Abstract
Writing training has shown clinical benefits in Parkinson’s disease (PD), albeit with limited retention and insufficient transfer effects. It is still unknown whether anodal transcranial direct current stimulation (atDCS) can boost consolidation in PD and how this interacts with medication. To investigate the effects of training + atDCS versus training + sham stimulation on consolidation of writing skills when ON and OFF medication. Second, to examine the intervention effects on cortical excitability. In this randomized sham-controlled double-blind study, patients underwent writing training (one session) with atDCS (N = 20) or sham (N = 19) over the primary motor cortex. Training was aimed at optimizing amplitude and assessed during online practice, pre- and post-training, after 24-h retention and after continued learning (second session) when ON and OFF medication (interspersed by 2 months). The primary outcome was writing amplitude at retention. Cortical excitability and inhibition were assessed pre- and post-training. Training + atDCS but not training + sham improved writing amplitudes at retention in the ON state (p = 0.017, g = 0.75). Transfer to other writing tasks was enhanced by atDCS in both medication states (g between 0.72 and 0.87). Also, training + atDCS improved continued learning. However, no online effects were found during practice and when writing with a dual task. A post-training increase in cortical inhibition was found in the training + atDCS group (p = 0.039) but not in the sham group, irrespective of medication. We showed that applying atDCS during writing training boosted most but not all consolidation outcomes in PD. We speculate that atDCS together with medication modulates motor learning consolidation via inhibitory processes (https://osf.io/gk5q8/, 2018-07-17).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-023-11669-3.
Keywords: Motor learning, Parkinson’s disease, Short interval intra-cortical inhibition, Transcranial direct current stimulation, Micrographia
Introduction
Parkinson’s disease (PD) is a progressive multisystem disorder characterized by the selective loss of dopaminergic neurons. Motor learning has been shown to exert some long-term training effects on micrographia and other dexterity problems in PD [1, 2]. However, the instability of these gains have also been demonstrated [3, 4]. A potentially effective tool for boosting motor learning in healthy people is anodal transcranial direct current stimulation (atDCS) [5]. A recent systematic review in PD on the effects of atDCS on upper limb motor performance revealed that the work to date suffers from low methodological quality [6]. As for learning, our own pilot work demonstrated that one session of writing training combined with atDCS improved retention and transfer [7] but this was not confirmed by a recent study on motor sequence learning [8]. These conflicting results inspired the current investigation relying on a power-based sample size, a more challenging writing task and a parallel design.
Striatal and cerebellar loops modulate motor learning by promoting neural plasticity via the induction of long-term potentiation (LTP) [9]. A deficit in these circuits, such as manifest in PD, alters motor learning processing [10, 11]. Motor learning involves different phases including skill acquisition and consolidation [12]. PD seems to particularly affect consolidation, as evidenced by reduced transfer, automatization (e.g., dual-task interference) and retention of learning [10, 13]. The primary motor cortex (M1) has a key role in the motor network and is, therefore, a primary region of interest for atDCS montages [14, 15]. Indeed, a single session of atDCS at M1 was found to induce LTP-like plasticity and repetitive stimulation resulted in more stable and longer lasting LTP in healthy subjects [16]. However, the influence of dopamine on motor learning [2, 17] and cortical excitability [18] is also inconsistent in PD and, therefore, the effects of atDCS when applied in conjunction with motor practice may also vary with the dopaminergic state.
To answer these open questions, we primarily aimed to investigate the effects of atDCS applied to M1 concurrently with writing training in comparison to sham. We hypothesized that training + atDCS would strengthen consolidation of writing skills as indicated by improved 24-h retention of the amplitude (primary outcome) compared to training + sham. As a secondary aim, we were interested in the assessment of three other outcomes of consolidation: (1) the ability to transfer learning to an untrained task; (2) the ability to withstand dual-task interference and (3) the ability to continue learning in a second session. We also investigated online-stimulation effects during actual practice. Finally, we examined the interactions between atDCS-augmented motor learning and dopaminergic medication by exploring motor cortex excitability outcomes using transcranial magnetic stimulation (TMS) pre- and post-learning while ON and OFF medication.
