single reaction, using a single set of amplification and sequencing primers. The compatibility of these two display modalities lowers the barrier to leveraging their synergy.
Variations of the MIPSA system.
A key aspect of MIPSA involves the conjugation of a protein to its associated UCI in cis, compared to another library member’s UCI in trans. Here we have used covalent conjugation via the HaloTag/HaloLigand system, but others could work as well. For instance, the SNAP-tag (a 20 kDa mutant of the DNA repair protein O6-alkylguanine-DNA alkyltransferase) forms a covalent bond with benzylguanine (BG) derivatives.47 BG could thus be used to label the RT primer in place of the HaloLigand. A mutant derivative of the SNAP-tag, the CLIP-tag, binds O2-benzylcytosine derivatives, which could also be adapted to MIPSA.48
The rate of HaloTag maturation and ligand binding is critical to the relative yield of cis versus trans UCI conjugation. A previous study determined that the rate of HaloTag protein production is about fourfold higher than the rate of HaloTag functional maturation.49 Considering a typical protein size is <1,000 amino acids in the ORFeome library, these data predict that most proteins should be released from the ribosome before HaloTag maturation, and thus before cis HaloLigand conjugation could occur, thereby favoring unwanted trans barcoding. However, we observed ~50% of protein-UCI conjugates are formed in cis, thereby enabling excellent assay performance in the setting of a complex library. During optimization experiments, we found the rate of cis barcoding to be slightly improved by excluding release factors from the translation mix, which stalls ribosomes on their stop codons and allows HaloTag maturation to continue in proximity to its UCI. Alternative approaches to promote controlled ribosomal stalling could include stop codon removal/suppression or use of a dominant negative release factor. Ribosome release could then be induced via addition of the chain terminator puromycin.
Since UCI cDNAs are formed on the 5’ UTR of the IVT-RNA, eukaryotic ribosomes would be unable to scan from the 5’ cap to the initiating Kozak sequence. The MIPSA system described here is therefore incompatible with cap-dependent eukaryotic cell-free translation systems. If cap-dependent translation is desired, however, two alternative methods could be developed. First, the current 5’ UCI system could be used if an internal ribosome entry site (IRES) were to be placed between the RT primer and the Kozak sequence. Second, the UCI could instead be introduced at the 3’ end of the RNA, provided that the RT was prevented from extending into the ORF. In an extension of eukaryotic MIPSA, RNA-cDNA hybrids could potentially be transfected into living cells or tissues, where UCI-protein formation could take place in situ, enabling many additional applications.
The ORF-associated UCIs can be embodied in a variety of ways. Here, we have stochastically assigned indexes to the human ORFeome at ~10x representation. This approach has two main benefits: first, a single degenerate oligonucleotide pool is low cost; second, multiple independent measurements are reported by the ensemble of UCIs associated with each ORF. We have designed our library of UCIs with uniform GC-content, and thus uniform PCR amplification efficiency. For simplicity, we have opted not to incorporate unique molecular identifiers (UMIs) into the RT primer, but this approach is compatible with MIPSA UCIs, and may potentially enhance quantitation. One disadvantage of stochastic indexing is the potential for ORF dropout, and thus the need for relatively high UCI representation; this increases the depth of sequencing required to quantify each UCI, and thus the overall per-sample cost. A second disadvantage is the requirement to construct a UCI-ORFeome matching dictionary. With short-read sequencing, we were unable to disambiguate a fraction of the library, comprised mostly of alternative isoforms. Using a long-read sequencing technology, such as PacBio or Oxford Nanopore Technologies, instead of or in addition to short-read sequencing technology could surmount incomplete disambiguation. As opposed to stochastic barcoding, individual UCI-ORF cloning is possible but costly and cumbersome. However, a smaller UCI set would provide the advantage of lower per-assay sequencing cost. We have previously developed a methodology to clone ORFeomes using Long Adapter Single Stranded Oligonucleotide (LASSO) probes.50 LASSO cloning of ORFeome libraries thus naturally synergizes with MIPSA-based applications.
MIPSA readout via qPCR.
A useful feature of appropriately designed UCIs is that they can also serve as qPCR readout probes. The degenerate UCIs that we have designed and used here (Fig. 1b) comprise 18 nt base-balanced forward and reverse primer binding sites. The low cost and rapid turnaround time of a qPCR assay can thus be leveraged in combination with MIPSA. For example, incorporating assay quality control measures, such as the TRIM21 IP, can be used to qualify a set of samples prior to a costlier sequencing run. Troubleshooting and optimization can similarly be expedited by employing qPCR as a readout, rather than relying exclusively on NGS. qPCR testing of specific UCIs may theoretically also provide enhanced sensitivity compared to sequencing, and may be more amenable to analysis in a clinical setting.
