Abstract

Asthma is a chronic respiratory disease. Bergamot essential oil (BEO) is extracted from the bergamot peel, which is widely used as a medicinal and food plant in China. Modern pharmacological studies have confirmed that BEO has anti-inflammatory properties, suggesting potential in treating asthma. First, the main active ingredients of BEO were detected and analyzed by gas chromatography–mass spectrometry (GC-MS). Network pharmacology methods were used to explore the possible core targets and main pathways of BEO in asthma treatment. Then ovalbumin (OVA)-induced in vivo and lipopolysaccharide (LPS)-induced in vitro models were established to investigate the antiasthmatic effects of BEO. BEO showed a good antiasthmatic effect by improving lung inflammation and inhibiting collagen deposition. Then, enzyme-linked immunosorbent assay (ELISA) and quantitative real-time polymerase chain reaction (qPCR) were used to explore the possible mechanism of BEO in asthma treatment. Furthermore, experimental verification showed that BEO could suppress the release of inflammatory factors in vitro and inhibit the activation of MAPK and JAK-STAT signaling pathways. This study demonstrated the anti-inflammatory effects of BEO against asthma. Moreover, it supplies a theoretical basis for the clinical application of BEO.
1. Introduction
Asthma is a chronic inflammatory airway disease that can develop onset in all age groups and is characterized by airway inflammation and hyperresponsiveness. During the second half of the past century, the number of asthma cases rapidly increased worldwide, causing at least 495000 deaths per year.1,2 The factors that play a role in asthma pathogenesis are mainly viral or bacterial respiratory tract infections, allergy or defective antiviral immunity, allergen exposure, and environmental factors.3 Childhood asthma is more common in boys, while adult asthma is more common in women, which might may be related to sex hormones.4 It is currently believed that the pathogenesis of asthma is related to inflammation and immune regulation.5 Current asthma treatments utilize inhaled corticosteroids, long-acting β-agonists, and leukotriene antagonists to control and reduce asthma exacerbations.3 However, these approaches do not eliminate all exacerbations, particularly in patients with severe asthma.6 Targeted biological therapy is also being developed, such as anti-IgE (omalizumab) and anti-interleukin (IL)-5 medications.3 Moreover, the current treatment methods are insufficient due to drug resistance and side effects.7 Thus, because existing approaches are insufficient for asthma management, the need for new interventions is urgent.
Traditional Chinese medicine (TCM) has a long history in the treatment of asthma, called “Xiao Zheng”. Numerous clinical cases have confirmed the therapeutic effect of Chinese medicine on asthma due to the synergistic effect of each component and target of Chinese medicine and the low level of toxic side effects.8
Natural products have potential advantages in anti-inflammation. As an increasing number of studies are performed, more and more mechanisms of anti-inflammatory action of herbal medicines are being discovered.9−12 In recent years, essential oils have been greatly developed for their remarkable biological activity and health benefits, playing a huge role as a safe alternative therapy.13 Bergamot (Citrus medica L. var. sarcodactylis Swingle) is an important edible and medicinal plant.14 Bergamot essential oil (BEO), extracted from the bergamot peel, has significant antiallergic, anti-inflammatory, analgesic, and anticancer effects.15,16
Terpenoids such as d-limonene and γ-pinene are the main components of BEO. d-Limonene relaxes the tracheal smooth muscle of guinea pigs.17 Inhaling d-limonene reduces peribronchial and perivascular inflammatory cell infiltration and prevents bronchial obstruction.18 Molecular docking and animal studies have revealed that d-limonene reduces airway reactivity and inflammation through the activation of A2A and A2B.19 Combined with the anti-inflammatory effect of BEO, we speculate that d-limonene has a certain role in treating asthma and relieving airway inflammation.
TCM offers distinct advantages in disease treatment because it emphasizes the holistic concept. The composition of TCM drugs is complicated, and there has been a lack of effective research methods.20 Network pharmacology is an effective method for us to understand Chinese medicine treating different diseases.21 Gene targets predicted by chemical components of TCM drugs have been associated with disease genes by building a relevant disease gene library. Constructing protein–protein interaction (PPI) networks to identify protein–drug relationships and selecting components and regulatory gene targets that play a role in a disease can be used to understand the mechanisms of action of herbal medicines in treating that disease.22,23 With network pharmacology, we can understand and utilize drugs more precisely to intervene in diseases.24 Cyber pharmacology is an effective way to understand how we can treat diseases with TCM. By building a library of relevant disease genes, gene targets predicted by the chemical composition of TCM are associated with disease genes.
In this study, gas chromatography–mass spectrometry (GC-MS) was used to qualitatively and quantitatively analyze the chemical composition of BEO. GC-MS is a more precise and accurate method of compositional analysis than high-performance liquid chromatography (HPLC) and liquid chromatography–mass spectrometry (LC-MS).25 Then, a network pharmacology strategy was applied to obtain the potential targets, underlying pathways, and the therapeutic mechanism of BEO against asthma. Finally, we validated the predicted pharmacologic mechanism of BEO in vivo and in vitro. Our study is the first to demonstrate the effect of BEO on asthma using a network pharmacology method combined with experiments. Currently, there is a limited number of drugs for the treatment of asthma, which cannot fully meet the needs of clinical treatment and have many side effects after prolonged use. Thus, there is an urgent need for new drugs, and BEO from natural plants is a safer and effective option, providing prospects for the development of new drugs.
2. Results
2.1. Identification of Main Components of BEO and Comparison with the Standard
There was a total of 27 components identified, the majority of which were terpene components. The proportion of d-limonene and γ-terpinene in BEO was 42.5% and 18.9%, respectively. The main components were compared with the standard d-limonene (S1) and γ-pinene (S2) to ensure the quality of the BEO, and the main components and total ion chromatogram monitored are shown in Figure S1 and Table S1.
2.2. Obtained BEO Component Targets and Asthma Disease Targets
After removing duplicates, 365 putative targets of BEO components were obtained from the Swiss Target Prediction and Drugbank databases. By intersecting disease targets with the constituent targets of BEO, 224 targets were obtained. These are thought to be potential targets for BEO asthma treatment.
