SUMMARY
Despite intensive long-term efforts, with very few exceptions, the development of effective vaccines against parasitic infections has presented considerable challenges, given the complexity of parasite life cycles, the interplay between parasites and their hosts, and their capacity to escape the host immune system and to regulate host immune responses. For many parasitic diseases, conventional vaccine platforms have generally proven ill suited, considering the complex manufacturing processes involved and the costs they incur, the inability to posttranslationally modify cloned target antigens, and the absence of long-lasting protective immunity induced by these antigens. An effective antiparasite vaccine platform is required to assess the effectiveness of novel vaccine candidates at high throughput. By exploiting the approach that has recently been used successfully to produce highly protective COVID mRNA vaccines, we anticipate a new wave of research to advance the use of mRNA vaccines to prevent parasitic infections in the near future. This article considers the characteristics that are required to develop a potent antiparasite vaccine and provides a conceptual foundation to promote the development of parasite mRNA-based vaccines. We review the recent advances and challenges encountered in developing antiparasite vaccines and evaluate the potential of developing mRNA vaccines against parasites, including those causing diseases such as malaria and schistosomiasis, against which vaccines are currently suboptimal or not yet available.
KEYWORDS: mRNA vaccine, parasitic infections, antiparasite vaccines, malaria, schistosomiasis
INTRODUCTION
Vaccination is one of the most highly effective public health measures we have in the battle against infectious diseases. Since Edward Jenner—the father of vaccination—used live cowpox virus to protect against smallpox in 1798, we now have vaccines to prevent ~30 life-threatening diseases, saving 6 million lives annually and helping people of all ages to live longer and enjoy healthier lives through immune protection from a spectrum of different pathogens (1–3). Vaccination can reduce disease treatment costs, effectively decreasing the economic burden on health care systems and lowering the necessity for antibiotic treatment of bacterial infections, thereby decreasing the possibility of antimicrobial resistance developing (4); vaccination is also used increasingly for cancer prevention and treatment (5). However, licensed vaccines are still not available against a number of serious chronic and debilitating communicable diseases. Among these are the 20 neglected tropical diseases (NTDs) which typically affect people, especially women and children, living in considerable poverty. Disproportionately affecting the world’s most resource-poor populations, NTDs often result in considerable levels of morbidity. All low-income countries are areas of endemicity for at least five NTDs simultaneously (https://www.cdc.gov/globalhealth/newsroom/topics/ntds/index.html). Notably, six of the top 10 diseases ranked by Vaccine Nation, against which vaccines are urgently required, are caused by parasites. These include malaria, schistosomiasis, leishmaniasis, intestinal hookworm disease, dengue, and Chagas disease (6).
The Bill and Melinda Gates Foundation (TBMGF), the World Health Organization (WHO), and a number of other agencies and nongovernment organizations (NGOs) provide support to help marginalized communities gain access to low-cost drug treatment for parasitic infections and to facilitate monitoring and public health surveillance in areas of endemicity. To date, $1.02 billion has been committed by the TBMGF to various groups that are developing new tools and delivery methods to improve drug availability (https://www.gatesfoundation.org/our-work/programs/global-health/neglected-tropical-diseases). However, despite these notable efforts and those by pharmaceutical companies to provide better access to medical care in low-income areas in the developing world, there remains a geographical and economic disconnect that has resulted in relatively limited attention being paid to the control of parasitic diseases and suboptimal efforts aimed at reducing their transmission to improve health standards globally.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, a global health crisis of unparalleled magnitude, led to an unprecedented expansion in vaccine pipeline development. Among the many diagnostic, therapeutic, and vaccine platforms developed recently with comparative rapidity, the mRNA-based technologies (including RNA biology, chemistry, stability, and delivery) were rapidly deployed and clinically tested. This new era saw the approval of highly protective SARS-Cov-2 mRNA vaccines for use in the European Union and the United States, among many other countries, culminating in wide-scale global immunization rollouts, saving countless lives. The mRNA vaccines could be manufactured using generic and low-cost processes, generating well-defined products, synthesized for stability. These vaccines have proven to evoke safe and persistent immune responses in both animal models and in human clinical trials (7–10). Compared with conventional vaccine platforms, these features make mRNA-based vaccines highly attractive, providing a vehicle to streamline vaccine discovery and development against a spectrum of disease pathogens. Since the emergence of coronavirus disease 2019 (COVID-19) in late 2019, many thousands of research papers have appeared in the field of mRNA vaccinology, with many detailing the extraordinary rapid advances that have been made and emphasizing the future opportunities and the challenges of this novel approach. Here, we review the state of the art of mRNA vaccine development and discuss the advantages mRNA vaccination has over conventional methods. We highlight the potential of the mRNA approach in development and manufacture of next-generation vaccines against parasitic infections. We discuss the development of vaccines against the parasitic protozoa, exemplified by the agent of malaria, the most deadly of the parasitic diseases, with 500 million infected globally and five times that number currently at risk of infection (11). In addition, we discuss the parasitic helminths, using as an example the schistosome (trematode) blood flukes, which cause schistosomiasis, an affliction that is second only to malaria in terms of devastating parasitic diseases, with over 250 million infected in 77 countries (12). We consider that this revolutionary technology will be equally suitable and extendable to the development/deployment of vaccines effective against a spectrum of other pathogens as well.
CURRENT VACCINE DEVELOPMENT APPROACHES AGAINST PARASITIC INFECTIONS
The development of safe and protective antiparasitic vaccines has proven to be highly problematic, given that that the interactions between parasites and their hosts are intricate and complex and that in many parasites’ life cycles, the parasites can present antigens specific to their stage of development (13). In addition, parasites are able, by damping down and regulating host immunity, to effectively evade the host immune response (14) through a variety of mechanisms, some of which are poorly understood. Current antiparasitic vaccines can be generally categorized into conventional protein-based and gene-based vaccines (15) and vaccines comprising attenuated/irradiation-killed parasites, parasite secretions, or recombinant protein antigens that can activate host protective immune responses (15–18).
Attenuated Parasite Vaccines
Promising host protection has been shown to be induced by exposure of hosts to attenuated parasites followed by parasite challenge (18–20). In 1967, the X-irradiated sporozoites of Plasmodium berghei was first used in mice to successfully generate protective immunity (21), and whole-sporozoite-based immunization in humans was developed in 1973, after the finding that a volunteer exposed to X-irradiated Plasmodium falciparum sporozoites was able to generate protection against challenge with a homologous strain of this malaria agent (22). However, obtaining consistently acceptable quality and quantity of attenuated parasites for vaccine application had been considered impractical and unattainable. Until the 2000s, remarkable progress was achieved in the clinical assessment of whole-sporozoite malaria vaccines (23). A number of clinical trials (24–26) have been undertaken with the attenuated sporozoite vaccine of P. falciparum (PfSPZ). Recently, a phase 2 clinical trial was conducted in 336 infants (5 to 12 months old) in areas of Kenya where rates of malaria transmission are high (27), showing 28.6 to 45.8% protection against clinical malaria at different times after immunization.
An alternative to attenuated parasitic vaccination is infection and treatment (I&T), whereby susceptible parasites are killed within the host after infection and before disease develops. With I&T, host immunity is elicited by antigens released from drug-treated parasites or those that die naturally in the host (28). A feature observed in Plasmodium and Schistosoma infections, I&T can provide the catalyst to improve our appreciation of induced rather than acquired antiparasite immunity (29, 30).
Protein-Based Vaccines
Extracellular-vesicle-based vaccines.
Recent extensive studies with a number of different parasites have shown that the immunogenicity of native molecules that they excrete and/or secrete or that are present in extracellular vesicles (EVs) they release is crucial in promoting resistance to infection (31–36). EVs containing proteins, lipids, or nucleic acids derived from parasites (37) act as the transmitters of information in the early phase of infection prior to host cell colonization. It has been demonstrated that EVs of pathogenic protozoans can functionally reflect the biochemical features of the parasite origin by eliciting a differential host cytokine expression pattern and activating different stimuli depending on the cell or tissues that inhabit (38). Thus, EVs containing numerous antiworm vaccine candidates are crucial in modulation of the host immune response and in induction of pathogenesis (37), providing novel immunotherapeutic approaches. Accordingly, there is increased interest in focusing on the composition of EVs and the role they play in the etiology of parasite diseases, such as protozoan diseases (39) (including malaria [40, 41] and Chagas disease [42]), schistosomiasis (43, 44), leishmaniasis (45, 46), fascioliasis (47), and echinococcosis (48). Studies on characteristics of microRNAs (miRNAs) targeting broad cellular gene networks further revealed that the immunoregulatory factors present in helminth EVs also have the potential to provide a pharmacopoeia for inflammatory diseases (38, 49). However, in addition to the difficulty in obtaining sufficient EVs, we have limited knowledge of the molecular mechanisms governing EV formation and the fusion and function of EVs in host-parasite interplay (50). This is due in part to inconsistent definitions and poor standardization of laboratory procedures for the identification, isolation, and characterization of different parasite-derived EVs (51), with the result that studies of EV biology in parasites are still in their infancy.
Recombinant-protein vaccines.
Generating effective protective immune responses against particular pathogen-derived antigens (52), and building on the success of the licensed recombinant hepatitis B vaccine (53), specific parasite antigens can be identified and produced by heterologous prokaryote (bacterial) expression, by eukaryotic expression (such as in Saccharomyces cerevisiae), by stable transfection of adherent CHO cells or nonadherent lymphoid cell lines, and by tagged recombinant baculovirus transfection of insect cells (54). However, these expression systems are costly, the methods are generally time-consuming, and there are often considerable challenges associated with the purification of recombinantly derived proteins and with avoiding the copurification of undesired contaminants (52). The key issue in the development of effective recombinant protein-based vaccines (or, indeed, any type of vaccine) is the identification of the specific antigens produced by a pathogen that can generate an effective and robust immune response that prevents an infection or eradicates an existing infection (55). Vaccine efficacy is often restricted by a lack of appropriate immune stimulation, so selection of a suitable adjuvant, coupled with multiple immunizations, is often critical to effectively elicit and sustain an adequate level of protective immunity (56, 57).
The RTS,S/AS01 vaccine for malaria.
It is a salient fact, but it took close to 40 years from the initial studies in the laboratory to the first commercial human recombinant vaccine effective against Plasmodium falciparum malaria—Mosquirix (the RTS,S/AS01 vaccine). Created in 1987 and developed in 2001 by a public-private partnership between PATH’s Malaria Vaccine Initiative (supported by the Bill and Melinda Gates Foundation to PATH) and GSK, pilot testing of Mosquirix did not commence until 2019 in areas where malaria is endemic (58). Although showing relatively modest efficacy, this first-generation malaria vaccine has promise as a public health intervention, particularly for children residing in areas where malaria is highly endemic and where mortality is high (58, 59). Indeed, despite the limited protection it provides against malaria in children (56% in those 5 to 17 months old; 31% in those 6 to 12 weeks old) (60), it is the only human malaria vaccine recommended by the WHO and approved by the European Medicines Agency to vaccinate children in sub-Saharan Africa. It is recognized that even a partially effective vaccine could have a substantial public health impact given that, of 627,000 deaths from malaria in 2013, some 90%, mainly of children (82%) less than 5 years of age, occurred in Africa (http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/07/WC500190447.pdf).
The commercially produced RTS,S purified bulk antigen vaccine involves fed-batch yeast (Saccharomyces cerevisiae RIX4397 strain) fermentation and recombinant expression and purification of two RTS and S protein components of the circumsporozoite protein (CSP) of P. falciparum; the purified bulk antigen is mixed with AS01 adjuvant, resulting in the RTS,S/AS01 vaccine (58). It is important to note that a successful experimental I&T study (61) involving the exposure of volunteers receiving chloroquine prophylaxis for P. falciparum sporozoites showed that I&T could promote strong protective responses lasting 1 year longer than those generated by the RTS,S/AS01 vaccine, based on the results of the first phase 3 trial in African children (62). This reinforces the concept that I&T studies can provide novel insight for a deeper understanding of induced immunity compared with acquired immunity against parasitic infections, thereby providing a novel platform to design long-lasting vaccines.
Recombinant protein vaccines for Taenia cestodes in veterinary animals.
Ground-breaking success was achieved in developing recombinant veterinary vaccines against several Taenia cestodes, achieving up to 94% protection against Taenia ovis infection in sheep (63) and almost 100% protection against Taenia saginata in cattle (64) and against Taenia solium (65, 66) in pigs. Using a similar strategy, the successful EG95 vaccine was also developed against Echinococcus granulosus infection in sheep (67). This work provided the impetus that stimulated researchers to explore the development and testing of additional recombinant protein helminth vaccines in animal vaccine/challenge trials (60, 61), some of which have now moved forward to clinical testing (16).
Human helminth vaccines.
A recent article in Nature emphasized that only vaccines can eradicate parasitic worms (68), which infect two billion of the world’s poorest people. However, despite decades of research and development, no vaccine against any helminth infection is licensed for human use (28), although a number of recombinant-based vaccines are at different clinical developmental phases. These include vaccines against the human hookworm Necator americanus, one (currently in phase 1) targeting the glutathione S-transferase-1 (Na-GST-1) and the other, the aspartic protease-1 (Na-APR-1) (69, 70). Four schistosomiasis vaccine candidates are undergoing clinical trials, including one targeting the 28-kDa glutathione S-transferase of Schistosoma haematobium (Sh28GST/Alhydrogel; completed phase 3) (71, 72) and one against Schistosoma mansoni proteins, namely, the 14-kDa fatty acid-binding protein (Sm14/GLA-SE; phase 2) (73), a tegument-linked tetraspanin (Sm-TSP-2/Alhydrogel; phases 1 and 2b) (74), and a calpain (Sm-p80/GLA-SE [SchistoShield]; phase 1) (75). Furthermore, several hundred additional vaccine candidates have been tested against at least one of the three major schistosomes that infect humans (Schistosoma japonicum, S. haematobium, and S. mansoni) in mice and nonhuman primates or, in the case of S. japonicum, bovines (16). Disappointingly, very few of these vaccines have developed beyond preclinical testing, and no vaccine candidates have yet advanced to the preclinical trial stage against the zoonotic pathogen S. japonicum, which is endemic to Asia and is the most pathogenic of the major schistosomes that infect humans (17, 18).
Development of vaccines against other parasitic infections.
A brief update of recent progress in the development of vaccines against some other high-prevalence parasitic infections, including Chagas disease, onchocerciasis, and diseases caused by trematode liver flukes, is presented below.
(i) Chagas disease, a zoonosis caused by the protozoan Trypanosoma cruzi, can lead to cardiomyopathy and death. As reviewed by Camargo et al. (76), although many excellent vaccine candidates (including the active trans-sialidase [aTS] family, amastigote surface antigen 2 [ASP-2], and the αGal epitope) and different strategies (recombinant vaccine, DNA vaccine, attenuated vaccine and viral vector vaccine) have been developed, limited progress in clinical vaccine trials has been made. The major point to consider is how to induce sterile immunity (exemplified by vaccines against yellow fever and measles) or long-term immunity by the vaccine, which is a difficult aim to achieve. As a novel strategy, a plan for development of an mRNA vaccine against Chagas disease was put forward by Versteeg et al. (77), but without any recent updates.
(ii) Onchocerciasis, a human zoonotic disease arising from infection with the filarial nematode Onchocerca volvulus, results in visual impairment and blindness in humans. As reviewed by Zhan et al. (78), after decades of efforts to identify and develop prophylactic and therapeutic vaccine candidates against onchocerciasis, two recombinant proteins, Ov-103 and Ov-RAL-2, were eventually promoted for use in a bivalent vaccine, which is currently being tested in naturally infected cattle in Cameroon. Clearly, more efforts are needed to strengthen the immunogenicity and protective efficacy by optimizing adjuvants, developing suitable product development, and exploring new-generation technologies.