Experimental procedures
Participants
Inclusion criteria for PD patients were: (i) a diagnosis of PD, based on the UK brain bank criteria [19]; (ii) able to participate in testing during a stable ON-medication period; (iii) Hoehn & Yahr (H&Y) stage II–III and stage I if right-dominant symptoms; (iv) Mini-Mental State Examination (MMSE) score ≥ 24 [20]; (v) no other interfering disorders or implants; (vi) absence of visuospatial deficits (Rey-Osterrieth Complex Figure Test score ≥ 32) [21]; and (vii) right-handedness determined by the Edinburgh Inventory [22]. For the TMS-protocol, the following additional inclusion criteria were applied to reduce variability: (i) Resting motor threshold (RMT) < 62% and (ii) short-interval intra-cortical inhibition (SICI) between 30 and 70%. Therefore, cortical excitability was not measured in all participants, resulting in different group sizes for the behavioral and TMS outcomes (see Supplemental Matrial).
The study was approved by the local Ethics Committee Research UZ/KU Leuven (S60893) and pre-registered in the Open Science Framework (OSF) (https://osf.io/gk5q8/). Sample size calculation was based on previous pilot work and included a dropout rate of 10% (see Supplemental Matrial) [7]. The sample was estimated to include 40 subjects. Due to the COVID-19 pandemic, the final sample size was N = 39 (Fig. 1). We recruited 39 healthy control subjects (HC) enabling comparison with PD at baseline (see Supplemental Matrial). All protocol deviations due to the pandemic are described in the Supplemental Matrial.
Fig. 1.
CONSORT flowchart. After the first retention session, one participant dropped out due to unexpected side effects of the intervention. Due to the COVID-19 pandemic, two participants discontinued the TMS assessments (no 1.5 m distance possible)
Experimental design
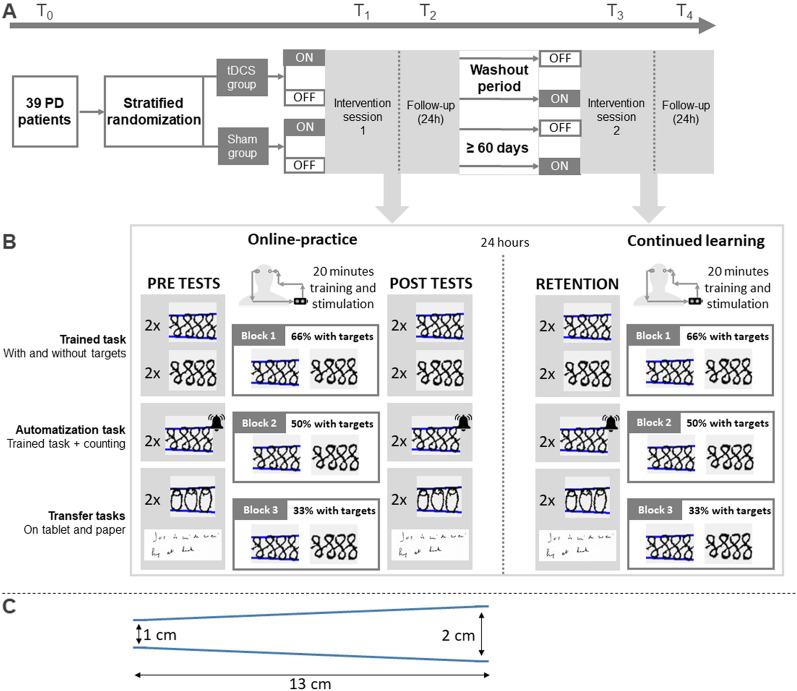
Figure 2A shows that a randomized double-blind design with parallel groups was applied in accordance with the CONSORT guidelines [23]. However, medication was manipulated within the atDCS and sham groups in a randomized cross-over design with a 2-month washout. Participants underwent five testing sessions. During the first familiarization session (T0) ON medication, the protocols were explained and clinical testing was conducted. At T0 hotspot localization was determined with TMS and standardized using a head mould [7]. After T0, an independent researcher carried out a concealed randomization procedure to assign subjects to either the active atDCS or sham stimulation (online tool: Sealed Envelope Ltd. 2017. Simple randomization service, https://www.sealedenvelope.com/simple-randomiser/v1/). Randomization was done in permuted blocks of four and H&Y stage (I–III) and age (< 65 or ≥ 65 years) were included as strata. There was one dropout (atDCS group) because of unexpected side effects after the intervention (Fig. 1). At the first intervention session (T1), writing training was combined with 20 min of sham or atDCS. Pre- and post-tests of writing without tDCS were performed. Cortical excitability tests were also conducted pre-post and this before the writing tests. At T2, 24 h later, retention tests were performed without tDCS and TMS assessment. After a washout of 30 min, they received a second learning and tDCS stimulation protocol to examine the ability for continued learning. The same protocol was repeated (T3 and T4) in the alternate medication state (Fig. 2).
