Summary
Background:
Early-life exposure to tobacco is associated with obesity, but the most susceptible developmental periods are unknown.
Objective:
To explore windows of susceptibility in a cohort of 568 mother–child pairs.
Methods:
We measured seven measures of tobacco exposure (five self-reported and two biomarkers) spanning from pre-conception to age 5 years. Mothers self-reported active smoking (pre-conception, 17 weeks, and delivery) and household smokers (5 and 18 months postnatally). Cotinine was measured in maternal urine (27 weeks) and child urine (5 years). Adiposity (fat mass percentage) was measured at birth and 5 years via air displacement plethysmography. Using a multiple informant approach, we tested whether adiposity (5 years) and changes in adiposity (from birth to 5 years) differed by the seven measures of tobacco exposure.
Results:
The associations may depend on timing. For example, only pre-conception (β = 3.1%; 95% CI: 1.0–5.1) and late gestation (β = 4.0%; 95% CI: 0.4–7.6) exposures influenced adiposity accretion from birth to 5 years (p for interaction = 0.01). Early infancy exposure was also associated with 1.7% higher adiposity at 5 years (95% CI: 0.1–3.2). Mid-pregnancy and early childhood exposures did not influence adiposity.
Conclusions:
Pre-conception, late gestation, and early infancy exposures to tobacco may have the greatest impact on childhood adiposity.
Keywords: adiposity, cotinine, developmental origins of health and disease, maternal smoking, obesity, secondhand smoke, tobacco, windows of susceptibility
1 |. INTRODUCTION
Early-life exposure to tobacco has been consistently linked to obesity. Two meta-analyses have reported that maternal smoking during pregnancy may increase the risk of obesity in childhood by at least 50%.1,2 While most studies have focused on the prenatal period, there is a growing body of evidence reporting exposures before3,4 and after pregnancy4–12 as risk factors for obesity in childhood. Although the associations are fairly consistent, no published studies have formally evaluated windows of heightened susceptibility to tobacco. Therefore, the most susceptible developmental periods are unknown.
The standard approach for disentangling the effects of prenatal and postnatal exposure has been to include a single polytomous exposure variable in models (e.g., no exposure, prenatal exposure only, postnatal exposure only)8,12 or to employ simultaneously adjusted models.4,13 Researchers typically draw conclusions about the most important developmental period by comparing the magnitude of the effect sizes. Although this approach is intuitive, it does not allow for formal testing of differences across the developmental periods.14 Other limitations of traditional approaches include multi-collinearity between exposure periods, the use of multiple testing, and the loss of information due to missing data.14
To address this gap in knowledge, we leveraged data from the Healthy Start study, a pre-birth cohort in Colorado. We utilized a multiple informant approach14 to identify the windows of heightened susceptibility to tobacco in relation to adiposity in childhood, using pre-conception, prenatal, and postnatal measurements of the exposure.
2 |. METHODS
2.1 |. Study population
The Healthy Start study recruited 1410 pregnant women aged ≥16 years with singleton pregnancies before 24 weeks of gestation from the obstetrics clinics at the University of Colorado Hospital between 2010 and 2014. Participants completed two research visits in pregnancy (median 17 and 27 weeks of gestation) and at delivery (median 1 day post-delivery). Women were excluded if they were expecting multiple births, had a previous stillbirth or pre-term birth before 25 weeks of gestation, or had pre-existing diabetes, asthma, cancer, or psychiatric illness. Mother–child pairs were included in this analysis if infants were born full-term (≥37 weeks gestation), had complete body composition measures at birth and at age 5, and had at least one exposure assessment. The Healthy Start study protocol was approved by the Colorado Multiple Institutional Review Board. All women provided written informed consent before the first study visit.
2.2 |. Tobacco exposure assessment
Healthy Start collected seven measures of tobacco exposure (five self-reported measures and two biomarkers) spanning from pre-conception through age 5 years. At enrollment (17 weeks gestation), women were asked in separate questions if they had smoked 100 cigarettes in their lifetime (yes or no) and if they had smoked in the 3 months prior to learning of their pregnancies (yes or no). Self-report of maternal smoking pre-conception was dichotomized as no (if they answered no to both questions) or yes (if they answered yes to the second question). If the participant self-reported smoking during the pre-conception period, participants were asked if they currently smoked cigarettes (yes or no) at the enrollment visit. Otherwise, they did not respond to this question, hence the large proportion of missingness at the enrollment visit. At delivery, women were also asked if they had smoked any cigarettes since their last research visit (yes or no). Self-report of household smokers (none, any) was ascertained through questionnaires completed at 5 and 18 months postnatally.
Cotinine, the major metabolite of nicotine,15 was measured in a convenience sample of women with available, stored urine samples collected at 27 weeks of gestation and children with available, stored urine samples collected at age 5 years. Cotinine was measured via solid phase competitive ELISA, with a sensitivity of 1 ng/ml (Calbiotech Cotinine ELISA CO096D). The limit of detection (LOD) was 0.05 ng/ml. Cotinine was categorized as no exposure (<LOD) and any exposure (≥LOD).
