Abstract
Background:
Most studies on the association of in utero exposure to cigarette smoking and childhood overweight or obesity (OWO) were based on maternal self-reported smoking status, and few were based on objective biomarkers. The concordance of self-report smoking, and maternal and cord blood biomarkers of cigarette smoking as well as their effects on children's long-term risk of overweight and obesity are unclear.
Methods:
In this study, we analyzed data from 2351 mother-child pairs in the Boston Birth Cohort, a sample of US predominantly Black, indigenous, and people of color (BIPOC) that enrolled children at birth and followed prospectively up to age 18 years. In utero smoking exposure was measured by maternal self-report and by maternal and cord plasma biomarkers of smoking: cotinine and hydroxycotinine. We assessed the individual and joint associations of each smoking exposure measure and maternal OWO with childhood OWO using multinomial logistic regressions. We used nested logistic regressions to investigate the childhood OWO prediction performance when adding maternal and cord plasma biomarkers as input covariates on top of self-reported data.
Results:
Our results demonstrated that in utero cigarette smoking exposure defined by self-report and by maternal or cord metabolites was consistently associated with increased risk of long-term child OWO. Children with cord hydroxycotinine in the fourth quartile (vs. first quartile) had 1.66 (95% confidence interval [CI] 1.03–2.66) times the odds for overweight and 1.57 (95% CI 1.05–2.36) times the odds for obesity. The combined effect of maternal OWO and smoking on offspring risk of obesity is 3.66 (95% CI 2.37–5.67) if using self-reported smoking. Adding maternal and cord plasma biomarker information to self-reported data improved the prediction accuracy of long-term child OWO risk.
Conclusions:
This longitudinal birth cohort study of US BIPOC underscored the role of maternal smoking as an obesogen for offspring OWO risk. Our findings call for public health intervention strategies to focus on maternal smoking – as a highly modifiable target, including smoking cessation and countermeasures (such as optimal nutrition) that may alleviate the increasing obesity burden in the United States and globally.
Keywords: Maternal prenatal smoking, Childhood obesity, Smoking biomarkers, Cotinine, Hydroxycotinine, Plasma, Cord blood, In utero exposure
Introduction
The prevalence of child and adolescent obesity has been on the rise for the past four decades in the United States.[1] Childhood obesity is associated with a myriad of adverse health consequences throughout lifetime, including higher risk of cardiovascular diseases, type 2 diabetes, certain cancers, and mental health issues.[2–6] In utero is a critical period when the fetus may be most vulnerable to environmental insults and susceptible to impacting multiple systems; such influence may extend into childhood and adulthood.[7] Multiple in utero environmental factors, such as endocrine disrupting chemicals,[8] ambient air pollutants,[9] toxic heavy metals,[10] and antibiotics,[11] have been linked with higher child body mass index (BMI) and/or higher risk of child overweight or obesity (OWO). Maternal cigarette smoking during pregnancy is another important and potentially modifiable environmental risk factor of child OWO.[12]
This study aims to address both methodological challenges and critical research questions related to in utero exposure to cigarette smoking. Methodologically, although the exposure-response association has been extensively examined,[13–15] most studies were based on self-reported cigarette smoking.[16–18] Characterizing in utero exposure to cigarette smoking based on self-report, however, is subject to potential recall bias, single-assessment bias, underreporting, and deliberate misreporting, all of which have been found to significantly introduce information bias.[19] In contrast, maternal biomarker measurements are objective. Also, since they are measured in mother’s circulation,[20] it is more related to our goal of investigating to what extent they reflect fetal exposure compared to other measurements, such as cotinine levels in urine.[21,22] There is evidence that cotinine was detectable in fetal fluids in both active and passive smokers.[23] An average cigarette yields about 2mg of absorbed nicotine. Metabolized by cytochrome P450 enzymes and FMO3, nicotine converts to metabolites such as cotinine and 2-hydroxynicotine.[24] After smoking cessation, cotinine can remain in the blood for up to 4 days[25] and, in the meantime, be further metabolized as hydroxycotinine whose plasma elimination half-life time is 6.6 hours.[26] However, most studies measured cotinine or its metabolites in the maternal compartment, while some others measured fetal fluids (amniotic fluid and blood).[23] Few studies[27] have simultaneously measured smoking biomarkers in both maternal and fetal compartments to evaluate their correlations and consistency with maternal self-reported data. Such data are particularly lacking in high-risk US minority populations. Cord biomarker measurements provide direct evidence of the fetal and prenatal exposure to smoking. Scientifically, it is necessary to clarify the concordance and utility of maternal vs. fetal blood biomarkers of cigarette smoking, given the growing number of birth cohort studies in the United States and around the world and the availability of both maternal and cord blood samples. A related research question is which sources of samples (maternal vs. fetal) should be obtained and what types of biomarkers (cotinine vs. hydroxycotinine) should be measured to best reflect fetal in utero smoking exposure and to best estimate its long-term influence on child health outcomes.
Using the unique database of the Boston Birth Cohort (BBC; one of the largest and longest-running high-risk US minority birth cohorts), this is the first study that has all three measurements (maternal self-report, maternal plasma biomarkers, cord plasma biomarkers) analyzed simultaneously to characterize the in utero exposure to cigarette smoking. Specifically, we aimed to investigate the association of the three sources of measures of in utero exposure to cigarette smoking with child OWO from childhood to adolescence. Findings from this study open the door for further investigation into maternal and fetal metabolism of nicotine and transplacental passage of nicotine metabolites.
Methods
Study population
This study included participants in the BBC who were enrolled at birth and followed prospectively at the Boston Medical Center (BMC). A detailed description of the BBC can be found in our previous studies.[28–30] Mothers were invited to participate in the BBC study 24 to 72 hours after delivery. With signed written informed consent, mothers were interviewed by trained research staff using a standardized questionnaire. Six months after birth, enrolled children who still received pediatric primary or special care from the BMC were invited to further participate in the postnatal follow-up study from birth up to age 21 years old. This analysis included 2351 mother-child pairs who had data on self-reported smoking and child OWO data up to 18 years of age. A flowchart of the participant selection process for the analysis is shown in Supplementary Figure 1, http://links.lww.com/PN9/A10. The study protocol for the baseline and follow-up studies was approved by the Institutional Review Boards of the BMC and the Johns Hopkins Bloomberg School of Public Health. No identifying information or images was included in this manuscript.
Exposure: in utero exposure to cigarette smoking
We used three types of in utero cigarette smoking exposure assessment: maternal self-reported cigarette smoking, maternal plasma biomarkers (cotinine and hydroxycotinine metabolites), and cord plasma biomarkers (cotinine and hydroxycotinine metabolites). Maternal self-reported cigarette smoking was collected by the standardized questionnaire administered at enrollment. Trained staff collected non-fasting maternal blood samples within 24 to 72 hours after delivery and umbilical cord blood samples at birth. We measured the peak intensity of cotinine and hydroxycotinine in maternal and cord plasma using liquid chromatography-tandem mass spectrometry techniques at the Broad Institute Metabolite Profiling Laboratory at Massachusetts Institute of Technology.
Outcome: childhood OWO
Trained medical staff measured the child's weight and height using the same clinical protocol and equipment at each well-child visit. Before the analyses, we cleaned child weight and height data by removing extreme values and outliers according to the growth curve. We used the US national reference data and calculated child age- and sex-specific BMI percentiles[31] and defined child overweight as BMI ≥ 85th percentile and child obesity as BMI ≥ 95th percentile for age and sex.[32] We used the last visit as the end point of child OWO, since older age obesity is more likely to persist into later life; this is consistent with our previous publications.
