Abstract
Introduction:
Development and implementation of effective treatments for opioid use disorder (OUD) and prevention of overdose are urgent public health needs. Though existing medications for OUD (MOUD) are effective, barriers to initiation and retention in treatment persist. Therefore, development of novel treatments, especially those that may complement existing treatments, is needed.
Areas covered:
This review provides an overview of vaccines for substance use disorders (SUD) and mechanisms underlying their function and efficacy. Next, we focus on existing preclinical and clinical trials of SUD Vaccines. We focus briefly on related strategies before providing an expert opinion on prior, current, and future work on vaccines for OUD. We included published findings from preclinical and clinical trials found on Pubmed and ScienceDirect as well as ongoing or initiated trials listed on ClinicalTrials.gov.
Expert opinion:
The present opioid overdose and OUD crises necessitate urgent development and implementation of effective treatments, especially those that offer protection from overdose and can serve as adjuvants to existing medications. Promising preclinical trial results paired with careful efforts to develop vaccines that account for prior SUD vaccine shortcomings offer hope for current and future clinical trials of opioid vaccines. Clinical advantages of opioid vaccines appear to outnumber disadvantages which may result in improved treatment options.
Keywords: Vaccines, Immunotherapies, Opioid Use Disorder, Substance Use Disorders, Heroin, Oxycodone, Fentanyl, Opioids, Nicotine, Cocaine
1.0. Introduction
Approximately 1.6 million Americans displayed symptoms consistent with Opioid Use Disorder [OUD] in 2019 [1]. In the United States, OUD is a major contributor to premature death, with 80,590 opioid-overdose related deaths in 2021, the highest number ever recorded [2]. During the COVID-19 pandemic, the incidence of overdoses increased substantially [3–5]. Though its origins are complex and multi-faceted, the current opioid epidemic presents a public health crisis, and efforts to understand and effectively treat OUD are critical [6–7].
Currently, several medications are approved by the U.S. Food and Drug Administration [FDA] to treat OUD, including methadone, buprenorphine, naltrexone, naloxone, and their combination [e.g., Suboxone] or extended-release formulations [e.g., Vivitrol]. Though the effectiveness of these medications has strong empirical support, barriers to implementation and high relapse rates among those who do start treatment contribute to increased risk for opioid overdose [8–10]. Further complicating efforts to treat those with OUD is the recent proliferation of high-potency synthetic opioids [HPSO; fentanyl, carfentanil, and related analogs and, more recently, benzimidazole opioids known as “nitazenes”] into opioid and other drug supplies [7]. HPSO are opioid analogues estimated to be more potent than heroin and therefore markedly increase the risk of opioid-related overdose given their rapid onset of action and narrow “therapeutic window” [the dose that produces analgesic response compared to the dose that produces respiratory depression [11–13]. The presence of HPSO in illicit opioid supplies appears to be complicating effective use of MOUD [2, 14–16]. Further, the presence of HPSO in opioid and other drug supplies appears likely to persist given high profit margins for drug producers and ease of distribution [17–18].
Together, these factors necessitate urgent exploration of novel interventions to treat OUD. One such approach, opioid vaccines, will be explored in this review. First, we will provide a brief overview on the mechanisms underlying vaccines for treating substance use disorders [SUD] more broadly. Next, this review will provide a brief overview of existing preclinical and clinical work on vaccines for nicotine, cocaine, and methamphetamine, with an emphasis on limitations of prior work, followed by a summary of preclinical and clinical work with vaccines for OUD. Finally, we will review literature on factors influencing response to vaccines for SUD, related work with monoclonal antibodies [mAb], and future directions. Finally, this review will provide an expert opinion on the current status of opioid vaccines and related interventions and outline future directions. We searched Pubmed and ScienceDirect databases between August 3rd, 2022 and October 30th, 2022 and included published findings from preclinical and clinical trials as well as ongoing or initiated trials listed on ClinicalTrials.gov. Keywords used for this search included “vaccines for SUD”, “vaccines for cocaine”, “vaccines for nicotine”, “vaccines for methamphetamines”, “vaccines for opioids”, “immunotherapies for SUD”, “monoclonal antibodies” and “biologics for SUD”. One-hundred-and-twenty-five sources are included in this review.
2.0. Vaccines for SUD: mechanisms of action and definitions