2.3. PPI Network Construction and Key Target Selection
The PPI network was visualized and analyzed by Cytoscape software, and the values of Betweenness Centrality, Closeness Centrality, and Degree were calculated by using plug-in network analysis. The median values were calculated separately, and the genes with topological feature values greater than the median were selected as the core genes (Betweenness Centrality > 0.001018, Closeness Centrality > 0.301724, and Degree > 3). The PPI networks are shown in Figures 1 and 2.
Figure 1.
PPI network diagram with interaction score set to 0.9.
Figure 2.

Diagram of PPI key targets. Larger circles and darker red in the figure represent more important targets.
2.4. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
The above-mentioned 46 key targets were recorded into the DAVID database for Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment to investigate the potential mechanism of BEO in asthma treatment. As shown in the graph, biological process (BP), cellular component (CC), and molecular function (MF) were among the top ten processes. Thus, the biological processes involved in BEO action in asthma were the JAK-STAT cascade reaction involved in the growth hormone signaling pathway, the positive regulation of transcription by RNA polymerase II promoter, the cellular response to lipopolysaccharide, signal transduction, cell differentiation, intracellular signaling, protein phosphorylation, the cellular response to reactive oxygen species, the interleukin-6 mediated signaling pathway.
The data from the KEGG enrichment analysis obtained in the DAVID database were processed. As the disease was caused by an underlying biological dysfunction, the enriched disease fraction was removed. The top 20 pathways were selected as the main pathways for the treatment of asthma with BEO. The smaller the value of P adjusted, the more significant the pathway, suggesting a higher correlation with the disease. The enriched pathways were classified into inflammatory immune-related pathways (JAK-STAT signaling pathway, IL-17 signaling pathway, tumor necrosis factor signaling pathway, T-cell receptor signaling pathway, nuclear factor-kappa B signaling pathway, Fc epsilon RI signaling pathway, chemokine signaling pathway, natural killer cell-mediated cytotoxicity, nod-like receptor signaling pathway, adipocytokine signaling pathway, and toll-like receptor signaling pathway), signal transduction-related pathways (PI3K-Akt signaling pathway, FoxO signaling pathway, Ras signaling pathway, ErbB signaling pathway, MAPK signaling pathway, Rap1 signaling pathway, and cAMP signaling pathway), and circulatory system-related pathways (VEGF signaling pathway and HIF-1 signaling pathway), as shown in Figures 3 and 4.
Figure 3.

Bubble diagram of KEGG enrichment analysis. Analyses of the 46 key targets using the KEGG database.
Figure 4.
Histogram of GO enrichment analyses. GO enrichment analyses of 46 key targets by the DAVID database. Green represented the biological process (BP), orange represented the cellular component (CC), and blue represented the molecular function (MF).
2.5. Compound-Target-Pathway Network Analysis
Compounds, gene targets, and the top 20 pathways were imported into Cytoscape software, and a network was drawn for visual analysis. The compounds and targets were sorted by Degree value, from the highest to the lowest, with higher values indicating greater importance in the network. Among them, the key targets were mainly concentrated in the MAPK and JAK-STAT signaling pathways. The top ten key active ingredients, as predicted by the network pharmacology, were linoleic acid, α-linolenic acid, methyl linoleate, 11,14,17-eicosatrienoic acid, methyl ester, neryl acetate, n-hexadecanoic acid, dodecanoic acid, tetradecanoic acid, α-bisabolene, and α-terpineol, as shown in Figure 5.
Figure 5.
Compound-target-pathway network diagram. The green octagon represents the 27 compounds in BEO, the pink circular represents the key targets, the orange target represents the gene targets, and the violet diamond represents the top 20 pathways The key signaling pathways, i.e., MAPK and JAK-STAT signaling pathways, are listed separately in the blue diamond.
2.6. BEO Can Alleviate OVA-Induced Asthma In Vivo
An ovalbumin (OVA)-induced mouse model was constructed to verify the effect of inhaled BEO on asthma relief. Figure 6 shows a significant infiltration of inflammatory cells around the bronchi, narrowing of the airways, thickened bronchial airway walls, and collagen deposition around the airways in the model group compared to the control group. In the control group, lung tissue structure was normal, with no obvious inflammatory changes. After inhaling BEO, different degrees of improvement in lung inflammation and airway narrowing were observed by staining the inflammatory cells in bronchoalveolar lavage fluid (BALF). As shown in Figure 7, the amount of inflammatory cells in the model group was significantly higher than that in the control group. There were significantly fewer positive cells in the BEO and budesonide groups. These findings suggested that inhaling BEO could significantly reduce lung inflammation. IL-4, IL-5, and IL-13 levels in BALF and lung tissue were measured using enzyme-linked immunosorbent assay (ELISA) and quantitative polymerase chain reaction (qPCR). When compared to the model group, different doses of BEO could reduce cytokine release and mRNA expression as shown in Figure 8 A-F. IL-6, IL-1β, and tumor necrosis factor (TNF)-α were common inflammatory factors. The ameliorating effect of BEO on airway inflammation in asthma could be revealed by measuring the release of inflammatory factors by ELISA as shown in Figure 8G–I. The detection of immunoglobulin (Ig)E in serum revealed that the BEO group had markedly decreased serum IgE levels as shown in Figure 8J. These findings provide additional evidence that BEO could be used to treat asthma.
Figure 6.
