Abstract
Aims/Introduction
In Japan, the increasing frequency of underweight among women of reproductive age and the accompanying increase in the rate of low birth weight (LBW) are social issues. The study aimed to establish a prospective registry system for gestational diabetes mellitus (GDM) in Japan and to clarify the actual status of GDM according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria.
Materials and Methods
Pregnant women with gestational diabetes mellitus and those in the normal glucose tolerance (NGT) group were enrolled in the Diabetes and Pregnancy Outcome for Mother and Baby study from October 2015. Pregnant women with positive glucose screening in early and mid‐to‐late pregnancy underwent a 75 g oral glucose tolerance test by gestational week 32. Gestational diabetes mellitus was diagnosed according to IADPSG criteria. Women with a positive glucose screening test at mid‐to‐late pregnancy but NGT were enrolled as references (NGT group). Treatment for gestational diabetes mellitus and maternal and neonatal pregnancy data were prospectively collected on outcomes.
Results
In total 1,795 singleton pregnancies (878 women with GDM and 824 NGT women) were analyzed. The risk of LBW and small‐for‐gestational age in the GDM group was significantly higher than in the NGT group. A similar relationship was found for LBW risk in the non‐overweight/obese group but not in the overweight/obese group.
Conclusions
We established a prospective GDM registry system in Japan. In the management of GDM in Japan, suppression of maternal weight gain may be associated with reduced fetal growth, especially in non‐overweight/obese women with GDM; however, further investigation is required.
Keywords: Gestational diabetes, Large‐for‐gestational age, Low birth weight
The gestational diabetes mellitus group had a higher rate of low birth weight and small‐for‐gestational age infants than the screening‐positive normal glucose tolerance (SPNGT group), possibly attributed to the suppression of weight gain during pregnancy.
INTRODUCTION
Consistent with the increase in obesity, the prevalence of gestational diabetes mellitus is increasing globally 1 , augmenting the transfer of non‐communicable diseases to the next generation 2 , 3 . Pregnant women who are obese and have gestational diabetes mellitus are at high risk of having large‐for‐gestational age (LGA) babies 4 . In the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, maternal obesity and maternal hyperglycemia additionally increased the birth weight of the infants, as most of the pregnant women were overweight or obese 5 . In addition, offspring of mothers with gestational diabetes mellitus were associated with increased adolescent adiposity 6 and impaired glucose tolerance 7 compared with those of non‐gestational diabetes mellitus mothers. The risk is further enhanced, especially in LGA babies 8 .
In Asia, diabetes and gestational diabetes are more frequent 9 , 10 despite a lower degree of obesity compared with non‐Asians, which is thought to be due to a low insulin secretory capacity 11 . The rapid increase in diabetes and gestational diabetes in Asia in recent years is worrisome, and the increase in adiposity due to improved nutritional status resulting from economic growth is thought to be one of the major factors 12 . On the other hand, the situation in Japan is unique. In Japan, the proportion of underweight women of reproductive age has increased since 1995, and the average birth weight in Japan has declined in synchrony with the increase in the proportion of underweight women. Moreover, the proportion of low birth weight (LBW) babies has remained at approximately 10% since 2000 with no sign of decrease 13 .
In 2010, Japan adopted the International Association of Diabetes and Pregnancy Study Group (IADPSG) diagnostic criteria, which were developed based on the results of the HAPO study and approved by the World Health Organization (WHO) 14 . Thereafter, the incidence of gestational diabetes mellitus increased four‐fold, from 2.1% to 8.5% 15 . To clarify the current status of gestational diabetes mellitus diagnosed by IADPSG criteria and its pregnancy outcome in Japan, a different context than in other Asian countries, the Japanese Society of Diabetes and Pregnancy established a prospective registry system of pregnant women with gestational diabetes mellitus and started enrolling such patients in 2015. By simultaneously enrolling pregnant women with a normal glucose tolerance (NGT group), we compared pregnancy outcomes between the gestational diabetes mellitus and NGT groups to evaluate the effect of the intervention on gestational diabetes mellitus.
MATERIALS AND METHODS
Study design and participants
A prospective cohort study was conducted in centers that belonged to the Japan Diabetes and Pregnancy Society and had delivery information registered in the Japan Society of Obstetrics and Gynecology (JSOG) Successive Pregnancy Birth Registry System. In this system, approximately 280 secondary and tertiary hospitals registered information on successful deliveries of ≥22 weeks gestational age, approximately 25% of all deliveries in Japan.
Pregnant women with positive glucose screening tests during early and mid‐to‐late pregnancy underwent a 75 g oral glucose tolerance test (OGTT) by gestational week 32 and were diagnosed with gestational diabetes mellitus according to IADPSG criteria. Pregnant women at high risk of developing gestational diabetes were sometimes omitted from screening and administered this test. Those who underwent a 75 g OGTT by mid‐to‐late pregnancy and had a NGT were enrolled as a control group for the gestational diabetes group. Both the gestational diabetes mellitus and the NGT groups completed a questionnaire in Japanese at the time of enrollment. All gestational diabetes mellitus cases were enrolled at the participating facilities successively; the decision to enroll in the NGT group was made by each facility, and all NGT cases in a facility were enrolled successively. This study was conducted after obtaining approval from the ethics committee of the National Center for Child Health and Development and at each facility (approval date of registry and the registration number of the study/trial: August 1, 2015 and UMIN0000023420). Written informed consent was obtained from all participants. The analysis in this study was limited to singleton pregnant women in the Diabetes and Pregnancy Outcome for Mother and Baby (DREAMBee) study.