2.3 |. Outcome assessment
Offspring fat mass and fat-free mass were measured by trained study staff using whole body air displacement plethysmography within ~72 h of birth (PEA POD, COSMED, Rome, Italy) and at age 5 years (BOD POD, COSMED, Rome, Italy). The PEA POD system measures body mass and volume, calculates body density, and estimates fat mass (g) and fat-free mass (g). The BOD POD was used with a paediatric algorithm. Fat mass and fat-free mass were measured twice. If the percent fat mass differed by more than 2.0%, a third measurement was taken. The average of the two closest readings was used in this analysis. Neonatal and childhood adiposity (fat mass percentage) was calculated as a proportion of the fat mass divided by the total mass. Changes in body composition from birth to age 5 years were calculated by subtracting the fat mass, fat-free mass, or adiposity measurement taken at birth from the fat mass, fat-free mass, or adiposity measurement taken at age 5 years.
2.4 |. Covariates
Mother and child characteristics were collected during the research visits and abstracted from medical records. Maternal age at delivery was calculated by subtracting the participant’s date of birth from the date of delivery. Gravidity, maternal race/ethnicity, maternal education, and annual household income were self-reported via study questionnaires. Maternal height was measured using a stadiometer during the first pregnancy research visit. Pre-pregnancy weight was obtained from medical records or self-reported at the first pregnancy research visit. Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy weight (kg) divided by height squared (m2). Gestational weight gain was calculated as the difference between the last available weight measurement during pregnancy (measured by research staff or medical personnel) and pre-pregnancy weight.
Maternal diet was measured throughout pregnancy using the Automated Self-Administered 24-hour Dietary Recall (ASA24). Maternal diet quality was determined by the Healthy Eating Index-2010 (HEI-2010), a valid and reliable measure of diet quality.16 As previously described,17 HEI-2010 scores were calculated based on the following foods/nutrients: total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, empty calories, total energy, saturated fat, mono- and polyunsaturated fats, and sodium.
The duration of exclusive breastfeeding was assessed through a questionnaire. At the 5-month postpartum visit, women were asked in separate questions if they had ever breastfed their infant, were currently feeding their infant any breast milk, had ever fed their infant formula, or were currently feeding their infant formula. A measure of breast milk–months was developed that incorporated duration and exclusivity, as previously described.18 The metric takes periods of mixed feeding into account and is interpreted continuously. For example, five breast milk months could be 5 months of exclusive breastfeeding or 4 months of exclusive breastfeeding followed by 2 months of 50:50 mixed feeding.
2.5 |. Statistical methods
We used a multiple informant approach14 within generalized estimating equations (GEEs) to estimate differences in adiposity, fat mass, and fat-free mass at age 5 years and the change in adiposity, fat mass, and fat-free mass from birth to 5 years according to the timing of exposure to tobacco. The multiple informant approach has been used in environmental epidemiology to identify windows of susceptibility to various environmental chemicals on childhood neurodevelopment and thyroid levels.19–21 This approach combines separate linear regression models for each time point into a single estimating Equation. A distinct advantage of the multiple informant approach is that it allows for the exposure data to be sparsely collected from multiple sources (e.g., biomarkers and self-report).14 Mean differences and 95% confidence intervals were calculated to compare body composition measurements between exposed and unexposed offspring at each time period. The reference category for each time point is the unexposed group. To formally evaluate whether the association depended on the timing of the exposure, an interaction term between the dichotomous tobacco exposure and the timing of the exposure measurement was included in all models. We considered an interaction p < 0.05 as evidence that the exposure-outcome association depended on the timing of the exposure. All models adjusted for a priori confounders that were associated with the exposure and the outcome, including infant sex, maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (<high school degree, high school degree, some college), pre-pregnancy body mass index (kg/m2), maternal diet quality during pregnancy (HEI-2010 scores), duration of exclusive breastfeeding (breast milk-months), and age at body composition (years). All statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, TX).
3 |. RESULTS
Of the 1410 participants enrolled in the Healthy Start cohort study, 1338 children were born at or after 37 weeks of gestation. Of these, 665 children had body composition measurements at birth and at age 5 years. Although all mothers and their children were invited to participate in the research visits, not all attended or completed every assessment. Thus, some of the participants are missing data on maternal diet quality during pregnancy (n = 29) and the duration of exclusive breastfeeding (n = 68). After excluding those with missing covariate data, a total of 568 mother–child pairs were included in this analysis. The participants in our analytical sample and the overall cohort were similar with respect to maternal age, maternal pre-pregnancy BMI, gestational weight gain, gravidity, maternal race/ethnicity, maternal education, household income, and maternal diet quality during pregnancy (Supplemental Table S1).
Participants in our analytical sample were racially and ethnically diverse, with 60% non-Hispanic white, 22% Hispanic, 12% non-Hispanic black, and 6% from all other racial and ethnic groups combined (Table 1). Most of the women had some college education (75%). Household income was evenly spread across the categories of <$40 000 (26%), $40 001 to $70 000 (19%), and ≥$70 000 (40%), with an additional 16% choosing not to answer or reporting they did not know their household income.
TABLE 1.