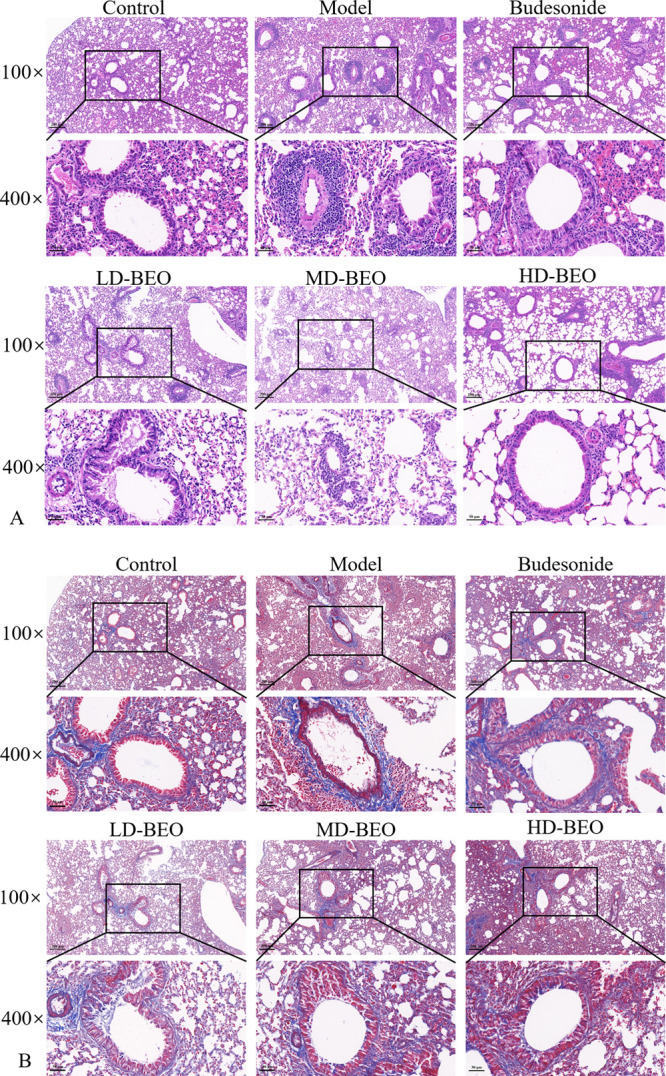
Lung tissue sections of each group with hematoxylin–eosin (HE) (A) and Masson staining (B).
Figure 7.

Total inflammatory cells in mice BALF were isolated and quantified using a Diff-Quick-stained reagent.
Figure 8.
Cytokines detected by qPCR and ELISA. Effect of inhaled BEO on the levels of IL-4, IL-5, and IL-13 in BALF (A–F) and IL-6, IL-1β, and TNF-α in BALF (G–I). Measurement of IgE levels in serum (J). Data are presented as the mean ± standard deviation (n = 3). #p < 0.05 and ###p < 0.001 vs the control group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs the model group.
2.7. Exploration of the Potential Mechanism of BEO in the Treatment of Asthma
We then performed a further experimental analysis of the core targets, combining the above-demonstrated network pharmacology results, to investigate the possible mechanism of BEO against asthma. BEO concentrations above 100 μg/mL could not be completely dissolved in the solvent, and large doses could also be considered ineffective drugs. Therefore, a BEO concentration of 100 μg/mL was chosen as the highest dose for the experiment. The CCK8 experiment demonstrated that there was no significant effect of BEO on MH-S at concentrations of 0–100 μg/mL, no significant decrease in cell viability, and no statistical difference between the groups, as shown in Figure 9A. Chronic inflammation is the underlying cause of asthma, and IL-6, IL-1β, and TNF-α are key proinflammatory factors. Thus, after lipopolysaccharide (LPS) treatment, there was a considerable increase in the mRNA and protein expression of IL-6, IL-1β, and TNF-α. According to results from qPCR and ELISA, the mRNA and protein expression of IL-6, IL-1β, and TNF-α were dramatically reduced after the addition of BEO. BEO could therefore effectively prevent the increase in of inflammatory factors, as shown in Figure 9B–G.
Figure 9.
(A) Effect of BEO on cell viability of MH-S cells. MH-S cells were incubated with absolute ethanol (solvents for BEO) for 24 h as the control group. MH-S cells were incubated with different concentrations (0.01, 0.1, 1, 10, and 100 μg/mL) of BEO for 24 h as the administration group. CCK-8 assay was used to detect cell viability. Data are presented as the mean ± SD (n = 10) and compared with the control group. (B–D) Effect of BEO on mRNA expression of IL-6, IL-1β, and TNF-α in MH-S cells as detected by qPCR. (E–G) The effect of BEO on the expression of inflammatory factors IL-6, IL-1β, and TNF-α in MH-S cells was detected by ELISA. MH-S cells were incubated with absolute ethanol as the control group. MH-S cells were incubated with BEO (1, 10, and 100 μg/mL) and LPS (0.1 μg/mL) for 24 h as the administration group. Data are presented as the mean ± SD (n = 3). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs the control group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs the model group.
2.8. Verification of Targets
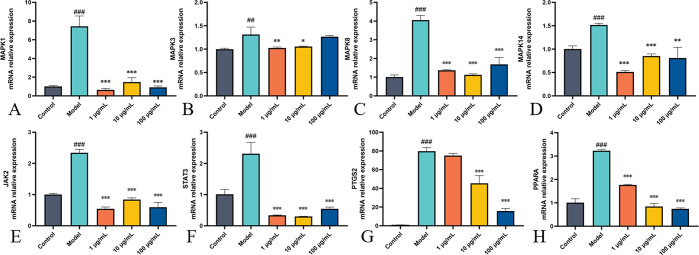
By using qPCR, the major targets of BEO for asthma treatment that network pharmacology predicted were validated. MAPK1, MAPK3, MAPK8, MAPK14, PPARA, PTGS2, STAT3, and JAK2 had a significantly greater mRNA expressions in the cells following LPS stimulation compared to the control group, and all mRNA expressions of the above-mentioned genes decreased to varying degrees after BEO administration. The findings showed that TCM has multi-pathway effects in asthma treatment and that the targets predicted by network pharmacology were trustworthy, as shown in Figure 10.
Figure 10.
Effects of BEO on mRNA expression of key genes in MH-S cells. (A–H) The mRNA expressions of MAPK1, MAPK3, MAPK8, MAPK14, JAK2, STAT3, PTGS2, and PPARA were detected by qPCR. MH-S cells were incubated with absolute ethanol as a control group. MH-S cells were incubated with BEO (1, 10, and 100 μg/mL) and LPS (0.1 μg/mL) for 24 h as the administration group. Data are present the mean ± SD (n = 3). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs the control group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs the LPS group.
