Abstract
BACKGROUND
Exercise therapy, self-management and education are recommended interventions for hand osteoarthritis (OA), but new delivery systems are needed to solve lack of adherence.
AIM
To determine the effects on hand function and pain related measures of a mobile app-delivered intervention, compared with usual care, in patients with symptomatic hand OA.
DESIGN
A pragmatic, multicenter, two-group parallel randomized controlled trial.
SETTING
Community health centers in rural southern Spain.
POPULATION
Eighty-three participants with unilateral or bilateral symptomatic hand OA were proposed to participate, and finally 74 were included and randomized.
METHODS
Participants received a home multimodal treatment (exercise, education, and self-management recommendations) with the CareHand mobile app or usual care (written exercises) over 12 weeks. Monthly telephone calls were performed to monitor adherence. The primary outcome was hand physical function (Australian/Canadian Hand Osteoarthritis Index, AUSCAN) at 3- and 6-months. Secondary measures included hand pain intensity and morning stiffness, upper limb function, hand dexterity, and grip and pinch strength.
RESULTS
The CareHand group showed significant within-group changes in hand function at 6-months (-3.0, 95% CI -5.1 to -0.9 vs. usual care: -0.9, 95% CI -3.3 to 1.5). Neither group showed improvements in hand function at 3-months (CareHand: -1.5, 95% CI -3.1 to 0.1; usual care: -0.5, 95% CI -2.7 to 1.7). For the secondary outcomes, the CareHand group showed better results on upper limb function both at 3- and 6-months, and on pain both at 1- and 3-months compared to usual care group. Linear regression models indicated that baseline scores of pain intensity, hand status, and upper limb function were associated with a greater improvement in hand pain and physical function.
CONCLUSIONS
A mobile app-delivered intervention is effective for improving hand function, and better than usual care for upper limb function and pain. Further research is warranted to understand the impact of mobile health (mHealth) in people with hand OA.
CLINICAL REHABILITATION IMPACT
mHealth interventions are a feasible and secure multimodal delivery approach in older adults with hand OA in rural primary care setting. Baseline pain and upper limb function might predict functional hand outcomes.
Key words: Exercise therapy, Hand, Telemedicine, Osteoarthritis, Self-management
Symptomatic hand osteoarthritis (OA) is a complex and prevalent disease in adults older than 45 years,1 with an overall lifetime risk of 40%.2 Patients with hand OA experience joint pain, stiffness, and impaired function, e.g., reduced pinch and grip strength,3 and hindered gross dexterity.4 Despite hand OA poses serious socioeconomic implications, it has been poorly investigated compared to the vast amount of research in hip or knee OA.5
Clinical guidelines for a comprehensive management of OA recommend some form of exercise and education, in addition to pharmacological treatment, as a core part of standard care.6-8 Lack of adherence to intervention is common in people with rheumatic diseases and has been associated with higher costs and worse outcomes.9 To ensure adherence, exercise therapy needs to be individually tailored,10 monitored, and combined with self-management strategies.11 For this purpose, digital tools12, 13 can be feasible,14 accessible,15 and cost-effective solutions,14 although aspects such as users’ readiness, motivation, and awareness may compromise their clinical effect.13, 16 The quality of the evidence on the impact of digital interventions for OA is low to moderate,14 thus further research is warranted.
New technologies have an enormous potential to save time and resources during rehabilitation.13 Mobile app-delivered interventions (mHealth) are the most effective e-health modality to decrease pain interference for chronic pain.17 A feedback-guided exercise program performed on a tablet touchscreen has shown to increase function and strength, and reduce healthcare usage, in people with wrist, hand or fingers injuries.18 mHealth is purported to be useful to deliver home exercises for individuals with rheumatic hand disorders.19 This has been demonstrated, to some extent, to enhance hand function,20 self-monitoring,21 and self-management behaviors in patients with rheumatoid arthritis of the hands.22 To date, the effects of mHealth have been barely investigated in hand OA.14 The aim of the study was to determine if a mobile app-delivered intervention combining exercise, education, and self-management recommendations was more effective at improving hand physical function and pain related measures than usual care in patients with symptomatic hand OA. As an additional aim, we explored which factors may be associated with greater changes in hand physical function and pain intensity. We hypothesized better results for those who received the mHealth approach.
Materials and methods
Study design
This was a pragmatic, multi-center, and two-group parallel randomized controlled trial planned to conform to the CONSORT guidelines and the ethical principles of the Helsinki Declaration. The study was approved by the Biomedical Research Committee of Andalusia, Spain (PI_RH_2018) and registered prior to initiation at ClinicalTrials.gov (NCT04263974, only the information referring to hand OA is pertinent to the study). All participants provided written informed consent.
Deviations from intended protocol
Due to the onset of the COVID-19 pandemic, to preserve the health of participants and the research team and respect patients’ preferences, self-reported measures were collected by phone or during in-person visits.
Setting and participants
Patients were recruited from two community health centers in rural southern Spain. The inclusion criteria were: older than 18 years; a clinical diagnosis of unilateral or bilateral hand OA according to the American College of Rheumatology;23 a history of hand pain for at least 6 months; current self-reported pain intensity ≥2 in a Numeric Rating Scale (NRS); and having a smartphone or tablet with internet access. Exclusion criteria included: upper limb surgery, fracture, or severe trauma within the previous 6 months;24 anticipated hand surgery;25 a diagnosed cognitive dysfunction; having received steroid injections in the past 2 months;26 or suffering from another rheumatic disease.26 Participants were asked not to engage in any new treatment, including manual or exercise therapy, during the study period but were allowed to continue with their regular medication intake. For those with bilateral symptoms, both hands were selected if eligibility criteria were fulfilled.20
Treatment allocation and blinding
An external staff member performed randomization using a computer-generated random numbers table with permuted block design and considering a 1:1 distribution ratio. After baseline assessment, sealed opaque envelopes were used to mask treatment allocation. Outcome assessors were blinded to allocation.
Interventions
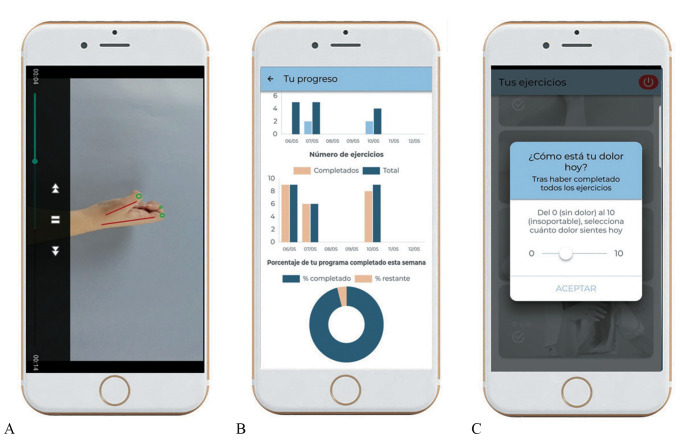
All participants completed a home exercise program (4 times a week) over 12 weeks, delivered with the CareHand mobile app (Healthinn, Seville, Spain) or using a paper sheet (usual care) that included pictures and explanations of exercises and dosage. For those who received usual care, information about the disease was provided by the physician during regular visits. The latter is the regular approach for rehabilitation of hand OA at the public health system where the study was conducted. All exercises were first explained in an introductory session. After that, home sessions were planned to last between 15 to 20 minutes depending on symptoms and training phase, with the number of repetitions gradually increased every 2 weeks. In case of a flare up, participants were instructed to reduce the number of sets and repetitions. The CareHand app uses a dosing algorithm, based on a daily report of pain intensity before and after exercising, to progress throughout training.27 The app also includes self-management recommendations, joint protection material,28 general information about the disease, videos with demonstrations, and a “follow-up diary” with graphical representations of the exercises performed every session (Figure 1).