Screening and diagnosis of gestational diabetes mellitus
Gestational diabetes mellitus screening in Japan is conducted according to the JSOG guidelines (Obstetrics and Gynecology Guidelines 2017), diagnosed in a two‐step method in many institutions, not based on universal screening.
In early pregnancy stages, a random plasma glucose level was measured, with cutoff values of 95 or 100 mg/dL.
In the mid‐pregnancy stages, a 50 g glucose challenge test (GCT) or random glucose screening was performed (considered positive at ≥140 or ≥100 mg/dL, respectively).
All pregnant women with positive glucose screening underwent a 75 g OGTT. Mid‐pregnancy screening was performed if early‐stage screening was either negative or showed normal OGTT results as a general rule. A mid‐to‐late gestational diabetes mellitus diagnosis was defined when a 75 g OGTT was performed between 24 and 32 weeks of gestation.
Early and mid‐to‐late gestational diabetes mellitus diagnoses were defined when a 75 g OGTT was performed at either <20 or between 24 and 32 weeks of gestation, respectively, for the subgroup analysis.
Management after diagnosis with gestational diabetes mellitus
After the gestational diabetes mellitus diagnosis, diet and/or weight advice was given according to the JSOG Obstetrics Practice Guidelines 15 . Target glucose fasting, pre‐meal, and 2 h postprandial values (mg/dL [mmol/L]) were ≤95 (5.3), ≤100 (5.6), or ≤120 (6.7), respectively. Insulin was introduced when the target blood glucose level was difficult to achieve even after diet and exercise therapy.
Predictor and outcome variables
The glucose screening and 75 g OGTT results and maternal and neonatal outcomes were recorded prospectively. For pregnancy outcomes, JSOG Successive Pregnancy Birth Registry System data were collected from each facility. Maternal and neonatal outcomes were birth weight >90th percentile for LGA, birth weight <10th percentile for small‐for‐gestational age (SGA), birth weight >4,000 g (macrosomia), birth weight <2,500 g (LBW), emergency cesarean delivery, preeclampsia, preterm birth (delivery before 37 weeks of gestation), shoulder dystocia, neonatal intensive care unit (NICU) admission, perinatal mortality, clinically diagnosed neonatal hypoglycemia. Upon transfer to a different hospital, the pregnancy outcome was obtained from the registered physician based on the delivery report submitted to the registered facility. For cases transferred at delivery, data were collected at transfer. For gestational diabetes mellitus, insulin administration during pregnancy, state of glycemic control during pregnancy, and the result of 75 g OGTT 3 months after delivery were also collected. Details of the whole collected data in the DREAMBee study are shown in Appendix S2.
Statistical analysis
Data are presented as n (%) and mean ± standard deviation for categorical and continuous variables, respectively. A chi‐square (χ2) test was performed for binary variables and a t‐test for continuous variables. Multivariate logistic regression was performed using dichotomous outcomes and reported as odds ratios (ORs) and 95% confidence intervals (CIs) for each pregnancy outcome. Covariate adjustments included maternal age, parity, maternal pre‐pregnancy body mass index (BMI), educational background, and income. For preterm birth, SGA, LGA, macrosomia, LBW, and maternal weight gain during pregnancy were considered covariates. Although Asian people with a high risk of type 2 diabetes and cardiovascular disease have BMIs lower than the current WHO cutoff point for overweight (≥25 kg/m2), a BMI of ≥25 kg/m2 was defined as overweight/obesity in the present study.
All statistical analyses were performed using Stata (StataCorp, College Station, TX, USA).
RESULTS
Of the 21 participating centers from October 2015 to March 2019, 16 registered both gestational diabetes mellitus and NGT, and five registered only gestational diabetes mellitus. Of 2,115 enrolled pregnant women in the DREAMBee study, 121 were excluded, including one patient who withdrew consent, six who had a missing questionnaire at registration, one who had missing OGTT data at gestational diabetes mellitus diagnosis, and 113 who were diagnosed with NGT via OGTT in early pregnancy. Additionally, 120 patients who were diagnosed after 33 weeks of gestation were excluded. Finally, the 1,874 participants in the DREAMBee study (957 gestational diabetes mellitus and 917 NGT) included 104 multiple (45 gestational diabetes mellitus and 59 NGT) and 1,770 singleton (912 gestational diabetes mellitus and NGT 858) pregnancies.
All 104 multiple (45 gestational diabetes mellitus and 59 NGT) pregnancies were followed up until the pregnancy outcome. In 1,770 singleton pregnancies, one patient had a miscarriage, and 66 had an unknown pregnancy outcome because of delivery at a transferred facility. Overall, 1,703 (878 gestational diabetes mellitus and 825 NGT) women were followed up until the pregnancy outcome (Figure 1). As the first report from the DREAMBee study, data of pregnancy outcomes of 878 gestational diabetes mellitus singleton pregnancies were analyzed, comparing them with those of 825 NGT singleton pregnancies.