3. Discussion
The inflammatory response is an important molecular mechanism in asthma, and controlling the inflammatory response is the main treatment approach for asthma.26−28 Currently, steroid hormones are considered the best way to control asthma inflammation, but they also do not cure or alter disease progression and have side effects with long-term use.8 There is a lack of experimental validation and mechanistic exploration of the mechanisms of Chinese herbal medicine in asthma treatment. BEO is a sesquiterpene compound derived from bergamot—a traditional Chinese herb commonly used to treat asthma, cough, and other respiratory disorders. BEO has demonstrated good anti-inflammatory effects in asthmatic mice.29
In this study, we verified the effect of BEO in the treatment of OVA-induced asthma in mice. After OVA induction, mice showed increased inflammatory exudation and collagen deposition around the airways. We found that BEO effectively ameliorated the inflammatory changes around the airways and reduced the levels of inflammatory factors in BALF and IgE levels in serum. BEO also reduced the expression levels of asthma-related cytokines IL-4, IL-5, and IL-13. Combining compositional analysis, network pharmacology, and in vitro experiments, we explored the potential mechanisms of BEO for asthma treatment. The predicted relevant pathways and targets were mainly focused on MAPK and JAK-STAT signaling pathways. In asthma, the MAPK signaling pathway is primarily involved in inflammatory cell infiltration and immune response activation. Activation of the MAPK signaling pathway stimulates immune cells to produce a response releasing inflammatory factors and activating goblet cells to produce mucus, leading to airway hyperresponsiveness and mucus obstruction of the airway.30−35 Bai et al. have found that OVA-induced inflammatory response to asthma could be attenuated by inhibiting MAPK activation.36 JAK-STAT is involved in inflammatory responses and immune regulation in asthma.37 A large body of clinical evidence also supports the idea that inhibiting the activation of targets in the JAK-STAT pathway can reduce asthma airway inflammation and hyperresponsiveness.38,39 Calbet et al. have demonstrated that the inhalation of Pan-JAK inhibitor reduced airway inflammation and improved lung function in a rat OVA model40 Southworth et al. have demonstrated that JAK inhibitors reduce the production of inflammatory cytokines in asthma.41 Prostaglandin endoperoxide synthase 2 (PTGS2)/COX-2 is a key enzyme in prostaglandin biosynthesis.42−44 Reducing the expression of PTGS2 further reduces the production of prostaglandin E2, decreases the inflammatory response, and reduces the possibility of inducing airway spasms and airway hyperresponsiveness.45,46 Peroxisome proliferator-activated receptor alpha (PPARA) influences the expression of target genes involved in immune and inflammatory responses.47,50 Previous studies have shown that PPARA is involved in lipid mediators common in the inflammatory response.48,49 PPARA plays an important role in controlling the duration of the inflammatory response by regulating the metabolism of fatty acids and oxidative degradation of derivatives, which can subside.50,51 Based on the experimental results, we found that BEO may have a positive effect on the treatment of asthma by downregulating MAPK, JAK-STAT pathway, and key genes PPARA and PTGS2.
Figure 11.
Description of the main putative target of BEO for the treatment of airway inflammation of asthma. Through this analysis, the predicted targets in this project all regulate immune response and inflammatory factor secretion. The key targets are colored with yellow, and the pink prisms represent the BEO. BEO alleviates asthma by inhibiting these targets.
4. Conclusion
In summary, the animal experiment has shown that BEO can be used to treat asthma. As the key targets predicted by network pharmacology and experimental validation, BEO might suppress the inflammation in asthma by modulating the MAPK pathway, JAK-STAT pathway PPARA, and PTGS2. However, there are shortcomings in our study, and further research is needed to determine whether there is a link between the active ingredient content of BEO and its antiasthma effects. Additionally, the safety of inhaled BEO needs to be investigated over the long term. BEO, a plant essential oil derived from a natural product, has great potential in relieving asthma. Our study provides a useful theoretical basis for investigating the antiasthma mechanism of BEO and offers new ideas for the development of asthma drugs.
5. Materials and Methods
5.1. Preparation of Bergamot Essential Oil
Bergamot was purchased from Lingnan Chinese Medicine (Guangzhou, China) and identified by Doctor Ding Qi (Shenzhen Research Institute, Beijing University of Chinese Medicine, Beijing, China) as the dried fruit of Citrus medica L. var. sarcodactylis Swingle. Bergamot was crushed, soaked in 10 times the amount of purified water for 30 min, and then distilled by hydrodistillation for 2 h to obtain BEO. BEO was stored at −4 °C and used for detection and experimental validation.
5.2. GC-MS
Component identification was performed by gas chromatography–mass spectrometry. GC conditions in a Shimadzu gas chromatograph (GC 2010 plus, Kyoto, Japan) were column DB-5MS, carrier gas high-purity helium, flow rate inlet temperature 280 °C, 15 °C/min until 150 °C, and 20 °C/min until 280 °C. Injection method: split; detector FID1 split ratio: 10:1. MS conditions were ion source temperature 230 °C, interface temperature 280 °C, and solvent delay time 1.5 min.
5.3. Target Prediction of Identified Components in BEO
The 2D structure of the chemical composition was obtained by searching the composition of BEO in Pubchem (https://pubchem.ncbi.nlm.nih.gov/).52 The structures were obtained by searching in Swiss target prediction (http://swisstargetprediction.ch/),53 and Drugbank (https://go.drugbank.com)54 was used to obtain the targets. Swiss Target Prediction is based on the known 2D and 3D structural similarities of compounds to predict the targets of compounds. Prediction can be performed in three different species: humans, rats, and mice. DrugBank is a bioinformatics and cheminformatics database provided by the University of Alberta that combines detailed drug data with comprehensive drug target information to predict drug targets and chemical composition. Detailed information is provided in Table S2.
5.4. Target Prediction in Asthma
By searching the keywords “asthma” in the Genecard and CTD databases, a total of 3829 asthma-related targets were identified. The Genecards database (https://www.genecards.org/)55 contains disease relationships, gene expression, gene function, and protein–protein interactions and is a comprehensive database providing human genetic predictions. The CTD database (http://ctdbase.org/)56 is an integrated database of a large number of chemicals, genes, functional phenotypes, and disease interactions. Detailed information is provided in Table S3.