Figure 1.
—The CareHand mobile app: A) exercise videos; B) self-monitoring system; C) visual display of self-reported pain intensity.
In both groups, follow-up calls were made once a month to monitor adherence and solve possible issues. Interventions are described in detailed in Supplementary Digital Material 1, Supplementary Tables I-III, following the intervention description and replication (TIDieR) checklist.29
Outcome measures
Clinical and demographic variables were obtained from all participants. Outcomes were collected by physicians or physical therapists trained in the use of the measurement tools.
Hand physical function
The primary outcome was self-reported hand physical function at 3- and 6-months postintervention, using the physical function subscale (9 items) of the Australian/Canadian Hand Osteoarthritis Index (AUSCAN), Likert version.30 The AUSCAN has shown high internal consistency and content validity, and acceptable test-retest reliability in individuals with hand OA.31 A 8% change has been established as clinically meaningful for AUSCAN physical function.32
Secondary outcomes
Secondary outcomes included self-reported measures of hand overall status, pain intensity and morning stiffness, and upper limb function. We used the AUSCAN Global Score and the stiffness (1 item) and pain (5 items: at rest, and while gripping, lifting, turning, and squeezing objects) subscales of AUSCAN at 3 and 6-months, and the short-form of the Disabilities of the Arm, Shoulder, and Hand questionnaire (QuickDASH),33 together with a 11-point NRS at 1, 3, and 6 months postintervention. The QuickDASH is a highly recommended tool in people with hand conditions,33 and the NRS is easy to administer and has demonstrated excellent psychometric properties.34 Additionally, performance-based function was assessed considering hand dexterity (Nine Hole Peg Test, NHPT),35 and grip and pinch strength using a dynamometer (Saehan SH5001, Saehan Corp., South Korea) and a Baseline® mechanical pinch gauge (Fabrication Enterprises, NY, USA).36
Sample size estimation
Based on previous research,20 sample size was estimated for the number of treated hands per group to detect a clinically relevant difference between groups in AUSCAN physical function (>4 points) at 3-months postintervention.32 We considered an alpha level of 0.05 with 80% power and a correlation among repeated measures of 0.5 (G*power software, v. 3.1.9.7, Kiel University, Kiel, Germany). A total of 122 hands were needed to account for a 20% attrition rate.
Statistical analysis
Statistical analysis was performed with the IBM Statistics Package for Social Science® software, v.26 (IBM Corp, NY, USA), using an intention-to-treat approach. The normality of variables was tested with the Shapiro-Wilk Test. Data are reported as mean±SD, mean and 95% confidence interval (CI), or in absolute numbers (frequency percentages). Baseline clinical and demographic data were analyzed with the independent sample t-test or Mann-Whitney tests for continuous variables, and the Fisher’s Exact Test for categorical variables.
A linear mixed model for repeated measures was conducted to compare the differences between groups in the changes of hand function and pain related measures from baseline to postintervention, with group (CareHand vs. usual care) as the between-subjects factor, time as the within-subjects factor, and baseline score of the NHPT as a covariate. The time variable included three points (baseline, 3 months, and 6 months postintervention) for AUSCAN and morning stiffness, four points (baseline, 1 month, 3 months, and 6 months) for the QuickDASH and pain intensity with the NRS, and two time points (baseline, 3 months) for performance-based function outcomes. Statistical significance was set to a P value <.05.
Multivariable linear regression of factor associated with changes in function and pain
We examined the association between changes in hand physical function and pain intensity from baseline to 3-months postintervention with patients’ demographics (gender and age), baseline scores of primary and secondary measures, and mean changes in the AUSCAN, NRS, and QuickDASH. We analyzed the correlation coefficients between each of the individual factors with hand function and pain intensity, and only those with r ≥ 0.3 were considered in the linear multivariable regression model.
Results
A total of 83 patients with hand OA were screened for eligibility between March 2020 and February 2021. Of those, 74 individuals (50 females, 67.5%) provided consent to participate and were randomized to the CareHand group (N.=34, 66 symptomatic hands) or usual care (N.=40, 78 symptomatic hands). Sixty-three participants completed assessments at the 3- and 6-months follow-ups (Figure 2).
Figure 2.
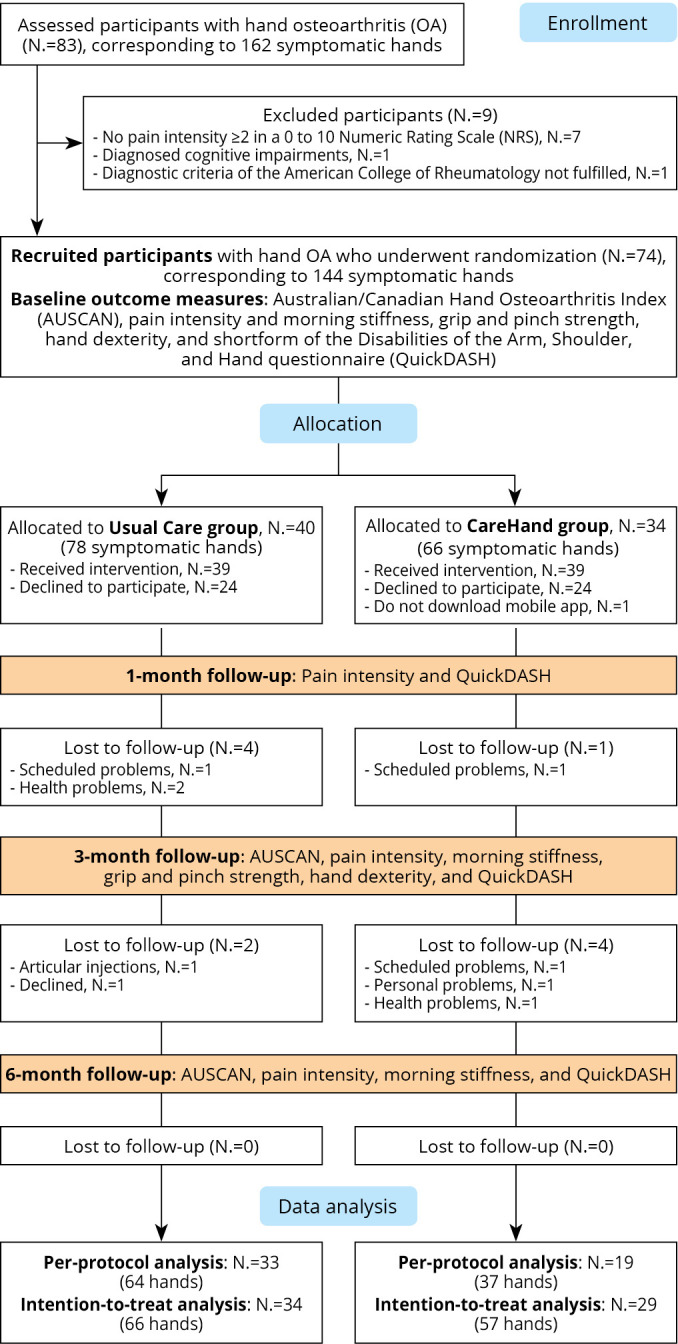
—CONSORT Flow chart diagram.
