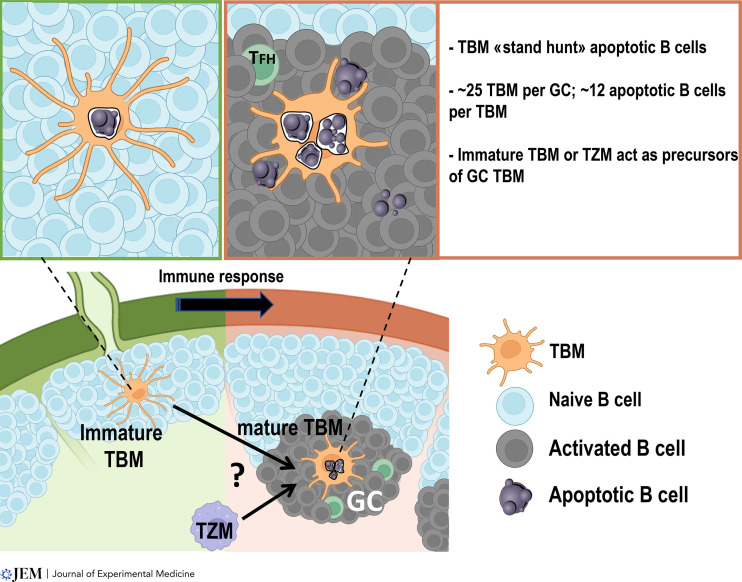
Tingible body macrophages in lymph node clear apoptotic B cell debris. Gurwisz et al. and Grootveld et al. report how tingible body macrophages, originating from tissue-resident macrophages, clear apoptotic B cells in the germinal center using a “stand-hunting” strategy.
Abstract
Tingible body macrophages in lymph node are involved in cleaning up debris from apoptotic B cells. Gurwisz et al. (2023. J. Exp. Med. https://doi.org/10.1084/jem.20222173) and Grootveld et al. (2023. Cell. https://doi.org/10.1016/j.cell.2023.02.004) report how tingible body macrophages, originating from tissue-resident macrophages, clear apoptotic B cells in the germinal center using a “stand-hunting” strategy.
Germinal centers (GCs) are transient lymphoid structures in which B cells clonally proliferate and mature their antibody affinity (Victora and Nussenzweig, 2022). Within GCs, B cells undergo iterative cycles of somatic hypermutation of antibody genes in conjunction with multiple Darwinian selection processes. B cells with a nonfunctional B cell receptor or deleterious mutations are removed from the GC (Mayer et al., 2017; Stewart et al., 2018). Debris and apoptotic B cells in GCs are removed by specialized phagocytic cells known as tingible body macrophages (TBMs; Swartzendruber and Congdon, 1963; Rahman et al., 2010). TBMs were first described by W. Flemming in the 19th century as phagocytic cells containing stainable (tingible) lymphocyte remnants (Flemming, 1885). Impaired functions of TBMs lead to the accumulation of apoptotic B cells in GC, formation of self-reactive antibodies, and eventually autoimmune diseases (Baumann et al., 2002). Although the dynamics of B cells in GCs have been largely documented in previous studies (Victora and Nussenzweig, 2022), the origin and behavior of TBMs remain unclear.

Insights from Elisa Madeleine Baudon and Marc Bajenoff.