Characteristics of mother–child pairs enrolled in the Healthy Start study, 2009–2014, and included in this analysis
| Included mother–child pairs (n = 568) | Entire cohort (n = 1410) | |
|---|---|---|
| Mother characteristics | ||
| Age (years) | 29 ± 6 | 28 ± 6 |
| Pre-pregnancy body mass index (kg/m2) | 26 ± 6 | 26 ± 6 |
| Gestational weight gain (kg) | 14 ± 6 | 13 ± 7 |
| Previous pregnancies (any) | 1 ± 1 | 1 ± 2 |
| Race/Ethnicity | ||
| Non-Hispanic white | 60% | 53% |
| Non-Hispanic black | 12% | 15% |
| Hispanic | 22% | 25% |
| Other | 6% | 6% |
| Highest level of education | ||
| <High school | 11% | 14% |
| High school degree | 14% | 18% |
| Some college or more | 75% | 67% |
| Household income | ||
| <$40 000 | 26% | 30% |
| $40 001 to $70 000 | 19% | 20% |
| ≥$70 000 | 40% | 32% |
| Do not know | 16% | 18% |
| Diet quality during pregnancy (Healthy Eating Index) | 62 ± 10 | 64 ± 29 |
| Child characteristics | ||
| Male | 52% | 53% |
| Gestational age at birth (weeks) | 40 ± 1 | 39 ± 1 |
| Birthweight (kg) | 3.3 ± 0.4 | 3.2 ± 0.5 |
| Duration of exclusive breastfeeding (breast milk-months) | 3.7 ± 2.0 | |
| Exposure to secondhand smoke | ||
| Self-report of smoking pre-conception (n = 568) | ||
| No | 89% | |
| Yes | 11% | |
| Self-report of smoking at 17 weeks gestation (n = 63) | ||
| No | 65% | |
| Yes | 35% | |
| Maternal cotinine at 27 weeks gestation (n = 360) | ||
| <LOD | 78% | |
| ≥LOD | 23% | |
| Self-report of smoking at delivery (n = 563) | ||
| Yes | 96% | |
| No | 4% | |
| Self-report of household smokers at age 5 months (n = 568) | ||
| None | 85% | |
| Any | 15% | |
| Self-report of household smokers at age 18 months (n = 517) | ||
| None | 87% | |
| Any | 13% | |
| Child cotinine at age 5 years (n = 518) | ||
| <LOD | 72% | |
| ≥LOD | 28% | |
| Neonatal body composition | ||
| Adiposity (percent fat mass) | 9.3 ± 3.9 | |
| Fat mass (kg) | 0.3 ± 0.2 | |
| Fat-free mass (kg) | 2.9 ± 0.3 | |
| Child body composition | ||
| Adiposity (percent fat mass) | 20.1 ± 6.8 | |
| Fat mass (kg) | 3.7 ± 1.8 | |
| Fat-free mass (kg) | 14.6 ± 2.6 | |
| Change in body composition (birth to 5 years) | ||
| Adiposity (percent fat mass) | 10.7 ± 7.3 | |
| Fat mass (kg) | 3.4 ± 1.8 | |
| Fat-free mass (kg) | 11.7 ± 2.5 | |
Most women reported no smoking in their lifetime or in the 3 months prior to pregnancy (n = 506, 89%). Of the women who reported no current smoking at enrollment (n = 41), most indicated that they had quit smoking after learning of their pregnancies (n = 32). At mid-gestation, 23% of women had cotinine levels at or above the LOD (indicating maternal active or secondhand smoking). Only 4% of the women reported smoking at the time of delivery. In the early postnatal period, few women reported living with a household smoker at 5 months or 18 months of age (prevalence of 13% and 15%, respectively). At age 5 years, approximately 28% of the children had detectable levels of cotinine levels (indicating tobacco exposure).
3.1 |. Early-life exposure to tobacco
The seven measures of tobacco appeared to be moderately concordant (Table 2). In general, the self-reported and biomarker-confirmed categorization of “no exposure” tended to agree. For instance, 98% of the women with cotinine levels indicating no exposure at 27 weeks gestation also self-reported no smoking pre-conception. Discordance was most notable when comparing the “exposed” category. For instance, only 46 (66%) of mothers who reported living with a household smoker at 5 months also reported living with a household smoker at 18 months.
TABLE 2.
