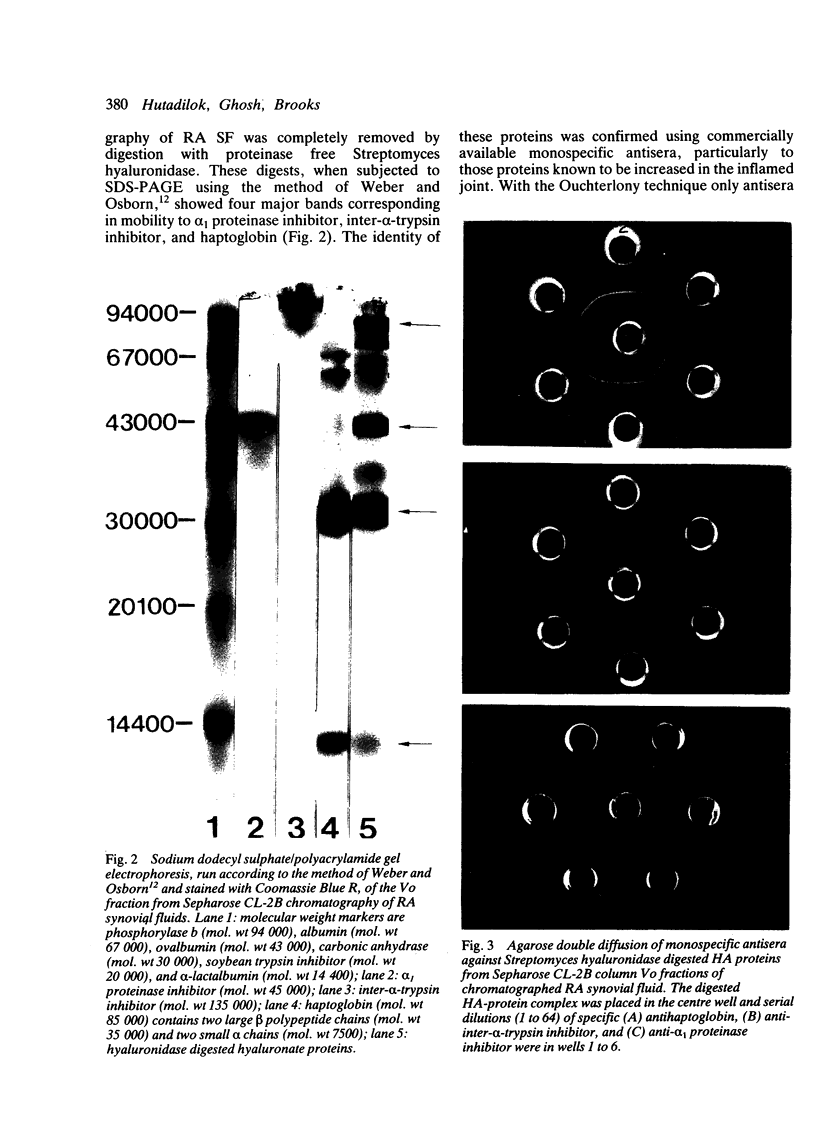
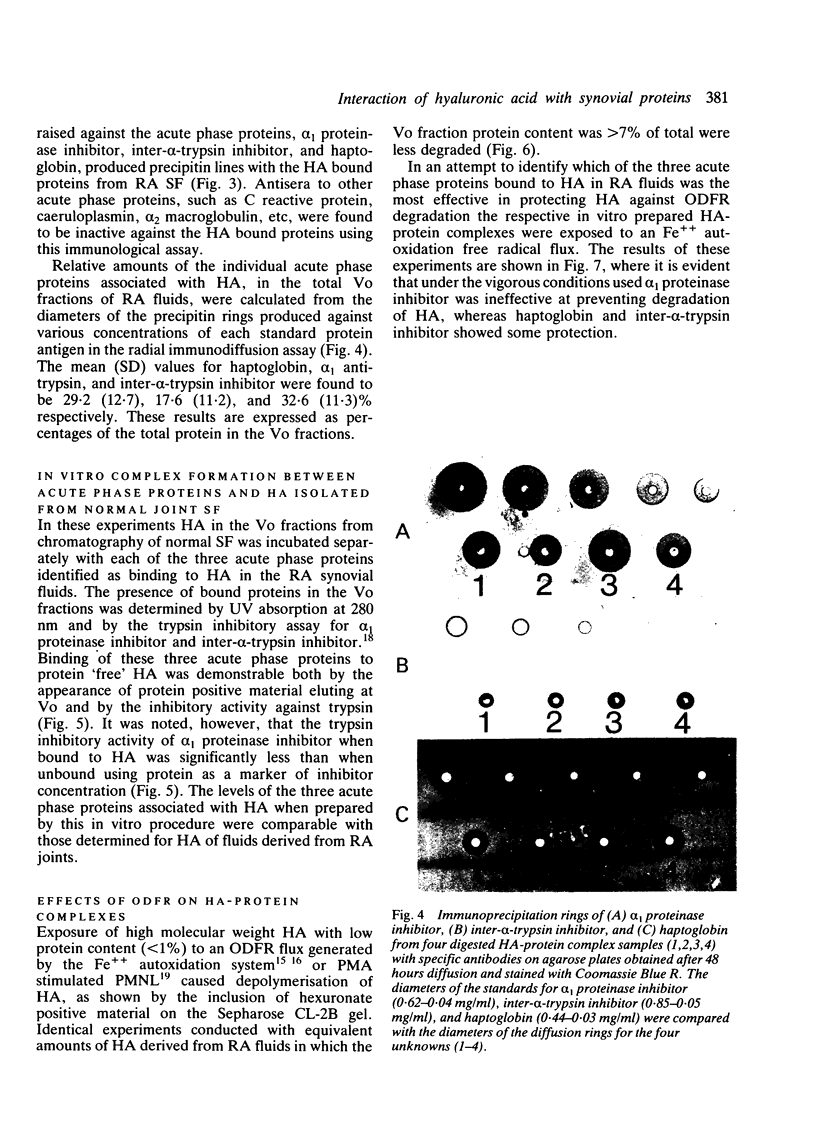
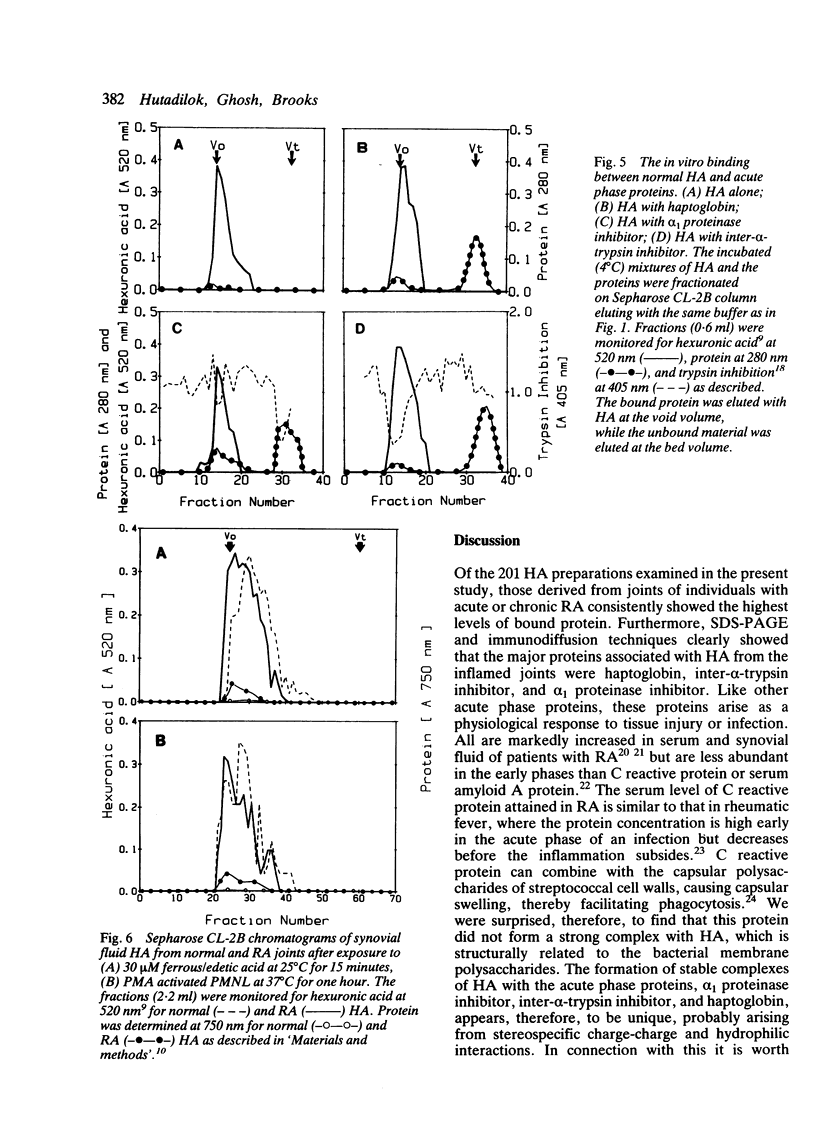
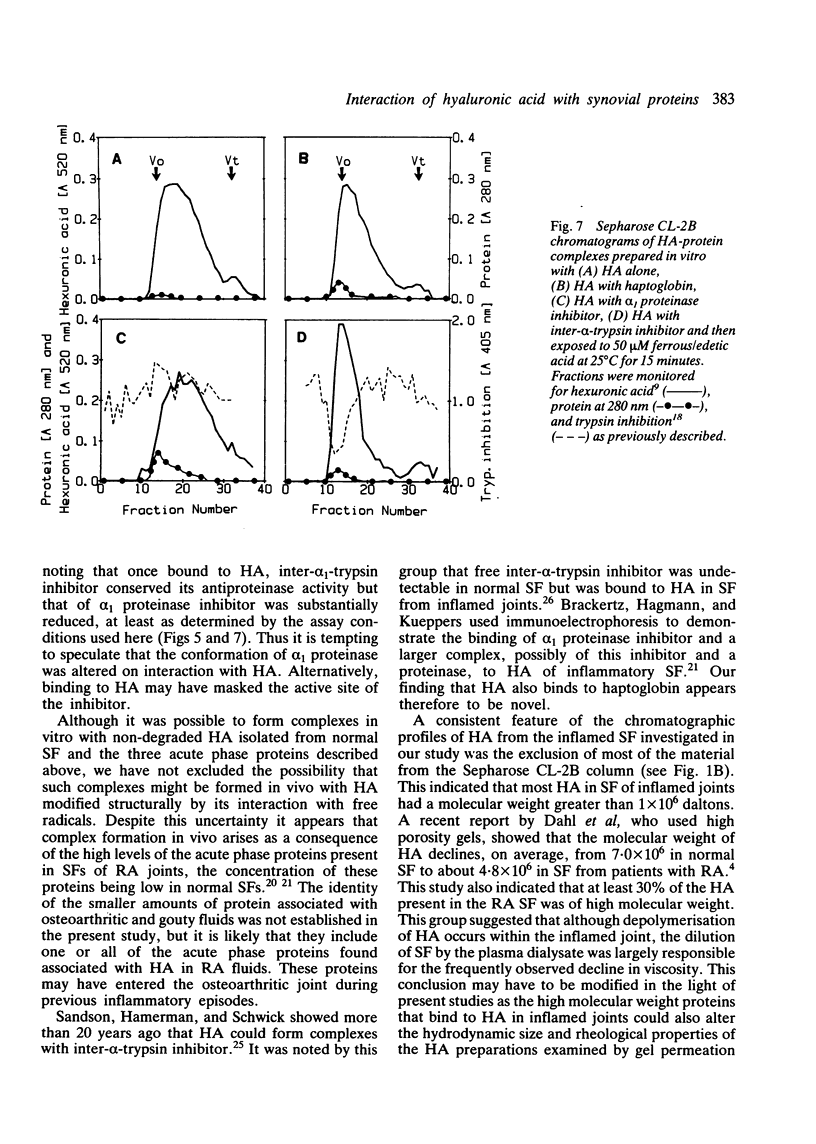
Abstract
Synovial fluid from 201 normal and pathological knee joints was subjected to gel filtration by Sepharose CL-2B chromatography to separate hyaluronic acid (HA) from unbound proteins, which were retarded on this column. HA from all normal fluids was excluded from the gel and contained 1% or less bound protein. Synovial fluids taken from joints of patients with rheumatoid arthritis (RA) contained considerably more protein bound to HA. In 46% of RA samples the level of protein was greater than 4%, whereas only one fluid examined from osteoarthritic joints contained this amount. The proteins bound to HA from RA joints were identified by sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS-PAGE) and immunodiffusion techniques as the acute phase proteins alpha 1 proteinase inhibitor, inter-alpha-trypsin inhibitor, and haptoglobin. The average relative percentages of these proteins bound to HA were 17.6%, 32.6%, and 29.2% respectively. These HA-protein complexes could be generated in vitro by mixing normal (low protein) HA with any one of the three acute phase proteins. The HA-protein complexes formed in vitro with inter-alpha-trypsin inhibitor or haptoglobin, and those isolated from RA synovial fluids, were more resistant to degradation by oxygen derived free radicals (ODFR) than HA from normal fluids. From these findings we conclude that certain acute phase proteins diffusing into synovial fluid during inflammatory episodes may play an important part in protecting HA from depolymerisation by activated phagocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON H. C., McCARTY M. The occurrence in the rabbit of an acute phase protein analogous to human C reactive protein. J Exp Med. 1951 Jan;93(1):25–36. doi: 10.1084/jem.93.