Figure 1.
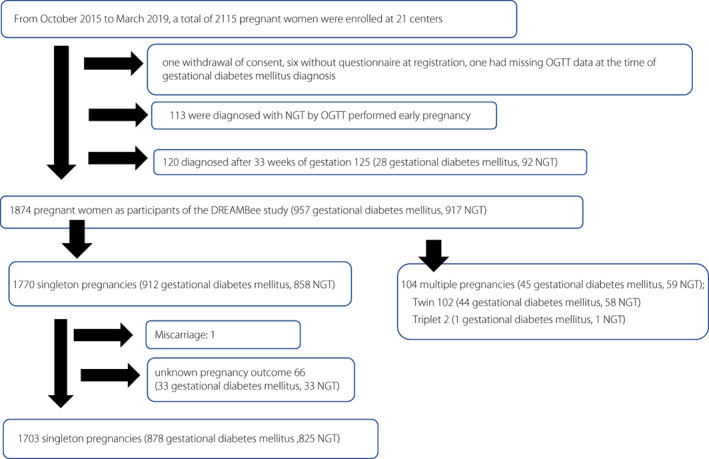
Flow diagram of DREAMBee study participants.
Characteristics and pregnancy outcome of gestational diabetes mellitus and NGT
Table 1 shows the characteristics of those with singleton pregnancies. Compared with the NGT group, the gestational diabetes mellitus group had a higher maternal age (36.0 ± 4.6 vs 35.5 ± 5.2 years [P = 0.04]), pre‐pregnancy BMI (22.7 ± 4.6 kg/m2 vs 21.4 ± 3.5 kg/m2 [P < 0.001]), were more likely to have a family history of diabetes (P < 0.001), medical history of gestational diabetes mellitus (10.5% vs 2.9% [P < 0.001]), lower rate of primipara (39.6% vs 45.0% [P = 0.02]), and earlier OGTT date at diagnosis (23.3 ± 6.5 weeks vs 27.0 ± 2.2 weeks [P < 0.001]).
Table 1.
The characteristics of the study participants in gestational diabetes mellitus and NGT groups with singleton pregnancies
| Characteristic | Gestational diabetes mellitus (n = 878) | NGT (n = 825) | P value |
|---|---|---|---|
| Maternal age, years | 36.0 ± 4.6 | 35.5 ± 5.2 | 0.04 |
| Primipara, n (%) | 348 (39.6) | 372 (45.1) | 0.02 |
| Family history of diabetes, n (%) | 276 (31.5) | 200 (24.2) | <0.001 |
| Pre pregnancy BMI, kg/m2 | 22.7 ± 4.6 | 21.4 ± 3.5 | <0.001 |
| Pre pregnancy obesity/overweight, n (%) | 205 (23.5) | 103 (12.5) | <0.001 |
| Smoking, n (%) | |||
| None | 746 (87.0) | 733 (89.0) | 0.47 |
| Stop after pregnancy | 103 (11.7) | 82 (10.0) | |
| Yes | 11 (1.3) | 9 (1.1) | |
| Academic, n (%) | |||
| High school graduate | 155 (17.8) | 130 (16.0) | 0.08 |
| Graduated from a vocational school/junior college | 309 (35.5) | 259 (31.8) | |
| University graduate or above | 406 (46.7) | 426 (52.3) | |
| Household income, yen/year, n (%) | |||
| Less than 3 million | 47 (5.8) | 61 (8.0) | 0.07 |
| 3–5 million | 188 (23.2) | 161 (21.1) | |
| 5–7 million | 190 (23.2) | 148 (19.3) | |
| 7–9 million | 137 (16.9) | 131 (17.2) | |
| More than 9 million | 250 (30.8) | 264 (34.5) | |
| Folic acid intake, n (%) | 518 (60.2) | 481 (58.8) | 0.54 |
| Past history | |||
| Polycystic ovarian syndrome, n (%) | 98 (11.2) | 74 (9.0) | 0.14 |
| Hypertension, n (%) | 63 (7.4) | 42 (5.1) | 0.06 |
| Thyroid disease, n (%) | 108 (12.6) | 105 (12.8) | 0.94 |
| Other disease, n (%) | 173 (19.7) | 179 (20.6) | 0.63 |
| Gestational diabetes mellitus, n (%) | 92 (10.5) | 24 (2.9) | <0.001 |
| 75 g glucose tolerance test at the time of gestational diabetes milieus diagnosis | |||
| Date, weeks | 23.3 ± 6.5 | 27.0 ± 2.2 | <0.001 |
| Fasting plasma glucose, mg/dL | 85.7 ± 9.8 | 80.4 ± 5.4 | <0.001 |
| 1 h plasma glucose, mg/dL | 177.7 ± 28.6 | 134.0 ± 23.6 | <0.001 |
| 2 h plasma glucose, mg/dL | 156.0 ± 27.0 | 117.1 ± 18.5 | <0.001 |
| HbA1c, mg/dL | 5.4 ± 0.31 (n = 475) | 5.2 ± 0.27 (n = 620) | <0.001 |
Data are mean (SD), or n (%). Obesity/overweight BMI ≧25.