Comparison of exposure to secondhand smoke variables
| Self-report of smoking pre-conception (n = 568) |
Self-report of smoking at 17 weeks gestation (n = 63) |
Maternal cotinine at 27 weeks gestationa (n = 360) |
Self-report of smoking at delivery (n = 563) |
Self-report of household smokers at 5 months (n = 568) |
Self-report of household smokers at 18 months (n = 517) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | <LOD | ≥LOD | No | Yes | None | Any | None | Any | |
| Self-report at 17 weeks gestation (n = 63) | ||||||||||||
| No | 1 (2%) | 40 (98%) | ||||||||||
| Yes | 0 (0%) | 22 (100%) | ||||||||||
| Maternal cotinine at 27 weeks gestation (n = 360) | ||||||||||||
| <LOD | 274 (98%) | 5 (2%) | 5 (100%) | 0 (0%) | ||||||||
| ≥LOD | 57 (70%) | 24 (30%) | 15 (63%) | 9 (37%) | ||||||||
| Self-report at delivery (n = 563) | ||||||||||||
| No | 500 (92%) | 41 (8%) | 36 (86%) | 6 (14%) | 277 (79%) | 72 (21%) | ||||||
| Yes | 1 (5%) | 21 (95%) | 5 (24%) | 16 (76%) | 1 (11%) | 8 (89%) | ||||||
| Self-report at age 5 months (n = 568) | ||||||||||||
| No | 456 (95%) | 25 (5%) | 53 (88%) | 23 (88%) | 266 (85%) | 48 (15%) | 474 (99%) | 3 (1%) | ||||
| Yes | 50 (57%) | 37 (43%) | 49 (48%) | 18 (49%) | 13 (29%) | 33 (72%) | 67 (78%) | 19 (22%) | ||||
| Self-report at age 18 months (n = 517) | ||||||||||||
| No | 427 (95%) | 21 (5%) | 51 (86%) | 18 (86%) | 260 (84%) | 50 (16%) | 441 (99%) | 2 (1%) | 424 (95%) | 24 (5%) | ||
| Yes | 43 (62%) | 26 (38%) | 35 (47%) | 14 (52%) | 15 (36%) | 27 (64%) | 54 (78%) | 15 (22%) | 23 (33%) | 46 (66%) | ||
| Child cotinine at age 5 yearsb (n = 518) | ||||||||||||
| <LOD | 359 (97%) | 12 (3%) | 9 (75%) | 3 (25%) | 220 (91%) | 22 (9%) | 363 (99%) | 3 (1%) | 349 (94%) | 22 (6%) | 334 (95%) | 17 (5%) |
| ≥LOD | 103 (70%) | 44 (30%) | 28 (62%) | 17 (38%) | 35 (40%) | 53 (60%) | 128 (87%) | 19 (13%) | 90 (61%) | 57 (39%) | 75 (61%) | 47 (39%) |
Abbreviations: LOD, limit of detection.
The cotinine categories were defined as follows: no exposure (<0.05 ng/ml [LOD]) and maternal active/secondhand smoker during pregnancy (≥0.5 ng/ml).
The cotinine categories were defined as follows: no exposure (<0.05 ng/ml [LOD]) and any exposure to secondhand smoke (≥0.5 ng/ml).
3.2 |. Tobacco exposure and childhood body composition
We observed strong evidence that the association between tobacco and changes in adiposity or fat mass from birth to age 5 years depends on the timing of exposure (Table 3, Figure 1; p for interaction = 0.01 and 0.02, respectively). For example, maternal smoking pre-conception and at delivery were associated with increased adiposity accretion from birth to age 5 years (3.1% [95% CI: 1.0, 5.1] and 4.0% [95% CI: 0.4, 7.6], respectively) as compared to offspring with no exposure during these exposure windows. By contrast, tobacco exposures at other time periods during pregnancy (17 weeks or 27 weeks gestation) were not associated with any change in adiposity or fat mass from birth to age 5 years.
TABLE 3.
Adjusteda mean differences [95% confidence intervals (CI)s] in body composition at age 5 years and change in body composition from birth to 5 years by pre-conception, prenatal, and postnatal exposure to secondhand smoke
| Exposure window | n | Adjusted mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Adiposity at 5 years (% fat mass) | ||||
| 1 | Pre-conception | 568 | 1.7 (0.1, 3.6) | 0.07 |
| 2 | 17 weeks gestation | 63 | −0.7 (−4.3, 2.9) | 0.70 |
| 3 | 27 weeks gestation | 360 | −0.3 (−1.8, 1.1) | 0.63 |