Fig. 2.
A Patients completed one baseline, two intervention and two retention sessions in ON and OFF (randomized). B Performance was assessed during training (online practice), offline (pre, post, retention) and continued learning. C Tablet tasks consisted of writing sequences with increasing size
Online practice and tests
Online practice and testing was done on a custom-made writing tablet (sampling frequency = 200 Hz; spatial resolution = 32.5 μm). Tasks consisted of writing sequences with increasing size (from 1.0 to 2.0 cm, reflecting usual writing sizes) over a trajectory of 13 cm with and without visual target zones, indicating the requested writing size (Fig. 2C). The task was sensitive to medication effects [24] and required producing a figure-8 pattern with increasing amplitudes [25]. Subjects were instructed to reproduce the targeted writing size at a comfortable speed for 45 s. A rest period (10 s) was applied in-between each trial. During online practice with atDCS or sham, patients performed three blocks of six trials per condition with decreasing feedback (i.e. visual targets) over time to optimize learning [26]. In the first block, 66% of the trials had target zones, followed by 50% and 33% during the second and the third block. The order of trials with and without targets was randomized per block. Trained and untrained tasks on the tablet were assessed without atDCS and consisted of 2 trials per time point (pre-, post- and 24 h follow-up).
The primary outcome was the amplitude of the trained sequence without targets measured at 24-h retention compared to pre- and post-performance. The amplitude of an untrained ‘O’-sequence, of equal difficulty, was used to test near-transfer. To examine far-transfer, daily life writing on paper was assessed with the Systematic Screening of Handwriting Difficulties (SOS) test [27] (Fig. 2B). To test the ability to withstand dual-task interference, subjects had to count high and low tones when writing at the same time. Online acquisition was quantified by comparing repeated measures of the trained task without targets. Other secondary measures included writing with targets, writing speed (cm/s), error (mm) and the writing performance index (WPI) (see Supplemental Matrial).
Transcranial direct current stimulation
During online practice, atDCS or sham stimulation was applied for 20 min on the left M1, determined via a TMS procedure (see Supplemental Material), and with the cathodal electrode positioned over the contralateral supraorbital area (mean contact impedance of 5.5 ± 1.1 kΩ for the atDCS and 8.6 ± 2.3 kΩ for the sham group). Stimulation intensity of 1 mA was applied, as this dose proved effective in previous work [7]. tDCS was administered via two saline-soaked sponge electrodes with a 5 cm × 7 cm surface area (i.e. current density of 0.03 mA/cm2). The participants and the experimenter were blinded to the type of stimulation (anodal or sham) through the ‘study mode’ of the tDCS stimulator (DC-Stimulator, NeuroConn GmbH, Germany). In this mode, the blinded researcher used a file-digit number provided by an independent researcher, which encoded the type of stimulation. Stimulation was administered by the same researcher in all sessions. The tDCS questionnaire [28] was used before and after each stimulation to monitor adverse effects. A visual analogue scale (VAS) was applied after each session to check blinding. After study completion, participants were asked to guess whether they underwent active tDCS or sham.
Cortical excitability assessment
The Supplemental Material provides a detailed description of TMS procedures, including hotspot identification and data processing. According to standard definitions, the resting motor threshold (RMT) was specified for each participant at the start of each TMS measurement [29]. Single-pulse TMS was used to determine motor evoked potentials (MEP) from peak-to-peak amplitudes. These measurements included 20 pulses applied with a test stimulus of 130% of the RMT determined at T0. Changes in excitability were examined by calculating a ratio of the post-TMS/pre-TMS MEP sizes at 130% of the pre-RMT values. Paired pulse TMS was used to measure pre-post SICI, applying 20 single-pulses and 20 paired-pulses in a randomized order. The following parameters were used: (1) 130% of the RMT as the test stimulus; (2) the stimulator intensity that brought the inhibition level most closely to 50% based on the SICI curve measured at baseline as the conditioning stimulus; and (3) the inter-stimulus interval at 2 ms.
Data processing and statistical analysis
For all tablet tasks, writing amplitude (cm) was extracted as described previously using Matlab R2020a (The MathWorks) [30]. The relative effect of the dual-task was estimated by calculating the dual-task interference (DTI) for writing amplitude (DTIamplitude = ((Dual task performance − single task performance)/Single task performance) × 100).
