Abstract
Background
Modern multistrand repairs can withstand forces present in active flexion exercises, and this may improve the outcomes of flexor tendon repairs. We developed a simple home-based exercise regimen with free wrist and intrinsic minus splint aimed at facilitating the gliding of the flexor tendons and compared the outcomes with the modified Kleinert regimen used previously in the same institution.
Methods
We searched the hospital database to identify flexor tendon repair performed before and after the new regimen was implemented and invited all patients to participate. The primary outcome was total active range of motion, and secondary outcomes were Disabilities of Arm, Shoulder, and Hand; grip strength; globally perceived function; and the quality of life.
Results
The active range of motion was comparable between the groups (mean difference = 14; 95% confidence interval [CI], −8 to 36; P = .22). Disabilities of Arm, Shoulder, and Hand; grip strength; global perceived function; and health-related quality of life were also comparable between the groups. There was 1 (5.3%) rupture in the modified Kleinert group and 4 (15.4%) in the early active motion group (relative risk = 0.3; 95% CI, 0.04-2.5; P = .3).
Conclusions
Increasing active gliding with a free wrist and intrinsic minus splint did not improve the clinical outcomes after flexor tendon injury at a mean of 38-month follow-up.
Keywords: flexor tendon, tendon injuries, finger injuries, early mobilization, postoperative period, rehabilitation, outcome
Introduction
Flexor tendon injuries are most frequently seen in young working-aged men. 1 Rehabilitation takes a long time, and thus, these injuries cause substantial economic burden to society. 2 Excellent results are difficult to achieve, although repair techniques and materials have evolved greatly during recent years. 3
Although differences likely exist, both active and passive flexor tendon rehabilitation seem to produce acceptable results with small rupture rate.4-6 However, stiffness of the finger is common, and modern multistrand repair techniques are capable to withstand tension beyond what is present in active flexion of the fingers. Thus, we hypothesized that a more aggressive active motion regimen could further improve the clinical outcomes without increase in the rupture rate.
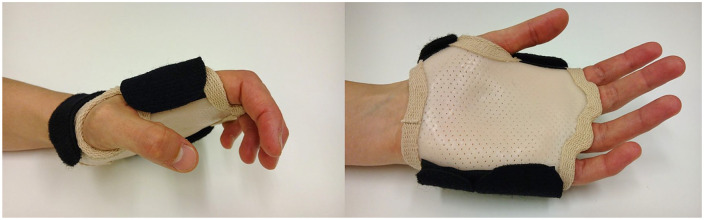
Knowing that the repairs can withstand active motions, and to overcome challenges related to long distances between homes and hand therapy, we developed a simple active rehabilitation regimen, which aims at facilitating flexor tendon gliding. Our aim was to encourage patients to do relatively aggressive active flexor tendon gliding exercises throughout the day without constant supervision. The gliding exercises were performed throughout the day with the help of splint blocking the metacarpophalangeal (MP) joints in extension (intrinsic minus splint, Figure 1) and allowed free motion of the wrist. Before the implementation of the new active regimen, our patients used modified Kleinert regimen.
Figure 1.
Daytime splint in the active protocol.
The purpose of this study was to compare the clinical outcomes of new active motion regimen with a modified Kleinert regimen in 2 cohorts of consecutive patients operated in a single center.
Material and Methods
This study was a retrospective comparison of clinical outcomes in 2 consecutive cohorts of patients who suffered a flexor tendon injury and were repaired between 2011 and 2015. The institution serves a population of 255 000 in an area with a diameter of 300 km, with many patients having to travel greater than 2 hours to get to the hospital. The hospital gathers most of the flexor tendon injuries in the region because it is the only hospital providing hand surgical service in the area. Consequently, the sample was population-based, including most of the injuries occurred during the time period.
The inclusion criteria were patients with flexor tendon injury with or without concomitant nerve injuries. We excluded patients younger than 16 years at the time of operation, noncompliant patients who did not participate in rehabilitation at all or who could not speak Finnish, patients with fracture in the same hand (eg, finger fracture), soft tissue defect repaired with a flap or skin graft, neurological diseases affecting upper limb functionality, or rheumatoid arthritis.
We identified 61 eligible patients by running a search in the hospital electronic database. Ten patients refused to take part in the study, 2 could not be contacted, and 3 patients did not arrive to the study follow-up visit. A total of 46 patients with 57 injured fingers attended the follow-up visits.
