Abstract
The kappa-opioid receptor (KOR)/dynorphin (DYN) system is involved in dysphoria and negative emotional states. Dysregulation of KOR function promotes maladaptive behavioral regulation during withdrawal associated with alcohol dependence. Mesolimbic dopaminergic (DA) projections from the ventral tegmental area (VTA) innervate the extended amygdala circuitry and presynaptic KORs attenuate DA in these regions leading to an excessive alcohol consumption and negative affective-like behavior, whereas mesocortical KOR-regulated DA projections have been implicated in executive function and decisionmaking. Thus, the neuroadaptations occurring in KOR/DYN systems are important aspects to consider for the development of personalized therapeutic solutions. Herein, we study the contribution of the VTA dopaminergic neuron Oprk1 (KOR gene) in excessive alcohol consumption, negative emotional state, and executive function. To do so, Oprk1 mRNA expression and KOR function were characterized to confirm alcohol dependence-induced dysregulation in the VTA. Then, a transgenic Cre-Lox rat model (male and female TH::Cre rats) was used to allow for conditional and inducible overexpression of Oprk1 in VTA DA neurons. The effect of this overexpression was evaluated on operant alcohol self-administration, negative emotional states, and executive function. We found that VTA Oprk1 overexpression recapitulates some phenotypes of alcohol dependence including escalated alcohol self-administration and depressive-like behavior. However, working memory performance was not impacted following VTA Oprk1 overexpression in TH::Cre rats. This supports the hypothesis that dysregulated KOR signaling within the mesolimbic DA system is an important contributor to symptoms of alcohol dependence and shows that understanding Oprk1-mediated contributions to alcohol use disorder (AUD) should be an important future goal.
Keywords: alcohol use disorder, alcohol self-administration, dopamine, dynorphin, elevated plus-maze, ethanol vapor exposure, forced swim test, kappa opioid receptor, mesolimbic, mesocortical, Oprk1, TH::Cre rats, ventral tegmental area
1. Introduction
Alcohol use disorder (AUD) involves an impaired ability to stop or control alcohol use despite the negative social, occupational and health consequences and is the third-most preventable cause of death in the United States (Mokdad et al., 2004). Furthermore, the costs of AUD for society are profound and routinely surpass over 200 billion USD annually (Sacks et al., 2015). In 2019, 14.5 million people were diagnosed with AUD which represents 5.3% of the US adults (SAMHSA, 2019), but current therapies are marginally effective (Akbar et al., 2018; Edwards et al., 2011; Heilig and Egli, 2006; Walker et al., 2012). AUD is associated with increased risk of injuries and disease including affective disorders such as anxiety and depression (Boden and Fergusson, 2011; Driessen et al., 2001; Sánchez-Peña et al., 2012; Weiss et al., 2001). One explanation for continuous use despite negative consequences is that alcohol’s positive reinforcing effects are reduced over time and negative reinforcement mechanisms during dependence and withdrawal become prominent. A negative emotional state during withdrawal can promote excessive drinking through self-medication processes via alcohol’s negative reinforcing effects (Khantzian, 1990; Koob and Le Moal, 1997, 2008; Markou et al., 1998; Walker, 2012).
The endogenous opioid peptide system is involved in the regulation of motivation and emotion and has been linked to the consumption of drugs of abuse (Bodnar, 2022). Classically, several opioid receptors were identified, each with a preferred endogenous ligand and include the mu-, delta-, and kappa-opioid receptors (MOR, DOR, and KOR) that preferentially bind β-endorphin, enkephalin and dynorphin (DYN), respectively (Chavkin et al., 1982; Fowler and Fraser, 1994). Subsequently, the nociceptin opioid receptor (NOP) and its ligand, nociceptin/orphaninFQ (N/OFQ) were included as a fourth opioid receptor / peptide system (Meunier et al., 1995; Mollereau et al., 1994; for review, see Toll et al., 2016). Endogenous opioid peptide release and receptor activation can have opposing effects on neurotransmission and behavior within motivational / emotional domains. For instance, in the conditioned placed preference paradigm (CPP) activation of the MOR and DOR is rewarding (Amalric et al., 1987; Herz, 1997; Mucha and Herz, 1985) whereas activation of KORs produces place aversions (Burgdorf et al., 2001; Mucha and Herz, 1985; Shippenberg and Herz, 1986) with these opposing behaviors involving opposing dopaminergic signaling in the nucleus accumbens (Acb) that is increased by MOR agonists and decreased by KOR agonists (Pan, 1998; Spanagel et al., 1992). Acute alcohol intake induces endogenous opioid peptide release in humans and rats (Dai et al., 2005; Gianoulakis et al., 1996; Marinelli et al., 2005; Marinelli et al., 2006; Marinelli et al., 2003, 2004) while chronic alcohol exposure has been shown to trigger changes in the levels of the opioid peptides (Gianoulakis et al., 1996; Lindholm et al., 2000), receptor densities/function (Chen and Lawrence, 2000; Economidou et al., 2008; Kissler et al., 2014; Turchan et al., 1999; Wei et al., 2022), as well as modifications of mRNA coding for both the peptides and receptors (Aujla et al., 2013; Cowen and Lawrence, 2001; Przewlocka et al., 1997; Rosin et al., 1999) that are often reflective of a compensatory response to the acute effects of alcohol (Walker et al., 2012). Collectively, these alcohol-induced alterations could explain the shift from primarily hedonic opioid peptide signaling to more prominent KOR-mediated aversive signaling in AUD (Walker et al., 2012).
The endogenous opioid system has long been a target in the treatment of AUD (June et al., 1998; Volpicelli et al., 1992). For example, nalmefene, an opioid receptor ligand with both MOR antagonist and KOR partial agonist effects, was shown to decrease alcohol self-administration in non-dependent and alcohol-dependent rats (Kissler et al., 2014; Nealey et al., 2011). However, in recent years, it has become apparent that the link between the KOR/DYN system and alcohol’s negative reinforcing effects could play a major role in the treatment of AUD (Walker et al., 2012). Indeed, contrary to nalmefene, the selective KOR antagonist nor-binaltorphimine (nor-BNI) was shown to reduce alcohol self-administration in dependent animals but not in non-dependent animals (Kissler et al., 2014; Nealey et al., 2011; Walker and Koob, 2008). This was the first evidence showing a specific role for the KOR/DYN system in alcohol dependence and its potential as an AUD therapeutic. Indeed, KOR activation is involved in aversion and dysphoria (Al-Hasani et al., 2015; Koob, 2014; Land et al., 2008; McLaughlin et al., 2003) and has been shown to promote depressive-like behavior (Bruchas et al., 2010; Carlezon et al., 2006; Carlezon and Miczek, 2010; Knoll and Carlezon, 2010; Nestler and Carlezon, 2006; Todtenkopf et al., 2004), while KOR antagonists have antidepressant properties (Bruchas et al., 2011; Carr et al., 2010; Mague et al., 2003; Pliakas et al., 2001) and dysregulation of the KOR/DYN system is involved in motivational, negative emotional and executive function psychopathologies associated with AUD (for reviews, see Karkhanis and Al-Hasani, 2020; Sirohi et al., 2012; Walker et al., 2012).
Within nuclei of the extended amygdala (Alheid and Heimer, 1988; Koob and Volkow, 2016), a functionally interconnected brain network comprised of the Acb shell, central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST), alcohol can increase DYN release and induce neuroadaptations in the DYN/KOR system with site-specific Acb, CeA and BNST KOR antagonism ameliorating alcohol dependence and withdrawal-induced escalation of alcohol self-administration (Erikson et al., 2018; Kissler et al., 2014; Kissler and Walker, 2016; Lindholm et al., 2000; Marinelli et al., 2006; Nealey et al., 2011; Olive et al., 2001; Przewlocka et al., 1997). One common feature of these extended amygdala nuclei is their innervation by mesolimbic DA initiating in the ventral tegmental area (VTA). The mesolimbic dopaminergic pathway connects the VTA to limbic nuclei including those of the extended amygdala (Beier et al., 2015; Volkow and Morales, 2015) and is involved in appetitive / aversive motivational processes (Koob and Nestler, 1997; Mogenson et al., 1980) and the reinforcing / rewarding properties of drugs of abuse (Hipolito et al., 2015; Koob and Nestler, 1997; Oliva and Wanat, 2016; Salamone and Correa, 2012; Volkow et al., 2017; Walker and Ettenberg, 2005). Within mesolimbic DAergic circuitry, KORs are anatomically positioned to presynaptically inhibit DA release, whereas KORs within VTA-originating mesocortical circuitry have been shown to have both somatic and presynaptic DA regulatory properties (Margolis et al., 2003; Margolis et al., 2006a; Morales and Margolis, 2017; Siciliano et al., 2016; Tejeda et al., 2013) with mesocortical DA and cortical KORs in the medial prefrontal cortex (mPFC) shown to be involved in cognitive control and executive functions such as working memory (Abraham et al., 2021; Liu et al., 2014; Miller, 2000; Tejeda et al., 2013; Tejeda et al., 2021; Wei et al., 2022).
