Abstract
Aim/Introduction
To investigate the differences in the clinical significance and glutamic acid decarboxylase autoantibody (GADA) affinity between RIA (RIA‐GADA) and ELISA (ELISA‐GADA) in patients with type 1 diabetes.
Methods
A total of 415 patients with type 1 diabetes were enrolled, including 199 acute‐onset type 1 diabetes, 168 slowly progressive type 1 diabetes (SPIDDM), and 48 fulminant type 1 diabetes. GADA affinity was measured by a competitive binding experiment using unlabeled recombinant human GAD65 protein, and the diagnostic performance of both assays and the relationship between GADA affinity and the decline of fasting C‐peptide (F‐CPR) were examined.
Results
While the ELISA‐GADA displayed a higher sensitivity than the RIA method in diagnosing type 1 diabetes in acute‐onset patients, about 40% of SPIDDM patients with low‐titer RIA‐GADA were determined as negative by the ELISA method. Patients with type 1 diabetes with RIA‐GADA alone had an older age of onset, less diabetic ketoacidosis, a higher BMI, and a higher F‐CPR compared with patients positive for both RIA‐GADA and ELISA‐GADA. Additionally, 36% of RIA‐GADA‐positive patients had low‐affinity GADA (<1010 L/mol), which was significantly higher than in the ELISA‐GADA‐positive patients (4%, P < 0.0001). Furthermore, over a 3 year monitoring period, F‐CPR levels decreased in ELISA‐GADA‐positive SPIDDM, whereas it was maintained in patients with RIA‐GADA alone, regardless of GADA affinity.
Conclusions
These results suggest that bivalent ELISA for GADA is superior to the RIA method in diagnosing type 1 diabetes. Moreover, the diagnostic superiority of the ELISA‐GADA made possible the concurrent identification of SPIDDM patients at high‐risk of early progression, and allowed for more accurate clinical diagnosis and management.
Keywords: Affinity, Autoantibodies, GAD
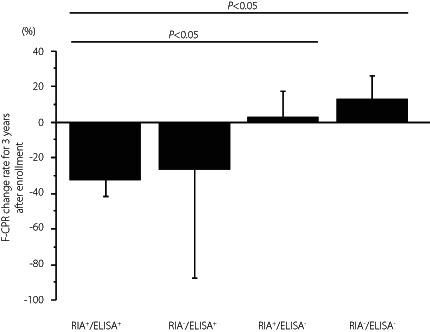
The present study showed that bivalent ELISA for GADA is superior to the RIA method, not only in diagnostic performance of type 1 diabetes but also in identifying patients at high‐risk of early progression in SPIDDM. There was a statistically significant difference in F‐CPR among the four groups of SPIDDM (P = 0.04). The mean F‐CPR in patients with RIA+/ELISA+ (−33.2 ± 9.5%) was significantly greater than that of patients who were positive for RIA‐GADA alone (2.6 ± 14.6%, P < 0.05) and negative for both assays (13.3 ± 12.9%, P < 0.05), but not so when compared with patients with ELISA‐GADA alone.
INTRODUCTION
Type 1 diabetes is an organ‐specific autoimmune disease characterized by pancreatic islet β‐cell destruction, which results in absolute insulin deficiency. Type 1 diabetes is classified into autoimmune‐mediated and idiopathic types in the etiological classification, but clinically, it is divided into three subtypes: acute‐onset type 1 diabetes, slowly progressive type 1 diabetes (SPIDDM), and fulminant type 1 diabetes based on the mode of onset 1 . Both acute‐onset type 1 diabetes and SPIDDM fall into the autoimmune‐mediated type 1 diabetes, whereas fulminant type 1 diabetes is classified as idiopathic type 1 diabetes. One of the hallmarks of autoimmune involvement in type 1 diabetes is the presence of anti‐islet autoantibodies which are important markers for predicting and diagnosing type 1 diabetes. Among them, autoantibodies to GAD (GADA) are most frequently detected in patients with either acute‐onset type 1 diabetes and SPIDDM and are, therefore, the first to be measured in general clinical practice. In line with the various GADA assays that have been developed over the years, the Diabetes Autoantibody Standardization Program or Islet Autoantibody Standardization Program (IASP) was established to improve assay performance and concordance of results among laboratories 2 , 3 . However, current assay performance varies markedly between laboratories 3 , 4 , and even thoroughly validated, frequently used radioligand‐binding assays pick up both disease‐relevant and disease‐irrelevant signals 5 . Other assays include the RIA and ELISA by RSR Ltd (Cardiff, UK), both well‐established tests for the analysis of GADA and widely distributed throughout the world as commercial kits.
For more than two decades, RSR‐RIA has been used in measuring GADA in Japan; however, recently, the distributor of this kit (Cosmic Corporation, Tokyo, Japan) has changed from RSR‐RIA to RSR‐ELISA, making it necessary to revise the interpretation and correspondence, including the positive/negative judgment of the RIA method. Furthermore, it has been reported that there are some patients who are diagnosed as having SPIDDM by the RIA method but who do not progress to an insulin‐dependent state 6 . Therefore, the present study was carried out as a research project under the Committee of Type 1 Diabetes of the Japan Diabetes Society (JDS) to investigate the differences in clinical significance between RSR‐RIA and RSR‐ELISA in Japanese patients with type 1 diabetes, especially concerning patients who showed discrepant results using the two methods. Furthermore, we examined the GADA affinity using a competitive binding assay, and investigated the difference in the GADA affinity identified by the RIA and ELISA methods. Besides this, the clinical usefulness of GADA affinity in predicting the progression of SPIDDM was thoroughly verified using the longitudinal data and samples from the Japanese Type 1 Diabetes Database (TIDE‐J) study 7 ; a multi‐center prospective study in Japan.
MATERIALS AND METHODS
Subjects
Four‐hundred and fifteen patients with type 1 diabetes were recruited from the contributing institutes through the Committee of Type 1 Diabetes of the JDS (n = 187) and the TIDE‐J study (n = 228). The TIDE‐J study is a multi‐center prospective study with the following inclusion criteria: (1) a duration of type 1 diabetes <5 years, and (2) the presence of one or more anti‐islet autoantibodies and/or fasting serum C‐peptide (F‐CPR) levels <1.0 ng/mL 7 . This study comprised 199 patients with acute‐onset type 1 diabetes, 168 with SPIDDM, and 48 with fulminant type 1 diabetes. A diagnosis of type 1 diabetes was made based on the criteria set by the Committee of the JDS 8 , 9 , 10 , while a diagnosis of SPIDDM, irrespective of anti‐islet autoantibody status at the time of the study, was given if patients were positive for GADA and/or islet cell antibodies at any time during the disease course. Details of the patients’ clinical characteristics are shown in Table 1. HLA‐DRB1 and DQB1 data were available in 149 patients with acute‐onset type 1 diabetes, 124 with SPIDDM, and 40 with fulminant type 1 diabetes. Of the 288 patients from the TIDE‐J study, 198 patients (117 acute‐onset type 1 diabetes and 81 SPIDDM) with available C‐peptide data were used in follow‐up studies. The study protocols were approved by the ethics committee of the JDS and each institute participating in this project, and informed consent was obtained from all participants. Serum samples were stored at −20°C until use.