The patients were informed about the study and asked to give informed consent to the study. The research ethics committee approved this study (KSSHP 13U/2015).
Postoperative Rehabilitation Regimens
From spring 2011 until autumn 2012, patients were rehabilitated with an active modified Kleinert regimen as per our department’s standard protocol. Patients from autumn 2012 until December 2015 were rehabilitated with new early active motion regimen.
In both protocols, a hand therapist fabricated the splints, and supervised exercises 3 to 5 days after surgery. The patients were instructed not to use the operated hand in daily activities, to wear the splints all the time, and to do the exercises precisely the way they were instructed during the first 6 weeks of rehabilitation. The patients received written information sheets with pictures of the exercises and splints.
Patients were encouraged to contact the rehabilitation personnel immediately if they had any questions or problems performing the exercises and additional follow-up visits were arranged if needed. An appointment with the hand surgeon was arranged 5 to 6 weeks postoperative and the visits to hand therapists depending on the regimen. Further follow-up visits were arranged depending on the patient’s progression.
In the modified Kleinert regimen, the splint enabled active extension of finger joints and rubber bands produced passive flexion via palmar pulley, modification presented by May et al. 7 The wrist was in neutral position, MP joints in 60° flexion, and, overnight, interphalangeal (IP) joints were resting in full extension. Same splinting principles were applied to thumb flexor repairs.
Hourly exercises included active extension exercises of IP joints with a splint. In addition, place-and-hold exercises were performed 4 times per day without the splint with the wrist extended. The splint was removed after 4 weeks, but the rubber band traction was applied until week 6 with a wrist cuff, and active flexion exercises with synergistic wrist motion were added to the exercise program. The traction was removed completely after 6 weeks, and full use of the hand was permitted by 8 weeks. Patients attended weekly appointments with a physiotherapist and an occupational therapist in the hospital outpatient clinic until 6 weeks postoperatively. Scar management and edema control remained constant throughout the study period.
In the early aggressive motion regimen, 2 different splints were fabricated. A volar extrinsic minus splint was designed to allow the wrist movement and direct the flexion force to IP joints by blocking MP joint flexion (Figure 1), and simultaneously it prevented inadvertent gripping. It was worn during the day, and hourly exercises were performed with the splint (Figure 1).
A dorsal splint with free wrist movement, MP joints in 60° flexion, and IP joints in full extension was used during nights and when extra protection was needed. Same splinting principles were applied to thumb flexor repairs.
The patients in the aggressive early active motion group were specifically encouraged to flex the fingers in as wide range of motion (ROM) as they could, but any gripping was forbidden. They were told that stiffness was more common than repair rupture and repair rupture would be easier to treat compared with adhesions. Hourly exercises with the volar splint included active extension of IP joints and active IP joint flexion without resistance with synergistic wrist motion. In addition, isolated IP joint flexion exercises and place-and-hold exercises with an extended wrist were performed 5 times per day without the splint. Splints were removed 5 to 6 weeks postoperatively. After the initial follow-up visit, patients attended 1 follow-up visit during the 6-week period with a physiotherapist, and an occupational therapist was consulted only if required.
Surgical Technique
The operation was scheduled to our day surgery unit within 10 days from the injury and the initial consultation. A total of 35 injuries were repaired using a 6-strand modified Lim-Tsai core repair with a 4-0 Fiberloop (Arthrex, Naples, Florida) suture. Two fellowship-trained hand surgeons performed all repairs. End-to-end repairs were reinforced with epitendinous repairs using a 5 to 0 polypropylene (Ethicon, Somerville, New Jersey) suture. Four injuries were repaired using bone anchors (Arthrex); all these were in zone I. One tendon was repaired using 8-strand suture with 4-0 Fiberloop. Three injuries were repaired using 4-strand core sutures.
Outcome Measures
Primary outcome measure was active range of motion (AROM; sum of AROM in MP, proximal interphalangeal [PIP], and distal interphalangeal [DIP] joints, and MP and interphalangeal [IP] joints in the thumb) of the injured finger measured with a Baseline Digit goniometer (Fabrication Enterprises Inc, White Plains, New York). One experienced hand therapist measured the ROM and collected all other data.
Secondary outcomes were grip strength (kg; Jamar, 2. position; mean of 3 tries) of both hands; Disabilities of Arm, Shoulder, and Hand (DASH) questionnaire score; global perceived function (functional Visual Analog Scale (f-VAS); 0 worst possible function, and 10 normal finger); quality of life VAS (0 = worst possible quality of life and 10 = best possible quality of life); and rupture rate.