5.5. Protein–Protein Interaction Network Construction and Core Target Selection
The above-mentioned potential targets of BEO for asthma were imported into the String database (https://cn.string-db.org/)57 to construct PPI protein interaction networks. Then the obtained PPI networks were imported into Cytoscape 3.9.0 software (Boston, MA) to construct visual networks. A network analyzer plug-in was used to calculate Betweenness Centrality, Closeness Degree, and Degree. Detailed information is provided in Table S4.
5.6. GO and KEGG Signaling Pathway Analysis
The collected potential targets were imported into the DAVID database (https://david.ncifcrf.gov/)58 for GO enrichment and KEGG enrichment analyses. The GO enrichment analysis included BP, CC, and MF. We selected P(adjusted) < 0.05 as the confidence gene enrichment threshold setting.
5.7. Animal Experiments
Balb/c female mice aged 6–8 weeks were purchased from Guangzhou Ruige Biological Technology Co., Ltd. (Guangzhou, China; animal license: SCXK (Yue) 2021-0059). Mice were divided into six groups 1 week after acclimatization: control, model, budesonide (AstraZeneca Pty Ltd. H20140475, North Ryde, Australia, 0.2 mg/mL), BEO low dose (20 mg/kg), BEO medium dose (40 mg/kg), and BEO high dose (80 mg/kg). The method of administration was inhalation. Each group had five mice. During the experiment, the mice were fed and watered as needed and kept in a 12/12 h light/dark cycle. Except for the control group, mice were sensitized on days 0, 7, and 14 via intraperitoneal injection of 200 μL phosphate-buffered saline (PBS) (Gibco, New York) containing 10 μg of OVA (Sigma, St. Louis, MO) and 1 mg of aluminum hydroxide (Aladdin, Shanghai, China).59 On days 21–27, mice were nebulized for 30 min per day with 1% OVA and budesonide, with three BEO dose groups administered 30 min before each nebulization. On the 28th day, mice were sacrificed, and lung tissue, BALF, and blood were collected. The modeling method is shown in Figure 12.
Figure 12.
Process of OVA-induced allergic asthma.
5.8. Histopathology and Stained Inflammatory Cells in BALF
Lung tissue samples were fixed in a 4% paraformaldehyde (Biosharp, Shanghai, China) solution for 48 h and then embedded in paraffin wax for fixation. Paraffin-embedded sections were stained using hematoxylin–eosin (HE) and Masson trichrome staining to assess pathological changes in the lungs. The cell precipitate obtained after centrifugation of the collected BALF was resuspended in 100 μL of PBS to make smears, which were then stained with Diff-Quick Stain (Solarbio, Beijing, China) according to the manufacturer’s instructions. Inflammatory cells were observed under a light microscope.
5.9. Cell Culture and Treatment
Mouse alveolar macrophages (MH-S) were purchased from Shanghai Fu Heng Bio, (Shanghai, China). MH-S were cultured in 1640 medium (Gibco, New York) with 10% fetal bovine serum (FBS) (Gibco, New York) at 37 °C and 5% CO2 in an incubator. The inflammation model was induced using LPS (Sigma, St. Louis, MO, 0.1 mg/mL).60,61
5.10. BEO Toxicity Test
The Cell Counting Kit-8 (CCK-8, MCE, Monmouth Junction, NJ) assay was used to detect the effect of BEO on the growth of MH-S cells and test whether BEO has toxic effects on MH-S cells. MH-S cells were inoculated in 96-well plates with 10000 cells per well at 100 μL medium. After 24 h of inoculation and 4 h of starvation, the cells were treated with 0.01, 0.1, 1, 10, and 100 μg/mL of BEO, and 10 μL of CCK-8 solution was added to each well after 24 h of incubation. The cells were incubated for 1 h at 37 °C in a 5% CO2 incubator, and the absorbance was measured at 450 nm on an enzyme marker.
5.11. Quantitative Real-Time Polymerase Chain Reaction
MH-S cells were inoculated in the six-well plate at 4 × 105 per well, cultured overnight, starved for 4 h, and then administered at 0, 1, 10, and 100 μg/mL. After stimulating the cells with LPS (0.1 μg/mL) for 24 h, lung tissue samples were extracted using an RNA extraction kit. Total RNA was extracted with an RNA extraction kit (Tian Gen, Beijing, China) from the MH-S cells, and RNA concentration was measured before reverse transcription (HiScript II Q RT SuperMix, Vazyme, Nanjing, China). Total RNA (1000 ng) was further reverse transcribed into cDNA. The reversed samples were cyclically amplified with ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) in a RocheLightCycler 480 (Roche, Basel, Switzerland) using GAPDH as the internal reference gene. The relative expression levels of the target genes were determined using the 2–ΔΔct calculation method. Primers were purchased from Sangon Biotech (Shanghai, China), and detailed information is shown in Table 1.
Table 1. Primers Used for Quantitative Real-Time Polymerase Chain Reaction (QPCR) Assay.