| 4 | Delivery | 563 | 2.5 (−0.9, 5.9) | 0.14 |
| 5 | 5 months of age | 568 | 1.7 (0.1, 3.2) | 0.04 |
| 6 | 18 months of age | 517 | 0.9 (−0.6, 2.5) | 0.24 |
| 7 | 5 years of age | 518 | −0.3 (−1.5, 0.9) | 0.62 |
| p for interaction = 0.17 | ||||
| Fat mass (kg) | ||||
| 1 | Pre-conception | 568 | 0.3 (−0.1, 0.8) | 0.15 |
| 2 | 17 weeks gestation | 63 | −0.2 (−1.1, 0.7) | 0.63 |
| 3 | 27 weeks gestation | 360 | −0.3 (−0.7, 0.1) | 0.14 |
| 4 | Delivery | 563 | 0.5 (−0.3, 1.3) | 0.21 |
| 5 | 5 months of age | 568 | 0.3 (−0.2, 0.7) | 0.21 |
| 6 | 18 months of age | 517 | 0.1 (−0.3, 0.5) | 0.53 |
| 7 | 5 years of age | 518 | −0.1 (−0.5, 0.2) | 0.41 |
| p for interaction = 0.22 | ||||
| Fat-free mass (kg) | ||||
| 1 | Pre-conception | 568 | −0.2 (−0.7, 0.3) | 0.38 |
| 2 | 17 weeks gestation | 63 | −0.3 (−1.2, 0.6) | 0.49 |
| 3 | 27 weeks gestation | 360 | −0.4 (−0.9, 0.0) | 0.08 |
| 4 | Delivery | 563 | −0.8 (−1.5, −0.1) | 0.03 |
| 5 | 5 months of age | 568 | −0.3 (−0.8, 0.1) | 0.14 |
| 6 | 18 months of age | 517 | −0.2 (−0.7, 0.3) | 0.45 |
| 7 | 5 years of age | 518 | −0.2 (−0.5, 0.2) | 0.31 |
| p for interaction = 0.48 | ||||
| Change in adiposity from birth to 5 years (% fat mass) | ||||
| 1 | Pre-conception | 517 | 3.1 (1.0, 5.1) | <0.01 |
| 2 | 17 weeks gestation | 56 | −2.5 (−6.7, 1.7) | 0.24 |
| 3 | 27 weeks gestation | 331 | 0.5 (−1.1, 2.2) | 0.53 |
| 4 | Delivery | 517 | 4.0 (0.4, 7.6) | 0.03 |
| p for interaction = 0.01 | ||||
| Change in fat mass from birth to 5 years (kg) | ||||
| 1 | Pre-conception | 517 | 0.5 (0.0, 1.0) | 0.05 |
| 2 | 17 weeks gestation | 56 | −0.5 (−1.4, 0.5) | 0.33 |
| 3 | 27 weeks gestation | 331 | −0.2 (−0.6, 0.2) | 0.23 |
| 4 | Delivery | 517 | 0.6 (−0.1, 1.4) | 0.11 |
| p for interaction = 0.02 | ||||
| Change in fat-free mass from birth to 5 years (% fat mass) | ||||
| 1 | Pre-conception | 517 | −0.2 (−0.8, 0.3) | 0.40 |
| 2 | 17 weeks gestation | 56 | −0.2 (−1.1, 0.7) | 0.68 |
| 3 | 27 weeks gestation | 331 | −0.3 (−0.8, 0.1) | 0.18 |
| 4 | Delivery | 517 | −0.7 (−1.4, 0.1) | 0.09 |
| p for interaction = 0.46 | ||||
Adjusted for infant sex, maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (<high school degree, high school degree, some college), pre-pregnancy body mass index (kg/m2), maternal diet quality during pregnancy (according to continuous Healthy Eating Index scores), duration of exclusive breastfeeding (<5 months, >5 months), and age at body composition (years).
Note: The bolded text signifies statistical significance (p < 0.05).
FIGURE 1.
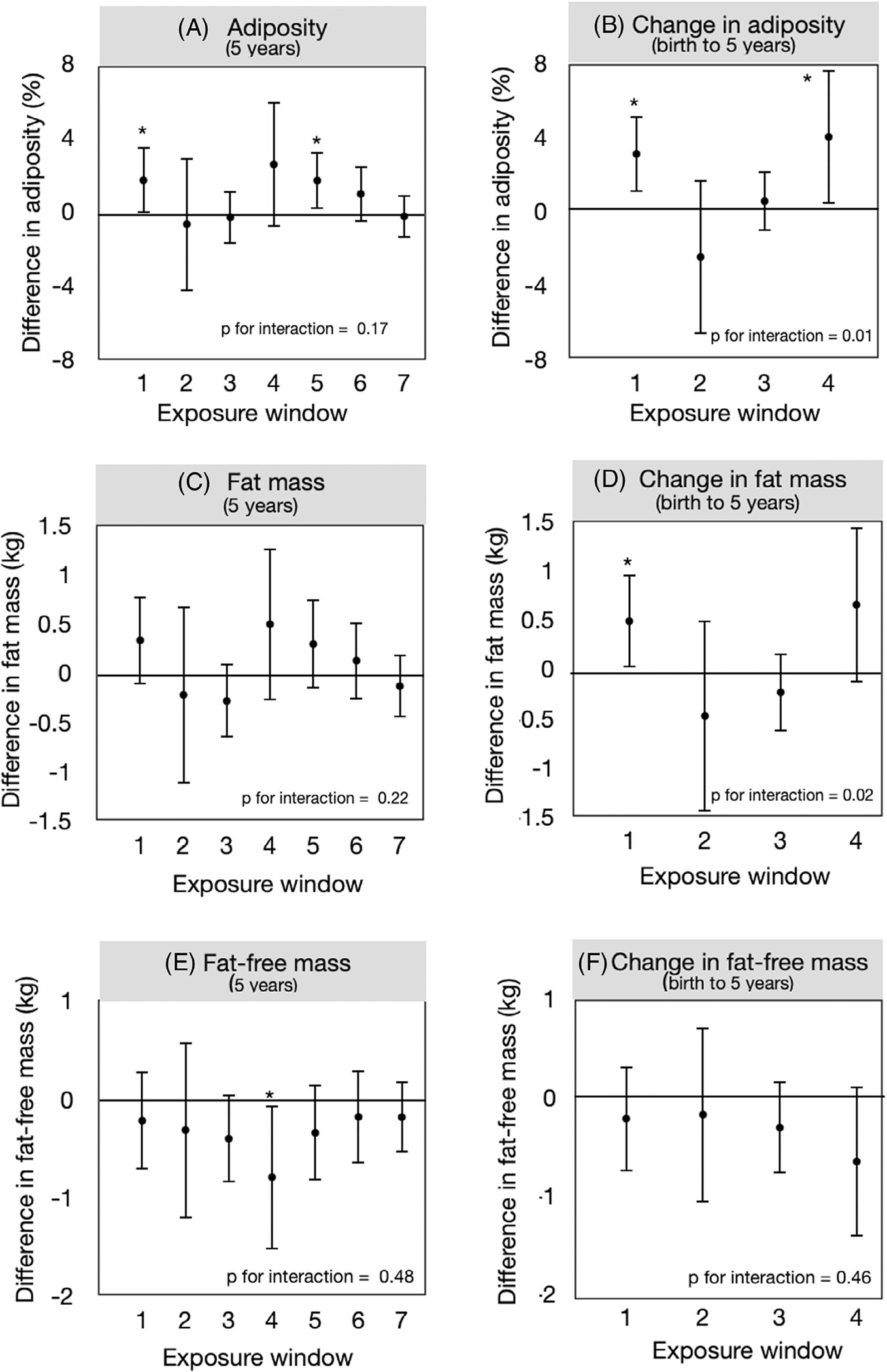
Adjusted mean differences [95% confidence intervals (CIs)] in body composition at age 5 years and change in body composition from birth to 5 years by pre-conception, prenatal, and postnatal exposure to tobacco. Exposure windows are as follows: 1 (self-report of smoking pre-conception), 2 (self-report of smoking at 17 weeks gestation), 3 (maternal cotinine at 27 weeks gestation), 4 (self-report of smoking at delivery), 5 (self-report of household smokers at age 5 months), 6 (self-report of household smokers at age 18 months), and 7 (child cotinine at age 5 years). All models adjusted for time infant sex, maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), maternal education (<high school degree, high school degree, some college), pre-pregnancy body mass index (kg/m2), maternal diet quality during pregnancy (according to continuous Healthy Eating Index scores), duration of exclusive breastfeeding (<5 months, >5 months), and age at body composition (years).