Sample Size and Statistical Analysis
Without prior knowledge of minimal important difference in AROM, we used a value of 30°. To detect greater than or equal to 30° difference in AROM, the sample size was calculated to be 21 per group (α = 0.05; β = 0.10; SD = 30).
To account for clustering in participants with several affected rays, we used a linear mixed model to compare the total active motion (TAM) (in ray level, n = 57) between the groups. Treatment group and affected ray were entered as fixed factors and patient as a random factor to the model.
For secondary outcomes (grip strength, DASH, f-VAS, and quality of life), we performed all analyses at the participant level (n = 49). We estimated the effect using a generalized linear model adjusting for the number of affected rays, presence of nerve injury (yes/no), affected ray, and zone of injury.
To compare rupture rates, we calculated relative risk (RR) with 95% confidence intervals. To compare baseline characteristics and time from the injury, we used Student t test for continuous variables with normal distribution, Mann-Whitney U test with skewed data, and χ2 test for binary variables.
Results
Twenty-one patients with 26 digits underwent modified Kleinert regimen and 28 patients with 31 digits underwent early active motion (49 participants with 57 injured fingers). The groups were otherwise comparable regarding their participant characteristics, but due to using 2 consecutive cohorts, the Kleinert regimen group had a significantly longer time between surgery and measurement (mean difference = 24 months, 95% confidence interval [CI], 19-29 months; P < .001) (Table 1). The range of follow-up was 9 to 71 months; 2 patients in the early active motion group were assessed less than 15 months from the injury (9 and 11 months).
Table 1.
Participant and Injury Characteristics.
| Variable | Modified Kleinert (N = 21) | Early active motion (N = 28) | P value a |
|---|---|---|---|
| Digits, n | 26 | 31 | |
| Age, mean (range) | 40 (19-59) | 37 (18-65) | .56 |
| Males, n | 14 | 19 | .93 |
| Injured hand right, n | 12 | 14 | .62 |
| Injured digit, n | |||
| Thumb | 9 | 7 | |
| Index | 7 | 5 | |
| Middle | 3 | 5 | |
| Ring | 2 | 6 | |
| Little | 5 | 8 | |
| Injury zone, n | |||
| I | 3 | 6 | |
| II | 15 | 22 | |
| III | 4 | 2 | |
| IV | 1 | 1 | |
| V | 3 | 0 | |
| Concomitant nerve injury, n (%) | 10 | 13 | .862 |
| Time since surgery, mo, mean (SD) | 52.1 | 27.8 | <.001 |
| Number of follow-up visits, mean (SD) | 7.6 | 5.8 | .07 |
Student’s T test; Mann-Whitney U-test; or Chi-Square test.
We did not find significant difference between the groups in the primary or secondary outcomes (Table 2). Regarding the primary outcome (TAM), the unadjusted values were 190° (SD = 59) in the modified Kleinert versus 184° (SD = 52) in the AROM group for the fingers 2 to 4, and 111° (SD = 24) versus 85° (SD = 31) in the thumb, respectively. The average ROM corresponded with 79% of the uninjured finger (fingers 2-5) and 78% of the uninjured thumb in the whole sample. According to the Strickland classification, 12 fingers were excellent, 8 good, 8 fair, and 3 poor. In modified Kleinert, the numbers were 14 excellent, 11 good, and 1 fair.
Table 2.
Comparison of the Clinical Outcomes.
| Variable | Active group | Kleinert group | Mean difference | 95% CI | P value |
|---|---|---|---|---|---|
| AROM a | 187 | 201 | 14 | −8 to 36 | .22 |
| Grip b | 39.3 | 43.5 | 4.2 | −3.1 to 11.5 | .59 |
| DASH c | 6.3 | 8.2 | 1.9 | −1.9 to 5.7 | .33 |
| F-VAS d | 7.3 | 8.4 | 1.1 | −0.2 to 2.5 | .1 |
| Quality of life e | 7.9 | 8.2 | 0.4 | −0.7 to 1.4 | .47 |
Note. CI = confidence interval; AROM = active range of motion; DASH = Disabilities of Arm, Shoulder, and Hand.
AROM in degrees, including thumbs.
Measured in kilograms, Jamar position 2.
Higher score indicates worse function (scale 0-100).
Functional visual Analog Scale; higher value indicates better perceived function (scale 0-10).
Higher value indicates better quality of life (scale 0-10).