| target | forward (5′–3′) | reverse (5′–3′) |
|---|---|---|
| GADPH | AAATGGTGAAGGTCGGTGTGAAC | CAACAATCTCCACTTTGCCACTG |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| IL-6 | AGTCCTTCCTACCCCAATTTCC | TGGTCTTGGTCCTTAGCCAC |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| MAPK1 | GGTTGTTCCCAAATGCTGACT | CAACTTCAATCCTCTTGTGAGGG |
| MAPK3 | TCCGCCATGAGAATGTTATAGGC | GGTGGTGTTGATAAGCAGATTGG |
| PPARA | AGAGCCCCATCTGTCCTCTC | ACTGGTAGTCTGCAAAACCAAA |
| PTGS2 | TTCAACACACTCTATCACTGGC | AGAAGCGTTTGCGGTACTCAT |
| MAPK8 | AGCAGAAGCAAACGTGACAAC | GCTGCACACACTATTCCTTGAG |
| JAK2 | TTGTGGTATTACGCCTGTGTATC | ATGCCTGGTTGACTCGTCTAT |
| MAPK14 | TGACCCTTATGACCAGTCCTTT | GTCAGGCTCTTCCACTCATCTAT |
| STAT3 | CAATACCATTGACCTGCCGAT | GAGCGACTCAAACTGCCCT |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| IL-5 | CTCTGTTGACAAGCAATGAGACG | TCTTCAGTATGTCTAGCCCCTG |
| IL-13 | CCTGGCTCTTGCTTGCCTT | GGTCTTGTGTGATGTTGCTCA |
5.12. Enzyme-Linked Immunosorbent Assay
MH-S cells were inoculated in a 12-well plate at 1 × 105 per well, cultured overnight, and starved of cells for 4 h. Then, BEO was administered at 0, 1, 10, and 100 μg/mL after the cells were stimulated with LPS (0.1 μg/mL) for 24 h prior to cell supernatant collection. BALF was processed as required by the ELISA kit. The cytokine content was measured using an ELISA kit (ABclonal double antibody sandwich, Wuhan, China; Proteintech, ELISA kit, Chicago, IL; Biolegend, ELISA kit, California).
5.13. Statistical Analysis
All experimental data for this study were obtained from the standard deviation of three independent experiments. Statistical analysis was performed using GraphPad Prism 8 (GraphPad, San Diego, CA). One-way ANOVA was used for multiple samples. A p of <0.05 indicated a statistically significant difference between the groups.
Acknowledgments
The authors thank Dongyang Gao for his technical assistance.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07366.
Figure S1: (A) total ion chromatograms and major component identification chromatograms; (B) Standards d-limonene (S1) and γ-terpinene (S2); Table S1: main components of BEO were identified by GC-MS analysis; Table S2: drug targets; Table S3: disease targets; Table S4: details of the PPI network (PDF)
This study was supported by the Basic Research Project of the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20210324135410028), Science, Technology and Innovation Commission of Shenzhen Municipality (JSGG20220226090203006), and Nebulized inhalation herbal product development (HSZY0002-01).
The authors declare no competing financial interest.
Supplementary Material
References
- Papi A.; Brightling C.; Pedersen S. E.; Reddel H. K. Asthma. Lancet 2018, 391 (10122), 783–800. 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- Crisford H.; Sapey E.; Rogers G. B.; Taylor S.; Nagakumar P.; Lokwani R.; Simpson J. L. Neutrophils in asthma: the good, the bad and the bacteria. Thorax 2021, 76 (8), 835–44. 10.1136/thoraxjnl-2020-215986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J. R.; Peters S. P.; Busse W. W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin Immunol Pract. 2017, 5 (4), 918–927. 10.1016/j.jaip.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J.; Pier J.; Litonjua A. A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42 (1), 5–15. 10.1007/s00281-020-00785-1. [DOI] [PubMed] [Google Scholar]
- Finotto S. Resolution of allergic asthma. Semin Immunopathol. 2019, 41 (6), 665–674. 10.1007/s00281-019-00770-3. [DOI] [PubMed] [Google Scholar]
- Haldar P.; Brightling C. E.; Hargadon B.; Gupta S.; Monteiro W.; Sousa A.; Marshall R. P.; Bradding P.; Green R. H.; Wardlaw A. J.; Pavord I. D. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J. Med. 2009, 360 (10), 973–84. 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsahai J. M.; Hansbro P. M.; Wark P. A. B. Mechanisms and Management of Asthma Exacerbations. Am. J. Respir Crit Care Med. 2019, 199 (4), 423–432. 10.1164/rccm.201810-1931CI. [DOI] [PubMed] [Google Scholar]
- Wang W.; Yao Q.; Teng F.; Cui J.; Dong J.; Wei Y. Active ingredients from Chinese medicine plants as therapeutic strategies for asthma: Overview and challenges. Biomed. Pharmacother. 2021, 137, 111383. 10.1016/j.biopha.2021.111383. [DOI] [PubMed] [Google Scholar]
- Yi T.; Zhao Z.-Z.; Yu Z.-L.; Chen H.-B. Comparison of the anti-inflammatory and anti-nociceptive effects of three medicinal plants known as “Snow Lotus” herb in traditional Uighur and Tibetan medicines. J. Ethnopharmacol. 2010, 128 (2), 405–11. 10.1016/j.jep.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Guo H.; Zhang Y.; Cheng B. C.-Y.; Lau M.-Y.; Fu X.-Q.; Li T.; Su T.; Zhu P.-L.; Chan Y.-C.; Tse A. K.-W.; Yi T.; Chen H.-B.; Yu Z.-L. Comparison of the chemical profiles and inflammatory mediator-inhibito ry effects of three Siegesbeckia herbs used as Herba Siegesbeckiae (Xi xiancao). BMC Complement Altern Med. 2018, 18 (1), 141. 10.1186/s12906-018-2205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P.; Zhang Z.; Yi T.; Zhang H.; Liu X.; Mo Z. Anti-inflammatory activity of the extracts and fractions from Erigeron multiradiatus through bioassay-guided procedures. J. Ethnopharmacol. 2008, 119 (2), 232–7. 10.1016/j.jep.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Luo P.; Li J.; Yi T.; Wang J.; An J.; Zhang H. Comparison of the antiinflammatory activities of three medicinal plant s known as “meiduoluomi” in Tibetan folk medicine. Yakugaku Zasshi 2008, 128 (5), 805–10. 10.1248/yakushi.128.805. [DOI] [PubMed] [Google Scholar]
- Ni Z.-J.; Wang X.; Shen Y.; Thakur K.; Han J.; Zhang J.-G.; Hu F.; Wei Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and in dustrial applications of plant essential oils. Trends in Food Science & Technology. 2021, 110, 78–89. 10.1016/j.tifs.2021.01.070. [DOI] [Google Scholar]