The goal of vaccines for treating SUD is to elicit production of antibodies that bind to the target drug molecules in serum. This binding of drug molecules to antibodies creates a drug: antibody complex that is too large to pass through the blood-brain barrier [BBB; Figure 1; 19–21]. In preventing transfer across the BBB, vaccines decrease the amount of unbound [free] drug circulating in and activating receptors in the brain, thus reducing any neurobiological or behavioral changes that typically occur with drug administration [19, 21]. In addition, vaccine-induced antibodies have been shown to reduce free drug circulation in organs such as lungs or heart which may further contribute to the protective effects of vaccines against respiratory depression and bradycardia [22]. Generally, vaccines for SUD are composed of several components that are intramuscularly or subcutaneously injected [see Figure 2]. Drug-derived haptens are small molecules that do not produce an immune response on their own but are designed to model the chemical structure of the target drug. Typically, a carrier protein or immunogenic carrier of viral, bacterial, or other foreign origin is attached to the hapten to elicit an immune response. Finally, the conjugate immunogens consisting of the hapten and carrier protein combination are paired with an adjuvant, which further enhances the strength and durability of the immune response [Figure 2]. Currently, there are 6 existing adjuvants that have been developed and approved in licensed vaccines for human use [23], and many others are at different stages of clinical development. After vaccination, usually with several doses delivered over time, generation of drug-specific polyclonal antibodies is triggered.
Figure 1:
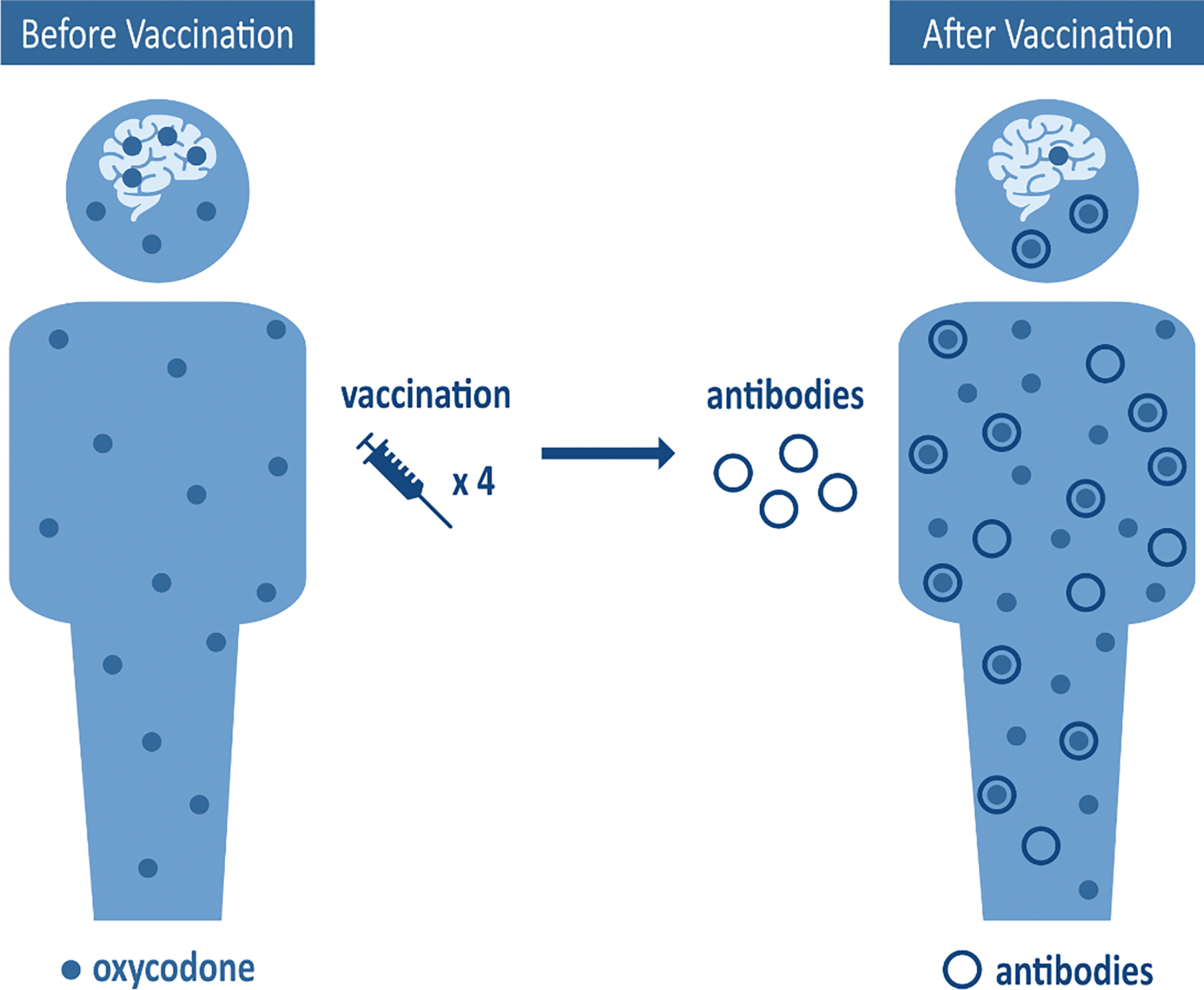
Oxycodone Vaccine Mechanism
Figure 2:
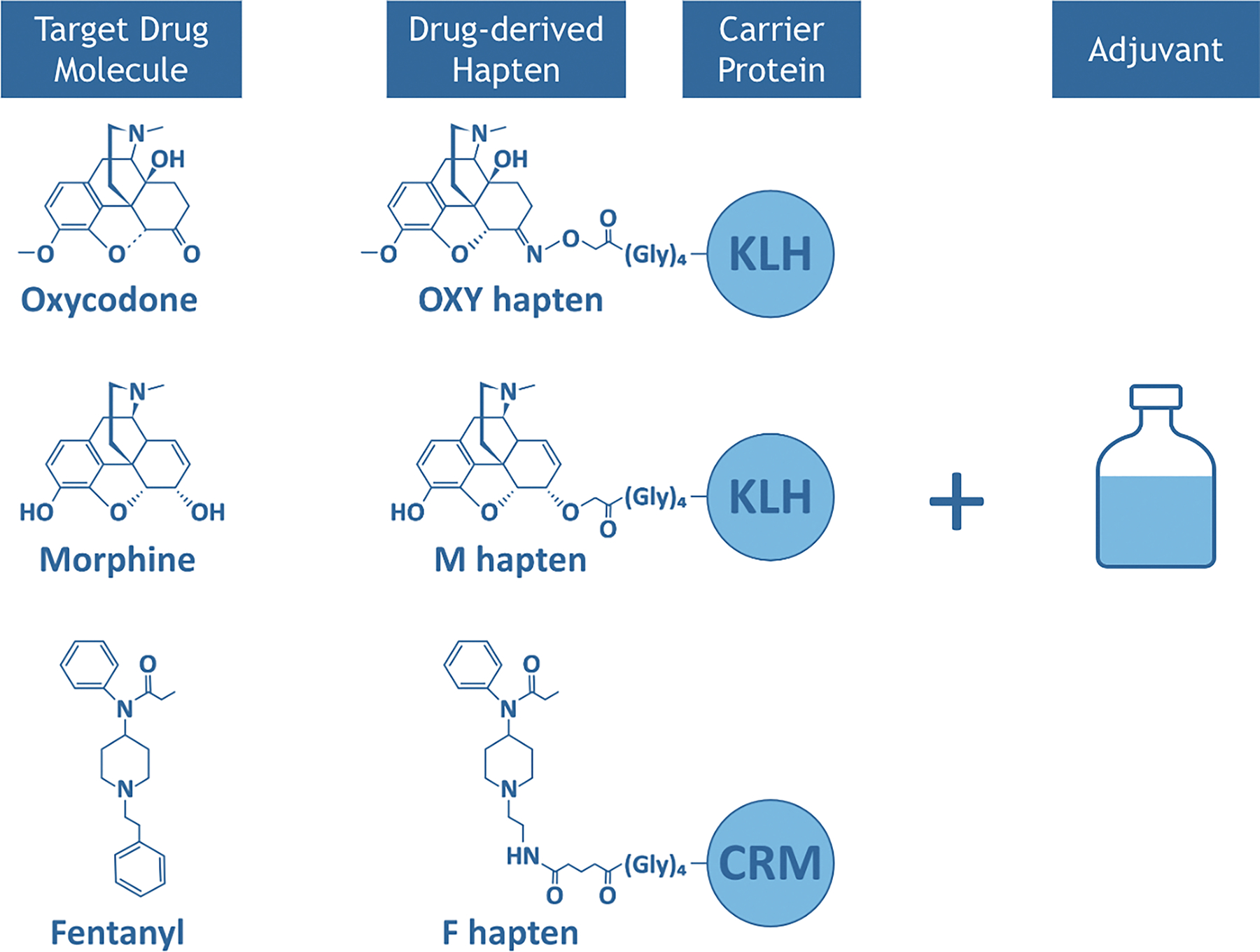
Substance Use Disorder (SUD) Vaccine Components
Generally, immune response to vaccines is mediated by a variety of interconnected cellular and molecular processes. B cell lymphocytes, which are white blood cells that produce antibodies, work with T cells to recognize and mount an immune response to antigens [21, 24]. Several types of T cells play a role in the immune response against different categories of antigens. Generally, specialized subsets of helper T cells [Th] work with B cells in producing a humoral response involving production of antibodies against the target. Vaccines for SUD are conjugate immunogens that stimulate both hapten-specific B cells and carrier-specific T cells. Hence these vaccines rely on CD4 + T cell-dependent activation of B cells, which occurs when either B cells bind the hapten on the hapten-carrier conjugate, or antigen-presenting cells such as macrophages or dendritic cells present the antigen on their surface via a major histocompatibility [MHC] process. Once this occurs, T cells recognize the carrier-derived T cell epitopes, bind to the MHC with support from CD4+ co-receptors, and activate B cell response. Once activated, B cells produce drug-specific antibodies that bind and neutralize their pharmacological effects. Use of carrier proteins appears to recruit carrier-specific CD4+ T cells to trigger the necessary CD4+ T cell-dependent B cell activation required to produce antibody responses [19, 25–26]. The immune response to vaccines is generally measured by analysis of blood samples for the presence of drug-specific antibodies. Recent advances have allowed for more comprehensive analyses including measurement of drug-specific B cell lymphocytes and carrier-specific T cells and characterization of antigen-presenting cells [APC] such as dendritic cells or macrophages. Efforts to characterize hapten-specific B cells and carrier-specific T cells and their relation to anti-drug antibody production in SUD vaccines may help to elucidate individual differences in vaccine response [21].
One of the clinical shortcomings that can be associated with use of therapeutic vaccines is variability in individual response to active immunization. Variability appears to stem from several factors, including specific vaccine components, vaccine dose and schedule of vaccination, individual and/or baseline differences in immune response, sex, age, genotype, and individual drug use patterns [20–21, 27–28]. These factors, along with drug-specific [e.g., stoichiometry of target drug versus antibody binding site mole ratios] factors must be considered in developing effective vaccines for SUD. Another important consideration is cross-reactivity, or an observable immune response to pathogens/molecules not specifically targeted by the vaccine.
2.1. Nicotine vaccines
To date, a number of nicotine vaccines have been tested in preclinical and clinical models and vary based on choice of carrier [virus linked particles [VLP], bacterial toxin components] and nicotine derivatives [29]. Vaccines targeting nicotine stimulate production of antibodies that bind to nicotine and reduce or block its ability to cross the BBB. As highlighted above, the strength of a nicotine vaccine’s efficacy is generally measured by the concentration of nicotine-specific IgG antibodies or antibody titers produced post-vaccination [30]. More recent evidence indicates that other factors affecting vaccine efficacy may be related to the IgG subclasses [e.g., IgG1-3] and affinity for the target compound [22, 31–32].
