Abstract
Aim:
To determine if robotic gait training for individuals with cerebral palsy is more effective than the standard of care for improving function.
Method:
PubMed, Embase, Scopus, and Cochrane databases were searched from 1980–January, 2022 for articles that investigated robotic gait training versus standard of care (i.e., physical therapy or standard gait training) for individuals with cerebral palsy. Articles were included if a randomized controlled trial design was used, and excluded if robotic gait training was combined with another neuromuscular intervention, such as functional electrical stimulation. A meta-analysis of outcomes measured in at least four studies was conducted.
Results:
Eight citations met all criteria for full-text review and inclusion in the meta-analysis. A total of 188 individuals with cerebral palsy, ages four to 35, and Gross Motor Function Classification System levels I – IV were studied. Level of evidence ranged from 2b – 1b. All studies utilized a tethered, assistive device for robotic gait training. The overall effect was not significantly different between the robotic gait training and control interventions for six minute walk test performance (95% CI: −0.17, 0.73; P = 0.22), free walking speed (95% CI: −0.18, 0.57; P = 0.30), or Gross Motor Function Measures D (Standing) (95% CI: −0.29, 0.39; P = 0.77) and E (Walking, Running and Jumping) (95% CI: −0.11, 0.57; P = 0.19).
Conclusion:
Tethered robotic devices that provide assistive gait training for individuals with cerebral palsy do not provide a greater benefit for improving mobility than the standard of care.
Introduction
Walking is difficult for an overwhelming majority of individuals with cerebral palsy, the most common physical disability of childhood1. Most individuals with cerebral palsy will experience a decline in mobility as they age, with many requiring the use of a wheelchair by adulthood2. This unfortunate, yet typical progression in cerebral palsy has been a driving motivation for developing robotic gait training interventions, which have sought to address the current gaps in care for this population. These devices are developed on the premise that their technology can augment rehabilitative efforts beyond those possible with traditional treatments. For example, robotic gait training was found to significantly increase the odds of independent walking after a stroke compared to the standard of care alone3.
A systematic review of robotic gait training for individuals with cerebral palsy was previously conducted, which supported this intervention for improving walking speed, endurance, and gross motor function, despite a non-significant meta-analysis result4. In addition, at the time of the review, only two randomized controlled trials had been conducted, with the majority of supporting evidence coming from uncontrolled cohort studies. No comprehensive review has provided an update on the evidence from randomized controlled robotic gait training interventions in cerebral palsy. Without this information, it is unclear whether the current paradigms available for robotic gait training in this population are truly offering benefits beyond those of traditional treatments.
The goal of this systematic review was to compile all randomized controlled trials that have specifically studied robotic gait training for individuals with cerebral palsy, and conduct a meta-analysis of common outcome variables to evaluate the most recent evidence of this intervention’s effectiveness. Our specific research question was, “what is the effectiveness of robotic gait training for improving function in individuals with cerebral palsy relative to the standard of care as indicated by randomized controlled trials?”.
Methods
A systematic review, prospectively registered with PROSPERO (ID # CRD42021236195), was conducted by following PRISMA-P guidelines5. Using a date range of 1980 – January 25, 2022, the databases PubMed, Embase, Scopus, and Cochrane were used to complete a search of the available literature. The detailed search strategy can be found in Appendix A.
Results of the database search were combined and duplicates were removed. The inclusion criteria for articles was: 1) study participants had a diagnosis of cerebral palsy, 2) robotic gait training was compared to either traditional gait training or functional exercises typical for a child with cerebral palsy receiving physical therapy, 3) a randomized controlled trial design was used; if a study used a randomized crossover design and met all other inclusion criteria, the pre-crossover data was used, 4) written in or translated to English language, and 5) it was an original research article (although secondary sources, such as other systematic reviews, were utilized to locate additional articles). Articles were excluded if 1) robotic gait training was combined with another neuromuscular intervention (i.e., functional electrical stimulation), 2) greater than 30% of participants did not meet the inclusion criteria, 3) only an abstract was written (i.e., conference presentations or papers), and 4) we were unable to access the full text.
The title of each article was read as an initial screening. If an article’s title was clearly not within the scope of our research question, it was removed. Following this, two independent authors reviewed the abstracts of remaining articles against our inclusion and exclusion criteria to screen articles for full-text review. Disputes on article selection were settled by a third, independent author. If an article was removed during full review, the reason for doing so was noted in Table 1. Finally, during review, if a citation was discovered that was not included in the original search, but was related to the research question, it was considered for review.
Table 1.
Rationale for removal of articles considered for full-text review
| Study | Reason |
|---|---|
| Wu 201710 | Control group was not a standard of care |
| Yazici 201911 | Non-RCT study design |
| Kawasaki 202012 | Only 5 minutes with device (not considered training) |
| Sucuoglu 202013 | Non-RCT study design |
| Pool 202114 | Robotic gait training was combined with functional electrical stimulation |
RCT: Randomized controlled trial
Articles selected for full review were critically appraised using the Critical Review Form for Quantitative studies, which provides a numerical score based on the number of key components within an article, with a maximum score of 156 (Supp Table 1). The level of evidence of each article was also evaluated using the Oxford Centre for Evidence-Based Medicine Levels of Evidence7. Finally, demographics of study participants, details on the robotic device used, outcome measures, and results were extracted from each article. If results necessary for the meta-analysis (see below) were not available in the published article, respective corresponding authors were contacted for this data.