Outlook
MIPSA is a protein-display technology that has key advantages over alternative approaches. It has properties that complement techniques such as PhIP-Seq, and the MIPSA ORFeome libraries can be conveniently screened in the same reactions with phage-display libraries. The MIPSA protocol requires cap-independent cell-free translation, but future adaptations may overcome this limitation. Applications for MIPSA-based studies include protein–protein, protein–antibody, and protein–small-molecule interaction studies, as well as analyses of post-translational modifications. We used MIPSA to detect known autoantibodies and to discover neutralizing IFN-λ3 autoantibodies, among many other potentially pathogenic autoreactivities (Supplementary Table 2) that may contribute to life-threatening COVID-19 in a subset of at-risk individuals.
Methods
MIPSA Destination vector construction.
The MIPSA vector was constructed using the Gateway pDEST15 vector as a backbone. A gBlock fragment (Integrated DNA Technologies) encoding the RBS, Kozak sequence, N-terminal HaloTag fusion protein, and FLAG tag, followed by an attR1 sequence was cloned into the parent plasmid. A 150 bp poly(A) sequence was also added after attR2 site. The TRIM21 and GAPDH ORF sequences used for characterizing and optimizing the two-component system included native stop codons that were retained in the final MIPSA construct.
UCI barcode library construction.
A 41 nt barcode oligo was generated within a gBlock Gene Fragment (Integrated DNA Technologies) with alternating mixed bases (S: G/C; W: A/T) to produce the following sequence: (SW)18-AGGGA-(SW)18. The sequences flanking the degenerate barcode incorporated the standard PhIP-Seq PCR1 and PCR2 primer binding sites.51 Eighteen nanograms of the starting UCI library was used to run 40 cycles of PCR to amplify the library and incorporate BglII and PspxI restriction sites. The MIPSA vector and amplified UCI library were then digested with the restriction enzymes overnight, column purified, and ligated at 1:5 vector-to-insert ratio. The ligated MIPSA vector was used to transform electrocompetent One Shot ccdB 2 T1R cells (Thermo Fisher Scientific). Six transformation reactions yielded ~800,000 colonies to produce the pDEST-MIPSA UCI library.
Human ORFeome recombination into the pDEST-MIPSA UCI plasmid library.
150 ng of each pENTR-hORFeome subpool (L1-L5) from the hORFeome v8.1 was individually combined with 150 ng of the pDEST-MIPSA UCI library plasmid and 2 μl of Gateway LR Clonase II mix (Life Technologies) for a total reaction volume of 10 μl. The reaction was incubated overnight at 25°C. The entire reaction was transformed into 50 μl of One Shot OmniMAX 2 T1R chemical competent E. coli (Life Technologies). In aggregate, the transformations yielded ~120,000 colonies, which is ~10-fold the complexity of the hORFeome v8.1. Colonies were collected and pooled by scraping, followed by purification of the barcoded pDEST-MIPSA-hORFeome plasmid DNA (human ORFeome MIPSA library) using the Qiagen Plasmid Midi Kit (Qiagen). The human hORFeome v8.1 collection was cloned without stop codons; the displayed proteins may therefore contain poly-lysine C-termini resulting from translation of the polyA tail. A more recent version of the MIPSA destination vector includes a stop codon in frame with recombined ORFs.
HaloLigand conjugation to RT oligo and HPLC purification.
100 μg of a 5’ amine modified oligo HL-32_ad (Supplementary Table 3) was incubated with 75 μl (17.85 μg/μl) of the HaloTag Succinimidyl Ester (O2) (Promega Corporation), the HaloLigand, in 0.1 M sodium borate buffer for 6 hours at room temperature following Gu, et al.19 3 M NaCl and ice-cold ethanol was added at 10% (v/v) and 250% (v/v), respectively, to the labeling reaction and incubated overnight at −80°C. The reaction was centrifuged for 30 minutes at 12,000 x g. The pellet was rinsed once in ice-cold 70% ethanol and air-dried for 10 minutes.
HaloLigand-conjugated RT primer was HPLC purified using a Brownlee Aquapore RP-300 7u, 100×4.6 mm column (Perkin Elmer) using a two-buffer gradient of 0–70% CH3CN/MeCN (100 mM triethylamine acetate to acetonitrile) over 70 minutes. Fractions corresponding to labeled oligo were collected and lyophilized (Supplementary Fig. 1). Oligos were resuspended at 1 μM (15.4 ng/µl) and stored at −80°C.
MIPSA library IVT-RNA preparation.