2.1.1. Preclinical studies of nicotine vaccines
Preclinical studies examining nicotine vaccines suggest promising effects, including greater serum nicotine concentrations in vaccinated versus unvaccinated rodents, evidence of sequestration of nicotine by antibodies, and lower concentrations of nicotine in the brain of vaccinated rodents [30, 33–37]. Over time, repeated nicotine dosing appears to attenuate the results of vaccination in animals, but even with repeated dosing nicotine reaches the brain more slowly and therefore produces less reinforcing effects in vaccinated animals [30, 38]. With regard to acquisition and maintenance of nicotine self-administration, animal models have shown vaccines can block or significantly attenuate these behaviors [30, 39]. Broadly, findings from preclinical studies of nicotine vaccines have been replicated across laboratories and across specific vaccine compounds, bolstering the strength of findings [30]. One important caveat is that most animal models of vaccines involve intravenous or subcutaneous injection of nicotine by itself, which clearly differs from inhalation of nicotine, tobacco, and the numerous other chemicals present in cigarettes smoked by humans [30]. Yet, vaccines against nicotine have shown pre-clinical efficacy in rats exposed to cigarette smoke in inhalation models [36]. Preclinical studies have largely utilized Fruend’s adjuvant, which though typically linked to a strong immune response, has an unfavorable safety profile in humans, limiting use in clinical trials [35]. Despite promising efficacy in various pre-clinical models, nicotine vaccines have shown a relatively low threshold of efficacy against nicotine doses equivalent to more than 2 cigarettes [40].
2.1.2. Clinical studies of nicotine vaccines
In spite of promising preclinical findings, clinical trials of nicotine vaccines have been less successful, and have generally not replicated preclinical findings. To date, five nicotine vaccines have been tested across 16 Phase I-III clinical trials [see 21, 29, 41 for overview]. A proof-of-concept study on one candidate vaccine [TA-Nic [NCT00633321; 42]] was initiated between 2006 and 2007, but no peer-reviewed findings were ever published and development was halted. A Phase II trial of Niccine, a nicotine-hapten conjugate vaccine, utilized a relapse-prevention model, and failed to find significant effects of vaccination on rates of relapse, smoking status, time to relapse, or abstinence across 1 year. Though anti-nicotine antibody levels increased in the vaccinated group, antibody level was not associated with relapse rate, and further development of Niccine was halted [43]. A third candidate vaccine, Nic-002, was evaluated across three clinical trials. An initial trial evaluated the safety and tolerability of Nic-002 in 40 nonsmoking volunteers and demonstrated a favorable safety profile with a high antibody response [35]. A larger Phase II study in 341 adult smokers suggested similar rates of abstinence between active vaccine and placebo groups, though antibody titer level at month 2 appeared to be an important moderator, such that those deemed to have a high antibody response demonstrated significantly higher rates of abstinence between 2 and 6 months post-vaccination and at 12 months post-vaccination [44]. As inflammatory, influenza-like symptoms were reported among those receiving Nic-002, efforts were made to reformulate the vaccine for a subsequent Phase IIb trial. However, results from this trial were not published or reported, and further development appears to have been halted [41].
NicVAX is the most thoroughly tested candidate vaccine in human trials [see 45–47]. Initial studies assessed dosage and vaccination schedule and suggested NicVAX was well tolerated with an acceptable safety and side-effect profile. A subsequent Phase IIb trial [46] suggested that antibody response was reliably and significantly associated with smoking cessation and long-term abstinence rates, with those demonstrating the highest antibody response demonstrating the most robust effects. However, two subsequent Phase III trials of NicVAX failed to replicate these findings. Though NicVAX appeared safe and well-tolerated across Phase I and II trials, Phase III trials demonstrated identical rates of abstinence between active and placebo vaccine conditions [11%; 48]. A nicotine vaccine consisting of nicotine-CRM197 adjuvanted in alum/CpG was tested by Pfizer [48–49], but results are not available. One final vaccine candidate, SEL-068, was tested in a clinical trial that recruited 82 participants in Belgium between 2011 and 2013 but no results have been made publicly available to date and development appears to have been halted [NCT01478893; 51].
Together, promising preclinical findings have failed to replicate in the vast majority of clinical trials of nicotine vaccines [21, 29–30] and one 2012 Cochrane review thus concluded that no current evidence supports nicotine vaccine-related enhancement of long-term smoking cessation rates [48]. Prior work suggests that human trials of nicotine vaccines have failed to replicate preclinical findings due to insufficient anti-nicotine antibody production, insufficient specificity or affinity of antibodies produced from vaccination, or weaknesses in study design that may have contributed to low motivation to quit [41]. Further, across preclinical and clinical trials of nicotine vaccines, high individual variability in antibody titer concentration post-vaccination, both a facet of general variability of immune response and variability in tested products, makes development of an effective vaccine quite challenging. Future work must identify factors driving individual variability in immune response in order to develop vaccines that result in fewer non-responders, either by increasing mean antibody response or reducing variability [30].
2.2. Cocaine vaccines
To date, several preclinical and clinical trials have been conducted to develop and test a therapeutic vaccine for cocaine. Unlike with nicotine vaccines, just two candidate vaccines have progressed to human trials, with published data only available for one.
2.2.1. Preclinical studies of cocaine vaccines
Preclinical trials of cocaine vaccines began in the early 1990’s. Between 1992 and 2005, several groups of researchers developed and tested several vaccine iterations utilizing different haptens, carrier proteins, and adjuvants across species [52]. Early work by Janda and colleagues developed and tested two haptens [GNC and GND] in rat models. This work demonstrated suppression of cocaine-induced locomotor activity and lower levels of cocaine in the brains of vaccinated rats compared to controls [53–54] and prevention of cocaine reinstatement in vaccinated rats [55]. Controls in these studies included animals injected with monoclonal antibodies, somewhat distinguishing results from prior studies utilizing placebo-vaccines as controls.
Subsequent preclinical work by Fox and colleagues testing a distinct conjugate [TA-CD] vaccine that was later adapted for human trials, demonstrated lower cocaine levels in the brains of vaccinated mice [56] and high enough antibody response to diminish cocaine self-administration in rats [57–58]. Exploration of a different cocaine vaccine in rhesus monkeys suggested a robust antibody response to vaccination [59]. Further work replicated prior findings that vaccination reduced cocaine levels in the serum, brain, and olfactory bulbs of vaccinated mice compared to controls [60]. A more recent examination of an additional cocaine vaccine [dAd5GNE] suggested similar findings with vaccination associated with reduced cocaine levels in the brains of vaccinated non-human primates [61–62] and reduced cocaine-induced hyperactivity among vaccinated animals [63].
2.2.2. Clinical trials of cocaine vaccines
To date, two cocaine vaccines have progressed from preclinical to clinical trials, with published data presently available only for TA-CD. The first clinical trial of TA-CD tested 3 vaccine doses in 24 participants vaccinated once per month for three months. TA-CD was well tolerated, anti-cocaine antibodies were detected following the second vaccination and higher mean antibody response in those receiving higher doses of TA-CD was observed [64–65]. Subsequent trials with TA-CD suggested that individuals demonstrating a higher antibody response post-vaccination appeared less likely to use cocaine or experience euphoric effects from cocaine during follow up [66]. Unfortunately, as with nicotine vaccines, high variability in antibody response was noted, and in one study 25–30% of participants demonstrated low antibody response [66]. In a subsequent larger trial only 38% of participants demonstrated a sufficient antibody response [767]. As observed in trials with nicotine vaccines, participants demonstrating a high antibody level appear to experience clinically meaningful results. For example, those with a high antibody response demonstrated fewer cocaine positive urine samples at follow up [67] and reported less reinforcing effects from smoked cocaine in a laboratory paradigm [68]. Still, this high variability made for difficult interpretation of findings and subsequent development of TA-CD was not pursued [20]. A Phase I clinical trial examining the safety and immunogenicity of dAd5GNE was initiated in 2015 but no results have been published to date, and the anticipated study completion date is December 2025 [NCT02455479; 69].