Pooled effect sizes of outcome measures used by at least four studies were calculated for the meta-analysis. To specifically calculate the effect sizes of changes with robotic gait training relative to a control intervention, we calculated Cohen’s d by the following equation8:
where ΔRGT is the change in outcome measure with robotic gait training, ΔControl is the change in outcome measure with the control intervention, RGT baseline SD is the baseline standard deviation of the outcome measure for the robotic gait training group, and Control baseline SD is the baseline standard deviation of the outcome measure for the control group.
Cochran’s RevMan software9 (v5.4.1; The Cochrane Collaboration, London, UK) was then used to calculate 95% confidence intervals of each effect size (standardized by mean difference), test for heterogeneity based on the I2 statistic (indicating low, moderate, or high heterogeneity with an I2 statistic of <25%, 25 – 75%, or >75%, respectively), and calculate the overall effect (Z-test) and 95% confidence interval. Forest plots for each outcome measure were generated to visually inspect results and heterogeneity. We used a random effects model to account for the expected differences in study protocols.
Results
A total of 114 articles were screened from the four databases after duplicates were removed, 13 of which were fully reviewed against our inclusion and exclusion criteria. Of these 13 articles, five were removed for reasons outlined in Table 1. The resulting eight articles were used for both qualitative and quantitative synthesis (Fig.1; Table 2).
Figure 1.
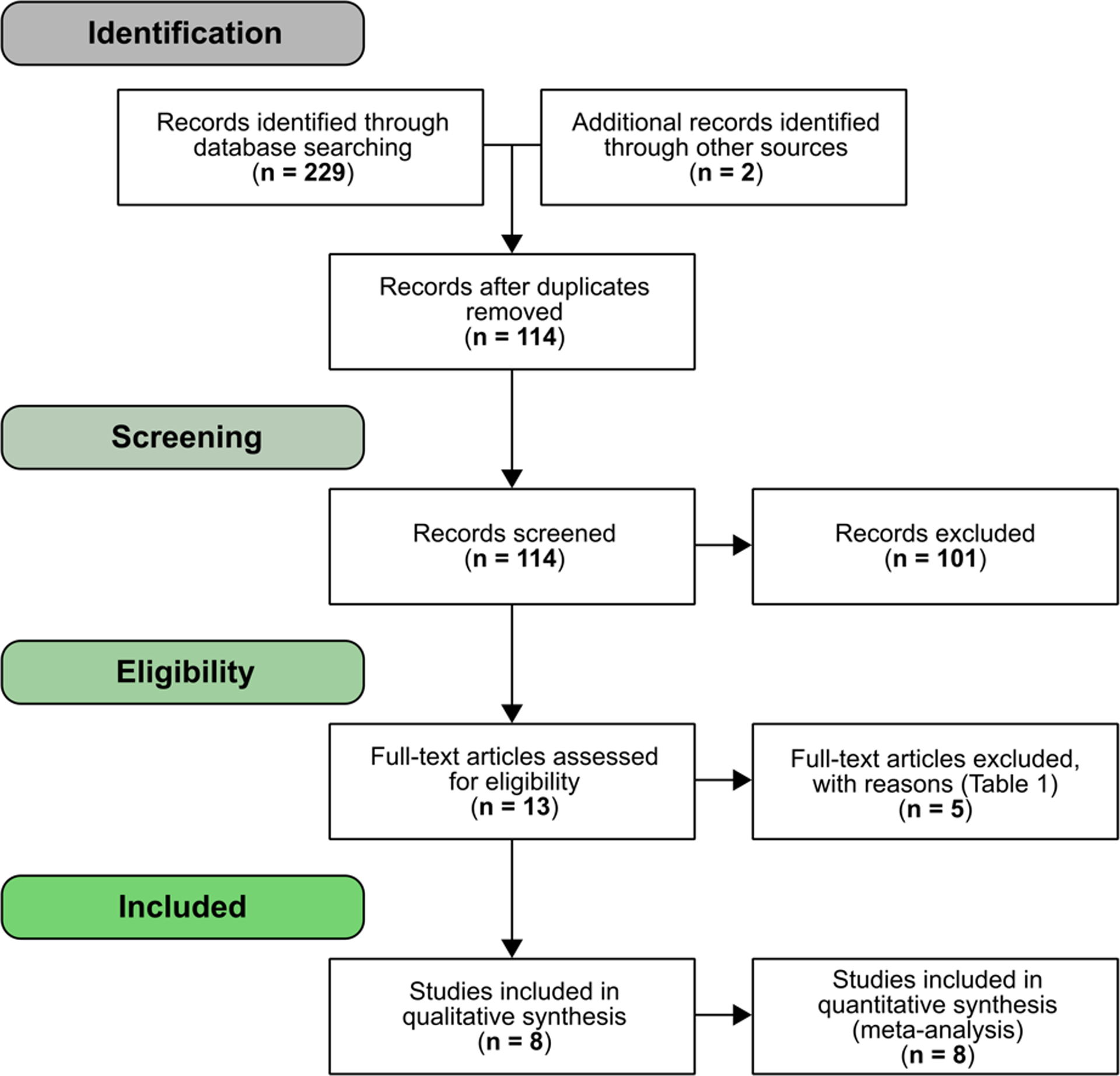
Article screening and selection flowchart
Table 2.