The human ORFeome MIPSA library plasmid (4 μg) was linearized with the I-SceI restriction endonuclease (New England Biolabs) overnight. The product was column-purified with the NucleoSpin Gel and PCR Clean Up kit (Macherey-Nagel). A 40 μl in vitro transcription reaction using the HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs) was used to transcribe 1 μg of the purified, linearized pDEST-MIPSA plasmid library. The product was diluted with 60 μl molecular biology grade water, and 1 μl of DNAse I was added. The reaction was incubated for another 15 minutes at 37°C. Then 50 μl of 1 M LiCl was added to the solution and incubated at −80°C overnight. A centrifuge was cooled to 4°C, and the RNA was spun at maximum speed for 30 minutes. The supernatant was removed, and the RNA pellet washed with 70% ethanol. The sample was spun down at 4°C for another 10 minutes, and the 70% ethanol removed. The pellet was dried at room temperature for 15 minutes, and subsequently resuspended in 100 μl water. To preserve the sample, 1 μl of 40 U/μl RNAseOUT Recombinant Ribonuclease Inhibitor (Life Technologies) was added.
MIPSA library IVT-RNA reverse transcription and translation.
A reverse transcription reaction was prepared using SuperScript IV First-Strand Synthesis System (Life Technologies). First, 1 μl of 10 mM dNTPs, 1 μl of RNAseOUT (40 U/μl), 4.17 μl of the RNA library (1.5 μM), and 7.83 μl of the HaloLigand-conjugated RT primer (1 μM, Supplementary Table 3) were combined in a single 14 μl reaction and incubated at 65°C for 5 minutes followed by a 2-minute incubation on ice. Four microliters of 5X RT buffer, 1 μl of 0.1 M DTT, and 1 μl of SuperScript IV RT Enzyme (200 U/μl) was added to the 14 μl reaction on ice and incubated for 20 minutes at 42°C. A single 20 μl RT reaction received 36 μl of RNAClean XP beads (Beckman Coulter) and was incubated at room temperature for 10 minutes. The beads were collected by magnet and washed five times with 70% ethanol. The beads were air-dried for 10 minutes at room temperature and resuspended in 7 μl of 5 mM Tris-HCl, pH 8.5. The product was analyzed with spectrophotometry to measure the RNA yield. A translation reaction was set up on ice using the PURExpress ΔRibosome Kit (New England Biolabs).52 The reaction was modified such that the final concentration of ribosomes was 0.3 μM. For each 10 μl translation reaction, 4.57 μl of the RT reaction was added to 4 μl Solution A, 1.2 μl Factor Mix, and 0.23 μl ribosomes (13.3 μM). This reaction was incubated at 37°C for two hours, diluted to a total volume of 45 μl with 35 μl 1X PBS, and used immediately or stored at −80°C after addition of glycerol to a final concentration of 25% (v/v).
Immunoprecipitation of the translated MIPSA hORFeome library.
5 μl of plasma, diluted 1:100 in PBS, is mixed with the 45 μl of diluted MIPSA library translation reaction (see above) and incubated overnight at 4ºC with gentle agitation. For each IP, a mixture of 5 μl of Protein A Dynabeads and 5 μl of Protein G Dynabeads (Life Technologies) was washed 3 times in 2X their original volume with 1X PBS. The beads were then resuspended in 1X PBS at their original volume, and added to each IP. The antibody capture proceeded for 4 hours at 4°C. Beads were collected on a magnet and washed 3 times in 1X PBS, changing tubes or plates between washes. The beads were then collected and resuspended in a 20 μl PCR master mix containing the T7-Pep2_PCR1_F forward and the T7-Pep2_PCR1_R+ad_min reverse primers (Supplementary Table 3) and Herculase-II (Agilent). PCR cycling was as follows: an initial denaturing and enzyme activation step at 95ºC for 2 min, followed by 20 cycles of: 95ºC for 20 s, 58ºC for 30 s, and 72ºC for 30 s. The final extension step was performed at 72ºC for 3 minutes. Two microliters of the PCR1 amplification product were used as input to a 20 μl dual-indexing PCR reaction with the PhIP_PCR2_F forward and the PhIP_PCR2_R reverse primers, each containing 10 nt barcodes (i5 and i7, respectively). PCR cycling was as follows: an initial denaturing step at 95ºC for 2 min, followed by 20 cycles of: 95ºC for 20 s, 58ºC for 30 s, and 72ºC for 30 s. The final extension step was performed at 72ºC for 3 min. i5/i7 indexed libraries were pooled and column purified (NucleoSpin columns, Takara). Libraries were sequenced on an Illumina NextSeq 500 using a 1×50 nt SE or 1×75 nt SE protocol. MIPSA_i5_NextSeq_SP and Standard_i7_SP primers were used for i5/i7 sequencing (Supplementary Table 3) The output was demultiplexed using i5 and i7 without allowing any mismatches.