Two studies by Gurwisz et al. (2023) and Grootveld et al. (2023) investigated the dynamics of TBMs using two-photon (2P) intravital imaging. They first identified CX3CR1GFP and Siglec1 (Cd169) Cre/+.Rosa26LSL-Tdtomato/+ mice as reliable tools for tracking TBMs in intact LNs. Fluorescent antigen-specific B cells were adoptively transferred in those mice and immunized with the appropriate antigens. 7 d later, when GCs are formed, the dynamics of fluorescent TBM and B cells were recorded using 2P imaging. The authors observed that each GC contained an average of 25 immobile TBMs that constantly probed their immediate environment with dynamic cellular processes. Surprisingly, the number of TBMs, their distribution, and their behavior within GCs were very close to the optimal prediction calculated by in silico analysis to efficiently clear the debris and apoptotic B cells present in GCs. Indeed, the authors calculated that few macrophages with low mobility would be the optimal strategy in a very dynamic cellular graveyard, where half of all GC B cells are removed every 6 h (Mayer et al., 2017). Gurwisz et al. (2023) went one step further and investigated the specificity of TBM efferocytosis by adoptively transferring fluorescent control B cells or T follicular helper cells at the peak of the GC response. The authors revealed that, despite their presence in GC structures, none of these cell types were captured by TBM, demonstrating the high specificity of TBM for apoptotic cells. These results suggest that dying GC B cells emit unique signals that can be detected by TBMs. It is worth noting that apoptotic B cells secrete fractalkine (CX3CL1; Truman et al., 2008) in vitro, which serves as a “find-me” signal for macrophages (Ravichandran, 2011). The extent to which this process facilitates B cell efferocytosis by CX3CR1-expressing TBMs remains to be determined. Owing to their insatiable appetite and stand-hunting strategy, TBM can be compared to gargantuan chameleons catching motile prey with extensible appendages.
TBMs play a crucial role in the immune response by phagocytosing apoptotic B cells in the GC. While immature TBMs are responsible for engulfing apoptotic naive B cells, mature TBMs, which arise from tissue-resident macrophages, exhibit specificity toward activated dying B cells following immunization. Despite their limited number (∼25 TBMs per GC) and immobility, TBMs have an efficient strategy for debris clearance from B cells, as highlighted by recent studies by Gurwisz et al. (2023) and Grootveld et al. (2023). TFH, T follicular helper cell. Created with Biorender.com.
Next, the authors investigated the origin and turnover of the TBMs. Many tissue-resident macrophages are long-lived cells that originate in the embryo and are slowly replaced by blood monocytes derived from definitive hematopoiesis (Ginhoux and Guilliams, 2016). Indeed, recent studies have shown that tissue-resident macrophages present in LN, such as T cell zone macrophages (TZMs) and subcapsular sinusoidal macrophages (SSMs), are long-lived macrophages that are seeded in utero and gradually diluted by blood monocytes after birth (Baratin et al., 2017; Bellomo et al., 2018). To explore whether TBMs originated from bone marrow (BM)–derived monocytes, the authors used monocyte fate mapper (Ccr2CreER/+.Rosa26LSL-Tdtomato/+) mice and BM chimeras. These experiments demonstrated that the physiological turnover of TBMs does not depend on a continuous influx of BM-derived cells, but rather favors a model in which TBMs are derived from a population of resident macrophages that are already present in the LN before immunization.
Grootveld et al. (2023) sought to identify the local precursors of TBM. To this end, they took advantage of the photoconvertible Cd169Cre/+ Rosa26LSL-Kikume reporter mice. The authors color-stamped CD169-lineage cells of inguinal LN cells with white light and immunized the animals. As 98% of TBM remained photoconverted 7 d later, the authors elegantly demonstrated that TBMs were derived from LN-resident CD169+ progenitor cells. These results were confirmed using partially shielded BM chimeras. Deletion of tissue-resident macrophages CD169+ SSM and medullary sinusoidal macrophages (MSM) in CD169DTR mice treated with diphtheria toxin did not alter TBM, ruling out a direct precursor–product relationship between SSM/MSM and TBM. Interestingly, injection of colony-stimulating factor 1 receptor (CSF1R) blocked antibody-deleted SSM and MSM, but did not alter TBMs, suggesting that an alternative trophic factor regulates TBM survival. Grootveld et al. (2023) concluded that TBMs might arise from the few macrophages present in nonimmunized follicles, whereas Gurwisz et al. (2023) hypothesized that TZMs might act as TBM precursors. The latter hypothesis is supported by functional, phenotypic, and transcriptomic similarities between TBMs and TZMs. In addition, TBMs and TZMs are both involved in the clearance of apoptotic lymphocytes in LN and upregulate efferocytotic genes, such as Merkt, Cx3cr1, and Cd68. Notably, during the immune response, B cell follicles have been observed to trespass within the adjacent T cell zone (Mionnet et al., 2013). Since GCs usually develop near the T/B boundary, it is possible that annexed TZMs carry out their efferocytic function in the newly formed GC. In this scenario, TBMs and TZMs would represent transient and versatile forms of a single cellular entity. TZMs lineage tracing models or in vivo photoactivation could be used to test this hypothesis.