- Navarra M.; Mannucci C.; Delbò M.; Calapai G. Citrus bergamia essential oil: from basic research to clinical application. Front Pharmacol. 2015, 6, 36. 10.3389/fphar.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoky N. S.; Setzer W. N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19 (7), 1966. 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani A. R.; Yadav K.; Khursheed A.; Rather M. A. An updated and comprehensive review of the antiviral potential of esse ntial oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. J. Microb Pathog. 2021, 152, 104620. 10.1016/j.micpath.2020.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete A.; Ávila-Rosas N.; Majín-León M.; Balderas-López J. L.; Alfaro-Romero A.; Tavares-Carvalho J. C. Mechanism of action of relaxant effect of Agastache mexicana ssp.mexic ana essential oil in guinea-pig trachea smooth muscle. J. Pharm. Biol. 2017, 55 (1), 96–100. 10.1080/13880209.2016.1230140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinan E.; Alt A.; Amir G.; Bentur L.; Bibi H.; Shoseyov D. Natural ozone scavenger prevents asthma in sensitized rats. Bioorganic & Medicinal Chemistry 2005, 13 (2), 557–562. 10.1016/j.bmc.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Patel M.; Narke D.; Kurade M.; Frey K. M.; Rajalingam S.; Siddiquee A.; Mustafa S. J.; Ledent C.; Ponnoth D. S. Limonene-induced activation of A2A adenosine receptors reduces airway inflammation and reactivity in a mouse model of asthma. Purinergic Signal. 2020, 16 (3), 415–426. 10.1007/s11302-020-09697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T.-T.; Lu Y.; Yan S.-K.; Xiao X.; Rong X.-L.; Guo J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin J. Integr Med. 2020, 26 (1), 72–80. 10.1007/s11655-019-3064-0. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Zhu X.; Bai H.; Ning K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front Pharmacol. 2019, 10, 123. 10.3389/fphar.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J. Nat. Med. 2013, 11 (2), 110–20. 10.3724/SP.J.1009.2013.00110. [DOI] [PubMed] [Google Scholar]
- Li X.-L.; Zhang X.-X.; Ma R.-H.; Ni Z.-J.; Thakur K.; Cespedes-Acuña C. L.; Zhang J.-G.; Wei Z.-J. Integrated miRNA and mRNA omics reveal dioscin suppresses migration an d invasion via MEK/ERK and JNK signaling pathways in human endometrial carcinoma in vivo and in vitro. J. Ethnopharmacol 2023, 303, 116027. 10.1016/j.jep.2022.116027. [DOI] [PubMed] [Google Scholar]
- Nogales C.; Mamdouh Z. M.; List M.; Kiel C.; Casas A. I.; Schmidt H. H. H. W. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022, 43 (2), 136–150. 10.1016/j.tips.2021.11.004. [DOI] [PubMed] [Google Scholar]
- Yi T.; Li S.-M.; Fan J.-Y.; Fan L.-L.; Zhang Z.-F.; Luo P.; Zhang X.-J.; Wang J.-G.; Zhu L.; Zhao Z.-Z.; Chen H.-B. Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC-MS. Lipids in Health and Disease 2014, 13 (1), 190. 10.1186/1476-511X-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B. N.; Hammad H.; Fahy J. V. The Cytokines of Asthma. Immunity 2019, 50 (4), 975–991. 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Cellular and molecular mechanisms of asthma and COPD. Clin Sci. (London) 2017, 131 (13), 1541–1558. 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- Koczulla A. R.; Vogelmeier C. F.; Garn H.; Renz H. New concepts in asthma: clinical phenotypes and pathophysiological mechanisms. Drug Discov Today 2017, 22 (2), 388–396. 10.1016/j.drudis.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Changhun S.; Jianying W.; Wanping Z. Effects of volatile oil of Pterophil on eosinophils in peripheral blood, alveolar lavage fluid and lung tissue of asthmatic mice. Chinese Traditional and Herbal Drugs 2009, 40 (01), 99–101. [Google Scholar]
- Johnson G. L.; Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298 (5600), 1911–2. 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Pelaia C.; Vatrella A.; Crimi C.; Gallelli L.; Terracciano R.; Pelaia G. Clinical relevance of understanding mitogen-activated protein kinases involved in asthma. Expert Rev. Respir Med. 2020, 14 (5), 501–510. 10.1080/17476348.2020.1735365. [DOI] [PubMed] [Google Scholar]
- Chung K. F. p38 mitogen-activated protein kinase pathways in asthma and COPD. J. Chest. 2011, 139 (6), 1470–1479. 10.1378/chest.10-1914. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. New drugs for asthma. Nat. Rev. Drug Discovery 2004, 3 (10), 831–44. 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- Newton R.; Holden N. Inhibitors of p38 mitogen-activated protein kinase: potential as anti-inflammatory agents in asthma?. J. BioDrugs. 2003, 17 (2), 113–29. 10.2165/00063030-200317020-00004. [DOI] [PubMed] [Google Scholar]
- Baines K. J.; Fricker M.; McDonald V. M.; Simpson J. L.; Wood L. G.; Wark P. A. B.; Macdonald H. E.; Reid A.; Gibson P. G. Sputum transcriptomics implicates increased p38 signalling activity in severe asthma. Respirology 2020, 25 (7), 709–718. 10.1111/resp.13749. [DOI] [PubMed] [Google Scholar]
- Bai D.; Sun T.; Lu F.; Shen Y.; Zhang Y.; Zhang B.; Yu G.; Li H.; Hao J. Eupatilin Suppresses OVA-Induced Asthma by Inhibiting NF-κB and MAPK and Activating Nrf2 Signaling Pathways in Mice. Int. J. Mol. Sci. 2022, 23 (3), 1582. 10.3390/ijms23031582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino A. V.; Kanno Y.; Ferdinand J. R.; O’Shea J. J. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 2015, 194 (1), 21–7. 10.4049/jimmunol.1401867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georas S. N.; Donohue P.; Connolly M.; Wechsler M. E. JAK inhibitors for asthma. J. Allergy Clin Immunol. 2021, 148 (4), 953–963. 10.1016/j.jaci.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athari S. S. Targeting cell signaling in allergic asthma. Signal Transduct Target Ther. 2019, 4, 45. 10.1038/s41392-019-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet M.; Ramis I.; Calama E.; Carreño C.; Paris S.; Maldonado M.; Orellana A.; Calaf E.; Pauta M.; De Alba J.; Bach J.; Miralpeix M. Novel Inhaled Pan-JAK Inhibitor, LAS194046, Reduces Allergen-Induced A irway Inflammation, Late Asthmatic Response, and pSTAT Activation in Brown Norway Rats. Pharmacol Exp Ther. 2019, 370 (2), 137–147. 10.1124/jpet.119.256263. [DOI] [PubMed] [Google Scholar]
