Abstract
AIM
This study aimed to compare the effects of myofascial release (MFR) on upper extremity volume in patients with breast cancer-related lymphedema (BCRL).
DESIGN
A randomized, single-blinded, cross-over, controlled trial.
SETTING
An outpatient rehabilitation clinical setting.
POPULATION
Thirty patients with BCRL.
METHODS
Within a crossover design with randomized treatment sequences, fifteen subjects received MFR for 4 weeks, followed by 4 weeks of washout period, and then received placebo MFR and the other fifteen subjects received interventions in the reverse order. Each session had a 60 min process including either MFR or placebo MFR for 30 min, followed by complete decongestive therapy for 30 min twice a week. Upper limb volume as the primary outcome and subjective pain, shoulder range of motion (ROM), chest mobility, shoulder function, and quality of life as secondary outcomes were assessed before and at the end of each intervention period.
RESULTS
There were significant differences in upper limb volume after both MFR and placebo MFR (P<0.05) while no significant difference between MFR and placebo MFR treatments was found (P>0.05). MFR-based treatment also achieved a greater improvement than placebo MFR-based treatment in subjective pain and shoulder ROM (P<0.05), except for internal rotation, and shoulder function.
CONCLUSIONS
MFR-based treatment showed clinical improvement in shoulder function, induced by decreased edema volume and pain, and improved ROM and chest mobility. However, a further study with parallel randomized controlled trials to confirm what was achieved in the present study.
CLINICAL REHABILITATION IMPACT
MFR-based treatment is considered an important part of BCRL rehabilitation. Moreover, MFR-based treatment may be safe for patients with BCRL.
Key words: Breast cancer lymphedema; Myofascial release therapy; Pain; Range of motion, articular; Shoulder; Quality of life
Recently, according to statistics in Global Cancer Statistics 2020,1 the incidence of breast cancer has been on the rise worldwide, ranking the first among cancers in female in South Korea (23.7%) and the world (24.5%). With recent developments in medical technology, the overall 5-year relative survival rate for breast cancer patients has reached 80%, but survival is affected by various sequelae.2 Secondary lymphedema is one of the various side effects of breast cancer, and it occurs in up to 40% of patients with breast cancer, which would lead to developing into a chronic disease.3
Postoperative myofascial restriction causes high tensile strength, which has negative effects on secondary lymphedema by slowing lymphatic flow.4 In addition, an unspecified inflammatory response to the lungs by radiation therapy or local muscular defense caused by reduced movement of the affected arm to protect the surgical site cause poor respiratory function and reduced chest mobility in patients with breast cancer.5 Decreased respiratory volume due to chest mobility restriction reduces the central flow of venous and lymphatic fluid, increasing peripheral lymphedema volume.6 For these reasons, recent postoperative rehabilitation approaches for breast cancer patients have focused on reducing pain and improving shoulder ROM, shoulder function, and QoL by releasing the adhesions in fibrous thoracic and upper extremity myofascia and loosening surgical wound tissue.7, 8
Different management strategies for breast cancer-related lymphedema (BCRL) are found in the scientific literature, such as manual lymphatic drainage (MLD), compression therapy, physical exercise, and risk factor management (infection management, wound care, etc.).9 Compression therapies, such as compression sleeve, bandages, and intermittent pneumatic compression pumps, can be used to increase the fluid filtered from the capillary through external pressure instead of damaged lymphatic circulation.10 MLD has been widely used as a common treatment for lymphedema related to breast cancer.11 This technique is used to drain out stacked lymph into unaffected lymph nodes by stimulating lymph nodes under the skin with low pressure to increase rhythmic contractions of the lymphatic system, thereby promoting the flow of lymphatic fluid.10
The fascia is made up of fibrous connective tissue that glides back to its original length under load, and decreased muscle and joint movements cause fascia contracture.12
Because the brachial fascia is a distal extension of the shoulder girdle muscles, breast cancer surgery disturbs the glide of the brachial fascia, causing pain and leading to restrictions in daily activities.13 It is known that changes in the superficial fascia may cause lymphedema, so any intervention for the fascia can improve symptoms related to lymphedema.14
Myofascial release (MFR) is a manual technique in which a therapist feels for stiff or tightened points and applies manual pressure to the trigger points until they are certain that the tension is fully released.12 Research on the effect of MFR in breast cancer patients after surgery has been widely conducted, and the importance of MFR has been emphasized since MFR showed physical benefits in patients with breast cancer surgery.15, 16 MFR significantly reduced persistent upper extremity pain,16 improved shoulder ROM and function,17 and improved QoL18 in breast cancer. In addition, since lymphatic flow can be promoted by fascia movement, fascial restriction may lead to lymphatic system dysfunction that blocks fluid flow can be applied for helping lymph to return the system in lymphedema.19 However, there is no study on the effect of MFR on the reduction of edema in patients diagnosed with BCRL. Therefore, this study aims to compare the effects of MFR primarily on upper extremity volume in patients with BCRL.
Materials and methods
Study design
This randomized cross-sectional study was conducted in accordance with the Consolidated Standards of Reporting Trial (CONSORT) recommendations and was registered on a cris.org website (KCT0007156). The intervention was reported based on the template for the intervention description and replication (TIDieR) checklist and guide. The study protocol was approved by the Gachon University Institutional Review Board (1044396-202112-HR-237-01) and conducted in accordance with the Declaration of Helsinki. Before the study was conducted, the participants were informed about the study’s aim and measures were undertaken to protect their privacy. Participants who agreed to participate in the study signed an informed consent form.