There were no baseline differences between groups in their clinical and demographic characteristics, except for hand dexterity (P=0.001) (Table I).
Table I. —Baseline clinical and demographic characteristics of participants.
| Baseline characteristics | CareHand group (N.=34, 66 hands) | Usual care (N.=40, 78 hands) | P value |
|---|---|---|---|
| Age | 62.2±8.8 | 64.3±7.7 | 0.242 |
| Sex: female, N. (%) | 25 (73%) | 25 (62%) | 0.417 |
| Dominant hand: right; left; both, N. (%) | 32 (94%); 1 (3%); 1 (3%) | 37 (92%); 0 (0%); 3 (8%) | 0.389 |
| Affected hand: right; left, N. (%) | 33 (50%); 33 (50%) | 39 (50%); 39 (50%) | 1.000 |
Normally distributed data are reported as mean±standard deviation. Categorical data are presented as absolute number and percent (%).
Hand physical function
The CareHand group showed significant within-group improvements in hand physical function at 6-months postintervention (mean difference, 95% CI: -3.0, 95% CI -5.1 to -0.9 vs. usual care: -0.9, 95% CI -3.3 to 1.5). There were no changes in this outcome for neither group at 3-months (CareHand: -1.5, 95% CI -3.1 to 0.1; usual care: -0.5, 95% CI -2.7 to 1.7) (Table II).
Table II. —Primary and secondary outcomes (baseline scores and within-groups mean differences at each timepoint).
| Variable | Group | Baseline | Change to 1-month | Change to 3-months | Change to 6-months | P value | |
|---|---|---|---|---|---|---|---|
| Group | Time | ||||||
| Primary outcome | |||||||
| AUSCAN function | CareHand (N.=66 hands) | 17.8±8.2 (15.7 to 19.8) |
NA | -1.5±5.8 (-3.1 to 0.1) |
-3.0±7.8 (-5.1 to -0.9)* |
0.252 | 0.034 |
| Usual care (N.=78 hands) | 16.9±8.3 (15.1 to 18.8) |
NA | -0.5±8.7 (-2.7 to 1.7) |
-0.9±9.5 (-3.3 to 1.5) |
|||
| Secondary measures | |||||||
| QuickDASH | CareHand (N.=66 hands) | 45.0±23.2 (36.9 to 53.1) |
-8.1±14.6 (-13.7 to -2.4)* |
-7.9±16.1 (-14.1 to -1.6)* |
-8.9±18.2 (-15.8 to -1.9)* |
0.041 | 0.048 |
| Usual care (N.=78 hands) | 46.9±22.2 (37.2 to 52.5) |
-6.3±16.2 (-12.2 to -0.5)* |
1.1±24.9 (-7.7 to 9.9) |
-1.2±28.0 (-11.1 to 8.8) |
|||
| AUSCAN Pain | CareHand (N.=66 hands) | 8.8±4.3 (7.7 to 9.8) |
NA | -1.3±4.6 (-2.5 to -0.1)* |
-2.0±4.6 (-3.2 to -0.8)* |
0.543 | 0.002 |
| Usual care (N.=78 hands) | 9.1±4.6 (8.1 to 10.1) |
NA | -0.4±5.1 (-1.6 to 0.9) |
-1.1±5.4 (-2.4 to -0.3) |
|||
| AUSCAN Stiffness | CareHand (N.=66 hands) | 1.3±1.1 (1.1 to 1.6) |
NA | -0.1±1.2 (-0.4 to 0.3) |
-0.3±1.4 (-0.7 to 0.1) |
0.246 | 0.035 |
| Usual care (N.=78 hands) | 1.4±1.2 (1.1 to 1.7) |
NA | 0.3±1.2 (0.0 to 0.6)* |
-0.1±1.5 (-0.4 to 0.3) |
|||
| AUSCAN Total | CareHand (N.=66 hands) | 27.9±12.4 (24.8 to 30.9) |
NA | -2.9±9.1 (-5.3 to -0.4)* |
-5.3±11.7 (-8.4 to -2.2)* |
0.271 | 0.004 |
| Usual care (N.=78 hands) | 27.4±13.2 (24.5 to 30.4) |
NA | -0.5±13.3 (-3.9 to 2.8) |
-2.1±14.3 (-5.6 to 1.5) |
|||
| NRS Pain | CareHand (N.=66 hands) | 5.7±2.6 (5.1 to 6.4) |
-0.8±2.5 (-1.5 to -0.1)* |
-0.7±2.4 (-1.3 to 0.0)* |
0.0±2.6 (-0.7 to 0.7) |
0.004 | 0.576 |
| Usual care (N.=78 hands) | 4.9±2.8 (4.3 to 5.6) |
0.9±3.1 (0.2 to 1.7)* |
1.0±3.2 (0.2 to 1.8)* |
0.4±3.5 (-0.4 to 1.3) |
|||
| NRS Stiffness | CareHand (N.=66 hands) | 4.5±3.4 (3.7 to 5.4) |
NA | -0.7±2.6 (-1.4 to 0.0)* |
-1.7±3.6 (-2.6 to -0.7)* |
0.193 | 0.001 |
| Usual care (N.=78 hands) | 4.8±3.2 (4.1 to 5.5) |
NA | 0.3±3.2 (-0.5 to 1.1) |
-0.8±3.9 (-1.8 to 0.1) |
|||
| Grip Strength | CareHand (N.=66 hands) | 40.8±21.9 (35.4 to 46.2) |
NA | -1.2±18.9 (-6.8 to 4.3) |
NA | 0.057 | 0.084 |
| Usual care (N.=78 hands) | 43.5±18.5 (39.4 to 47.7) |
NA | 3.4±15.1 (-0.7 to 7.5) |
NA | |||
| Pinch Strength | CareHand (N.=66 hands) | 10.3±4.7 (9.1 to 11.5) |
NA | -0.4±4.0 (-1.6 to 0.8) |
NA | 0.661 | 0.530 |
| Usual care (N.=78 hands) | 11.2±4.7 (10.1 to 12.3) |
NA | -0.1±3.1 (-0.9 to 0.8) |
NA | |||
| NHPT | CareHand (N.=66 hands) | 34.2±13.4 (30.9 to 37.5) |
NA | -3.8±11.8 (-7.3 to -0.3)* |
NA | 0.239 | 0.006 |
| Usual care (N.=78 hands) | 27.9±5.9 (26.6 to 29.3) |
NA | -1.6±6.9 (-3.4 to -0.3) |
NA | |||
Data are reported as mean±SD (95% confidence interval). *Significant intragroup mean difference P value<0.05. NRS: numeric rating scale; QuickDASH: short form of the Disabilities of the Arm, Shoulder, and Hand questionnaire; AUSCAN: Australian/Canadian Hand Osteoarthritis Index.