(iii) Diseases caused by liver flukes include fascioliasis (Fasciola infection), opisthorchiasis (Opisthorchis infection), and clonorchiasis (Clonorchis infection), which cause liver and bile duct disease in humans. As recently reviewed by McManus (79), recent vaccine progress is highlighted by the following. (i) Lead vaccine candidates against Fasciola hepatica, including fatty acid binding proteins (FABP), cathepsin L1 (CatL1), peroxiredoxin (Prx), and the gut-associated exopeptidase leucine aminopeptidase (LAP), were tested in animal vaccine/challenge experiments (79), showing a large range of variability in vaccine efficacy (33 to 89%) in different trials, possibly due to variation of the antigen source, the adjuvant, and the animal models vaccinated. (ii) Opisthorchis viverrini exosome-like extracellular vesicles and recombinant tetraspanin proteins (TSPs) can elicit antibody responses in the hamster vaccine model, and these were effective in inhibiting the uptake of EVs by cells of the bile duct, resulting in partial protective efficacy against O. viverrini challenge (80, 81). (iii) Genome and transcriptome analyses of the liver fluke Clonorchis sinensis (82, 83) has resulted in some critical tegumental molecules, excretory/secretory products, and metabolic enzymes being identified as potential vaccine targets (84). The area was further advanced by recent vaccine/challenge trials in rats showing highly significant vaccine efficacy (60 to 67% worm reduction) generated by vaccination with several recombinant proteins, including cathepsin B cysteine protease 3, enolase (Bacillus subtilis spore based), and soinRho GTPase (79).
Gene-Based Vaccines
Nucleic acid (gene-based) vaccines comprise plasmid DNA or mRNA encoding a target antigen(s) that is injected directly into an animal or human recipient, with the antigen expressed subsequently in situ by cells of the vaccinated individual (85, 86).
DNA vaccines.
Gene-based vaccination came of age in 1993, with the use of a plasmid construct encoding the nucleoprotein of influenza A virus (87). This construct induced the production of nucleoprotein-specific cytotoxic T lymphocytes in vaccinated mice and generated protection against challenge infection of a heterologous strain of the virus (87). Since then, the synthetic DNA approach to vaccine development has advanced considerably, and more potent vaccine platforms have resulted due to improvements in delivering the DNA into host cells, improved tolerability, and numerous changes in genetic design and the use of different formulations. These advances incorporate a number of key characteristics required to ensure rapid vaccine development, including robust generation of durable T-cell-mediated and mucosal immunity against a variety of emerging infectious agents (88). These pathogens include Zika virus (89), Ebola virus (90), and SARS-CoV-2 (91, 92).
The emergency use authorization of a COVID-19 DNA vaccine in India in August 2021 was historic, as this was the first DNA vaccine approved for clinical application in humans (93, 94). In partnership with the Indian Department of Biotechnology, the pharmaceutical company Zydus Cadila developed ZyCov-D, a 3-dose (given 28 days apart) intradermal vaccine, subsequently authorized for use in people aged 12 years and over (93). According to Zydus Cadila, its phase 3 trial of >28,000 volunteers indicated that the vaccine demonstrated 67% protection against symptomatic infections. No severe cases or COVID-19 deaths were observed after the second dose, and no moderate cases were evident after subjects were given the third vaccination (95). Until the deployment of ZyCov-D, DNA vaccines had proved successful only in the veterinary medicine field; these included an equine vaccine against West Nile virus infection, a vaccine (Oncept) for canine melanoma targeting tyrosinase, and two vaccines against viral infections (hematopoietic necrosis virus and salmonid alphavirus subtype 3) in salmon (96). Several DNA vaccines have also shown some promise against parasites, including against Plasmodium (97, 98), Leishmania (99), Ancylostoma (hookworm) (100), Schistosoma (101, 102), and Onchocerca (103), although none have been developed sufficiently to be tested clinically.
There are some historic inefficiencies with DNA vaccines that need to be overcome. In particular there are concerns regarding potential risks of genotoxicity due to chromosomal integration which may lead to cancer (104). However, their ease of manufacture, high stability, and durability in maintaining an effective immune response make DNA vaccines potentially useful alternatives to recombinant-protein-, adenovirus-, or mRNA-based vaccines, with DNA vaccination providing a suitable means to deploy vaccines in low-income countries on a wide scale (93). Indeed, this premise gained support from the initial studies testing DNA vaccines in larger animal models and humans, which demonstrated that DNA was safe and well tolerated (104). Some inherent drawbacks, however, remain that have delayed the development of effective DNA vaccines (105); these include different biological barriers (cell and nuclear membranes and endosomes) that hamper the delivery of DNA plasmids to the cytoplasm or cell nucleus, resulting in low protein expression and inefficient transport of antigen to antigen-presenting cells (APCs), with suboptimal protective immune responses generated. Another concern is that continuous expression of a target antigen through DNA vaccination may induce host tolerance to the pathogen in question (79). A number of approaches have been used in efforts to increase the immunogenicity of DNA vaccines such as DNA encapsulation, coexpression with cytokine molecular adjuvants, and heterologous prime-boost regimens (106, 107).
mRNA vaccines.
In general, the development of vaccines using protein-based or DNA-based methods has failed to induce effective protective immunity against parasites such as Plasmodium and Schistosoma and other pathogens, including Mycobacterium tuberculosis and HIV. Many of the current conventional vaccine platforms cannot satisfy the need for a rapid, safe, efficient, and cost-effective response to epidemic pathogen outbreaks. The COVID-19 pandemic strengthened the case for producing and implementing vaccines formulated using in vitro mRNA transcription and in-host mRNA translation. With the most-promising results, BNT162b2, developed by BioNTech and Pfizer, was further used for testing on a human population cohort (108). This trial represented a critical milestone and advance both in science and in public health. Possessing features similar to those of the production of DNA vaccines (involving a rapid, simple, and cell-free manufacturing process), mRNA vaccines have been shown to be safe (e.g., they do not carry any integration risks) and are highly effective in that they stimulate robust and potent humoral and T-cell immune responses (10, 109). These features make mRNA an excellent candidate for development as an effective prophylactic or therapeutic vaccine that can bridge the gap between an emerging pandemic and an effective public health response (10).
Properties of mRNA-Based Vaccines Compared with Recombinant-Protein and DNA-Based Vaccines
Table 1 presents a comparison of the characteristics of mRNA vaccines with those of DNA and recombinant protein vaccines. To date, research efforts have been initiated to develop mRNA vaccines against three parasitic protozoa (Plasmodium spp. [110, 111], Leishmania donovani [112], and Toxoplasma gondii [113]) and the black-legged tick, Ixodes scapularis (114). Further details of these mRNA vaccines are presented below. However, no mRNA vaccines have yet been developed against any of the parasitic helminths. Improvements in our understanding of how mRNA vaccines work will be necessary to extend this new platform to target other pathogens, including the parasitic helminths.
TABLE 1.
Characteristics of mRNA vaccines, DNA vaccines, and recombinant protein vaccinesa
| Characteristic | mRNA vaccine | DNA vaccine | Recombinant protein vaccine |
|---|---|---|---|
| Advantages | |||
| Stimulation of humoral and cellular responses | + | +/− | +/− |
| Both MHC-I and -II presentation | + | + | +/− |
| Ability to polarize/balance Th1/Th2 responses | + | + | +/− |
| Native structural/posttranslational modification | + | + | − |
| Simplicity of formulation and production | + | + | − |
| Speed of purification and modification | + | + | − |
| Low cost | + | + | − |
| Stability | − | + | +/− |
| Safety | + | +/− | + |
| Disadvantages | |||
| Lower vaccine efficacy | − | +/− | +/− |
| Low immunogenicity | − | +/− | +/− |
| Necessity for repeat doses | +/− | + | + |
| Risk of autoimmune reactions | +/− | − | +/− |
| Risk of host genome integration | − | + | − |
+/−, the characteristic is present in some vaccines but not others.
OVERVIEW AND HISTORY OF mRNA VACCINES
Research on mRNA vaccines was initiated in 1990, when the in vivo expression of 3 proteins (β-galactosidase, luciferase, and chloramphenicol acetyltransferase) was successfully revealed after the separate injection of the 3 enzyme-coding mRNAs into mouse skeletal muscle (86). However, due to concerns at the time about the instability of the mRNA product and the potential for an unwanted innate immune response triggered by the mRNA, this initial report failed to attract much attention from the research community. A major breakthrough occurred, however, in 2005, when the group led by Drew Weissman and Katalin Kariko discovered that chemically modifying mRNA by replacing uridine with its isomer pseudouridine could greatly increase protein expression and damp any unwanted innate immune responses (115). This major advance totally changed attitudes regarding the pharmaceutical potential of mRNA vaccination. Another key feature of Weissman and Kariko’s discovery was that the modified mRNA with increased stability could potentially be used in a broad range of interventions, including vaccination. The modifications to the molecular structure and manufacture of mRNA can effectively avoid causing inflammation in the hosts, which can be induced generally by unmodified mRNA molecules. As a consequence, the modified mRNAs are active longer inside target cells and effectively produce key antigens that stimulate the host to fight disease.
A substantial amount of development followed this landmark study, which led subsequently to regulatory approvals of the COVID-19 mRNA vaccine in 2020/2021. Two major pharmaceutical companies, Pfizer-BioNTech and Moderna, successfully developed these first COVID-19 mRNA vaccines with ~95% protective efficacy (116); both vaccines superseded the efficacy of 15 mRNA vaccines developed against other pathogens that, by the end of 2019, had entered clinical trials, although none had advanced to phase 3 (117). These two COVID-19 mRNA vaccines utilized the nonreplicating-mRNA approach with the mRNA chemically modified (115) to prevent unwanted immune activation (118).
Structure of mRNA Vaccines
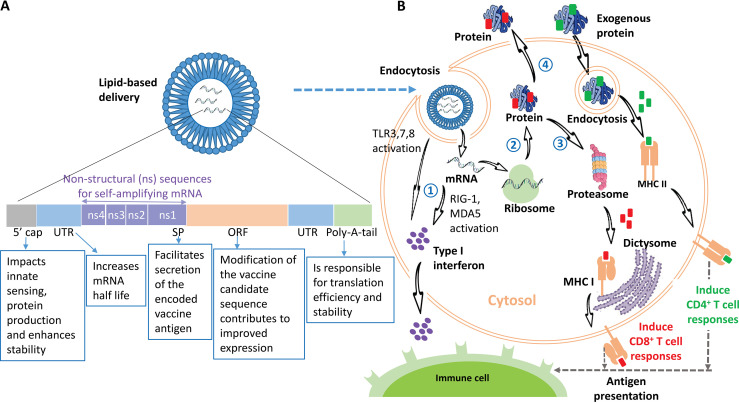
The main principle of any mRNA vaccine is to transport a transcript of interest, coding for an immunogen(s), into the cytoplasm, where the mRNA is translated by ribosomes in vivo. The transcript can be encoded to translate a protein of interest, be it an intracellular, transmembrane, or secreted component (119). Two categories of mRNA constructs have been classified: self-amplifying mRNA (SAM) and nonreplicating mRNA (NRM) (120) (Fig. 1A). SAMs are generally larger molecules than NRMs and can encode four nonstructural proteins (NSPs) in addition to the target transcript. These NSPs facilitate RNA transcription by amplifying the entire original strand of RNA and then the encoded antigen strand, resulting in much higher protein expression. Thus, SAMs require a lower dose of the construct, at least in preclinical models (121, 122). This feature of SAM has been evidenced in multiple animal models and humans against a wide variety of infectious diseases, such as those cause by rabies virus, influenza virus, respiratory syncytial virus, HIV, and Ebola virus (15).
FIG 1.
(A) A typical mRNA vaccine is composed of a protein-encoding open reading frame (ORF) flanked by two untranslated regions (UTRs), which are responsible for regulating transcription and increasing mRNA stability. The ORF normally starts with a signal peptide (SP), which can promote the secretion of the targeted coded vaccine antigen. The four extra nonstructural proteins (NSPs) and sequences of self-amplifying mRNA (SAM) are located before the SP. The transcript should contain a 5′ cap to facilitate protein production and a 3′ poly(A) tail so that translational efficiency and intracellular stability and are improved. The mRNA construct is formulated in LNPs to facilitate cellular uptake and to offset degradation. (B) Induction of an immune response triggered by the mRNA vaccine. The in vitro-transcribed mRNA is formulated with LNPs and is subsequently internalized via endocytosis by APCs, including macrophages or DCs. The entrapped mRNA undergoes endosomal escape and is delivered into the cytosol. (Step 1) The released mRNA activates TLRs (TLR-3, -7, and -8) and the RIG-1-like receptors (RIG-1 and MDA5), subsequently triggering type I interferon production (77). (Step 2) The mRNA is translated into protein by ribosomes in the host cell. (Step 3) If the protein does not contain an SP, the antigenic protein is produced in the cytoplasm and degraded by the proteasomes; there, the produced antigenic epitopes are delivered into the endoplasmic reticulum, where they bind to major histocompatibility complex class I (MHC-I) molecules. The loaded MHC-I-peptide epitope complexes presented on the cell surface activate antigen-specific CD8+ T-cell responses subsequent to T-cell receptor recognition and appropriate costimulation. (Step 4) However, if the protein contains an SP, the mature proteins are either membrane anchored or secreted from the host cell (depending on the mRNA design). The exogenous proteins are internalized via endocytosis by APCs, the generated peptide epitopes then bind to MHC-II molecules, and the complex of MHC-II-peptide epitopes, which are cell surface presented, are able to induce antigen-specific CD4+ T-cell responses (150), providing help to CD8+ T cells and B cells. Cross loading or presentation to MHC-I can also occur (239). Both MHC-I and MHC-II are transported to plasma membranes, where they are presented as antigens to immune cells, including B and T cells (15). Secreted type I interferons can stimulate or inhibit T-cell activation depending on the intensity and timing of the type I IFN induced (77, 240). It has been shown that the humoral immune response is the major mechanism induced by a mRNA vaccine through the activation of B cells (241).
Immune Response Induced by mRNA Vaccination
The initiation of an immune response triggered by mRNA vaccination is illustrated in Fig. 1B. mRNA vaccines elicit adaptive and innate immunity (123). It is critical to understand how cells react to and defend themselves against nonself mRNA, thereby stimulating innate immunity, and how they can trigger cascades of signaling pathways prior to the induction of adaptive immunity. It is known that the pathogen-associated molecular patterns (PAMPs) contained in the mRNA tridimensional structure can be recognized and bound by pattern recognition receptors (PRRs) on the surface of cells (124). The ligand-receptor (PAMP-PRR) complex activates signaling pathways, subsequently stimulating distinct trans-acting factors to foster the induction of proinflammatory cytokines and chemokines (125). Endosomal Toll-like receptors (TLRs) and the RIG-I (retinoic acid-inducible gene 1)-like receptor family have been identified as PRRs (RNA sensors). Three TLRs (TLR3, TLR7, and TLR8), which are present in endosomal compartments of APCs, play important roles in identifying RNAs. TLR3 recognizes double-stranded RNA (dsRNA), and TLR7 recognizes dsRNA and single-stranded RNA (ssRNA), while TLR8 binds only ssRNA (126). Stimulation of TLR3, TLR7, and TLR8 leads to the induction of type I interferons (IFN-α/β) (127). The RIG-I-like receptor family includes RIG-I and melanoma differentiation-associated protein 5 (MDA5) (128). RIG-I recognizes ssRNA and dsRNA, while MDA5 binds dsRNA. Both receptors can activate IRF3 (interferon regulatory factor 3) and NF-κB (nuclear factor kappa B), and this leads to increased levels of type I IFN being produced (77, 129). Recent findings show that NOD2 (nucleotide-binding oligomerization domain 2) can be stimulated by ssRNA (130), activating IRF3 to produce IFN-β. The IFN response, induced by mRNA vaccination through stimulation of TLR3, -7, and -8, RIG-I, and MDA5, is reliant on the quality of the mRNA that is transcribed in vitro, mRNA modification, the means of delivery, and the administration route (10).