Covariates: maternal and child characteristics
We collected covariates including maternal age at delivery, race/ethnicity, education, parity, pre-pregnancy weight and height, perceived stress during pregnancy, child age, gestational age, and birth weight from the standardized questionnaire administered at enrollment. We abstracted maternal gestational or preexisting diabetes from the electronic medical records. We collected infant breastfeeding data from the first 2-year follow-up questionnaire. We calculated child birthweight for gestational age (BW-GA) and categorized BW-GA into small for gestational age (SGA) (BW-GA <10th percentile), appropriate for gestational age (AGA) (BW-GA between 10th to 90th percentile), and large for gestational age (LGA) (BW-GA > 90th percentile). We grouped children into exclusively breastfeeding, exclusively formula feeding, or both breast and formula feeding. Gestational age was obtained either based on the first day of the last menstrual period or early prenatal ultrasonographic results.[28] We calculated maternal pre-pregnancy BMI as pre-pregnancy weight (in kg) divided by squared height (in m) and further dichotomized into non-OWO (BMI < 25 kg/m2) and OWO (BMI ≥ 25 kg/m2). Metabolite levels in maternal plasma and cord plasma were inverse normal transformed from the original relative concentrations, which were calculated as the total signal of the metabolite divided by the internal standard, as described in a previous study.[33]
Statistical analysis
We first assessed the concordance of maternal self-reported smoking (three categories: continuous smoking, never smoker, quit smoking) vs. metabolite biomarkers of smoking (continuous) using the analysis of variance (ANOVA) F test. We also examined the correlations of maternal and cord plasma cotinine and hydroxycotinine. We then used multinomial logistic regression models to examine the independent effect of in utero smoking exposure (self-reported, maternal blood biomarkers, cord blood biomarkers) on levels of child OWO (normal weight, overweight, obese). We modeled maternal and cord biomarkers (cotinine, hydroxycotinine, and their sum) as categorical variables (in quartiles) and as continuous variables (z-score). We used multinomial logistic regression models to examine the joint effects of in utero smoking exposure and maternal OWO on child OWO. We modeled maternal and cord biomarkers as binary variables (first to third quartile vs. fourth quartile). We examined the associations of cigarette biomarkers (continuous) and risk of childhood OWO (yes, no) by different levels of maternal OWO using a generalized additive model (GAM). In this analysis, we considered values beyond −2 and 2 as outliers and excluded them in model fitting. We investigated the child OWO prediction performance when adding information of maternal and cord plasma biomarkers on top of self-report smoking to the input covariates by three nested logistic regression models. The averaged area under the receiver operating characteristic curve (AUROC) from ten-fold cross-validation (train:test = 9:1) was used as the performance statistics.
In all regression analyses, we adjusted for confounders including maternal age at delivery, race/ethnicity, education level, parity, perceived stress during pregnancy, child age, gestational or preexisting diabetes, and breastfeeding. In a sensitivity analysis, we additionally adjusted for fetal growth restriction (SGA, AGA, LGA). We considered potential effect modifiers including child sex (male, female), preterm birth status (yes, no), and BW-GA (SGA, AGA, LGA). We conducted subgroup analyses by these potential effect modifiers and included interaction terms in the regression models to assess the statistical significance of these effect modifications.
We performed causal mediation test to examine whether the association of in utero exposure to cigarette smoking and child OWO was mediated by fetal growth restriction. For each comparison (childhood OWO, both were compared to non-OWO as a referent), probit regression models were fitted to regress SGA (yes or no) on maternal smoking, and to regress child OWO on SGA and smoking. We tested the significance of the indirect effect using the quasi-Bayesian Monte Carlo simulation with 1000 iterations. Unstandardized indirect effects were computed for each of the 1000 Monte Carlo draws, and the 95% confidence intervals were computed by determining the indirect effects at the 2.5th and 97.5th percentiles.
For all analyses, we considered P-value <0.05 as statistically significant. We used R packages mice (v.3.12.0),[34] tableone (v.0.12.0),[35] nnet (v.7.3.114),[36] mediation (v.4.5.0),[37] mgcv (v.1.8.31), PerformanceAnalytics (v.2.0.4),[38] and ggplot (v.3.3.2)[39] in this analysis. We imputed missing values using Multivariate Imputation by Chained Equations methods.[34] All methods were performed in accordance with the relevant guidelines and regulations.
Results
Population characteristics
This study included 2351 mother-child pairs (Tables 1 and 2). The prevalence of childhood overweight and obesity was 17.01% and 28.16%, respectively. Mothers of children with OWO were older and had higher pre-pregnancy BMI. Children with OWO had higher birthweight in measures including absolute weight in grams, standard birthweight in z-score, and BW-GA.
Table 1.
Characteristics of participating mothers, overall and by child overweight or obesity status.
| Variables | Overall (N = 2351) | Non-OWO* (N = 1289) | Overweight (N = 400) | Obesity (N = 662) | P value† |
|---|---|---|---|---|---|
| Maternal age (year) (%) | |||||
| <20 | 226 (9.6) | 144 (11.2) | 30 (7.5) | 52 (7.9) | 0.039 |
| ≥20 and <35 | 1686 (71.7) | 921 (71.5) | 292 (73.0) | 473 (71.5) | |
| ≥35 | 439 (18.7) | 224 (17.4) | 78 (19.5) | 137 (20.7) | |
| Race/Ethnicity (%) | |||||
| African American | 1612 (68.6) | 892 (69.2) | 272 (68.0) | 448 (67.7) | 0.116 |
| Hispanic | 459 (19.5) | 228 (17.7) | 81 (20.2) | 150 (22.7) | |
| Others | 153 (6.5) | 92 (7.1) | 26 (6.5) | 35 (5.3) | |
| White | 127 (5.4) | 77 (6.0) | 21 (5.2) | 29 (4.4) | |
| Education (%) | |||||
| Above college | 803 (34.2) | 457 (35.5) | 135 (33.8) | 211 (31.9) | 0.161 |
| Below college | 1531 (65.1) | 825 (64.0) | 259 (64.8) | 447 (67.5) | |
| Unknown | 17 (0.7) | 7 (0.5) | 6 (1.5) | 4 (0.6) | |
| Parity (%) | |||||
| Multiparous | 1367 (58.1) | 736 (57.1) | 246 (61.5) | 385 (58.2) | 0.297 |
| Nulliparous | 984 (41.9) | 553 (42.9) | 154 (38.5) | 277 (41.8) | |
| Smoking (%) | |||||
| Continuous smoking | 233 (9.9) | 117 (9.1) | 42 (10.5) | 74 (11.2) | 0.296 |
| Never smoker | 1930 (82.1) | 1072 (83.2) | 331 (82.8) | 527 (79.6) | |
| Quit smoking | 188 (8.0) | 100 (7.8) | 27 (6.8) | 61 (9.2) | |
| Perceived stress during pregnancy (%) | |||||
| High | 425 (18.1) | 226 (17.5) | 71 (17.8) | 128 (19.3) | 0.873 |
| Low | 895 (38.1) | 489 (37.9) | 156 (39.0) | 250 (37.8) | |
| Medium | 1031 (43.9) | 574 (44.5) | 173 (43.2) | 284 (42.9) | |
| Pre-pregnancy BMI category (%) | |||||
| <25 | 1109 (47.2) | 732 (56.8) | 176 (44.0) | 201 (30.4) | <0.001 |
| 25–25.99 | 662 (28.2) | 350 (27.2) | 120 (30.0) | 192 (29.0) | |
| ≥30 | 580 (24.7) | 207 (16.1) | 104 (26.0) | 269 (40.6) | |
| Gestational or preexisting diabetes | |||||
| No | 2248 (95.6) | 1244 (96.5) | 379 (94.8) | 625 (94.4) | 0.065 |
| Yes | 103 (4.4) | 45 (3.5) | 21 (5.2) | 37 (5.6) | |
Child non-OWO, overweight, and obesity are defined as child BMI <85 percentile, ≥85th percentile, and ≥95th percentile for age and sex,[32] respectively, where the percentile is calculated using US national reference data.[31]
P values are calculated using Pearson χ2 test for categorical variables and analysis of variance test for continuous variables. BMI, body mass index; OWO, overweight or obesity.