2.3. Methamphetamine vaccines
To date, a number of preclinical studies have examined the utility of methamphetamine vaccines and have demonstrated somewhat promising results. Broadly, this work has demonstrated that mice vaccinated against methamphetamines demonstrate a reliable and robust antibody response and appear to experience less reinforcing effects of methamphetamines [70]. Subsequent animal testing with a vaccine more viable in humans [using alum as an adjuvant] suggest reduced acquisition and reinstatement of methamphetamine self-administration in mice [71]. Additional conjugate vaccines have demonstrated reduced psychomotor effects, reduced self-administration of methamphetamines, and robust antibody response in vaccinated rats and mice [72–75]. Additional testing with alternative adjuvants has sought to bolster antibody response and demonstrated promising results [76–78]. Still, in spite of these promising initial findings no methamphetamine vaccines have progressed to human trials.
2.4. Opioid vaccines
Efforts to develop a vaccine targeting opioids dates back to the 1970’s, with early work suggesting strong antibody response and high specificity from three vaccines targeting morphine derivatives in a rabbit model [79] and stimulation of antibodies associated with reduced heroin self-administration in non-human primates [80]. In spite of these early efforts, continued development of opioid vaccines declined with the advent of methadone as a treatment for OUD, and interest in vaccines for SUD did not return until the 1990’s with a focus on cocaine vaccines [52]. Therefore, to date, few clinical trials have been carried out with opioid vaccines. There are some distinctions to note in the development of candidate vaccines for opioids in contrast to candidate vaccines for other drugs. Specifically, because opioids can have very different chemical structures [e.g., fentanyl vs morphine], and because many with OUD use a range of distinct opioids simultaneously and across time, a multivalent vaccine is considered necessary. Multivalent vaccines target a number of distinct target molecules simultaneously as they contain multiple individual hapten-carrier conjugates or multiple haptens conjugated to the same carrier. The advantages and disadvantages of this approach, paired with distinct clinical considerations associated with opioid vaccines will be discussed below.
2.4.1. Preclinical opioid vaccines
Though clinical trials exploring opioid vaccines are few, a wealth of recent preclinical data supports the utility and progression to clinical trials of vaccines targeting opioids. To date, several distinct candidate vaccines have been tested in animal models targeting heroin and its metabolites, hydrocodone, oxycodone, fentanyl, carfentanil, and other fentanyl analogs. Candidate vaccines have differed in terms of target opioid molecule and hapten. Early work using the KLH-6-SM vaccine [which consists of 6-succinylmorphine linked to lysine groups on KLH] reduced morphine-induced response and brain morphine levels in rats [81]. Given heroin’s metabolism to three distinct compounds [6-mono-acetylmorphine [6-MAM], morphine, and morphine-6-glucuronide], some research groups have targeted candidate vaccines that take a multivalent approach, targeting multiple compounds at once. This multivalent approach has the capacity to address real-world drug use patterns as many with OUD use a variety of opioids either simultaneously based on what is available in the illicit drug supply, or due to alterations in drug use patterns over time [switching from prescription opioids to heroin for example; 20, 82]. Conjugate vaccines for opioids have broadly demonstrated robust antibody response to heroin and its metabolites, reduced opioid concentration in the brains of vaccinated animals, and reduced heroin-induced motor and behavioral activity, including declines in heroin-seeking behavior and self-administration [26, 83]. Some of this work suggests that cue- and stress-induced relapse appear less responsive to vaccination, though rats exposed to stress- and cue-induced self-administration paradigms after a period of abstinence appear to reinstate use in a less rapidly escalating manner compared to unvaccinated rats [84].
A conjugate vaccine targeting oxycodone demonstrated high specificity, protection against the respiratory effects of oxycodone, and low cross-reactivity to other opioids [85]. Fentanyl vaccines have also demonstrated promising effects in preclinical work, offering protection from overdose and attenuating the analgesic and respiratory effects of fentanyl in rhesus monkeys [86–87]. Sublingual and intranasal fentanyl vaccines have also been tested, with data suggesting reduced fentanyl levels in the brains of vaccinated mice [88].
Efforts to develop and test fentanyl and fentanyl-analog vaccines present unique challenges and further work is needed to determine whether a single vaccine can effectively target several molecules in the fentanyl chemical family or if multiple vaccines targeting each analog is necessary. One group demonstrated the efficacy of a fentanyl vaccine in reducing fentanyl-induced pharmacological and behavioral effects of fentanyl on its own [86] and heroin contaminated with fentanyl [89]. A second group of researchers showed reduced distribution of fentanyl, reduced fentanyl-induced analgesia, and reduced respiratory depression in vaccinated mice and rats [90]. Importantly, this vaccine did not appear to interfere with naloxone’s ability to reverse respiratory depression [90]. More recent work to refine one fentanyl vaccine and test alternative adjuvants [LTA1 or dmLT] compared to alum suggests a more robust antibody response and more marked reductions in CNS distribution and fentanyl-induced analgesia with these adjuvants [88]. This study also supported intranasal vaccine administration [88]. Given evolution in drug supply over time, including increased synthesis of fentanyl analogs, a successful vaccine must demonstrate efficacy for fentanyl and related compounds, which may complicate development. To date, preclinical work with fentanyl vaccines suggests that they can effectively target fentanyl and carfentanil, eliciting high-affinity antibodies against both drugs in mice, and offering protection against opioid-induced respiratory depression [22,91]. Related work suggests preclinical efficacy against some fentanyl analogs [sufentanil, acetylfentanyl], but not others [alfentanil] suggesting that more work is needed to refine this approach [92]. In an ever-shifting landscape of HPSO, these compounds present somewhat of a moving target for vaccine developers, necessitating further work to develop and test vaccines that are highly targeted while also offering selective cross-reactivity to related HPSO compounds.
Efforts to increase efficacy of opioid vaccines have focused on manipulation of vaccine components in developing more effective, specific, and reliable candidates. Research conducted at Walter Reed Army Institute of Research on a heroin vaccine [Army Liposome Formulation [ALF]] found promising effects of ALF on analgesia and motor activity in vaccinated mice and rats [93–94]. Importantly, subsequent studies suggested increased efficacy of ALF with use of aluminum hydroxide as an adjuvant [95]. Similarly, researchers at Scripps Research Institute compared conjugation techniques and found greater efficacy with carbodiimide chemistry such that this technique produced greater reductions in heroin-induced antinociception in mice and heroin self-administration in non-human primates compared to a maleimide-based conjugate [96].
Work on a morphine-based hapten conjugated to KLH [M-KLH] has shed light on the potential development of multivalent opioid vaccines. On its own, M-KLH has demonstrated efficacy in reducing heroin, morphine, and 6-MAM distribution to the brains of vaccinated rodents, attenuating heroin-induced analgesia and respiratory depression, and reducing reinstatement of heroin self-administration [97–99]. Further work to deliver M-KLH simultaneously with an oxycodone vaccine suggests combined effects, such that co-administration of both vaccines reliably blocked 6-AM and oxycodone distribution [99].
Though somewhat less abundant, work testing oxycodone and hydrocodone vaccines largely replicates findings with heroin and morphine vaccines. Specifically, two separate research groups have developed and tested oxycodone- and hydrocodone-specific vaccines that generate antibodies specific to oxycodone and hydrocodone [100]. This research in rodents demonstrated vaccine-induced reductions in: distribution of target molecules to the brain [100–102], analgesia [100–102], self-administration [101–103], and respiratory depression [85, 101–102, 104]. Both groups developing and testing oxycodone and hydrocodone vaccines have also found that vaccine-generated antibodies do not bind to off-target opioids, do not interfere with naloxone in reversing overdose, and are not affected by maintenance on other off-target opioids or opioid antagonists [85, 101–102].