Replacement of uridine with pseudouridine in nonself mRNA, as well as purification of the mRNA via high-performance liquid chromatography (HPLC), can reduce any undesirable innate immune response and enhance antigen stability and expression (131, 132). Further, encapsulation of the modified mRNA into lipid nanoparticles (LNPs) provides the potential to induce a strong T helper response and to produce a considerable quantity of long-living germinal center B cells and high-titer antibodies (133). mRNA vaccines induce robust levels of humoral immunity by activating naive B cells (123). Specific antibodies are generated by germinal centers (GCs) in B-cell follicles of secondary lymphoid organs. When B cells mature with the help of T cells (134), they produce an antibody response on receiving nonself antigen presented by dendritic cells (DCs) (135). If the antigen is consistently presented during the initiation of GCs, both the antiantigen antibody responses and B cell/T follicular helper cell responses are increased (136), and this subsequently fosters sustained neutralizing antibody responses. It has been shown in animal models that mRNA vaccines, even with only one or two low-dose immunizations, are able to generate robust neutralizing-antibody responses (109). Furthermore, SARS-CoV-2 mRNA vaccines are capable of eliciting multicomponent immune memory, maintaining long-lasting memory B- and T-cell responses even when antibody levels decline (137). The immune memory induced by mRNA vaccination is rapid after antigen re-exposure, promoting continued protection against reinfection (137).
Advantages of mRNA Vaccines
mRNA technology exhibits a number of advantages, making it a highly attractive alternative to current conventional approaches for vaccine development. First, mRNA vaccines can be generated rapidly, employing relatively straightforward manufacturing procedures (138). The RNA synthesis is based on the sequencing information available for a target pathogen, and this can be carried out rapidly (within weeks or even days), unlike recombinant protein-based vaccines, which normally require a deep understanding of the target organism and can take years to develop and produce. Furthermore, if a different encoded antigen is required, the mRNA vaccine process requires only slight platform changes during the new mRNA formulation and manufacture, as such modifications do not impact the physical and chemical features of the mRNA backbone (139), making production easy to standardize and rendering it relatively inexpensive. In addition, mRNA production is cell free and scalable due to the high quantities that result from the in vitro transcription process (140).
Second, mRNA vaccines are safe and can generate a robust level of immunity, highlighted by four key features. (i) mRNA vaccine production involves a nonintegrating platform, and this leads to improved biosafety compared with DNA-based vaccines, since the delivery of mRNA requires only cytoplasmic involvement for activity, and mRNA vaccines cannot randomly integrate into the genomes of host cells (141). (ii) mRNA vaccines utilize a noninfectious platform and thus do not harbor infectious elements that would elicit risks for replication and disadvantageous mutagenesis (109). Safety issues regarding cell-derived impurities and culture contaminants, which are typically concerns with other platforms, are easy to avoid during the process of cell-free transcription of mRNA. Unlike attenuated or inactivated vaccines, mRNA vaccines express only a specific encoded antigen(s) that subsequently generates effective and specific immune responses. This is illustrated by the success of mRNA vaccines in inducing cellular immune responses against infectious agents where conventional (“classical”) technology has failed (142). (iii) mRNA, following injection into host muscle, is translated into protein by the host. This results in correct native protein conformation and posttranslational modifications (such as glycosylation and phosphorylation) similar to those occurring in the live pathogen, thereby favoring the generation of correct antibody specificities (143). As indicated earlier, recombinant proteins are normally expressed in prokaryotic (Escherichia coli or Bacillus subtilis) or eukaryotic expression systems (144). Thus, the yield, production, and stability of the purified recombinant proteins, together with posttranslational modifications of the target antigens, have to be considered, and this makes vaccine manufacture complex, expensive and time-consuming. (iv) Modification of mRNAs makes them more stable and highly translatable, allowing for increased dose tolerance and eliciting high antibody responses (145). The modified mRNA is able to elicit a strong immune response by promoting both cellular and humoral immunity, as evidenced both in clinical trials and with animal models (121, 142). Even a single dose of mRNA has been shown to elicit a high level of neutralizing antibodies, a feature that is vital to provide long-term protection against infection (121, 146).
Third, an important advantage of mRNA vaccines is that they allow the codelivery of multiple mRNAs eliciting synergistic effects and subsequently fostering protective immunity (147). With the rapid spread of new coronavirus variants, such as Delta and Omicron, a critical strategy was to update COVID mRNA vaccines by producing multivalent vaccines against multiple spike mutations of the coronavirus so they maintain their protective efficacy (148). Progress has also been made in the development of a multivalent mRNA vaccine targeting influenza virus by establishing a well-controlled scalable platform that can be utilized to include additional vaccine candidates in short lead times (149). This approach, which is aimed at developing multivalent mRNA vaccines, can be pivotal for generating multivalent vaccines against complex parasites, including large, clinically important macroparasitic helminths such as schistosomes and hookworms.
LNPs as Delivery Systems for mRNA Vaccines
Selection of an appropriate system for mRNA delivery is crucial for preventing the mRNA from degrading, promoting its cellular uptake and its effector presentation, and leading to the induction of a strong and effective immune response (150, 151). The mRNA delivery system should be designed according to biomedical demands of biocompatibility and biosafety. To date, multiple procedures to deliver mRNA vaccines have been investigated, including physical delivery approaches, ex vivo loading of dendritic cells, and the use of cationic LNPs. Nanoparticles have proved the most appealing, having been used with success for the delivery of nucleic acids, including mRNA, in the clinic (108, 109, 152–156). The delivery of mRNA with LNPs elicits durable, protective immune responses against pathogens and against cancer after minimal doses (121, 157). LNPs protect the mRNA from degradation through surface decoration with ligands, can be potentially targeted to a desired cell type, and, if needed, can be codelivered with adjuvants. LNPs promote self-assembly into particles ~100 nm in size in order to cross the negatively charged phospholipid bilayer of cell membranes and to facilitate the endosomal release of the mRNA into the cytoplasm, where protein translation occurs (109). LNPs that are less than 150 nm efficiently drain to lymph nodes via afferent lymphatic vessels (158), while larger LNPs can be phagocytosed by immune cells and transferred to the lymph nodes. LNPs generally consist of four constituents: (i) cationic (ionizable) lipids, which are able to effectively increase the entrapment of mRNA, enhance the efficiency of cellular uptake, and facilitate endosomal escape; (ii) cholesterol, which stabilizes LNP complexes; (iii) lipid-anchored polyethylene glycol (PEG), which can reduce the size of particles, prevents their aggregation, promotes circulation time, and decreases uptake by the reticuloendothelial system; and (iv) phospholipids, which are crucial to retain lipid bilayer structure (109). Whereas LNPs have received extensive investigation, additional research of other potential delivery platforms to enhance efficient transfection and specific targeting, to increase the stability of encapsulated mRNA, and to reduce reactogenic side effects will remain a priority to guide and advance the development and optimization of intracellular delivery systems generally to improve basic health care and medical treatment.
Safety Profiles of mRNA Encapsulated with LNPs
Early reports of the safety of Pfizer-BioNTech’s COVID-19 vaccine recorded circa 11 anaphylaxis cases/million vaccinations (including less severe nonanaphylactic reactions) following the first dose (159). The majority of the side effects (e.g., ache at the injection point, fatigue, headaches, fever, chills, and Bell’s palsy) attributed to mRNA vaccination in clinical trials are not life-threatening (138, 160). It has been shown that some components, such as PEGylated lipids, which are included to stabilize and to ensure the biocompatibility of LNP-mRNA vaccines, may contribute to severe allergy-like reactions potentially resulting from preexisting PEG allergy in vaccinated subjects (161). Indeed, there are reports that PEG lipids can activate the complement system, inducing hypersensitivity reactions (162, 163). An unexpected immunogenic response—accelerated blood clearance (ABC) phenomenon—has also been observed during the repeated administration of PEGylated nanoparticles (164), which may modify the bioavailability and biodistribution of the encapsulated mRNA, leading to side effects (162). This raises concerns for immunotherapy applications when multiple dosing is required to maintain high levels of protective antibodies. Given these safety concerns, numerous natural or synthetic components (such as heparin, dextran, chitosan, and biodegradable/nonbiodegradable polymers) have been considered as alternatives to PEG (162), but research in this area is still in its infancy. It is critical to optimize suitable mRNA delivery methods that can enhance effective protective immunity outcomes while at the same time minimizing vaccine-associated risks (165).
Transport and Storage of mRNA-LNP Formulations
Originally, it was thought that the Pfizer-BioNTech COVID-19 mRNA vaccine required temperatures between −80°C and −60°C for shipping, that it could be kept in a −20°C freezer for only about 2 weeks, and that it could be stored at 2 to 8°C for only 5 days (https://www.gavi.org/vaccineswork/change-cold-storage-conditions-could-make-pfizer-covid-19-vaccine-more-widely). This meant that only a limited number of countries could manage a vaccine requiring such ultracold storage. Subsequently, however, data submitted to the regulators by Pfizer indicated that thawed and undiluted vials of their vaccine could be stored at 2 to 8°C for up to 1 month. From the outset, it was clear that Moderna’s Spikevax mRNA vaccine had less stringent cold chain requirements: requiring shipment at −20°C, it could be stored for up to 6 months at −20°C, and after thawing, it too could be kept at 2 to 8°C for up to 30 days (https://www.gavi.org/vaccineswork/change-cold-storage-conditions-could-make-pfizer-covid-19-vaccine-more-widely). Nevertheless, it is clear that each step in handling mRNA vaccines needs diligent care and coordination (15), significantly impacting vaccine accessibility (138). Such a requirement represents a substantial technical hurdle and considerable economic burden if mRNA vaccines are to be delivered, distributed, and stored in appropriate facilities in developing countries. Accordingly, systematic strategies to determine the key physicochemical mechanism(s) of degradation in formulated mRNA vaccines will be required, given our current limited understanding of these processes (166). In addition, further research is required to determine the optimal design of formulations for LNP-mRNA vaccines to ensure that they maintain stability at refrigeration or ambient temperatures during transport, storage, and administration (166). Strategies may involve the selection of excipients (such as the use of preservatives and stabilizers), formulation milieu (such as tonicifying agents and/or the appropriate pH), improvements in lipid nanoparticle technologies and production methods, and optimal delivery procedures (such as the use of lyophilized or liquid vaccine doses) (166). Ideally these factors would enable shipment and storage of mRNA at realistic temperatures throughout the vaccine supply chain (167), with the improvements in stability facilitating better access to basic health care and medical treatment by residents of developing countries and remote areas.
POTENTIAL OF mRNA VACCINES FOR COMBATING PARASITIC INFECTIONS
The mRNA vaccine platform offers immense potential for developing vaccines against a range of infectious agents (88). Important targets of mRNA vaccines include influenza virus (168, 169), with phase 1 trials recently initiated and led by Moderna, Pfizer, and Sanofi (170); Zika virus (171–173), with a vaccine undergoing concurrent phase 1 and 2 clinical trials led by Moderna (174, 175); respiratory syncytial virus (RSV), with Moderna having initiated the phase 3 portion of its pivotal clinical trial as a step toward its ultimate goal of combining the mRNA vaccine for RSV (mRNA-1345) with boosters for SARS-CoV-2 and influenza virus into a single vaccine dose (https://www.biopharma-reporter.com/Article/2022/02/22/moderna-initiates-phase-3-trial-for-rsv-vaccine-candidate); and HIV, with results of a preclinical study having recently been announced (176). Furthermore, it is notable that anticancer mRNA vaccines that target tumor-specific neo-epitopes and tumor-associated antigens (177, 178) have also been developed, and these are undergoing evaluation in phase 1/2 clinical trials for the treatment of solid tumors (179). The “warp-speed” development and remarkable deployment of mRNA vaccines against COVID-19 provide the impetus to employ this unique technology platform to tackle the more complex and demanding task of developing vaccines against parasitic infections.
mRNA Vaccines for Protozoan Parasites
The concept of successfully applying mRNA platform technology to produce effective vaccines against parasites is supported by some recent successes, albeit not extensive ones, in utilizing the approach to develop mRNA vaccines against parasitic protozoan infections, including malaria (110, 111) and those caused by L. donovani (112) and T. gondii (113), discussed below.
Malaria.
Plasmodium spp. produce PMIF, an ortholog of the mammalian cytokine macrophage migration inhibitory factor (MIF), which modulates inflammation against malaria parasites. A novel PMIF mRNA vaccine was able to promote the control of blood-stage and liver Plasmodium infection and elicit a remarkable level of protection against P. berghei, which causes lethal malaria in mice, upon challenge with either a blood-stage parasite or a sporozoite (the mosquito-transmitted stage) (111). Specifically, the anti-PMIF vaccine led to a delay in blood-stage patency following sporozoite infection, decreased expression levels of IFN-γ, interleukin 12 (IL-12), and tumor necrosis factor alpha (TNF-α) during the blood-stage infection, increased germinal center and CD4 T follicular helper (Tfh) cell responses, enhanced anti-Plasmodium IgG antibody levels and the differentiation of memory CD4+ T cells and liver-resident CD8+ T cells (89). Notably, the PMIF mRNA vaccine further enhanced parasite control in a primary infection while generating 100% protection against subsequent challenge (111). In addition, this protection against new infection could be recaptured when CD8+ or CD4+ T cells were transferred from mice that had received the PMIF mRNA vaccine (111). The study is noteworthy because, as the authors suggest, MIF inhibition may be an applicable method for stimulating protective immunity not only to malaria but also potentially to other parasitic protozoan and helminthic species that produce protein orthologues of MIF (111).
Another recent study showed that an mRNA vaccine encoding the P. falciparum circumsporozoite protein (PfCSP) (the immunodominant surface protein of the invasive sporozoite stage of the Plasmodium parasite), encapsulated with LNPs, also achieved sterile protective immunity against malaria in mice (110). The authors of the study first showed good expression of the PfCSP mRNA vaccine, which was cell associated in mammalian cell transfection experiments. Then, a vaccine formulation of PfCSP mRNA-LNP generated sterile protective immunity in mice following challenge infection with two P. berghei PfCSP transgenic parasite strains; the vaccine dose and the interval between doses had the most effect on outcome (110). This study served well as a basis to assess the pre-erythrocytic PfCSP mRNA vaccine, with the reported outcomes making it a compelling candidate with considerable potential to improve on protective efficacy levels achieved with conventional approaches. These findings have led to plans by BioNTech to develop the first mRNA-based malaria vaccine, with clinical trials projected by the end of 2022 (https://www.technologyreview.com/2022/02/23/1045131/malaria-vaccine-save-lives/).
Leishmaniasis.
Leishmania donovani is an important human intracellular parasitic protozoan that causes visceral leishmaniasis (kala-azar), a severe and life-threatening form of the disease. LEISH-F2 has been shown to be an important vaccine candidate against this disease, and its genetic sequence has been used to design a RNA vaccine construct (112). When the LEISH-F2 mRNA vaccine was given to mice as a primary immunization and heterologously boosted by vaccination with recombinantly expressed LEISH-F2 (adjuvanted with the oil-in-water emulsion glucopyranosyl lipid A in SLA-SE (second generation glucopyranosyl lipid A in stable oil-in-water emulsion)), a significantly reduced parasite burden was evident in the livers of the vaccinated mice following L. donovani challenge. In contrast, compared with challenged control mice, no reductions in parasite numbers were evident following homologous vaccination with either the LEISH-F2 mRNA vaccine or LEISH-F2 SLA-SE. Furthermore, the heterologous vaccine approach induced in mouse splenocytes a very high level of secreted IFN-γ and strong antigen-specific Th1 responses (112). This study emphasizes the potential and utility of heterologous prime-boost immunization to generate robust antigen-specific T-cell responses for protection against intracellular pathogens.
Toxoplasmosis.
Toxoplasma gondii is an obligately intracellular parasite belonging to the phylum Apicomplexa. This species parasitizes a broad variety of warm-blooded animals and humans. Although infection with T. gondii is generally asymptomatic, it can be a severe threat to pregnant women and individuals who are immunocompromised. In a novel vaccine approach, a vaccine platform using modified dendrimer nanoparticles (MDNP) in which antigens are encoded by encapsulated mRNA replicons was developed by Chahal et al. (180). A single dose of the vaccine was able to generate both antibody and CD8+ T-cell responses that resulted in complete protection of mice following challenge with a range of dangerous pathogens, including T. gondii (180). With T. gondii, six specific antigens (surface antigen 1 [SAG1], SAG2A, rhoptry protein 18 [ROP18], ROP2A, apical membrane antigen 1 [AMA1], and dense granule protein 6 [GRA6]), which are expressed in different life cycle stages and parasite strains, were multiplexed into the (hexaplex) MDNP vaccine. In the vaccination study, animals received the hexaplex MDNP vaccine prior to challenge with T. gondii type II strain Prugniaud (PRU), which is known to be lethal to mice. Vaccinated mice survived longer than 6 months without any clinical indications, whereas all the nonvaccinated controls were killed by the infection. This MDNP vaccine platform, with its ability to rapidly produce viable, contaminant-free vaccines comprising single or multiple antigens, may be widely applicable against a variety of pathogens, including parasites, and the diseases they cause.