While alcohol dependence-induced dysregulation of KOR/DYN in the mesocorticolimbic circuit terminal regions has been shown to promote motivational, negative- emotional and executive function phenotypes of alcohol withdrawal, the role of VTA KORs on AUD-related phenotypes remains elusive. In the present study, we characterized VTA KOR gene (Oprk1) mRNA levels and KOR function in alcohol-dependent Wistar rats and subsequently tested the hypothesis that dysregulation of Oprk1 expression levels in the VTA contributes to phenotypes of AUD such as escalated alcohol self-administration, depressive-and/or anxiety-like symptoms and altered working memory. To do so, we leveraged Cre-lox technology and utilized TH::Cre (Cre-recombinase inserted at tyrosine hydroxylase promotor region) transgenic rats (Witten et al., 2011) in combination with site-specific intra-VTA floxed (flanked by lox P sites to promote recombination in the presence of Cre-recombinase) Oprk1 adeno-associated viral infusions to conditionally (selectively in tyrosine hydroxylase positive neurons) and inducibly (once the TH::Cre rats had developed into adults and learned to self-administer alcohol) increase Oprk1 expression. We found that Oprk1 mRNA was increased in VTA of alcohol-dependent rats, concomitant with increased KOR function. When replicating such a VTA Oprk1 profile in non-dependent TH::Cre rats through floxed Oprk1 viral infusions, VTA overexpression of Oprk1 mRNA in the VTA of non-dependent rats was sufficient to trigger phenotypes of alcohol dependence such as escalated alcohol self-administration and depressive-like states. These results support that VTA KOR dysregulation is a central component of AUD phenomenology and should be considered an important target for AUD therapeutic development.
2. Methods and Materials
2.1. Animals
Twenty-two adult (>PND 70) male Wistar rats (Charles River Labs, Raleigh, NC) and 142 male and female adult (>PND 70) heterozygous TH::Cre rats (Witten et al., 2011; LE-Tg(TH-Cre)3.1Deis; courtesy of Karl Deisseroth via RRRC, Columbia, MO) were housed (2–3 rats / cage) in an environmentally controlled vivarium on a 12-hr reverse light cycle (lights off at 6 AM; all behavioral tests were conducted during the dark cycle) with food and water provided ad libitum (unless specified below). All work complied with the National Research Council’s Guide for the Care and Use of Laboratory Animals (2011) and the Institutional Animal Care and Use Committee guidelines.
2.2. Experiment 1: Oprk1 mRNA and KOR function in alcohol dependence
2.2.1. Chronic intermittent ethanol vapor exposure
Chronic intermittent ethanol vapor exposure (14 h on/10 h off; 7 days/week) was carried out in custom designed vapor chambers for 12 weeks. 95% ethanol was vaporized and mixed with the airflow of the sealed environmental chambers in which animals were housed. The rate of 95% ethanol vaporized was controlled by the experimenter such that the blood alcohol level (BAL) of the animals was titrated within a desired range (175–250mg%). The BAL was determined by collecting blood from the tails (~50 μl) twice a week. After centrifugation, plasma samples were tested for alcohol content using the Analox GL5 (Analox Instruments, Lunenburg, MA).
2.2.2. Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
At a time-point consistent with acute withdrawal for the vapor-exposed animals (i.e., 6-10 hours after the daily vapor-exposure terminated), both air- and vapor-exposed rats (n=8/grp) had their brains extracted following decapitation and immediately snap frozen in 2-methylbutane cooled by dry ice. Snap-frozen brains were stored at −80°C until histology examination was carried out. As described previously (Erikson et al., 2018), the VTA was bilaterally micropunched (1.0 mm diameter) and placed in RNA later (50ul) in 4°C for 24 hours , then stored in −80°C freezer until RNA extraction occurred according to the Purelink RNA extraction protocol (Life Technologies, Carlsbad, CA). The quantity of the RNA extracted was measured using a NanoDrop (Thermo-Fisher Scientific, Waltham, MA) system whereas the RNA quality was assessed using a total RNA fragment analysis (Agilent 5200 Fragment Analyzer, Santa Clara, CA) Advanced Technologies, Ankeny, IA). Only samples with RNA quality greater or equal to 9.0 were used for RT-qPCR assessment of Oprk1 mRNA expression. The primers for Gapdh and Oprk1 were taken from (Vats et al., 2008) or designed using Primer3 (Untergasser et al., 2012) and the specificity verified using NCBI BLAST (Altschul et al., 1990). To evaluate the primers through amplification efficiency, five different concentrations were used in triplicate. The melt curves were also analyzed to confirm the absence of primer-dimerization. RNA was transcribed into cDNA using the Invitrogen Superscript IV VILO Master Mix protocol (Thermo Fischer Scientific, Waltham, MA). The quantification and relative expression of the gene transcripts were calculated using the comparative CT method (Livak and Schmittgen, 2001).
2.2.2. GTPγS Assay
The assay was conducted as described previously (Kissler et al., 2014) and measures guanine nucleotide exchange at G proteins in response to ligand binding to G protein-coupled receptors. The principle relies on monitoring the accumulation of a radiolabeled, [35S] GTPyS in the presence of unlabeled GDP, as described in detail elsewhere (Harrison and Traynor, 2003). Snap-frozen rat VTA tissue from air- and intermittent ethanol vapor-exposed (n = 3/grp, in triplicate) animals was micro-dissected using a brain matrix and tissue punches at −20°C according to the coordinates of Paxinos and Watson (2005) and homogenized in 1.5 ml of membrane buffer (pH 7.4, 50.0-mM Tris–HCl, 3.0-mM MgCl2 and 1.0-mM EGTA). The homogenate was centrifuged (21,000 g, 4°C for 30 min), resuspended in 1.5 ml of membrane buffer, homogenized, and centrifuged again. The pellet was re-homogenized in 1.5 ml of assay buffer (pH 7.4, 50.0-mM Tris–HCl, 3.0-mM MgCl2, 0.2-mM EGTA, 100.0-mM NaCl). The estimation of protein concentration was assessed using a BCA protein assay (Pierce). Prior to the addition of 3 μg of protein homogenate, the samples were homogenized. DYN A (0.0–1.0 μM) was purchased from Tocris Biosciences (Minneapolis, MN) and incubated in assay buffer in triplicate (90 min; 25°C) with 10 μM GDP and 0.05 nM [35S] GTPγS in 1.0-ml. The reaction was terminated by filtration using a cell harvester, washed three times in phosphate buffer (pH 7.2) and liquid scintillation spectrophotometry was used to quantify filter disc bound radioactivity.
2.3. Experiment 2: Viral optimization and effect of VTA Oprk1 overexpression on alcohol self-administration
2.3.1. Transgenic TH::Cre rat operant alcohol self-administration
Male and female TH::Cre rats were trained to self-administer a 10% ethanol (w/v) solution using a sweetener-fade method during 30-min sessions (Walker and Koob, 2007). Standard two-lever operant chambers (Med Associates, St. Albans, VT) were utilized with custom dual fluid receptacles (Behavioral Pharma, La Jolla, CA), allowing the animals to press a single lever and receive 0.1 ml of the solution or water. Stability of responding, corresponding to three days with a maximum of 10% deviation on the ethanol-paired lever, was required prior to advancement to subsequent experimental phases. Two experiments were conducted: The first experiment was a TH::Cre phenotyping experiment involving air and ethanol vapor exposure (see section 2.2.1) to confirm that male and female TH::Cre rats (N=36) self-administered alcohol normally when non-dependent and showed typical escalation once alcohol-dependent and in withdrawal. For the alcohol dependence and withdrawal component of this first experiment, rats were tested in the operant chambers for alcohol self-administration during daily 30-min self-administration sessions twice weekly (Mon/Thurs or Tues/Fri) at a time-point consistent with acute withdrawal (6 h following the termination of the daily vapor exposure for the vapor-exposed animals). The second experiment involved non-dependent male and female TH::Cre rats (N=36) receiving a floxed Oprk1 viral construct or control viral construct (see section 2.3.2) to assess operant alcohol self-administration following intra-VTA Oprk1 overexpression. The non-dependent viral-infused animals resumed 30-min operant alcohol/water self-administration sessions following 28 days of viral incubation and were tested Mon-Fri for two weeks.
2.3.2. Stereotaxic Viral infusions
All surgeries were conducted under 2% isoflurane gas anesthesia. Viral infusions targeted the VTA with the following coordinates from bregma (AP: −6 & ML: ±0.5 for both males and females, and DV: −8.3 and −7.9 for females and DV: −8.5 and −8.1 for males). 0.3 μl of either the active Oprk1 (AAV5-Ef1a-OPRK1-DIO-EYFP; 2.05E12 vg/ml titer, adapted from (Nygard et al., 2016), courtesy of Dr Michael R, Bruchas) or control construct (AAV5-Ef1a-DIO-EYFP, UNC Viral Core, Chapel Hill, NC) was infused at a rate of 0.15μl/min. Initial pilot studies to optimize the infusion parameters utilized unilateral infusions. A subsequent pilot experiment to determine viral efficacy by RT-qPCR in male and female TH::Cre rats (N=18), as well as the full experiments (described below) utilized bilateral viral infusions, at two different depths per hemisphere (see above for the coordinates), amounting to 1.2 μl per animal. Microinjectors were left in place for 5 min after each injection to allow for diffusion. A postoperative analgesic (flunixin) was administered daily for 5 days following surgery. Following 28 days of viral incubation, animals were tested in the behavioral paradigms as described.