For quantification of MIPSA experiments by qPCR, the PCR1 product (above) was analyzed as follows. A 4.6 μl of 1:1,000 dilution of the PCR1 reaction was added to 5 μl of Brilliant III Ultra Fast 2X SYBR Green Mix (Agilent), 0.2 μl of 2 μM reference dye and 0.2 μl of 10 μM forward and reverse primer mix (specific to the target UCI). PCR cycling was as follows: an initial denaturing step at 95ºC for 2 min, followed by 45 cycles of: 95ºC for 20 s, 60ºC for 30. Following completion of thermocycling, amplified products were subjected to melt-curve analysis. The qPCR primers for MIPSA immunoprecipitation experiments were: BT2_F and BT2_R for TRIM21, BG4_F and BG4_R for GAPDH, and NT5C1A_F and NT5C1A_R for NT5C1A (Supplementary Table 3).
Plasma Samples.
All samples were collected from subjects that met protocol eligibility criteria, as described below. All studies protected the rights and privacy of the study participants and were approved by their respective Institutional Review Boards for original sample collection and subsequent analyses.
Pre-pandemic and healthy control plasma samples.
All human samples were collected prior to 2017 at the National Institutes of Health (NIH) Clinical Center under the Vaccine Research Center’s (VRC)/National Institutes of Allergy and Infectious Diseases (NIAID)/NIH protocol “VRC 000: Screening Subjects for HIV Vaccine Research Studies” (NCT00031304) in compliance with NIAID IRB approved procedures.
COVID-19 Convalescent Plasma (CCP) from non-hospitalized patients.
Eligible non-hospitalized CCP donors were contacted by study personnel, as previously described.53 All donors were at least 18 years old and had a confirmed diagnosis of SARS-CoV-2 by detection of RNA in a nasopharyngeal swab sample. Basic demographic information (age, sex, hospitalization with COVID-19) was obtained from each donor; initial diagnosis of SARS-CoV-2 and the date of diagnosis were confirmed by medical chart review.
Severe COVID-19 plasma samples.
The study cohort was defined as inpatients who had: 1) a confirmed RNA diagnosis of COVID-19 from a nasopharyngeal swab sample; 2) survival to death or discharge; and 3) remnant specimens in the Johns Hopkins COVID-19 Remnant Specimen Biorepository, an opportunity sample that includes 59% of Johns Hopkins Hospital COVID-19 patients and 66% of patients with length of stay ≥3 days.54,55 Patient outcomes were defined by the World Health Organization (WHO) COVID-19 disease severity scale. Samples from severe COVID-19 patients that were included in this study were obtained from 17 patients who died, 13 who recovered after being ventilated, 22 who required oxygen to recover, and 3 who recovered without supplementary oxygen. This study was approved by the JHU Institutional Review Board (IRB00248332, IRB00273516), with a waiver of consent because all specimens and clinical data were de-identified by the Core for Clinical Research Data Acquisition of the Johns Hopkins Institute for Clinical and Translational Research; the study team had no access to identifiable patient data.
Sjogren’s Syndrome and Inclusion body myositis (IBM) plasma samples.
Sjogren’s syndrome samples were collected under protocol NA_00013201. All patients were >18 years old and gave informed consent. IBM patient samples were collected under protocol IRB00235256. All patients met ENMC 2011 diagnostic criteria56 and provided informed consent.
Immunoblot analysis.
Laemmli buffer containing 5% β-ME was added to samples, boiled for 5 min, and analyzed on NuPage 4–12% Bis-Tris polyacrylamide gels (Life Technologies). Following transfer to PVDF membranes, blots were blocked in 20 mM Tris-buffered saline, pH 7.6, containing 0.1% Tween 20 (TBST) and 5% (wt/vol) non-fat dry milk for 30 minutes at room temperature. Blots were subsequently incubated overnight at 4°C with primary anti-FLAG antibody (#F3165, MilliporeSigma) at 1:2,000 (v/v), followed by a 4-hour incubation at room temperature in anti-mouse IgG, HRP-linked secondary antibody (#7076, Cell Signaling) at 1:4,000 (v/v).
Construction of the UCI-ORF dictionary.