Table 2.
| Variables | Overall (N = 2351) | Non-OWO (N = 1289) | Overweight (N = 400) | Obesity (N = 662) | P value |
|---|---|---|---|---|---|
| Child sex | |||||
| Female | 1174 (49.9) | 651 (50.5) | 195 (48.8) | 328 (49.5) | 0.806 |
| Male | 1177 (50.1) | 638 (49.5) | 205 (51.2) | 334 (50.5) | |
| Children’s age, mean (SD) | 10 (4.0) | 9.4 (4.0) | 9.9 (4.0) | 10.0 (3.9) | 0.003 |
| Birth weight, mean (SD) | 2920 (818.0) | 2833.2 (805.6) | 2981.4 (805.1) | 3052.3 (828.4) | <0.001 |
| Standard birthweight (SBW) z-score, mean (SD) | −0 (1.0) | −0.2 (1.0) | −0.1 (1.0) | 0.2 (1.1) | <0.001 |
| Gestational age, mean (SD) | 38 (3.0) | 37.6 (3.6) | 38.0 (3.3) | 37.8 (3.3) | 0.13 |
| Preterm birth† | |||||
| No | 1708 (72.6) | 922 (71.5) | 306 (76.5) | 480 (72.5) | 0.149 |
| Yes | 643 (27.4) | 367 (28.5) | 94 (23.5) | 182 (27.5) | |
| Birth weight for gestational age (%) | |||||
| Appropriate | 1829 (77.8) | 1011 (78.4) | 309 (77.2) | 509 (76.9) | <0.001 |
| Large | 228 (9.7) | 84 (6.5) | 38 (9.5) | 106 (16.0) | |
| Small | 294 (12.5) | 194 (15.1) | 53 (13.2) | 47 (7.1) | |
| Breastfeeding (%) | |||||
| Both | 1579 (67.2) | 873 (67.7) | 276 (69.0) | 430 (65.0) | 0.172 |
| Bottle fed | 597 (25.4) | 313 (24.3) | 94 (23.5) | 190 (28.7) | |
| Exclusive breastfed | 175 (7.4) | 103 (8.0) | 30 (7.5) | 42 (6.3) | |
The definitions of non-OWO, overweight, obesity, and P value calculation are the same as those in Table 1.
Preterm is defined as gestational age <37 weeks. OWO, overweight or obesity; SD, standard deviation.
Concordance of self-reported smoking and biomarkers
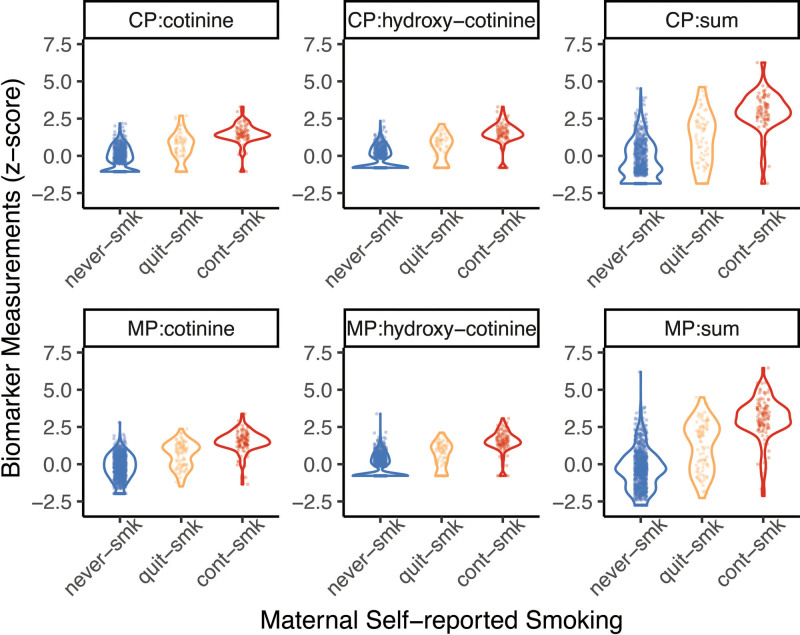
The concordance of self-reported smoking and biomarkers was found in this data. Maternal self-reported cigarette smoking status was consistent with measures of maternal and cord plasma cotinine and hydroxycotinine, meaning the self-reported smoking data was reliable. In general, plasma cotinine, hydroxycotinine or their sum correlated well with maternal self-reported smoking status (Figure 1). Self-reported smoking status was significantly associated with cotinine, hydroxycotinine, and their sum: ANOVA F test p-values were , , in maternal plasma, and and in cord blood for cotinine, hydroxycotinine, and their sum, respectively. Concentrations of cotinine, hydroxycotinine, and their sum did not differ by maternal OWO status in either maternal or cord plasma (Supplementary Figure 2, http://links.lww.com/PN9/A10). The pairwise correlations among cotinine, hydroxycotinine, and their sum in maternal and cord plasma are shown in Supplementary Figure 3, http://links.lww.com/PN9/A10. Metabolites measured within maternal or cord plasma were highly correlated; however, the correlations between maternal and cord metabolites measures were weak. This raised the question of which source of markers was more strongly associated with childhood OWO.
Figure 1:
Continuous distribution of biomarker measures in maternal plasma (MP) and cord plasma (CP). X-axis shows maternal self-reported smoking status: never smoker (never-smk), quit smoking (quit-smk), and continuous smoking (cont-smk). Dots show the measured values, and the violin plots show the distribution of these values (the width shows the probability density of the data at different values, smoothed by a kernel density estimator). The P values obtained from ANOVA test in all the six measurements in the above subplots are <2.2 × 10−16. Sum = sum of cotinine and hydroxycotinine. ANOVA, analysis of variance.
Association of self-reported cigarette smoking and childhood OWO
Maternal self-reported smoking was not significantly associated with child OWO by itself, but its combined effect with maternal OWO enhanced children’s long-term risk of OWO (Table 3). Such an association was additive with maternal OWO to enhance the intergenerational link of maternal-childhood OWO. Compared to children born to non-OWO mothers who did not smoke, those born to non-OWO mothers who continued smoking had 1.73 (95% confidence interval [CI] 1.01–2.97) times the odds of having overweight, and those born to OWO mothers who did not smoke, quit smoking, and continued smoking had 2.98 (95% CI 2.73–3.75), 3.63 (95% CI 2.32–5.68), and 3.66 (95% CI 2.37–5.67) times the odds of having obesity, after adjusting for potential confounders (Table 3). Stratification by child sex shows that this risk was stronger for overweight in female children and obesity in male children (Supplementary Table 2, http://links.lww.com/PN9/A10). Findings were consistent with the overall results when stratified by preterm birth status (Supplementary Table 3, http://links.lww.com/PN9/A10) or fetal growth restriction (Supplementary Table 4, http://links.lww.com/PN9/A10).