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaza E. A., Davies J. V., Phillips G. O., Young M. D. Transient intermediates in the radiolysis of hyaluronic acid. Radiat Res. 1967 Jun;31(2):243–255. [PubMed] [Google Scholar]
- Balazs E. A., Watson D., Duff I. F., Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967 Aug;10(4):357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Harper G. S., Lowther D. A., Preston B. N. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984 May;8(5):629–637. [PubMed] [Google Scholar]
- Bates E. J., Johnson C. C., Lowther D. A. Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta. 1985 Feb 15;838(2):221–228. doi: 10.1016/0304-4165(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Handley C. J. Oxygen free-radicals mediate an inhibition of proteoglycan synthesis in cultured articular cartilage. Ann Rheum Dis. 1984 Jun;43(3):462–469. doi: 10.1136/ard.43.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Johnson C. C. Hyaluronic acid synthesis in articular cartilage: an inhibition by hydrogen peroxide. Biochem Biophys Res Commun. 1985 Oct 30;132(2):714–720. doi: 10.1016/0006-291x(85)91191-x. [DOI] [PubMed] [Google Scholar]
- Becker A., Sandson J. The source of the inter-alpha trypsin inhibitor in pathologic hyaluronateprotein. Arthritis Rheum. 1971 Nov-Dec;14(6):764–766. doi: 10.1002/art.1780140613. [DOI] [PubMed] [Google Scholar]