2.3.3. Viral site- and cell type fluorescent microscopy
The fluorescent immunohistological approach was adapted from Witten et al. (2011) and Kissler et al. (2014). In brief, rats were anesthetized with isoflurane and perfused for 5 min with phosphate buffer saline (PBS, 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4) followed by 5 min of 4% paraformaldehyde perfusion. The whole brains were immediately extracted and stored in 4% paraformaldehyde at 4 °C overnight. Subsequently, the brains were transferred to 30% sucrose in PBS and stored in 4 °C until the brains sank to the bottom of the collection tubes before slicing occurred. The brains were sliced (40 μm thickness) using a cryostat (Leica 1850, Bannockburn, IL) and washed with PBS 3 times and blocked for 1h in 1% serum with PBS and 0.1% Triton X-100. After blocking, the sections were incubated with the primary antibody (anti-Tyrosine hydroxylase (TH), 1:1000, Aves Labs, Davis, CA, ref: TYH) and 1% serum overnight at 4 °C. On the next day, the sections were washed 3 times in PBS + 1% Serum and incubated in the secondary antibody (goat anti-chicken antibody coupled to Alexa fluor 568, 1:1000; ThermoFisher Scientific, Waltham, MA, ref: A-11041) for 1 h at room temperature. Finally, sections were washed and mounted on slides using an anti-fade mounting medium and coverslipped. The coverslip edge was ringed with clear fingernail polish and the slides were examined under a microscope using the corresponding fluorescent filters (i.e., red = TH and green = EYFP) using an Olympus BX53 upright microscope with a U-HGLGPS fluorescent light source.
2.4. Experiment 3: Overexpression of VTA Oprk1, somatic signs of withdrawal and negative affective-like behavior
2.4.1. Experiment 3 test parameters
Three behaviors were measured in male and female TH::Cre rats (N=36) in this experiment: somatic signs reflecting dependence/withdrawal-like behavior, anxiety-like behavior using the elevated plus-maze (EPM), and depressive-like behavior using the forced swim test (FST). The order of testing was not counterbalanced but was instead scheduled based on an assessment of stress-inducing impact on the animals with the least stressful tests occurring on the first day of testing (somatic signs + EPM) and the FST occurring on following an intervening day. This was to avoid the potential confound of swim stress-induced bias (Bruchas et al., 2007) on somatic signs and EPM performance. Twenty-eight days after intra-VTA viral infusions using the established coordinates, male and female TH::Cre rats were transported to the test room in a container identical to their homecage and tested for somatic signs reflective of withdrawal and then tested in the EPM apparatus. Following an intervening day, testing in the FST occurred with subsequent brains extraction, snap frozen and histological assessment for VTA-specific viral infusions (see histology section below) and then micropunched for RT-qPCR to assess Oprk1 mRNA over-expression (see above).
2.4.2. Somatic signs of alcohol withdrawal
Somatic signs of alcohol withdrawal as those previously observed in alcohol dependent animals (Schulteis et al., 1995; Williams et al., 2012), were assessed in active viral- or control-infused TH::Cre animals using the following methods. Four behaviors were assessed and scored on a scale of 0–2 (2 indicating severe or persistent presence of the symptom): 1) hyperirritability upon touch, 2) presence of the ventromedial distal flexion response (measured by gently grasping the rat by the scruff of the neck and checking the retraction of the limbs towards the body), 3) tail stiffness/rigidity, and 4) abnormal posture or gait. Animals were observed for 3 min and given scores for all symptoms that were added to obtain a single final score for physiological withdrawal symptoms that can range from 0 to 8.
2.4.3. Elevated plus-maze
The EPM apparatus consists of a raised platform (50 cm high) with two open arms and two closed arms of equal length (47×10 cm each) and a 10×10-cm center platform. The floors and the walls of the closed arms were opaque (40 cm high). Each animal was placed in the center of the platform facing the same direction and allowed to explore the maze for 5 min. Illumination in all arms was approximately 50 lux. Each animal was recorded by video and the AnyMaze video tracking software (Stoelting Co,Wood Dale, IL) was used to score the amount of time spent in the open arm, closed arm, and center platform as well as entries into and distance traveled in those regions. The maze was cleaned with Oxivir® and dried between each animal.
2.4.4. Forced swim test
The FST apparatus was a custom-built clear Plexiglas® cylinder (diameter= 34 cm, height= 79.5 cm). The cylinder was filled to 53 cm with water temperature at 24±2 °C. The illumination at the surface of the water was 25 lux. Animals were gently placed in the water and a 5 min video was recorded and analyzed with AnyMaze video tracking software (Stoelting Co, Wood Dale, IL). Swimming was defined as active movement of the animals with all four paws while immobility was defined as a lack of active swimming with animals floating to maintain the head above water with only minor paw movement.
2.5. Experiment 3: Overexpression of VTA Oprk1 and working memory performance
The delayed non-matching-to-sample task (DNMST) used in the current experiment employed a T-maze apparatus (see Wei et al., 2022) to assess working memory performance in male and female TH::Cre rats (N=16). The walls of the T-maze were made of grey Plexiglas with three removable guillotine doors. One door separated the 30-cm compartment of the start arm and the two other doors marked the entrance of each goal arm. All animals were food restricted to 80%–85% of normal body weight as described previously (George et al., 2008). DNMST training was as follows: First, animals were habituated to the T-maze 10 min/day and could freely explore and eat the sucrose pellet in the two goal boxes. Once habituated, rats were trained for five trials/session. Each trial consisted of two runs: one forced choice run (which side was defined randomly), and one free choice run (with reinforcement dependent on an opposite arm entry). This was continued until the acquisition criteria (>80% correct choices during two consecutive days) were met. For the DNMST testing, a variable delay (0, 10, 70, 130 and 190 second) was introduced between the forced and free runs, with the delay sequence appearing at random with equal representation in each session. The number of trials to acquisition criterion and correct choices were recorded.
2.6. Post-behavioral viral histology and RT-qPCR
For all behavioral experiments involving viral infusions, assessment of the position of viral infusions was conducted after the brains were removed and immediately snap frozen in 2-methylbutane cooled by dry ice. Frozen brains were stored at −80°C until histology examination was carried out. On the day of histological examination, the brains were brought to −20°C and mounted on specimen discs to be sliced using a cryostat 1850 (Leica, Bannockburn, IL). 40 mm sections were obtained, mounted on slides and the viral injection sites indicated by fluorescent protein luminescence were evaluated for appropriate VTA placement with an Olympus BX53 with U-HGLGPS fluorescent light source. Following brain section collection indicating the position of infusion, the brain was micropunched at −20°C and RT-qPCR subsequently conducted (as described above) to evaluate post-viral infusion Oprk1 mRNA expression levels.
2.7. Statistics
IBM SPSS Statistics 27.0.1 was used to conduct all statistical analyses. In experiment 1, intra-VTA air- and ethanol vapor-exposed Oprk1 mRNA ΔΔCt values in the RT-qPCR assay and percent basal [35S] coupling in the DYN A-stimulated GTPγS assay were compared with a univariate analysis of variance (ANOVA) with exposure condition as the between-groups variable. For experiment 2, pilot viral infusion VTA Oprk1 mRNA ΔΔCt values were assessed in the RT-qPCR assay using two-way ANOVA with sex and viral condition as the between-group factors. In addition, for the TH::Cre alcohol self-administration (g/kg) phenotyping, a three-way mixed model ANOVA was used with sex and exposure condition (air vs vapor) as the between-group factors and session (i.e., pre- vs post-exposure) as the within-subject variables. Furthermore, alcohol self-administration (g/kg) was evaluated using a three-way mixed-model ANOVA with sex and viral condition as the between-group variables and session as the within-subject variable with VTA Oprk1 mRNA ΔΔCt values assessed the same animals. Experiment 3 used a multivariate ANOVA to compare the FST, EPM and somatic dependent variables with sex and viral condition as the between-group factors with VTA Oprk1 mRNA ΔΔCt values assessed the same animals. Finally, experiment 4 used a three-way mixed-model ANOVA to assess working memory performance in the DNMST with sex and viral condition as the between-group factors and delay as the within-subject variable with VTA Oprk1 mRNA ΔΔCt values assessed the same animals.
3. Results
3.1. Experiment 1: Oprk1 mRNA and KOR function in alcohol dependence
Wistar rats were exposed to air or ethanol vapor for twelve weeks and the expression of Oprk1 mRNA and KOR function in the VTA was assessed. Data from three animals was excluded from the analysis: two animals from the vapor-exposed RT-PCR group did not meet the fragment analysis inclusion threshold for the RT-qPCR experiment resulting in n=6-8/exposure condition for the RT-qPCR experiment. The univariate ANOVA showed that Oprk1 mRNA expression was significantly higher in the VTA of vapor-exposed group than in the air-exposed group (F (1,12) = 23.881, p = 0.000374; see Fig. 1A). The mixed-model ANOVA showed that DYN A-stimulated GTPγS coupling increased as the concentration of DYN increased (F (2,8) = 5.324, p = 0.034) and when comparing the ethanol vapor-exposed and air-exposed groups there was a trend towards a DYN A x Exposure Condition interaction (F (2,8) = 4.312, p = 0.054, see Fig. 1B) indicating that DYN A-stimulated KOR function was differentially increased in ethanol vapor-exposed animals as the concentration of DYN increased. These results show that chronic alcohol exposure led to increased Oprk1 mRNA expression and strong trend towards increased KOR function.