Study characteristics
| Level of evidence (OCEBM) | Critical appraisal score* | Participant characteristics | Intervention | Outcomes (for meta-analysis) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (RGT/Control) | CP type | GMFCS level | Age (yrs) | RGT | Control | Duration | ||||
| Smania 201115 | 2b | 12 | 9/9 | SD, ST | I – IV | 8 – 18 | Gait Trainer I | Physical therapy | 10, 40-min sessions (5x/wk) | 10m walk test (gait speed), 6MWT |
| Druzbicki 201316 | 2b | 13 | 26/9 | SD | II – III | 6 – 13 | Lokomat | Physical therapy | 20, 45-min sessions | Gait speed |
| Wu 201717 | 1b | 14 | 11/10 | SD | I – IV | 4 – 16 | 3DCaLT | Gait training | 18, 30 to 40-min sessions (3x/wk) | Gait speed, 6MWT, GMFM-D, GMFM-E |
| Peri 201718 | 2b | 13 | 12/10 | SD | I – III | 4 – 17 | Lokomat | Physical therapy | 40, 30-min sessions over 10 wks | 6MWT, GMFM-D, GMFM-E |
| Wallard 2017/1819,20 | 2b | 13 | 14/16 | SD | II | 8 – 10 | Lokomat | Physical therapy | 20, 40-min sessions (5x/wk) | GMFM-D and GMFM-E (2017), gait speed (2018) |
| Klobucka 202021 | 2b | 14 | 20/26 | SD | I – IV | 15 – 35 | Lokomat | Physical therapy | 20, 30 to 45 min sessions (2 – 5x/wk) | GMFM-D, GMFM-E |
| Ammann-Reiffer 202022 | 2b | 12 | 8/8 | SD | II – IV | 6 – 18 | Lokomat | Physical therapy | 15, 30 to 45-min sessions (1 – 3x/wk) | Gait speed, 6MWT, GMFM-E, GMFM-D |
OCEBM: Oxford Centre for Evidence-Based Medicine
See Supp Table 1 for details
RGT: Robotic gait training
CP type: cerebral palsy type; spastic diplegia (SD), spastic triplegia (ST)
GMFCS level: Gross Motor Function Classification System
6MWT: Six minute walk test
GMFM: Gross Motor Function Measure, D (Standing), E (Walking, Running and Jumping)
A total of 188 individuals with cerebral palsy were included across the eight randomized controlled trials, ranging in age from four to 35 and Gross Motor Function Classification System levels I – IV, with both spastic diplegia and triplegia. Robotic gait training ranged in duration from 400 – 1200 minutes of training, with control conditions comprised of traditional gait training with spotting from a physical therapist, or physical therapy. The physical therapy prescribed for the control condition was relatively homogenous across studies, with exercises aimed at improved range of motion, balance, and functional movement patterns. Critical appraisal scores ranged from 12 – 14, with the majority of studies lacking a sample size justification (i.e., prospective power analysis) or sufficient description of the exact robotic gait training parameters used (Supp. Table 1). Oxford Centre for Evidence-Based Medicine Level of Evidence was mainly 2b, indicating a lower quality randomized controlled trial design, which was a reflection of relatively small sample sizes and/or high dropout rates for the control group (Table 2). A summary of study characteristics can be found in Table 2.
The majority of studies16,18–22 utilized a Lokomat device (Figure 2b) for their robotic gait training. Briefly, the Lokomat is able to unload a user’s body weight while also providing assistive forces at the hips and knees. User-specific parameters can be set to control for things such as velocity and step length, and there is a visual display of performance for biofeedback23. One study15 used the Gait Trainer I (Figure 2a), which also provided body weight support and could be adapted to a user’s performance over time24. Another study17 used a custom cable-driven robotic device (3DCaLT, Figure 2c), which assisted with leg swing and pelvic motion25. All devices were tethered, and provided assistive forces during training.
Figure 2.