Table 3.
Associations of maternal overweight or obesity and smoking on child’s overweight or obesity*.
| Maternal OWO status | Smoking status | N | Child overweight | Child obesity | ||
|---|---|---|---|---|---|---|
| n 1 | OR (95% CI) | n 2 | OR (95% CI) | |||
| Individual associations | ||||||
| Non-OWO | 1109 | 176 | Referent | 201 | Referent | |
| OWO | 1242 | 224 | 1.61 (1.27–2.03) | 461 | 3.02 (2.46–3.71) | |
| Never smoker | 1930 | 331 | Referent | 527 | Referent | |
| Quit smoking | 188 | 27 | 0.90 (0.57–1.41) | 61 | 1.00 (0.88–1.75) | |
| Continuous smoking | 233 | 42 | 1.21 (0.81–1.80) | 74 | 1.28 (0.92–1.79) | |
| Joint associations | ||||||
| Non-OWO | Never smoker | 927 | 144 | Referent | 165 | Referent |
| Quit smoking | 75 | 8 | 0.68 (0.32–1.48) | 15 | 1.03 (0.56–1.90) | |
| Continuous smoking | 107 | 24 | 1.73 (1.01–2.97) | 21 | 1.29 (0.75–2.25) | |
| OWO | Never smoker | 1003 | 187 | 1.70 (1.31–2.20) | 362 | 2.98 (2.37–3.75) |
| Quit smoking | 113 | 19 | 1.68 (0.95–2.96) | 46 | 3.63 (2.32–5.68) | |
| Continuous smoking | 126 | 18 | 1.45 (0.81–2.58) | 53 | 3.66 (2.37–5.67) | |
| p for interaction (OWO × smoking) | 0.453 | 0.664 | ||||
Models were adjusted for maternal age at delivery, race, education, parity, perceived stress during pregnancy, child age, breastfeeding, gestational or preexisting diabetes. CI, confidence interval; N, number of children in overall samples (total = 2351); n1, number of children with overweight; n2, number of children with obesity.
Association of cigarette smoke biomarkers in maternal plasma and childhood OWO
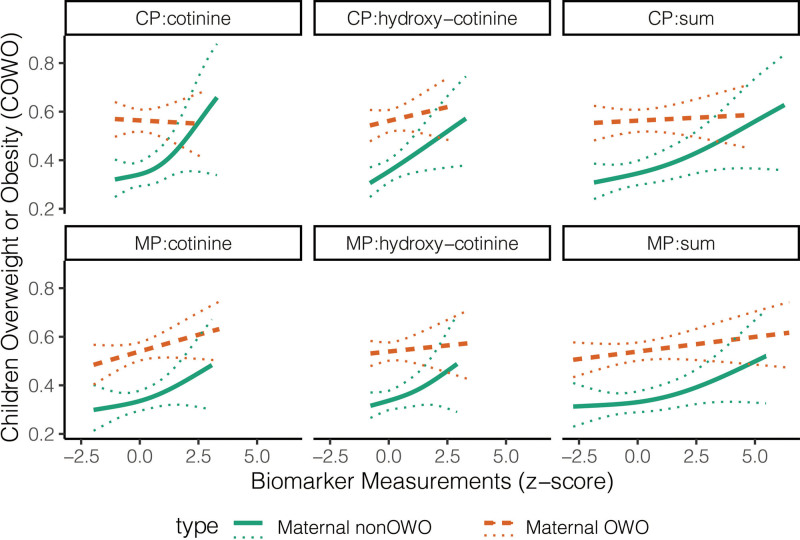
As an individual covariate, children with Q4 level of cotinine had 1.37 (95% CI 0.87–2.15) times the odds of being overweight and 1.51 (95% CI 1.03–2.21) times the odds of being obese. Children's OWO risk is highly differentiated between maternal OWO and maternal non-OWO groups (Figure 2). Compared to children of mothers who were non-OWO and had biomarker levels in Q1–Q3, the risk of overweight was higher for children whose mothers were OWO and were in Q1–Q3 levels of cotinine (odds ratio [OR] = 1.52, 95% CI 1.04–2.21), hydroxycotinine (OR = 1.69, 95% CI 1.17–2.45), or their sum (OR = 1.46, 95% CI 1.00–2.12). This association was stronger for children of mothers with biomarkers levels in Q4: compared to those of mothers with non-OWO, children whose mothers were OWO had 2.44 (95% CI 1.47–4.05), 1.59 (95% CI 0.95–2.68), and 2.40 (95% CI 1.47–3.92) times the odds of being overweight for cotinine, hydroxycotinine, and their sum, respectively (Table 4). Obesity risk was significantly higher among children whose mothers were OWO (vs. non-OWO) and in Q1–Q3 levels of cotinine (OR = 2.63, 95% CI 1.92–3.59), hydroxycotinine (OR = 3.02, 95% CI 2.20–4.14), and their sum (OR = 2.96, 95% CI 2.15–4.06); such risk was higher for children born to mothers who had Q4 level of cotinine (OR = 3.78, 95% CI 2.49–5.75), hydroxycotinine (OR = 3.03, 95% CI 2.00–4.59), and their sum (OR = 3.34, 95% CI 2.19–5.08) (Table 4). Children of whose mothers were OWO have higher odds of obesity from cotinine Q1-3 (OR = 2.63, 95% CI 1.92–3.59) to Q4 (OR = 3.78, 95% CI 2.49–5.75), but the odds are comparable from hydroxycotinine Q1–3 (OR = 3.02, 95% CI 2.20–4.14) to Q4 (OR = 3.03, 95% CI 2.00–4.59). In subgroup analyses, the combined effect of cotinine and maternal OWO was stronger for overweight male children, and the cotinine by itself increased the male children’s risk of obesity (Supplementary Table 5, http://links.lww.com/PN9/A10). The sex differences (generally stronger in female children but steeper increase in male children whose mothers were OWO) were also observed in the GAM that fitted childhood OWO risk vs. biomarker measures (first-row plots in Supplementary Figure 3, http://links.lww.com/PN9/A10).
Figure 2:
Assessment of child overweight or obesity risk by biomarkers of in utero exposure to cigarette smoke. Solid curves are GAM fitted values. Dotted curves denote 95% CI. CI, confidence interval; CP, cord plasma; GAM, generalized additive model; MP, maternal plasma; sum, sum of cotinine and hydroxycotinine.
Table 4.