Participants
Thirty patients with BCRL were recruited from the outpatient settings at local hospitals in the city of Incheon, Korea. The inclusion criteria were as follows: suffering from at least 6 months after mastectomy for breast cancer; being diagnosed with stage I-II lymphedema by physiatrist, as defined by the International Society of Lymphology; having present pain and limited shoulder function. Participants were excluded if they have any musculoskeletal injury in adjacent areas (shoulder or neck etc.), bilateral lymphadenectomy, open wounds on upper limbs, circulatory disorders, and history of shoulder surgery for reasons other than breast cancer. Participants were also excluded if they did not follow the rehabilitation procedure due to cognitive impairment.
The sample size was calculated using G Power 3.1.9.7 software (Heinrich Heine University, Dusseldorf, Germany). Since there were a few studies investigating the effect of MFR mainly targeting lymphedema volume, a medium effect size of d=0.5 was considered to calculate the sample size.20 Twenty-seven participants were required to detect statistical significance when a clinically significant difference was observed between two dependent means, with an effect size of d=0.5, significance level of 0.05, and power of 0.80. An additional 10% of the patients were recruited to provide unanticipated attrition.
Study procedure
This study was a randomized, single-blinded (participants), 2 × 2 cross-over, controlled trials. Simple randomization of the order of interventions was conducted by a study coordinator who was not involved in the assessment and data analysis process, and allocation was performed using Randomization.com website before data collection. The group A received MFR with complex decongestive therapy (CDT) for 4 weeks, followed by 4 weeks of washout period, then received placebo MFR with CDT for another 4 weeks with the group B receiving intervention in the reverse order. Both groups underwent intervention for 60 min (either MFR or placebo MFR for 30 min followed by CDT for 30 min) twice a week. In accordance with the crossover design, a sufficient washout period was provided to reverse the effect of the intervention through a 4-week recess between the two interventions.21, 22 To evaluate the effectiveness of the intervention, the outcomes were measured four times: week 0 before treatment (T0), week 4 after the first treatment (T1), week 8 after the washout period (T2), and week 12 after the second treatment (Figure 1).
Figure 1.
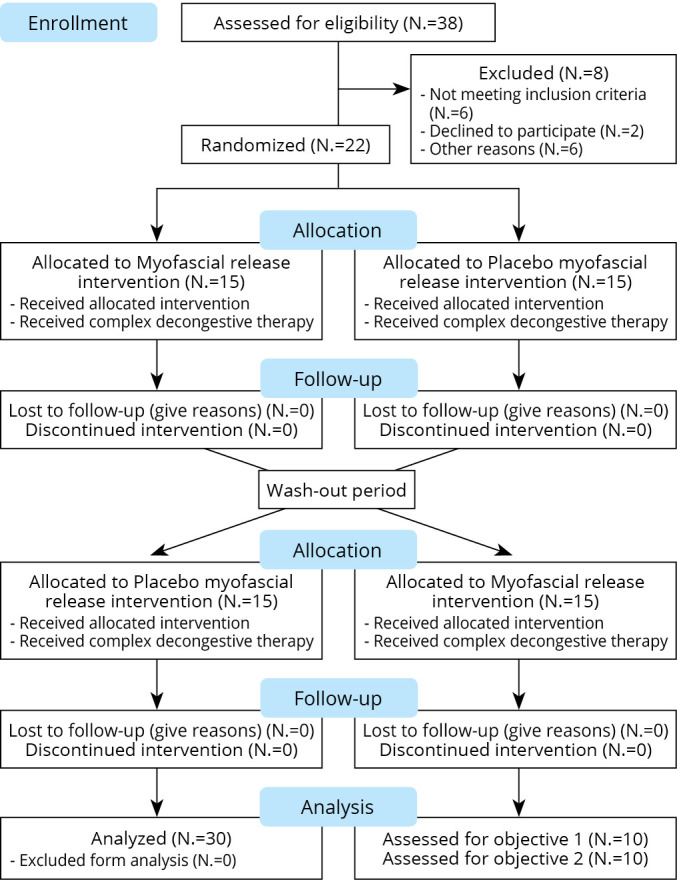
—Flowchart according to the CONSORT statement for the reporting of randomized trials.
Outcome measurements
Primary outcome - upper limb volume
Upper limb volume was measured using circumference measurements.23, 24 The anatomical positions of the arm were selected at 7 cm apart, at the wrist (the slenderest part of the wrist), 7 cm above the wrist (lower arm), 7 cm below the elbow (lower arm), at the elbow, 7 cm above the elbow (upper arm), and at the same level as the axilla (upper arm). To minimize measurement error, all measurements were taken at a constant pressure with sitting position in which the hips and knees were bent at 90 degrees.25 The volume of a truncated cone is used as a circumference measurement for calculating the upper limb volume (V = h * (Ct2 + Ct*Cb + Cb2)/ (12 * π)), where V is the volume of a segment of the upper extremity, Ct is the circumferences (in cm) of the top of the cone, Cb is the circumferences (in cm) of the base of the cone, and h is the distance between circumferences (Ct, Cb) in each segment (h=7 cm was used). Total volume of the upper extremity was measured by sum of each V (volume of a segment) (Figure 2). The minimal clinically important difference (MCID) for upper limb volume is 2.39% or 42.9 mL.26
Figure 2.