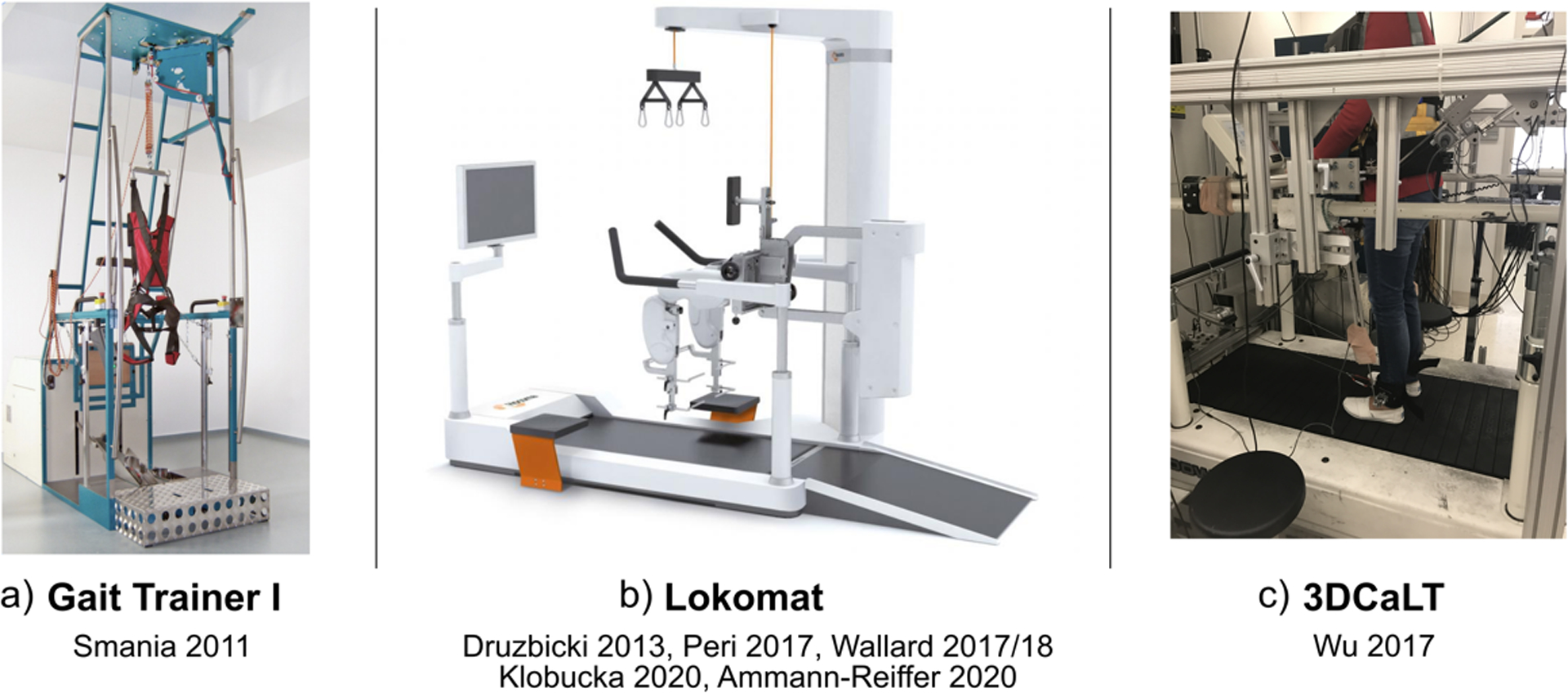
Robotic gait training devices utilized in each study, including a) the Gait Trainer I, b) the Lokomat, and c) the 3DCaLT.
Six minute walk test performance, free walking speed, Gross Motor Function Measure-D (Standing), and Gross Motor Function Measure-E (Walking, Running and Jumping) were assessed by four or more studies and therefore, included in the meta-analysis.
Four studies15,17,18,22, with no significant heterogeneity (P = 0.77, I2 = 0%) and comprising 40 robotic gait training participants and 37 control participants, assessed change in six minute walk test performance. The overall effect was not significantly different between the robotic gait training and control intervention (95% CI: −0.17, 0.73; P = 0.22; Figure 3).
Figure 3.
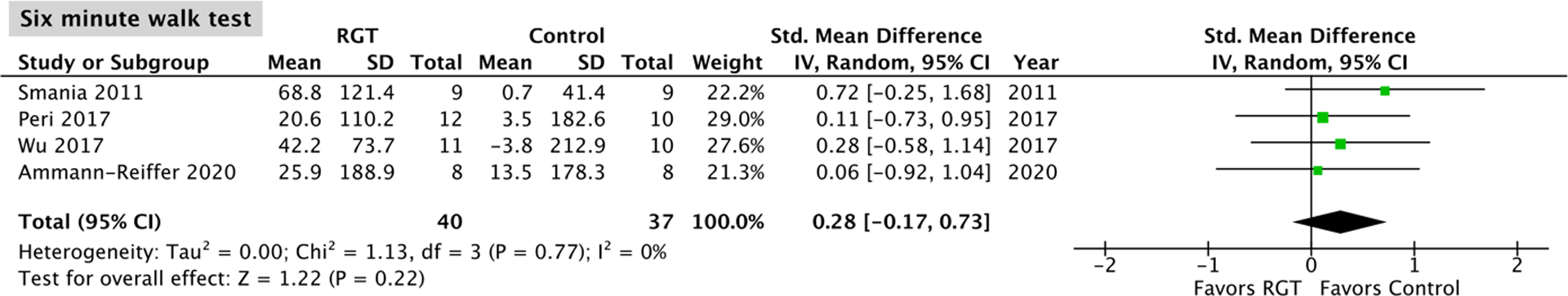
Meta-analysis outcomes and forest plot; six minute walk test; RGT: robotic gait training.