Table 1.
Clinical characteristics
| Acute‐onset type 1 diabetes | SPIDDM | Fulminant type 1 diabetes | |
|---|---|---|---|
| n | 199 | 168 | 48 |
| Female | 121 (61%) | 90 (54%) | 22 (46%) |
| Onset age (years) | 40.3 ± 17.5 | 49.7 ± 14.9 | 43.9 ± 16.4 |
| Duration (years) | 3.9 ± 5.4 | 7.6 ± 9.3 | 3.2 ± 3.7 |
| BMI (kg/m2) | 20.8 ± 3.2 | 22.8 ± 4.1 | 21.5 ± 2.7 |
| DKA at onset (+/−) † | 138/43 | 29/128 | 43/4 |
| Insulin therapy (+/−) | 199/0 | 116/52 | 48/0 |
| HLA‐DRB1*04:05 (+) | 87/298 (29%) | 58/248 (23%) | 26/80 (33%) |
| HLA‐DRB1*09:01 (+) | 112/298 (38%) | 67/248 (27%) | 19/80 (24%) |
| GADA group | |||
| RIA+/ELISA+ | 138 (69%) | 92 (55%) | 1 (2%) |
| RIA+/ELISA− | 9 (4.5%) | 32 (19%) | 7 (15%) |
| RIA−/ELISA+ | 15 (7.5%) | 14 (8%) | 3 (6%) |
| RIA−/ELISA− | 37 (19% | 30 (18%) | 37 (77%) |
Data are n (%) or mean ± SD.
BMI, body mass index; DKA, diabetic ketoacidosis; GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen; SPIDDM, slowly progressive type 1 diabetes.
Data for DKA at onset were unavailable in 18 patients with acute‐onset type 1 diabetes, 11 with SPIDDM, and one with fulminant type 1 diabetes.
Anti‐islet autoantibody measurement
RSR‐RIA‐GADA (RIA‐GADA) were determined by liquid‐phase RIA using 125I‐labeled recombinant human GAD65 as a tracer reagent as described previously 11 . Antigen–antibody complexes were adsorbed onto solid‐phase protein A, and the results were read from a calibration curve constructed in the same run with the calibrators and expressed in U/mL. RSR‐ELISA‐GADA (ELISA‐GADA) were determined using bivalent ELISA using biotinylated GAD65 as described previously 12 . The results were read from a calibration curve constructed in the same run with the calibrators and expressed in U/mL. The cut‐off value for the RIA‐GADA was 1.5 and 5.0 U/mL for ELISA‐GADA. The intra‐ and inter‐assay coefficients of variation for RIA‐GADA were 3.6–3.7% and 5.5–6.9%, respectively, whereas those for ELISA‐GADA were 3.5–8.5% and 5.2–6.4%, respectively 13 . In the 2015 IASP workshop, the assay sensitivities and specificities achieved were 78% and 94% for RIA‐GADA and 86% and 98% for ELISA‐GADA, respectively.
GADA affinity measurement
The GADA affinity assay was carried out by competitive binding experiments with unlabeled recombinant human GAD65 with an assay format identical to that of the RSR‐RIA and RSR‐ELISA. Serum samples (100 μL) were incubated with different concentrations of unlabeled recombinant human GAD65 (10 μL, RSR Ltd) varying from 1.9 × 10−9 to 1.9 × 10−5 mol/L for RIA‐GADA and 7.6 × 10−10 to 3.8 × 10−8 mol/L for ELISA‐GADA for 1 h at RT before GADA measurements. The IC50 and K d values were calculated by nonlinear regression analysis using SigmaPlot software (version 14.5; Systat Software Inc., San Jose, CA, USA). The K d values were determined using the dissociation constants in GADA‐positive patients and ligand concentration for each assay, and the GADA affinity was expressed as the reciprocal of the K d value (L/mol). Subsequently, the displacement curves were computed from the U/mL for each competition reaction with a one‐site binding model. This experiment comprised a total of 155 samples obtained from 133 patients (n = 43 from the JDS committee and n = 70 from the TIDE‐J study) consisting of 42 patients with acute‐onset type 1 diabetes (8 RIA+/ELISA−, 11 RIA−/ELISA+, and 23 RIA+/ELISA+), 62 patients with SPIDDM (29 RIA+/ELISA−, 13 RIA−/ELISA+, and 20 RIA+/ELISA+), and 9 patients with fulminant type 1 diabetes (6 RIA+/ELISA− and 3 RIA−/ELISA+). The titers of RIA‐GADA and ELISA‐GADA were selected to be similar among the subtypes of type 1 diabetes for affinity comparison.
Statistical analysis
All results were expressed as either mean ± SD or ±SE, and the categorical variables were compared using the Chi‐square test and Fisher's exact test where appropriate. Differences in nonparametric data were tested using the Mann–Whitney U test or Kruskal‐Wallis test followed by the Dunn's multiple comparison test, and the correlation between autoantibody titer was analyzed using the Spearman's rank correlation test. Agreement between the RIA‐GADA and ELISA‐GADA was assessed with the κ statistic, which measures the strength of agreement between two raters on a scale from 0 to 1 14 . A value of P less than 0.05 was considered statistically significant. Statistical analysis for this study was performed using StatView statistical software (version 5.0; SAS Institute, Cary, NC, USA) and SigmaPlot ver. 14.5 software.