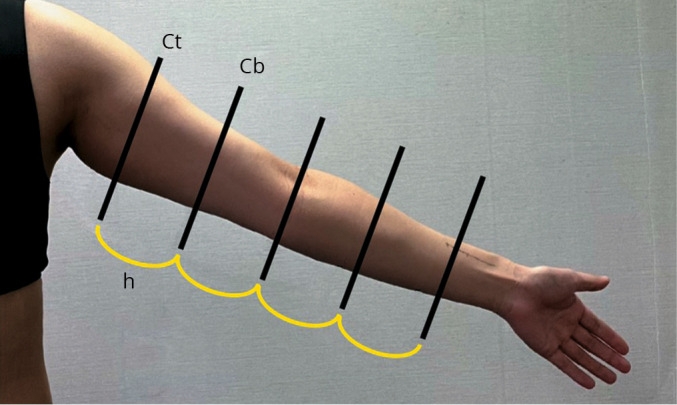
—Upper limb volume measured by a circumference measurement. Ct: circumferences of the top of the cone; Cb: circumferences of the base of the cone; h: distance between circumferences in each segment (h=7 cm).
Secondary outcomes
Pain
Subjective pain was measured using the Numerical Rating Scale (NRS), which is the simplest and most commonly used scale. The patients drew a line from 0 (no pain) to 10 (worst pain), to describe their pain. The MCID of pain for determining the effectiveness of MFR is 16-19%.27
Shoulder range of motion
Shoulder ROM was measured using a stainless-steel goniometer (JAMAR Co., Pakistan) for flexion, extension, abduction, adduction, internal rotation, and external rotation. Shoulder ROM was measured in a standing position or in a prone position28 with the arms spread wide. The goniometer was specified by setting the stationary arm parallel to the torso at the center point of the glenohumeral joint and elbow joint, and then positioning the moving arm towards the distal end of the upper extremity, aligning with the humerus or forearm.29 All measurements were performed twice, and the average value was used. The MCID of shoulder flexion is 12°, extension, abduction, adduction, internal rotation, and external rotation were 11°, 11°, 12°, 14°, and 12°, respectively.30
Chest mobility
Chest mobility was measured as the difference in circumference between maximum inhalation and maximum exhalation at the xiphoid process level. Measurements were taken three times and the difference between the largest circumference and the smallest circumference during maximum exhalation was used.5
Shoulder function
Shoulder function was measured using the Korean version of the Disabilities of the Arm, Shoulder, and Head (DASH). The DASH consists of 30 items evaluating the degree of pain, stiffness, and muscular atrophy during daily life, and each item was evaluated on a 5-point Likert Scale (1 to 5), indicating that the higher the score, the better the shoulder function.31 The score ranges from 0 to 100 points. The MCID of the DASH Score for determining the effectiveness of MFR is 15.91 points.32
Quality of life
QoL was measured using the Korean version of the Functional Assessment of Cancer Therapy for Breast Cancer Patients (FACT-B). The FACT-B consists of 37 items evaluating physical, social/family, emotional and functional well-being, and each item was evaluated on a 5-point Likert Scale (0 to 4), indicating that the higher the score, the better the QoL.33 The total FACT-B Score ranges from 0 to 148. The MCID of FACT-B Score of patients with breast cancer is 11.2 points.34
Intervention
Myofascial release
Myofascial release (MFR) was performed by extending the myofascia with one hand or finger fixed to the starting point of the muscle and the opposite hand or finger in the direction of the muscle and fascia or by gliding both hands or fingers away from each other in the middle of the fascia.8 MFR was applied to the sites according to the severity of fascial adhesion for 30 min by pressing and focusing the pressure on the adhesion point of the fascia with the therapist’s hand or finger in the direction of the fascia until the therapist felt that the tension was fully released.35 It was applied to the axillary and breast surgical scar, the pectoralis major, the subscapularis, the latissimus dorsi, the deltoid, and the intercostal muscles that reduce chest mobility5 (Supplementary Digital Material 1: Supplementary Text File 1, Supplementary Figure 1, Supplementary Table I).
Placebo myofascial release
Placebo myofascial release (placebo MFR) was applied for 30 min in the same manner as well the same regions as that of MFR.5 However, placebo MFR stimulated only the skin surface with light pressure, which did not reach the fascia layer.36
Complex decongestive therapy
Complex decongestive therapy (CDT) consists of manual lymphatic drainage (MLD), compression, patient education, and upper-extremity muscle strengthening. MLD was applied for 15 min in the order of the patient’s neck, affected chest, and affected upper extremity.37 Various techniques such as the stationary cycle, pump, scoop, or rotatory with light pressure have been used to induce rhythmic lymphatic flow.37 A 10-minute upper extremity muscle strengthening exercise was performed using a yellow-color TheraBand (Theraband, Performance Health Inc., Warrenville, IL, USA) for shoulder flexion, abduction, external rotation, and internal rotation within the patient’s possible range of motion.38 Finally, a 5-minute patient education session, including wound care and application of compression garments were conducted39 and all subjects were recommended to wear compression garments every day.