Associations of maternal overweight or obesity and plasma cotinine/hydroxycotinine on child’s overweight or obesity*.
| Maternal OWO status | Biomarkers of cigarette smoking | N | Child overweight | Child obesity | ||
|---|---|---|---|---|---|---|
| n 1 | OR (95% CI) | n2 (%) | OR (95% CI) | |||
| Individual associations | ||||||
| Non-OWO | 609 (0.46) | 91 (0.15) | Referent | 117 (0.19) | Referent | |
| OWO | 706 (0.54) | 117 (0.17) | 1.53 (1.11–2.10) | 264 (0.374) | 2.70 (2.06–3.54) | |
| Cotinine Q1 | 331 (0.25) | 57 (0.17) | Referent | 85 (0.26) | Referent | |
| Cotinine Q2 | 328 (0.25) | 44 (0.13) | 0.73 (0.46–1.14) | 93 (0.284) | 1.02 (0.71–1.47) | |
| Cotinine Q3 | 327 (0.25) | 46 (0.14) | 0.81 (0.52–1.26) | 95 (0.291) | 1.08 (0.75–1.55) | |
| Cotinine Q4 | 329 (0.25) | 61 (0.19) | 1.37 (0.87–2.15) | 108 (0.328) | 1.51 (1.03–2.21) | |
| Hydroxycotinine Q1 | 576 (0.44) | 96 (0.17) | Referent | 159 (0.28) | Referent | |
| Hydroxycotinine Q2 | 96 (0.07) | 14 (0.15) | 0.86 (0.46–1.63) | 24 (0.250) | 0.85 (0.50–1.42) | |
| Hydroxycotinine Q3 | 314 (0.24) | 44 (0.14) | 0.84 (0.56–1.25) | 94 (0.299) | 1.05 (0.77–1.45) | |
| Hydroxycotinine Q4 | 329 (0.25) | 54 (0.16) | 1.08 (0.73–1.62) | 104 (0.316) | 1.23 (0.89–1.70) | |
| Joint associations | ||||||
| Non-OWO | Cotinine Q1–3 | 468 (0.36) | 65 (0.14) | Referent | 87 (0.19) | Referent |
| Cotinine Q4 | 141 (0.11) | 26 (0.18) | 1.63 (0.95–2.79) | 30 (0.213) | 1.34 (0.82–2.20) | |
| OWO | Cotinine Q1–3 | 518 (0.39) | 82 (0.16) | 1.52 (1.04–2.21) | 186 (0.359) | 2.63 (1.92–3.59) |
| Cotinine Q4 | 188 (0.14) | 35 (0.19) | 2.44 (1.47–4.05) | 78 (0.415) | 3.78 (2.49–5.75) | |
| Non-OWO | Hydroxycotinine Q1–3 | 465 (0.35) | 66 (0.14) | Referent | 83 (0.18) | Referent |
| Hydroxycotinine Q4 | 144 (0.11) | 25 (0.17) | 1.43 (0.84–2.45) | 34 (0.236) | 1.56 (0.97–2.52) | |
| OWO | Hydroxycotinine Q1–3 | 521 (0.40) | 88 (0.17) | 1.69 (1.17–2.45) | 194 (0.372) | 3.02 (2.20–4.14) |
| Hydroxycotinine Q4 | 185 (0.14) | 29 (0.16) | 1.59 (0.95–2.68) | 70 (0.378) | 3.03 (2.00–4.59) | |
| Non-OWO | Sum Q1–3 | 468 (0.36) | 67 (0.14) | Referent | 83 (0.18) | Referent |
| Sum Q4 | 141 (0.11) | 24 (0.17) | 1.49 (0.86–2.57) | 34 (0.241) | 1.63 (1.01–2.65) | |
| OWO | Sum Q1–3 | 518 (0.39) | 79 (0.15) | 1.46 (1.00–2.12) | 194 (0.375) | 2.96 (2.15–4.06) |
| Sum Q4 | 188 (0.14) | 38 (0.20) | 2.40 (1.47–3.92) | 70 (0.372) | 3.34 (2.19–5.08) | |
| p for interaction (smoking × SGA) | ||||||
| Continuous smoking | SGA | 0.091 | 0.569 | |||
| Never smoker | Non-SGA | 0.637 | 0.786 | |||
| Never smoker | SGA | 0.867 | 0.04 | |||
| Quit smoking | Non-SGA | 0.898 | 0.981 | |||
| Quit smoking | SGA | 0.726 | 0.129 | |||
Only samples with maternal cotinine measurements are considered. Sum, sum of cotinine and hydroxycotinine. Models were adjusted for maternal age at delivery, race, education, parity, perceived stress during pregnancy, child age, breastfeeding, gestational or preexisting diabetes. CI, confidence interval; N, number of children (1315); n1, number of children with overweight; n2, number of children with obesity.
Association of cigarette smoke biomarkers in cord blood and childhood OWO
As an individual covariate, children with hydroxycotinine levels in Q4 had 1.66 (95% CI 1.03–2.66) times the odds of being overweight and 1.57 (95% CI 1.05–2.36) times the odds of being obese, compared to those had hydroxycotinine levels in Q1 (Table 5). Children OWO risk is highly differentiated between maternal OWO and maternal non-OWO groups (Figure 2). The combined effect of maternal OWO and biomarkers (cotinine, hydroxycotinine) were significantly associated with both children overweight and obesity. For children born to non-OWO mothers, their risk of being overweight was significantly higher for those with cord cotinine (OR = 2.07, 95% CI 1.09–3.94) and hydroxycotinine (OR = 2.13, 95% CI 1.13–4.03) levels in Q4 (vs. Q1–3). Compared to children of non-OWO mothers and with cord hydroxycotinine levels in Q1–3, the odds of overweight were significantly increased in children of mothers with OWO and with cord hydroxycotinine levels in Q1–3 (OR = 2.10, 95% CI 1.36–3.26) and Q4 (OR = 2.57, 95% CI 1.41–4.68). Risk of child obesity was also significantly higher with higher cord plasma cotinine levels, regardless of mothers OWO status. For children of non-OWO mothers, those with cord cotinine levels in Q4 (vs. Q1–3) had 1.71 (95% CI 0.94–3.10) times the odds of obesity. Results were consistent for hydroxycotinine (OR = 1.52, 95% CI 0.83–2.78) and their sum (OR = 1.71, 95% CI 0.96–3.06). Obese risk was higher in children of mothers with OWO with odds significantly increased in cotinine from Q1–Q3 (OR = 2.97, 95% CI 2.02–4.37) to Q4 (OR = 3.49, 95% CI 2.10–5.79), hydroxycotinine from Q1–3 (OR = 2.79, 95% CI 1.90–4.09) to Q4 (OR = 3.70, 95% CI 2.24–6.11), and their sum from Q1–3 (OR = 2.93, 95% CI 1.99–4.31) to Q4 (OR = 3.74, 95% CI 2.25–6.23), all compared to children of mothers with non-OWO and the corresponding biomarker in Q1–Q3 (Table 5). This is different from the biomarker measurements in maternal plasma, where such increasing trend of OR was not observed for hydroxycotinine (Table 4). Stratification by child sex showed that the cotinine’s effect was mainly for obesity in male children and overweight in female children (Supplementary Table 6, http://links.lww.com/PN9/A10). The sex difference (generally higher risk in female children) was similar to what we observed in the maternal plasma (second-row plots in Supplementary Figure 4, http://links.lww.com/PN9/A10).
Table 5.