RESULTS
Assay performance and concordance of RIA‐GADA and ELISA‐GADA
First, the prevalence and concordance of RIA‐GADA and ELISA‐GADA were compared in patients with three subtypes of type 1 diabetes. The distributions are shown in Table 2, more patients were identified as ELISA‐GADA‐positive than RIA‐GADA‐positive in acute‐onset type 1 diabetes, but the prevalence of ELISA‐GADA was lower than that of RIA‐GADA in SPIDDM and fulminant type 1 diabetes. The lower frequency of ELISA‐GADA in SPIDDM was due to patients who were positive for GADA by RIA but were negative with the ELISA kit. This lower frequency was particularly evident in those with lower RIA‐GADA titers. Forty‐two percent (32/76) of SPIDDM patients showed RIA+/ELISA− in RIA‐GADA titers of ≤20 U/mL, which was significantly higher than that in patients with acute‐onset type 1 diabetes in the same titer range (9/72, 13%, P < 0.0001). The agreement between the RIA‐GADA and ELISA‐GADA was 87.9% (κ statistic = 0.675, 95% CI 0.556–0.795) for acute‐onset type 1 diabetes, and 72.6% for SPIDDM (κ statistic = 0.374, 95% CI 0.230–0.519), and 79.2% (κ statistic = 0.063, 95% CI −0.240 to 0.365) for fulminant type 1 diabetes (Table 2). Furthermore, the titers of GADA by the RIA kit were significantly correlated with those by the ELISA kit in patients with acute‐onset type 1 diabetes (r = 0.78, P < 0.0001) and SPIDDM (r = 0.90, P < 0.0001), but not in patients with fulminant type 1 diabetes, when excluding patients whose GADA titers exceeded the assay range as well as those negative for both RIA‐GADA and ELISA‐GADA (Figure 1a–c).
Table 2.
Agreement between the RIA‐GADA and the ELISA‐GADA with sera from type 1 diabetes
| Patients | n | RIA+ (%) | ELISA+ (%) | Agreement (%) | κ statistics (95% CI) |
|---|---|---|---|---|---|
| Acute‐onset type 1 diabetes | 199 | 147 (74) | 153 (77) | 87.9 | 0.675 (0.556–0.795) |
| SPIDDM | 168 | 124 (74) | 106 (63) | 72.6 | 0.374 (0.230–0.519) |
| Fulminant type 1 diabetes | 48 | 8 (17) | 4 (8) | 79.2 | 0.063 (−0.240–0.365) |
Data are n (%).
CI, confidence interval; ELISA, enzyme‐linked immunosorbent assay; RIA, radioimmunoassay; SPIDDM, slowly progressive type 1 diabetes.
Figure 1.
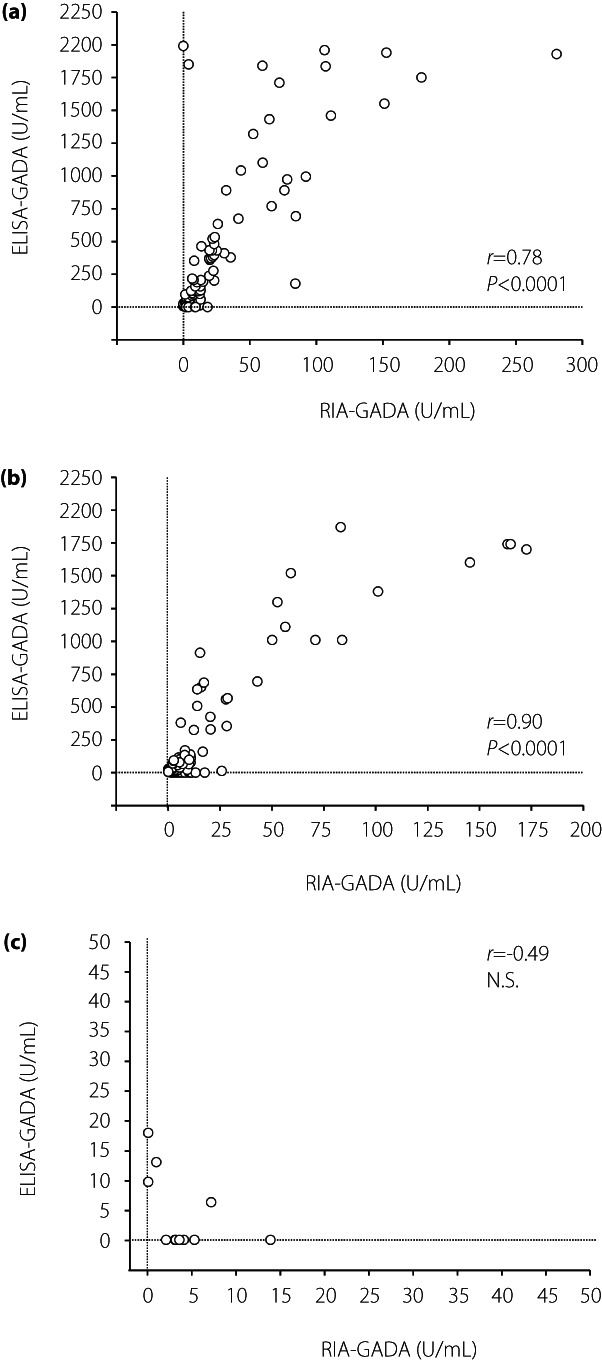
Correlation between the titer of GADA by RIA and ELISA in patients with acute‐onset type 1 diabetes (a), slowly progressive type 1 diabetes (b), and fulminant type 1 diabetes (c). Autoantibody‐positive sera by either assay and those within the assay range were used in this analysis. N.S., not significant.
Patients with RIA‐GADA alone have different clinical characteristics
Patients were divided into four groups according to their RIA/ELISA results for GADA (Table 1) and the clinical and immunogenetic data were compared in patients with acute‐onset type 1 diabetes and SPIDDM, especially in subjects with discrepant GADA results, to examine the clinical significance of RIA‐GADA and ELISA‐GADA. Data were analyzed for acute‐onset type 1 diabetes and SPIDDM combined (Table 3), and then for both subtypes separately (Tables S1 and S2). As shown in Table 3, patients with RIA‐GADA alone had an older age at onset, less diabetic ketoacidosis at onset, were more obese, and had higher F‐CPR levels compared with the other three groups. The former two results were mainly attributed to the clinical features found in SPIDDM (Table S2). Furthermore, the mean GADA level in patients with RIA+/ELISA+ was significantly higher than that in the RIA+/ELISA− group or RIA−/ELISA+ group regardless of subtypes of type 1 diabetes (Tables S1 and S2; P < 0.0001).
Table 3.