- Betts W. H., Cleland L. G. Effect of metal chelators and antiinflammatory drugs on the degradation of hyaluronic acid. Arthritis Rheum. 1982 Dec;25(12):1469–1476. doi: 10.1002/art.1780251213. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brackertz D., Hagmann J., Kueppers F. Proteinase inhibitors in rheumatoid arthritis. Ann Rheum Dis. 1975 Jun;34(3):225–230. doi: 10.1136/ard.34.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl L. B., Dahl I. M., Engström-Laurent A., Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985 Dec;44(12):817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Greenwald R. A., Moak S. A. Degradation of hyaluronic acid by polymorphonuclear leukocytes. Inflammation. 1986 Mar;10(1):15–30. doi: 10.1007/BF00916037. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- HAMERMAN D., SANDSON J. UNUSUAL PROPERTIES OF HYALURONATEPROTEIN ISOLATED FROM PATHOLOGICAL SYNOVIAL FLUIDS. J Clin Invest. 1963 Dec;42:1882–1889. doi: 10.1172/JCI104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Kar N. C., Cracchiolo A., 3rd, Mirra J., Pearson C. M. Acid, neutral, and alkaline hydrolases in arthritic synovium. Am J Clin Pathol. 1976 Feb;65(2):220–228. doi: 10.1093/ajcp/65.2.220. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Matsumura G., Herp A., Pigman W. Depolymerization of hyaluronic acid by autoxidants and radiatiions. Radiat Res. 1966 Aug;28(4):735–752. [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McNeil J. D., Wiebkin O. W., Betts W. H., Cleland L. G. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann Rheum Dis. 1985 Nov;44(11):780–789. doi: 10.1136/ard.44.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi N., Mori I. The effect of synovial fluid proteins in the degradation of hyaluronic acid induced by ascorbic acid. J Inorg Biochem. 1985 May;24(1):69–74. doi: 10.1016/0162-0134(85)85015-7. [DOI] [PubMed] [Google Scholar]