Figure 1: Alcohol dependence-induced modification of Oprk1 mRNA expression and KOR function in the ventral tegmental area (VTA).
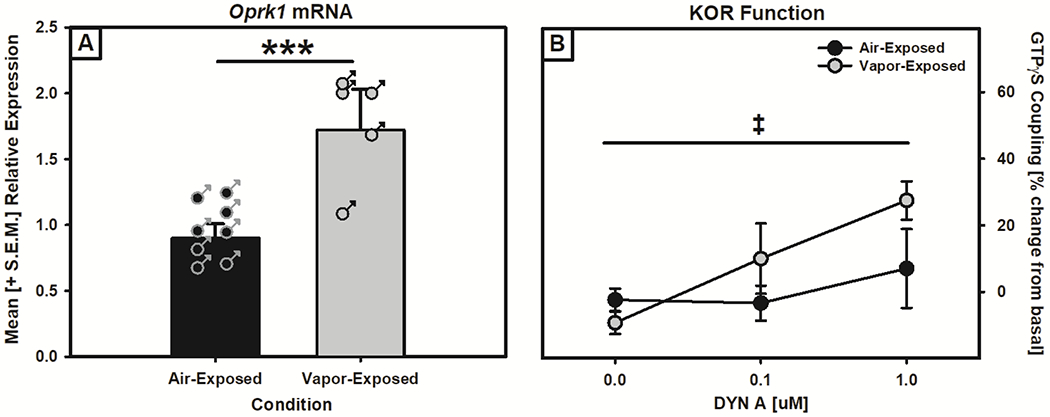
Panel A: Oprk1 mRNA expression in the VTA of air- and vapor-exposed rats as measured by RT-qPCR. Oprk1 mRNA showed increased expression in the VTA of ethanol vapor-exposed rats (***p < 0.01, main effect of exposure condition). Panel B: KOR function in the VTA of air- and vapor-exposed rats as measured by DYN-A stimulated GTPγS coupling. There was a trend towards increased KOR function in ethanol vapor-exposed compared to air-exposed rats (trend towards a DYN A x Exposure interaction, ‡p = 0.054).
3.2. Experiment 2: TH::Cre phenotyping, viral optimization, and effect of VTA Oprk1 overexpression on alcohol self-administration
In the TH::Cre alcohol phenotyping assessment, three females (two air-exposed and one vapor-exposed) were unable to complete the entire regimen which resulted in n=16 and n=17 for the air-and vapor-exposed conditions respectively. Both male and female TH::Cre rats acquired alcohol self-administration and showed ‘typical’ ethanol vapor-induced escalation (Williams et al., 2012; main effects of Session (F (1, 29) = 18.932, p = 0.000153), Exposure (F (1, 29) = 6.849, p = 0.014), and an Exposure x Session interaction (F (1, 29) = 12.725, p = 0.00127), but no sex differences (F (1, 29) = 1.479, p = 0.23; see Supplemental Fig. S1). In the pilot viral infusion and efficacy experiments, to initially confirm VTA-specific TH+/AAV colocalization, unilateral intra-VTA viral infusions were conducted, and fluorescent immunohistochemistry and microscopy were utilized to visualize TH+ neurons (red) with viral infection (green) that showed colocalization (yellow) when overlayed (see Fig. 2). Once the VTA viral infusion parameters were established, male and female TH::Cre rats were bilaterally infused with an active (AAV5-Ef1a-OPRK1-DIO-EYFP) or control (AAV5-Ef1a-DIO-EYFP) viral construct in the VTA so that Oprk1 mRNA expression could be assessed by RT-qPCR. Two males (one from each viral condition) were lost during surgery yielding n = 7-9/sex. A significant main effect of Viral Condition (F (1, 12) = 47.943, p = 0.00016; see Supplemental Fig. S2) was observed in which the active viral construct increased Oprk1 mRNA expression with no sex differences in Oprk1 mRNA expression (F (1,12) = 0.942, p = 0.35).
Figure 2: Viral Expression in TH Positive Neurons.
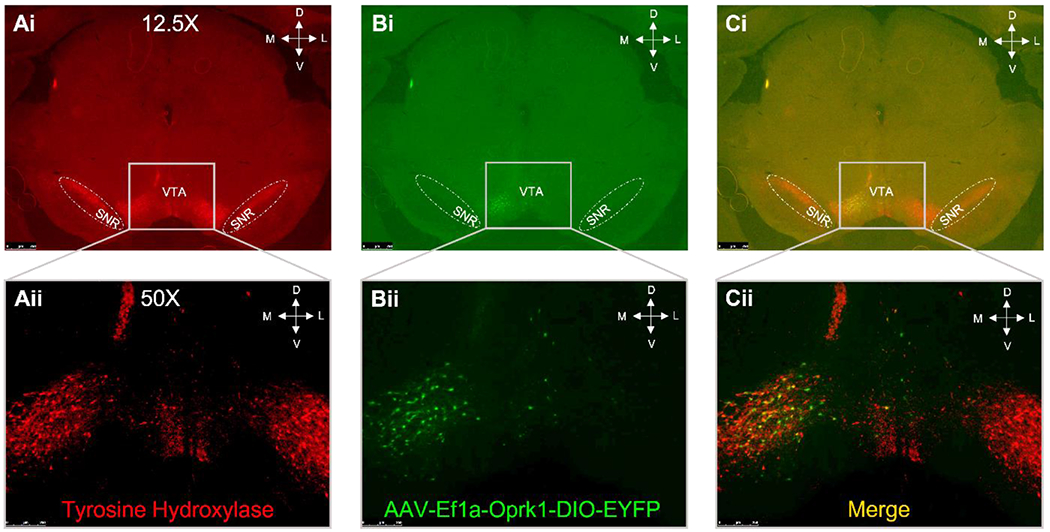
Unilateral infusion of a floxed Oprk1 AAV into the VTA of TH::Cre rats showed VTA specificity. Panel A: TH positive neurons were visualized using fluorescent immunochemistry (red color). Panel B: The viral spread was visualized by evaluating YFP fluorescence (green color). Panel C: The merged images demonstrate specificity in the VTA that is distinct from the TH positive substantia nigra neurons (SNR).
Of the animals that completed the entire Oprk1 overexpression and alcohol self-administration experiment regimen (N=32; two males and two females were lost due to complications during surgery or prior to the sixth week post-virus infusion experimental completion point), the histological assessment resulted in five males infused with the active viral construct being removed from the analysis due to infusion misplacement outside of the VTA, but those misplacement animals were included as a negative control in the behavioral data. Following histological removal, both male (n=6) and female (n=7) TH::Cre rats receiving the active viral construct in the VTA showed a significant increase in Oprk1 mRNA in the VTA compared to the male (n=6) and female (n=8) TH::Cre rats that received the control virus (F (1,23) = 447.421, p = 1.42xE−16; see Fig. 3A) with no sex differences (F (1,27) = 9.0xE−6, p=0.998) or Viral Condition x Sex interaction F (1,23) = 0.043, p = 0.837). The complementary self-administration of the animals showed that after 28 days of viral incubation and testing during the fifth and sixth week, as apparent in Fig. 3B, alcohol self-administration was significantly increased in the active viral vs control viral infusion group as confirmed by the mixed-model ANOVA showing main effects of Session (F (9, 207) = 2.521, p = 0.009) and Viral Condition (F (1, 23) = 7.259, p = 0.013), as well as a trend towards a Session x Viral Condition interaction (F (9, 207) = 1.905, p = 0.053) which indicated that the active viral group increased their self-administration over the sessions differentially from the control condition. Interestingly, there was a main effect of Sex (F (1,23) = 11.245; p = 0.006) on alcohol self-administration, but no Sex x Viral Condition interaction (F (1,27) = 2.034, p = 0.167) indicating that overall, females self-administered more alcohol (see Supplemental Fig. S3), but not when accounting for viral condition. When assessing water self-administration under the same parameters, the mixed model ANOVA showed there were no main effects or interactions (see Supplemental Fig. S4) due to viral condition or sex over the post-viral self-administration sessions. Taken together, these data indicate that VTA Oprk1 overexpression can induce escalated alcohol self-administration in non-dependent animals.
Figure 3: Oprk1 mRNA expression and alcohol self-administration in TH::Cre rats following VTA viral infusions.