Clinical characteristics according to groups in patients with acute‐onset and slowly progressive type 1 diabetes
| RIA+/ELISA+ | RIA+/ELISA− | RIA−/ELISA+ | RIA−/ELISA− | P value* | |
|---|---|---|---|---|---|
| n | 230 | 41 | 29 | 67 | |
| Subtype of type 1 diabetes | |||||
| Acute‐onset | 138 (60%) | 9 (22%) | 15 (52%) | 37 (55%) | <0.0001 |
| SPIDDM | 92 (40%) | 32 (78%) | 14 (48%) | 30 (45%) | |
| Female | 142 (62%) | 23 (56%) | 12 (41%) | 34 (51%) | N.S. |
| Onset age (years) | 42.9 ± 16.2 | 51.4 ± 14.3a | 40.8 ± 18.4 | 47.6 ± 19.1 | 0.0061 |
| Duration (years) | 4.9 ± 7.0 | 8.3 ± 10.2 | 6.0 ± 7.6 | 5.9 ± 8.1 | N.S. |
| DKA at onset (+/−) † | 119/93 | 7/31a | 11/13 | 30/34 | 0.0003 |
| BMI (kg/m2) | 21.3 ± 3.5 | 26.0 ± 4.3a,b,c | 20.6 ± 3.6 | 22.4 ± 3.6 | <0.0001 |
| F‐CPR (ng/mL) | 0.79 ± 0.79 | 2.09 ± 1.40a,b,c | 0.93 ± 0.94 | 1.01 ± 1.23 | <0.0005 |
| RIA‐GADA (U/mL) | 67.2 ± 84.5 | 5.3 ± 4.1 | N.A. | N.A. | <0.0001 |
| ELISA‐GADA (U/mL) | 898.7 ± 863.4 | N.A. | 82.2 ± 367.0 | N.A. | <0.0001 |
| HLA‐DRB1*04:05 (+) | 98/352 (28%) | 8/56 (14%) | 11/46 (24%) | 27/92 (29%) | N.S. |
| HLA‐DRB1*09:01 (+) | 119/352 (34%) | 14/56 (25%) | 16/46 (35%) | 30/92 (33%) | N.S. |
Data are n (%) or mean ± SD. Autoantibody levels were determined in patients positive for corresponding autoantibody.
BMI, body mass index; DKA, diabetic ketoacidosis; ELISA, enzyme‐linked immunosorbent assay; F‐CPR, fasting C‐peptide; GADA, glutamic acid decarboxylase autoantibody; HLA, human leukocyte antigen; N.A., not applicable; N.S., not significant; RIA, radioimmunoassay; SPIDDM, slowly progressive type 1 diabetes.
*P value by Kruskal‐Wallis test or Chi‐square test; a P < 0.005 vs RIA+/ELISA+, b P < 0.001 vs RIA−/ELISA+, c P < 0.001 vs RIA−/ELISA− by Dunn's multiple comparison.
Data for DKA at onset were unavailable in 18 patients with RIA+/ELISA+, 3 with RIA+/ELISA−, 5 with RIA−/ELISA+, and 3 with RIA−/ELISA−.
A higher BMI and higher F‐CPR in patients with RIA‐GADA alone were observed in both patients with acute‐onset type 1 diabetes and SPIDDM (Figures 2 and 3). However, regarding gender difference, the duration of diabetes, and the frequencies of disease‐susceptible HLA‐DRB1*04:05 and ‐DRB1*09:01, there were no significant differences among the four groups, even when analyzed separately for the acute‐onset type 1 diabetes and SPIDDM (Tables S1 and S2). In contrast, the clinical and immunological characteristics in patients with ELISA‐GADA alone were similar to those in patients positive for both RIA‐GADA and ELISA‐GADA (Table 3, Figures 2 and 3).
Figure 2.
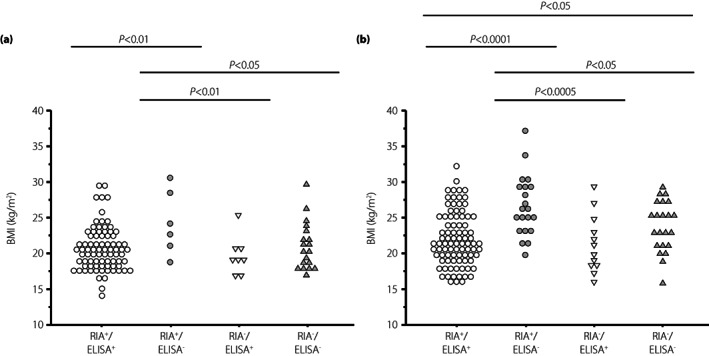
Comparison of BMI values in four groups of patients with acute‐onset type 1 diabetes (a) and slowly progressive type 1 diabetes (b): GADA+ in both RIA and ELISA, RIA‐GADA+ only, ELISA‐GADA+ only, and GADA− in both RIA and ELISA.
Figure 3.
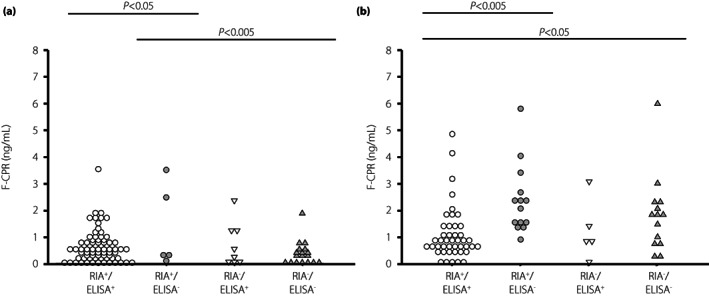
Comparison of fasting C‐peptide levels in four groups of patients with acute‐onset type 1 diabetes (a) and slowly progressive type 1 diabetes (b): GADA+ in both RIA and ELISA, RIA‐GADA+ only, ELISA‐GADA+ only, and GADA− in both RIA and ELISA. F‐CPR, fasting C‐peptide.