We observed 1 repair failure in the Kleinert regimen group and 4 failures in the early active motion group (RR = 0.3; 95% CI, 0.04-2.5; P = .27). The presumed reason for the rupture in the Kleinert regimen group was failure to comply with splinting regimen, and for the early active motion group, the reasons were noncompliance with the splinting regimen in 1 patient and spontaneous ruptures in 3 patients. One participant refused further surgery, and the other 3 had successful rerepair. None of the participants needed tenolysis in this sample. All participants returned to their previous work.
Discussion
We did not achieve improved results by implementing more aggressive exercise regimen as we hypothesized. Both regimens resulted in acceptable clinical outcomes with differences smaller than what we considered clinically meaningful, also confirmed by comparable results in the secondary outcomes.
The AROM results are comparable with previous reports that use AROM or place and hold exercises.8-10 Two recent randomized controlled trials indicate that early active movement regimens may produce better ROM when compared with passive motion regimens.8,11 Several authors advocate the use of early active motion regimens in flexor tendon rehabilitation because modern techniques can withstand the forces present in nonresisted active flexion. 12
Synergistic wrist movement could, in theory, facilitate achieving better ROM, if the repair is strong enough to endure wrist movement. 13 In this study, no benefit was found from free wrist motion. Based on biomechanical studies, there seems to be no rationale to limit wrist extension.14-16 However, controlling biology may be more important than controlling biomechanics to further improve the outcomes.
The mean disability measured by DASH score was comparable between the groups. This is plausible as the difference in ROM was clinically unimportant. Furthermore, we found no difference in the quality of life or global satisfaction with perceived finger function measured in VAS scale. Trumble et al used a similar method to assess function, and they found a 1.2 point (0-10 point scale) difference between the groups in favor of the active motion group corresponding well with the magnitude of difference found in our study. 11 However, it should be remembered that our modified Kleinert regimen was not a true passive regimen.
Our rupture rate in the active regimen was relatively high, but this might also be due to random variation. The sample is not sufficient to estimate differences due to a low event rate, and regarding ruptures, our study is inconclusive. Prowse et al 17 found a 4-fold increase in the rupture rate in their controlled active motion regimen and noted that previous active motion studies have reported rupture rates up to 46%. 18 A systematic review reported a mean rate of 4%. 19
Recognizing the potential bias related to nonrandomized study design, 20 we acknowledge that the analyses should be considered as exploratory rather than explanatory. However, we also believe that large effects would have been obvious if the new protocol was clearly superior. Furthermore, due to sampling from 2 different time periods, the follow-up time was different between the groups. We also cannot isolate the effect of reduced number of follow-up visits from the effect of different exercises and splints but speculate that the number of follow-up (7.6 vs 5.8) visits has little effect. Finally, the study participation rate was 70%, and a higher participation rate may yield different estimates. The strength of the study is that population-based sampling results in representable group of participants during both time periods.
To conclude, increasing active aggressive extrinsic gliding by free wrist and intrinsic minus splint did not provide meaningful benefits in patients with acute flexor tendon injury compared with modified Kleinert protocol. Regarding harms, the study was underpowered, and we cannot conclude whether a more aggressive regimen results in a higher rupture rate.
Acknowledgments
Teemu Karjalainen has worked under grants from Finnish Medical Foundation and Gyllenberg Foundation during the preparation of this article.
Footnotes
Ethical Approval: This study was approved by our institutional review board (KSSHP 13U/2015).
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and with the Helsinki Declaration of 1975, as revised in 2008).
Statement of Informed Consent: All participants gave informed consent to participate in this study.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T.L. reports personal fees from AO Foundation, the Finnish Society for Surgery of the Hand, the Finnish Trauma Association, Arthrone Inc, and Articular Inc, during the conduct of the study. T.K. reports grants from Gyllenberg Foundation and the Finnish Medical Foundation, during the conduct of the study. The other authors declare no conflict of interest.
Funding: The author(s) received no financial support for the financial support for the research, authorship, and/or publication of this article.