The Nextera XT DNA Library Preparation kit (Illumina) was used for tagmentation of 150 ng of the pDEST-MIPSA hORFeome plasmid library to yield the optimal size distribution centered around 1.5 kb. Tagmented libraries were amplified using Herculase-II (Agilent) with T7-Pep2_PCR1_F forward and Nextera Index 1 Read primer. PCR cycling was as follows: an initial denaturing step at 95ºC for 2 minutes, followed by 30 cycles of: 95ºC for 20 s, 53.5ºC for 30 s, 72ºC for 30 s. A final extension step was performed at 72ºC for 3 minutes. PCR reactions were run on a 1% agarose gel followed by excision of ~1.5kb products and purification using the NucleoSpin Gel and PCR Clean-up columns (Macherey-Nagel). The purified product was then amplified for another 10 cycles with PhIP_PCR2_F forward and P7.2 reverse primers (see Supplementary Table 3 for list of primer sequences). The product was gel-purified and sequenced on a MiSeq (Illumina) using the T7-Pep2.2_SP_subA primer for read 1 and the MISEQ_MIPSA_R2 primer for read 2. Read 1 was 60 bp long to capture the UCIs. The first index read, I1, was substituted with a 50 bp read into the ORF. I2 was used to identify the i5 index for sample demultiplexing.
The hORFeome v8.1 DNA sequences were truncated to the first 50 nt, and the ORF names corresponding to non-unique sequences were concatenated with a “|” delimiter. The demultiplexed output of the 50 nt R2 (ORF) read from an Illumina MiSeq was aligned to the truncated human ORFeome v8.1 library using the Rbowtie2 package with the following parameters: options = “-a --very-sensitive-local”.57 The unique FASTQ identifiers were then used to extract corresponding sequences from the 60 bp R1 (UCI) read. Those sequences were then truncated using the 3’ anchor ACGATA, and sequences that did not have the anchor were removed. Additionally, any truncated R1 sequences that had fewer than 18 nucleotides were removed. The ORF sequences that still had a corresponding UCI post-filtering were retained using the FASTQ identifier. The names of ORFs that had the same UCI were concatenated with a “&” delimiter, and this final dictionary was used to generate a FASTA alignment file composed of ORF names and UCI sequences.
Informatic analysis of MIPSA sequencing data.
Illumina output FASTQ files were truncated using the constant ACGAT anchor sequence following all UCI sequences. Next, perfect match alignment was used to map the truncated sequences to their linked ORFs via the UCI-ORF lookup dictionary. A read count matrix was constructed, in which rows correspond to individual UCIs and columns correspond to samples. We next used the edgeR software package58 which, using a negative binomial model, compares the signal detected in each sample against a set of negative control (“mock”) IPs that were performed without plasma, to return a maximum likelihood fold-change estimate and a test statistic for each UCI in every sample, thus creating fold-change and -log10(p-value) matrices. By comparison of EdgeR output data from replicate IPs, we established that significantly enriched UCIs (“hits”) should require a read count of at least 15, a p-value less than 0.001, and a fold change of at least 3. Hits fold-change matrices report the fold-change value for “hits” and report a “1” for UCIs that are not hits.
Protein sequence similarity.
To evaluate sequence homology among proteins in the hORFeome v8.1 library, a blastp alignment was used to compare each protein sequence against all other library members (parameters: “-outfmt 6 -evalue 100 -max_hsps 1 -soft_masking false -word_size 7 -max_target_seqs 100000”). To evaluate sequence homology among reactive peptides in the human 90-aa phage display library, the epitopefindr59 software was employed.
Phage ImmunoPrecipitation Sequencing (PhIP-Seq) analyses.
PhIP-Seq was performed according to a previously published protocol.51 Briefly, 0.2 μl of each plasma was individually mixed with the 90-aa human phage library and immunoprecipitated using protein A and protein G coated magnetic beads. A set of 6–8 mock immunoprecipitations (no plasma input) were run on each 96 well plate. Magnetic beads were resuspended in PCR master mix and subjected to thermocycling. A second PCR reaction was employed for sample barcoding. Amplicons were pooled and sequenced on an Illumina NextSeq 500 instrument using a 1×50 nt SE or 1×75 nt SE protocol. PhIP-Seq with the human library was used to characterize autoantibodies in a collection of plasma from healthy controls. For fair comparison to the severe COVID-19 cohort, we first determined the minimum sequencing depth that would have been required to detect the IFN-λ3 reactivity in both of the positive individuals. We then only considered the 423 data sets from the healthy cohort with sequencing depth greater than this minimum threshold. None of these 423 individuals were found to be reactive to any peptide from IFN-λ3.
Type I/III interferon neutralization assay.