- NIEDERMEIER W., CROSS R., BEETHAM W. P., Jr THE CONCENTRATION OF HAPTOGLOBIN IN SYNOVIAL FLUID OF PATIENTS WITH RHEUMATOID ARTHRITIS. Arthritis Rheum. 1965 Jun;8:355–360. doi: 10.1002/art.1780080303. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- PIGMAN W., RIZVI S., HOLLEY H. L. Depolymerization of hyaluronic acid by the ORD reaction. Arthritis Rheum. 1961 Jun;4:240–252. doi: 10.1002/art.1780040303. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rowley G., Antonas K. N., Hilbert B. J. Quantitation of hyaluronic acid in equine synovia. Am J Vet Res. 1982 Jun;43(6):1096–1099. [PubMed] [Google Scholar]
- Salier J. P., Martin J. P., Lambin P., McPhee H., Hochstrasser K. Purification of the human serum inter-alpha-trypsin inhibitor by zinc chelate and hydrophobic interaction chromatographies. Anal Biochem. 1980 Dec;109(2):273–283. doi: 10.1016/0003-2697(80)90649-1. [DOI] [PubMed] [Google Scholar]
- Sandson J., Hamerman D., Schwick G. Altered properties of pathological hyaluronate due to a bound inter-alpha trypsin inhibitor. Trans Assoc Am Physicians. 1965;78:304–313. [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Somorin O., Tokura S., Nishi N., Noguchi J. The action of trypsin on synthetic chromogenic arginine substrates. J Biochem. 1979 Jan;85(1):157–162. doi: 10.1093/oxfordjournals.jbchem.a132305. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Ghosh P., Taylor T. K., Gale C. A., Swann J. C., Robinson R. G., Webb J. The origins and relative distribution of polysaccharidases in rheumatoid and osteoarthritic fluids. J Rheumatol. 1975 Dec;2(4):393–400. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]