- Southworth T.; Plumb J.; Gupta V.; Pearson J.; Ramis I.; Lehner M. D.; Miralpeix M.; Singh D. Anti-inflammatory potential of PI3Kδ and JAK inhibitors in asthma patients. Respir Res. 2016, 17 (1), 124. 10.1186/s12931-016-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J.; Chow J.; Ives D.; Chiou M.; Mackenzie R.; Osen E.; Nguyen B.; Tsing S.; Bach C.; Freire J.; et al. Purification, characterization and selective inhibition of human prost aglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta 1994, 1209 (1), 130–9. 10.1016/0167-4838(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Kulmacz R. J.; Wang L. H. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin H synthase-1 and −2. J. Biol. Chem. 1995, 270 (41), 24019–23. 10.1074/jbc.270.41.24019. [DOI] [PubMed] [Google Scholar]
- Kim S. F.; Huri D. A.; Snyder S. H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005, 310 (5756), 1966–70. 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- Ou G.; Liu Q.; Yu C.; Chen X.; Zhang W.; Chen Y.; Wang T.; Luo Y.; Jiang G.; Zhu M.; Li H.; Zeng M. The Protective Effects of Maresin 1 in the OVA-Induced Asthma Mouse Model. Mediators Inflamm. 2021, 2021, 4131420. 10.1155/2021/4131420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-S.; Cho D.-H.; Kim K.-S.; Kim K.-H.; Park J.; Kim Y.; Jung J. H.; Kim K.; Jung H.-J.; Jang H.-J. Anti-inflammatory effects of embelin in A549 cells and human asthmatic airway epithelial tissues. Immunopharmacol Immunotoxicol. 2018, 40 (1), 83–90. 10.1080/08923973.2017.1414836. [DOI] [PubMed] [Google Scholar]
- Grabacka M.; Pierzchalska M.; Płonka P. M.; Pierzchalski P. The Role of PPAR Alpha in the Modulation of Innate Immunity. Int. J. Mol. Sci. 2021, 22 (19), 10545. 10.3390/ijms221910545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin Immunol. 2017, 33, 44–51. 10.1016/j.smim.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusznak M.; Peebles R. S. Jr. Eosinophils Express LTA4 Hydrolase and Synthesize LTB4: Important for Asthma Pathogenesis?. Am. J. Respir Cell Mol. Biol. 2019, 60 (4), 375–376. 10.1165/rcmb.2018-0367ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand P. R.; Keller H.; Peters J. M.; Vazquez M.; Gonzalez F. J.; Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 1996, 384 (6604), 39–43. 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Yu K.; Bayona W.; Kallen C. B.; Harding H. P.; Ravera C. P.; McMahon G.; Brown M.; Lazar M. A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995, 270 (41), 23975–83. 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- Kim S. Getting the most out of PubChem for virtual screening. Expert Opin Drug Discovery 2016, 11 (9), 843–55. 10.1080/17460441.2016.1216967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller D.; Grosdidier A.; Wirth M.; Daina A.; Michielin O.; Zoete V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32. 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S.; Feunang Y. D.; Guo A. C.; Lo E. J.; Marcu A.; Grant J. R.; Sajed T.; Johnson D.; Li C.; Sayeeda Z.; Assempour N.; Iynkkaran I.; Liu Y.; Maciejewski A.; Gale N.; Wilson A.; Chin L.; Cummings R.; Le D.; Pon A.; Knox C.; Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46 (D1), D1074–D1082. 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer G.; Rosen N.; Plaschkes I.; Zimmerman S.; Twik M.; Fishilevich S.; Stein T. I.; Nudel R.; Lieder I.; Mazor Y.; Kaplan S.; Dahary D.; Warshawsky D.; Guan-Golan Y.; Kohn A.; Rappaport N.; Safran M.; Lancet D. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc Bioinformatics. 2016, 54, 1.30.1–1.30.33. 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- Davis A. P.; Grondin C. J.; Johnson R. J.; Sciaky D.; Wiegers J.; Wiegers T. C.; Mattingly C. J. Comparative Toxicogenomics Database (CTD): update 2021. Nucleic Acids Res. 2021, 49 (D1), D1138–D1143. 10.1093/nar/gkaa891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino E.; Chiarugi S.; Margheriti F.; Garau G. Mapping, Structure and Modulation of PPI. Front Chem. 2021, 9, 718405. 10.3389/fchem.2021.718405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G. Jr.; Sherman B. T.; Hosack D. A.; Yang J.; Gao W.; Lane H. C.; Lempicki R. A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4 (5), P3. 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- Brewer J. P.; Kisselgof A. B.; Martin T. R. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am. J. Respir Crit Care Med. 1999, 160 (4), 1150–6. 10.1164/ajrccm.160.4.9806034. [DOI] [PubMed] [Google Scholar]
- Jang Y. J.; Back M. J.; Fu Z.; Lee J. H.; Won J. H.; Ha H. C.; Lee H. K.; Jang J. M.; Choi J. M.; Kim D. K. Protective effect of sesquiterpene lactone parthenolide on LPS-induced acute lung injury. Arch Pharm. Res. 2016, 39 (12), 1716–1725. 10.1007/s12272-016-0716-x. [DOI] [PubMed] [Google Scholar]
- Immanuel C. N.; Teng B.; Dong B.; Gordon E. M.; Kennedy J. A.; Luellen C.; Schwingshackl A.; Cormier S. A.; Fitzpatrick E. A.; Waters C. M. Apoptosis signal-regulating kinase-1 promotes inflammasome priming in macrophages. Am. J. Physiol Lung Cell Mol. Physiol. 2019, 316 (3), L418–L427. 10.1152/ajplung.00199.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.