Washout period
The 4-week washout period was determined based on a previous study.21 Since the present study was conducted on active patients with BCRL, an untreated washout period is not ethical, MLD and patient education was applied during the washout period. Also, strengthening exercises were applied for the lower extremity instead of the upper extremity to minimize the treatment effect on the upper extremity.
Statistical analysis
IBM SPSS software (version 26.0; IBM, Armonk, NY, USA) was used for statistical analyses. Data were summarized as means and standard deviations (SDs) for continuous variables and frequencies and percentages for categorical variables. The Shapiro-Wilk Test was used to test the normality of the distributions. A paired t-test was performed to confirm the treatment effects and period effects of the two different interventions. An independent t-test was conducted to determine whether the carry-over effects (treatment by period interaction) between the first (T0-T1) and second (T2-T3) treatments are presented. The level of significant was set at α=0.05.
Results
General characteristics of subjects
All thirty participants completed the experimental procedures without dropouts. No significant difference was found in general characteristics of patients between the two groups. The general characteristics of the participants are listed in Table I.
Table I. —General characteristics of the participants.
| Group A (N.=15) | Group B (N.=15) | |
|---|---|---|
| Age (years) | 47.8±5.2 | 48.0±8.3 |
| Height (cm) | 161.4±4.5 | 161.6±5.3 |
| Weight (kg) | 60.8±7.5 | 62.4±7.2 |
| BMI (kg/m2) | 23.3±2.7 | 23.8±2.1 |
| Duration with lymphedema (month) | 17.6±8.26 | 18.93±8.92 |
| Affected side, right | 7 (46.7) | 9 (60.0) |
| Lymphedema stage | ||
| Stage 1 | 10 (66.7) | 10 (66.7) |
| Stage 2 | 5 (33.3) | 5 (33.3) |
BCRL: breast cancer related lymphedema.
Upper limb volume
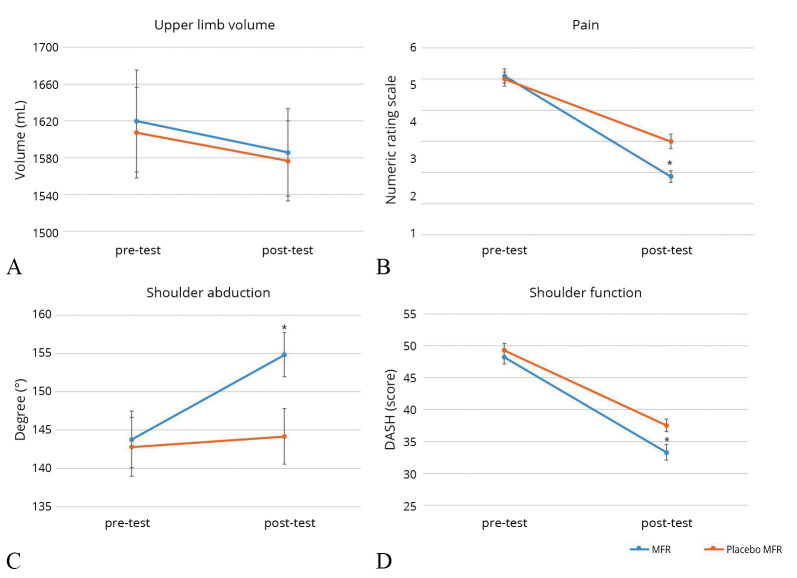
There were significant differences in upper limb volume change in both MFR (1619.78±522.96 to 1585.69±479.93 mL, P<0.05) and placebo MFR (1607.29±488.82 to 1576.53±457.53 mL, P<0.05) after the intervention. However, there was no significant difference between MFR and placebo MFR interventions (P>0.05). The period effect was significant with the change in volume being —23.82±51.10 mL between the first application of intervention (T0-T1) and the second (T2-T3) (P<0.05) (Table II, Figure 3A).
Table II. —Changes in upper limb volume pre- and postintervention.
| Pre-test | Post-test | MD (95% CI) | |
|---|---|---|---|
| MFR | 1619.78±522.96 | 1585.69±479.93 | 34.08 (10.85 to 57.31) |
| Placebo MFR | 1607.29±488.82 | 1576.53±457.53 | 30.76 (9.27 to 52.26) |
| MFR | Placebo MFR | ||
| Treatment effect | 34.08±62.21 | 30.08±62.21 | 3.32 (-17.70 to 24.40) |
| T0-T1 | T2-T3 | ||
| Period effect | 20.51±41.42 | 44.34±71.97 | -23.82 (-42.90 to -4.70) |
T0-T1 was measured between T0 and T1. T2-T3 was measured between T2 and T3. MD: mean difference; 95% CI: 95% confidence interval; MFR: myofascial release.
Figure 3.
—Changes in (A) upper limb volume (B) pain (C) shoulder abduction (D) shoulder function between pre- and postintervention. DASH: Disabilities of the Arm, Shoulder, and Head. *Significant changes pre-test to post-test.
Pain
There was a significant difference in subjective pain in the upper limb before and after MFR from 5.1 to 1.8 (P<0.05). There was a significant difference in subjective pain in the upper limb before and after placebo MFR from 5.0 to 3.0 (P<0.05). The average change in pain score was 1.26±1.36, and there was a significant difference between the two interventions (P<0.05). To see the period effect, the change in pain score was 0.13±1.87 between the first application of the intervention (T0-T1) and the second (T2-T3) and there was no significant difference (P>0.05) (Table III, Figure 3B).