A subsequent study (113) targeted the nucleoside triphosphate hydrolase II (NTPase II) protein from T. gondii, which has been shown to elicit Th1 cell-mediated protective immunity against T. gondii infection. This study, undertaken by Luo et al. (113), involved the development of a self-amplifying RNA vaccine (RREP-NTPase-II) which was delivered to mice intramuscularly in LNPs. The vaccinated mice generated potent immunity, with robust levels of IgG antibodies and IFN-γ produced. In an acute mouse infection, compared with phosphate-buffered-saline (PBS) controls, vaccinated animals challenged with the RH strain of T. gondii (103 tachyzoites) survived longer and had reduced parasite burdens in their brains. In addition, in a chronic mouse model of T. gondii infection, animals vaccinated with the RREP-NTPase-II encapsulated in LNPs and subsequently challenged with 20 tissue cysts of the lethal Prugniaud strain had significantly decreased numbers of brain cysts compared with the control animals (113). Overall, the mixture of self-amplifying RNA encapsulated in LNPs was shown to successfully induce a high level of protective immunity and offers promise for future development as a safe and long-lasting vaccine against T. gondii.
Multivalent mRNA vaccine against Ixodes scapularis.
Further support for utilizing a multivalent RNA approach for antiparasite vaccine development is provided by the recent success of a mRNA vaccine encoding 19 salivary proteins (19ISP) expressed by I. scapularis (114) This multicellular ectoparasitic arthropod is able to transmit a number of pathogens, including the Lyme disease agent (Borrelia burgdorferi), Borrelia miyamotoi, Anaplasma phagocytophilum, Babesia microti, and other disease agents that are currently on the increase in Europe and North America (181). Naturally acquired resistance to I. scapularis is generally considered the outcome of repeated tick exposure, and the resultant host immune responses (referred to as tick immunity or acquired tick resistance) are postulated as being generated against the complex mixture of salivary protein antigens that are released into the tick bite site (114). Sajid et al. (114) used published information on the tick’s salivary gland transcriptome/proteome (sialome) and its dynamic changes during the process of feeding to identify a panel of proteins in the saliva that enabled them to generate a cocktail of antigens. The 19 antigens (19ISPs) were chosen based on their high level of immunogenicity and, for the majority, their known mode of action. Nucleoside-modified mRNAs that encoded the 19ISP were encapsulated in LNPs. Subsequent immunization of guinea pigs with the LNP-19ISP vaccine induced a robust specific antibody response that was protective against tick challenge. The vaccinated guinea pigs presented with erythema (redness or irritation), a key feature of acquired tick resistance, at the site of tick attachment; in addition, due to poor feeding and early tick detachment, the engorgement weights of the arthropods that fed on the vaccinated animals were significantly lower than those of ticks that fed on control animals.
Developing Vaccines for Parasitic Helminths: Some Challenges
The development of effective vaccines against multicellular helminth parasites has generally proved to be extremely daunting and has proved a more significant challenge than developing antibacterial or antiviral vaccines. Helminth parasites are complex organisms that have evolved a range of mechanisms, including immune escape, utilization of efficient nutrient uptake mechanisms, and exploitation of host hormones and growth factors for worm development, maturation, and egg production, that allow them to survive in different hosts, often for many years (182). It is likely that blocking and/or inhibiting a single gene or gene product by vaccination may stimulate a helminth parasite, such as a schistosome, to compensate by a switch to an alternative metabolic pathway to ensure that it maintains the ability to acquire essential nutrients from the host or the difficult external environment in order to survive (183). Further major challenges on the path to developing effective vaccines against parasitic helminths, as indicated above, are the facts that they generally have multiple life cycle stages, with each often presenting antigens that are stage specific, and they have the notorious ability to damp and regulate the host immune response (14). Moreover, helminths possess complex genomes and proteomes that add to the challenge of identifying suitable antigenic targets, a process that is crucial for the development of efficacious vaccines (14, 184). By employing the schistosome flatworm parasites as models, cutting-edge techniques have been used for schistosome antigen discovery and for deciphering nuclear genomes of S. japonicum, S. mansoni, S. haematobium, and other schistosome species (185–188) and major advances made in transcriptomics (189–191), proteomics (192, 193), immunomics (194, 195), and exosomics (196, 197). This novel information provides a vantage point for understanding the functions of thousands of gene products with potential as critical vaccine targets, enabling rapid future progress in antischistosomiasis vaccine development (198).
With the development of novel vaccine strategies such as immunotherapeutic approaches (199) utilizing the strategies of EV proteins (200) and genetic elements (201), numerous constructs are being defined for a wide range of helminth parasites. However, a better understanding of immunological mechanisms induced by helminth vaccines is necessary for the development of potent vaccine platforms and their optimal design. Immunopathological reactions in schistosomiasis are mainly induced by eggs laid by female adults living in blood vessels of the definitive hosts (202). Eggs are released from feces into the environment to continue the parasite life cycle, while a large proportion of eggs are trapped in the host liver and intestine (S. mansoni and S. japonicum) or bladder and genital tissues (S. haematobium). These eggs secrete various components (such as proteolytic enzymes and glycoproteins) which are able to induce inflammatory immune responses mediated by CD4+ T-helper-2 lymphocytes, subsequently resulting in formation of granulomas when inflammatory cells (macrophages, lymphocytes, neutrophils, and eosinophils) clump around the eggs (203). The result is fibrotic lesions in host tissues, eventually resulting in inflammatory and hepatosplenic schistosomiasis (S. mansoni and S. japonicum) or urogenital disease (S. haematobium) (204).
The potential factors affecting vaccination include the stage of parasitic infection and the time of relative infection and/or vaccination. It has been argued that the most vulnerable period for an immune response generated by vaccine is 72 h after cercarial skin penetration, when the parasite has attained the early developmental stage of the schistosomula in the lungs (205). In addition, existing parasite infections at the time of vaccination may also result in worse immunization outcomes. Meanwhile, it was also found that the natural immunity evidenced in residents living in areas of endemicity can be normally obtained by repeated infections and that its maintenance might be stage specific depending on different antibody classes or T-cell responses (206). Therefore, it is critical to determine whether there is a match between the type of immune response generated by a parasitic infection and the type of protective response required for vaccine against the infection. The vaccine factors also include vaccine formulation and route of administration, which are critical to determine whether a vaccine is susceptible to interference by parasite infections (207).
In terms of generating vaccine-induced protective immune responses to parasitic helminths, there is extensive research documenting the requirement for a solid Th2-biased immune response in animal models (14, 20, 184). The cytokine profile analysis was investigated in mice vaccinated with radiation-attenuated cercariae followed by challenge with normal cercariae (208), the current best model in achieving protection levels corresponding to a >75% reduction in worm burden. It was found that early upregulated pathways were highly involved with Th2-skewed response and polarization of IgG antibody in the mice vaccinated with radiation-attenuated cercariae (208). The results also showed high expression levels of the proinflammatory cytokines IFN-γ and TNF-α, which are critical in activation of macrophages in the pulmonary effector response (209), and IL-10, which plays an important role in regulating excessive inflammation by depressing a polarized Th1 response (210). This also supports evidence from human studies showing that immunity to worm infections requires the generation of a robust Th2 immune response and the production of Th2-associated cytokines such as IL-4, IL-5, IL-6, IL-9, and IL-13 (14, 184). Thus, there is compelling evidence from numerous studies in humans and animal models that any helminth vaccine should induce Th2 immunity, but the development of a balanced Th reaction is also important in order to prevent unwanted effects, since both Th1 and Th2 components, if one-sided and extreme, can cause damaging pathology (184). The deployment of any vaccine dependent on IgE, which is associated with allergic responses, might be problematic and held back by safety and regulatory issues (211). Indeed, finding the right balance of Th1/Th2 responses poses a challenge for any future antihelminth vaccine development.
Can mRNA Vaccines Induce Long-Lasting Protective Immunity against Parasitic Infections?
It is important to re-emphasize that studies on mRNA vaccines tested both in human clinical trials and in animal models have demonstrated that these vaccines are able to elicit potent and durable immune responses and that the lasting protection can be maintained by immune memory (10, 137). A key point for the development of successful antiparasite vaccines is obtaining long-lasting protective immunity by producing long-lived memory B and T cells to provide effector responses that exceed regulatory responses induced during a natural infection (28). A natural helminth infection can induce not only an effector response that is protective and a nonpathological immune response but also a regulatory response. Regulatory responses are known to suppress bystander vaccination (212) and are characterized by repressive cytokines (transforming growth factor β [TGF-β] and IL-10) generated by regulatory T (Treg) cells (213), which may contribute to the depressed resistance to parasite (e.g., a schistosome) reinfection in animal models (214). A balanced acquired immune response between effector CD4+ T-cell subsets (Th1, Th2, and Th17), including effector cytokines (IFN-γ, IL-4, and IL-21), has been implicated in protecting against helminth reinfection (215). As discussed above, mRNA vaccines are characterized by their ability not only to promote humoral immunity, producing type I IFN responses and downstream Th1-polarized responses, but also to stimulate a cellular immune response, activating CD4+ T cells that are needed to support B-cell differentiation and develop memory responses (9, 142, 216). These immunological characteristics make mRNA vaccines highly suitable for combating helminthic infections.
Another critical point in effective vaccination is to retain the vaccine-induced protection even when antibody levels decline or vanish (the half-life of an antibody is ~30 days). Acting as an important mechanism in maintaining long-lasting immunity induced by mRNA vaccines (135, 136), antigen retention by follicular DCs in peripheral lymph nodes has also been shown to be crucial in generating the protective immunity stimulated in the I&T approaches against malaria (61) and schistosomiasis (29). As indicated by the recent debate on the efficacy of SARS-Cov-2 mRNA vaccines versus natural immunity (217), it is assumed that frequent (re)exposure to parasites in areas of endemicity postvaccination may help to maintain long-lasting immunity generated by the vaccination. A recent report further indicated that the host immune response induced by an antischistosomiasis vaccine can be enhanced by repeated treatment with praziquantel, which remains the only effective drug for large-scale population treatment against schistosomiasis in regions of endemicity. Due to the capacity of praziquantel to kill parasites, its destruction of the worm tegument and the subsequent release of worm molecules into the host provide an excellent antigen source to boost the host immune response (218). These features further highlight the potential of mRNA vaccines in generating protective immune responses that are long lasting against a parasitic infection.
The Argument for Developing Multivalent mRNA Vaccines against Helminth Parasites: Time to Reboot
There is now strong evidence to promote the development of multivalent anthelmintic vaccines as essential and effective tools if the goal of controlling or even eliminating parasitic worm infections is to be achieved (14). Moreover, this approach could be advanced even further, as researchers have proposed the development of single pan-anthelmintic vaccines that could be deployed against several parasitic helminth species in a host at the same time to stimulate potent and enduring immune responses with minimal side effects (14). The development of a pan-anthelmintic vaccine that is built from multiple cross-protective antigens may provide a novel alternative to whole-antigen vaccines. Furthermore, such an approach is timely, given that coinfections with two or more worm pathogens are common in individuals from marginalized communities in many developing countries (219). Given the fact that the versatile mRNA platform is amenable to multiple targets (220) and in vivo synergistic effects of multiple mRNAs can significantly foster protective immunity (147), the strategy of developing multivalent mRNA vaccines against helminth parasites has the potential to lead to a quantum leap in the control of the diseases they cause, resulting in a clear positive public health impact. This scenario is strengthened by the fact that codelivery of multiple mRNAs has already been shown to promote synergistic effects that enhance and broaden protective immunity, exemplified by the hexaplex MDNP vaccine developed for T. gondii (180) discussed above. It is noteworthy that the multivalent mRNA platform has also been successfully optimized for multiple antigen combinations with potential for the development of multivalent mRNA vaccines against COVID-19 variants (148) and seasonal or pandemic influenza viruses (149).
Test Case Scenario: Developing a Transmission-Blocking mRNA Vaccine against Zoonotic Schistosomiasis Caused by Schistosoma japonicum
When the complexity of the schistosome life cycle and the zoonotic nature of schistosomiasis japonica are taken into consideration, the development of a mRNA-based transmission blocking veterinary vaccine targeting S. japonicum could provide a unique approach to parasite control in areas where it is endemic (184). Studies performed in China (211, 221) and the Philippines (222) demonstrated that bovines, especially water buffalo (called carabao in the Philippines), are major host reservoirs for S. japonicum and are likely responsible for circa 90% of environmental egg contamination. Transmission of S. japonicum through the passage of eggs in the stools of infected buffalo and cattle in waterways is a significant problem, with the result that nearby human populations are frequently exposed to cercariae released from infected freshwater snails (acting as intermediate hosts), resulting in high levels of schistosomiasis. Quite recent studies undertaken in the Philippines revealed an extraordinarily high schistosome prevalence (70 to 100%) in the carabao residing in areas where schistosomiasis is endemic (222). A mathematical model of S. japonicum transmission has predicted that a bovine vaccine for schistosomiasis, capable of reducing stool egg numbers in water buffalo by 45%, in combination with praziquantel treatment, would lead to a significant decrease in transmission, almost to the point of elimination within 10 years (223, 224). The model further predicts that increasing vaccine efficacy above this threshold would substantially reduce the time required to achieve elimination (178). With this in mind, much research has focused on development of transmission-blocking vaccines (recombinant-protein or DNA vaccines) (17, 102, 225–227) effective against S. japonicum in buffalo. An experimental randomized double-blind trial undertaken in China of a DNA vaccine encoding the triose phosphate isomerase enzyme of S. japonicum (SjTPI) fused with bovine heat shock protein 70 (Hsp70) (223) induced responses in water buffalo that were able to reduce worm burdens by 51% and miracidial hatching by 52%, outcomes that were higher than those induced by the DNA vaccine coding for SjTPI alone. Subsequently, two double-blind, phase 3 cluster randomized control trials (RCTs) were recently completed in China (102, 225) and the Philippines (submitted for publication). The RCTs were designed to determine the effect of an integrated, multicomponent strategy of treatment, snail (intermediate host) control, and vaccination of bovines with the SjTPI vaccine on schistosome transmission. This framework of transmission underpins the current efforts toward a vaccine deployed in animals against S. japonicum as an effective and feasible public health objective, an outcome that aligns well with the concept of One Health, which aims to achieve optimal health outcomes for people, animals, and the environment (20, 102, 184, 228).
The application of anthelmintic treatments is currently more advanced in the veterinary field than the human one (229). The development of a veterinary vaccine provides a number of advantages over a human vaccine, including the costs and issues of safety. Indeed, it has been asserted that the safety profile of a transmission-blocking veterinary vaccine against schistosomiasis japonica is less stringent than that required for clinical application (24). Furthermore, the differentiation of Th1/Th2 responses to antigen in bovines tends to be more closely related to human responses than that of responses generated in mice (230), which are currently extensively used as a model for schistosomiasis. Indeed, much current knowledge of schistosomiasis immunology is based on murine studies, which may not reflect the true picture of immune responses induced in natural, outbred mammalian, including human, hosts in areas where the disease is endemic (230). These arguments apply to vaccine trials with S. japonicum, a robust parasite that is difficult to kill via acquired protective immunity in the murine model (231); indeed, a number of key differences between mice and natural schistosome hosts are evident (232). Consequently, once appropriate vaccine candidates have been identified, it is critical as a next step in translation to assess their protective efficacy in more relevant, natural hosts of infection—in the case of S. japonicum, cattle and buffalo.
The mRNA vaccine platform makes it feasible to develop veterinary-based transmission-blocking multivalent vaccines targeting multiple helminth and other major zoonotic diseases spread between animals and humans, which would be acceptable and appealing to farmers in areas of endemicity. A chronic schistosome infection impact on host metabolism, immunity, and the composition of the microbiome, which can modulate and/or enhance host susceptibility to infection with other diseases (212, 233). A prime example is bovine tuberculosis (BTB) caused by the bacterium Mycobacterium bovis, another major zoonotic chronic respiratory disease spread between bovines and humans. It continues to be a serious problem in animals and impacts human health in many poor rural communities in a number of African and Southeast Asian countries (234), where schistosomiasis may be coendemic. It has been shown that chronic schistosome infection enhances host susceptibility to BTB (235) and that BTB infection can also increase the occurrence of parasites in buffalo (236). Given the advantages and feasibility of the multivalent mRNA vaccine approach, developing a transmission-blocking multivalent mRNA vaccine for bovines that targets both schistosomiasis and BTB has the potential to lead to a quantum improvement in the control of these zoonotic infectious diseases, with a clear future public health impact.