BMI, body mass index; NGT, normal glucose tolerance.
Table 2 shows the pregnancy outcome of those with singleton pregnancies. Compared with the NGT group, the gestational diabetes mellitus group gained less weight during pregnancy (7.4 ± 5.0 kg vs 9.6 ± 4.7 kg [P < 0.001]), had earlier deliveries (38.4 ± 1.8 vs 38.7 ± 1.6 gestational weeks [P < 0.001]), higher proportion of preterm birth (8.8% vs 5.7% [P = 0.02]), lower infant height (48.9 ± 2.8 cm vs 49.3 ± 2.4 cm [P = <0.001]), and lower infant birth weight (2992.7 ± 480.0 g vs 3039.7 ± 442.8 g [P = 0.03]). The gestational diabetes mellitus group had a higher proportion of LBW infants (12.2% vs 8.4% [P = 0.01]), NICU admission (17.9% vs 12.8% [P = 0.04]), and neonatal hypoglycemia (1.3% vs 0.24% [P = 0.02]). LGA proportions were nearly equal for both the gestational diabetes mellitus (16.0%) and NGT (15.9%) groups. There were no significant between‐group differences for other pregnancy outcomes. However, regarding neonatal hypoglycemia, blood glucose levels were not measured in all infants in the NGT group.
Table 2.
The pregnancy outcomes of the study participants in gestational diabetes mellitus and NGT groups with singleton pregnancies
| Gestational diabetes mellitus (n = 878) | NGT (n = 825) | P value | |
|---|---|---|---|
| Insulin use, n (%) | 213 (24.3) | 1 (0.12) | 0.02 |
| Gestational weight gain, kg | 7.4 ± 5.0 | 9.6 ± 4.7 | <0.001 |
| Gestational week, week | 38.4 ± 1.8 | 38.7 ± 1.6 | <0.001 |
| Preterm birth, n (%) | 77 (8.8) | 47 (5.7) | 0.02 |
| Mode of delivery | |||
| Vaginal | 433 (49.4) | 419 (50.9) | 0.34 |
| Suction | 95 (10.8) | 101 (12.3) | |
| Forceps | 24 (2.7) | 20 (2.4) | |
| Scheduled cesarean section | 160 (18.3) | 149 (18.1) | |
| Emergency cesarean section | 158 (18.0) | 136 (16.5) | |
| Others | 6 (0.69) | 0 (0.0) | |
| Neonatal death, n (%) | 3 (0.34) | 2 (0.24) | 1.00 |
| Sex (women), n (%) | 413 (47.0) | 406 (49.2) | 0.38 |
| High, cm | 48.9 ± 2.8 | 49.3 ± 2.4 | <0.001 |
| Birth weight, g | 2992.7 ± 480.0 | 3039.7 ± 442.8 | 0.03 |
| Small‐for‐gestational age, n (%) | 69 (7.9) | 48 (5.8) | 0.10 |
| Large‐for‐gestational age, n (%) | 140 (16.0) | 131 (15.9) | 0.98 |
| Macrosomia, n (%) | 10 (1.1) | 13 (1.6) | 0.43 |
| Low‐birth‐weight, n (%) | 107 (12.2) | 69 (8.4) | 0.01 |
| Hypertensive disorders of pregnancy, n (%) | 69 (9.7) | 57 (6.9) | 0.45 |
| Placental abruption, n (%) | 6 (0.69) | 4 (0.49) | 0.59 |
| HELLP syndrome, n (%) | 3 (0.34) | 3 (0.36) | 1.00 |
| Maternal death, n (%) | 0 (0.0) | 0 (0.0) | |
| Neonatal morphological abnormality, n (%) | 50 (5.9) | 32 (3.9) | 0.06 |
| NICU admission, n (%) | 118 (17.0) | 77 (12.8) | 0.04 |
| Apgar score 1 ≥ 7, n (%) | 816 (93.3) | 770 (93.3) | 0.96 |
| Apgar score 5 ≥ 7, n (%) | 859 (98.1) | 810 (98.2) | 0.86 |
| Shoulder dystocia, n (%) | 3 (0.34) | 1 (0.12) | 0.63 |
| Neonatal hypoglycemia, n (%) | 11 (1.3) | 2 (0.24) | 0.02 |
| Intravenous glucose administration (%) | 25 (2.9) | 14 (1.7) | 0.11 |
| Fracture, n (%) | 0 (0.0) | 0 (0.0) | – |
| Nerve plexus paralysis, n (%) | 0 (0.0) | 0 (0.0) | – |
Data are mean (SD), or n (%).
HELLP, hemolytic anemia elevated liver enzymes low platelet count; NGT, normal glucose tolerance; NICU, neonatal intensive care unit.