Table III. —Treatment effects and period effects between MFR and placebo MFR.
| Outcome (units) | Treatment effect | Period effect | ||
|---|---|---|---|---|
| MD | 95% CI | MD | 95% CI | |
| Pain (NRS) | 1.27 | 0.76 to 1.78 | 0.13 | -0.57 to 0.83 |
| Shoulder ROM - flexion | -8.30 | -12.98 to -3.62 | -3.37 | -8.86 to 2.13 |
| Shoulder ROM - extension | -3.47 | -5.90 to -1.03 | 1.13 | -1.60 to 3.87 |
| Shoulder ROM - abduction | -9.70 | -12.31 to -7.09 | 0.77 | -3.74 to 5.27 |
| Shoulder ROM - adduction | -2.07 | -3.81 to -0.32 | 0.80 | -1.09 to 2.69 |
| Shoulder ROM - internal rotation | -0.30 | -2.54 to 1.94 | 1.37 | -0.82 to 3.55 |
| Shoulder ROM - external rotation | -5.23 | -6.83 to -3.64 | 0.03 | -2.51 to 2.58 |
| Chest mobility | -0.22 | -0.43 to -0.02 | -0.43 | -0.27 to 0.18 |
| Shoulder function (DASH) | 3.19 | 1.47 to 4.90 | -1.46 | -3.49 to 0.57 |
| Quality of life (FACT-B) | 1.73 | -0.25 to 3.72 | 3.47 | -1.84 to 5.09 |
MD: mean difference; 95% CI: 95% confidence interval; MFR: myofascial release; ROM: range of motion; DASH: Disabilities of the Arm: Shoulder and Head; FACT-B: Functional Assessment of Cancer Therapy for Breast Cancer Patients.
Shoulder ROM
There was a significant difference in shoulder ROM before and after MFR, including shoulder flexion, extension, abduction, adduction, and external rotation, except for internal rotation (P<0.05). There was a significant decrease in shoulder ROM after placebo MFR, including shoulder extension and adduction (P<0.05). In addition, the results showed a significant difference in all types of shoulder ROM between the two interventions, except for internal rotation, and the greatest change was found in shoulder abduction (P<0.05) (Figure 3C). To see the period effect, there was no significant difference in all types of shoulder ROM between the first application of the first intervention (T0-T1) and the second (T2-T3) (P>0.05) (Table III).
Chest mobility
There was a significant difference in chest circumference before and after MFR from 3.3 cm to 3.5 cm (P<0.05). There was no significant difference before and after placebo MFR from 3.1 cm to 3.1 cm (P>0.05). The average value of the change in chest circumference was -0.22±0.55 cm, and there was a statistically significant difference between two groups (P<0.05). To see the period effect, the change in chest circumference was -0.04±0.59 cm between the time of applying the first intervention (T0-T1) and the second (T2-T3) and it showed no significant difference in chest mobility (P>0.05) (Table III).
Shoulder function (DASH)
There was a significant difference in DASH Score before and after MFR from 48.2 to 33.2, and placebo MFR from 49.2 to 37.5 (P<0.05). The average value of the change in the DASH Score was 3.18±4.59, which was a statistically significant difference between the two groups (P<0.05). To see the period effect, the change in DASH Score was -1.46±5.42 between the time of applying the first intervention (T0-T1) and the second (T2-T3) and it showed no significant difference in chest function (p >0.05) (Table III, Figure 3D).
QoL (FACT-B)
There was a significant difference in FACT-B scores before and after MFR, from 53.8 to 51.2 (P<0.05). There was no significant difference before and after placebo MFR from 58.1 to 57.2 (P>0.05). However, the average value of the change in FACT-B was 1.73±5.31 between two intervention groups, which was not statistically significant (P>0.05). To see the period effect, the change in FACT-B Score was 3.46±4.34 between the first application of the intervention (T0-T1) and the second (T2-T3) and the difference was statistically significant (P<0.05) (Table III).
Discussion
This study observed changes in upper limb volume, pain, shoulder ROM, chest circumference for chest mobility, shoulder function, and QoL in patients with BCRL after myofascial release (MFR) in addition to CDT. The analysis revealed a significant reduction in upper limb volume after four weeks of both MFR and placebo MFR; however, no significant treatment effect (between MFR and placebo MFR intervention) was found. One possible explanation for the findings related to upper limb volume may be the composition of the interventions. The present study provides 30-minute of CDT followed by 30-minute of either MFR or placebo MFR. The MLD, which is a part of the 30-minute CDT as supportive care, might influence the upper limb volume in lymphedema. It is also possible that this is due to the measurement method used in this study. Although this circumference measurement has been found to correlate well with the volumetric measurement of water, which is the gold standard method for estimating arm volume,40 it may not be sensitive enough to detect changes. However, a previous study showed improved lymph microcirculation around the soft tissue which has a damaged lymphatic system due to burn trauma, after applying myofascial technique combined with MLD;41 therefore, myofascial technique combined with MLD, might be also effective on secondary lymphedema caused by impaired ability of lymphatic flow due to lymphadenectomy.