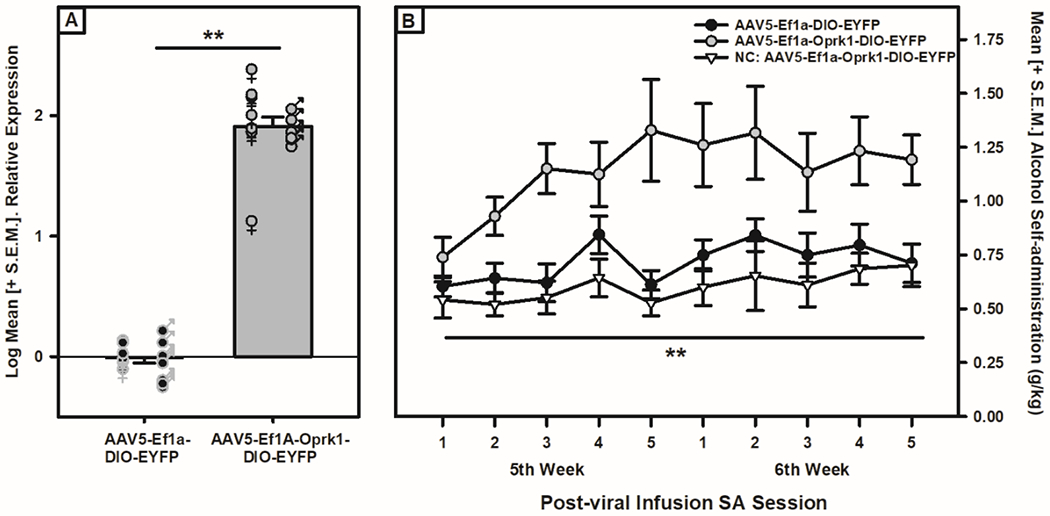
Panel A: Oprk1 mRNA expression in VTA of male and female TH::Cre rats. Oprk1 mRNA expression in the VTA of both male and female rats was increased when exposed to the active AAV5-Ef1a-OPRK1-DIO-EYFP virus when compared to the control AAV5-Ef1a-DIO-EYFP virus (main effect of Viral Condition, **p < 0.01). Panel B: Alcohol self-administration (g/kg) in male and female transgenic TH::Cre rats. Alcohol self-administration levels following 28 days of viral incubation during the 5th and 6th week post-viral infusion demonstrated a significant increase (main effect of Viral Condition, **p < 0.01) in the active (AAV5-Ef1a-OPRK1-DIO-EYFP) viral infusion group when compared to the control (AAV5-Ef1a-DIO-EYFP). The negative control group (NC, misplaced active virus infusions outside the VTA) was included as a comparison.
3.3. Experiment 3: Overexpression of VTA Oprk1, somatic signs of withdrawal and negative affective-like behavior
Following viral infusion and a 28-day incubation period, male and female TH::Cre rats were tested for somatic signs of withdrawal, as well as depressive-like and anxiety-like behavior in the FST and EPM, respectively. Four animals (two males and two females from the control conditions) did not survive the viral infusion surgery or were removed due to post-surgical complications. Upon completion of the behavioral testing, the multivariate ANOVA assessment of RT-qPCR VTA Oprk1 mRNA ΔΔCt values indicted that the active viral (n=18) group showed significantly elevated Oprk1 mRNA expression when compared to the control infusion (n=14) group (F (1, 28) = 824.69, p = 2.57xE−22; see Fig. 4A) with no sex differences in viral efficacy (F (1, 28) = 0.297, p = 0.59). The assessment of somatic signs of withdrawal showed a lack of a Viral Condition-mediated main effect (F (1, 28) = 0.13, p=0.721; see Fig. 4B) and no effect of sex or a Sex x Viral Condition interaction (F (1, 28) = 1.370, p= 0.252 and F (1, 28) = 0.338, p= 0.566, respectively). In the FST examination, significant increases in immobility were substantiated using a multivariate ANOVA by the main effect of Viral Condition on immobility (F (1, 28) = 4.843, p = 0.036; see Fig. 4C), but no differences in distance traveled (F (1, 28) = 0.003, p=0.957) and no sex differences in immobility (F (1,28) = 2.18, p=0.15) were observed. Although the total distance traveled was not affected by viral condition, there was a main effect of Sex on distance traveled (F (1, 32) = 12.168, p = 0.002) reflecting female TH::Cre rats showing more total distance traveled than male TH::Cre rats (see Supplemental Fig. S5). Finally, when tested in the EPM, the multivariate ANOVA showed that rats infused with the active virus had no differences in percent open-arm time, open-arm entries or closed-arm entries (F (1, 28) = 0.166, p = 0.687; F (1, 28) = 0.624, p = 0.436; F (1, 28) = 0.043, p = 0.836, respectively; see Supplemental Fig. S6), although there was a significant decrease in the total distance traveled (F (1, 28) = 16.881, p = 0.000314; see Fig. 4D). In the EPM, there were no sex differences in percent open-arm time, open-arm entries, closed-arm entries or distance traveled (F (1, 28) = 0.366, p = 0.550; F (1, 28) = 0.138, p = 0.713; F (1, 28) = 2.194, p = 0.150; F (1,28) = 1.517, p= 0.228, respectively) or Sex x Viral Condition interactions (F (1, 28) = 0.027, p = 0.871; F (1, 28) = 1.418, p = 0.244; F (1, 28) = 2.526, p = 0.123; F (1,28) = .049, p= 0.827, respectively). These data support the interpretation VTA Oprk1 overexpression-induced increased depressive-like behavior in the FST, but not anxiety-like behavior in the EPM, in non-dependent TH::Cre rats.
Figure 4: VTA Oprk1 overexpression induces depressive-like behavior in non-dependent TH::Cre rats.
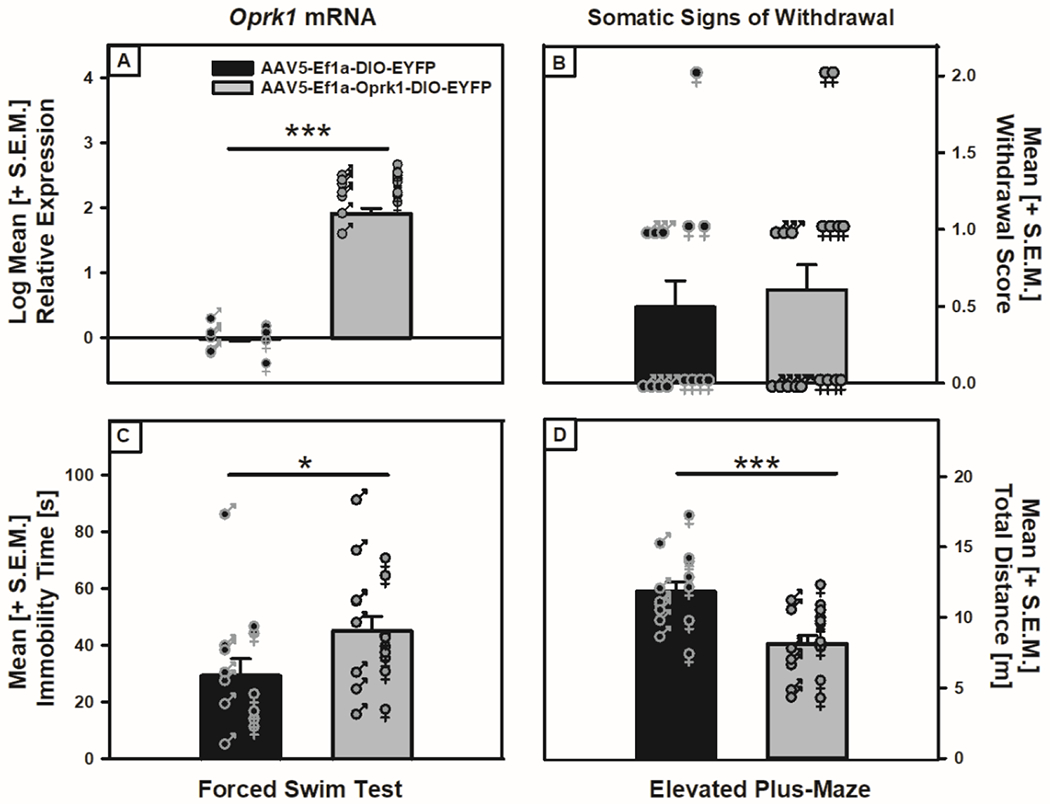
Panel A: Oprk1 mRNA in the VTA was increased (main effect of Viral Condition, ***p < 0.001) following infusion of the active viral construct (AAV5-Ef1a-Oprk1-DIO-EYFP) compared to the control virus (AAV5-Ef1a-DIO-EYFP). Panel B: Somatic withdrawal scores for animals infused with the active virus or control virus showed no differences between conditions. Panel C: Rats infused with the active viral construct showed increased immobility when compared to those receiving control viral infusions (main effect of Viral Condition, *p < 0.05). Panel D: TH::Cre rats infused with the active viral construct decreased their distance traveled when compared to those receiving control viral infusions (main effect of Viral Condition, ***p < 0.001).
3.4. Experiment 3: Overexpression of VTA Oprk1 and working memory performance
When evaluating whether overexpression of Oprk1 in the VTA could impact executive function and induce a change in working memory performance, three rats (one male and two females) from the control viral group did not complete the experimental regimen due to surgical or post-surgical complications which resulted in n=8 for the active viral group and n=5 for the control group. The two-way ANOVA utilized to assess VTA Oprk1 mRNA expression showed a main effect of Viral Condition (F (1, 9) = 10.694, p = 0.01; see Fig. 5A) with no effect of Sex (F (1, 9) = 0.061, p = 0.81). When assessing working memory performance, there were no Viral Condition or Sex differences in the number of sessions to reach criterion (F (1, 9) = 0.388, p = 0.549 and F (1,9) = 0.002, p = 0.963, respectively) for advancing to the multiple delay challenge. There was a main effect of Delay (F (4, 36) = 4.419, p = 0.005; see Fig. 5B) reflecting that as the delays between the forced and free choice runs increased, both control and active virus groups showed a decrease in correct responses. However, there were no main effects of Viral Condition (F (1, 9) = 0.866, p = 0.376) or Sex (F (1, 9) = 0.283, p = 0.608) or interactions on performance for any of the delays. These data imply that VTA Oprk1 expression levels are not involved in working memory performance.