IFN-α2 (catalog no. 11100–1), IFN-λ1 (catalog no. 1598-IL-025) and IFN-λ3 (catalog no. 5259-IL-025) were purchased from R&D Systems. Twenty microliters of plasma were incubated for 1 hour at room temperature with either 100 U/ml IFN-α2 or 1 ng/ml IFN-λ3, and 180 μl DMEM in a total volume of 200 µl before addition into 7.5×104 A549 cells in 48-well tissue culture plates. After 4-hour incubation, the cells were washed with 1x PBS and cellular mRNA was extracted and purified using RNeasy Plus Mini Kit (Qiagen). Six hundred nanograms of extracted mRNA was reverse transcribed using the SuperScript III First-Strand Synthesis System (Life Technologies) and diluted 10-fold for qPCR analysis on a QuantStudio 6 Flex System (Applied Biosystems). PCR consisted of 95°C for 3 minutes, followed by 45 cycles of the following: 95°C for 15 seconds and 60°C for 30 seconds. MX1 expression was chosen as a measure of cell stimulation by the interferons, and the relative mRNA expression was normalized to GAPDH expression. The qPCR primers for GAPDH and MX1 were obtained from Integrated DNA Technologies (Supplementary Table 3). Anti-hIFN-α2-IgG (cat # mabg-hifna-3) and anti-hIL-28b-IgG (cat # mabg-hil28b-3) were purchased from InvivoGen. Manufacturer’s note about mabg-hifna-3: “This antibody reacts with hIFN-α1, hIFN-α2, hIFN-α5, hIFN-α8, hIFN-α14, hIFN-α16, hIFN-α17 and hIFN-α21; it reacts very weakly with hIFN-α4 and IFN-α10; it does not react with hIFN-α6 or hIFN-α7.” The Manufacturer’s note about mabg-hil28b-3: “Reacts with human IL-28A and human IL-28B.”
Supplementary Material
Acknowledgements
This study was made possible by a Johns Hopkins University Provost Research Grant made through the Johns Hopkins COVID-19 Research Response Program, by a National Institute of General Medical Sciences (NIGMS) grant R01 GM127353 (to H.B.L. and B.P.), by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL151826 (to E.M.B.), by grants from the Sjögren’s Syndrome Foundation and the Jerome L. Greene Foundation (to A.N.B., H.B.L.), and by funding from the intramural research program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We would like to thank Steve Elledge for generously providing the human ORFeome library and the 90-aa human peptidome T7 phage display library. We thank Rachel Green, Marco Catipovic, Allen Buskirk, Tim O’Donnell, Priya Duggal and Janet Markle for helpful discussions. We also thank Jodie Franklin and the Johns Hopkins Synthesis and Sequencing Core for HPLC purification of the HaloLigand conjugated RT-primer, as well as Linda Orzolek and Haiping Hao of the Johns Hopkins Transcriptomics and Deep Sequencing Core Facility. We would also like to thank Corinna Tuckey at New England Biolabs for assistance with Translation kits. The severe COVID-19 specimens used for this work were part of the Johns Hopkins Biospecimen Repository, which relies on the contribution of many patients, research teams and clinicians. We thank the members of the NIH Vaccine Research Center for pre-pandemic sample collection: Barney Graham, Laura Novick, Joseph Casazza, Julie Ledgerwood, Uzma Sarwar, LeeJah Chang, Cynthia Starr Hendel, Lasonji Holman, Sarah Plummer, Pam Costner, Ingelise Gorden, Brenda Larkin, Floreliz Mendoza, Jamie Saudners, Kathy Zephir, Mary E Enama, Galina Yamshchikov, Iris Pittman and Pernell Williams. Parts of some figures were created with BioRender.com.
Footnotes
Additional information
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Competing interests
H.B.L., J.J.C., J.G. and P.S. are listed as inventors on a patent application filed by Johns Hopkins University that covers the MIPSA technology. H.B.L. is a founder of Portal Bioscience, Alchemab and ImmuneID, and an advisor to TScan Therapeutics. The other authors declare no competing interests.
Peer review information Nature Biomedical Engineering thanks the anonymous, reviewer(s) for their contribution to the peer review of this work.
Code availability
The MIPSAlign package for alignment and UCI-ORF matching, implemented in R v 4.0.2, is available on github at https://github.com/jgunn123/MIPSAlign.
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are provided with this paper. The raw and analysed datasets are available from the corresponding author on request.