MFR is known to reduce pain in breast cancer patients with stage 1 or undiagnosed lymphedema.16 Our result is consistent with these studies showing significantly greater pain reduction in the upper extremity after applying MFR compared to that of placebo MFR, but pain was also decreased after placebo MFR. A randomized controlled trial of breast cancer patients with axillary web syndrome showed the pain-reducing effect of CDT (MLD with stretching and strengthening exercise).42 Considering these results, it is thought that both MFR and MLD influence pain reduction. However, 63% of pain decreased after the MFR (effect size (es), d=2.83) and 39% after the placebo MFR (es, d=1.56); the effect on pain reduction can be maximized with MFR-based treatment.
We found that there was a more significant increase in all shoulder movements, except internal rotation, when comparing the effects of MFR and placebo MFR. Significant improvement beyond the MCID in shoulder abduction and external rotation might be due to a positive effect of MFR on the muscles and fascia performing shoulder adduction and internal rotation, where direct adhesion occurred due to breast cancer surgery.43-45
Deacon et al. reported that improvements in chest mobility followed by respiratory volume increase accelerated lymphatic flow and blood flow towards the central pathway; thus, it was confirmed that it led to a decrease in the lymphedema volume of the peripheral limbs.6 In this study, chest mobility during maximum inhalation and exhalation significantly increased after MFR. This increase in chest mobility may have affected the reduction in edema volume. However, a significant decrease in edema volume was affected by CDT based on the result that edema volume in the placebo group showed a statistically significant decrease, although there was no significant increase in chest mobility. Therefore, additional studies are needed to determine the effects of MFR without CDT on chest mobility in patients with BCRL.
There was a statistically significant increase in DASH scores for shoulder function when both interventions were applied, but the DASH Score changed beyond the MICD after MFR. This seems to have changed clinical shoulder function due to increased shoulder ROM and chest mobility, as well as reduced upper limb volume and pain. With increased shoulder function, QoL significantly improved after MFR but not after placebo MFR.
This study found that MFR-based treatment decreased upper limb volume and pain, and increased shoulder ROM, shoulder function, and QoL in patients with BCRL. In addition, MFR can be used as a safe and reliable intervention without causing lymphatic damage in patients diagnosed with BCRL.
To the best of our knowledge, this is the first study to compare the effects of MFR primarily on upper extremity volume in patients with BCRL.
Limitations of the study
This study had some limitations. First, there might have been a carry-over effect from the previous intervention even though the washout period was set to remove the carry-over effect. The treatment effect might not only be due to the treatment itself but also due to interactions between treatment and study period or sequence, so type II errors may persist. In addition, in the case of lymphedema caused by breast cancer that can easily turn into a chronic disease, it is quite difficult to prove the long-term effect of MFR on the volume of lymphedema. In addition, although placebo MFR is a manual therapy technique that is limited to the skin, it may have been indirectly influenced by the thermal effect transferred from the therapist’s hand.46 Therefore, parallel randomized controlled trials to analyze the effects of MFR on upper limb volume are needed in future studies.
Conclusions
Our results indicate that MFR-based treatment might have a positive effect on upper limb volume, pain, shoulder ROM, shoulder function, and QoL in patients with BCRL. Particularly, in terms of functionality, there was a clinically significant improvement in shoulder function, induced by decreased edema volume and pain, improved ROM, and chest mobility. Moreover, MFR-based treatments may be safe in BCRL rehabilitation settings. However, a further study with parallel randomized controlled trials to confirm what was achieved in the present study.
Supplementary Digital Material 1
Supplementary Text File 1
Myofascial release techniques
Supplementary Figure 1
Myofascial release techniques. A) Breast and axillary scar; B) pectoralis major (diagonal fiber); C) subscapularis; D) latissimus dorsi; E) deltoid anterior fiber; F) intercostalis.
Supplementary Table I
Changes in secondary outcomes between pre- and post-intervention.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33538338&dopt=Abstract 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA 2019;321:288–300. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30667505&dopt=Abstract 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- 3.Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 2018;7:379–403. [P] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30175055&dopt=Abstract 10.21037/gs.2017.11.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krull J. Fascia is the Heart of the Lymphatic System. Myofascial Release Mississauga; 2021 [Internet]. Available from: https://www.myofascialmississauga.com/blog/fascia-is-the-heart-of-the-lymphatic-system [cited 2022, Dec 14].