Associations of maternal overweight or obesity and cord plasma cotinine/hydroxycotinine on child’s overweight or obesity*.
| Maternal OWO status | Cord biomarkers of cigarette smoking | N | Child overweight | Child obesity | ||
|---|---|---|---|---|---|---|
| n1 (%) | OR (95% CI) | n2 (%) | OR (95% CI) | |||
| Individual associations | ||||||
| Non-OWO | 421 (0.47) | 67 (0.16) | Referent | 83 (0.20) | Referent | |
| OWO | 472 (0.53) | 93 (0.20) | 1.85 (1.27–2.70) | 173 (0.367) | 2.74 (1.97–3.82) | |
| Cotinine Q1 | 239 (0.27) | 41 (0.17) | Referent | 70 (0.29) | Referent | |
| Cotinine Q2 | 208 (0.23) | 48 (0.23) | 1.31 (0.80–2.15) | 45 (0.216) | 0.72 (0.45–1.14) | |
| Cotinine Q3 | 223 (0.25) | 32 (0.14) | 0.81 (0.47–1.38) | 64 (0.287) | 0.91 (0.59–1.40) | |
| Cotinine Q4 | 223 (0.25) | 39 (0.17) | 1.26 (0.73–2.18) | 77 (0.345) | 1.28 (0.82–2.01) | |
| Hydroxycotinine Q1 | 376 (0.42) | 64 (0.17) | Referent | 99 (0.26) | Referent | |
| Hydroxycotinine Q2 | 71 (0.08) | 15 (0.21) | 1.28 (0.66–2.51) | 18 (0.254) | 0.99 (0.53–1.85) | |
| Hydroxycotinine Q3 | 223 (0.25) | 36 (0.16) | 1.01 (0.63–1.62) | 63 (0.283) | 1.14 (0.77–1.69) | |
| Hydroxycotinine Q4 | 223 (0.25) | 45 (0.20) | 1.66 (1.03–2.66) | 76 (0.341) | 1.57 (1.05–2.36) | |
| Joint associations | ||||||
| Non-OWO | Cotinine Q1–3 | 325 (0.36) | 47 (0.14) | Referent | 59 (0.18) | Referent |
| Cotinine Q4 | 96 (0.11) | 20 (0.21) | 2.07 (1.09–3.94) | 24 (0.250) | 1.71 (0.94–3.10) | |
| OWO | Cotinine Q1–3 | 345 (0.39) | 74 (0.21) | 2.34 (1.52–3.61) | 120 (0.348) | 2.97 (2.02–4.37) |
| Cotinine Q4 | 127 (0.14) | 19 (0.15) | 1.77 (0.93–3.37) | 53 (0.417) | 3.49 (2.10–5.79) | |
| Non-OWO | Hydroxycotinine Q1–3 | 328 (0.37) | 47 (0.14) | Referent | 61 (0.19) | Referent |
| Hydroxycotinine Q4 | 93 (0.10) | 20 (0.22) | 2.13 (1.13–4.03) | 22 (0.237) | 1.52 (0.83–2.78) | |
| OWO | Hydroxycotinine Q1–3 | 342 (0.38) | 68 (0.20) | 2.10 (1.36–3.26) | 119 (0.348) | 2.79 (1.90–4.09) |
| Hydroxycotinine Q4 | 130 (0.15) | 25 (0.19) | 2.57 (1.41–4.68) | 54 (0.415) | 3.70 (2.24–6.11) | |
| Non-OWO | Sum Q1–3 | 323 (0.36) | 48 (0.15) | Referent | 58 (0.18) | Referent |
| Sum Q4 | 98 (0.11) | 19 (0.19) | 1.77 (0.94–3.36) | 25 (0.255) | 1.71 (0.96–3.06) | |
| OWO | Sum Q1–3 | 347 (0.39) | 74 (0.21) | 2.22 (1.44–3.42) | 119 (0.343) | 2.93 (1.99–4.31) |
| Sum Q4 | 125 (0.14) | 19 (0.15) | 1.80 (0.94–3.43) | 54 (0.432) | 3.74 (2.25–6.23) | |
| p for interaction (smoking × SGA) | ||||||
| Continuous smoking | SGA | 0.388 | 0.357 | |||
| Never smoker | Non-SGA | 0.431 | 0.764 | |||
| Never smoker | SGA | 0.395 | 0.008 | |||
| Quit smoking | Non-SGA | 0.377 | 0.881 | |||
| Quit smoking | SGA | 0.795 | 0.368 | |||
Only samples with cord cotinine measurements are considered. Sum, sum of cotinine and hydroxycotinine. Models were adjusted for maternal age at delivery, race, education, parity, perceived stress during pregnancy, child age, breastfeeding, gestational or preexisting diabetes. CI, confidence interval; N, number of children (893); n1, number of children with overweight; n2, number of children with obesity.
Improved prediction performance when adding maternal and cord plasma biomarkers
The AUROC in the prediction of child non-OWO vs. obesity (mean = 0.633) was overall higher than that of child non-OWO vs. overweight (mean = 0.583). In the prediction model of child non-OWO vs. obesity, the AUROC was 0.622 when including self-reported smoking as the smoking information (model 1); after adding maternal plasma biomarkers information (model 2), the AUROC increased to 0.637; and after adding cord plasma biomarkers information (model 3), AUROC increased to 0.638 (Supplementary Figure 5, http://links.lww.com/PN9/A10). The AUROC in the prediction of child non-OWO vs. overweight also monotonically increased from model 1 to model 3. This monotonic increasing pattern of AUROC demonstrated the contribution of both maternal and cord plasma biomarkers in the prediction accuracy of child OWO. In addition, maternal biomarkers have more additive prediction power than cord plasma biomarkers.
Mediation analyses
SGA mediated 47.3% of the total effect of maternal continuous smoking during pregnancy on child obesity. The regression coefficients between continuous smoking and child obesity and between SGA and child obesity were statistically significant (P = 0.01 and , respectively). The average causal mediation effect (ACME) was −0.03 (95% CI −0.05 to −0.02; P < ). The average direct effect was 0.10 (95% CI 0.03–0.18; P = 0.08). The total effect was 0.07 (95% CI 0.00–0.15, P = 0.05). The coefficient of smoking when regressed against SGA was 0.70 (CI 0.47–0.93, P = ) and the coefficient of SGA when regressed on child obesity was −0.55 (CI −0.76 to −0.34, P = ), suggesting that continuous smoking led to a higher chance of SGA, which further led to a decreased chance of obesity, resulting in a negative ACME. While in other comparison, the causal mediation effects were not statistically significant. In both sub-cohorts of 1315 mother-child pairs with maternal plasma metabolites and 893 mother-child pairs with cord plasma metabolites, BW-GA (a variable significantly associated with child OWO) showed a significant interaction with never smoker in children with obesity (Tables 4 and 5).
Discussion
Most previous studies assessed in utero exposure to cigarette smoking using maternal self-reported data, which might introduce information bias due to recall bias, single-assessment bias, underreporting, and deliberate misreporting.[17–19,39,40] Biomarkers such as cotinine levels in maternal urine[21,22] and serum[20] may not necessarily reflect fetal exposure. Maternal smoking metabolites such as cotinine and hydroxycotinine could potentially cross the placenta and accumulate in fetal tissues.[27] In this study, we examined in utero exposure to cigarette smoking using cotinine and its metabolite hydroxycotinine in cord blood, in addition to self-report or biomarkers in maternal plasma. As shown in Supplementary Figure 3, http://links.lww.com/PN9/A10, the paired correlation among cotinine, hydroxycotinine, and the sum of both was high within maternal and cord plasma, respectively. However, they were poorly correlated between maternal and cord measurements, which could be due to several possibilities: different half-life of cotinine and hydroxycotinine metabolism and elimination rate between maternal and fetal compartment; cord blood was obtained at birth, while maternal blood was obtained within 2 to 3 days after delivery. Despite the lack of correlation between maternal and cord plasma nicotine metabolites, it is assuring that both maternal and cord plasma nicotine metabolites had a high concordance with maternal self-reported smoking status. Our findings imply that future studies can use either maternal or cord blood samples to detect in utero cigarette smoking exposure. In addition, findings from this study opened the door for further investigation into maternal and fetal metabolism of nicotine and transplacental passage of nicotine metabolites, which may serve as targets for developing pharmaceutical interventions.