Figure 5: Effect of VTA Oprk1 overexpression on working memory performance of non-dependent TH::Cre rats.
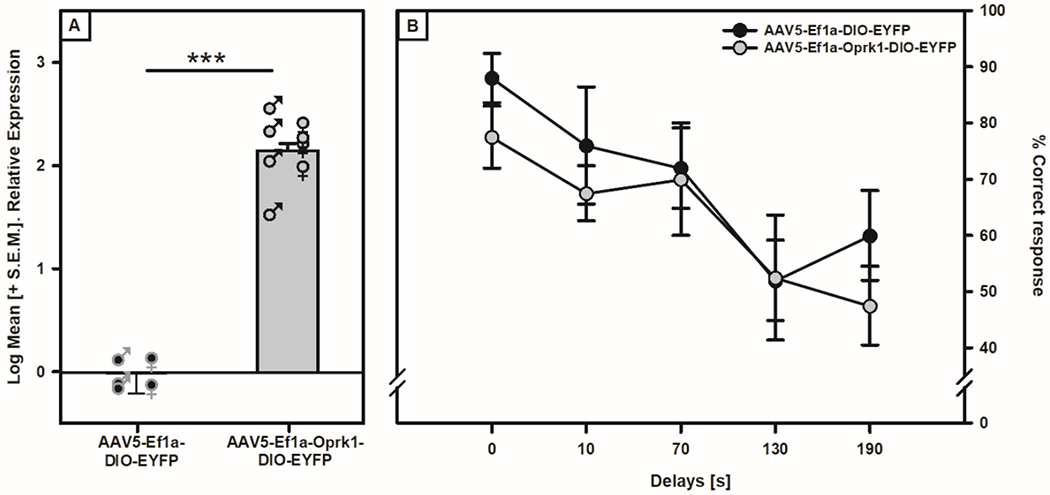
Panel A: Oprk1 mRNA expression in VTA of TH::Cre rats was increased (main effect of Viral Condition, ***p< 0.001) when exposed to the active virus (AAV5-Ef1a-Oprk1-DIO-EYFP) compared to the control virus (AAV5-Ef1a-DIO-EYFP). Panel B: Four weeks after viral infusions the animals were tested in the DNMST. Compared with the animals receiving the control viral infusions, the animals receiving active viral infusion in the VTA showed no significant difference in working memory performance at any delay tested.
4. Discussion
Mesocorticolimbic DA projections originating in the VTA are heavily involved in motivation, emotion, and executive function and are a substrate of addictive behaviors (Morales and Margolis, 2017). These mesocorticolimbic DA pathways can be regulated by KORs through presynaptically and somatodendritic mechanisms (Margolis et al., 2003; Margolis et al., 2006a; Rose et al., 2016; Spanagel et al., 1992) and have been implicated in alcohol use disorder (Karkhanis et al., 2017; Sirohi et al., 2012; Walker et al., 2012). To test the hypothesis that VTA KORs could be dysregulated in alcohol dependence and putatively contribute to the expression of AUD phenotypes, alcohol dependence was induced using chronic intermittent ethanol vapor exposure (O’Dell et al., 2004; Rogers et al., 1979; Williams et al., 2012). To accomplish this, the VTA collected during acute withdrawal from air- and vapor-exposed male rats and Oprk1 mRNA expression and KOR function were assessed using RT-qPCR and DYN A-stimulated GTPγS assays, respectively. Oprk1 mRNA expression was significantly increased in the VTA of vapor-exposed animals compared to air-exposed animals (Fig. 1A) with a concomitant trend towards increased DYN A-induced [35S] GTPγS coupling (Fig. 1B). This indicates that both Oprk1 gene expression and KOR function were enhanced in alcohol dependence. It must be noted that these VTA Oprk1 mRNA and KOR function data were only determined in male rats and that it is unknown whether such increases also occur in female alcohol-dependent rats during withdrawal. However, given that effects observed in the present experiments tested hypotheses related to intra-VTA Oprk1 overexpression in both males and females and saw complementary significant effects, one could assume that the changes observed in the VTA of male rats would also be observed in female rats.
To investigate the functional implications of increased intra-VTA Oprk1 mRNA expression in alcohol dependence-like phenotypes, namely altered motivational, emotional/negative affective and executive function domains that are hallmarks of alcohol dependence (Erikson et al., 2018; Kissler et al., 2014; Nealey et al., 2011; Walker and Koob, 2008; Wei et al., 2022), we leveraged Cre-Lox gene manipulation technology. More specifically, to attempt to recapitulate phenotypes of alcohol dependence we utilized non-dependent transgenic TH::Cre rats in combination with VTA-specific infusions of a floxed Oprk1 viral construct (AAV5-Ef1a-OPRK1-DIO-EYFP) that overexpressed Oprk1 in the presence of Cre-recombinase that is specifically located in neurons containing the TH promotor region (e.g., in the VTA, dopamine neurons). Use of this approach allows for conditional (cell type specific based on presence of TH) and inducible (initiation of transcriptional regulation upon adulthood once viral construct is infused) regulation of gene expression.
The initial behavioral phenotyping of male and female TH::Cre rats showed that they acquired alcohol self-administration and displayed escalated alcohol self-administration during acute withdrawal following alcohol dependence induction using an intermittent ethanol vapor exposure paradigm (Fig. S1) at levels consistent with the literature (e.g., Williams et al., 2012). Furthermore, when TH::Cre rats were infused in the VTA (using the parameters identified in the pilot VTA infusion targeting experiments; Fig. 2) with the active (AAV5-Ef1a-OPRK1-DIO-EYFP) or control (AAV5-Ef1a-DIO-EYFP) viral constructs, significant overexpression of Oprk1 mRNA was observed in the VTA (see Supplemental Fig. S3). These bilateral infusion parameters were used in the subsequent experiments evaluating whether VTA Oprk1 overexpression could recapitulate phenotypes of alcohol dependence in the form of escalated alcohol self-administration, increased negative emotional / affective-like behavior and compromised executive function in non-dependent TH::Cre rats. Although the correlation between mRNA and receptor protein levels can vary, with some estimates suggesting up to 40% of protein production can be correlated with mRNA levels, specific mRNA’s coding for a particular protein are typically considered an excellent representation for the coded protein (Vogel and Marcotte, 2012). Therefore, the viral infusions carried out in these experiments should have translated into observable phenotypes if VTA Oprk1 mRNA expression underlies specific phenotypes of alcohol dependence.
After initial operant alcohol self-administration training and acquiring stability, male and female TH::Cre rats were stereotaxically infused with either an active floxed Oprk1 or control viral construct. Four and five weeks after viral infusion the animals resumed limited access (30-min) operant alcohol self-administration sessions. As there were no Sex x Viral Condition interactions, male and female data for the different viral conditions was pooled. Compared to the control infused TH::Cre rats, the floxed Oprk1 group showed a progressive escalation of operant alcohol self-administration (Fig. 3B) that directly parallels plasticity-dependent operant self-administration test sessions conducted during acute withdrawal after alcohol dependence induction in rats (Smith et al., 2011; see Walker, 2012 for detailed review). Of interest was the inclusion of a negative control group of TH::Cre rats that had misplaced (outside of the VTA) active viral construct infusions and displayed a self-administration profile consistent with the control viral group and indicates the site-specificity of the VTA Oprk1 overexpression effect. Furthermore, Oprk1 overexpression in the VTA did not impact water self-administration when comparing the active and control levels (Fig. S4). When evaluating VTA Oprk1 mRNA expression following the completion of the self-administration component, the active viral group showed significantly increased Oprk1 expression compared to control (Fig. 3A). Therefore, when non-dependent animals have Oprk1 overexpressed selectively in the VTA to mimic the Oprk1 profile produced by intermittent ethanol vapor-exposure (Fig. 1A), they behave in an alcohol dependence-like manner. However, it must be noted that in the present experiments, the level of viral-induced increases in Oprk1 exceeded that identified in alcohol dependent Wistar rats during withdrawal. That said, the overarching hypothesis that increased Oprk1 expression in the VTA can underlie certain phenotypes of alcohol dependence was supported, although future work using multiple dilutions of the viral construct could be conducted to achieve overexpression levels with greater consistency. Moreover, when using KOR antagonists centrally or in mesolimbic DA terminal regions of the extended amygdala in alcohol dependent rats during acute withdrawal, escalated alcohol self-administration is completely ameliorated (Erikson et al., 2018; Kissler et al., 2014; Nealey et al., 2011; Walker and Koob, 2008).