A total of five studies15–17,20,22, with no significant heterogeneity (P = 0.76, I2 = 0%) and comprising 68 robotic gait training participants and 52 control participants, assessed change in free walking speed. The overall effect was not significantly different between the robotic gait training and control intervention (95% CI: −0.18, 0.57; P = 0.30; Figure 4).
Figure 4.
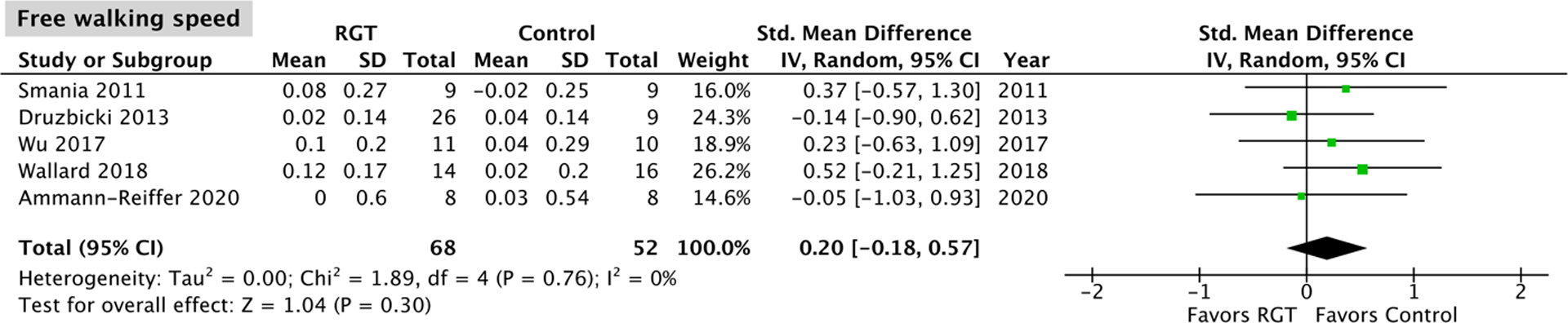
Meta-analysis outcomes and forest plot; free walking speed; RGT: robotic gait training.
Five studies17–19,21,22, with no significant heterogeneity (Gross Motor Function Measure-D: P = 0.76, I2 = 0%; Gross Motor Function Measure-E: P = 0.83, I2 = 0%) and comprising 65 robotic gait training participants and 70 control participants, assessed changes in Gross Motor Function Measure-D and -E scores. The overall effect was not significantly different between the robotic gait training and control intervention for both Gross Motor Function Measure-D (95% CI: −0.29, 0.39; P = 0.77; Figure 5) and -E (95% CI: −0.11, 0.57; P = 0.19; Figure 6). Four of the five studies18,19,21,22 evaluating Gross Motor Function Measures-D and -E used a Lokomat device, and a Lokomat-specific pooled effect on these measures was also not significantly different from the control interventions tested (GMFM-D 95% CI: −0.25, 0.49; P = 0.52; GMFM-E 95% CI: −0.10, 0.65; P = 0.15; Appendix B).
Figure 5.
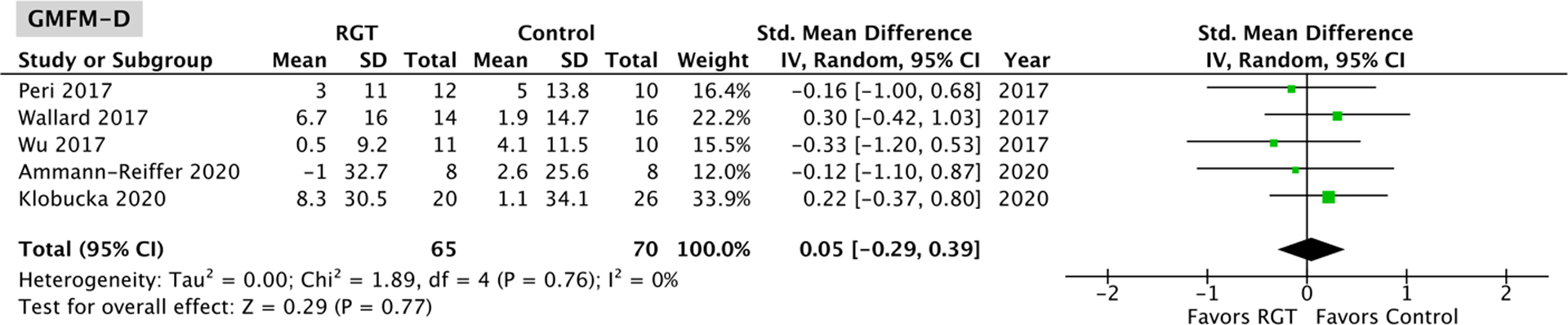
Meta-analysis outcomes and forest plot; Gross Motor Function Measure-D (Standing); RGT: robotic gait training.