CONCLUSION
The mRNA platform is a notable novel technology that has already reformed vaccine development. mRNA vaccines can be simply developed and rapidly deployed, they are reproducible and inexpensive to manufacture, and, importantly, they are capable of stimulating broadly protective and long-lasting humoral and cellular immunity (220). The warp-speed development and the subsequent successful deployment of the highly effective SARS-CoV-2 mRNA vaccines were a result of the unparalleled early expansion of the COVID-19 pandemic. The accelerated development of mRNA vaccines currently in different phases of clinical trials targeting a wide range of pathogens (168–179) has stimulated considerable interest in enlisting the approach to develop effective and long-lasting antiparasite vaccines; these include vaccines that target the parasitic helminths, which are the cause of considerable morbidity and infect more than a quarter of the global population, the majority of whom live in extreme poverty (237).
Sitting at the top of the list, arguably, is a vaccine against schistosomiasis, although limitations in the development and production of an mRNA vaccine will require considerable future exploration. This disease continues to be a poverty-promoting, stigmatizing disease occurring mainly in the poor rural areas of developing countries. Indeed, no human or animal schistosomiasis vaccine is available, although the journal Science ranked an antischistosome vaccine as one of the top 10 vaccines that are urgently needed to improve global public health (238). To date, whereas a considerable number of antischistosome vaccine candidates have been identified and tested, very few have proceeded to clinical trials, since the level of protective immunity induced was insufficient. What is urgently required is a revolutionary and effective antischistosome vaccine platform to foster the development and testing of vaccine candidates at high throughput via cutting-edge, novel technology. By taking advantage of the mRNA vaccine approach which has proven so successful in the development of the highly effective COVID mRNA vaccines, together with the use of suitably appropriate animal models that will allow evaluation of the protective efficacy of candidate vaccines and determination of immune correlates of protection, this can be achieved. Antiparasite mRNA vaccines provide a clear prospect for improving the health conditions of human and animals in areas of endemicity and preventing exposure of travelers and military personnel to infection, all of which are potential market populations. Further refinement and optimization of the multivalent mRNA vaccine technology against schistosomiasis and other parasitic diseases could result in significant improvements in the accessibility of advanced vaccine techniques and the deployment of effective vaccines in the most parasite-affected regions globally.
ACKNOWLEDGMENTS
Our studies on schistosomiasis and other NTDs received support from an NHMRC (National Health and Medical Research Council) of Australia Investigator Grant to D.P.M. (APP1194462) and QIMR Berghofer Medical Research Institute seed grants (2021 and 2022) to H.Y. C.W.P. and H.A.-W. acknowledge financial research support from the NHMRC, Medical Research Future Fund (MRFF), mRNA Victoria, and Monash University for their mRNA research.
We declare that we have no conflicts of interest.
Biographies

Hong You, B.Sc., Ph.D., is a Senior Research Officer at QIMR Berghofer Medical Research Institute and Adjunct Associate Professor at University of Queensland. Her research is focused on identification/characterization of vaccine or drug targets against schistosome infections by exploiting new, cutting-edge technologies for gene functional studies, including CRISPR-based systems (CRISPR/Cas9, Cas13, and CRISPR interference/activation), transcriptomics, proteomics, and RNA interference. Her research also extends to development of new-generation point-of-care diagnostics tools (by using CRISPR-based system) for schistosomiasis. She has published 54 peer-reviewed articles, including 21 articles highly relevant for developing effective vaccines against schistosome infection. Dr. You has accumulated extensive experience and skills in performing immunological assessment of vaccine efficacy in animal vaccine/challenge trials both in the laboratory and in the field.

Malcolm K. Jones is Professor of Parasitology in the School of Veterinary Science at University of Queensland and an Affiliate Scientist at QIMR Berghofer Medical Research Institute in Brisbane, Australia. His research interests lie in molecular and cell biological approaches to understanding the biology and control of pathogenic helminth (worm) infections of humans and animals, notably schistosomiasis, echinococcosis, and angiostrongyliasis. Professor Jones has special interests in applications of advanced microscopy methods to investigating complex biological questions and has experience with many analytical tools, including electron microscopy, confocal microscopy, laser microdissection microscopy, and X-ray fluorescence microscopy. Professor Jones is President of the International Federation for Tropical Medicine and is Past President and a Fellow of the Australian Society for Parasitology. Professor Jones has authored over 240 scientific publications in the fields of parasitology, microscopy, and virology. He is currently Editor in Chief of One Health.

Catherine A. Gordon, B.Sc., Ph.D., is a senior postdoctoral researcher at the QIMR Berghofer Medical Research Institute and lecturer in parasitology at the University of Queensland and the Queensland University of Technology. Her research is focused on using molecular tools to understand the biology and epidemiology of a range of parasitic diseases, including schistosomiasis, soil-transmitted helminths, and intestinal protozoa. She is particularly focused on developing rapid point-of-care diagnostics for use in the field where these parasitic diseases occur in order to make rapid treatment possible and to acquire accurate prevalence data to better understand epidemiology.

Alexa E. Arganda holds a bachelor’s degree in advanced science (biomedical science) at the University of Queensland, graduating with class I honors in 2020. She completed her honors project at Mater Research Institute, with her thesis entitled “Investigating the Effects of IL-22 on Different Subsets of Intestinal Epithelial Cells under Homeostasis.” Her research background and interests pertain specifically to the fields of immunology, infectious disease, and vaccine development. She is now working as a reporter with The Medical Republic and has written and proofread for the limbic. She has also produced media releases and product descriptions for pharmaceuticals.

Pengfei Cai, B.Sc., Ph.D., is a Senior Research Officer at QIMRB and Adjunct Associate Professor at University of Queensland and Queensland University of Technology. He received his Ph.D. from Peking Union Medical College (PUMC), China, in 2008. He worked as a research assistant in the Laboratory of Parasitology, Institute of Pathogen Biology, Chinese Academy of Medical Science (CAMS) from 2008 to 2013. He has researched parasitic helminths, with a particular focus on schistosomiasis, for the past two decades. He has developed considerable expertise in immunology, molecular biology, omics, diagnostics, and field-based studies. His current research aims to develop innovative point-of-care diagnostic tools for schistosomiasis, and he also focuses on the use of helminth-derived products for treating/preventing immunological disorders. He has established productive collaborations with scientists in China, the Philippines, Madagascar, and Uganda. He has published over 60 peer-reviewed articles.

Harry Al-Wassiti is an early career innovator (ECI) at the Monash Institute of Pharmaceutical Sciences (MIPS) with expertise in nucleic acid development and delivery. Dr. Al-Wassiti is an inventor of a number of key nucleic acid technologies and has worked collaboratively to develop mRNA research, products, and intellectual properties for various therapeutic applications, including vaccines. In addition to working closely with industry, he has developed products that were translated into human clinical trials. He has a growing research profile career investigating the role of novel delivery vehicles.

Colin W. Pouton is Professor of Pharmaceutical Biology at the Monash Institute of Pharmaceutical Sciences, Melbourne, Australia. Professor Pouton contributes to the research fields of nucleic acid delivery, stem cell technology, drug delivery, and vaccine design. During the COVID-19 pandemic, his research group applied their experience in nucleic acid delivery to design and deliver candidate mRNA COVID-19 vaccines. One of the vaccine candidates progressed to phase 1 clinical trials, the first Australian mRNA product to enter the clinic. The group is now engaged in a range of collaborations to evaluate new mRNA products as vaccines or therapeutics.

Donald P. McManus, B.Sc., Ph.D., D.Sc., was a National Health and Medical Research Senior Leadership Fellow, and Senior Principal Research Fellow and Senior Scientist at QIMR Berghofer Medical Research Institute, Professor of Tropical Health, University of Queensland, and Professor, Australian National University. He researched the molecular biology, immunology, diagnosis, and epidemiology of parasitic worms. Professor McManus published over 650 articles, cited more than 38,000 times. He was an Honorary International Fellow of the American Society of Tropical Medicine and Hygiene, honorary member of the American and British Societies of Parasitologists, and Fellow of the Royal Society of Biology (United Kingdom) and the Australian Academy of Health and Medical Sciences. He was recipient of the Ralph Doherty QIMR Berghofer Prize for Outstanding Achievement and Leadership in Medical Research, the 2018 Sornchai Looareesuwan Medal “for outstanding achievements in experimental and clinical topical medicine research,” and the 2020 NHMRC Peter Doherty Investigator Grant Award for the top ranked application in the Leadership category.
Footnotes
[This article was published on 10 January 2023 with incorrect information in a sentence in the “mRNA vaccines” paragraph in the “Current Vaccine Development Approaches against Parasitic Infections” section. The sentence was updated in the current version, posted on 23 March 2023.]
REFERENCES
- 1.Standaert B, Rappuoli R. 2017. Towards a more comprehensive approach for a total economic assessment of vaccines? 1. The building blocks for a health economic assessment of vaccination. J Mark Access Health Policy 5:1335162. 10.1080/20016689.2017.1335162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin S. 2014. History of vaccination. Proc Natl Acad Sci USA 111:12283–12287. 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenner E. 1798. An inquiry into the causes and effects of the variolae vaccinae: a disease discovered in some of the western counties of England, particularly Gloucestershire, and known by the name of the cow pox. Samuel Cooley, Publisher, Springfield, MA. [Google Scholar]
- 4.Bloom DE, Black S, Salisbury D, Rappuoli R. 2018. Antimicrobial resistance and the role of vaccines. Proc Natl Acad Sci USA 115:12868–12871. 10.1073/pnas.1717157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollingsworth RE, Jansen K. 2019. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 4:7. 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottazzi M, Hotez P. 2013. Vaccine Nation: 10 most important diseases without a licensed vaccine. https://blogs.bcm.edu/2013/09/03/vaccine-nation-10-most-important-diseases-without-a-licensed-vaccine/. Accessed 16 August 2013.
- 7.Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. 2021. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine 39:2190–2200. 10.1016/j.vaccine.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heine A, Juranek S, Brossart P. 2021. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer 20:52. 10.1186/s12943-021-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagigi A, Lore K. 2021. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines (Basel) 9:61. 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Maruggi G, Shan H, Li J. 2019. Advances in mRNA vaccines for infectious diseases. Front Immunol 10:594. 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley EA, Pyae Phyo A, Woodrow CJ. 2018. Malaria. Lancet 391:1608–1621. 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 12.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. 2002. Schistosomiasis. N Engl J Med 346:1212–1220. 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 13.Tomazic ML, Marugan-Hernandez V, Rodriguez AE. 2021. Next-generation technologies and systems biology for the design of novel vaccines against apicomplexan parasites. Front Vet Sci 8:800361. 10.3389/fvets.2021.800361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zawawi A, Else KJ. 2020. Soil-transmitted helminth vaccines: are we getting closer? Front Immunol 11:576748. 10.3389/fimmu.2020.576748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JW, Lagniton PNP, Liu Y, Xu RH. 2021. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci 17:1446–1460. 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molehin AJ, McManus DP, You H. 2022. Vaccines for human schistosomiasis: recent progress, new developments and future prospects. Int J Mol Sci 23:2255. 10.3390/ijms23042255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You H, McManus DP. 2015. Vaccines and diagnostics for zoonotic schistosomiasis japonica. Parasitology 142:271–289. 10.1017/S0031182014001310. [DOI] [PubMed] [Google Scholar]
- 18.McManus DP, Bergquist R, Cai P, Ranasinghe S, Tebeje BM, You H. 2020. Schistosomiasis—from immunopathology to vaccines. Semin Immunopathol 42:355–371. 10.1007/s00281-020-00789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meeusen EN, Piedrafita D. 2003. Exploiting natural immunity to helminth parasites for the development of veterinary vaccines. Int J Parasitol 33:1285–1290. 10.1016/s0020-7519(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 20.Tebeje BM, Harvie M, You H, Loukas A, McManus DP. 2016. Schistosomiasis vaccines: where do we stand? Parasit Vectors 9:528. 10.1186/s13071-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nussenzweig RS, Vanderberg J, Most H, Orton C. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of plasmodium berghei. Nature 216:160–162. 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 22.Clyde DF, Most H, McCarthy VC, Vanderberg JP. 1973. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 266:169–177. 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Nunes-Cabaco H, Moita D, Prudencio M. 2022. Five decades of clinical assessment of whole-sporozoite malaria vaccines. Front Immunol 13:977472. 10.3389/fimmu.2022.977472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, Chakravarty S, Gunasekera A, Chattopadhyay R, Li M, Stafford R, Ahumada A, Epstein JE, Sedegah M, Reyes S, Richie TL, Lyke KE, Edelman R, Laurens MB, Plowe CV, Sim BKL. 2010. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 6:97–106. 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 25.Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, Samake Y, Sissoko K, Mwakingwe-Omari A, Lane J, Imeru A, Mohan R, Thera I, Guindo CO, Dolo A, Niare K, Koita F, Niangaly A, Rausch KM, Zeguime A, Guindo MA, Bah A, Abebe Y, James ER, Manoj A, Murshedkar T, Kc N, Sim BKL, Billingsley PF, Richie TL, Hoffman SL, Doumbo O, Duffy PE. 2022. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis 22:377–389. 10.1016/S1473-3099(21)00332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jongo SA, Church LWP, Nchama V, Hamad A, Chuquiyauri R, Kassim KR, Athuman T, Deal A, Natasha KC, Mtoro A, Mpina M, Nyakarungu E, Bidjimi GO, Owono MA, Maye ERM, Mangue MEO, Okomo GNN, Pasialo BEN, Mandumbi DMO, Mikue MAL, Mochomuemue FL, Obono MO, Besaha JCM, Bijeri JR, Abegue GM, Veri YR, Bela IT, Chochi FC, Lima Sanchez JE, Pencelli V, Gayozo G, Nlang J, Schindler T, James ER, Abebe Y, Lemiale L, Stabler TC, Murshedkar T, Chen MC, Schwabe C, Ratsirarson J, Rivas MR, Ayekaba MO, Milang DVN, Falla CC, Phiri WP, Garcia GA, Maas CD, Nlavo BM, Tanner M, et al. 2022. Multi-dose priming regimens of PfSPZ vaccine: safety and efficacy against controlled human malaria infection in Equatoguinean adults. Am J Trop Med Hyg 106:1215–1226. 10.4269/ajtmh.21-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oneko M, Steinhardt LC, Yego R, Wiegand RE, Swanson PA, Kc N, Akach D, Sang T, Gutman JR, Nzuu EL, Dungani A, Kim Lee Sim B, Oloo PN, Otieno K, Bii DK, Billingsley PF, James ER, Kariuki S, Samuels AM, Jongo S, Chebore W, Abdulla S, Daubenberger C, Mpina M, Styers D, Potter GE, Abarbanell G, Richie TL, Hoffman SL, Seder RA. 2021. Safety, immunogenicity and efficacy of PfSPZ Vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med 27:1636–1645. 10.1038/s41591-021-01470-y. [DOI] [PubMed] [Google Scholar]