Adiposity at age 5 years was 1.7% (95% CI: 0.1, 3.2) higher for those living with household smokers during the first 5 months of life, as compared to those with no exposure during these exposure windows. However, the interaction p-value is not consistent with the hypothesis that these associations depend on the timing of the exposure (p for interaction = 0.17). Tobacco exposures during other time periods during pregnancy (17 weeks or 27 weeks gestation) or in early childhood (18 months or 5 years of age) were not significantly associated with any increase in adiposity or fat mass at age 5 years.
We did not detect any associations between tobacco exposure and fat-free mass at age 5 years or the change in fat-free mass from birth through age 5 years.
4 |. DISCUSSION
We demonstrate that the association between early-life exposure to tobacco and childhood adiposity and fat mass depends on the timing of the exposure. Tobacco exposures during pre-conception, late gestation, and early infancy represent the windows of greatest susceptibility for increased adiposity in early childhood. Our results may provide novel insights about the underlying biological mechanisms as well as potential opportunities for targeted smoking cessation interventions.
The pre-conception period is the earliest sensitive window for human development. Smoking before conception may influence offspring adiposity via epigenetic changes to the oocytes.22 These epigenetic changes may contribute to metabolic abnormalities in the offspring via transgenerational epigenetic inheritance,23 a mechanism in which certain loci on the oocyte deoxyribonucleic acid (DNA) escape epigenetic reprogramming after fertilization.24 However, some research suggests that the long-term effects of this exposure may result from sustained maternal smoking throughout pregnancy rather than epigenetic inheritance.25 Maternal smoking before and during early pregnancy (when many women are not yet aware of being pregnant) may influence offspring growth by hindering the differentiation of specialized epithelial cells of the placenta known as cytotrophoblasts.26 This morphological change may result in a reduction in the blood flow to the fetus,27 which is known to cause intrauterine growth restriction. After birth, these growth-restricted offspring often experience rapid and excessive catch-up growth28–33 followed by an increased risk for obesity in early childhood.1,2 This pattern of growth is consistent with our findings, which revealed that the effect estimates for the change in adiposity from birth to 5 years were stronger than the effect estimates for the absolute difference in adiposity at 5 years.
Although there is consistent evidence linking prenatal exposure to tobacco with obesity in childhood,1,2 not much is known about the potential trimester-specific effects. Our study revealed that maternal active smoking at the time of delivery, but not in mid-gestation, was considered a critical window of exposure. This finding is consistent with previous research in three population-based cohorts, which reported that maternal smoking during the third trimester is associated with the greatest risk of obesity among children.34–36 Maternal smoking during the third trimester may contribute to intrauterine growth restriction and subsequent metabolic changes in the offspring through a number of mechanisms, including a drastic decrease in placental vascularization.37 An alternative explanation is that our study had insufficient statistical power to detect an association at the other prenatal windows. Furthermore, since there was moderate concordance between exposure measurements, our findings may be a result of differences between individuals rather than differences within individuals. Although some uncertainty remains about the most susceptible prenatal windows, smoking cessation at any stage in pregnancy continues to be beneficial for the prevention of obesity in childhood.38
Infants may be more susceptible to tobacco exposure as compared to older children. From a biological perspective, infants may experience a relatively higher dose of nicotine than older children due to faster ventilation rates,39 slower nicotine clearance,40,41 and immature detoxification and metabolic pathways.42 Furthermore, infants may spend more time in the home, whereas older children may spend more time in childcare or school settings and away from household smokers.43 Finally, the relative importance of other factors, such as postnatal nutrition and physical activity, may shift throughout childhood, such that these factors play a more important role in the development of obesity than tobacco exposure.
Our study may be limited by selection bias, as not all of the mother–child pairs had complete exposure, outcome, or covariate data. We did not detect any meaningful differences in sociodemographic characteristics between the included and excluded mother–child pairs. Nevertheless, selection bias may have impacted our results if loss to follow-up was associated with the exposure, outcome, or other covariates.