Statistical analyses were conducted using SPSS software (version 28 SPSS, Inc., USA) or SAS software (OnDemand for Academics: User’s Guide. Cary, NC: SAS Institute Inc) with significance levels of p < 0.05. Parametric or non-parametric tests were performed after checking data distribution. Mann–Whitney U tests were used for investigating blinding of the participants as assessed by VAS scores. Cross-tabulation was used to analyze whether the participants believed active tDCS or sham was administered. Group differences during pre-tests were checked with between-group statistics. Potential carry-over effects after the cross-over of medication state and medication effects on pre-writing performance per group were checked with within-group statistics (see Supplemental Matrial).
Side-effects, average writing performance and cortical excitability outcomes were analyzed with Linear Mixed Models with unstructured covariance matrices. Significant interactions were explored by Bonferroni multiple comparison post hoc tests. We controlled for within-subject differences by including participant as a random effect. Hedges g based on pooled standard deviations was used to calculate effect sizes. Scores for side effects were analyzed for each session separately (intervention or retention). Medication effects were analyzed with GROUP (atDCS or sham) and TIME (pre or post) as fixed factors. Comparisons for retention, transfer and dual tasking included GROUP, TIME (pre, post or offline retention) and MEDICATION (OFF or ON) as fixed factors. For outcomes obtained during online practice and continued learning, the between-participant factor was GROUP and within-participant factors included BLOCK (block 1, 2 or 3) and MEDICATION. The post/pre-ratios of MEP sizes were analyzed with GROUP and MEDICATION as fixed factors. For other TMS outcomes, the fixed factors GROUP, TIME (pre or post) and MEDICATION were used. An explorative ANCOVA analysis between changes in writing performance and cortical excitability is provided in the Supplemental Matrial.
Results
Blinding and clinical outcomes
Side-effects did not differ across groups over time (no significant interactions for GROUP*TIME). Similarly, VAS scores of stimulation intensity after atDCS or sham showed no significant group differences (InterventionOFF ; RetentionOFF ; InterventionON ; RetentionON ) (Fig. S1). There were also no significant differences in the assessments of blinding for both participants and researchers (Table S1). Table 1 shows that groups were similar for all clinical outcomes, except for disease duration which was significantly longer for the sham group (). Table 2 also illustrates that at baseline groups were similar for all writing and cortical excitability outcomes. Medication significantly improved the amplitude of the untrained task in the tDCS group, but no other medication effects were found (Fig. 3B).
Table 1.
Subject characteristics
| HC (N = 39) | Sham group (N = 19) | tDCS group (N = 20) | Sham vs. tDCS (p value) | |
|---|---|---|---|---|
| Age (years) | 68.0 (± 8.2) | 63.5 (± 8.5) | 62.9 (± 8.3) | 0.80 |
| Gender (M/F) | 18/21 | 14/5 | 17/3 | 0.45 |
| Edinburgh handedness Inventory (%) | 100 (95.0, 100) | 100 (90.0, 100) | 100 (88.3, 100) | 0.51 |
| Education (years) | 15.0 (12.0, 17.0) | 15.0 (14.0, 15.0) | 16.0 (14.0, 16.8) | 0.17 |
| MoCA (0–30) | 27.0 (25.5, 28.0) | 28.0 (25.0, 29.0) | 28.0 (25.3, 29.0) | 0.95 |
| HADS-Anxiety (0–21) | 3.5 (± 2.7) | 5.6 (± 4.1) | 6.3 (± 3.4) | 0.55 |
| HADS-Depression (0–21) | 2.4 (± 2.3) | 5.6 (± 3.4) | 5.7 (± 3.4) | 0.99 |
| DEXTQ-24 (24–96) | 24.0 (24.0, 24.0) | 35.0 (30.0, 48.0) | 32.0 (25.5, 37.8) | 0.08 |
| Disease duration (years) | – | 6.0 (4.0, 9.0) | 3.5 (2.0, 8.0) | 0.03* |
| Disease dominance (R/L) | – | 11/8 | 15/5 | 0.32 |
| MDS-UPDRS III (0–132) | – | 29.1 (± 12.1) | 23.4 (± 12.1) | 0.15 |
| H&Y (0–5) | – | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.48 |
| LEDD (mg/24 h) | – | 748.8 (± 381.8) | 588.4 (± 379.0) | 0.20 |
Results are presented as the mean (± standard deviation) for normally distributed variables and as the median (1st quartile, 3rd quartile) for non-normally distributed variables. *atDCS and sham group significantly different at p < 0.05
DEXTQ Dexterity Questionnaire, H&Y stage Hoehn and Yahr stage, HADS Hospital anxiety and depression questionnaire, HC healthy control subjects, LEDD Levodopa Equivalent Daily Dose, MDS-UPDRS Movement Disorders Society Unified Parkinson’s disease rating scale, MoCA Montreal Cognitive Assessment
Table 2.