References
- 1.Larman HB et al. Cytosolic 5’-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Annals of neurology 73, 408–418, doi: 10.1002/ana.23840 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Xu GJ et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 348, aaa0698, doi: 10.1126/science.aaa0698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrock E et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 370, doi: 10.1126/science.abd4250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monaco DR et al. Profiling serum antibodies with a pan allergen phage library identifies key wheat allergy epitopes. Nat Commun 12, 379, doi: 10.1038/s41467-020-20622-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingsmore SF Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov 5, 310–320, doi: 10.1038/nrd2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodadek T Protein microarrays: prospects and problems. Chem Biol 8, 105–115, doi: 10.1016/s1074-5521(00)90067-x (2001). [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran N, Hainsworth E, Demirkan G & LaBaer J On-chip protein synthesis for making microarrays. Methods Mol Biol 328, 1–14, doi: 10.1385/1-59745-026-X:1 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Rungpragayphan S, Yamane T & Nakano H SIMPLEX: single-molecule PCR-linked in vitro expression: a novel method for high-throughput construction and screening of protein libraries. Methods Mol Biol 375, 79–94, doi: 10.1007/978-1-59745-388-2_4 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Zhu J et al. Protein interaction discovery using parallel analysis of translated ORFs (PLATO). Nat Biotechnol 31, 331–334, doi: 10.1038/nbt.2539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liszczak G & Muir TW Nucleic Acid-Barcoding Technologies: Converting DNA Sequencing into a Broad-Spectrum Molecular Counter. Angew Chem Int Ed Engl 58, 4144–4162, doi: 10.1002/anie.201808956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Los GV et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3, 373–382, doi: 10.1021/cb800025k (2008). [DOI] [PubMed] [Google Scholar]
- 12.Yazaki J et al. HaloTag-based conjugation of proteins to barcoding-oligonucleotides. Nucleic Acids Res 48, e8, doi: 10.1093/nar/gkz1086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Sawalha AH & Lu Q COVID-19 and autoimmune diseases. Curr Opin Rheumatol 33, 155–162, doi:10.1097 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight JS et al. The intersection of COVID-19 and autoimmunity. J Clin Invest 131, doi: 10.1172/JCI154886 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang EY et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288, doi: 10.1038/s41586-021-03631-y (2021). [DOI] [PubMed] [Google Scholar]
- 16.Bastard P et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol 6, doi: 10.1126/sciimmunol.abl4340 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abers MS et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol Cell Biol 99, 917–921, doi: 10.1111/imcb.12495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammad F, Green R & Buskirk AR A systematically-revised ribosome profiling method for bacteria reveals pauses at single-codon resolution. Elife 8, doi: 10.7554/eLife.42591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu L et al. Multiplex single-molecule interaction profiling of DNA-barcoded proteins. Nature 515, 554–557, doi: 10.1038/nature13761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X et al. A public genome-scale lentiviral expression library of human ORFs. Nat Methods 8, 659–661, doi: 10.1038/nmeth.1638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Consiglio CR et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 183, 968–981 e967, doi: 10.1016/j.cell.2020.09.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastard P et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, doi: 10.1126/science.abd4585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo Y et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 12, doi: 10.1126/scitranslmed.abd3876 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casciola-Rosen L et al. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. medRxiv, doi: 10.1101/2020.10.13.20211664 (2020). [DOI]
- 25.Woodruff MC, Ramonell RP, Lee FE & Sanz I Broadly-targeted autoreactivity is common in severe SARS-CoV-2 Infection. medRxiv, doi: 10.1101/2020.10.21.20216192 (2020). [DOI]
- 26.Wang D et al. AAgAtlas 1.0: a human autoantigen database. Nucleic Acids Res 45, D769–D776, doi: 10.1093/nar/gkw946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd TE et al. Cytosolic 5’-Nucleotidase 1A As a Target of Circulating Autoantibodies in Autoimmune Diseases. Arthritis Care Res (Hoboken) 68, 66–71, doi: 10.1002/acr.22600 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Nakabo S, Chu J, Hasni S & Kaplan MJ Association between anti-interferon-alpha autoantibodies and COVID-19 in systemic lupus erythematosus. medRxiv, doi: 10.1101/2020.10.29.20222000 (2020). [DOI]
- 29.Xu GJ et al. Systematic autoantigen analysis identifies a distinct subtype of scleroderma with coincident cancer. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1615990113 (2016). [DOI] [PMC free article] [PubMed]