2.4.2. Clinical trials of opioid vaccines
One clinical study testing a morphine-based conjugate vaccine was conducted in Iran and supported the safety and tolerability of this vaccine. This trial examined a morphine-6-succinate hapten conjugated to bovine serum albumin [BSA] administered to 347 individuals with OUD. Though the authors report that the vaccine was well tolerated with minimal side effects, detailed reports on adverse events are not available. Similarly, no efficacy data from this trial have been made available for peer review, limiting interpretation of findings [105–106]. Currently, there is one ongoing clinical trial examining safety, tolerability, and preliminary efficacy of an oxycodone vaccine. This work, conducted by our group, is part of a multi-phase grant to explore an opioid vaccine targeting oxycodone [NCT04458545; 107]. Other opioid vaccines in development by our group will target heroin and fentanyl. Given promising preclinical findings, further clinical testing is a crucial next step in developing and refining opioid vaccines for clinical use.
3.0. Other approaches [passive immunization]
Alternate efforts to elicit antibody response to target drug molecules have focused on passive immunization with monoclonal antibodies [mAb; see 21]. Passive immunization involves a similar mechanism as vaccination, but through direct delivery of high-affinity, anti-drug mAb. It has been proposed that this delivery system may reduce the individual variability seen with prior vaccinations and elicits a more rapid and reliable antibody response. Preclinical findings appear to support this hypothesis as shown in the use of anti-nicotine [108–109], anti-cocaine [56, 110–111], anti-methamphetamine [112], anti-opioid [113–115], and anti-phencyclidine [116] mAb. One clinical trial examining a mAb targeting methamphetamines showed an acceptable safety profile and mean half-life of mAb between 17 and 19 days [117]. A more recent clinical trial exploring a different anti-methamphetamine mAb [IXT-m200, NCT03336866; 118] was recently completed, though peer-reviewed findings are not yet available. Pre-clinical studies show that mAbs are effective in reversing the effects of fentanyl and carfentanil in mice and rats [119]. Findings that mAb may reverse opioid-related toxicity were somewhat unexpected, but if replicated, may provide rationale for further development of mAb as overdose-reversal agents. Additional preclinical and clinical studies of mAb are needed to further understand their efficacy and therapeutic utility.
Successful development of an opioid mAb may pose several advantages and disadvantages. As noted above, passive immunization with mAb may provide less variability in time course, including onset, duration, and offset of effects than vaccine-elicited antibody levels. Therefore, clinicians may have greater confidence in using an opioid mAb alone if the clinical effect is immediate, robust, and of known duration. As with vaccines, opioid mAb are expected to have low cross-reactivity to off-target opioids so they could be used in combination with another MOUD which provides greater flexibility for clinical use. Another potential advantage of an opioid mAb versus vaccine relates to the potential need for opioids to treat pain. The duration of antibody response with an anti-oxycodone vaccine is unclear both within and across patients, making it difficult to determine the exact course of action for someone who may recover from an OUD but later require treatment with an opioid [for example post-operatively]. An opioid mAb, however, may have a much shorter and more predictable duration of action within and between patients. One possible disadvantage of this approach is that mAb development is quite expensive. Additionally, currently mAb must be intravenously infused in a large volume for immediate effect, limiting utility in emergency [overdose-response] settings. Still, this approach appears promising and future efforts to develop and refine the use of mAb, especially in cost-effective ways, appears warranted.
4.0. Conclusion
Current rates of OUD and opioid-related overdose necessitate critical development and implementation of novel, effective treatments. Though prior clinical trials with vaccines for SUD have been somewhat disappointing, there is reason to believe that current efforts to develop and test vaccines for OUD can accomplish what previous vaccine candidates have not. Arming clinicians and patients with multi-faceted, cost-effective, and easy-to-deliver treatment options is of the utmost importance and adding an opioid vaccine to current MOUD options could improve therapeutic outcomes.
5.0. Expert opinion
Though stagnant for many years, development of opioid vaccines appears to be accelerating, and researchers may be in a unique position to develop and test candidate vaccines that account for prior shortcomings of other SUD vaccines. For example, numerous preclinical studies of opioid vaccines have sought to refine and test a number of adjuvants, haptens, and carrier proteins [21, 26]. These efforts may help to ensure that subsequent clinical trials are utilizing the most promising candidates. Further, efforts to learn from prior work, and account for and more effectively measure individual differences in vaccine response [and potential drivers of this variability] can aid in more effective vaccine development. Efforts to better understand and account for B-cell and T-cell activity, for example, can help to more accurately identify those most likely to demonstrate a good response to candidate vaccines [21, 32, 120]. In our current oxycodone vaccine trial, years of preclinical work to understand B and T cells, Fc effector functions, microbiome factors, and cytokine levels contributed to development of our vaccine [21, 32, 100, 103, 121–122]. In our ongoing clinical trial [NCT04458545; 107] efforts to characterize B cell activity as a potential biomarker of vaccine response is therefore an important aim.
An effective vaccine for OUD would confer notable advantages and function as a valuable adjunct to existing treatments. As stated, though there are several FDA-approved medications for treating OUD, medication adherence and retention in treatment remain critical challenges with estimates suggesting that 50–75% of patients entering treatment for OUD drop out of treatment within the first year [123–125]. This places individuals with OUD who relapse at high risk of opioid overdose [10], especially in light of a growing presence of HPSOs in illicit drug supplies. An effective, multivalent opioid vaccine could confer long-lasting protection from overdose among individuals with OUD at risk for relapse. Unlike other approved medications for OUD, vaccines do not require detoxification prior to their use, do not pose a risk of misuse or diversion, and because of their high specificity, appear unlikely to have cross-reactivity with other off-target opioids. Therefore, an opioid vaccine could effectively be integrated into ongoing treatment with methadone, buprenorphine, or naltrexone. Unlike treatment with oral methadone or sublingual/buccal formulations of buprenorphine, vaccination would not require daily dosing to confer protection. Another potential application for opioid vaccines is use in individuals who use opioids recreationally, and are therefore not candidates for medications like methadone, buprenorphine, or naltrexone. Furthermore, recent estimates suggest that individuals who use psychostimulants like cocaine and amphetamines may be at risk of opioid-related overdose due to contamination of stimulants with HPSOs [2]. Individuals who primarily use these psychostimulants are not tolerant to opioids and are therefore at risk of overdose should they inadvertently use stimulants adulterated with an HPSO [6]. Still, more work would be needed to explore the utility and acceptability of an opioid vaccine in this population. As discussed, disadvantages of vaccines for OUD lie in the potential for variable antibody response, the need for several doses over time, and thus far unclear onset and duration of action. Ongoing and future clinical trials testing opioid vaccines must address these questions in order to offer a promising and effective therapeutic option.
Article highlights.
Vaccines and immunotherapies offer some advantages as standalone and adjuvant interventions for opioid use disorder [OUD]
Extensive preclinical data support current candidate vaccines for OUD
Further work is needed to extend preclinical findings to clinical trials, and efforts to address prior Substance Use Disorder [SUD] vaccine shortcomings are important
Funding
Funding was in the form of salary support for R Luba and S Martinez through NIDA T32 (DA007294-22) and salary support for M Pravetoni and SD Comer from NIDA (UG3DA047711).
List of Abbreviations
- APC
Antigen Presenting Cell
- BBB
Blood Brain Barrier
- CDC
Centers for Disease Control and Prevention
- FDA
Food and Drug Administration
- HPSO
High-Potency Synthetic Opioids
- IgG
Immunoglobulin G
- mAb
Monoclonal Antibodies
- OUD
Opioid Use Disorder
- SUD
Substance Use Disorders
- VLP
Virus Linked Particles
Footnotes
Declaration of interest
R Luba discloses a NIDA T32 Post-Doctoral Fellowship in the Division of Substance Use Disorders at NYSPI/CUIMC.
S Martinez discloses a NIDA T32 Post-Doctoral Fellowship in the Division of Substance Use Disorders at NYSPI/CUIMC.
J Jones discloses serving as a consultant to Alkermes; receiving an investigator-initiated grant from Merck Pharmaceuticals and the Peter McManus Charitable Trust; and receiving an honorarium from the World Health Organization.
M Pravetoni has filed patents disclosing composition and methods of use of vaccines and monoclonal antibodies for substance use disorders.