Means (standard deviations) of writing and cortical excitability outcomes measured during pre-tests
| Sham group | tDCS group | Sham vs. tDCS, p value | |
|---|---|---|---|
| Trained task (tablet) | |||
| Amplitude (cm) | |||
| OFF | 1.38 (0.19) | 1.40 (0.26) | 0.86 |
| ON | 1.45 (0.26) | 1.47 (0.29) | 0.83 |
| p value | 0.29 | 0.17 | |
| Untrained task (tablet) | |||
| Amplitude (cm) | |||
| OFF | 1.30 (0.10) | 1.27 (0.14) | 0.34 |
| ON | 1.34 (0.10) | 1.31 (0.11) | 0.35 |
| p value | 0.15 | 0.06 | |
| Dual task (tablet) | |||
| DTIamplitude (%) | |||
| OFF | − 4.63 (5.65) | − 4.67 (5.78) | 0.98 |
| ON | − 3.30 (5.45) | − 1.98 (4.52) | 0.43 |
| p value | 0.48 | 0.13 | |
| SOS-test (paper) | |||
| Writing quality score (0–10) | |||
| OFF | 4.53 (1.35) | 4.60 (1.67) | 0.88 |
| ON | 3.89 (1.91) | 4.42 (1.80) | 0.39 |
| p value | 0.18 | 0.58 | |
| Cortical excitability | |||
| RMT (%) | |||
| OFF | 47.80 (7.80) | 47.63 (6.06) | 0.94 |
| ON | 47.07 (7.03) | 47.25 (5.96) | 0.94 |
| p value | 0.10 | 0.14 | |
| MEP size (mV) | |||
| OFF | 1.79 (1.13) | 2.07 (2.09) | 0.66 |
| ON | 2.29 (1.62) | 1.89 (1.65) | 0.52 |
| p value | 0.33 | 0.62 | |
| SICI (%) | |||
| OFF | 53.97 (26.62) | 55.01 (35.12) | 0.93 |
| ON | 51.47 (27.17) | 58.29 (38.35) | 0.59 |
| p value | 0.67 | 0.93 | |
DTI dual-task interference, MEP motor evoked potentials, RMT resting motor threshold, SICI short-interval intra-cortical inhibition, SOS-test the ‘Systematic Screening of Handwriting Difficulties’-test, tDCS transcranial direct current stimulation
Fig. 3.
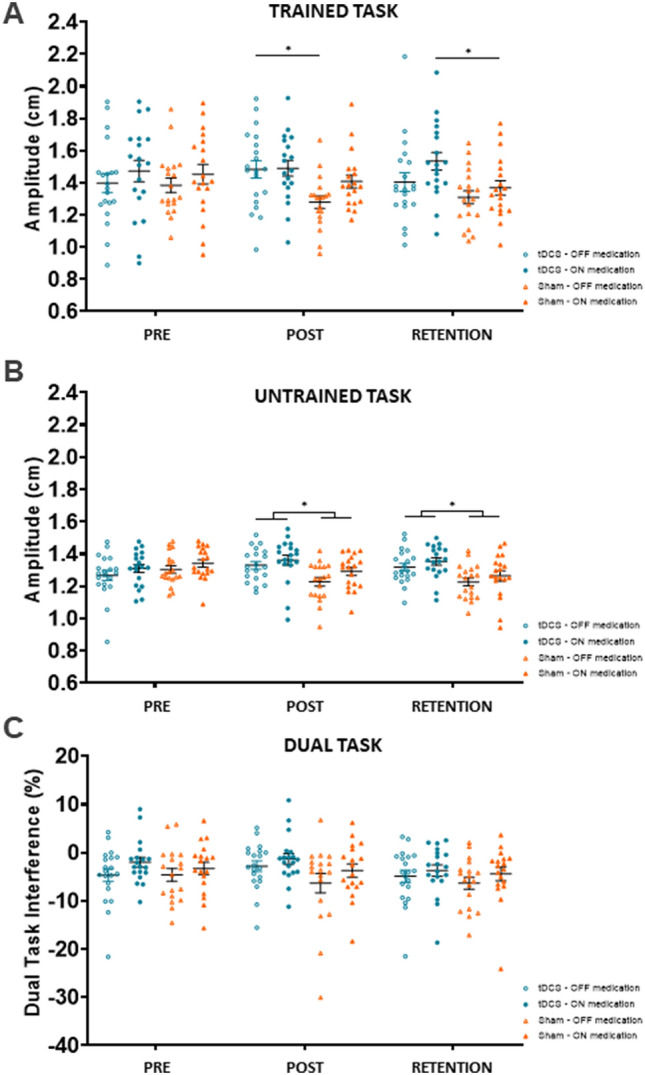
A Trained task writing amplitude (cm) (primary outcome), B amplitude (cm) of the untrained tablet task and C dual-task interference (%). *p value < 0.05, indicated for between group differences only
Consolidation of writing
A significant GROUP*TIME*MEDICATION interaction for amplitude of the trained task without targets was found ). OFF medication, there was a significant GROUP*TIME interaction ). Post hoc analysis showed that the training + atDCS group wrote significantly larger at the post-test compared to training + sham (). ON medication, there was a non-significant GROUP*TIME interaction ). Because of its clinical significance, we explored this result post hoc, revealing that patients wrote significantly larger at retention after training + atDCS compared to training + sham ( (Fig. 3A).
