Abstract
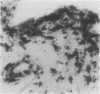
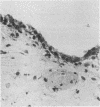
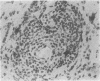
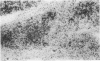
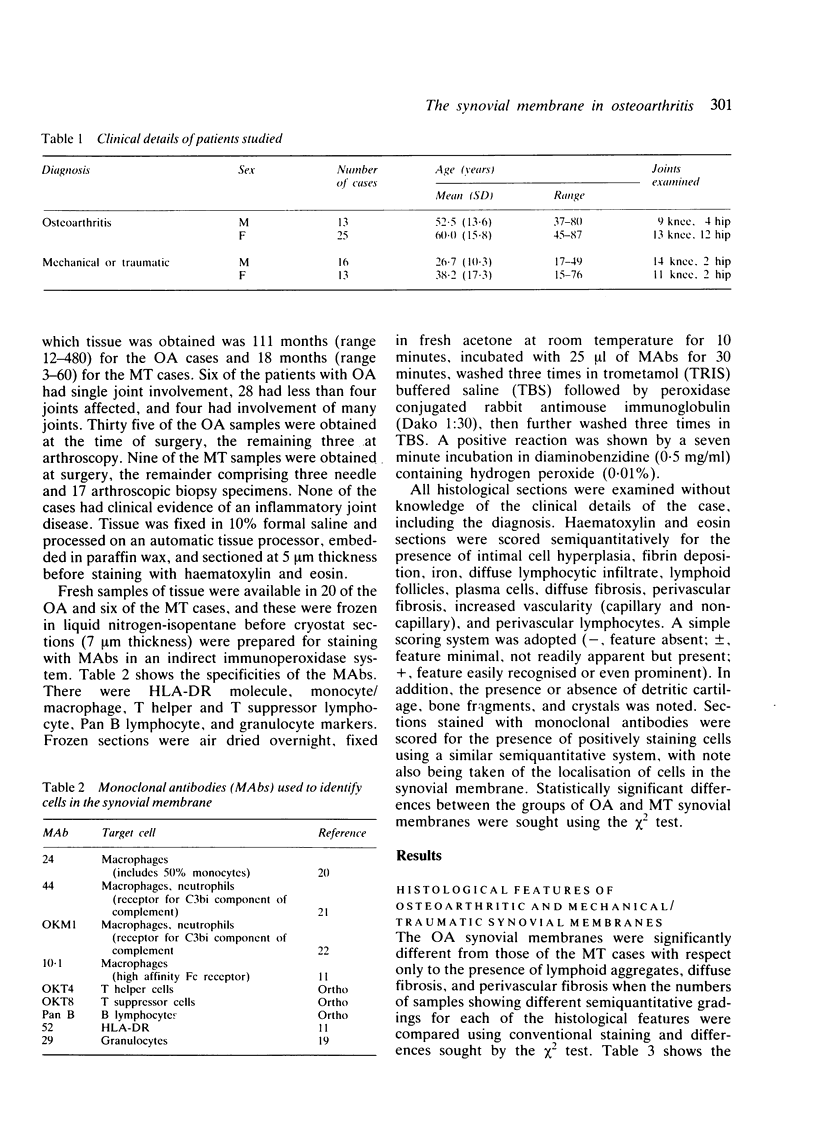
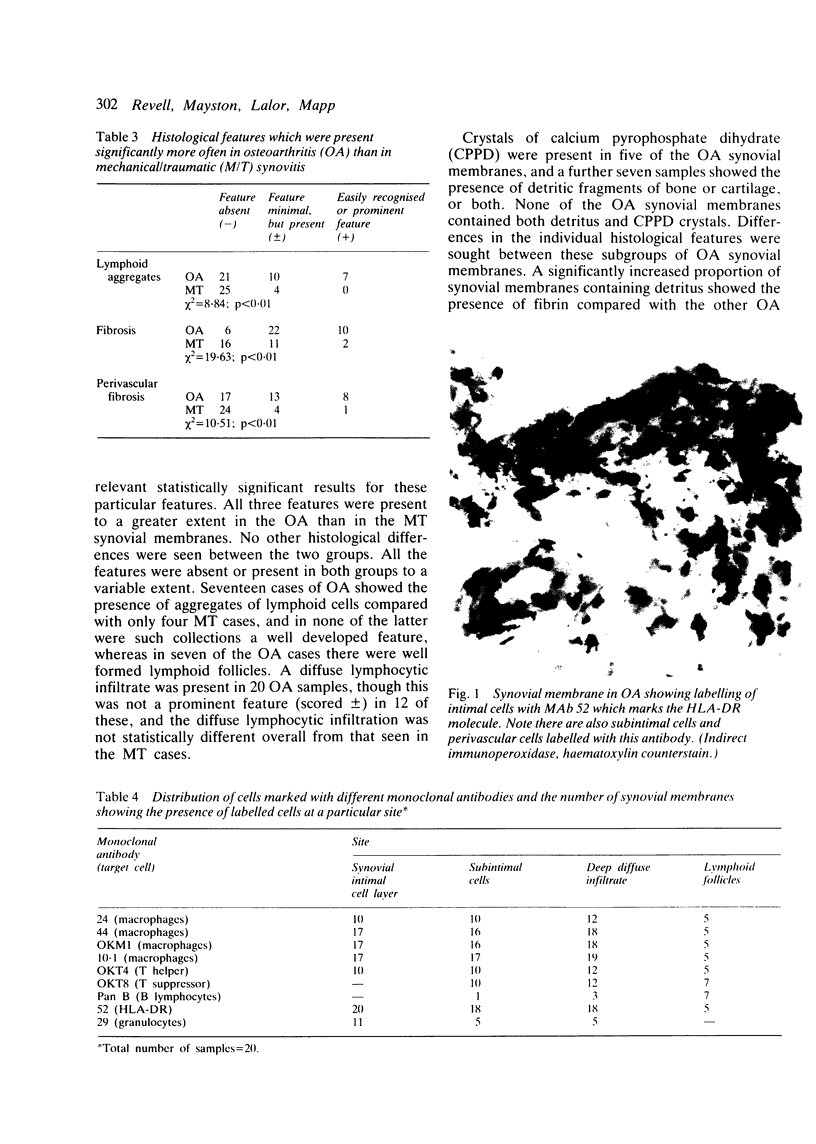
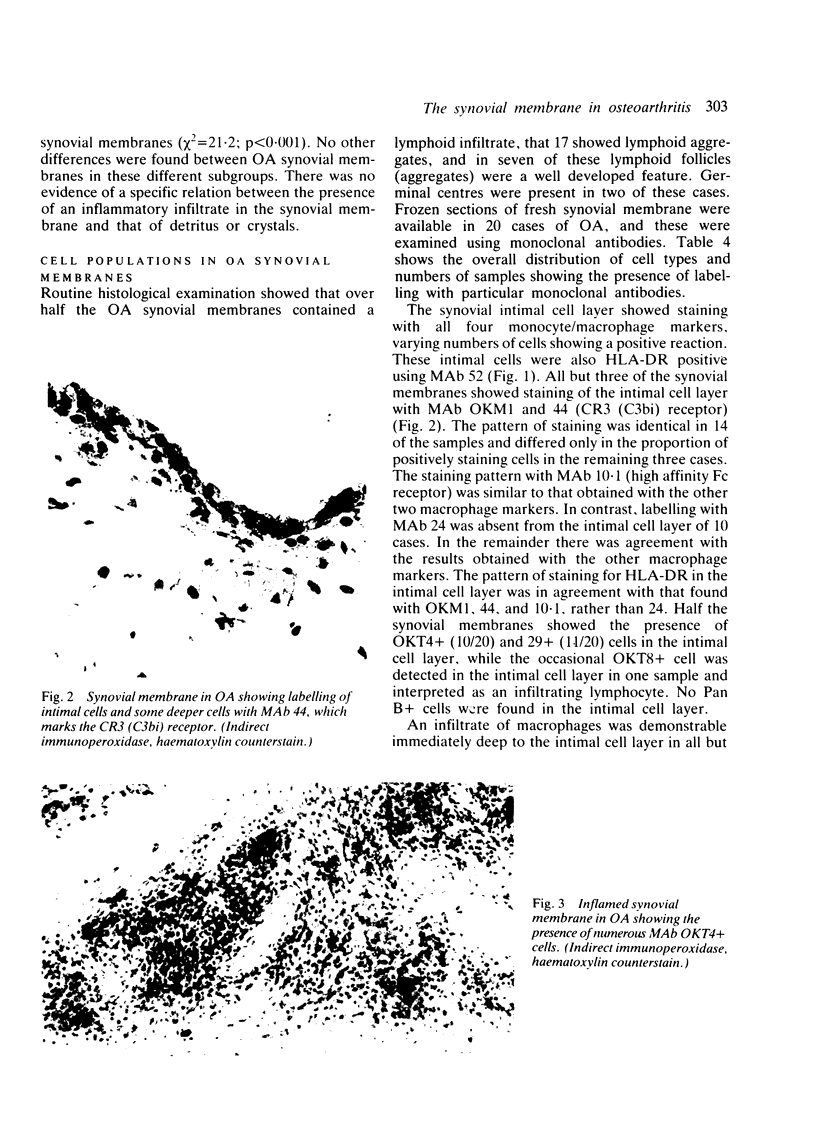
Inflammatory infiltration of the synovial membrane has been described in a proportion of cases of osteoarthritis (OA). Using conventional histology, lymphoid follicles, diffuse fibrosis, and perivascular fibrosis were shown to be present to a significantly greater extent and in more synovial membranes in osteoarthritis than in those cases where there was a mechanical or traumatic background to the joint disease. Calcium pyrophosphate dihydrate crystals (five patients) and detritus fragments of bone and cartilage (seven patients) were present in small numbers of the total cases of OA (38) studied. Neither of these features was related to the presence of an inflammatory infiltrate. Examination of 20 osteoarthritic synovial membranes using monoclonal antibodies showed the presence of lymphoid follicles containing T helper and T suppressor lymphocytes, B lymphocytes, and macrophages expressing HLA-DR in five cases. The T helper:suppressor ratio varied between 1:1 and 2.5:1 in these follicles. In addition, half of the OA samples, including these five cases, showed the presence of a diffuse cellular infiltrate containing T and B lymphocytes and macrophages, which were HLA-DR positive. Granulocytes were present in this diffuse infiltrate in those cases containing lymphoid follicles. The results confirm the presence of an inflammatory form of osteoarthritis