AUD and depression are highly comorbid (Boden and Fergusson, 2011). KOR/DYN signaling can have pro-depressive effects (Berger et al., 2013; McLaughlin et al., 2003) and alcohol dependence dysregulation of KOR/DYN is involved in depressive-like behavior (Jarman et al., 2018). Thus, using the forced swim test, we looked at a depressive-like phenotype following Oprk1 overexpression (Lucki, 1997). Male and female TH::Cre rats receiving the active viral construct showed increased immobility during a 5-minutes test (Fig. 4C). This increased immobility was not confounded by a viral Oprk1-mediated reduction in distance traveled although a sex difference was found with females traveling an overall greater distance than males regardless of the viral condition. Thus, we concluded that the increased immobility induced by the overexpression of Oprk1 in the VTA was a sign of a depressive-like state (Duman, 2010). When tested for anxiety-like behavior in the EPM (Pellow et al., 1985) we found that there were no viral condition-induced changes in percentage open-arm time, open-arm entries or closed-arm entries (Fig. S6), but there was a reduction in distance traveled for animals receiving the active viral construct (Fig. 4D). Given that KOR agonists have been shown to reduce locomotor activity in rats (e.g., Leyton and Stewart, 1992) there was a concern about the potential of non-specific locomotor effects from increasing Oprk1 in the VTA. However, the distance traveled during the FST was not different between active viral and control groups (Fig. S5) and more importantly, those TH::Cre rats infused with the active virus increased alcohol self-administration, which requires active participation of the animals to press the levers. Collectively, this evidence suggests that general locomotion was not impaired by the increased Oprk1 expression and putative increase in KOR signaling. Therefore, it could be that the reduction in distance traveled in the EPM in the active viral group could instead be reflective of an anxiety-like behavioral state (i.e., analogous to minor freezing behavior), although this is speculative. Taken together we concluded that an overexpression of VTA Oprk1 in non-dependent animals was sufficient to induce depressive-like, but not necessarily anxiety-like, behavior and that this effect was modest. Further tests could be conducted in the future to more broadly assess Oprk1 overexpression-induced increases in negative affective-like behavior (comprising both depressive- and anxiety-like behavior) that have been observed in alcohol-dependent animals (Baird et al., 2021; Boden and Fergusson, 2011; Williams et al., 2012).
Somatic signs of withdrawal can be used as a marker of alcohol dependence (Schulteis et al., 1995, Williams et al., 2012). Dependent animals show higher withdrawal scores than non-dependent animals (Williams et al., 2012, Wei et al 2022). Evidence has been shown that dissociates KOR-mediated contributions to somatic vs psychiatric symptoms of dependence and withdrawal depending on the brain site. For example, in the CeA, KOR signaling dissociated motivational from somatic signs of withdrawal (Kissler and Walker, 2016), whereas in the BNST that dissociation was less evident (Erikson et al., 2018). With that being said, since both of those extended amygdala regions are innervated by mesolimbic DA projections originating in the VTA, it was important to assess VTA Oprk1 overexpression on somatic withdrawal signs. Although the animals for which VTA Oprk1 was overexpressed showed a negative emotional / affective-like state, they did not show signs of somatic withdrawal (Fig. 4B). This is consistent with the fact that KOR antagonism in alcohol dependent animal failed to ameliorate a somatic withdrawal state (Kissler et al 2016) but ameliorated dependence-induced escalation of self-administration. Therefore, in the present study, motivational and negative affective-like behavior is dissociated from somatic withdrawal signs following Oprk1 overexpression in the VTA.
Executive function can be impaired by chronic ethanol exposure (Ambrose et al., 2001; Bechara et al., 2001; Noel et al., 2005) and dynorphin release in the PFC was shown to impair cognitive function (Abraham et al., 2021). Recently, Wei and colleagues (Wei et al., 2022) showed that working memory in alcohol-dependent rats was compromised when tested in a DNMST and that KOR/DYN dysregulation was involved and could serve as a therapeutic target to treat those deficits. Since VTA KORs can inhibit dopaminergic projection to the mPFC (Margolis et al., 2006b; Morales and Margolis, 2017) and VTA DA projections to PFC are important for updating working memory (D’Ardenne et al., 2012), we hypothesized that an exacerbated KOR signaling originating from the VTA could induce working memory impairment in non-dependent animals by reducing DA release in the mPFC. However, no difference of performance in the DNMST was measured between animals infused with active virus in comparison to the control group (Fig. 5B) even though VTA Oprk1 mRNA overexpression was confirmed by RT-qPCR (Fig. 5A). This implies that VTA Oprk1-containing DA neurons projecting to mPFC are not involved in the working memory deficits measured in alcohol dependent animals (Wei et al., 2022). However, DA acting through D1 receptor subtypes in the mPFC have been shown to be essential in the retrieval-induced forgetting process (Gallo et al., 2022). Even though mPFC KORs can alter place conditioning via alterations in DA (Tejeda et al., 2013), it is possible that mPFC DA involved with working memory performance is not affected by VTA Oprk1 overexpression. Indeed, in the Acb, D2 dopamine receptor were involved in avoidance learning (while D1 were not) and regulated by VTA KOR (Hikida et al., 2013; Robble et al., 2020). Al-Hasani and colleagues (Al-Hasani et al., 2015) demonstrated that two subpopulations of dynorphin neurons can promote aversion and reward, showing that circuit and region-specific signaling can impact KOR-dependent behaviors. Whether such a distinction of receptor subtype and/or pathway involving VTA Oprk1-regulated working memory process takes place here is currently unknown.
In conclusion we showed that following intra-VTA infusion of a floxed Oprk1 viral construct, TH::Cre rats displayed increased Oprk1 mRNA expression consistent with that observed in alcohol dependence. In non-dependent TH::Cre rats, intra-VTA Oprk1 overexpression induced escalated alcohol self-administration and depressive-like phenotypes that are observed in individuals suffering from AUD. These findings emphasis the role of VTA in the expression of AUD phenotypes and show the potential of the KOR/DYN system as a therapeutic target in the treatment of AUD.
Supplementary Material
Highlights:
Chronic intermittent ethanol vapor increased Oprk1 (kappa opioid receptor gene) mRNA expression and kappa opioid receptor function in the ventral tegmental area (VTA) of Wistar rats.
In transgenic TH::Cre rats, inducible and tyrosine hydroxylase-containing neuron (TH+) conditional overexpression of VTA Oprk1 resulted in escalated operant alcohol self-administration without impacting water self-administration.
Inducible Oprk1 overexpression conditionally in TH+ VTA neurons promoted increased depressive-like, but not anxiety-like, behavior in TH::Cre rats.
Working memory performance was not impacted by inducible and conditional VTA Oprk1 overexpression in TH::Cre rats.
Acknowledgements
Support for this research was provided by R01AA020394 from the National Institute on Alcohol Abuse and Alcoholism awarded to BMW, grants from the Washington State University Alcohol and Drug Abuse Research Program according to the State of Washington Initiative Measure No. 171 awarded to BMW, and R37-DA033396 and P30-DA048736 from the National Institute on Drug Abuse to MRB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, National Institutes of Health, or the State of Florida. The authors do not have any biomedical financial interests or potential conflicts of interest to report in relation to the present manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Credit Statement: BMW, GS, GW, and GL were responsible for the study concept and design. GW, BG, GS, GC, and GL optimized experimental procedures and collected the animal data. MRB and AS developed, created, and provided the viral constructs and participated in the behavioral optimization of those constructs. SS performed the GTPγS experiment. GL and BMW conducted the final data analysis and interpreted the findings. GL and BMW drafted the manuscript. GL, GS, GW, AS, GC, SS, BG, MRB and BMW contributed to revisions of the manuscript. All authors reviewed the content and approved the final version for publication.