- 5.Domaszewska K, Pieńkowski T, Janiak A, Bukowska D, Laurentowska M. The Influence of Soft Tissue Therapy on Respiratory Efficiency and Chest Mobility of Women Suffering from Breast Cancer. Int J Environ Res Public Health 2019;16:5092. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31847158&dopt=Abstract 10.3390/ijerph16245092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deacon R, de Noronha M, Shanley L, Young K. Does the speed of aquatic therapy exercise alter arm volume in women with breast cancer related lymphoedema? A cross-over randomized controlled trial. Braz J Phys Ther 2019;23:140–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30471966&dopt=Abstract 10.1016/j.bjpt.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley AL, Piller NB, Carati CJ. The effect of gentle arm exercise and deep breathing on secondary arm lymphedema. Lymphology 2005;38:136–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16353491&dopt=Abstract [PubMed] [Google Scholar]
- 8.Manheim CJ. The Myofascial Release Manual. Third edition. West Deptford, NJ:Slack Inc.;2006. [Google Scholar]
- 9.Fu MR. Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J Clin Oncol 2014;5:241–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25114841&dopt=Abstract 10.5306/wjco.v5.i3.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin 2009;59:8–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19147865&dopt=Abstract 10.3322/caac.20001 [DOI] [PubMed] [Google Scholar]
- 11.Liang M, Chen Q, Peng K, Deng L, He L, Hou Y, et al. Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020;99:e23192. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33285693&dopt=Abstract 10.1097/MD.0000000000023192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remvig L, Ellis RM, Patijn J. Myofascial release: an evidence-based treatment approach? Int Musculoskelet Med 2008;30:29–35. 10.1179/175361408X293272 [DOI] [Google Scholar]
- 13.Stecco C, Porzionato A, Macchi V, Stecco A, Vigato E, Parenti A, et al. The expansions of the pectoral girdle muscles onto the brachial fascia: morphological aspects and spatial disposition. Cells Tissues Organs 2008;188:320–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18349526&dopt=Abstract 10.1159/000121433 [DOI] [PubMed] [Google Scholar]
- 14.Stecco A, Stern R, Fantoni I, De Caro R, Stecco C. Fascial Disorders: implications for Treatment. PM R 2016;8:161–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26079868&dopt=Abstract 10.1016/j.pmrj.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Stubblefield MD, Keole N. Upper body pain and functional disorders in patients with breast cancer. PM R 2014;6:170–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24360839&dopt=Abstract 10.1016/j.pmrj.2013.08.605 [DOI] [PubMed] [Google Scholar]
- 16.Serra-Añó P, Inglés M, Bou-Catalá C, Iraola-Lliso A, Espí-López GV. Effectiveness of myofascial release after breast cancer surgery in women undergoing conservative surgery and radiotherapy: a randomized controlled trial. Support Care Cancer 2019;27:2633–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30470892&dopt=Abstract 10.1007/s00520-018-4544-z [DOI] [PubMed] [Google Scholar]
- 17.De Groef A, Van Kampen M, Dieltjens E, Christiaens MR, Neven P, Geraerts I, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil 2015;96:1140–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25595999&dopt=Abstract 10.1016/j.apmr.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Rao MS, Pattanshetty RB. Effect of myofascial release, stretching, and strengthening on upper torso posture, spinal curvatures, range of motion, strength, shoulder pain and disability, and quality of life in breast cancer survivors. Physiother Res Int 2022;27:e1939. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35044712&dopt=Abstract 10.1002/pri.1939 [DOI] [PubMed] [Google Scholar]
- 19.Remien K, Vilella RC. Osteopathic manipulative treatment: lymphatic procedures. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioral sciences: London: Routledge; 2013. [Google Scholar]
- 21.Castro-Martín E, Galiano-Castillo N, Ortiz-Comino L, Cantarero-Villanueva I, Lozano-Lozano M, Arroyo-Morales M, et al. Effects of a Single Myofascial Induction Session on Neural Mechanosensitivity in Breast Cancer Survivors: A Secondary Analysis of a Crossover Study. J Manipulative Physiol Ther 2020;43:394–404. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32703613&dopt=Abstract 10.1016/j.jmpt.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 22.Pajero Otero V, García Delgado E, Martín Cortijo C, Romay Barrero HM, de Carlos Iriarte E, Avendaño-Coy J. Kinesio taping versus compression garments for treating breast cancer-related lymphedema: a randomized, cross-over, controlled trial. Clin Rehabil 2019;33:1887–97. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31495192&dopt=Abstract 10.1177/0269215519874107 [DOI] [PubMed] [Google Scholar]
- 23.Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther 2006;86:205–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16445334&dopt=Abstract 10.1093/ptj/86.2.205 [DOI] [PubMed] [Google Scholar]
- 24.Chen YW, Tsai HJ, Hung HC, Tsauo JY. Reliability study of measurements for lymphedema in breast cancer patients. Am J Phys Med Rehabil 2008;87:33–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17993983&dopt=Abstract 10.1097/PHM.0b013e31815b6199 [DOI] [PubMed] [Google Scholar]
- 25.Casley-Smith JR. Measuring and representing peripheral oedema and its alterations. Lymphology 1994;27:56–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8078362&dopt=Abstract [PubMed] [Google Scholar]