- 28.Mutapi F, Billingsley PF, Secor WE. 2013. Infection and treatment immunizations for successful parasite vaccines. Trends Parasitol 29:135–141. 10.1016/j.pt.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, Loukas A, Petri W, Reed S, Valenzuela JG, Hotez PJ. 2011. Vaccines to combat the neglected tropical diseases. Immunol Rev 239:237–270. 10.1111/j.1600-065X.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roestenberg M, Bijker EM, Sim BKL, Billingsley PF, James ER, Bastiaens GJH, Teirlinck AC, Scholzen A, Teelen K, Arens T, van der Ven A, Gunasekera A, Chakravarty S, Velmurugan S, Hermsen CC, Sauerwein RW, Hoffman SL. 2013. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 88:5–13. 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lightowlers MW. 1996. Vaccination against cestode parasites. Int J Parasitol 26:819–824. 10.1016/s0020-7519(96)80048-8. [DOI] [PubMed] [Google Scholar]
- 32.Abuzeid AMI, Zhou X, Huang Y, Li G. 2020. Twenty-five-year research progress in hookworm excretory/secretory products. Parasit Vectors 13:136. 10.1186/s13071-020-04010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harnett W. 2014. Secretory products of helminth parasites as immunomodulators. Mol Biochem Parasitol 195:130–136. 10.1016/j.molbiopara.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Coakley G, McCaskill JL, Borger JG, Simbari F, Robertson E, Millar M, Harcus Y, McSorley HJ, Maizels RM, Buck AH. 2017. Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep 19:1545–1557. 10.1016/j.celrep.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sanchez del Pino MM, Munoz-Antoli C, Toledo R, Bernal D. 2012. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One 7:e45974. 10.1371/journal.pone.0045974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lightowlers MW. 2006. Cestode vaccines: origins, current status and future prospects. Parasitology 133(Suppl):S27–S42. 10.1017/S003118200600179X. [DOI] [PubMed] [Google Scholar]
- 37.Carrera-Bravo C, Koh EY, Tan KSW. 2021. The roles of parasite-derived extracellular vesicles in disease and host-parasite communication. Parasitol Int 83:102373. 10.1016/j.parint.2021.102373. [DOI] [PubMed] [Google Scholar]
- 38.Olajide JS, Cai J. 2020. Perils and promises of pathogenic protozoan extracellular vesicles. Front Cell Infect Microbiol 10:371. 10.3389/fcimb.2020.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Chen J, Zheng J. 2022. The state of the art of extracellular vesicle research in protozoan infection. Front Genet 13:941561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, Grau GE, Riley EM, de Souza JB. 2010. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog 6:e1000744. 10.1371/journal.ppat.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos FM, Franklin BS, Teixeira-Carvalho A, Filho AL, de Paula SC, Fontes CJ, Brito CF, Carvalho LH. 2010. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J 9:327. 10.1186/1475-2875-9-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira ACS, Rezende L, Gorshkov V, Melo-Braga MN, Verano-Braga T, Fernandes-Braga W, Guadalupe JLM, de Menezes GB, Kjeldsen F, de Andrade HM, Andrade LO. 2021. Biological and molecular effects of Trypanosoma cruzi residence in a LAMP-deficient intracellular environment. Front Cell Infect Microbiol 11:788482. 10.3389/fcimb.2021.788482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Li Z, Shen J, Liu Z, Liang J, Wu X, Sun X, Wu Z. 2015. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage. Parasitol Res 114:1865–1873. 10.1007/s00436-015-4373-7. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Yu Z, Wan S, Wu F, Chen W, Zhang B, Lin D, Liu J, Xie H, Sun X, Wu Z. 2017. Exosomes derived from dendritic cells treated with Schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol 8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atayde VD, Hassani K, da Silva Lira Filho A, Borges AR, Adhikari A, Martel C, Olivier M. 2016. Leishmania exosomes and other virulence factors: impact on innate immune response and macrophage functions. Cell Immunol 309:7–18. 10.1016/j.cellimm.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE. 2010. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022. 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 47.Bennett APS, de la Torre-Escudero E, Oliver NAM, Huson KM, Robinson MW. 2020. The cellular and molecular origins of extracellular vesicles released by the helminth pathogen, Fasciola hepatica. Int J Parasitol 50:671–683. 10.1016/j.ijpara.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Siles-Lucas M, Sanchez-Ovejero C, Gonzalez-Sanchez M, Gonzalez E, Falcon-Perez JM, Boufana B, Fratini F, Casulli A, Manzano-Roman R. 2017. Isolation and characterization of exosomes derived from fertile sheep hydatid cysts. Vet Parasitol 236:22–33. 10.1016/j.vetpar.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 49.Eichenberger RM, Sotillo J, Loukas A. 2018. Immunobiology of parasitic worm extracellular vesicles. Immunol Cell Biol 96:704–713. 10.1111/imcb.12171. [DOI] [PubMed] [Google Scholar]
- 50.Wilson RA, Jones MK. 2021. Fifty years of the schistosome tegument: discoveries, controversies, and outstanding questions. Int J Parasitol 51:1213–1232. 10.1016/j.ijpara.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z, Wang L, Li J, Wang L, Wu Z, Sun X. 2018. Extracellular vesicle-mediated communication within host-parasite interactions. Front Immunol 9:3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nascimento IP, Leite LC. 2012. Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res 45:1102–1111. 10.1590/s0100-879x2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michel ML, Tiollais P. 2010. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris) 58:288–295. 10.1016/j.patbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Shrestha MP, Scott RM, Joshi DM, Mammen MP, Jr, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, David MP, Seriwatana J, Vaughn DW, Safary A, Endy TP, Innis BL. 2007. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 356:895–903. 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 55.Noon JB, Aroian RV. 2017. Recombinant subunit vaccines for soil-transmitted helminths. Parasitology 144:1845–1870. 10.1017/S003118201700138X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez O, Batista-Duharte A, Gonzalez E, Zayas C, Balboa J, Cuello M, Cabrera O, Lastre M, Schijns VE. 2012. Human prophylactic vaccine adjuvants and their determinant role in new vaccine formulations. Braz J Med Biol Res 45:681–692. 10.1590/s0100-879x2012007500067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephenson R, You H, McManus DP, Toth I. 2014. Schistosome vaccine adjuvants in preclinical and clinical research. Vaccines (Basel) 2:654–685. 10.3390/vaccines2030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laurens MB. 2020. RTS,S/AS01 vaccine (Mosquirix): an overview. Hum Vaccin Immunother 16:480–489. 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King A. 2019. Building a better malaria vaccine. Nature 575:S51–S54. 10.1038/d41586-019-03639-5. [DOI] [PubMed] [Google Scholar]
- 60.RTS,S Clinical Trials Partnership. 2015. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386:31–45. 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377:1770–1776. 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 62.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BGNO, Doucka Y, Flamen A, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D’Alessandro U, Sorgho H, Valea I, Tahita MC, Kaboré W, Ouédraogo S, Sandrine Y, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Odero C. RTS,S Clinical Trials Partnership, et al. 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365:1863–1875. [DOI] [PubMed] [Google Scholar]
- 63.Johnson KS, Harrison GB, Lightowlers MW, O’Hoy KL, Cougle WG, Dempster RP, Lawrence SB, Vinton JG, Heath DD, Rickard MD. 1989. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature 338:585–587. 10.1038/338585a0. [DOI] [PubMed] [Google Scholar]
- 64.Lightowlers MW, Rolfe R, Gauci CG. 1996. Taenia saginata: vaccination against cysticercosis in cattle with recombinant oncosphere antigens. Exp Parasitol 84:330–338. 10.1006/expr.1996.0121. [DOI] [PubMed] [Google Scholar]
- 65.Flisser A, Gauci CG, Zoli A, Martinez-Ocana J, Garza-Rodriguez A, Dominguez-Alpizar JL, Maravilla P, Rodriguez-Canul R, Avila G, Aguilar-Vega L, Kyngdon C, Geerts S, Lightowlers MW. 2004. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect Immun 72:5292–5297. 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez AE, Gauci CG, Barber D, Gilman RH, Tsang VC, Garcia HH, Verastegui M, Lightowlers MW. 2005. Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg 72:837–839. [PubMed] [Google Scholar]
- 67.Heath DD, Lawrence SB. 1996. Antigenic polypeptides of Echinococcus granulosus oncospheres and definition of protective molecules. Parasite Immunol 18:347–357. 10.1046/j.1365-3024.1996.d01-114.x. [DOI] [PubMed] [Google Scholar]
- 68.King A. 2019. Only vaccines can eradicate parasitic worms. Nature 575:S54. 10.1038/d41586-019-03643-9. [DOI] [PubMed] [Google Scholar]
- 69.Diemert D, Campbell D, Brelsford J, Leasure C, Li G, Peng J, Zumer M, Younes N, Bottazzi ME, Mejia R, Pritchard DI, Hawdon JM, Bethony JM. 2018. Controlled human hookworm infection: accelerating human hookworm vaccine development. Open Forum Infect Dis 5:ofy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diemert DJ, Freire J, Valente V, Fraga CG, Talles F, Grahek S, Campbell D, Jariwala A, Periago MV, Enk M, Gazzinelli MF, Bottazzi ME, Hamilton R, Brelsford J, Yakovleva A, Li G, Peng J, Correa-Oliveira R, Hotez P, Bethony J. 2017. Safety and immunogenicity of the Na-GST-1 hookworm vaccine in Brazilian and American adults. PLoS Negl Trop Dis 11:e0005574. 10.1371/journal.pntd.0005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boulanger D, Warter A, Sellin B, Lindner V, Pierce RJ, Chippaux JP, Capron A. 1999. Vaccine potential of a recombinant glutathione S-transferase cloned from Schistosoma haematobium in primates experimentally infected with an homologous challenge. Vaccine 17:319–326. 10.1016/s0264-410x(98)00202-3. [DOI] [PubMed] [Google Scholar]
- 72.Riveau G, Schacht AM, Dompnier JP, Deplanque D, Seck M, Waucquier N, Senghor S, Delcroix-Genete D, Hermann E, Idris-Khodja N, Levy-Marchal C, Capron M, Capron A. 2018. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: a phase 3 randomized, controlled trial in Senegalese children. PLoS Negl Trop Dis 12:e0006968. 10.1371/journal.pntd.0006968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santini-Oliveira M, Coler RN, Parra J, Veloso V, Jayashankar L, Pinto PM, Ciol MA, Bergquist R, Reed SG, Tendler M. 2016. Schistosomiasis vaccine candidate Sm14/GLA-SE: phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine 34:586–594. 10.1016/j.vaccine.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 74.Keitel WA, Potter GE, Diemert D, Bethony J, El Sahly HM, Kennedy JK, Patel SM, Plieskatt JL, Jones W, Deye G, Bottazzi ME, Hotez PJ, Atmar RL. 2019. A phase 1 study of the safety, reactogenicity, and immunogenicity of a Schistosoma mansoni vaccine with or without glucopyranosyl lipid A aqueous formulation (GLA-AF) in healthy adults from a non-endemic area. Vaccine 37:6500–6509. 10.1016/j.vaccine.2019.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panzner U, Excler J-L, Kim JH, Marks F, Carter D, Siddiqui AA. 2021. Recent advances and methodological considerations on vaccine candidates for human schistosomiasis. Front Trop Dis 2. 10.3389/fitd.2021.719369. [DOI] [Google Scholar]
- 76.Camargo EP, Gazzinelli RT, Morel CM, Precioso AR. 2022. Why do we still have not a vaccine against Chagas disease? Mem Inst Oswaldo Cruz 117:e200314. 10.1590/0074-02760200314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Versteeg L, Almutairi MM, Hotez PJ, Pollet J. 2019. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines 7:122. 10.3390/vaccines7040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhan B, Bottazzi ME, Hotez PJ, Lustigman S. 2022. Advancing a human onchocerciasis vaccine from antigen discovery to efficacy studies against natural infection of cattle with Onchocerca ochengi. Front Cell Infect Microbiol 12:869039. 10.3389/fcimb.2022.869039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McManus DP. 2020. Recent progress in the development of liver fluke and blood fluke vaccines. Vaccines (Basel) 8:553. 10.3390/vaccines8030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaiyadet S, Sotillo J, Krueajampa W, Thongsen S, Brindley PJ, Sripa B, Loukas A, Laha T. 2019. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS Negl Trop Dis 13:e0007450. 10.1371/journal.pntd.0007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thi Phung L, Chaiyadet S, Hongsrichan N, Sotillo J, Dinh Thi Dieu H, Quang Tran C, Brindley PJ, Loukas A, Laha T. 2020. Partial protection with a chimeric tetraspanin-leucine aminopeptidase subunit vaccine against Opisthorchis viverrini infection in hamsters. Acta Trop 204:105355. 10.1016/j.actatropica.2020.105355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Chen W, Huang Y, Sun J, Men J, Liu H, Luo F, Guo L, Lv X, Deng C, Zhou C, Fan Y, Li X, Huang L, Hu Y, Liang C, Hu X, Xu J, Yu X. 2011. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol 12:R107. 10.1186/gb-2011-12-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang D, Korhonen PK, Gasser RB, Young ND. 2018. Improved genomic resources and new bioinformatic workflow for the carcinogenic parasite Clonorchis sinensis: biotechnological implications. Biotechnol Adv 36:894–904. 10.1016/j.biotechadv.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Qian MB, Utzinger J, Keiser J, Zhou XN. 2016. Clonorchiasis. Lancet 387:800–810. 10.1016/S0140-6736(15)60313-0. [DOI] [PubMed] [Google Scholar]
- 85.Ulmer JB, Liu MA. 2021. Path to success and future impact of nucleic acid vaccines: DNA and mRNA. Mol Front J 5:38–57. 10.1142/S2529732521400022. [DOI] [Google Scholar]
- 86.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465–1468. 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 87.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745–1749. 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 88.Gary EN, Weiner DB. 2020. DNA vaccines: prime time is now. Curr Opin Immunol 65:21–27. 10.1016/j.coi.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, Boyer J, Park YK, Trottier S, Remigio C, Krieger D, Spruill SE, Bagarazzi M, Kobinger GP, Weiner DB, Maslow JN. 2021. Safety and Immunogenicity of an anti-Zika virus DNA vaccine. N Engl J Med 385:e35. 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tebas P, Kraynyak KA, Patel A, Maslow JN, Morrow MP, Sylvester AJ, Knoblock D, Gillespie E, Amante D, Racine T, McMullan T, Jeong M, Roberts CC, Park YK, Boyer J, Broderick KE, Kobinger GP, Bagarazzi M, Weiner DB, Sardesai NY, White SM. 2019. Intradermal SynCon(R) Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis 220:400–410. 10.1093/infdis/jiz132. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Bi Y, Xiao H, Yao Y, Liu X, Hu Z, Duan J, Yang Y, Li Z, Li Y, Zhang H, Ding C, Yang J, Li H, He Z, Liu L, Hu G, Liu S, Che Y, Wang S, Li Q, Lu S, Cun W. 2021. A novel DNA and protein combination COVID-19 vaccine formulation provides full protection against SARS-CoV-2 in rhesus macaques. Emerg Microbes Infect 10:342–355. 10.1080/22221751.2021.1887767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Motamedi H, Ari MM, Dashtbin S, Fathollahi M, Hossainpour H, Alvandi A, Moradi J, Abiri R. 2021. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int Immunopharmacol 96:107763. 10.1016/j.intimp.2021.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sheridan C. 2021. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat Biotechnol 39:1479–1482. 10.1038/d41587-021-00023-5. [DOI] [PubMed] [Google Scholar]
- 94.Khobragade A, Bhate S, Ramaiah V, Deshpande S, Giri K, Phophle H, Supe P, Godara I, Revanna R, Nagarkar R, Sanmukhani J, Dey A, Rajanathan TMC, Kansagra K, Koradia P. ZyCo VDpSIG. 2022. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 399:1313–1321. 10.1016/S0140-6736(22)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abbasi J. 2021. India’s New COVID-19 DNA vaccine for adolescents and adults is a first. JAMA 326:1365. 10.1001/jama.2021.16625. [DOI] [PubMed] [Google Scholar]