This is the first study to simultaneously examine three measures of in utero cigarette smoking exposure in relation to childhood OWO: maternal self-reported smoking and measures of maternal and cord plasma biomarkers (cotinine, hydroxycotinine). A recent study applied a similar way of combining information from maternal smoking and cord cotinine to assess the effect of smoking on child celiac disease, another type of childhood disease associated with intrauterine smoking exposure,[41] which served as one of the rationales to conduct similar analyses for child OWO. In this study, regardless of methods of exposure assessment, we found consistent evidence that in utero exposure to cigarette smoking appears to be an obesogen—additively with maternal OWO—that increases child long-term risk of OWO up to 18 years of age. Such association was consistently demonstrated by three different measures of exposure, and the biomarkers allowed us to assess the dose-response relationships and improve the association estimates.
Consistently, previous studies have also shown that in utero exposure to cigarette smoking was associated with higher risk of child OWO.[42] The biological mechanisms underlying the association are not completely clear. One possibility is fetal re-programming in response to the disruption by environmental insults such as cigarette smoking exposure in order to adapt to the environmental changes. Many experimental studies highlighted the phenotypic consequences of fetal-placental changes that predispose to obesity, diabetes, hypertension, and cardiovascular disease in adulthood, often known as fetal origins of adult disease, proposed by Dr. David Barker.[43–47] These changes in phenotype can become permanent in adulthood and give rise to metabolic memory,[48] fetal primed,[49] and developmental plasticity.[50] Besides, researchers have also found evidence of maternal smoking associated with DNA methylation,[51] an epigenetic modification that can modulate gene expression and carry inheritable information capable of shaping phenotypes and causing diseases.[52]
This study has many strengths. First, we used data from a large prospective cohort rich in low-income, minority populations; this high-risk group has been under-represented in previous research. Second, we assessed in utero cigarette smoking exposure using self-reported data and biomarkers in maternal and cord plasma (ie, direct evidence of fetal exposure). Third, our longitudinal design and robust findings further underscored the role of smoking as an obesogen by simultaneously modeling its metabolites in maternal and cord plasma in relation to child OWO. A limitation of this study is that postnatal smoking exposures were not considered. In addition, biomarkers were only measured at one time point. Measuring biomarkers longitudinally may reveal the association between cotinine/hydroxycotinine and child OWO with more granularity at different life stages. It is also worth exploring in the future what other biomarkers are associated with child OWO. Taking these biomarkers together, we may be able to predict childhood obesity at an earlier life stage and preemptive interventions that can be taken to prevent childhood obesity.
Conclusions
This study has made the following new contributions to the field. First, this is the first large prospective birth cohort study of US high-risk minority mother-child dyads, in which we characterized fetal in utero exposure to cigarette smoking in three ways: maternal self-reported smoking status, and two nicotine biomarkers (ie, cotinine, hydroxycotinine) measured in both maternal and cord plasma samples obtained at birth. Second, we examined the association of each of the three sources of exposure measures with child long-term risk of OWO. In this US minority birth cohort, we found that in utero cigarette smoking exposure defined by self-report, maternal or cord metabolites were all consistently associated with increased child long-term risk of OWO. Adding maternal and cord plasma biomarker information slightly improved the prediction accuracy of long-term child OWO risk compared to self-report alone.
Our findings provided a strong argument for smoking cessation before pregnancy and highlighted the need for public health intervention strategies to focus on maternal smoking—as a highly modifiable target, including smoking cessation and countermeasures (such as optimal nutrition) that may alleviate the increasing obesity burden in the United States and globally.
Acknowledgments
We would like to acknowledge all teammates related to Boston Birth Cohorts and the nursing staff at Boston Medical Center.
Funding
The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R21HD066471, R01HD086013, R01HD098232, R01ES031272, R01ES031521, U01ES034983); and the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (UT7MC45949). Dr. Ji is supported by grants from NIH/NHGRI (R01HG009518; R01HG010889). Dr. Hou is supported by grants from NIH/NHGRI (K99HG011468, R00HG011468). The content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by any funding agencies.
Author contributions
Designed research, WH, YJ; conducted research, XH, GW, WH, YJ, LL; analyzed data or performed statistical analysis, WH; wrote the article – original draft preparation, WH; wrote the article – review and editing, MZ and other authors. All authors discussed, read, and approved the manuscript.
Institutional review board statement
The Boston Birth Cohort has received initial and continuation approval from the institutional review board (IRB) of the Boston Medical Center and Johns Hopkins University Bloomberg School of Public Health (IRB#: 3966).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data sharing
Data described in the manuscript, code book, and analytic code will be made available upon request pending IRB review and approval. Data and related materials are available upon reasonable request to the corresponding author after the IRB review and approval.