Differences in the GADA affinity detected by RIA‐GADA and ELISA‐GADA
In confirming the previous preliminary finding that the RIA‐GADA kit identifies both high‐ and low‐affinity GADA, whereas the ELISA‐GADA kit identifies only high‐affinity GADA 12 , GADA affinity was analyzed in a total of 113 patients, including 42 patients with acute‐onset type 1 diabetes, 62 with SPIDDM, and 9 with fulminant type 1 diabetes. As shown in Figure 4, wide variations were observed in the competition curve, and GADA affinities ranged from 1.1 × 105 to 1.2 × 1011 L/mol. In patients with acute‐onset type 1 diabetes and SPIDDM, high‐affinity GADA (>1010 L/mol) was observed in 24% (9/37) of RIA+/ELISA− sera, 92% (22/24) in RIA−/ELISA+ sera and 98% (42/43) in RIA+/ELISA+ sera (Figure 5). Thus, 36% (29/80) of RIA‐GADA‐positive sera had low‐affinity GADA, which was significantly higher than that of ELISA‐GADA‐positive sera (4%, 3/67, P < 0.0001). Of note, three of nine patients with fulminant type 1 diabetes, which is considered non‐autoimmune type 1 diabetes, also had high‐affinity GADA (Figure 5), although their GADA titer was low; ELISA‐GADA 9.8 and 13.1 U/mL for RIA−/ELISA+ sera, and RIA‐GADA 2.1 U/mL for RIA+/ELISA− sera (Figure 1c). The results confirmed that while the RIA‐GADA kit identifies both low‐ and high‐affinity GADA, the ELISA‐GADA kit identifies high‐affinity GADA exclusively. In addition, our data also revealed the presence of high‐affinity GADA in patients with fulminant type 1 diabetes.
Figure 4.
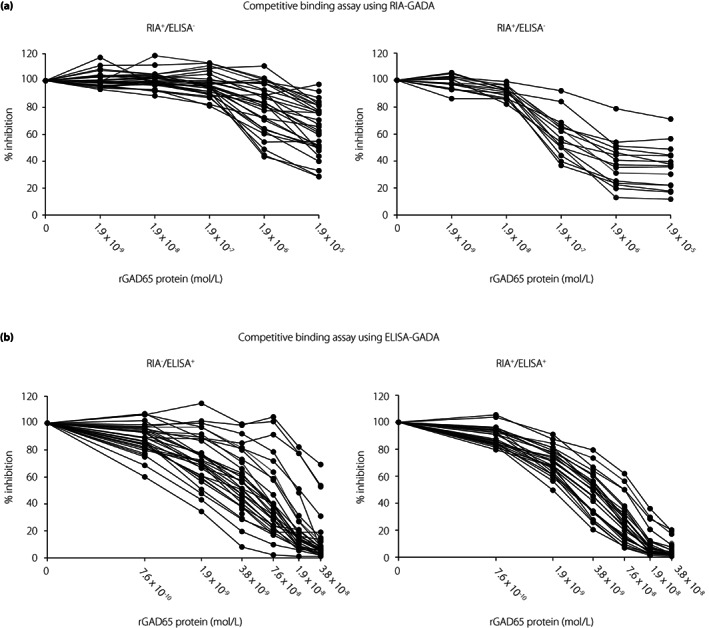
GADA‐competitive binding curve using RIA‐GADA assay (a) and ELISA‐GADA assay (b).
Figure 5.
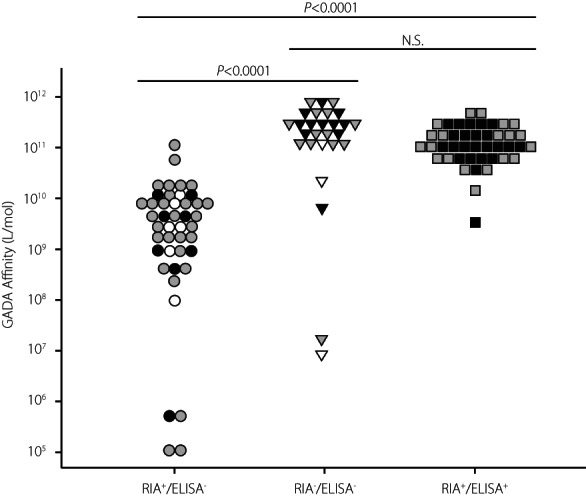
Comparison of GADA affinity in three groups: RIA‐GADA+ only, ELISA‐GADA+ only, and GADA+ in both RIA and ELISA. The closed, shaded, and open symbols indicate acute‐onset type 1 diabetes, slowly progressive type 1 diabetes, and fulminant type 1 diabetes, respectively. N.S., not significant.
Besides this, we analyzed the serial changes in GADA affinities in five samples from RIA−/ELISA+, (three acute‐onset type 1 diabetes and two SPIDDM), 10 from RIA+/ELISA− (two acute‐onset type 1 diabetes, four SPIDDM, and four fulminant type 1 diabetes), and 9 from RIA+/ELISA+ (five acute‐onset type 1 diabetes and four SPIDDM) in which the serum volume was sufficient for affinity analysis (Figure S1). On follow‐up, GADA affinity remained relatively constant in most patients, with affinity changes >1 log observed in only one SPIDDM patient with RIA‐GADA alone. While this patient initially had high‐affinity GADA, it became low‐affinity GADA over follow‐up periods.
ELISA‐GADA predicts the early decline in endogenous insulin secretion in SPIDDM patients
To clarify the association of the difference in GADA kits and GADA affinities with the progression of type 1 diabetes, we examined the rate of change in F‐CPR for 3 years from the baseline (∆F‐CPR) in 133 patients (73 acute‐onset type 1 diabetes and 60 SPIDDM), while excluding those in an insulin‐dependent state (F‐CPR <0.6 ng/mL) at the time of enrollment. Since the patients with RIA‐GADA alone showed higher F‐CPR levels in both acute‐onset type 1 diabetes and SPIDDM, we investigated whether the ∆F‐CPR differed between RIA‐positive and ELISA‐positive patients. As shown in Figure 6, the ∆F‐CPR among the four groups was not statistically significant in patients with acute‐onset type 1 diabetes (P = 0.13 by Kruskal‐Wallis test; Figure 6a), which contrasted with the statistically significant difference in ΔF‐CPR among the four groups of SPIDDM (P = 0.04 by Kruskal‐Wallis test; Figure 6b). The mean ΔF‐CPR in patients with RIA+/ELISA+ (−33.2 ± 9.5%) was significantly greater than that of patients who were positive for RIA‐GADA alone (2.6 ± 14.6%, P < 0.05) and negative for both assays (13.3 ± 12.9%, P < 0.05), but not so when compared with patients with ELISA‐GADA alone (Figure 6b).
Figure 6.