SD Comer has received research funding from Alkermes, BioXcel (NIDA grant and company-sponsored grant), Go Medical (NIDA grant), Intra-cellular Therapies (NIDA grant), Janssen, and Lyndra (NIDA grants); and in the past 3 years has consulted for Alkermes, Nektar, Opiant, and Otsuka.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Abuse S Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health 2020. Accessed at: https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf August 2022
- 2.Centers for Disease Control and Prevention. State Unintentional Drug Overdose Reporting System (SUDORS). Atlanta, GA: US Department of Health and Human Services, CDC; [2022, June 27]. Accessed at: https://www.cdc.gov/drugoverdose/fatal/dashboard [Google Scholar]
- 3.Appa A, Rodda LN, Cawley C, Zevin B et al. Drug overdose deaths before and after shelter-in-place orders during the COVID-19 pandemic in San Francisco. JAMA network open, 2021; 4(5), e2110452–e2110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiGennaro C, Garcia GGP, Stringfellow EJ, Wakeman S et al. Changes in characteristics of drug overdose death trends during the COVID-19 pandemic. International Journal of Drug Policy, 2021; 98, 103392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanz LJ, Dinwiddie AT, Snodgrass S, O’Donnell J et al. Drug overdose deaths in 28 states and the District of Columbia: 2020 data from the State Unintentional Drug Overdose Reporting System 2022 [Google Scholar]
- 6.Centers for Disease Control and Prevention. Understanding the Opioid Overdose Epidemic. Atlanta, GA: US Department of Health and Human Services, CDC; [2022, June 1]. Accessed at: https://www.cdc.gov/opioids/basics/epidemic.html August 2022 [Google Scholar]
- 7.Ciccarone D The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Current Opinion in Psychiatry, 2021; 34(4), 344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IMS Institute for Healthcare Informatics. Use of opioid recovery medications. IMS Institute for Healthcare Informatics; [2016, September]. Accessed at: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/use-of-opioid-recovery-medications.pdf in August 2022 [Google Scholar]
- 9.Park TW, Larochelle MR, Saitz R, Wang N et al. Associations between prescribed benzodiazepines, overdose death and buprenorphine discontinuation among people receiving buprenorphine. Addiction, 2020; 115(5), 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AR, Samples H, Crystal S, & Olfson M Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. American Journal of Psychiatry, 20020; 177(2), 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comer SD, & Cahill CM Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neuroscience & Biobehavioral Reviews, 2019; 106, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armenian P, Vo KT, Barr-Walker J, & Lynch KL Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology, 2018; 134, 121–132. [DOI] [PubMed] [Google Scholar]
- 13.Schmid CL, Kennedy NM, Ross NC, Lovell KM et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell, 2017; 171(5), 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson CL, Tanz LJ, Quinn K, Kariisa M et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. Morbidity and Mortality Weekly Report, 2021; 70(6), 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo B, Caulkins JP, Kilmer B, Pacula RL et al. The synthetic opioid surge in the United States. 2019. Santa Monica, CA: RAND Corporation. [Google Scholar]
- 16.Reuter P, Pardo B, & Taylor J Imagining a fentanyl future: Some consequences of synthetic opioids replacing heroin. International Journal of Drug Policy, 2021; 94, 103086. [DOI] [PubMed] [Google Scholar]
- 17.US Drug Enforcement Administration. 2020 National Drug Threat Assessment, DEA DCT-DIR-008-21. 2021. Available at: https://www.dea.gov/documents/2021/03/02/2020-national-drug-threat-assessment. accessed August 2022.
- 18.Gilbert M, & Dasgupta N Silicon to syringe: Cryptomarkets and disruptive innovation in opioid supply chains. International Journal of Drug Policy, 2017; 46, 160–167. [DOI] [PubMed] [Google Scholar]
- 19.Gefen T, Vaya J, Khatib S, Rapoport I et al. The effect of haptens on protein-carrier immunogenicity. Immunology, 2015; 144(1), 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heekin RD, Shorter D, & Kosten TR Current status and future prospects for the development of substance abuse vaccines. Expert review of vaccines, 2017; 16(11), 1067–1077. [DOI] [PubMed] [Google Scholar]
- 21. Pravetoni M Biologics to treat substance use disorders: Current status and new directions. Human vaccines & immunotherapeutics, 2016; 12(12), 3005–3019. *Provides a thorough and concise review
- 22.Crouse B, Hicks D, & Pravetoni M Investigating the role of interleukin-4 in anti-opioid vaccine efficacy. 2022
- 23.Pulendran B, S Arunachalam P, & O’Hagan DT (2021). Emerging concepts in the science of vaccine adjuvants. Nature Reviews Drug Discovery, 20(6), 454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cano RLE, & Lopera HDE Introduction to T and B lymphocytes. In Autoimmunity: From Bench to Bedside [Internet]. 2013. El Rosario University Press. [PubMed] [Google Scholar]
- 25.Pichichero ME Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Human vaccines & immunotherapeutics, 2013; 9(12), 2505–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pravetoni M, & Comer SD Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology, 2019; 158, 107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen XY, Orson FM, & Kosten TR Vaccines against drug abuse. Clinical Pharmacology & Therapeutics, 2012; 91(1), 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann P, & Curtis N Factors that influence the immune response to vaccination. Clinical microbiology reviews, 2019; 32(2), e00084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudary S, Kaur A, Atri M, Sharma M et al. Recent advancements in the development of nicotine vaccines: A systematic review. Annals of Public Health Research, 2020; 7(2), 205–450. [Google Scholar]
- 30.Pentel PR, & LeSage MG New directions in nicotine vaccine design and use. Advances in pharmacology, 2014; 69, 553–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelcher AMH, Baehr CA, Hamid FA, Hart GT et al. Contribution of antibody-mediated effector functions to the mechanism of efficacy of vaccines for opioid use disorders. The Journal of Immunology, 2021; 207(3), 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laudenbach M, Baruffaldi F, Vervacke JS, Distefano MD et al. The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. The Journal of Immunology, 2015; 194(12), 5926–5936. * Provides a useful introduction to relevant work on biomarkers related to immune response
- 33.Cerny EH, Levy R, Mauel J, Mpandi M et al. Preclinical development of a vaccine ‘against smoking’. Oncology Research and Treatment, 2002; 25(5), 406–411. [DOI] [PubMed] [Google Scholar]
- 34.Desai RI, & Bergman J Effects of the nanoparticle-based vaccine, SEL-068, on nicotine discrimination in squirrel monkeys. Neuropsychopharmacology, 2015; 40(9), 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer P, Jennings GT, Willers J, Rohner F et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. European journal of immunology, 2005; 35(7), 2031–40. [DOI] [PubMed] [Google Scholar]
- 36.Pravetoni M, Keyler DE, Raleigh MD, Harris AC et al. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochemical pharmacology, 2011; 81(9), 1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoskar SD, Keyler DE, LeSage MG, Raphael DE et al. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. International immunopharmacology, 2003; 3(7), 957–970. [DOI] [PubMed] [Google Scholar]
- 38.Hieda Y, Keyler DE, Ennifar S, Fattom A et al. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. International journal of immunopharmacology, 2000; 22(10), 809–819. [DOI] [PubMed] [Google Scholar]
- 39.Lindblom N, De Villiers SHL, Kalayanov G et al. Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration, 2002; 69(3), 254–260. [DOI] [PubMed] [Google Scholar]
- 40.LeSage MG, Keyler DE, Hieda Y, Collins G et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology, 2006; 184(3), 409–16. [DOI] [PubMed] [Google Scholar]
- 41.Fahim RE, Kessler PD, & Kalnik MW (2013). Therapeutic vaccines against tobacco addiction. Expert review of vaccines, 12(3), 333–342. * [DOI] [PubMed] [Google Scholar]
- 42.ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of Medicine. 2000. Feb 29. Identifier NCT00633321, Study of TA-NIC to Assess the Efficacy and Safety of a Vaccine to aid in Smoking Cessation. and Pharmacodynamics of SEL-068 Vaccine in Smokers and Non-Smokers, 2008, Mar 12 [cited August 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT00633321 [Google Scholar]
- 43.Tonstad S, Heggen E, Giljam H, Lagerbäck et al. Niccine®, a nicotine vaccine, for relapse prevention: a phase II, randomized, placebo-controlled, multicenter clinical trial. nicotine & tobacco research, 2013; 15(9), 1492–1501. [DOI] [PubMed] [Google Scholar]