but also show that the proportions of lymphoid cells are not the same as those considered to be typical of rheumatoid arthritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Locher P., Koch B., Winchester R. J., Dimitriu-Bona A., Kalden J. R., Mohr W. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol Int. 1983;3(4):173–181. doi: 10.1007/BF00541597. [DOI] [PubMed] [Google Scholar]
- Cooper N. S., Soren A., McEwen C., Rosenberger J. L. Diagnostic specificity of synovial lesions. Hum Pathol. 1981 Apr;12(4):314–328. doi: 10.1016/s0046-8177(81)80141-4. [DOI] [PubMed] [Google Scholar]
- Dieppe P. A., Doherty M., Macfarlane D. G., Hutton C. W., Bradfield J. W., Watt I. Apatite associated destructive arthritis. Br J Rheumatol. 1984 May;23(2):84–91. doi: 10.1093/rheumatology/23.2.84. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Førre O., Thoen J., Lea T., Dobloug J. H., Mellbye O. J., Natvig J. B., Pahle J., Solheim B. G. In situ characterization of mononuclear cells in rheumatoid tissues, using monoclonal antibodies. No reduction of T8-positive cells or augmentation in T4-positive cells. Scand J Immunol. 1982 Oct;16(4):315–319. doi: 10.1111/j.1365-3083.1982.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Cohen A. S. Synovial membrane histopathology in the differential diagnosis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease. Medicine (Baltimore) 1978 May;57(3):239–252. doi: 10.1097/00005792-197805000-00004. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Egan M. S., Cohen A. S. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982 Mar-Apr;9(2):204–209. [PubMed] [Google Scholar]