A second limitation is the potential for residual confounding. Tobacco exposure is strongly correlated with socioeconomic position, early-life nutrition, and many other unmeasured genetic or epigenetic factors. Although we adjusted for many covariates, residual confounding may have biased our results either towards the null or, in the most extreme case, away from the null when no such association between tobacco exposure and increased adiposity exists.44 Furthermore, it is likely that genetic45 or epigenetic25 mechanisms may mediate, or confound, these associations. Future studies are needed to clarify the mechanisms.
Finally, our study may be limited by the exposure assessment. First, we lacked data on paternal exposure or use of tobacco during the pre-conception period, which may have played a role in these associations. Second, early-life exposure to tobacco was measured differently across the seven exposure windows. Thus, it is not clear whether the discordance of the exposure across time can be attributed to differences in measurement or other factors, such as changes in behaviour or household composition. Third, both self-report and cotinine may underestimate exposure. Parents tend to underestimate tobacco use or exposure, particularly during pregnancy. Cotinine may be a more objective measure of early-life exposure to tobacco than self-report. However, since the half-life is relatively short (~16 h), cotinine may fail to capture intermittent exposure. This exposure misclassification would likely be non-differential with respect to the outcome, resulting in a bias towards the null. Despite these limitations, our use of several measures of self-report and cotinine over time may have reduced the potential for exposure misclassification.46
Our study has several strengths, including the use of air displacement plethysmography to directly measure fat mass and fat-free mass.47 This method has been validated in neonates and children47–49 and is being increasingly used in clinical and research settings. The effect sizes noted in our study (e.g., pre-conception exposure to tobacco is associated with a 1.70% increase in childhood adiposity) are similar to the effect sizes for other early-life risk factors. For instance, one published study reported that childhood adiposity (as measured via air displacement plethysmography) was 1.40% higher among offspring born to mothers with gestational diabetes mellitus.50
4.1 |. Conclusions
The associations between tobacco exposure and increased adiposity and fat mass from birth to age 5 years may depend on the timing of the exposure. Exposure immediately before pregnancy, during late gestation, and in early infancy may have the greatest impact on childhood adiposity. The plausibility of our results is strengthened by the fact that we detected interactions between the exposure and the timing of the exposure despite the relatively low statistical power in our models.14 Our results provide novel insights about the underlying mechanisms, which may include a combination of epigenetic inheritance/modifications,23,24 structural and functional changes to the placenta,26,27,37 and postnatal changes in metabolism39–42 and behaviour.43 Furthermore, our results emphasize the need for smoking cessation efforts to be tailored specifically to non-pregnant women of childbearing age for the prevention of increased adiposity in childhood, and to remain in place during the early postpartum period when smoking relapse is common.
Supplementary Material
Funding information
National Institutes of Health, Grant/Award Numbers: R00ES025817, R00ES028711, R01DK076648, R01ES022934, UH3OD023248
Footnotes
CONFLICT OF INTEREST
No conflict of interest was declared.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Ino T Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52(1):94–99. [DOI] [PubMed] [Google Scholar]
- 2.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) (2005). 2008;32(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen GTM, Dharmage S, Janson C, et al. Parents’ smoking onset before conception as related to body mass index and fat mass in adult offspring: findings from the RHINESSA generation study. PloS ONE. 2020;15(7):e0235632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raum E, Kupper-Nybelen J, Lamerz A, Hebebrand J, Herpertz-Dahlmann B, Brenner H. Tobacco smoke exposure before, during, and after pregnancy and risk of overweight at age 6. Obesity (Silver Spring, md). 2011;19(12):2411–2417. [DOI] [PubMed] [Google Scholar]
- 5.Mangrio E, Lindstrom M, Rosvall M. Early life factors and being overweight at 4 years of age among children in Malmo, Sweden. BMC Publ Health. 2010;10:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell R, Shen E, Gilliland FD, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s health study. Environ Health Perspect. 2015;123(4):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagani LS, Nguyen AK, Fitzpatrick C. Prospective associations between early long-term household tobacco smoke exposure and subsequent indicators of metabolic risk at age 10. Nicotine Tob Res. 2015;18:1250–1257. [DOI] [PubMed] [Google Scholar]
- 8.von Kries R, Bolte G, Baghi L, Toschke AM, GMES G. Parental smoking and childhood obesity--is maternal smoking in pregnancy the critical exposure? Int J Epidemiol. 2008;37(1):210–216. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Decker A, Kramer MS. Exposure to parental smoking and child growth and development: a cohort study. BMC Pediatr. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaakkola JM, Rovio SP, Pahkala K, et al. Childhood exposure to parental smoking and life-course overweight and central obesity. Ann Med. 2021;53(1):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braithwaite I, Stewart AW, Hancox RJ, Beasley R, Murphy R, Mitchell EA. Maternal post-natal tobacco use and current parental tobacco use is associated with higher body mass index in children and adolescents: an international cross-sectional study. BMC Pediatr. 2015;15:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Møller SE, Ajslev TA, Andersen CS, Dalgård C, Sørensen TI. Risk of childhood overweight after exposure to tobacco smoking in prenatal and early postnatal life. PloS ONE. 2014;9(10):e109184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore BF, Starling AP, Magzamen S, et al. Fetal exposure to maternal active and secondhand smoking with offspring early-life growth in the healthy start study. Int J Obes (Lond) (2005). 2019;43(4):652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and non-smokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. [DOI] [PubMed] [Google Scholar]