Figure 6.
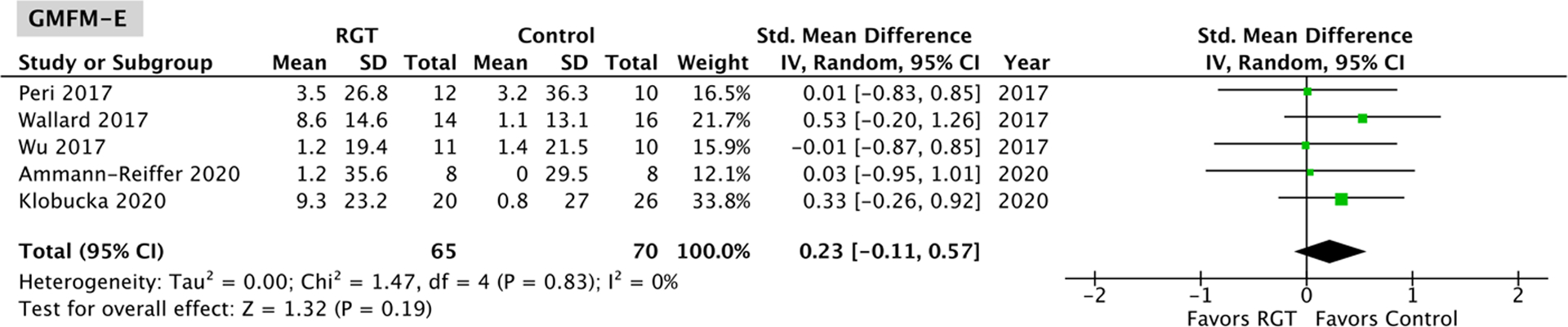
Meta-analysis outcomes and forest plot; Gross Motor Function Measure-E (Walking, Running and Jumping); RGT: robotic gait training.
Discussion
The purpose of this systematic review and meta-analysis was to determine if robotic gait training for individuals with cerebral palsy is effective beyond the standard of care (i.e., traditional gait training or functional exercises typically used in physical therapy). Based on the findings from this review, the devices and training protocols tested in these randomized controlled trials did not significantly improve six minute walk test performance, walking speed, or gross motor function compared to a dose-matched standard of care.
The findings here serve as an update to a previous review that incorporated data from uncontrolled cohort studies due to the low number of randomized controlled trials at the time of publication4. This former review concluded that robotic gait training could significantly improve walking speed and endurance for individuals with cerebral palsy based on the findings of individual studies, most of which were uncontrolled. However, the meta-analysis results from this former review for the overall effect of these parameters (i.e., walking endurance, walking speed, and gross motor function) were non-significant, which is in agreement with the findings of the present review. Additionally, the present review limited studies to those with a randomized controlled trial design, which allowed us to isolate the effect of robotic gait training and determine its true effectiveness beyond the standard of care.
The finding that the robotic gait training interventions in the reviewed studies are not more effective relative to non-robotic gait training interventions may be due to the inherent nature of the paradigms tested. A common feature of all of the robotic gait training devices and protocols was the assistive nature of the intervention. Through a combination of unloading a user’s body weight and robotically guiding the lower limbs, all studies utilized assistive mechanisms to train the gait of their participants with cerebral palsy. Specifically, six of the eight studies used a Lokomat device, which has gained popularity as a clinical gait training tool for individuals with cerebral palsy26 after demonstrating significant benefits on measures of mobility in uncontrolled studies27–29. As four of the studies reviewed here used a Lokomat device and assessed Gross Motor Function Measure-D and -E, we were able to calculate a Lokomat-specific pooled effect of these measures, which was not significantly different from the standard of care (Appendix B). This finding suggests that the significant cost of this gait training tool may not be justified by the current evidence of its benefit beyond standard of care treatments.
While the utility of assistive robotic gait training is understandable and likely effective for re-training the spinal pathways necessary for locomotion, explaining the success of these interventions for those with spinal cord injury30, it may not be efficacious for disorders due to brain injury like cerebral palsy, where motor learning and cortical reorganization rely on active neuromuscular engagement31,32. Alternatively, interventions that incorporate more volitional engagement and result in increased supraspinal drive may be more beneficial for improving mobility in individuals with cerebral palsy33. This is supported by the finding that resistive robotic gait training, necessitating active motor input, was more effective for improving locomotor function in children with cerebral palsy when compared to assistive robotic gait training in a randomized controlled trial34. In addition, recent pilot studies35,36 utilizing devices that increase neuromuscular activity during walking in children with cerebral palsy have had promising findings for improving mobility-related outcomes in this population. Another consideration is the tethered nature of the devices investigated, which may lack the ecological validity for translation to real-world performance, supporting future work in untethered, mobile devices.