- Halverson P. B., McCarty D. J. Identification of hydroxyapatite crystals in synovial fluid. Arthritis Rheum. 1979 Apr;22(4):389–395. doi: 10.1002/art.1780220412. [DOI] [PubMed] [Google Scholar]
- Hogg N., Palmer D. G., Revell P. A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology. 1985 Dec;56(4):673–681. [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Selvendran Y. An anti-human monocyte/macrophage monoclonal antibody, reacting most strongly with macrophages in lymphoid tissue. Cell Immunol. 1985 May;92(2):247–253. doi: 10.1016/0008-8749(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Koch B., Locher P., Burmester G. R., Mohr W., Kalden J. R. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. II. The localization of mononuclear cells as detected by monoclonal antibodies directed against T-lymphocyte subsets and natural killer cells. Rheumatol Int. 1984;4(2):79–85. doi: 10.1007/BF00541201. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Palmer D. G., Hogg N., Revell P. A. Lymphocytes, polymorphonuclear leukocytes, macrophages and platelets in synovium involved by rheumatoid arthritis. A study with monoclonal antibodies. Pathology. 1986 Oct;18(4):431–437. doi: 10.3109/00313028609087564. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Roy S. Ultrastructure of synovial membrane in osteo-arthritis. Ann Rheum Dis. 1967 Nov;26(6):517–527. doi: 10.1136/ard.26.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Schindler J., Kung P. C., Goldstein G., Symoens J., Van Wauwe J. Evaluation of T cell subsets with monoclonal antibodies in patients with rheumatoid arthritis. J Rheumatol. 1982 Jan-Feb;9(1):25–29. [PubMed] [Google Scholar]