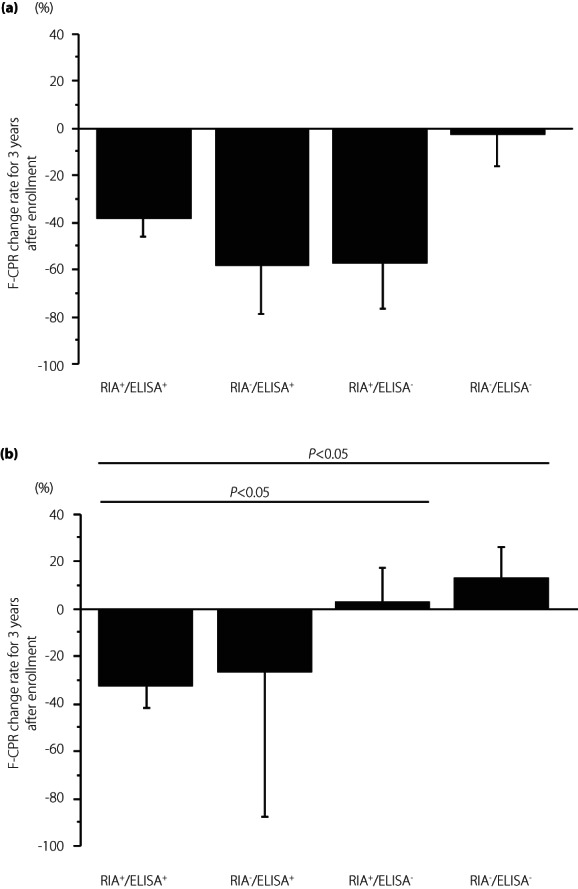
Comparison of fasting C‐peptide change rate for 3 years after enrollment in four groups of patients with acute‐onset type 1 diabetes (a) and slowly progressive type 1 diabetes (b): GADA+ in both RIA and ELISA, ELISA‐GADA+ only, RIA‐GADA+ only, and GADA− in both RIA and ELISA. Data are mean ± SE. F‐CPR, fasting C‐peptide.
We next analyzed the association between the serial change in F‐CPR and GADA affinity in 24 SPIDDM patients (Figure 7). Over 3 years, the F‐CPR gradually decreased in high‐affinity RIA−/ELISA+ and RIA+/ELISA+ patients, whereas it was maintained in patients with RIA‐GADA alone, regardless of GADA affinity. Furthermore, the F‐CPR levels were significantly higher over time in patients with RIA‐GADA alone than in those in the RIA+/ELISA+ group, regardless of GADA affinity (P < 0.05). Also, while there was no significant difference in the F‐CPR levels between patients with ELISA‐GADA alone and RIA+/ELISA+ group, the mean ∆F‐CPR in patients with RIA‐GADA alone was significantly higher than that in patients positive for ELISA‐GADA (9.1 ± 12.3% vs −22.2 ± 8.3%, P < 0.05).
Figure 7.
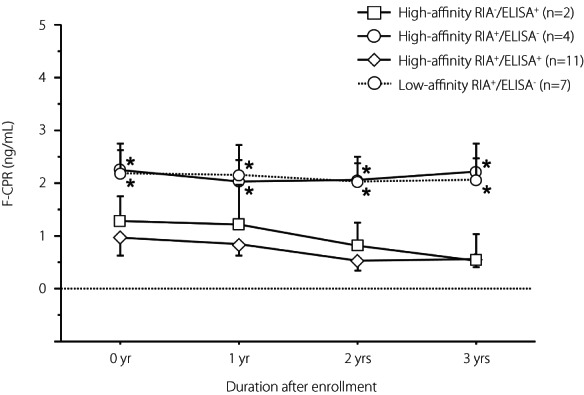
Comparison of serial change of fasting C‐peptide levels for 3 years in the four groups of patients with slowly progressive type 1 diabetes: High‐affinity ELISA‐GADA+ alone, High‐affinity RIA‐GADA+ alone, High‐affinity GADA+ in both RIA and ELISA, and Low‐affinity RIA‐GADA+ alone. Data are mean ± SE. Patients with low‐affinity ELISA‐GADA+ alone were not included, because C‐peptide data were unavailable. F‐CPR, fasting C‐peptide. *P < 0.05 vs High‐affinity RIA+/ELISA+ group.
DISCUSSION
In the present study, we showed that: (i) the diagnostic sensitivity of the ELISA method is higher than that of the RIA method in acute‐onset type 1 diabetes, whereas about 40% of SPIDDM patients with low‐titer RIA‐GADA were negative by ELISA; (ii) patients with RIA‐GADA alone showed different clinical characteristics compared with ELISA‐GADA‐positive patients; (iii) detectable GADA affinities differed between RIA and ELISA methods; (iv) the ELISA‐GADA was superior to the RIA‐GADA in identifying SPIDDM patients with early reduction in endogenous insulin secretion.
Evaluating the quantity (antibody titer) and quality (epitope, affinity) of anti‐islet autoantibodies is very important in the diagnosis and prediction of type 1 diabetes. Consequently, many studies have been conducted on the clinical significance of GADA, which has the highest prevalence among anti‐islet autoantibodies, resulting in an accumulation of evidence 15 , 16 . However, as GADA may not always reflect insulitis, it should be interpreted with caution especially when distinguishing between SPIDDM and type 2 diabetes, which can affect management strategies. Therefore, a GADA assay that can provide a more accurate diagnosis is desired.
The present study investigated the differences in the concordance rate and affinity between the RSR‐RIA, used for over 20 years as a standard kit in general clinical practice in Japan, and the new RSR‐ELISA in three subtypes of type 1 diabetes. In patients with acute‐onset type 1 diabetes, the diagnostic sensitivity of the ELISA‐GADA proved superior to that of the RIA‐GADA. Fourteen of 15 patients with acute‐onset type 1 diabetes who were ELISA‐positive but RIA‐negative had ELISA‐GADA titers of <37.5 U/mL, corresponding with the cut‐off value of RIA‐GADA 1.5 U/mL 17 . These results were similar to those reported in patients with childhood‐onset type 1 diabetes 18 . In addition, as with the patients with acute‐onset type 1 diabetes, all 14 SPIDDM patients positive for ELISA alone also had an ELISA‐GADA titer of <37.5 U/mL. These results indicate that the ELISA‐GADA is superior to the RIA method in detecting GADA in the low‐titer range.