Secondary outcomes
The CareHand group intervention was more effective than usual care at improving upper limb function (F=3.006, P=0.041, η2=0.052), both at 3-months (CareHand: -7.9, 95% CI -14.1 to -1.6; usual care: 1.1, 95% CI -7.7 to 9.9) and 6-months postintervention (CareHand: -8.9, 95% CI -15.8 to -1.9; usual care: -1.2, 95% CI -11.1 to 8.8). In addition, hand pain intensity decreased in the CareHand group, compared to usual care (F=4.601, P=0.004, η2=0.040), at 1-month (CareHand: -0.8, 95% CI -1.5 to -0.1; usual care: 0.9, 95% CI 0.2 to 1.8) and 3-months after intervention (CareHand: -0.7, 95% CI -1.3 to -0.0; usual care: 1, 95% CI 0.2 to 1.8). There were no changes in hand pain intensity for those who received usual care during follow-up measurements.
Further, there was no time*group effect for any of the other secondary measures (all, P>0.05). However, for those in the CareHand group: 1) the AUSCAN overall status improved at 3-months (CareHand: -2.9, 95% CI -5.3 to -0.4; usual care: -0.5, 95% CI -3.9 to 2.8) and 6-months (CareHand: -5.3, 95% CI -8.4 to -2.2; usual care: -2.1, 95% CI -5.6 to 1.5); 2) the AUSCAN pain significantly decreased at 3-months (CareHand: -1.3, 95% CI -2.5 to -0.1; usual care: -0.4, 95% CI -1.6 to 0.9) and 6-months (CareHand: -2.0, 95% CI -3.2 to -0.8; usual care: -1.1, 95% CI -2.4 to -0.3); and 3) hand morning stiffness, assessed with a NRS, was reduced after intervention at 3-months (CareHand: -0.7, 95% CI -1.4 to 0.0; usual care: 0.3, 95% CI -0.5 to 1.1) and 6-months (CareHand: -1.7, 95% CI -2.6 to -0.7; usual care: -0.8, 95% CI -1.8 to 0.1).
As regards performance-based function outcomes, neither mHealth nor usual care could increase grip or pinch strength. Significant changes were only observed at 3-months for hand dexterity in the CareHand group (CareHand: -3.8 seconds, 95% CI -7.3 to -0.3, usual care: -1.6 seconds, 95% CI -3.4 to -0.3).
Factors associated with changes in hand physical function and pain intensity
Model 1 explained 62.9% of the variance in changes in AUSCAN physical function from baseline to 3-months postintervention. A lower pain intensity (AUSCAN pain) at baseline, a worse baseline hand overall status, and a greater decrease of pain from baseline to 3-months were associated with a higher improvement in hand physical function. For every point less in AUSCAN pain at baseline, there was a one-point increase in hand function (Table III).
Table III. —Factors associated with changes in hand physical function and pain intensity from baseline to 3 months postintervention.
| Model 1: Changes in Hand physical function (AUSCAN function), r2=0.629 | ||
|---|---|---|
| Factor | β (95% Confidence Interval) | P value |
| Hand pain intensity (AUSCAN pain) at baseline | 0.995 (0.788 to2.639) | 0.001 |
| Hand overall status (AUSCAN total) at baseline | -1.054 (-1.060 to -0.206) | 0.005 |
| Change in AUSCAN pain from baseline to 3 months postintervention | 0.592 (0.406 to1.431) | 0.001 |
| Model 2: Changes in Hand Pain intensity (NRS), r2=0.573 | ||
|---|---|---|
| Factor | β (95% Confidence Interval) | P value |
| Hand pain intensity (AUSCAN pain) at baseline | -0.603 (-0.997 to -0.334) | 0.001 |
| Upper limb function (QuickDASH) at baseline | 0.433 (0.008 to 0.106) | 0.023 |
| Change in QuickDASH from baseline to 3 months postintervention | 0.469 (0.016 to 0.112) | 0.010 |
AUSCAN: Australian/Canadian Hand Osteoarthritis Index; NRS: Numeric Rating Scale; QuickDASH: short form of the Disabilities of the Arm, Shoulder, and Hand Questionnaire.
Model 2 explained 57.3% of the variance in changes of pain intensity (NRS) from baseline to 3-months. A higher pain intensity, a poorer upper limb function before intervention, and a greater improvement of upper limb function at the 3-months follow-up were associated with a greater reduction of pain intensity. Sex, group, age, and the rest of clinical measures were included in the models, but no significant associations were found.
Adverse events
There were no serious adverse events during the study. One participant receiving usual care complained of an inflammatory flare-up and was prescribed corticosteroids. Three participants (2 in the CareHand group) complained of minor hand pain flare-ups during follow-up and another 3 (2 in the usual care group) experienced transient shoulder or neck pain that did not require additional treatment.
Discussion
This is the first randomized controlled trial to determine if home exercise therapy is more effective when delivered with a mHealth app or on paper in patients with symptomatic hand OA. Hand physical function increased over time, with significant changes at the 6-month follow-up for those who used the CareHand app. In addition, the home exercise program was more effective when performed with the mobile app, compared to usual care, to improve upper limb function and reduce hand pain intensity. For the rest of secondary measures, hand overall status, morning stiffness, and dexterity improved over time, with better results for the CareHand app, and neither group showed changes for grip or pinch strength. For the secondary aim, our findings support the importance of baseline scores of pain intensity, hand overall status, and upper limb function for changes over time in hand physical function and pain intensity.
Hand physical function
Patients with hand OA rate function as one of the most important domains affecting their daily life.37 Overall, previous literature concludes that exercise leads to a beneficial, but small, effect on hand function,5, 38 with some contradictory results.39 However, this evidence is mostly supported by low-quality trials with high heterogeneity and risk of bias.5, 38, 40 Our findings were in line with studies where home exercise programs were conducted together with joint protection materials,28 or within a multimodal protocol including nutritional advice and strategies to enhance self-efficacy.41, 42 Noteworthy, better results have been reported following supervised exercise regimes,43, 44 which could explain that the positive effects of exercise may sometimes decrease when performed as a home program.45 Nevertheless, a face-to-face approach is far from the reality of clinical settings, particularly in primary care, where rehabilitation practice tends to be home-based. When interpreting these results, it is important to bear in mind that significant changes were shown at the 6-month follow-up, but not at our primary endpoint. However, these observed improvements surpassed the clinically relevant threshold,32 which suggests a beneficial effect of the CareHand app. Despite these promising findings, the differences between studies in exercise dose, treatment delivery, and training routine and duration supports the need of new research to understand the impact of exercise on hand OA outcomes,38, 46 especially with digital tools.