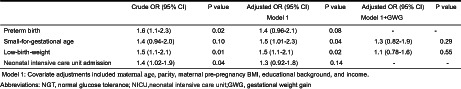
Table 3 shows the results of multiple logistic regression analysis for preterm birth, SGA, LBW, and NICU admission for women with gestational diabetes mellitus, using the data of NGT as a reference category. The ORs (95% CIs), after adjusting for maternal age, parity, maternal pre‐pregnancy BMI, academic background, and income, were significantly higher in SGA (OR 1.5; 95% CI 1.01–2.3; P = 0.04) and LBW (OR 1.5; 95% CI 1.1–2.1; P = 0.02) in the gestational diabetes mellitus using the data of the NGT group as a reference. However, the association disappeared after adjusting for maternal weight gain during pregnancy (SGA [OR 1.3, 95% CI 0.82–1.9, P = 0.29]; LBW [OR 1.1, 95% CI 0.78–1.6, P = 0.55]). In non‐obese women (pre‐pregnancy BMI <25 kg/m2), the adjusted OR for LBW of 1.5 (95% CI 1.1–2.2, P = 0.02) became non‐significant after adjusting for maternal weight gain during pregnancy (adjusted OR 1.2; 95% CI 0.79–1.7, P = 0.43), while the adjusted ORs for preterm birth, SGA, and LBW were not significant in obese women (pre‐pregnancy BMI ≥25 kg/m2) (Table 4).
Table 3.
The multiple logistic regression analysis in preterm north, small‐for‐gestational age, low‐birth‐weight and NICU admission with the gestational diabetes mellitus group using the NGT group as reference category
| Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Model 1 | Model 1 + GWG | |||||
| Preterm birth | 1.6 (1.1–2.3) | 0.02 | 1.4 (0.96–2.1) | 0.08 | – | – |
| Small‐for‐gestational age | 1.4 (0.94–2.0) | 0.10 | 1.5 (1.01–2.3) | 0.04 | 1.3 (0.82–1.9) | 0.29 |
| Low‐birth‐weight | 1.5 (1.1–2.1) | 0.01 | 1.5 (1.1–2.1) | 0.02 | 1.1 (0.78–1.6) | 0.55 |
| Neonatal intensive care unit admission | 1.4 (1.02–1.9) | 0.04 | 1.3 (0.92–1.8) | 0.14 | – | – |
Model 1: Covariate adjustments included maternal age, parity, maternal pre‐pregnancy BMI, educational background, and income.
GWG, gestational weight gain; NGT, normal glucose tolerance; NICU, neonatal intensive care unit.
Table 4.
The multiple logistic regression analysis in small‐for‐gestational age and ow‐birth‐weight with the gestational diabetes mellitus group using the NGT group as reference category by pre‐pregnancy BMI
| NGT n, % | Gestational diabetes mellitus n, % | Crude OR (95% CI) | P value | Adjusted OR (95% CI) Model 1 | P value | Adjusted OR (95% CI) Model 1 + GWG | P value | |
|---|---|---|---|---|---|---|---|---|
| BMI < 25 | ||||||||
| Small‐for‐gestational age | 42 (5.9) | 56 (8.4) | 1.5 (0.98–2.2) | 0.06 | 1.5 (0.99–2.4) | 0.05 | 1.3 (0.80–2.0) | 0.29 |
| Low‐birth‐weight | 59 (8.2) | 85 (12.8) | 1.6 (1.1–2.3) | 0.006 | 1.5 (1.1–2.2) | 0.02 | 1.2 (0.79–1.7) | 0.43 |
| BMI ≥25 | ||||||||
| Small‐for‐gestational age | 6 (5.7) | 13 (6.1) | 1.1 (0.40–3.0) | 0.87 | 1.4 (0.47–4.2) | 0.54 | 1.2 (0.38–3.7) | 0.77 |
| Low‐birth‐weight | 10 (9.5) | 22 (10.4) | 1.1 (0.51–2.4) | 0.79 | 1.3 (0.56–3.0) | 0.53 | 0.87 (0.36–2.1) | 0.76 |
Model 1: Covariate adjustments included maternal age, parity, maternal pre‐pregnancy BMI, educational background, and income.
GDM, gestational diabetes mellitus; GWG, gestational weight gain; SGA, small‐for‐gestational age; SPNGT, screening positive normal glucose tolerance; LBW, low‐birth‐weight; LGA, large‐for‐gestational age; NICU, neonatal intensive care unit.
Characteristics and pregnancy outcomes of mid‐to‐late pregnancy diagnosed with gestational diabetes mellitus and NGT
To exclude the impact of early intervention for gestational diabetes mellitus on gestational weight gain, we compared the characteristics and pregnancy outcomes of mid‐to‐late pregnancy diagnosed with gestational diabetes mellitus and NGT.