On the other hand, in SPIDDM patients, 32 of 124 patients (26%) were RIA‐positive but ELISA‐negative, and 42% were in the low‐titer range (RIA‐GADA ≤20 U/mL), which was significantly higher than that in patients with acute‐onset type 1 diabetes in the same titer range (13%, P < 0.0001). These results were consistent with recent studies which reported that 25–30% of GADA‐positive patients with SPIDDM initially diagnosed by RIA methods had been determined as negative by the ELISA method 19 , 20 . Several possible explanations exist for the greater number of cases of SPIDDM with RIA‐GADA positivity alone. First, we speculate that more patients with low‐affinity GADA, identified mainly by the RIA method, may exist in SPIDDM than in acute‐onset type 1 diabetes. However, as the frequency of low‐affinity GADA in patients positive for RIA alone was similar between acute‐onset type 1 diabetes and SPIDDM (88% vs 72%), this hypothesis is unlikely. Second, differences in GADA epitopes between acute‐onset type 1 diabetes and SPIDDM may be associated with these discordant results. Previous studies demonstrated that unique epitopes for SPIDDM lie in the N‐terminal region of GAD65, and RIA‐GADA assays using N‐terminally truncated GAD65 (aa 96–585) improved the clinical phenotyping of autoimmune type 1 diabetes and the prediction of future insulin therapy 21 , 22 . The GAD65 antigen used in RSR‐RIA and RSR‐ELISA was truncated and lacked aa 2–45 in the N‐terminal region and a full‐length protein, respectively. Therefore, it is assumed that the conformational change due to the removal of the N‐terminal 45 amino acids creates SPIDDM‐specific unique epitopes that can be detected only by the RIA method. Future studies investigating differential responses to the two N‐terminally truncated GAD65 antigens (aa 46–585 and aa 96–585) in the RSR‐RIA should resolve this issue. Third, GADA, which was positive for RIA alone, may result from immunization with a cross‐reactive molecule. Sera from both RIA+/ELISA− and RIA+/ELISA+ groups can be completely absorbed by native antigen molecules and thus are not biochemically false‐positive.
Taking advantage of the serial samples from the TIDE‐J study, a prospective follow‐up study of Japanese type 1 diabetes, we verified the significance of ELISA‐GADA and RIA‐GADA in the declining β‐cell function after onset. In acute‐onset type 1 diabetes, the reduction in endogenous insulin secretion for 3 years from the baseline (ΔF‐CPR) was more significant in three groups positive for either RIA‐GADA or ELISA‐GADA compared with GADA‐negative patients (Figure 6a). However, ΔF‐CPR in SPIDDM positive for RIA‐GADA alone was comparable to that of GADA‐negative patients with a phenotype more similar to type 2 diabetes (2.6 ± 14.6% vs 13.3 ± 12.9%, P = 0.66; Figure 6b). Since approximately 70% of SPIDDM patients with RIA‐GADA alone had low‐affinity GADA in this study, the results are consistent with previous reports that low‐affinity GADA has prolonged preservation of residual β‐cell function 23 . These results indicate that while the RIA‐GADA detects both disease‐irrelevant and disease‐relevant signals, ELISA‐GADA detect disease‐relevant signals exclusively. The exact reasons for the ELISA method detecting only high‐affinity antibodies are still unknown. As high‐affinity antibodies bind a greater amount of antigen in a shorter period than low‐affinity antibodies, further studies are required to examine the kinetics of an antigen–antibody reaction between the RIA and ELISA methods. Other possible reasons for this discrepancy may be related to different assay principles. The ELISA‐GADA assay is a bivalent assay in which autoantibodies in serum link to both the antigen on the ELISA plate and the biotinylated antigen, thereby potentially increasing the specificity. Furthermore, unlike RIA method, which detects only IgG, the ELISA method captures all immunoglobulins, including IgG, IgM, and IgA.
Of note, the serial changes of F‐CPR in SPIDDM patients who were positive for RIA‐GADA alone were superimposed between patients with high‐affinity and low‐affinity GADA (Figure 7). These results suggest that GADA affinity may not be associated with destructive insulitis in SPIDDM positive for RIA‐GADA alone. Another possible reason might be related to the titers of GADA. We and others have reported the association between the GADA titer and insulin dependence in patients with SPIDDM, namely SPIDDM patients with lower RIA‐GADA titers (<10 U/mL) who have a slower decline in endogenous insulin secretion than those with RIA‐GADA titers of ≥10 U/mL 24 , 25 , 26 . In fact, approximately 90% of patients with SPIDDM with RIA‐GADA alone had RIA‐GADA titers of <10 U/mL. However, about 80% of the RIA+/ELISA+ patients used in the affinity study also had RIA‐GADA titers of <10 U/mL, so the relationship between low titers of GADA and a reduction in endogenous insulin secretion is still unknown. Future studies with longer follow‐ups of these patients are required to confirm this hypothesis.
Importantly, our data demonstrated that about 10% or fewer of the fulminant type 1 diabetes were positive for GADA by either the RIA or ELISA method. Furthermore, we showed for the first time that high‐affinity GADA was detected in patients with fulminant type 1 diabetes, despite most of the patients with GADA‐positive fulminant type 1 diabetes having low titers and a low affinity of GADA and only one patient showing RIA+/ELISA+. Therefore, we project that the presence of GADA in fulminant type 1 diabetes may be incidental and thus have little clinical significance.
This study has several limitations to report. First, the number of subjects was relatively small, especially for a study concerning patients with fulminant type 1 diabetes, which could affect the concordance rate and correlation between the two assays. Second, most of the sera with discrepant RIA and ELISA results were obtained from long‐standing patients, so further investigations involving new‐onset patients are necessary. Third, the endpoint titers of GADA were not measured. As a possibility exists that endpoint titration could affect the correlation between the two assays, it would be helpful for future studies to examine this.
In summary, our study revealed that bivalent ELISA for GADA is superior to the RIA method, not only in diagnostic performance of type 1 diabetes but also in identifying patients at high‐risk of early progression in SPIDDM.
DISCLOSURE
Akihisa Imagawa received honoraria or consultation fees from Astellas Pharma Inc.; grants or research support from Astra Zeneca K.K., Taiho Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Merck Biopharma Co., Ltd, Parexel International Inc., Shionogi Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, and Takeda Pharmaceutical Co., Ltd; Daisuke Chujo received honoraria for lectures from Eli Lilly and Company; Tomoyasu Fukui received research funding from Cosmic Corp.; Junnosuke Miura received honorarium for lectures from Taisho Pharmaceutical Co., Ltd, Novo Nordisk Pharma Ltd, Eli Lilly Japan K.K., Sanofi K.K., Life Science K.K., Abbott Japan LLC, Terumo Corporation, Kowa Company, Ltd, Astra Zeneca K.K., and Astellas Pharma Inc.; manuscript fees from Novo Nordisk Pharma Ltd; Other authors have no conflict of interest to declare.
Approval of the research protocol: This study protocol was approved by the Ethics Committee of Japan Diabetes Society.
Informed consent: Informed consent was obtained from all participants.