It is important to note a few limitations of this review and meta-analysis. First, the meta-analysis was limited to outcome measures that were assessed in at least four studies, and it is possible that assistive robotic gait training is effective for improving an outcome measure that was not included here. Second, cerebral palsy has a highly heterogenous phenotype, and the studies reviewed here encompassed a relatively small number of individuals (n = 188), all of whom had a similar diagnosis (spastic diplegia/triplegia). For this reason, the finding that assistive robotic gait training does not improve the outcomes reviewed may not be applicable to all individuals with cerebral palsy and a small benefit may still be possible. Third, some assistive robotic gait trainers have biofeedback and virtual reality features, and several of the studies reviewed did not provide exact details on how these features were utilized when training their participants. Virtual reality37 and biofeedback38 have shown promise for improving motor function in individuals with cerebral palsy, so it is possible that greater incorporation of these modalities with assistive robotic gait training could improve outcomes. Finally, not all studies used the same robotic device or protocol for training. However, there was no significant heterogeneity between studies, supporting the overall findings from this review.
In conclusion, to date, assistive robotic gait training interventions do not appear to be effective for improving walking endurance, walking speed, or gross motor function in individuals with cerebral palsy. This likely stems from the passivity involved with assistive training, and basic need for active engagement to promote motor learning. These findings provide support for future studies to focus on resistive robotic gait training for individuals with cerebral palsy, which have already shown some promise, but need randomized controlled trial-level investigations to move forward with true clinical applications.
Supplementary Material
Clinical messages.
Assistive robotic gait training has not proven to be effective for improving mobility in individuals with cerebral palsy beyond the standard of care.
Early evidence hints that resistive robotic gait training may be more efficacious than assistive robotic gait training for driving the motor learning necessary for improved mobility in this population.
Acknowledgments
This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R03HD094583 and F30HD103318. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Systematic review registration info: Prospectively registered with PROSPERO (ID # CRD42021236195)
Conflict of Interest Statement
ZFL is a named inventor on a pending utility patent application that describes a robotic device similar to those reviewed here, as well as a co-founder of a company seeking to commercialize the device.
References
- 1.Graham HK, Rosenbaum P, Paneth N, Dan B, Lin J-P, Damiano DL, et al. Cerebral palsy. Nat Rev Dis Prim. 2016;2(1):15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy KP, Molnar GE, Lankasky K. Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol. 1995;37(12):1075–84. [DOI] [PubMed] [Google Scholar]
- 3.Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database of Syst Rev. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho I, Pinto SM, Chagas DDV, Praxedes dos Santos JL, de Sousa Oliveira T, Batista LA. Robotic Gait Training for Individuals With Cerebral Palsy: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2017;98(11):2332–44. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law M, Stewart D, Pollock N, Letts I, Bosch J, Westmorland M. Critical review form - Quantitative studies. 1998.
- 7.OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2.
- 8.Morris SB. Estimating Effect Sizes From Pretest-Posttest-Control Group Designs. Organ Res Methods. 2008;11(2):364–86. [Google Scholar]
- 9.Review Manager (RevMan). Copenhagen: The Cochrane Collaboration; 2020.
- 10.Wu M, Kim J, Gaebler-Spira DJ, Schmit BD, Arora P, et al. Robotic Resistance Treadmill Training Improves Locomotor Function in Children With Cerebral Palsy: A Randomized Controlled Pilot Study. Arch Phys Med Rehabil. 2017;98(11):2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazıcı M, Livanelioğlu A, Gücüyener K, Tekin L, Sümer E, Yakut Y. Effects of robotic rehabilitation on walking and balance in pediatric patients with hemiparetic cerebral palsy. Gait Posture. 2019;70:397–402. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki S, Ohata K, Yoshida T, Yokoyama A, Yamada S. Gait improvements by assisting hip movements with the robot in children with cerebral palsy: a pilot randomized controlled trial. J Neuroeng Rehabil. 2020;17(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sucuoglu H Effects of robot-assisted gait training alongside conventional therapy on the development of walking in children with cerebral palsy. J Pediatr Rehabil Med. 2020;13(2):127–35. [DOI] [PubMed] [Google Scholar]
- 14.Pool D, Valentine J, Taylor NF, Bear N, Elliott C. Locomotor and robotic assistive gait training for children with cerebral palsy. Dev Med Child Neurol. 2021;63(3):328–35. [DOI] [PubMed] [Google Scholar]
- 15.Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil. 2011;90(2):137–49. [DOI] [PubMed] [Google Scholar]