We found that baseline scores of hand pain and overall status were associated with changes in hand function. This agrees with results showing that pain intensity is a high determinant of hand functional limitations in people with hand OA.47 In contrast, we found no correlation between age and changes in hand function. Future studies could cluster participants based on different levels of hand pain and function and provide more information of the role of risk factors in this population.8, 48
Secondary outcomes
The CareHand app was better than usual care to reduce upper limb functional impairments, with changes within the 8-to-16-point range considered as clinically meaningful.49 Hand exercise therapy, alone or together with education and assistive devices, has demonstrated a similar magnitude of improvement for upper limb function in previous research in hand OA.50, 51 However, evidence is still conflicting,52, 53 and surgery, i.e., joint arthroplasty, may become suitable for those with advanced disease who do not improve with conservative care.54 Cognitive and psychological factors, such as self-efficacy,55 and pain catastrophizing,56 can also predict patients’ upper limb disability, which could explain the different results in the literature. The CareHand app was more beneficial than usual care to decrease pain intensity. There is moderate certainty of evidence that hand exercises reduce pain and joint stiffness, and improve hand overall status in hand OA,5, 38, 46, 57 albeit with contradictory findings.58, 59 Although hand status, morning stiffness, and dexterity improved over time in both groups, changes were higher for those who used the mHealth app. In fact, these within-group improvements surpassed the clinically relevant threshold for AUSCAN pain and global score (15% and 8% change, respectively)32 at 6-months postintervention, which seems encouraging. Additionally, the regression analysis showed that improvements in pain intensity can be influenced by the level of hand pain and upper limb function before intervention. This has been already observed for pain intensity in adults with thumb base OA.60 In patients with hip or knee OA, different clusters have been characterized to target groups for tailored exercise interventions.10 Our results suggest that self-reported measures of hand pain and upper limb function could be also useful to clinically subgroup patients with hand OA.
Clinical guidelines recommend rehabilitation programs involving hand exercises to increase hand strength and dexterity,57 but present evidence is ambiguous. Some systematic reviews suggest a small to moderate positive impact of exercise on hand strength,5, 38, 61 while others conclude otherwise.39 The effect of exercise does not seem to be influenced by how the intervention is delivered. In fact, positive and negative results have been observed after group-based sessions,42, 43, 50 individual face-to-face interventions,28, 44 home exercise programs,24, 41, 62 or a combination of those.26 Hand dexterity is essential for good performance in daily life activities,35 but it has been rarely included as an outcome measure in studies about exercise therapy in hand OA. The scarce literature on this topic has shown either a lack of effect of the exercise program26, 59 or slight improvements.53 In the latter case, the changes were attributed to the use of bimanual tasks and fine motor skills movements.53 In short, the heterogeneous literature on the topic,38 the differences among studies in how grip strength is measured,51 and other factors, i.e., patient’s age, sex, physical fitness, and comorbidities, can influence performance-based function measures in individuals with hand OA.63
Hand OA and mHealth
The growing burden of OA and the rapid advance of new technologies have raised the question whether e-health modalities may have a similar impact than usual care for OA.14 Digitally-delivered rehabilitation can lead to improvements in pain and function,14 and promote adherence to treatment64 in patients with hip or knee OA. However, this remains uncertain for hand OA.14 Most existing apps for the management of rheumatic diseases are low-quality and not evidence-based.65, 66 Therefore, a mHealth app combining exercise with video demonstrations and a self-monitoring system could support people with hand OA long term and reduce the costs associated with face-to-face sessions.19 The CareHand app has proven to be effective to increase hand function and work performance and reduce pain intensity in individuals with rheumatoid arthritis of the hands.20 Our current findings seem to support partially these results.
New technologies can be a solution to enhance access to health care in rural settings.67 Older age and living in a rural area are often cited as barriers for patients’ adoption of mHealth, together with social, organizational, health, and political factors.68 A proper balance between telehealth and in-person attention may increase engagement with digital primary care.69 In the present study, neither age nor the clinical setting were perceived as hindering factors for adherence to treatment. However, none of these aspects were specifically investigated and their exact role remains to be elucidated.
Strengths and limitations of the study
The main strength of this clinical trial is that, for the first time, the effect of a mHealth app has been compared with usual care in older adults with hand OA living in a rural area. Yet, this sample may not represent a wide spectrum of patients. Additionally, usual care did not include self-management recommendations. Although this may have influenced the results, our pragmatic approach aimed to reflect routine clinical practice. Those factors associated with changes in hand pain and function were assessed at the 3-month follow-up despite only changes in hand pain intensity were shown at this assessment point. Due to the onset of the COVID-19 pandemic, some participants reported a state of stress or anxiety during the study period, which could have influenced their approach to interventions and the findings. In addition, the mHealth app could not collect data for treatment adherence, which may be of clinical interest. Finally, a cost-utility analysis based on consumption of health care resources and the health-related quality of life of participants would be conducted in a future study.
Conclusions
A mobile app-delivered home exercise program including education and self-management recommendation is an effective intervention to increase hand physical function, and better than usual care to improve upper limb function and hand pain intensity. Baseline levels of hand pain and upper limb function can predict changes in hand physical function and pain intensity. Future research should confirm the clinical impact of mHealth in hand OA in different settings and populations.
Funding.—This study is part of the CareHand project, which has received funding from the Fundación Pública Andaluza Progreso y Salud, Consejería de Salud y Consumo, Junta de Andalucía, Spain (reference code AP-0149-2017).
Supplementary Digital Material 1
Supplementary Table I
Summary of the interventions included in the present study, following the TIDieR recommendations.
Supplementary Table II
Description of the home exercise program in the usual care group.
Supplementary Table III
Description of the home exercise program in the CareHand group.