Registry and the registration no. of the study/trial: Approval date of Registry 4 April 2018, and approval number 30‐006‐(4).
Animal studies: N/A.
Supporting information
Figure S1 Serial changes of GADA affinity in three groups positive for either RIA‐GADA or ELISA‐GADA.
Table S1 Clinical characteristics according to groups in patients with acute‐onset type 1 diabetes.
Table S2 Clinical characteristics according to groups in patients with slowly progressive type 1 diabetes.
ACKNOWLEDGMENTS
This research has not received any specific grants from funding agencies in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia 2006; 49: 828–836. [DOI] [PubMed] [Google Scholar]
- 2. Törn C, Mueller PW, Schlosser M, et al. Diabetes antibody standardization program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen‐2. Diabetologia 2008; 51: 846–852. [DOI] [PubMed] [Google Scholar]
- 3. Bingley PJ, Bonifacio E, Mueller PW. Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes 2003; 52: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 4. Lampasona V, Pittman DL, Williams AJ, et al. Islet autoantibody standardization program 2018 workshop: interlaboratory comparison of glutamic acid decarboxylase autoantibody assay performance. Clin Chem 2019; 65: 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid‐phase versus standard ELISA autoantibody assays. Clin Immunol 2007; 125: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasui J, Kawasaki E, Tanaka S, et al. Clinical and genetic characteristics of non‐insulin‐requiring glutamic acid decarboxylase (GAD) autoantibody‐positive diabetes: a nationwide survey in Japan. PLoS One 2016; 11: e0155643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chujo D, Imagawa A, Yasuda K, et al. Japanese type 1 diabetes database study (TIDE‐J): rationale and study design. Diabetol Int 2021; 13: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig 2012; 3: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawasaki E, Maruyama T, Imagawa A, et al. Diagnostic criteria for acute‐onset type 1 diabetes mellitus (2012): report of the Committee of Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus. J Diabetes Investig 2014; 5: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka S, Ohmori M, Awata T, et al. Diagnostic criteria for slowly progressive insulin‐dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the committee on slowly progressive insulin‐dependent (type 1) diabetes mellitus of the Japan diabetes society. Diabetol Int 2015; 6: 1–7. [Google Scholar]
- 11. Powell M, Prentice L, Asawa T, et al. Glutamic acid decarboxylase autoantibody assay using 125I‐labelled recombinant GAD65 produced in yeast. Clin Chim Acta 1996; 256: 175–188. [DOI] [PubMed] [Google Scholar]
- 12. Kawasaki E, Okada A, Uchida A, et al. Discrepancy of glutamic acid decarboxylase 65 autoantibody results between RSR radioimmunoassay and enzyme‐linked immunosorbent assay in patients with type 1 diabetes is related to autoantibody affinity. J Diabetes Investig. 2019; 10: 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawasaki E, Miwa M, Tanaka M. Basic and clinical evaluation of ELISA assay kits (cosmic) for GADAb and IA‐2Ab. Jpn J Med Pharm Sci 2011; 66: 345–352 (in Japanese). [Google Scholar]
- 14. Pottumarthy S, Morris AJ, Harrison AC, et al. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J Clin Microbiol 1999; 37: 3229–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawasaki E, Eisenbarth GS. High‐throughput radioassays for autoantibodies to recombinant autoantigens. Front Biosci 2000; 5: E181–E190. [DOI] [PubMed] [Google Scholar]
- 16. Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab 2010; 95: 25–33. [DOI] [PubMed] [Google Scholar]
- 17. Oikawa Y, Kondo T, Shimada A, et al. Actual condition survey regarding mismatch of measurements between radioimmunoassay and enzyme‐linked immunosorbent assay tests for anti‐glutamic acid decarboxylase antibody in real‐world clinical practice. J Diabetes Investig 2019; 10: 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugihara S, Yokota I, Mukai T, et al. Increased diagnosis of autoimmune childhood‐onset Japanese type 1 diabetes using a new glutamic acid decarboxylase antibody enzyme‐linked immunosorbent assay kit, compared with a previously used glutamic acid decarboxylase antibody radioimmunoassay kit. J Diabetes Investig 2020; 11: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oikawa Y, Tanaka H, Uchida J, et al. Slowly progressive insulin‐dependent (type 1) diabetes positive for anti‐GAD antibody ELISA test may be strongly associated with a future insulin‐dependent state. Endocr J 2017; 64: 163–170. [DOI] [PubMed] [Google Scholar]
- 20. Murata T, Tsuzaki K, Nirengi S, et al. Diagnostic accuracy of the anti‐glutamic acid decarboxylase antibody in type 1 diabetes mellitus: comparison between radioimmunoassay and enzyme‐linked immunosorbent assay. J Diabetes Investig 2017; 8: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin P, Huang G, Lin J, et al. Epitope analysis of GAD65 autoantibodies in adult‐onset type 1 diabetes and latent autoimmune diabetes in adults with thyroid autoimmunity. Acta Diabetol 2011; 48: 149–155. [DOI] [PubMed] [Google Scholar]
- 22. Achenbach P, Hawa MI, Krause S, et al. Autoantibodies to N‐terminally truncated GAD improve clinical phenotyping of individuals with adult‐onset diabetes: action LADA 12. Diabetologia 2018; 61: 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krause S, Landherr U, Agardh CD, et al. GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care 2014; 37: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 24. Kasuga A, Maruyama T, Nakamoto S, et al. High‐titer autoantibodies against glutamic acid decarboxylase plus autoantibodies against insulin and IA‐2 predicts insulin requirement in adult diabetic patients. J Autoimmun 1999; 12: 131–135. [DOI] [PubMed] [Google Scholar]
- 25. Kawasaki E, Nakamura K, Kuriya G, et al. Autoantibodies to insulin, insulinoma‐associated antigen‐2, and zinc transporter 8 improve the prediction of early insulin requirement in adult‐onset autoimmune diabetes. J Clin Endocrinol Metab 2010; 95: 707–713. [DOI] [PubMed] [Google Scholar]
- 26. Wada E, Onoue T, Kinoshita T, et al. Adult‐onset autoimmune diabetes identified by glutamic acid decarboxylase autoantibodies: a retrospective cohort study. Diabetologia 2021; 64: 2183–2192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Serial changes of GADA affinity in three groups positive for either RIA‐GADA or ELISA‐GADA.
Table S1 Clinical characteristics according to groups in patients with acute‐onset type 1 diabetes.
Table S2 Clinical characteristics according to groups in patients with slowly progressive type 1 diabetes.