- 16.Druzbicki M, Rusek W, Snela S, Dudek J, Szczepanik M, Zak E, et al. Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. J Rehabil Med. 2013;45(4):358–63. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Kim J, Arora P, Gaebler-Spira DJ, Zhang Y. Effects of the Integration of Dynamic Weight Shifting Training Into Treadmill Training on Walking Function of Children with Cerebral Palsy: A Randomized Controlled Study. Am J Phys Med Rehabil. 2017;96(11):765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peri E, Turconi AC, Biffi E, Maghini C, Panzeri D, Morganti R, et al. Effects of dose and duration of Robot-Assisted Gait Training on walking ability of children affected by cerebral palsy. Technol Heal Care. 2017;25:671–81. [DOI] [PubMed] [Google Scholar]
- 19.Wallard L, Dietrich G, Kerlirzin Y, Bredin J. Robotic-assisted gait training improves walking abilities in diplegic children with cerebral palsy. Eur J Paediatr Neurol. 2017;21(3):557–64. [DOI] [PubMed] [Google Scholar]
- 20.Wallard L, Dietrich G, Kerlirzin Y, Bredin J. Effect of robotic-assisted gait rehabilitation on dynamic equilibrium control in the gait of children with cerebral palsy. Gait Posture. 2018;60:55–60. [DOI] [PubMed] [Google Scholar]
- 21.Klobucká S, Klobucký R, Kollár B. Effect of robot-assisted gait training on motor functions in adolescent and young adult patients with bilateral spastic cerebral palsy: A randomized controlled trial. NeuroRehabilitation. 2020;47(4):495–508. [DOI] [PubMed] [Google Scholar]
- 22.Ammann-Reiffer C, Bastiaenen CHG, Meyer-Heim AD, van Hedel HJA. Lessons learned from conducting a pragmatic, randomized, crossover trial on robot-assisted gait training in children with cerebral palsy (PeLoGAIT). J Pediatr Rehabil Med. 2020;13(2):137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jezernik S, Colombo G, Keller T, Frueh H, Morari M. Robotic Orthosis Lokomat: A Rehabilitation and Research Tool. Neuromodulation Technol Neural Interface. 2003;6(2):108–15. [DOI] [PubMed] [Google Scholar]
- 24.Hesse S, Uhlenbrock D, Werner C, Bardeleben A. A mechanized gait trainer for restoring gait in nonambulatory subjects. Arch Phys Med Rehabil. 2000;81(9):1158–61. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Hornby TG, Landry JM, Roth H, Schmit BD. A cable-driven locomotor training system for restoration of gait in human SCI. Gait Posture. 2011;33(2):256–60. [DOI] [PubMed] [Google Scholar]
- 26.Aurich T, Warken B, Graser JV, Ulrich T, Borggraefe I, Heinen F, et al. Practical Recommendations for Robot-Assisted Treadmill Therapy (Lokomat) in Children with Cerebral Palsy: Indications, Goal Setting, and Clinical Implementation within the WHO-ICF Framework. Neuropediatrics. 2015;46(4):248–60. [DOI] [PubMed] [Google Scholar]
- 27.van Hedel HJA, Meyer-Heim A, Rüsch-Bohtz C. Robot-assisted gait training might be beneficial for more severely affected children with cerebral palsy. Dev Neurorehabil. 2016;19(6):410–5. [DOI] [PubMed] [Google Scholar]
- 28.Borggraefe I, Schaefer JS, Klaiber M, Dabrowski E, Ammann-Reiffer C, Knecht B, et al. Robotic-assisted treadmill therapy improves walking and standing performance in children and adolescents with cerebral palsy. Eur J Paediatr Neurol. 2010;14(6):496–502. [DOI] [PubMed] [Google Scholar]
- 29.Meyer-Heim A, Ammann-Reiffer C, Schmartz A, Schäfer J, Sennhauser FHH, Heinen F, et al. Improvement of walking abilities after robotic-assisted locomotion training in children with cerebral palsy. Arch Dis Child. 2009;94(8):615–20. [DOI] [PubMed] [Google Scholar]
- 30.Nam KY, Kim HJ, Kwon BS, Park JW, Lee HJ, Yoo A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid LB, Rose SE, Boyd RN. Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nat Rev Neurol. 2015;11(7):390–400. [DOI] [PubMed] [Google Scholar]
- 32.Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126(4):866–72. [DOI] [PubMed] [Google Scholar]
- 33.Yang JF, Gorassini M. Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist. 2006;12(5):379–89. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Kim J, Gaebler-Spira DJ, Schmit BD, Arora P, et al. Robotic Resistance Treadmill Training Improves Locomotor Function in Children With Cerebral Palsy: A Randomized Controlled Pilot Study. Arch Phys Med Rehabil. 2017;98(11):2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conner BC, Remec NM, Orum EK, Frank EM, Lerner ZF. Wearable adaptive resistance training improves ankle strength, walking efficiency and mobility in cerebral palsy: a pilot clinical trial. IEEE Open J Eng Med Biol. 2020;1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang J, Martelli D, Vashista V, Martinez-Hernandez I, Kim H, Agrawal SK. Robot-driven downward pelvic pull to improve crouch gait in children with cerebral palsy. Sci Robot. 2017;2(8):eaan2634. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Fanchiang HD, Howard A. Effectiveness of Virtual Reality in Children With Cerebral Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phys Ther. 2018;98(1):63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacIntosh A, Lam E, Vigneron V, Vignais N, Biddiss E. Biofeedback interventions for individuals with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41(20):2369–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