- 44.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PloS one, 2008; 3(6), e2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatsukami DK, Rennard S, Jorenby D, Fiore M et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology & Therapeutics, 2005; 78(5), 456–67. [DOI] [PubMed] [Google Scholar]
- 46.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clinical Pharmacology & Therapeutics, 2011; 89(3), 392–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagena EJ, de Vos A, Horwith G, & van Schayck CP The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo-controlled phase 1/2 trial. Nicotine & Tobacco Research, 2008; 10(1), 213–18. [DOI] [PubMed] [Google Scholar]
- 48. Hartmann-Boyce J, Stead LF, Cahill K, & Lancaster T Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction, 2013; 108(10), 1711–21. * Provides a thorough overview of prior work on nicotine vaccines
- 49.Pryde DC, Jones LH, Gervais DP, Stead DR et al. Selection of a novel anti-nicotine vaccine: influence of antigen design on antibody function in mice. PloS one, 2013; 8(10), e76557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCluskie MJ, Thorn J, Gervais DP, Stead DR et al. Anti-nicotine vaccines: Comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. International immunopharmacology, 2015; 29(2), 663–671. [DOI] [PubMed] [Google Scholar]
- 51.ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of Medicine. 2000. Feb 29. Identifier NCT01478893, Safety and Pharmacodynamics of SEL-068 Vaccine in Smokers and Non-Smokers, 2011, Nov 23 [cited August 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT01478893 [Google Scholar]
- 52.Kinsey BM, Kosten TR, & Orson FM Anti-cocaine vaccine development. Expert review of vaccines, 2010; 9(9), 1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carrera MRA, Ashley JA, Parsons LH, Wirsching P et al. Suppression of psychoactive effects of cocaine by active immunization. Nature, 1995; 378(6558), 727–30. [DOI] [PubMed] [Google Scholar]
- 54.Carrera MRA, Ashley JA, Wirsching P, Koob GF et al. A second-generation vaccine protects against the psychoactive effects of cocaine. Proceedings of the National Academy of Sciences, 2001; 98(4), 1988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carrera MRA, Ashley JA, Zhou B, Wirsching. et al. Cocaine vaccines: antibody protection against relapse in a rat model. Proceedings of the National Academy of Sciences, 2000; 97(11), 6202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fox BS, Kantak KM, Edwards MA, Black KM et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nature medicine, 1996; 2(10), 1129–1132. [DOI] [PubMed] [Google Scholar]
- 57.Kantak KM, Collins SL, Lipman EG, Bond J et al. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology, 2000; 148(3), 251–62. [DOI] [PubMed] [Google Scholar]
- 58.Kantak KM, Collins SL, Bond J, & Fox BS Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology, 2001; 153(3), 334–40. [DOI] [PubMed] [Google Scholar]
- 59.Koetzner L, Deng S, Sumpter TL, Weisslitz M et al. Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics, 2001; 296(3), 789–96. [PubMed] [Google Scholar]
- 60.Hrafnkelsdottir K, Valgeirsson J, & Gizurarson S Induction of protective and specific antibodies against cocaine by intranasal immunisation using a glyceride adjuvant. Biological and Pharmaceutical Bulletin, 2005; 28(6), 1038–42. [DOI] [PubMed] [Google Scholar]
- 61.Hicks MJ, Kaminsky SM, De BP, Rosenberg JB et al. Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anticocaine vaccine. Human Gene Therapy Clinical Development, 2014; 25(1), 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maoz A, Hicks MJ, Vallabhjosula S, Synan M et al. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology, 2013; 38(11), 2170–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wee S, Hicks MJ, De BP, Rosenberg JB et al. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology, 2012; 37(5), 1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosten TR, Gonsai K, St Clair Roberts J, Jack L et al. Phase II human study of cocaine vaccine TA-CD. 2002, June. In CPDD Annual Meeting, Quebec City. [Google Scholar]
- 65.Kosten TR, & Biegel D Therapeutic vaccines for substance dependence. Expert Review of Vaccines, 2002; 1(3), 365–71. [DOI] [PubMed] [Google Scholar]
- 66.Martell BA, Mitchell E, Poling J, Gonsai K et al. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biological psychiatry, 2005; 58(2), 158–64. [DOI] [PubMed] [Google Scholar]
- 67.Martell BA, Orson FM, Poling J, Mitchell E et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Archives of general psychiatry, 2009; 66(10), 1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haney M, Gunderson EW, Jiang H, Collins ED et al. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biological psychiatry, 2010; 67(1), 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of Medicine. 2000. Feb 29. Identifier NCT02455479, Safety Study of a Disrupted Adenovirus (Ad) Serotype Cocaine Vaccine for Cocaine-Dependent Individuals, 2015, May 27 7 [cited August 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT02455479 [Google Scholar]
- 70.Shen XY, Kosten TA, Lopez AY, Kinsey BM et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug and alcohol dependence, 2013; 129(1–2), 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haile CN, Kosten TA, Shen XY, O’Malley PW et al. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. The American Journal on Addictions, 2015; 24(8), 748–55. [DOI] [PubMed] [Google Scholar]
- 72.Keller CM, Spence AL, Stevens MW, Owens SM et al. Effects of a methamphetamine vaccine, IXT-v100, on methamphetamine-related behaviors. Psychopharmacology, 2020; 237(3), 655–67. [DOI] [PubMed] [Google Scholar]
- 73.Miller ML, Moreno AY, Aarde SM, Creehan KM et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biological psychiatry, 2013; 73(8), 721–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller ML, Aarde SM, Moreno AY, Creehan KM et al. Effects of active anti-methamphetamine vaccination on intravenous self-administration in rats. Drug and alcohol dependence, 2015; 153, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreno AY, Mayorov AV, & Janda KD Impact of distinct chemical structures for the development of a methamphetamine vaccine. Journal of the American Chemical Society, 2011; 133(17), 6587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collins KC, Schlosburg JE, Lockner JW, Bremer PT et al. Lipid tucaresol as an adjuvant for methamphetamine vaccine development. Chemical Communications, 2014; 50(31), 4079–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins KC, Schlosburg JE, Bremer PT, & Janda KD Methamphetamine vaccines: improvement through hapten design. Journal of medicinal chemistry, 2016; 59(8), 3878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevens MW, Gunnell MG, Tawney R, & Owens SM Optimization of a methamphetamine conjugate vaccine for antibody production in mice. International immunopharmacology, 2016; 35, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spector S, Berkowitz B, Flynn EJ, & Peskar B Antibodies to morphine, barbiturates, and serotonin. Pharmacological reviews, 1973; 25(2), 281–291. [PubMed] [Google Scholar]
- 80.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, et al. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature, 1974; 252(5485), 708–10. [DOI] [PubMed] [Google Scholar]
- 81.Kosten TA, Shen XY, O’Malley PW, Kinsey, et al. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2013. 45, 223–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raleigh MD, & Pentel PR Vaccines for opioid addiction. In Biologics to Treat Substance Use Disorders 2016; 37–63. [Google Scholar]
- 83.Truong TT, & Kosten TR Current status of vaccines for substance use disorders: A brief review of human studies. Journal of the neurological sciences, 2021; 120098. [DOI] [PubMed] [Google Scholar]
- 84.Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW Dynamic vaccine blocks relapse to compulsive intake of heroin. Proceedings of the National Academy of Sciences, 2013; 110(22), 9036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F et al. Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS One, 2017; 12(12), e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bremer PT, Kimishima A, Schlosburg JE, Zhou B et al. Combatting synthetic designer opioids: a conjugate vaccine ablates lethal doses of fentanyl class drugs. Angewandte Chemie, 2016; 128(11), 3836–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tenney RD, Blake S, Bremer PT, Zhou B et al. Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology, 2019; 158, 107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stone AE, Scheuermann SE, Haile CN, Cuny GD et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. npj Vaccines, 2021; 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hwang CS, Smith LC, Natori Y, Ellis B et al. Efficacious vaccine against heroin contaminated with fentanyl. ACS chemical neuroscience, 2018; 9(6), 1269–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Raleigh MD, Baruffaldi F, Peterson SJ, Le Naour M A fentanyl vaccine alters fentanyl distribution and protects against fentanyl-induced effects in mice and rats. Journal of Pharmacology and Experimental Therapeutics, 2019; 368(2), 282–91. ** Provides a useful overview of preclinical work on opioid vaccines