Posted history
This manuscript was previously posted as a preprint on Preprints: doi: 10.20944/preprints202110.0296.v1.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Supplementary Material
Footnotes
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
How to cite this article: Hou W, Zhang M, Ji Y, Hong X, Wang G, Xu R, Liang L, Saria S, Ji H. A prospective birth cohort study of maternal prenatal cigarette smoking assessed by self-report and biomarkers on childhood risk of overweight or obesity. Precis Nutr 2022;1(3):e00017. doi: 10.1097/PN9.0000000000000017
References
- [1].Skinner AC, Ravanbakht SN, Skelton JA, et al. Prevalence of Obesity and Severe Obesity in US Children, 1999-2016. Pediatrics 2018;141(3):e20173459. doi:10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet 2010;375(9727):1737–1748. doi:10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390(10113):2627–2642. doi:10.1016/s0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Singh AS, Mulder C, Twisk JW, et al. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9(5):474–488. doi:10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- [5].Park MH, Falconer C, Viner RM, et al. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012;13(11):985–1000. doi:10.1111/j.1467-789X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- [6].Quek YH, Tam WWS, Zhang MWB, et al. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes Rev 2017;18(7):742–754. doi:10.1111/obr.12535. [DOI] [PubMed] [Google Scholar]
- [7].Edwards M. The Barker Hypothesis. In: Preedy V, Patel VB, eds. Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy. Springer International Publishing; 2017:1–21. [Google Scholar]
- [8].Braun JM, Eliot M, Papandonatos GD, et al. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond) 2021;45(1):25–35. doi:10.1038/s41366-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mao G, Nachman RM, Sun Q, et al. Individual and joint effects of early-life ambient exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ Health Perspect 2017;125(6):067005. doi:10.1289/EHP261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang G, DiBari J, Bind E, et al. Association between maternal exposure to lead, maternal folate status, and intergenerational risk of childhood overweight and obesity. JAMA Netw Open 2019;2(10):e1912343. doi:10.1001/jamanetworkopen.2019.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang M, Differding MK, Benjamin-Neelon SE, et al. Association of prenatal antibiotics with measures of infant adiposity and the gut microbiome. Ann Clin Microbiol Antimicrob 2019;18(1):18. doi:10.1186/s12941-019-0318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu R, Hong X, Zhang B, et al. DNA methylation mediates the effect of maternal smoking on offspring birthweight: a birth cohort study of multi-ethnic US mother-newborn pairs. Clin Epigenetics 2021;13(1):47. doi:10.1186/s13148-021-01032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gorog K, Pattenden S, Antova T, et al. Maternal smoking during pregnancy and childhood obesity: results from the CESAR study. Matern Child Health J 2011;15(7):985–992. doi:10.1007/s10995-009-0543-5. [DOI] [PubMed] [Google Scholar]
- [14].von Kries R, Bolte G, Baghi L, et al. ; GME Study Group. Parental smoking and childhood obesity--is maternal smoking in pregnancy the critical exposure? Int J Epidemiol 2008;37(1):210–216. doi:10.1093/ije/dym239. [DOI] [PubMed] [Google Scholar]
- [15].Neovius K, Rasmussen F, Sundström J, et al. Forecast of future premature mortality as a result of trends in obesity and smoking: nationwide cohort simulation study. Eur J Epidemiol 2010;25(10):703–709. doi:10.1007/s10654-010-9485-x. [DOI] [PubMed] [Google Scholar]
- [16].Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R study. Am J Clin Nutr 2011;94(1):164–171. doi:10.3945/ajcn.110.009225. [DOI] [PubMed] [Google Scholar]
- [17].Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32(2):201–210. doi:10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oken E, Huh SY, Taveras EM, et al. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res 2005;13(11):2021–2028. doi:10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bakker R, Kruithof C, Steegers EA, et al. Assessment of maternal smoking status during pregnancy and the associations with neonatal outcomes. Nicotine Tob Res 2011;13(12):1250–1256. doi:10.1093/ntr/ntr117. [DOI] [PubMed] [Google Scholar]
- [20].Goudarzi H, Konno S, Kimura H, et al. Contrasting associations of maternal smoking and pre-pregnancy BMI with wheeze and eczema in children. Sci Total Environ 2018;639:1601–1609. doi:10.1016/j.scitotenv.2018.05.152. [DOI] [PubMed] [Google Scholar]
- [21].Wang X, Tager IB, Van Vunakis H, et al. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol 1997;26(5):978–988. doi:10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- [22].Vrijheid M, Fossati S, Maitre L, et al. Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ Health Perspect 2020;128(6):67009. doi:10.1289/EHP5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jauniaux E, Gulbis B, Acharya G, et al. Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet Gynecol 1999;93(1):25–29. doi:10.1016/s0029-7844(98)00318-4. [DOI] [PubMed] [Google Scholar]
- [24].Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005;57(1):79–115. doi:10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- [25].Jarvis MJ, Russell MA, Benowitz NL, et al. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health 1988;78(6):696–698. doi:10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Benowitz NL, Jacob P, 3rd. Trans-3’-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking. Br J Clin Pharmacol 2001;51(1):53–59. doi:10.1046/j.1365-2125.2001.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Machado Jde B, Chatkin JM, Zimmer AR, et al. Cotinine and polycyclic aromatic hydrocarbons levels in the amniotic fluid and fetal cord at birth and in the urine from pregnant smokers. PLoS One 2014;9(12):e116293. doi:10.1371/journal.pone.0116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang G, Divall S, Radovick S, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA 2014;311(6):587–596. doi:10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang X, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA 2002;287(2):195–202. doi:10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- [30].Pearson C, Bartell T, Wang G, et al. Boston Birth Cohort profile: rationale and study design. Precision Nutrition 2022;1(2):e00011. doi:10.1097/pn9.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Centers for Disease Control and Prevention. Overview of the CDC growth charts for use in the United States among children and teens aged 2 years to 20 years; 2015. Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/training/overview/index.html. [Accessed November 29, 2020].
- [32].Centers for Disease Control and Prevention. Defining childhood obesity; 2018. Available from: https://www.cdc.gov/obesity/childhood/defining.html. [Accessed November 29, 2020].
- [33].Zhang M, Buckley JP, Liang L, et al. A metabolome-wide association study of in utero metal and trace element exposures with cord blood metabolome profile: Findings from the Boston Birth Cohort. Environ Int 2022;158:106976. doi:10.1016/j.envint.2021.106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45(3):67. doi:10.18637/jss.v045.i03. [Google Scholar]
- [35].Pollard TJ, Johnson AEW, Raffa JD, et al. tableone: an open source Python package for producing summary statistics for research papers. JAMIA Open 2018;1(1):26–31. doi:10.1093/jamiaopen/ooy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. New York: Springer; 2002. ISBN 0-387-95457-0.. [Google Scholar]
- [37].Tingley D, Yamamoto T, Hirose K, et al. mediation: R package for causal mediation analysis. J Stat Softw 2014;59(5):38. doi:10.18637/jss.v059.i05. [Google Scholar]
- [38].Peterson BG, Carl P, Boudt K, et al. PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis. R package version 2.0.4. 2020. Available from: https://cran.r-project.org/package=PerformanceAnalytics. [Accessed November 29, 2020].
- [39].Wickham H. ggplot: An implementation of the Grammar of Graphics in R. 2006.
- [40].Dukic VM, Niessner M, Pickett KE, et al. Calibrating self-reported measures of maternal smoking in pregnancy via bioassays using a Monte Carlo approach. Int J Environ Res Public Health 2009;6(6):1744–1759. doi:10.3390/ijerph6061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shipton D, Tappin DM, Vadiveloo T, et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ 2009;339:b4347. doi:10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mårild K, Tapia G, Midttun O, et al. Smoking in pregnancy, cord blood cotinine and risk of celiac disease diagnosis in offspring. Eur J Epidemiol 2019;34(7):637–649. doi:10.1007/s10654-019-00522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Riedel C, Schönberger K, Yang S, et al. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking--a meta-analysis. Int J Epidemiol 2014;43(5):1593–1606. doi:10.1093/ije/dyu150. [DOI] [PubMed] [Google Scholar]
- [44].Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science 2004;305(5691):1733–1736. doi:10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- [45].Plagemann A. “Fetal programming” and “functional teratogenesis”: on epigenetic mechanisms and prevention of perinatally acquired lasting health risks. J Perinat Med 2004;32(4):297–305. doi:10.1515/JPM.2004.055. [DOI] [PubMed] [Google Scholar]
- [46].Stocker CJ, Arch JR, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc 2005;64(2):143–151. doi:10.1079/pns2005417. [DOI] [PubMed] [Google Scholar]
- [47].Barker DJP. Mothers, babies, and health in later life. Elsevier Health Sciences; 1998. [Google Scholar]
- [48].Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory.” Exp Diabetes Res 2011;2011:218598. doi:10.1155/2011/218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bruce KD, Hanson MA. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr 2010;140(3):648–652. doi:10.3945/jn.109.111179. [DOI] [PubMed] [Google Scholar]
- [50].McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005;85(2):571–633. doi:10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- [51].Chatterton Z, Hartley BJ, Seok MH, et al. In utero exposure to maternal smoking is associated with DNA methylation alterations and reduced neuronal content in the developing fetal brain. Epigenetics Chromatin 2017;10:4. doi:10.1186/s13072-017-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Feinberg AP. The key role of epigenetics in human disease prevention and mitigation. N Engl J Med 2018;378(14):1323–1334. doi:10.1056/NEJMra1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.