ORCID iD: Teemu Karjalainen  https://orcid.org/0000-0002-5650-895X
https://orcid.org/0000-0002-5650-895X
References
- 1. Manninen M, Karjalainen T, Määttä J, et al. Epidemiology of flexor tendon injuries of the hand in a Northern Finnish population. Scand J Surg. 2017;106(3):278-282. [DOI] [PubMed] [Google Scholar]
- 2. Rosberg HE, Carlsson KS, Höjgård S, et al. What determines the costs of repair and rehabilitation of flexor tendon injuries in zone II? a multiple regression analysis of data from southern Sweden. J Hand Surg Br. 2003;28(2):106-112. [DOI] [PubMed] [Google Scholar]
- 3. Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21(2):199-210. [DOI] [PubMed] [Google Scholar]
- 4. Dy C, Daluiski A, Do H, et al. The epidemiology of reoperation after flexor tendon repair. J Hand Surg Am. 2012;37:919-924. [DOI] [PubMed] [Google Scholar]
- 5. Starr HM, Snoddy M, Hammond KE, et al. Flexor tendon repair rehabilitation protocols: a systematic review. J Hand Surg Am. 2013;38(9):1712-1717. [DOI] [PubMed] [Google Scholar]
- 6. Thien TB, Becker JH, Theis JC. Rehabilitation after surgery for flexor tendon injuries in the hand. Cochrane Database Syst Rev. 2004;4:CD003979. [DOI] [PubMed] [Google Scholar]
- 7. May EJ, Silfverskiöld KL, Sollerman CJ. Controlled mobilization after flexor tendon repair in zone II: a prospective comparison of three methods. J Hand Surg Am. 1992;17(5):942-952. [DOI] [PubMed] [Google Scholar]
- 8. Farzad M, Layeghi F, Asgari A, et al. A prospective randomized controlled trials of controlled passive mobilization vs. place and active hold exercises after zone 2 flexor tendon repairs. Hand Surg. 2014;19(1):53-59. [DOI] [PubMed] [Google Scholar]
- 9. Frueh FS, Kunz VS, Gravestock IJ, et al. Primary flexor tendon repair in zones 1 and 2: early passive mobilization versus controlled active motion. J Hand Surg Am. 2014;39(7):1344-1350. [DOI] [PubMed] [Google Scholar]
- 10. Rigó IZ, Haugstvedt JR, Røkkum M. The effect of adding active flexion to modified Kleinert regime on outcomes for zone 1 to 3 flexor tendon repairs. A prospective randomized trial. J Hand Surg Eur Vol. 2017;42(9):920-929. [DOI] [PubMed] [Google Scholar]
- 11. Trumble TE, Vedder NB, Seiler JG, III, et al. Zone-II flexor tendon repair: a randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. J Bone Joint Surg Am. 2010;92(6):1381-1389. [DOI] [PubMed] [Google Scholar]
- 12. Starr HM, Snoddy M, Hammond KE, et al. Flexor tendon repair rehabilitation protocols: a systematic review. J Hand Surg Am. 2013;38(9):1712-1717. [DOI] [PubMed] [Google Scholar]
- 13. Peck F, Roe A, Ng C, et al. The Manchester short splint: a change to splinting practice in the rehabilitation of zone II flexor tendon repairs. Hand Ther. 2014;19(2):47-53. [Google Scholar]
- 14. Minamikawa Y, Peimer CA, Yamaguchi T, et al. Wrist position and extensor tendon amplitude following repair. J Hand Surg Am. 1992;17(2):268-271. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka T, Amadio PC, Zhao C, et al. Flexor digitorum profundus tendon tension during finger manipulation. J Hand Ther. 2005;18(3):330-338; quiz 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rappaport PO, Thoreson AR, Yang TH, et al. Effect of wrist and interphalangeal thumb movement on zone T2 flexor pollicis longus tendon tension in a human cadaver model. J Hand Ther. 2015;28(4):347-354; quiz 355. [DOI] [PubMed] [Google Scholar]
- 17. Prowse P, Nixon M, Constantinides J, et al. Outcome of zone 2 flexor tendon injuries: Kleinert versus controlled active motion therapy regimens. Hand Ther. 2011;16(4):102-106. [Google Scholar]
- 18. Peck FH, Bücher CA, Watson JS, et al. A comparative study of two methods of controlled mobilization of flexor tendon repairs in zone 2. J Hand Surg Br. 1998;23(1):41-45. [DOI] [PubMed] [Google Scholar]
- 19. Dy C, Hernandez-Soria A, Ma Y, et al. Complications after flexor tendon repair: a systematic review and meta-analysis. J Hand Surg Am. 2012;37(3):543-551. [DOI] [PubMed] [Google Scholar]
- 20. Ioannidis JP, Haidich AB, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA. 2001;286(7):821-830. [DOI] [PubMed] [Google Scholar]