- 96.Thorarinsson R, Wolf JC, Inami M, Phillips L, Jones G, Macdonald AM, Rodriguez JF, Sindre H, Skjerve E, Rimstad E, Evensen O. 2021. Effect of a novel DNA vaccine against pancreas disease caused by salmonid alphavirus subtype 3 in Atlantic salmon (Salmo salar). Fish Shellfish Immunol 108:116–126. 10.1016/j.fsi.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Penny MA, Camponovo F, Chitnis N, Smith TA, Tanner M. 2020. Future use-cases of vaccines in malaria control and elimination. Parasite Epidemiol Control 10:e00145. 10.1016/j.parepi.2020.e00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo K, Zhang H, Zavala F, Biragyn A, Espinosa DA, Markham RB. 2014. Fusion of antigen to a dendritic cell targeting chemokine combined with adjuvant yields a malaria DNA vaccine with enhanced protective capabilities. PLoS One 9:e90413. 10.1371/journal.pone.0090413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez-Rodrigo A, D SD, Ribeiro PAF, Roatt BM, Mas A, Carrion J, Coelho EAF, Dominguez-Bernal G. 2019. Immunization with the HisAK70 DNA vaccine induces resistance against Leishmania amazonensis infection in BALB/c mice. Vaccines (Basel) 7:183. 10.3390/vaccines7040183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wiśniewski M, Jaros S, Bąska P, Cappello M, Długosz E, Wędrychowicz H. 2016. Hamsters vaccinated with Ace-mep-7 DNA vaccine produced protective immunity against Ancylostoma ceylanicum infection. Exp Parasitol 163:1–7. 10.1016/j.exppara.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Li MJ, Lei JH, Wang T, Lu SJ, Guan F, Liu WQ, Li YL. 2011. Cimetidine enhances the protective effect of GST DNA vaccine against Schistosoma japonicum. Exp Parasitol 128:427–432. 10.1016/j.exppara.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 102.Williams GM, Li YS, Gray DJ, Zhao ZY, Harn DA, Shollenberger LM, Li SM, Yu X, Feng Z, Guo JG, Zhou J, Dong YL, Li Y, Guo B, Driguez P, Harvie M, You H, Ross AG, McManus DP. 2019. Field testing integrated interventions for schistosomiasis elimination in the People’s Republic of China: outcomes of a multifactorial cluster. Front Immunol 10:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steisslinger V, Korten S, Brattig NW, Erttmann KD. 2015. DNA vaccine encoding the moonlighting protein Onchocerca volvulus glyceraldehyde-3-phosphate dehydrogenase (Ov-GAPDH) leads to partial protection in a mouse model of human filariasis. Vaccine 33:5861–5867. 10.1016/j.vaccine.2015.07.110. [DOI] [PubMed] [Google Scholar]
- 104.Kutzler MA, Weiner DB. 2008. DNA vaccines: ready for prime time? Nat Rev Genet 9:776–788. 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Belakova J, Horynova M, Krupka M, Weigl E, Raska M. 2007. DNA vaccines: are they still just a powerful tool for the future? Arch Immunol Ther Exp (Warsz) 55:387–398. 10.1007/s00005-007-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Radosevic K, Rodriguez A, Lemckert A, Goudsmit J. 2009. Heterologous prime-boost vaccinations for poverty-related diseases: advantages and future prospects. Expert Rev Vaccines 8:577–592. 10.1586/erv.09.14. [DOI] [PubMed] [Google Scholar]
- 107.Saade F, Petrovsky N. 2012. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 11:189–209. 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC. C4591001 Clinical Trial Group. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pardi N, Hogan MJ, Porter FW, Weissman D. 2018. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov 17:261–279. 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mallory KL, Taylor JA, Zou X, Waghela IN, Schneider CG, Sibilo MQ, Punde NM, Perazzo LC, Savransky T, Sedegah M, Dutta S, Janse CJ, Pardi N, Lin PJC, Tam YK, Weissman D, Angov E. 2021. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines 6:84. 10.1038/s41541-021-00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baeza Garcia A, Siu E, Sun T, Exler V, Brito L, Hekele A, Otten G, Augustijn K, Janse CJ, Ulmer JB, Bernhagen J, Fikrig E, Geall A, Bucala R. 2018. Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat Commun 9:2714. 10.1038/s41467-018-05041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duthie MS, Van Hoeven N, MacMillen Z, Picone A, Mohamath R, Erasmus J, Hsu F-C, Stinchcomb DT, Reed SG. 2018. Heterologous immunization with defined RNA and subunit vaccines enhances T cell responses that protect against Leishmania donovani. Front Immunol 9:2420–2420. 10.3389/fimmu.2018.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo F, Zheng L, Hu Y, Liu S, Wang Y, Xiong Z, Hu X, Tan F. 2017. Induction of protective immunity against Toxoplasma gondii in mice by nucleoside triphosphate hydrolase-II (NTPase-II) self-amplifying RNA vaccine encapsulated in lipid nanoparticle (LNP). Front Microbiol 8:605–605. 10.3389/fmicb.2017.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sajid A, Matias J, Arora G, Kurokawa C, DePonte K, Tang X, Lynn G, Wu MJ, Pal U, Strank NO, Pardi N, Narasimhan S, Weissman D, Fikrig E. 2021. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci Transl Med 13:eabj9827. 10.1126/scitranslmed.abj9827. [DOI] [PubMed] [Google Scholar]
- 115.Kariko K, Buckstein M, Ni H, Weissman D. 2005. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23:165–175. 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 116.Pilkington EH, Suys EJA, Trevaskis NL, Wheatley AK, Zukancic D, Algarni A, Al-Wassiti H, Davis TP, Pouton CW, Kent SJ, Truong NP. 2021. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater 131:16–40. 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaudhary N, Weissman D, Whitehead KA. 2021. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov 20:817–838. 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dolgin E. 2021. The tangled history of mRNA vaccines. Nature 597:318–324. 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 119.Rinaldi A. 2020. RNA to the rescue: RNA is one of the most promising targets for drug development given its wide variety of uses. EMBO Rep 21:e51013. 10.15252/embr.202051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. 2020. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 5:11. 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang Q, Ji K, Tian S, Wang F, Huang B, Tong Z, Tan S, Hao J, Wang Q, Tan W, Gao GF, Yan J. 2021. A single-dose mRNA vaccine provides a long-term protection for hACE2 transgenic mice from SARS-CoV-2. Nat Commun 12:776. 10.1038/s41467-021-21037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flemming A. 2012. Vaccines: self-amplifying RNA in lipid nanoparticles: a next-generation vaccine? Nat Rev Drug Discov 11:748–749. 10.1038/nrd3854. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. 2021. mRNA vaccine: a potential therapeutic strategy. Mol Cancer 20:33. 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liehl P, Zuzarte-Luis V, Chan J, Zillinger T, Baptista F, Carapau D, Konert M, Hanson KK, Carret C, Lassnig C, Muller M, Kalinke U, Saeed M, Chora AF, Golenbock DT, Strobl B, Prudencio M, Coelho LP, Kappe SH, Superti-Furga G, Pichlmair A, Vigario AM, Rice CM, Fitzgerald KA, Barchet W, Mota MM. 2014. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med 20:47–53. 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jain A, Irizarry-Caro RA, McDaniel MM, Chawla AS, Carroll KR, Overcast GR, Philip NH, Oberst A, Chervonsky AV, Katz JD, Pasare C. 2020. T cells instruct myeloid cells to produce inflammasome-independent IL-1beta and cause autoimmunity. Nat Immunol 21:65–74. 10.1038/s41590-019-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ablasser A, Poeck H, Anz D, Berger M, Schlee M, Kim S, Bourquin C, Goutagny N, Jiang Z, Fitzgerald KA, Rothenfusser S, Endres S, Hartmann G, Hornung V. 2009. Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J Immunol 182:6824–6833. 10.4049/jimmunol.0803001. [DOI] [PubMed] [Google Scholar]
- 127.Chen N, Xia P, Li S, Zhang T, Wang TT, Zhu J. 2017. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life 69:297–304. 10.1002/iub.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kato H, Oh SW, Fujita T. 2017. RIG-I-like receptors and type I interferonopathies. J Interferon Cytokine Res 37:207–213. 10.1089/jir.2016.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738. 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 130.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. 2009. Activation of innate immune antiviral responses by Nod2. Nat Immunol 10:1073–1080. 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kariko K, Muramatsu H, Ludwig J, Weissman D. 2011. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res 39:e142. 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Avci-Adali M, Behring A, Steinle H, Keller T, Krajeweski S, Schlensak C, Wendel HP. 2014. In vitro synthesis of modified mRNA for induction of protein expression in human cells. J Vis Exp 2014:e51943. 10.3791/51943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, Moody MA, Verkerke HP, Myles A, Willis E, LaBranche CC, Montefiori DC, Lobby JL, Saunders KO, Liao HX, Korber BT, Sutherland LL, Scearce RM, Hraber PT, Tombacz I, Muramatsu H, Ni H, Balikov DA, Li C, Mui BL, Tam YK, Krammer F, Kariko K, Polacino P, Eisenlohr LC, Madden TD, Hope MJ, Lewis MG, Lee KK, Hu SL, Hensley SE, Cancro MP, Haynes BF, Weissman D. 2018. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med 215:1571–1588. 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Basso K, Dalla-Favera R. 2015. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol 15:172–184. 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 135.Heyman B. 2000. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol 18:709–737. 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 136.Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ. 2016. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA 113:E6639–E6648. 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, Lundgreen KA, Reynaldi A, Khoury DS, Pattekar A, Gouma S, Kuri-Cervantes L, Hicks P, Dysinger S, Hicks A, Sharma H, Herring S, Korte S, Baxter AE, Oldridge DA, Giles JR, Weirick ME, McAllister CM, Awofolaju M, Tanenbaum N, Drapeau EM, Dougherty J, Long S, D’Andrea K, Hamilton JT, McLaughlin M, Williams JC, Adamski S, Kuthuru O, Frank I, Betts MR, Vella LA, Grifoni A, Weiskopf D, Sette A, Hensley SE, Davenport MP, Bates P, Luning Prak ET, Greenplate AR, Wherry EJ, et al. 2021. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374:abm0829. 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao Y, Gao GF. 2021. mRNA vaccines: a matter of delivery. EClinicalMedicine 32:100746. 10.1016/j.eclinm.2021.100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Armbruster N, Jasny E, Petsch B. 2019. Advances in RNA vaccines for preventive indications: a case study of a vaccine against rabies. Vaccines (Basel) 7:132. 10.3390/vaccines7040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jouneau L, Lefebvre DJ, Costa F, Romey A, Blaise-Boisseau S, Relmy A, Jaszczyszyn Y, Dard-Dascot C, Dejean S, Versille N, Guitton E, Hudelet P, Curet M, De Clercq K, Bakkali-Kassimi L, Zientara S, Klonjkowski B, Schwartz-Cornil I. 2020. The antibody response induced FMDV vaccines in sheep correlates with early transcriptomic responses in blood. NPJ Vaccines 5:1. 10.1038/s41541-019-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sullenger BA, Nair S. 2016. From the RNA world to the clinic. Science 352:1417–1420. 10.1126/science.aad8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pollard C, De Koker S, Saelens X, Vanham G, Grooten J. 2013. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol Med 19:705–713. 10.1016/j.molmed.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 143.Espeseth AS, Cejas PJ, Citron MP, Wang D, DiStefano DJ, Callahan C, Donnell GO, Galli JD, Swoyer R, Touch S, Wen Z, Antonello J, Zhang L, Flynn JA, Cox KS, Freed DC, Vora KA, Bahl K, Latham AH, Smith JS, Gindy ME, Ciaramella G, Hazuda D, Shaw CA, Bett AJ. 2020. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 5:16. 10.1038/s41541-020-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jia B, Jeon CO. 2016. High-throughput recombinant protein expression in Escherichia coli: current status and future perspectives. Open Biol 6:160196. 10.1098/rsob.160196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim SC, Sekhon SS, Shin WR, Ahn G, Cho BK, Ahn JY, Kim YH. 2022. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol Cell Toxicol 18:1–8. 10.1007/s13273-021-00171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Laczko D, Hogan MJ, Toulmin SA, Hicks P, Lederer K, Gaudette BT, Castano D, Amanat F, Muramatsu H, Oguin TH, III, Ojha A, Zhang L, Mu Z, Parks R, Manzoni TB, Roper B, Strohmeier S, Tombacz I, Arwood L, Nachbagauer R, Kariko K, Greenhouse J, Pessaint L, Porto M, Putman-Taylor T, Strasbaugh A, Campbell TA, Lin PJC, Tam YK, Sempowski GD, Farzan M, Choe H, Saunders KO, Haynes BF, Andersen H, Eisenlohr LC, Weissman D, Krammer F, Bates P, Allman D, Locci M, Pardi N. 2020. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53:724–732.E7. 10.1016/j.immuni.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zeng C, Zhang C, Walker PG, Dong Y. 2020. Formulation and delivery technologies for mRNA vaccines. Current Topics in Microbiology and Immunology. Springer, Berlin, Heidelberg. 10.1007/82_2020_217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Callaway E, Ledford H. 2021. How to redesign COVID vaccines so they protect against variants. Nature 590:15–16. 10.1038/d41586-021-00241-6. [DOI] [PubMed] [Google Scholar]
- 149.Chivukula S, Plitnik T, Tibbitts T, Karve S, Dias A, Zhang D, Goldman R, Gopani H, Khanmohammed A, Sarode A, Cooper D, Yoon H, Kim Y, Yan Y, Mundle ST, Groppo R, Beauvais A, Zhang J, Anosova NG, Lai C, Li L, Ulinski G, Piepenhagen P, DiNapoli J, Kalnin KV, Landolfi V, Swearingen R, Fu TM, DeRosa F, Casimiro D. 2021. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 6:153. 10.1038/s41541-021-00420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. 2020. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 12:102. 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu T, Liang Y, Huang L. 2021. Development and delivery systems of mRNA vaccines. Front Bioeng Biotechnol 9:718753. 10.3389/fbioe.2021.718753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pardi N, Hogan MJ, Weissman D. 2020. Recent advances in mRNA vaccine technology. Curr Opin Immunol 65:14–20. 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 153.Hou X, Zaks T, Langer R, Dong Y. 2021. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6:1078–1094. 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. COVE Study Group. 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Corbett KS, Swanson PA, II, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH. mRNA-1273 Study Group. 2020. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383:2427–2438. 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schlake T, Thess A, Fotin-Mleczek M, Kallen K-J. 2012. Developing mRNA-vaccine technologies. RNA Biol 9:1319–1330. 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, Yuan N, Hope M, Cullis P, Tam Y. 2007. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother 56:1251–1264. 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. 2016. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv 7:319–334. 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.CDC COVID-19 Response Team, Food and Drug Administration. 2020. Allegic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep 70:46–51. 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kadali RAK, Janagama R, Peruru S, Malayala SV. 2021. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 106:376–381. 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Banerji A, Wickner PG, Saff R, Stone CA, Jr, Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E, Blumenthal KG. 2021. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 9:1423–1437. 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Knop K, Hoogenboom R, Fischer D, Schubert US. 2010. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl 49:6288–6308. 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 163.Yang Q, Lai SK. 2015. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:655–677. 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abu Lila AS, Kiwada H, Ishida T. 2013. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release 172:38–47. 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 165.Rauch S, Jasny E, Schmidt KE, Petsch B. 2018. New vaccine technologies to combat outbreak situations. Front Immunol 9:1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. 2021. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci 110:997–1001. 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei-Haas M. 2021. Future COVID-19 vaccines might not have to be kept so cold. https://www.nationalgeographic.com/science/article/future-covid-19-vaccines-might-not-have-to-be-kept-so-cold. Accessed 1 May 2021. [Google Scholar]
- 168.Hekele A, Bertholet S, Archer J, Gibson DG, Palladino G, Brito LA, Otten GR, Brazzoli M, Buccato S, Bonci A, Casini D, Maione D, Qi Z-Q, Gill JE, Caiazza NC, Urano J, Hubby B, Gao GF, Shu Y, De Gregorio E, Mandl CW, Mason PW, Settembre EC, Ulmer JB, Craig Venter J, Dormitzer PR, Rappuoli R, Geall AJ. 2013. Rapidly produced SAM() vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect 2:e52. 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen K-J, Stitz L, Kramps T. 2012. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol 30:1210–1216. 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 170.Dolgin E. 2021. mRNA flu shots move into trials. Nat Rev Drug Discov 20:801–803. 10.1038/d41573-021-00176-7. [DOI] [PubMed] [Google Scholar]