References
- 1.Eaton CB, Schaefer LF, Duryea J, Driban JB, Lo GH, Roberts MB, et al. Prevalence, Incidence, and Progression of Radiographic and Symptomatic Hand Osteoarthritis: The Osteoarthritis Initiative. Arthritis Rheumatol 2022;74:992–1000. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35077023&dopt=Abstract 10.1002/art.42076 [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Barbour KE, Murphy LB, Nelson AE, Schwartz TA, Helmick CG, et al. Lifetime Risk of Symptomatic Hand Osteoarthritis: The Johnston County Osteoarthritis Project. Arthritis Rheumatol 2017;69:1204–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28470947&dopt=Abstract https://doi.org/ 10.1002/art.40097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magni NE, McNair PJ, Rice DA. Impairments in grip and pinch force accuracy and steadiness in people with osteoarthritis of the hand: A case-control comparison. Musculoskelet Sci Pract 2021;55:102432. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34333399&dopt=Abstract 10.1016/j.msksp.2021.102432 [DOI] [PubMed] [Google Scholar]
- 4.Gracia-Ibáñez V, Agost MJ, Bayarri-Porcar V, Granell P, Vergara M, Sancho-Bru JL. Hand kinematics in osteoarthritis patients while performing functional activities. Disabil Rehabil 2022;1–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35298313&dopt=Abstract 10.1080/09638288.2022.2051082 [DOI] [PubMed]
- 5.Kroon FP, Carmona L, Schoones JW, Kloppenburg M. Efficacy and safety of non-pharmacological, pharmacological and surgical treatment for hand osteoarthritis: a systematic literature review informing the 2018 update of the EULAR recommendations for the management of hand osteoarthritis. RMD Open 2018;4:e000734. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30402266&dopt=Abstract 10.1136/rmdopen-2018-000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol 2020;72:220–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31908163&dopt=Abstract 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis 2019;78:16–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30154087&dopt=Abstract 10.1136/annrheumdis-2018-213826 [DOI] [PubMed] [Google Scholar]
- 8.Iolascon G, Ruggiero C, Fiore P, Mauro GL, Moretti B, Tarantino U. Multidisciplinary integrated approach for older adults with symptomatic osteoarthritis: SIMFER and SI-GUIDA Joint Position Statement. Eur J Phys Rehabil Med 2020;56:112–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31742367&dopt=Abstract 10.23736/S1973-9087.19.05837-4 [DOI] [PubMed] [Google Scholar]
- 9.Ritschl V, Stamm T, Aletaha D, Bijlsma JW, Boehm P, Dragoi R, et al. 2020 EULAR Points To Consider for the Detection, Assessment and Management of Non-Adherence in People With Rheumatic and Musculoskeletal Diseases. EULAR e-Congress; 2020. p. 1–7. [Google Scholar]
- 10.Krauss I, Katzmarek U, Rieger MA, Sudeck G. Motives for physical exercise participation as a basis for the development of patient-oriented exercise interventions in osteoarthritis: a cross-sectional study. Eur J Phys Rehabil Med 2017;53:590–602. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28215059&dopt=Abstract 10.23736/S1973-9087.17.04482-3 [DOI] [PubMed] [Google Scholar]
- 11.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken) 2020;72:149–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31908149&dopt=Abstract 10.1002/acr.24131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cronström A, Dahlberg LE, Nero H, Ericson J, Hammarlund CS. ‘I would never have done it if it hadn’t been digital’: a qualitative study on patients’ experiences of a digital management programme for hip and knee osteoarthritis in Sweden. BMJ Open 2019;9:e028388. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31129601&dopt=Abstract 10.1136/bmjopen-2018-028388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seron P, Oliveros MJ, Gutierrez-Arias R, Fuentes-Aspe R, Torres-Castro RC, Merino-Osorio C, et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys Ther 2021;101:1–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33561280&dopt=Abstract 10.1093/ptj/pzab053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safari R, Jackson J, Sheffield D. Digital self-management interventions for people with osteoarthritis: systematic review with meta-analysis. J Med Internet Res 2020;22:e15365. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32706657&dopt=Abstract 10.2196/15365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darlow B, Brown M, Hudson B, Frew G, Clark J, Vincent L, et al. Feasibility of a randomised controlled trial of two types of written information for people with knee osteoarthritis. Osteoarthr Cartil Open 2022;4:100254. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36475283&dopt=Abstract 10.1016/j.ocarto.2022.100254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niknejad N, Ismail W, Bahari M, Nazari B. Understanding Telerehabilitation Technology to Evaluate Stakeholders’ Adoption of Telerehabilitation Services: A Systematic Literature Review and Directions for Further Research. Arch Phys Med Rehabil 2021;102:1390–403. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33484693&dopt=Abstract 10.1016/j.apmr.2020.12.014 [DOI] [PubMed] [Google Scholar]
- 17.Slattery BW, Haugh S, O’Connor L, Francis K, Dwyer CP, O’Higgins S, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. J Med Internet Res 2019;21:e11086. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31317869&dopt=Abstract 10.2196/11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanquero J, Cortés-Vega MD, Rodríguez-Sánchez-Laulhé P, Corrales-Serra BP, Gómez-Patricio E, Díaz-Matas N, et al. Feedback-guided exercises performed on a tablet touchscreen improve return to work, function, strength and healthcare usage more than an exercise program prescribed on paper for people with wrist, hand or finger injuries: a randomised trial. J Physiother 2020;66:236–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33069608&dopt=Abstract 10.1016/j.jphys.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Hammond A, Prior Y. The effectiveness of home hand exercise programmes in rheumatoid arthritis: a systematic review. Br Med Bull 2016;119:49–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27365455&dopt=Abstract 10.1093/bmb/ldw024 [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez Sánchez-Laulhé P, Luque-Romero LG, Barrero-García FJ, Biscarri-Carbonero Á, Blanquero J, Suero-Pineda A, et al. An Exercise and Educational and Self-management Program Delivered With a Smartphone App (CareHand) in Adults With Rheumatoid Arthritis of the Hands: Randomized Controlled Trial. JMIR Mhealth Uhealth 2022;10:e35462. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35389367&dopt=Abstract 10.2196/35462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seppen BF, Wiegel J, L’ami MJ, Duarte Dos Santos Rico S, Catarinella FS, Turkstra F, et al. Feasibility of self-monitoring rheumatoid arthritis with a smartphone app: results of two mixed-methods pilot studies. JMIR Form Res 2020;4:e20165. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32955447&dopt=Abstract 10.2196/20165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollard E, Michaud K. A mobile app with optical imaging for the self-management of hand rheumatoid arthritis: pilot study. JMIR Mhealth Uhealth 2018;6:e12221. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30373732&dopt=Abstract 10.2196/12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein K, Brandt K, et al. the American College of Rheumatology Reporting O F Osteoarthritis of the Hand. Arthritis Rheum 1990;33:1601–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2242058&dopt=Abstract 10.1002/art.1780331101 [DOI] [PubMed] [Google Scholar]