Table S1 shows the characteristics of the participants with singleton pregnancies. Compared with the NGT group, the mid‐to‐late pregnancy diagnosed gestational diabetes mellitus group had a higher maternal age (36.0 ± 4.7 vs 35.5 ± 5.2 years, P = 0.006), pre‐pregnancy BMI (22.1 ± 3.9 kg/m2 vs 21.4 ± 3.5 kg/m2, P < 0.001), were more likely to have a family history of diabetes (P = 0.02), and medical history of gestational diabetes mellitus (7.9% vs 2.9%, P < 0.001). However, compared with the NGT group, the primipara rate was significantly lower in the gestational diabetes mellitus group (36.8% vs 45.0%, P = 0.002), and the OGTT date at diagnosis was earlier (27.5 ± 1.9 weeks vs 27.0 ± 2.2 weeks P < 0.001).
Table S2 shows the pregnancy outcome of mid‐to‐late singleton pregnancies diagnosed with gestational diabetes mellitus and NGT. Compared with the NGT group, the gestational diabetes mellitus group gained less weight during pregnancy (7.6 ± 4.6 kg vs 9.6 ± 4.7 kg, P < 0.001), had earlier deliveries (38.5 ± 1.7 vs 38.7 ± 1.6, P = 0.04), and had higher proportions of LBW (12.5% vs 8.4%, P = 0.02) than the NGT group.
Table S3 shows the results of multiple logistic regression analysis for preterm birth, SGA, LBW, and NICU admission of mid‐to‐late pregnancy diagnosed gestational diabetes mellitus women, using the data of NGT as a reference category. After adjusting for age at delivery, parity, maternal pre‐pregnancy BMI, academic background, and income, the ORs (95% CIs) were significantly higher in LBW (OR 1.2; 95% CI 1.03–1.3, P = 0.02) in the mid‐to‐late pregnancy diagnosed gestational diabetes mellitus using the data of the NGT group as a reference. However, the association disappeared after adjusting for maternal weight gain during pregnancy (adjusted OR 1.1, 95% CI = 0.94–1.2, P = 0.35).
In non‐obese women (pre‐pregnancy BMI <25 kg/m2), the adjusted OR for LBW of 1.2 (95% CI 1.0–1.3, P = 0.02) became non‐significant after adjusting for maternal weight gain during pregnancy (adjusted OR = 1.1; 95% CI 0.23–1.3, P = 0.23), while the adjusted ORs for preterm birth, SGA, and LBW were not significant in obese women (pre‐pregnancy BMI ≥25 kg/m2).
DISCUSSION
A prospective registry of pregnant women with gestational diabetes mellitus diagnosed by IADPSG criteria was established in Japan (DREAMBee study – gestational diabetes mellitus), where the number of LBW infants increased consistently with the increase in the number of underweight women of reproductive age. The results suggest that the risk of LBW and SGA is higher in the gestational diabetes mellitus group than in the NGT group and that the increased risk may be related to the suppression of maternal weight gain in the management of gestational diabetes mellitus. This fetal growth suppression in the gestational diabetes mellitus group was also found in mid‐to‐late pregnancy. No fetal growth suppression was found in the overweight/obese group, but in the non‐overweight/obese group, fetal growth suppression was found to be related to the suppression of maternal weight gain. Generally, the increase in LGA is a problem in gestational diabetes mellitus; however, in Japan, fetal growth suppression in non‐overweight/obese gestational diabetic women should also be taken into consideration in the management of gestational diabetes mellitus.
A retrospective multicenter study of 893 patients with gestational diabetes mellitus in singleton pregnancies at 30 centers was conducted in Japan from 2005 to 2010 16 ; however, the gestational diabetes mellitus diagnosis was not recent, and no control group was enrolled. The Japan Environment and Children's Study (JECS) is a large prospective national cohort study of births in Japan that included approximately 100,000 pregnant women between January 2011 and March 2014, tracking the health status of mothers and their children until age 13 17 . Although JECS was conducted after adopting the IADPSG criteria, the proportion of gestational diabetes mellitus was only 2.7% 18 , suggesting that gestational diabetes mellitus was not properly screened and diagnosed. In addition, data on the results of the 75 g OGTT for the diagnosis of gestational diabetes mellitus, treatment status for gestational diabetes mellitus, or postpartum 75 g OGTT results have not been collected. Therefore, it was not a useful database to examine gestational diabetes mellitus.
In the present study, the risk of LBW and SGA infants in the gestational diabetes mellitus group was significantly higher than that in the NGT group. However, these risks disappeared after adjusting for maternal weight gain during pregnancy, suggesting that suppression of weight gain during pregnancy may be associated with reduced fetal growth in gestational diabetes mellitus. This association was also observed in the mid‐to‐late diagnosis of gestational diabetes mellitus group, indicating that weight gain suppression may not have resulted from interventions during the entire gestational period, starting in early pregnancy. However, this association was not observed in the overweight/obesity group, although it was observed for the risk of increased LBW in the gestational diabetes mellitus group when the pre‐pregnancy BMI was <25 kg/m2, indicating the need to pay attention to the control of weight gain during pregnancy in gestational diabetes mellitus women who are not overweight/obese.