- 171.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu J-S, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang Y-J, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Karikó K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D. 2017. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543:248–251. 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Erasmus JH, Archer J, Fuerte-Stone J, Khandhar AP, Voigt E, Granger B, Bombardi RG, Govero J, Tan Q, Durnell LA, Coler RN, Diamond MS, Crowe JE, Jr, Reed SG, Thackray LB, Carnahan RH, Van Hoeven N. 2020. Intramuscular delivery of replicon RNA encoding ZIKV-117 human monoclonal antibody protects against Zika virus infection. Mol Ther Methods Clin Dev 18:402–414. 10.1016/j.omtm.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kose N, Fox JM, Sapparapu G, Bombardi R, Tennekoon RN, de Silva AD, Elbashir SM, Theisen MA, Humphris-Narayanan E, Ciaramella G, Himansu S, Diamond MS, Crowe JE, Jr.. 2019. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci Immunol 4:eaaw6647. 10.1126/sciimmunol.aaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fernandez E, Diamond MS. 2017. Vaccination strategies against Zika virus. Curr Opin Virol 23:59–67. 10.1016/j.coviro.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pattnaik A, Sahoo BR, Pattnaik AK. 2020. Current status of Zika virus vaccines: successes and challenges. Vaccines (Basel) 8:266. 10.3390/vaccines8020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morris L. 2021. mRNA vaccines offer hope for HIV. Nat Med 27:2082–2084. 10.1038/s41591-021-01602-4. [DOI] [PubMed] [Google Scholar]
- 177.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547:222–226. 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 178.Schumacher TN, Schreiber RD. 2015. Neoantigens in cancer immunotherapy. Science 348:69–74. 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 179.Miao L, Zhang Y, Huang L. 2021. mRNA vaccine for cancer immunotherapy. Mol Cancer 20:41. 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S, Ploegh HL, Anderson DG. 2016. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci USA 113:E4133–E4142. 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eisen RJ, Eisen L. 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol 34:295–309. 10.1016/j.pt.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.You H, Gobert GN, Jones MK, Zhang W, McManus DP. 2011. Signalling pathways and the host-parasite relationship: putative targets for control interventions against schistosomiasis: signalling pathways and future anti-schistosome therapies. Bioessays 33:203–214. 10.1002/bies.201000077. [DOI] [PubMed] [Google Scholar]
- 183.You H, Stephenson RJ, Gobert GN, McManus DP. 2014. Revisiting glucose uptake and metabolism in schistosomes: new molecular insights for improved schistosomiasis therapies. Front Genet 5:176. 10.3389/fgene.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.You H, Cai P, Tebeje BM, Li Y, McManus DP. 2018. Schistosome vaccines for domestic animals. Trop Med Infect Dis 3:68. 10.3390/tropicalmed3020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schistosoma japonicum Genome S, Functional Analysis C. 2009. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 460:345–351. 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream M-A, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, et al. 2009. The genome of the blood fluke Schistosoma mansoni. Nature 460:352–358. 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, Li Y, Cantacessi C, Hall RS, Xu X, Chen F, Wu X, Zerlotini A, Oliveira G, Hofmann A, Zhang G, Fang X, Kang Y, Campbell BE, Loukas A, Ranganathan S, Rollinson D, Rinaldi G, Brindley PJ, Yang H, Wang J, Wang J, Gasser RB. 2012. Whole-genome sequence of Schistosoma haematobium. Nat Genet 44:221–225. 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 188.Oey H, Zakrzewski M, Gravermann K, Young ND, Korhonen PK, Gobert GN, Nawaratna S, Hasan S, Martinez DM, You H, Lavin M, Jones MK, Ragan MA, Stoye J, Oleaga A, Emery AM, Webster BL, Rollinson D, Gasser RB, McManus DP, Krause L. 2019. Whole-genome sequence of the bovine blood fluke Schistosoma bovis supports interspecific hybridization with S. haematobium. PLoS Pathog 15:e1007513. 10.1371/journal.ppat.1007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wendt GR, Collins JN, Pei J, Pearson MS, Bennett HM, Loukas A, Berriman M, Grishin NV, Collins JJ, III.. 2018. Flatworm-specific transcriptional regulators promote the specification of tegumental progenitors in Schistosoma mansoni. Elife 7:e33221. 10.7554/eLife.33221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.King M, Carson J, Stewart MT, Gobert GN. 2021. Revisiting the Schistosoma japonicum life cycle transcriptome for new insights into lung schistosomula development. Exp Parasitol 223:108080. 10.1016/j.exppara.2021.108080. [DOI] [PubMed] [Google Scholar]
- 191.Pearson MS, McManus DP, Smyth DJ, Lewis FA, Loukas A. 2005. In vitro and in silico analysis of signal peptides from the human blood fluke, Schistosoma mansoni. FEMS Immunol Med Microbiol 45:201–211. 10.1016/j.femsim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 192.De Marco Verissimo C, Potriquet J, You H, McManus DP, Mulvenna J, Jones MK. 2019. Qualitative and quantitative proteomic analyses of Schistosoma japonicum eggs and egg-derived secretory-excretory proteins. Parasit Vectors 12:173. 10.1186/s13071-019-3403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Carson JP, Robinson MW, Hsieh MH, Cody J, Le L, You H, McManus DP, Gobert GN. 2020. A comparative proteomics analysis of the egg secretions of three major schistosome species. Mol Biochem Parasitol 240:111322. 10.1016/j.molbiopara.2020.111322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pearson MS, Tedla BA, Becker L, Nakajima R, Jasinskas A, Mduluza T, Mutapi F, Oeuvray C, Greco B, Sotillo J, Felgner PL, Loukas A. 2021. Immunomics-guided antigen discovery for praziquantel-induced vaccination in urogenital human schistosomiasis. Front Immunol 12:663041. 10.3389/fimmu.2021.663041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.de Assis RR, Ludolf F, Nakajima R, Jasinskas A, Oliveira GC, Felgner PL, Gaze ST, Loukas A, LoVerde PT, Bethony JM, Correa-Oliveira R, Calzavara-Silva CE. 2016. A next-generation proteome array for Schistosoma mansoni. Int J Parasitol 46:411–415. 10.1016/j.ijpara.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 196.Kifle DW, Chaiyadet S, Waardenberg AJ, Wise I, Cooper M, Becker L, Doolan DL, Laha T, Sotillo J, Pearson MS, Loukas A. 2020. Uptake of Schistosoma mansoni extracellular vesicles by human endothelial and monocytic cell lines and impact on vascular endothelial cell gene expression. Int J Parasitol 50:685–696. 10.1016/j.ijpara.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 197.Mu Y, McManus DP, Gordon CA, Cai P. 2021. Parasitic helminth-derived microRNAs and extracellular vesicle cargos as biomarkers for helminthic infections. Front Cell Infect Microbiol 11:708952. 10.3389/fcimb.2021.708952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cai P, Gobert GN, You H, McManus DP. 2016. The Tao survivorship of schistosomes: implications for schistosomiasis control. Int J Parasitol 46:453–463. 10.1016/j.ijpara.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 199.Pearson MS, Becker L, Driguez P, Young ND, Gaze S, Mendes T, Li XH, Doolan DL, Midzi N, Mduluza T, McManus DP, Wilson RA, Bethony JM, Nausch N, Mutapi F, Felgner PL, Loukas A. 2015. Of monkeys and men: immunomic profiling of sera from humans and non-human primates resistant to schistosomiasis reveals novel potential vaccine candidates. Front Immunol 6:213. 10.3389/fimmu.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mekonnen GG, Tedla BA, Pickering D, Becker L, Wang L, Zhan B, Bottazzi ME, Loukas A, Sotillo J, Pearson MS. 2020. Schistosoma haematobium extracellular vesicle proteins confer protection in a heterologous model of schistosomiasis. Vaccines (Basel) 8:416. 10.3390/vaccines8030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Buck JC, De Leo GA, Sokolow SH. 2020. Concomitant immunity and worm senescence may drive schistosomiasis epidemiological patterns: an eco-evolutionary perspective. Front Immunol 11:160. 10.3389/fimmu.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chulanetra M, Chaicumpa W. 2021. Revisiting the mechanisms of immune evasion employed by human parasites. Front Cell Infect Microbiol 11:702125. 10.3389/fcimb.2021.702125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. 2018. Schistosomiasis. Nat Rev Dis Primers 4:13. 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 204.Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet 383:2253–2264. 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bergquist R, Utzinger J, McManus DP. 2008. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis 2:e244. 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Knox DP. 2010. Parasite vaccines: recent progress in, and problems associated with their development. Open Infect Dis J 4:63–73. 10.2174/1874279301004010063. [DOI] [PubMed] [Google Scholar]
- 207.Wait LF, Dobson AP, Graham AL. 2020. Do parasite infections interfere with immunisation? A review and meta-analysis. Vaccine 38:5582–5590. 10.1016/j.vaccine.2020.06.064. [DOI] [PubMed] [Google Scholar]
- 208.Farias LP, Vitoriano-Souza J, Cardozo LE, Gama LDR, Singh Y, Miyasato PA, Almeida GT, Rodriguez D, Barbosa MMF, Fernandes RS, Barbosa TC, Neto A, Nakano E, Ho PL, Verjovski-Almeida S, Nakaya HI, Wilson RA, Leite LCC. 2021. Systems biology analysis of the radiation-attenuated schistosome vaccine reveals a role for growth factors in protection and hemostasis inhibition in parasite survival. Front Immunol 12:624191. 10.3389/fimmu.2021.624191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smythies LE, Pemberton RM, Coulson PS, Mountford AP, Wilson RA. 1992. T cell-derived cytokines associated with pulmonary immune mechanisms in mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Immunol 148:1512–1518. [PubMed] [Google Scholar]
- 210.Hogg KG, Kumkate S, Mountford AP. 2003. IL-10 regulates early IL-12-mediated immune responses induced by the radiation-attenuated schistosome vaccine. Int Immunol 15:1451–1459. 10.1093/intimm/dxg142. [DOI] [PubMed] [Google Scholar]
- 211.McManus DP, Loukas A. 2008. Current status of vaccines for schistosomiasis. Clin Microbiol Rev 21:225–242. 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maizels RM, McSorley HJ. 2016. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 138:666–675. 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. 2005. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol 174:4924–4933. 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 214.Wilson MS, Cheever AW, White SD, Thompson RW, Wynn TA. 2011. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog 7:e1002171. 10.1371/journal.ppat.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Allen JE, Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11:375–388. 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 216.Liang F, Lindgren G, Lin A, Thompson EA, Ols S, Rohss J, John S, Hassett K, Yuzhakov O, Bahl K, Brito LA, Salter H, Ciaramella G, Lore K. 2017. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther 25:2635–2647. 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pugh J, Savulescu J, Brown RCH, Wilkinson D. 2022. The unnaturalistic fallacy: COVID-19 vaccine mandates should not discriminate against natural immunity. J Med Ethics 48:371–377. 10.1136/medethics-2021-107956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Driciru E, Koopman JPR, Cose S, Siddiqui AA, Yazdanbakhsh M, Elliott AM, Roestenberg M. 2021. Immunological considerations for Schistosoma vaccine development: transitioning to endemic settings. Front Immunol 12:635985. 10.3389/fimmu.2021.635985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gordon CA, Krause L, McManus DP, Morrison M, Weerakoon KG, Connor MC, Olveda RM, Ross AG, Gobert GN. 2020. Helminths, polyparasitism, and the gut microbiome in the Philippines. Int J Parasitol 50:217–225. 10.1016/j.ijpara.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 220.Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. 2019. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther 27:757–772. 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu J, Zhu C, Shi Y, Li H, Wang L, Qin S, Kang S, Huang Y, Jin Y, Lin J. 2012. Surveillance of Schistosoma japonicum infection in domestic ruminants in the Dongting Lake region, Hunan province, China. PLoS One 7:e31876. 10.1371/journal.pone.0031876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gordon CA, Acosta LP, Gray DJ, Olveda RM, Jarilla B, Gobert GN, Ross AG, McManus DP. 2012. High prevalence of Schistosoma japonicum infection in carabao from Samar Province, the Philippines: implications for transmission and control. PLoS Negl Trop Dis 6:e1778. 10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Da’dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, Feng Z, Yu XL, Gray DJ, Harn DA. 2008. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine 26:3617–3625. 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Williams GM, Sleigh AC, Li Y, Feng Z, Davis GM, Chen H, Ross AG, Bergquist R, McManus DP. 2002. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the People’s Republic of China. Acta Trop 82:253–262. 10.1016/S0001-706X(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 225.Gray DJ, Li YS, Williams GM, Zhao ZY, Harn DA, Li SM, Ren MY, Feng Z, Guo FY, Guo JG, Zhou J, Dong YL, Li Y, Ross AG, McManus DP. 2014. A multi-component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: design and baseline results of a 4-year cluster-randomised intervention trial. Int J Parasitol 44:659–668. 10.1016/j.ijpara.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 226.You H, Gobert GN, Duke MG, Zhang W, Li Y, Jones MK, McManus DP. 2012. The insulin receptor is a transmission blocking veterinary vaccine target for zoonotic Schistosoma japonicum. Int J Parasitol 42:801–807. 10.1016/j.ijpara.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 227.McManus DP. 2005. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol 27:297–308. 10.1111/j.1365-3024.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- 228.Da’Dara AA, Li C, Yu X, Zheng M, Zhou J, Shollenberger LM, Li YS, Harn DA. 2019. Prime-boost vaccine regimen for SjTPI and SjC23 schistosome vaccines, increases efficacy in water buffalo in a field trial in China. Front Immunol 10:284. 10.3389/fimmu.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nixon SA, Welz C, Woods DJ, Costa-Junior L, Zamanian M, Martin RJ. 2020. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Int J Parasitol Drugs Drug Resist 14:8–16. 10.1016/j.ijpddr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lebens M, Sun JB, Czerkinsky C, Holmgren J. 2004. Current status and future prospects for a vaccine against schistosomiasis. Expert Rev Vaccines 3:315–328. 10.1586/14760584.3.3.315. [DOI] [PubMed] [Google Scholar]
- 231.Wilson RA, Li XH, Castro-Borges W. 2016. Do schistosome vaccine trials in mice have an intrinsic flaw that generates spurious protection data? Parasit Vectors 9:89. 10.1186/s13071-016-1369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Abdul-Ghani RA, Hassan AA. 2010. Murine schistosomiasis as a model for human schistosomiasis mansoni: similarities and discrepancies. Parasitol Res 107:1–8. 10.1007/s00436-010-1855-5. [DOI] [PubMed] [Google Scholar]
- 233.Vaumourin E, Vourc’h G, Gasqui P, Vayssier-Taussat M. 2015. The importance of multiparasitism: examining the consequences of co-infections for human and animal health. Parasit Vectors 8:545. 10.1186/s13071-015-1167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Devi KR, Lee LJ, Yan LT, Syafinaz AN, Rosnah I, Chin VK. 2021. Occupational exposure and challenges in tackling M. bovis at human-animal interface: a narrative review. Int Arch Occup Environ Health 94:1147–1171. 10.1007/s00420-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Elias D, Akuffo H, Thors C, Pawlowski A, Britton S. 2005. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin Exp Immunol 139:398–404. 10.1111/j.1365-2249.2004.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Beechler BR, Boersma KS, Buss PE, Coon CAC, Gorsich EE, Henrichs BS, Siepielski AM, Spaan JM, Spaan RS, Ezenwa VO, Jolles AE. 2019. Bovine tuberculosis disturbs parasite functional trait composition in African buffalo. Proc Natl Acad Sci USA 116:14645–14650. 10.1073/pnas.1903674116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Weatherhead JE, Gazzinelli-Guimaraes P, Knight JM, Fujiwara R, Hotez PJ, Bottazzi ME, Corry DB. 2020. Host immunity and inflammation to pulmonary helminth infections. Front Immunol 11:594520. 10.3389/fimmu.2020.594520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Siddiqui AA, Siddiqui SZ. 2017. Sm-p80-based schistosomiasis vaccine: preparation for human clinical trials. Trends Parasitol 33:194–201. 10.1016/j.pt.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kreiter S, Selmi A, Diken M, Sebastian M, Osterloh P, Schild H, Huber C, Tureci O, Sahin U. 2008. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J Immunol 180:309–318. 10.4049/jimmunol.180.1.309. [DOI] [PubMed] [Google Scholar]
- 240.De Beuckelaer A, Grooten J, De Koker S. 2017. Type I interferons modulate CD8(+) T cell immunity to mRNA vaccines. Trends Mol Med 23:216–226. 10.1016/j.molmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 241.Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, Tam YK, Kariko K, Barbosa CJ, Madden TD, Hope MJ, Krammer F, Hensley SE, Weissman D. 2018. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun 9:3361. 10.1038/s41467-018-05482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]