- 24.Hennig T, Hæhre L, Hornburg VT, Mowinckel P, Norli ES, Kjeken I. Effect of home-based hand exercises in women with hand osteoarthritis: a randomised controlled trial. Ann Rheum Dis 2015;74:1501–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24667900&dopt=Abstract 10.1136/annrheumdis-2013-204808 [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Robbins S, Eyles J, Fedorova T, Virk S, Deveza LA, et al. Efficacy and safety of a supplement combination on hand pain among people with symptomatic hand osteoarthritis an internet-based, randomised clinical trial the RADIANT study. Osteoarthritis Cartilage 2021;29:667–77. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33617972&dopt=Abstract 10.1016/j.joca.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Østerås N, Hagen KB, Grotle M, Sand-Svartrud AL, Mowinckel P, Kjeken I. Limited effects of exercises in people with hand osteoarthritis: results from a randomized controlled trial. Osteoarthritis Cartilage 2014;22:1224–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25008206&dopt=Abstract 10.1016/j.joca.2014.06.036 [DOI] [PubMed] [Google Scholar]
- 27.Sandal LF, Roos EM, Bøgesvang SJ, Thorlund JB. Pain trajectory and exercise-induced pain flares during 8 weeks of neuromuscular exercise in individuals with knee and hip pain. Osteoarthritis Cartilage 2016;24:589–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26564575&dopt=Abstract 10.1016/j.joca.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 28.Dziedzic K, Nicholls E, Hill S, Hammond A, Handy J, Thomas E, et al. Self-management approaches for osteoarthritis in the hand: a 2×2 factorial randomised trial. Ann Rheum Dis 2015;74:108–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24107979&dopt=Abstract 10.1136/annrheumdis-2013-203938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24609605&dopt=Abstract 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 30.Bellamy N, Campbell J, Haraoui B, Gerecz-Simon E, Buchbinder R, Hobby K, et al. Clinimetric properties of the AUSCAN Osteoarthritis Hand Index: an evaluation of reliability, validity and responsiveness. Osteoarthritis Cartilage 2002;10:863–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12435331&dopt=Abstract 10.1053/joca.2002.0838 [DOI] [PubMed] [Google Scholar]
- 31.Bobos P, MacDermid JC, Boutsikari EC, Lalone EA, Ferreira L, Grewal R. Evaluation of the content validity index of the Australian/Canadian osteoarthritis hand index, the patient-rated wrist/hand evaluation and the thumb disability exam in people with hand arthritis. Health Qual Life Outcomes 2020;18:302. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32907589&dopt=Abstract 10.1186/s12955-020-01556-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellamy N, Hochberg M, Tubach F, Martin-Mola E, Awada H, Bombardier C, et al. Development of multinational definitions of minimal clinically important improvement and patient acceptable symptomatic state in osteoarthritis. Arthritis Care Res (Hoboken) 2015;67:972–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25581339&dopt=Abstract 10.1002/acr.22538 [DOI] [PubMed] [Google Scholar]
- 33.Wormald JC, Geoghegan L, Sierakowski K, Price A, Peters M, Jain A, et al. Site-specific Patient-reported Outcome Measures for Hand Conditions: Systematic Review of Development and Psychometric Properties. Plast Reconstr Surg Glob Open 2019;7:e2256. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31333975&dopt=Abstract 10.1097/GOX.0000000000002256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castarlenas E, de la Vega R, Jensen MP, Miró J. Self-Report Measures of Hand Pain Intensity: Current Evidence and Recommendations. Hand Clin 2016;32:11–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26611384&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am J Occup Ther 2003;57:570–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14527120&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 36.Coldham F, Lewis J, Lee H. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J Hand Ther 2006;19:318–26, quiz 327. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16861131&dopt=Abstract 10.1197/j.jht.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 37.Leung YY, Li JC, Thumboo J. Domains rated as important by patients with hand osteoarthritis. Int J Rheum Dis 2019;22:2045–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31722448&dopt=Abstract 10.1111/1756-185X.13709 [DOI] [PubMed] [Google Scholar]
- 38.Østerås N, Kjeken I, Smedslund G, Rh M, Uhlig T, Kb H. Exercise for hand osteoarthritis (Review). Cochrane Database Syst Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magni NE, McNair PJ, Rice DA. The effects of resistance training on muscle strength, joint pain, and hand function in individuals with hand osteoarthritis: a systematic review and meta-analysis. Arthritis Res Ther 2017;19:131. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28610637&dopt=Abstract 10.1186/s13075-017-1348-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lue S, Koppikar S, Shaikh K, Mahendira D, Towheed TE. Systematic review of non-surgical therapies for osteoarthritis of the hand: an update. Osteoarthritis Cartilage 2017;25:1379–89. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28602781&dopt=Abstract 10.1016/j.joca.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 41.Stoffer-Marx MA, Klinger M, Luschin S, Meriaux-Kratochvila S, Zettel-Tomenendal M, Nell-Duxneuner V, et al. Functional consultation and exercises improve grip strength in osteoarthritis of the hand - a randomised controlled trial. Arthritis Res Ther 2018;20:253. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30413191&dopt=Abstract 10.1186/s13075-018-1747-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stukstette MJ, Dekker J, den Broeder AA, Westeneng JM, Bijlsma JW, van den Ende CH. No evidence for the effectiveness of a multidisciplinary group based treatment program in patients with osteoarthritis of hands on the short term; results of a randomized controlled trial. Osteoarthritis Cartilage 2013;21:901–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23583457&dopt=Abstract https://doi.org/ 10.1016/j.joca.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 43.Nery M, Natour J, Jennings F, Fernandes AD, Souza MC, Jones A. Effects of a progressive resistance exercise program in patients with hand osteoarthritis: A randomized, controlled trial with a blinded assessor. Clin Rehabil 2021;35:1757–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34240642&dopt=Abstract 10.1177/02692155211030622 [DOI] [PubMed] [Google Scholar]
- 44.Kang TW, Lee JH, Park DH, Cynn HS. Effects of a finger exercise program on hand function in automobile workers with hand osteoarthritis: A randomized controlled trial. Hand Surg Rehabil 2019;38:59–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30401613&dopt=Abstract 10.1016/j.hansur.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 45.Thacker J, Bosello F, Ridehalgh C. Do behaviour change techniques increase adherence to home exercises in those with upper extremity musculoskeletal disorders? A systematic review. Musculoskelet Care 2021;19:340–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33331093&dopt=Abstract 10.1002/msc.1532 [DOI] [PubMed] [Google Scholar]
- 46.Veronese N, Smith L, Bolzetta F, Cester A, Demurtas J, Punzi L. Efficacy of conservative treatments for hand osteoarthritis: an umbrella review of interventional studies. Wien Klin Wochenschr 2021;133:234–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32607645&dopt=Abstract 10.1007/s00508-020-01702-0 [DOI] [PubMed] [Google Scholar]
- 47.Bijsterbosch J, Watt I, Meulenbelt I, Rosendaal FR, Huizinga TW, Kloppenburg M. Clinical and radiographic disease course of hand osteoarthritis and determinants of outcome after 6 years. Ann Rheum Dis 2011;70:68–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20736393&dopt=Abstract 10.1136/ard.2010.133017 [DOI] [PubMed] [Google Scholar]