- 26.Tánori-Tapia JM, Romero-Pérez EM, Camberos NA, Horta-Gim MA, Núñez-Othón G, Medina-Pérez C, et al. Determination of the Minimum Detectable Change in the Total and Segmental Volumes of the Upper Limb, Evaluated by Perimeter Measurements. Healthcare (Basel) 2020;8:285. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32825744&dopt=Abstract 10.3390/healthcare8030285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrar JT, Polomano RC, Berlin JA, Strom BL. A comparison of change in the 0-10 numeric rating scale to a pain relief scale and global medication performance scale in a short-term clinical trial of breakthrough pain intensity. Anesthesiology 2010;112:1464–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20463579&dopt=Abstract 10.1097/ALN.0b013e3181de0e6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furness J, Johnstone S, Hing W, Abbott A, Climstein M. Assessment of shoulder active range of motion in prone versus supine: a reliability and concurrent validity study. Physiother Theory Pract 2015;31:489–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26360531&dopt=Abstract 10.3109/09593985.2015.1027070 [DOI] [PubMed] [Google Scholar]
- 29.Reese NB. Joint range of motion and muscle length testing-E-book. Amsterdam: Elsevier Health Sciences; 2016. [Google Scholar]
- 30.Muir SW, Corea CL, Beaupre L. Evaluating change in clinical status: reliability and measures of agreement for the assessment of glenohumeral range of motion. N Am J Sports Phys Ther 2010;5:98–110. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21589666&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 31.Hudak PL, Amadio PC, Bombardier C, Beaton D, Cole D, Davis A, et al. The Upper Extremity Collaborative Group (UECG) . Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. Am J Ind Med 1996;29:602–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8773720&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 32.Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther 2014;44:30–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24175606&dopt=Abstract 10.2519/jospt.2014.4893 [DOI] [PubMed] [Google Scholar]
- 33.Suh YO. [Predictors of quality of life in women with breast cancer]. Taehan Kanho Hakhoe Chi 2007;37:459–66. [Korean]. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17615467&dopt=Abstract 10.4040/jkan.2007.37.4.459 [DOI] [PubMed] [Google Scholar]
- 34.Li F, Liu Y, Wan C, Zhou J, Tan J, Chen H. Establishing Minimal Clinically Important Differences for the Quality of Life Instrument in Patients With Breast Cancer QLICP-BR (V2.0) Based on Anchor-Based and Distribution-Based Methods. Front Oncol 2022;12:753729. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35586490&dopt=Abstract 10.3389/fonc.2022.753729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nam H, Choi J, Cho N. [The Effect That the Application of Myofascial Release Has on Neck Pain of Adults and Joint Range of Motion]. J Orthop Sports Phys The 2019;15:117–26. [Korean]. 10.24332/aospt.2019.15.2.15 [DOI] [Google Scholar]
- 36.Ajimsha MS, Daniel B, Chithra S. Effectiveness of myofascial release in the management of chronic low back pain in nursing professionals. J Bodyw Mov Ther 2014;18:273–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24725797&dopt=Abstract 10.1016/j.jbmt.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 37.Wittlinger H. Dr. Vodder’s manual lymph drainage: a practical guide. Stuttgart, Germany: Thieme; 2011. [Google Scholar]
- 38.Luz RP, Simao Haddad CA, Rizzi SK, Elias S, Nazario AC, Facina G. Complex Therapy Physical alone or Associated with Strengthening Exercises in Patients with Lymphedema after Breast Cancer Treatment: a Controlled Clinical Trial. Asian Pac J Cancer Prev 2018;19:1405–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29802707&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vignes S, Porcher R, Arrault M, Dupuy A. Factors influencing breast cancer-related lymphedema volume after intensive decongestive physiotherapy. Support Care Cancer 2011;19:935–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20495983&dopt=Abstract 10.1007/s00520-010-0906-x [DOI] [PubMed] [Google Scholar]
- 40.Brorson H, Svensson H. Complete reduction of lymphoedema of the arm by liposuction after breast cancer. Scand J Plast Reconstr Surg Hand Surg 1997;31:137–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9232698&dopt=Abstract 10.3109/02844319709085480 [DOI] [PubMed] [Google Scholar]
- 41.Loskotová A, Loskotová J, Suchanek I, Brychta P, Lipový B. Myofascial-manual lymphatic drainage for burn trauma: a service evaluation. Br J Community Nurs 2017;22(Suppl 5):S6–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28467220&dopt=Abstract 10.12968/bjcn.2017.22.Sup5.S6 [DOI] [PubMed] [Google Scholar]
- 42.Cho Y, Do J, Jung S, Kwon O, Jeon JY. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Support Care Cancer 2016;24:2047–57. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26542271&dopt=Abstract 10.1007/s00520-015-3005-1 [DOI] [PubMed] [Google Scholar]
- 43.Sclafani LM, Baron RH. Sentinel lymph node biopsy and axillary dissection: added morbidity of the arm, shoulder and chest wall after mastectomy and reconstruction. Cancer J 2008;14:216–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18677128&dopt=Abstract 10.1097/PPO.0b013e31817fbe5e [DOI] [PubMed] [Google Scholar]
- 44.De Groef A, Van Kampen M, Verlvoesem N, Dieltjens E, Vos L, De Vrieze T, et al. Effect of myofascial techniques for treatment of upper limb dysfunctions in breast cancer survivors: randomized controlled trial. Support Care Cancer 2017;25:2119–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28197849&dopt=Abstract 10.1007/s00520-017-3616-9 [DOI] [PubMed] [Google Scholar]
- 45.Massingill J, Jorgensen C, Dolata J, Sehgal AR. Myofascial Massage for Chronic Pain and Decreased Upper Extremity Mobility After Breast Cancer Surgery. Int J Ther Massage Bodywork 2018;11:4–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30108667&dopt=Abstract 10.3822/ijtmb.v11i3.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonar J. Fascia: The Tensional Network of the Human Body. J Can Chiropr Assoc 2012;56:235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text File 1
Myofascial release techniques
Supplementary Figure 1
Myofascial release techniques. A) Breast and axillary scar; B) pectoralis major (diagonal fiber); C) subscapularis; D) latissimus dorsi; E) deltoid anterior fiber; F) intercostalis.
Supplementary Table I
Changes in secondary outcomes between pre- and post-intervention.