In Japan, underweight among women in their 20s has increased since 1995, exceeding 20% 19 , and underweight women gain less weight during pregnancy 20 , 21 , further contributing to the birth of LBW infants. In fact, birth weight in Japan has declined consistently with the increase in the number of underweight women 19 , and the proportion of LBW infants is still 9.4% in the 2019 survey 13 . The situation of children born with LBW is of great public health concern, as they are more likely to develop diabetes, hypertension, cerebral or coronary vascular disease, and other non‐communicable diseases 22 . In 2021, the Ministry of Health, Labor and Welfare and the Japan Obstetrician and Gynecologist Society switched their policies toward increasing the amount of weight gain during pregnancy 23 , 24 . This policy may change the situation of gestational diabetes mellitus in Japan in the future, and we would like to focus on the results of our future registry surveys.
Conversely, pregnancy outcomes of gestational diabetes mellitus were similar to those of NGT, except for SGA and LBW children. Although not simply comparable, the outcomes of pregnancies with gestational diabetes mellitus in the present study were not as good as those of the 58,670 singleton pregnancies in the JECS 25 . In contrast, the rates of preeclampsia, cesarean section, and instrumental delivery were 9.7%, 36.3%, and 13.6% in the gestational diabetes mellitus group in our study, compared with 3.0%, 10.3%, and 6.0%, respectively, in the JECS group. In our study, those with normal results of the 75 g OGTT were selected as the NGT group for reference. In our study, those with normal results of the 75 g OGTT were selected as the NGT group for reference. Since the 75 g OGTT was performed only on those who screened positive in mid‐to‐late pregnancy, it is possible that this group had subnormal glucose tolerance. In fact, the LGA rate in the NGT group (17.8%) was higher than the originally expected 10%. Recently, reports from a non‐participating center in Japan and a study in the US also reported that non‐gestational diabetes mellitus women with a positive 50 g GCT had a higher LGA rate than women in the NGT group 26 , 27 . Conversely, a report from Thailand showed no difference in pregnancy outcomes between the two groups 28 . Therefore, there is essentially a need to compare and examine pregnancy outcomes in the gestational diabetes mellitus group in the 75 g OGTT performed as a universal screening using the NGT group as a reference.
The study had several limitations. First, the participants were not representative of all Japanese pregnant women because the participating centers were secondary or tertiary centers. Second, although we compared pregnancy outcomes between the gestational diabetes mellitus and NGT groups, the NGT group may have had subnormal glucose tolerance and would not be considered a normal reference. Finally, for some pregnancy outcomes (e.g., shoulder dystocia and neonatal hypoglycemia), we expected unreported cases on data collection, and the results may not have been robust.
In conclusion, we established a prospective registry system of gestational diabetes mellitus in Japan (DREAMBee study‐gestational diabetes mellitus) and clarified the actual status of gestational diabetes mellitus. Gestational diabetes mellitus had a higher proportion of LBW and SGA infants than NGT, especially in non‐overweight/obese women with gestational diabetes mellitus. In particular, the suppression of gestational weight gain in non‐overweight/obese gestational diabetes mellitus women may be related to the suppression of fetal growth. In the management of gestational diabetes mellitus in Japan, it is necessary to focus on growth suppression as well as fetal overgrowth.
DISCLOSURE
The authors have no conflicts of interest directly relevant to the content of this article to declare.
Approval of the research protocol: This study was conducted after obtaining approval from the ethics committee of the National Center for Child Health and Development and at each facility (Approval date of Registry and the Registration No. of the study/trial: 868, August 1, 2015).
Informed consent: Written informed consent was obtained from all participants.
Registry and the registration no. of the study/trial: UMIN0000023420, August, 2016.
Animal studies: N/A.
Supporting information
Appendix S1 | DREAMBee Study Gestational Diabetes Mellitus Group.
Appendix S2 | Details of obtained data.
Table S1 | Characteristics of mid‐to‐late diagnosed gestational diabetes mellitus and NGT.
Table S2 | Pregnancy outcomes of mid‐to‐late‐diagnosed gestational diabetes mellitus and NGT.
Table S3 | The multiple logistic regression analysis in preterm north, small‐for‐gestational age, low‐birth‐weight, and NICU admission with the mid‐to‐late pregnancy diagnosed gestational diabetes mellitus group using the NGT group as a reference category.
ACKNOWLEDGMENTS
This work was supported by a grant from the Japanese Society of Diabetes and Pregnancy and the Manpei Suzuki International Prize for Diabetes and Research. The authors thank DREAMBee for the gestational diabetes mellitus group (Appendix S1). The authors thank Dr Rintaro Mori of the current United Nations Population Fund Asia Pacific Regional Office, Dr Erika Ota, a professor of the International Nursing Department of Seiroka International University, and Mr Kenji Takehara, National Center for Child Health and Development, for providing design advice and support for the secretariat. Mr Mikami, National Center for Child Health and Development Policy Science Research, for providing administrative assistance, such as data management. The authors thank Ms Sie Saito, Ms Momoko Shiizu, Ms Katusra Yasuda, Ms Yoko Kasachi, and Ms Chiharu Matsuzawa, National Center for Child Health and Development, for helping with data management, and the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
[Correction added on March 08 2023, after first online publication: Author name “Atsuo Itakura” has been corrected.]