- 48.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31034380&dopt=Abstract 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed]
- 49.Garcia AN, Thigpen CA, Lake AD, Martinez C, Myers H, Cook C. Do older adults with shoulder disorders who meet the minimal clinically important difference also present low disability at discharge? An observational study. Braz J Phys Ther 2020;24:152–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30885628&dopt=Abstract 10.1016/j.bjpt.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjurehed L, Brodin N, Nordenskiöld U, Björk M. Improved Hand Function, Self-Rated Health and Decreased Activity Limitations - results after a two month hand osteoarthritis group intervention. Arthritis Care Res (Hoboken) 2018;70:1039–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28973832&dopt=Abstract 10.1002/acr.23431 [DOI] [PubMed] [Google Scholar]
- 51.Tveter AT, Østerås N, Nossum R, Eide RE, Klokkeide Å, Matre KH, et al. Short-term effects of occupational therapy on hand function and pain in patients with carpometacarpal osteoarthritis: secondary analyses from a randomized controlled trial. Arthritis Care Res (Hoboken) 2022;74:955–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33338325&dopt=Abstract 10.1002/acr.24543 [DOI] [PubMed] [Google Scholar]
- 52.Davenport BJ, Jansen V, Yeandle N, Unit PH, Hospitals D, Trust F. Pilot randomized controlled trial comparing specific dynamic stability exercises with general exercises for thumb carpometacarpal joint osteoarthritis. Hand Ther 2012;17:60–7. 10.1258/ht.2012.012010 [DOI] [Google Scholar]
- 53.Pérez-Mármol JM, García-Ríos MC, Ortega-Valdivieso MA, Cano-Deltell EE, Peralta-Ramírez MI, Ickmans K, et al. Effectiveness of a fine motor skills rehabilitation program on upper limb disability, manual dexterity, pinch strength, range of fingers motion, performance in activities of daily living, functional independency, and general self-efficacy in hand osteoarthritis: A randomized clinical trial. J Hand Ther 2017;30:262–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28502698&dopt=Abstract 10.1016/j.jht.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 54.Gómez-Garrido D, Triviño-Mayoral V, Delgado-Alcala V, Cervera-Irimia J, Medina-Lorca M, Sánchez-Sánchez F, et al. Five year long term results of total joint arthroplasties in the treatment of trapeziometacarpal osteoarthritis. Acta Biomed 2019;90:451–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31910169&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Calderon J, Zamora-Campos C, Navarro-Ledesma S, Luque-Suarez A. The Role of Self-Efficacy on the Prognosis of Chronic Musculoskeletal Pain: A Systematic Review. J Pain 2018;19:10–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28939015&dopt=Abstract 10.1016/j.jpain.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 56.Cheng H, Novak CB, Veillette C, von Schroeder HP. Influence of psychological factors on patient-reported upper extremity disability. J Hand Surg Eur Vol 2020;45:71–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31272266&dopt=Abstract 10.1177/1753193419859373 [DOI] [PubMed] [Google Scholar]
- 57.Brosseau L, Thevenot O, MacKiddie O, Taki J, Wells GA, Guitard P, et al. The Ottawa Panel guidelines on programmes involving therapeutic exercise for the management of hand osteoarthritis. Clin Rehabil 2018;32:1449–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29911409&dopt=Abstract 10.1177/0269215518780973 [DOI] [PubMed] [Google Scholar]
- 58.Leonard G, Paquet N, Guitard P, Toupin-April K, Cavallo S, Paterson G, et al. The effects of an 8-week knitting program on osteoarthritis symptoms in elderly women: A pilot randomized controlled trial. J Bodyw Mov Ther 2021;27:410–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34391264&dopt=Abstract https://doi.org/ 10.1016/j.jbmt.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 59.Rogers MW, Wilder FV. Exercise and hand osteoarthritis symptomatology: a controlled crossover trial. J Hand Ther 2009;22:10–7, discussion 19–20, quiz 18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19013758&dopt=Abstract 10.1016/j.jht.2008.09.002 [DOI] [PubMed]
- 60.Wouters RM, Tsehaie J, Slijper HP, Hovius SE, Feitz R, Selles RW, Hand-Wrist Study Group . Exercise Therapy in Addition to an Orthosis Reduces Pain More Than an Orthosis Alone in Patients With Thumb Base Osteoarthritis: A Propensity Score Matching Study. Arch Phys Med Rehabil 2019;100:1050–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30543802&dopt=Abstract 10.1016/j.apmr.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 61.Ye L, Kalichman L, Spittle A, Dobson F, Bennell K. Effects of rehabilitative interventions on pain, function and physical impairments in people with hand osteoarthritis: a systematic review. Arthritis Res Ther 2011;13:R28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21332991&dopt=Abstract 10.1186/ar3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasapoğlu Aksoy M, Altan L. Short-term efficacy of paraffin therapy and home-based exercise programs in the treatment of symptomatic hand osteoarthritis. Turk J Phys Med Rehabil 2017;64:108–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31453499&dopt=Abstract 10.5606/tftrd.2018.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haugen IK, Aaserud J, Kvien TK. Get a Grip on Factors Related to Grip Strength in Persons With Hand Osteoarthritis: Results From an Observational Cohort Study. Arthritis Care Res (Hoboken) 2021;73:794–800. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32702166&dopt=Abstract 10.1002/acr.24385 [DOI] [PubMed] [Google Scholar]
- 64.Patten RK, Tacey A, Pile R, Parker A, De Gori M, Tran P, et al. Digital self-management interventions for osteoarthritis: a systematic scoping review of intervention characteristics, adherence and attrition. Arch Public Health 2022;80:103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35361270&dopt=Abstract 10.1186/s13690-022-00854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dantas LO, Carvalho C, Prando BC, McAlindon TE, da Silva Serrão PR. Mobile health technologies for the management of rheumatic diseases: a systematic review of online stores in Brazil. Clin Rheumatol 2021;40:2601–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33389312&dopt=Abstract 10.1007/s10067-020-05561-y [DOI] [PubMed] [Google Scholar]
- 66.Knitza J, Tascilar K, Messner EM, Meyer M, Vossen D, Pulla A, et al. German mobile apps in rheumatology: review and analysis using the mobile application rating scale (MARS). JMIR Mhealth Uhealth 2019;7:e14991. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31381501&dopt=Abstract 10.2196/14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batsis JA, DiMilia PR, Seo LM, Fortuna KL, Kennedy MA, Blunt HB, et al. Effectiveness of Ambulatory Telemedicine Care in Older Adults: A Systematic Review. J Am Geriatr Soc 2019;67:1737–49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31066916&dopt=Abstract 10.1111/jgs.15959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacob C, Sezgin E, Sanchez-Vazquez A, Ivory C. Socio-technical Factors Impacting Patients’ Adoption of Mobile Health Tools: Systematic Literature Review and Narrative Synthesis (Preprint). JMIR Mhealth Uhealth 2022;10:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindberg J, Bhatt R, Ferm A. Older people and rural eHealth: perceptions of caring relations and their effects on engagement in digital primary health care. Scand J Caring Sci 2021;35:1322–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33448031&dopt=Abstract 10.1111/scs.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Summary of the interventions included in the present study, following the TIDieR recommendations.
Supplementary Table II
Description of the home exercise program in the usual care group.
Supplementary Table III
Description of the home exercise program in the CareHand group.