- 30.Venkataraman T et al. Analysis of antibody binding specificities in twin and SNP-genotyped cohorts reveals that antiviral antibody epitope selection is a heritable trait. Immunity 55, 174–184 e175, doi: 10.1016/j.immuni.2021.12.004 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoeckius M et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods 14, 865–868, doi: 10.1038/nmeth.4380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setliff I et al. High-Throughput Mapping of B Cell Receptor Sequences to Antigen Specificity. Cell 179, 1636–1646 e1615, doi: 10.1016/j.cell.2019.11.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saka SK et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat Biotechnol 37, 1080–1090, doi: 10.1038/s41587-019-0207-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman-Melendez GD et al. Citrullination of a phage-displayed human peptidome library reveals the fine specificities of rheumatoid arthritis-associated autoantibodies. EBioMedicine 71, 103506, doi: 10.1016/j.ebiom.2021.103506 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman-Melendez GD, Venkataraman T, Monaco DR & Larman HB Protease Activity Profiling via Programmable Phage Display of Comprehensive Proteome-Scale Peptide Libraries. Cell Syst 11, 375–381 e374, doi: 10.1016/j.cels.2020.08.013 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Mordstein M et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol 84, 5670–5677, doi: 10.1128/JVI.00272-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ank N et al. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 80, 4501–4509, doi: 10.1128/JVI.80.9.4501-4509.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busnadiego I et al. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio 11, doi: 10.1128/mBio.01928-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderheiden A et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J Virol 94, doi: 10.1128/JVI.00985-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanifer ML et al. Critical Role of Type III Interferon in Controlling SARS-CoV-2 Infection in Human Intestinal Epithelial Cells. Cell Rep 32, 107863, doi: 10.1016/j.celrep.2020.107863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galani IE et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 22, 32–40, doi: 10.1038/s41590-020-00840-x (2021). [DOI] [PubMed] [Google Scholar]
- 42.Felgenhauer U et al. Inhibition of SARS-CoV-2 by type I and type III interferons. J Biol Chem 295, 13958–13964, doi: 10.1074/jbc.AC120.013788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien TR et al. Weak Induction of Interferon Expression by Severe Acute Respiratory Syndrome Coronavirus 2 Supports Clinical Trials of Interferon-lambda to Treat Early Coronavirus Disease 2019. Clin Infect Dis 71, 1410–1412, doi: 10.1093/cid/ciaa453 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreakos E & Tsiodras S COVID-19: lambda interferon against viral load and hyperinflammation. EMBO Mol Med 12, e12465, doi: 10.15252/emmm.202012465 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prokunina-Olsson L et al. COVID-19 and emerging viral infections: The case for interferon lambda. J Exp Med 217, doi: 10.1084/jem.20200653 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feld JJ et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med, doi: 10.1016/S2213-2600(20)30566-X (2021). [DOI] [PMC free article] [PubMed]
- 47.Jongsma MA & Litjens RH Self-assembling protein arrays on DNA chips by auto-labeling fusion proteins with a single DNA address. Proteomics 6, 2650–2655, doi: 10.1002/pmic.200500654 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Gautier A et al. An engineered protein tag for multiprotein labeling in living cells. Chem Biol 15, 128–136, doi: 10.1016/j.chembiol.2008.01.007 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Samelson AJ et al. Kinetic and structural comparison of a protein’s cotranslational folding and refolding pathways. Sci Adv 4, eaas9098, doi: 10.1126/sciadv.aas9098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tosi L et al. Long-adapter single-strand oligonucleotide probes for the massively multiplexed cloning of kilobase genome regions. Nat Biomed Eng 1, doi: 10.1038/s41551-017-0092 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohan D et al. Publisher Correction: PhIP-Seq characterization of serum antibodies using oligonucleotide-encoded peptidomes. Nature protocols 14, 2596, doi: 10.1038/s41596-018-0088-4 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Tuckey C, Asahara H, Zhou Y & Chong S Protein synthesis using a reconstituted cell-free system. Curr Protoc Mol Biol 108, 16 31 11–22, doi: 10.1002/0471142727.mb1631s108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein SL et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest 130, 6141–6150, doi: 10.1172/JCI142004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Correction: Patient Trajectories Among Persons Hospitalized for COVID-19. Ann Intern Med 174, 144, doi: 10.7326/L20-1322 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Zyskind I RA, Zimmerman J, Naiditch H, Glatt AE, Pinter A, Theel ES, Joyner MJ, Hill DA, Lieberman MR, Bigajer E, Stok D, Frank E, Silverberg JI. SARS-CoV-2 Seroprevalence and Symptom Onset in Culturally-Linked Orthodox Jewish Communities Across Multiple Regions in the United States. JAMA Open Network In Press (2021). [DOI] [PMC free article] [PubMed]
- 56.Rose MR & Group, E. I. W. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 23, 1044–1055, doi: 10.1016/j.nmd.2013.08.007 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Wei Z, Zhang W, Fang H, Li Y & Wang X esATAC: an easy-to-use systematic pipeline for ATAC-seq data analysis. Bioinformatics 34, 2664–2665, doi: 10.1093/bioinformatics/bty141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson MD, McCarthy DJ & Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140, doi: 10.1093/bioinformatics/btp616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. brandonsie.github.io/epitopefindr.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are provided with this paper. The raw and analysed datasets are available from the corresponding author on request.