REFERENCES
- 1. McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers 2019; 5: 47. [DOI] [PubMed] [Google Scholar]
- 2. Pirkola J, Pouta A, Bloigu A, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care 2010; 33: 1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dabelea D, Mayer‐Davis EJ, Lamichhane AP, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case‐control study. Diabetes Care 2008; 31: 1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA 2001; 286: 2516–2518. [DOI] [PubMed] [Google Scholar]
- 5. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 6. Lowe WL Jr, Scholtens DM, Lowe LP, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018; 320: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowe WL Jr, Scholtens DM, Kuang A, et al. Hyperglycemia and adverse pregnancy outcome follow‐up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019; 42: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnsson IW, Haglund B, Ahlsson F, et al. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes 2015; 10: 77–83. [DOI] [PubMed] [Google Scholar]
- 9. Hedderson M, Ehrlich S, Sridhar S, et al. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012; 35: 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boffetta P, McLerran D, Chen Y, et al. Body mass index and diabetes in Asia: a cross‐sectional pooled analysis of 900,000 individuals in the Asia cohort consortium. PLoS One 2011; 6: e19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fakhrul‐Alam M, Sharmin J, Mashfiqul H, et al. Insulin secretory defect may be the major determinant of GDM in lean mothers. J Clin Transl Endocrinol 2020; 20: 100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanson MA, Bardsley A, De‐Regil LM, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: "think nutrition first". Int J Gynaecol Obstet 2015; 131(Suppl 4): S213–S253. [DOI] [PubMed] [Google Scholar]
- 13. Association MaCsHaW . 2021.
- 14. Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morikawa M, Yamada T, Yamada T, et al. Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract 2010; 90: 339–342. [DOI] [PubMed] [Google Scholar]
- 16. Sugiyama T, Nagao K, Metoki H, et al. Pregnancy outcomes of gestational diabetes mellitus according to pre‐gestational BMI in a retrospective multi‐institutional study in Japan. Endocr J 2014; 61: 373–380. [DOI] [PubMed] [Google Scholar]
- 17. Michikawa T, Nitta H, Nakayama SF, et al. Baseline profile of participants in the Japan environment and Children's study (JECS). J Epidemiol 2018; 28: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sasaki H, Arata N, Tomotaki A, et al. Time course of metabolic status in pregnant women: the Japan Environment and Children's Study. J Diabetes Investig 2020; 11: 1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Itoh H, Aoyama T, Kohmura‐Kobayashi Y, et al. Editorial: a half‐century history of nutritional guidance for pregnant women in Japan: a promising research target of the DOHaD study. Front Endocrinol (Lausanne) 2022; 13: 942256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morisaki N, Nagata C, Jwa SC, et al. Pre‐pregnancy BMI‐specific optimal gestational weight gain for women in Japan. J Epidemiol 2017; 27: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enomoto K, Aoki S, Toma R, et al. Pregnancy outcomes based on pre‐pregnancy body mass index in Japanese women. PLoS One 2016; 11: e0157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science (New York, NY) 2004; 305: 1733–1736. [DOI] [PubMed] [Google Scholar]
- 23. Itakura AA, Umazume T, Sago H, et al. Guidelines for weight gain during pregnancy (commentary of the JSOG perinatal committee). Acta Obstet Gynaecol Jpn 2021; 73: 678–679. [Google Scholar]
- 24. Japanese Ministry of Health Law . Dietary guidelines during pregnancy and after childbirth. Available from: https://www.mhlw.go.jp/seisakunitsuite/bunya/kodomo/kodomo_kosodate/boshi‐hoken/ninpu‐02.html Accessed January 30, 2023.
- 25. Yang L, Yamamoto‐Hanada K, Ishitsuka K, et al. Medical and surgical complications in pregnancy and obstetric labour complications in the Japan environment and Children's study (JECS) cohort: a birth cohort study. J Obstet Gynaecol 2020; 40: 918–924. [DOI] [PubMed] [Google Scholar]
- 26. Shinohara S, Amemiya A, Takizawa M. Association between false positive glucose challenge test results and large‐for‐gestational‐age infants: a retrospective cohort study. BMJ Open 2020; 10: e034627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yee LM, Cheng YW, Liddell J, et al. 50‐gram glucose challenge test: is it indicative of outcomes in women without gestational diabetes mellitus? J Matern Fetal Neonatal Med 2011; 24: 1102–1106. [DOI] [PubMed] [Google Scholar]
- 28. Boriboonhirunsarn D, Sunsaneevithayakul P. Relationship between 50‐g glucose challenge test and large for gestational age infants among pregnant women without gestational diabetes. J Obstet Gynaecol 2019; 39: 141–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | DREAMBee Study Gestational Diabetes Mellitus Group.
Appendix S2 | Details of obtained data.
Table S1 | Characteristics of mid‐to‐late diagnosed gestational diabetes mellitus and NGT.
Table S2 | Pregnancy outcomes of mid‐to‐late‐diagnosed gestational diabetes mellitus and NGT.
Table S3 | The multiple logistic regression analysis in preterm north, small‐for‐gestational age, low‐birth‐weight, and NICU admission with the mid‐to‐late pregnancy diagnosed gestational diabetes mellitus group using the NGT group as a reference category.