To determine whether GCs are necessary for TBM formation, the authors used SLAM-associated protein–deficient mice, which cannot form GCs (Gurwicz et al., 2023). After immunization of these mice, the authors failed to detect infiltrated macrophages in the B cell follicles, implying that GCs are necessary for the development of TBMs. However, Grootveld et al. (2023) hypothesized that TBMs might already be present in naive follicles, waiting to fulfill their function. In this scenario, it is not the GC reaction per se, but its associated cellular graveyard, which would be necessary to unravel the presence of TBM. They tested this hypothesis by inducing acute B cell apoptosis in unimmunized mice by photoablation or diphtheria toxin–induced B cell apoptosis (Mb1Cre/+.Rosa26iDTR). They observed that massive apoptosis of naive B cells triggered the maturation of TBMs without GC formation and concluded that TBMs are present in naive follicles in an immature state with basal efferocytic activity.
These studies have shed new light on TBMs, a key member of the GC reaction, which has been largely unexplored. They also raised questions about the extraordinary appetites of these giant phagocytes. The high mortality rate of B cells in GCs, coupled with the small number of TBMs responsible for their elimination, suggests that TBMs are extraordinary cell grinders capable of recognizing, engulfing, digesting, and recycling organelles. Given the closed microarchitecture of GC, it would be interesting to determine whether the recycling capacity of TBMs can be used to meet the high metabolic demands of the GC reaction. The transient nature of GC also raises the question of the fate of TBMs upon completion of the GC reaction. Photoconversion or lineage-tracing experiments should reveal whether GC TBMs die, revert to an immature phenotype, or regain access to the T cell zone when enlarged B cell follicles retract into their original territory. Finally, the static nature of TBMs within a GC filled with swarming B cells implies that these macrophages are anchored to a structural element of the GC (stroma) that may act as a niche for TBMs and remains to be identified. According to the niche concept, a macrophage and its niche form a two-cell circuit from which each partner benefits (Zhou et al., 2018). Because Grootveld et al. (2023) found that TBMs, unlike most tissue-resident macrophages, are “blockade resistant” to CSFR1, the nature of the trophic factor of TBMs remains to be determined. Whether TBMs regulate the behavior of their stromal niche is also an interesting question that remains to be investigated.
References
- Baratin, M., et al. 2017. Immunity. 10.1016/j.immuni.2017.07.019 [DOI] [Google Scholar]
- Baumann, I., et al. 2002. Arthritis Rheum. [DOI] [PubMed] [Google Scholar]
- Bellomo, A., et al. 2018. Cell. Immunol. 10.1016/j.cellimm.2018.01.010 [DOI] [Google Scholar]
- Flemming, W. 1885.
- Ginhoux, F., and Guilliams M.. 2016. Immunity. 10.1016/j.immuni.2016.02.024 [DOI] [PubMed] [Google Scholar]
- Grootveld, A.K., et al. 2023. Cell. 10.1016/j.cell.2023.02.004 [DOI] [Google Scholar]
- Gurwicz, N., et al. 2023. J. Exp. Med. 10.1084/jem.20222173 [DOI] [Google Scholar]
- Mayer, C.T., et al. 2017. Science. 10.1126/science.aao2602 [DOI] [Google Scholar]
- Mionnet, C., et al. 2013. PLoS Biol. 10.1371/journal.pbio.1001672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, Z.S., et al. 2010. J. Immunol. 10.4049/jimmunol.1001187 [DOI] [Google Scholar]
- Ravichandran, K.S. 2011. Immunity. 10.1016/j.immuni.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, I., et al. 2018. Immunity. 10.1016/j.immuni.2018.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber, D.C., and Congdon C.C.. 1963. J. Cell Biol. 10.1083/jcb.19.3.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman, L.A., et al. 2008. Blood. 10.1182/blood-2008-06-162404 [DOI] [Google Scholar]
- Victora, G.D., and Nussenzweig M.C.. 2022. Annu. Rev. Immunol. 10.1146/annurev-immunol-120419-022408 [DOI] [Google Scholar]
- Zhou, X., et al. 2018. Cell. 10.1016/j.cell.2018.01.015 [DOI] [Google Scholar]