- 16.Guenther PM, Kirkpatrick SI, Reedy J, et al. The healthy eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for Americans. J Nutr. 2014;144(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro AL, Kaar JL, Crume TL, et al. Maternal diet quality in pregnancy and neonatal adiposity: the healthy start study. Int J Obes (Lond) (2005). 2016;40(7):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D. Predictors of infant body composition at 5 months of age: the healthy start study. J Pediatr. 2017;183:94–99.e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun JM, Chen A, Hoofnagle A, et al. Associations of early life urinary triclosan concentrations with maternal, neonatal, and child thyroid hormone levels. Horm Behav. 2018;101:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Papandonatos GD, Calafat AM, et al. Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities. Environ Res. 2019;172:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stacy SL, Papandonatos GD, Calafat AM, et al. Early life bisphenol a exposure and neurobehavior at 8years of age: identifying windows of heightened vulnerability. Environ Int. 2017;107:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigert M, Hofstetter G, Kaipl D, et al. The effect of smoking on oocyte quality and hormonal parameters of patients undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16(6):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panzeri I, Pospisilik JA. Epigenetic control of variation and stochasticity in metabolic disease. Mol Metab. 2018;14:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13(3):153–162. [DOI] [PubMed] [Google Scholar]
- 25.Joubert BR, Håberg SE, Bell DA, et al. Maternal smoking and DNA methylation in newborns: in utero effect or epigenetic inheritance? Cancer Epidemiol Biomarkers Prev. 2014;23(6):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genbacev O, Bass KE, Joslin RJ, Fisher SJ. Maternal smoking inhibits early human cytotrophoblast differentiation. Reprod Toxicol. 1995;9(3):245–255. [DOI] [PubMed] [Google Scholar]
- 27.Sabra S, Gratacós E, Gómez Roig MD. Smoking-induced changes in the maternal immune, endocrine, and metabolic pathways and their impact on fetal growth: a topical review. Fetal Diagn Ther. 2017;41(4):241–250. [DOI] [PubMed] [Google Scholar]
- 28.Braun JM, Daniels JL, Poole C, et al. Prenatal environmental tobacco smoke exposure and early childhood body mass index. Paediatr Perinat Epidemiol. 2010;24(6):524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedel C, Fenske N, Müller MJ, et al. Differences in BMI z-scores between offspring of smoking and nonsmoking mothers: a longitudinal study of German children from birth through 14 years of age. Environ Health Perspect. 2014;122(7):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2006;35(1):121–130. [DOI] [PubMed] [Google Scholar]
- 31.Haga C, Kondo N, Suzuki K, et al. Developmental trajectories of body mass index among Japanese children and impact of maternal factors during pregnancy. PLoS ONE. 2012;7(12):e51896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe LD, Matijasevich A, Tilling K, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol. 2012;41(3):722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13(11):2021–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durmus B, Heppe DH, Taal HR, et al. Parental smoking during pregnancy and total and abdominal fat distribution in school-age children: the generation R study. Int J Obes (Lond) (2005). 2014;38(7):966–972. [DOI] [PubMed] [Google Scholar]
- 35.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2005;35(1):121–130. [DOI] [PubMed] [Google Scholar]
- 36.Toschke AM, Montgomery SM, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. Am J Epidemiol. 2003;158(11):1068–1074. [DOI] [PubMed] [Google Scholar]
- 37.Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83(11):699–706. [DOI] [PubMed] [Google Scholar]
- 38.Reeves S, Bernstein I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev Obstet Gynecol. 2008;3(6):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet (London, England). 2011;377(9770):1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dempsey D, Jacob P 3rd, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67(5):458–465. [DOI] [PubMed] [Google Scholar]
- 41.Ginsberg G, Hattis D, Sonawane B, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci. 2002;66(2):185–200. [DOI] [PubMed] [Google Scholar]
- 42.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatzke-Kopp LM, Willoughby MT, Warkentien SM, O’Connor T, Granger DA, Blair C. Magnitude and chronicity of environmental smoke exposure across infancy and early childhood in a sample of low-income children. Nicotine Tob Res. 2019;21(12):1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–655. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Moon JY, Wu Y, et al. Pleiotropy of genetic variants on obesity and smoking phenotypes: results from the Oncoarray project of the international lung cancer consortium. PloS One. 2017;12(9):e0185660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckley JP, Hamra GB, Braun JM. Statistical approaches for investigating periods of susceptibility in Children’s environmental Health Research. Curr Environ Health Rep. 2019;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma G, Yao M, Liu Y, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79(4):653–660. [DOI] [PubMed] [Google Scholar]
- 48.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53(3):486–492. [DOI] [PubMed] [Google Scholar]
- 49.Yao M, Nommsen-Rivers L, Dewey K, Urlando A. Preliminary evaluation of a new pediatric air displacement plethysmograph for body composition assessment in infants. Acta Diabetol. 2003;40(Suppl 1):S55–S58. [DOI] [PubMed] [Google Scholar]
- 50.Josefson JL, Catalano PM, Lowe WL, et al. The joint associations of maternal BMI and Glycemia with childhood adiposity. J Clin Endocrinol Metab. 2020;105(7):2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