5. References
- Abraham AD, Casello SM, Schattauer SS, Wong BA, Mizuno GO, Mahe K, Tian L, Land BB, Chavkin C, 2021. Release of endogenous dynorphin opioids in the prefrontal cortex disrupts cognition. Neuropsychopharmacology 46, 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M, Egli M, Cho YE, Song BJ, Noronha A, 2018. Medications for alcohol use disorders: An overview. Pharmacol Ther 185, 64–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR, 2015. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L, 1988. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27, 1–39. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ, 1990. Basic local alignment search tool. J. Mol. Biol 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Amalric M, Cline EJ, Martinez JL Jr., Bloom FE, Koob GF, 1987. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 91, 14–19. [DOI] [PubMed] [Google Scholar]
- Ambrose ML, Bowden SC, Whelan G, 2001. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcoholism-Clinical and Experimental Research 25, 185–191. [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F, 2013. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol 18, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird MA, Hsu TY, Wang R, Juarez B, Zweifel LS, 2021. kappa Opioid Receptor-Dynorphin Signaling in the Central Amygdala Regulates Conditioned Threat Discrimination and Anxiety. eNeuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE, 2001. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39, 376–389. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L, 2015. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AL, Williams AM, McGinnis MM, Walker BM, 2013. Affective cue-induced escalation of alcohol self-administration and increased 22-kHz ultrasonic vocalizations during alcohol withdrawal: role of kappa-opioid receptors. Neuropsychopharmacology 38, 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, 2011. Alcohol and depression. Addiction 106, 906–914. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, 2022. Endogenous opiates and behavior: 2020. Peptides 151, 170752. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C, 2007. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J. Neurosci 27, 11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C, 2010. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C, 2011. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71, 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Shippenberg TS, 2001. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology (Berl) 155, 35–42. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM, 2006. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther 316, 440–447. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Miczek KA, 2010. Ascent of the kappa-opioid receptor in psychopharmacology. Psychopharmacology (Berl) 210, 107–108. [DOI] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I, 2010. Antidepressant-like effects of kappa-opioid receptor antagonists in wistar kyoto rats. Neuropsychopharmacology 35, 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A, 1982. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215, 413–415. [DOI] [PubMed] [Google Scholar]
- Chen F, Lawrence AJ, 2000. Effect of chronic ethanol and withdrawal on the mu-opioid receptor- and 5-Hydroxytryptamine(1A) receptor-stimulated binding of [(35)S]Guanosine-5′-O-(3-thio)triphosphate in the fawn-hooded rat brain: A quantitative autoradiography study. J. Pharmacol. Exp. Ther 293, 159–165. [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C. [Google Scholar]
- Cowen MS, Lawrence AJ, 2001. Alterations in central preproenkephalin mRNA expression after chronic free-choice ethanol consumption by fawn-hooded rats. Alcohol Clin. Exp. Res 25, 1126–1133. [PubMed] [Google Scholar]
- D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD, 2012. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A 109, 19900–19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C, 2005. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin. Exp. Res 29, 1965–1975. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K, 2001. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol and Alcoholism 36, 249–255. [DOI] [PubMed] [Google Scholar]
- Duman CH, 2010. Models of depression. Vitam. Horm 82, 1–21. [DOI] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M, 2008. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol. Psychiatry 64, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Kenna GA, Swift RM, Leggio L, 2011. Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Curr Pharm Des 17, 1323–1332. [DOI] [PubMed] [Google Scholar]
- Erikson CM, Wei G, Walker BM, 2018. Maladaptive behavioral regulation in alcohol dependence: Role of kappa-opioid receptors in the bed nucleus of the stria terminalis. Neuropharmacology 140, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Fraser GL, 1994. Mu-,Delta-,Kappa-Opioid Receptors and Their Subtypes - a Critical-Review with Emphasis on Radioligand Binding Experiments. Neurochemistry International 24, 401–426. [DOI] [PubMed] [Google Scholar]
- Gallo FT, Zanoni Saad MB, Silva A, Morici JF, Miranda M, Anderson MC, Weisstaub NV, Bekinschtein P, 2022. Dopamine Modulates Adaptive Forgetting in Medial Prefrontal Cortex. J Neurosci 42, 6620–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF, 2008. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33, 2474–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J, 1996. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch. Gen. Psychiatry 53, 250–257. [DOI] [PubMed] [Google Scholar]
- Harrison C, Traynor JR, 2003. The [35S]GTPgammaS binding assay: approaches and applications in pharmacology. Life Sci 74, 489–508. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, 2006. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol. Ther 111, 855–876. [DOI] [PubMed] [Google Scholar]
- Herz A, 1997. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 129, 99–111. [DOI] [PubMed] [Google Scholar]
- Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, Nakanishi S, 2013. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci U S A 110, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipolito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Moron JA, 2015. Inflammatory Pain Promotes Increased Opioid Self-Administration: Role of Dysregulated Ventral Tegmental Area mu Opioid Receptors. J. Neurosci 35, 12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman SK, Haney AM, Valdez GR, 2018. Kappa opioid regulation of depressive-like behavior during acute withdrawal and protracted abstinence from ethanol. PLoS One 13, e0205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, McCane S, Williams LS, Mason D, Cummings R, Lawrence A, 1998. The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: preclinical studies in ethanol-preferring and outbred Wistar rats. Alcohol Clin. Exp. Res 22, 2174–2185. [PubMed] [Google Scholar]
- Karkhanis A, Holleran KM, Jones SR, 2017. Dynorphin/Kappa Opioid Receptor Signaling in Preclinical Models of Alcohol, Drug, and Food Addiction. International review of neurobiology 136, 53–88. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Al-Hasani R, 2020. Dynorphin and its role in alcohol use disorder. Brain Res 1735, 146742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ, 1990. The Self Regulation and Self Medication Factor in alcoholism and the addictions. [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM, 2014. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Walker BM, 2016. Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala kappa-Opioid Receptors. Neuropsychopharmacology 41, 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA, 2010. Dynorphin, stress, and depression. Brain Res 1314, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2014. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb. Clin. Neurol 125, 33–54. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008. Addiction and the brain antireward system. Annu. Rev. Psychol 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ, 1997. The neurobiology of drug addiction. Journal of Neuropsychiatry and Clinical Neurosciences 9, 482–497. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C, 2008. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci 28, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Stewart J, 1992. The stimulation of central kappa opioid receptors decreases male sexual behavior and locomotor activity. Brain Res 594, 56–74. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I, 2000. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol 22, 165–171. [DOI] [PubMed] [Google Scholar]
- Liu D, Gu X, Zhu J, Zhang X, Han Z, Yan W, Cheng Q, Hao J, Fan H, Hou R, Chen Z, Chen Y, Li CT, 2014. Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 346, 458–463. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lucki I, 1997. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioural Pharmacology 8, 523–532. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr., Jones RM, Portoghese PS, Carlezon WA Jr., 2003. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305, 323–330. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL, 2003. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J. Neurosci 23, 9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL, 2006a. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103, 2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL, 2006b. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577, 907–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C, 2005. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin. Exp. Res 29, 1821–1828. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C, 2006. A microdialysis profile of dynorphin A(1-8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin. Exp. Res 30, 982–990. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C, 2003. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 169, 60–67. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C, 2004. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience 127, 777–784. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF, 1998. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18, 135–174. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C, 2003. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci 23, 5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. , 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535. [DOI] [PubMed] [Google Scholar]
- Miller EK, 2000. The prefrontal cortex and cognitive control. Nat Rev Neurosci 1, 59–65. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY, 1980. From Motivation to Action - Functional Interface between the Limbic System and the Motor System. Progress in Neurobiology 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL, 2004. Actual causes of death in the United States, 2000. JAMA 291, 1238–1245. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC, 1994. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341, 33–38. [DOI] [PubMed] [Google Scholar]
- Morales M, Margolis EB, 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18, 73–85. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A, 1985. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 86, 274–280. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM, 2011. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA, 2006. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, d’Acremont M, Colmant M, Hanak C, Pelc I, Verbanck P, Bechara A, 2005. Cognitive biases toward alcohol-related words and executive deficits in polysubstance abusers with alcoholism. Addiction 100, 1302–1309. [DOI] [PubMed] [Google Scholar]
- Nygard SK, Hourguettes NJ, Sobczak GG, Carlezon WA, Bruchas MR, 2016. Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala. J Neurosci 36, 9937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF, 2004. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin. Exp. Res 28, 1676–1682. [DOI] [PubMed] [Google Scholar]
- Oliva I, Wanat MJ, 2016. Ventral Tegmental Area Afferents and Drug-Dependent Behaviors. Front Psychiatry 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW, 2001. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 21, Rc184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, 1998. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci 19, 94–98. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2005. The rat brain in stereotaxic coordinatesPAXINOS2005. Elsevier, Academic Press, San Diego. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr., 2001. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci 21, 7397–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R, 1997. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci. Lett 238, 13–16. [DOI] [PubMed] [Google Scholar]
- Robble MA, Bozsik ME, Wheeler DS, Wheeler RA, 2020. Learned avoidance requires VTA KOR-mediated reductions in dopamine. Neuropharmacology 167, 107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE, 1979. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol 27, 466–486. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR, 2016. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin A, Lindholm S, Franck J, Georgieva J, 1999. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett 275, 1–4. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD, 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, 2012. The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA, 2019. Results from the 2019 National Survey on Drug Use and Health. Office of Applied Studies, DHHS Publication, Rockville, MD. [Google Scholar]
- Sánchez-Peña JF, Alvarez-Cotoli P, Rodríguez-Solano JJ, 2012. Psychiatric disorders associated with alcoholism: 2 year follow-up of treatment. Actas Esp Psiquiatr 40, 129–135. [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF, 1995. Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. U. S. A 92, 5880–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A, 1986. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res. Monogr 75, 563–566. [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, Helms CM, Grant KA, Jones SR, 2016. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology (Berl) 233, 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Bakalkin G, Walker BM, 2012. Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci 5, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Nealey KA, Wright JW, Walker BM, 2011. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem 96, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS, 1992. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. U. S. A 89, 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Backman CM, Chefer V, O’Donnell P, Shippenberg TS, 2013. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38, 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Wang H, Flores RJ, Yarur HE, 2021. Dynorphin/Kappa-Opioid Receptor System Modulation of Cortical Circuitry. Handb Exp Pharmacol 271, 223–253. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr., 2004. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172, 463–470. [DOI] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT, 2016. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev 68, 419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R, 1999. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience 91, 971–977. [DOI] [PubMed] [Google Scholar]
- Vats ID, Dolt KS, Kumar K, Karar J, Nath M, Mohan A, Pasha MA, Pasha S, 2008. YFa, a chimeric opioid peptide, induces kappa-specific antinociception with no tolerance development during 6 days of chronic treatment. J. Neurosci. Res 86, 1599–1607. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM, 2012. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R, 2017. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18, 741–752. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP, 1992. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatry 49, 876–880. [DOI] [PubMed] [Google Scholar]
- Walker BM, 2012. Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol 46, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A, 2005. Intra-ventral tegmental area heroin-induced place preferences in rats are potentiated by peripherally administered alprazolam. Pharmacol. Biochem. Behav 82, 470–477. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF, 2007. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin. Exp. Res 31, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF, 2008. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G, 2012. Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol 46, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Sirohi S, Walker BM, 2022. Dysregulated kappa-opioid receptors in the medial prefrontal cortex contribute to working memory deficits in alcohol dependence. Addict Biol 27, e13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR, 2001. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci 937, 1–26. [DOI] [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, Kloss ND, Bilimoria JL, Smith CE, Walker BM, 2012. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 223, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K, 2011. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