- 91.Eubanks LM, Blake S, Natori Y, Ellis B et al. A highly efficacious carfentanil vaccine that blunts opioid-induced antinociception and respiratory depression. ACS chemical biology, 2021; 16(2), 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baehr C, Robinson C, Kassick A, Jahan R et al. Preclinical Efficacy and Selectivity of Vaccines Targeting Fentanyl, Alfentanil, Sufentanil, and Acetylfentanyl in Rats. ACS omega, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sulima A, Jalah R, Antoline JF, Torres OB et al. A stable heroin analogue that can serve as a vaccine hapten to induce antibodies that block the effects of heroin and its metabolites in rodents and that cross-react immunologically with related drugs of abuse. Journal of medicinal chemistry, 2018; 61(1), 329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torres OB, Matyas GR, Rao M, Peachman KK et al. Heroin-HIV-1 (H2) vaccine: induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. npj Vaccines, 2017; 2(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beck Z, Torres OB, Matyas GR, Lanar DE et al. Immune response to antigen adsorbed to aluminum hydroxide particles: Effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex. Journal of controlled release, 2018; 275, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bremer PT, & Janda KD Conjugate vaccine immunotherapy for substance use disorder. Pharmacological reviews, 2017; 69(3), 298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK et al. Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. Journal of Pharmacology and Experimental Therapeutics, 2013; 344(2), 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raleigh MD, Pentel PR, & LeSage MG Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS One, 2014; 9(12), e115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raleigh MD, Laudenbach M, Baruffaldi F, Peterson SJ et al. Opioid dose-and route-dependent efficacy of oxycodone and heroin vaccines in rats. Journal of Pharmacology and Experimental Therapeutics, 2018; 365(2), 346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pravetoni M, Le Naour M, Tucker AM, Harmon TM et al. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. Journal of medicinal chemistry, 2013; 56(3), 915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kimishima A, Wenthur CJ, Zhou B, & Janda KD An advance in prescription opioid vaccines: overdose mortality reduction and extraordinary alteration of drug half-life. ACS chemical biology, 2017; 12(1), 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen JD, Hwang CS, Grant Y, Janda KD et al. Prophylactic vaccination protects against the development of oxycodone self-administration. Neuropharmacology, 2018; 138, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pravetoni M, Pentel PR, Potter DN, Chartoff EH et al. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PloS one, 2014; 9(7), e101807. ** Provides a helpful introduction to preclinical work that informs the current ongoing clinical trial
- 104.Laudenbach M, Baruffaldi F, Robinson C, Carter P Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Scientific reports, 2018; 8(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akbarzadeh A, Mehraby M, Zarbakhsh M, & Farzaneh H Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnology and applied biochemistry, 1999; 30(2), 139–45. [PubMed] [Google Scholar]
- 106.Akbarzadeh A, Norouzian D, Farhangi A, Mehrabi M et al. Immunotherapy of 347 volunteer outpatient morphine addicts by human therapeutic morphine vaccine in Kermanshah province of Iran. J Pharmacol Toxicol, 2009; 4(1), 30–35. [Google Scholar]
- 107.ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of Medicine. 2000. Feb 29. Identifier NCT04458545, Clinical Trials of Multivalent Opioid Vaccine Components, 2020, Jul 7 [cited August 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT04458545 [Google Scholar]
- 108.Carrera MRA, Ashley JA, Hoffman TZ, Isomura S et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorganic & medicinal chemistry, 2004; 12(3), 563–70. [DOI] [PubMed] [Google Scholar]
- 109.Keyler DE, Roiko SA, Benlhabib E, LeSage MG et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose-and affinity-response relationships. Drug metabolism and disposition, 2005; 33(7), 1056–61. [DOI] [PubMed] [Google Scholar]
- 110.Carrera MRA, Trigo JM, Wirsching P, Roberts AJ et al. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacology biochemistry and behavior, 2005; 81(4), 709–14. [DOI] [PubMed] [Google Scholar]
- 111.Norman AB, Norman MK, Buesing WR, Tabet MR et al. The effect of a chimeric human/murine anti-cocaine monoclonal antibody on cocaine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics, 2009; 328(3), 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gentry WB, Laurenzana EM, Williams DK, West JR et al. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. International immunopharmacology, 2006; 6(6), 968–77. [DOI] [PubMed] [Google Scholar]
- 113.Baehr C, Kelcher AH, Khaimraj A, Reed DE et al. Monoclonal antibodies counteract opioid-induced behavioral and toxic effects in mice and rats. Journal of Pharmacology and Experimental Therapeutics, 2020; 375(3), 469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bogen IL, Boix F, Nerem E, Mørland J et al. A monoclonal antibody specific for 6-monoacetylmorphine reduces acute heroin effects in mice. Journal of Pharmacology and Experimental Therapeutics, 2014; 349(3), 568–76. [DOI] [PubMed] [Google Scholar]
- 115.Kvello AMS, Andersen JM, Øiestad EL, Mørland J et al. Pharmacological effects of a monoclonal antibody against 6-monoacetylmorphine upon heroin-induced locomotor activity and pharmacokinetics in mice. Journal of Pharmacology and Experimental Therapeutics, 2016; 358(2), 181–89. [DOI] [PubMed] [Google Scholar]
- 116.Hardin JS, Wessinger WD, Wenger GR, Proksch JW et al. A single dose of monoclonal anti-phencyclidine IgG offers long-term reductions in phencyclidine behavioral effects in rats. Journal of Pharmacology and Experimental Therapeutics, 2002; 302(1), 119–26. [DOI] [PubMed] [Google Scholar]
- 117.Stevens MW, Henry RL, Owens SM, Schutz R et al. First human study of a chimeric anti-methamphetamine monoclonal antibody in healthy volunteers. In MAbs 2014; 6 (6) 1649–56). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.ClinicalTrials.Gov [Internet]. Bethesda (MD): National Library of Medicine. 2000. Feb 29. Identifier NCT03336866, Study of Antibody for Methamphetamine Outpatient Therapy (STAMPOUT), 2017, Nov 8 [cited September 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03336866 [Google Scholar]
- 119.Baehr CA, Wu MM, Pandit SG, Arias-Umana J et al. Pharmacological Profiling of Antifentanyl Monoclonal Antibodies in Combination with Naloxone in Pre-and Postexposure Models of Fentanyl Toxicity. Journal of pharmacology and experimental therapeutics, 2022; 381(2), 129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaffer L (2021). Using vaccines to harness the immune system and fight drugs of abuse. Proceedings of the National Academy of Sciences, 118(52), e2121094118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baruffaldi F, Kelcher AH, Laudenbach M, Gradinati V et al. Preclinical efficacy and characterization of candidate vaccines for treatment of opioid use disorders using clinically viable carrier proteins. Molecular pharmaceutics, 2018; 15(11), 4947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crouse B, Zhang L, Robinson C, Ban Y et al. Housing conditions and microbial environment do not affect the efficacy of vaccines for treatment of opioid use disorders in mice and rats. Human vaccines & immunotherapeutics, 2021; 17(11), 4383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Connor AM, Cousins G, Durand L, Barry J et al. Retention of patients in opioid substitution treatment: a systematic review. PloS one, 2020; 15(5), e0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timko C, Schultz NR, Cucciare MA, Vittorio L et al. Retention in medication-assisted treatment for opiate dependence: a systematic review. Journal of addictive diseases, 2016; 35(1), 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu LT, Zhu H, & Swartz MS Treatment utilization among persons with opioid use disorder in the United States. Drug and alcohol dependence, 2016; 169, 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