A similar but stronger pattern of results was found for near-transfer, expressed by the amplitude changes of the untrained ‘O’-task. A significant GROUP*TIME interaction () confirmed by post hoc testing showed that the training + atDCS group wrote significantly larger compared to the training + sham group, which wrote significantly smaller with time at post and retention (resp. and ). No interactions with medication were found, but subjects wrote with larger writing amplitudes when ON compared to OFF (main effect: ) (Fig. 3B).
Analyzing the results for far-transfer, as expressed by the SOS score for writing on paper, showed a significant GROUP*TIME interaction ). Post hoc analysis revealed significantly better writing quality at retention in the training + atDCS compared to the training + sham group , OFF and ON .
No significant interactions were found for dual-task writing. Overall, medication improved (lowered) the DTIamplitude (main effect: ) (Fig. 3C). Other secondary writing variables did not show important GROUP*TIME changes. (Table S2). Together, these results also showed that training + atDCS improved retention and transfer of writing with large effect sizes (g > 0.75) irrespective of medication.
Online practice and continued learning
ON medication, patients wrote with larger amplitudes during the first online-practice session compared to OFF, irrespective of stimulation mode (main effect: ). General improvements over time were found for all participants (main effect: whereby the writing size increased significantly during block 2 () and non-significantly during block 3 ) compared to the first block. No significant GROUP effects were demonstrated (Fig. S2).
As for continued learning, evaluated after the 24-h retention test showed a significant GROUP*TIME interaction for the writing amplitude (). Post hoc tests revealed that the training + atDCS group wrote with larger sizes during block 2 and 3 compared to the training + sham group (resp. and (Fig. S2). Taken together, although no beneficial effects of atDCS were apparent for the initial online-practice session, continued learning was enhanced by tDCS.
Cortical excitability
For the RMT and single pulse MEP peak-to-peak amplitudes, the statistical analysis did not reveal significant interactions (RMT: and MEP: ) (Fig. S3A, Table S2). However, analysis of the cortical excitability outcomes showed significant interaction effects for SICI (GROUP*TIME: ). Post hoc analysis showed increased inhibition (a decrease in SICI values) within the training + atDCS group at post- compared to pre-test ), while the sham group exhibited a non-significant decrease ). In addition, the training + atDCS group tended to show more post-inhibition compared to training + sham ) (Fig. S3B). TMS parameters were not different in ON and OFF and interactions with medication were in significant (Fig. S3). However, exploratory correlation analysis revealed a weak but significant negative association between lower SICI and greater amplitude gains (pre-retention) in the training + atDCS group (β = − 0.002, p = 0.022) ON medication only and not apparent in the sham group (see Supplemental Matrial).
Discussion
This is the first power-based randomized double-blind study that investigated the effects of writing training boosted by atDCS versus sham in PD. In line with our hypothesis, one session of training + atDCS resulted in better 24-h retention, transfer and continued learning compared to training + sham. Online practice remained unaffected and resilience to DT was not enhanced by tDCS-supplementation. Medication had an impact on writing performance but did not improve learning. The effects of stimulation were mostly similar in both medication states, except that training + atDCS improved retention only in the ON state. As for mechanisms, we found an increased inhibitory signal (decreased SICI) after training in the atDCS group, not present in the sham group.
The presents findings suggest that atDCS + training facilitated early plasticity at 24 h after training and facilitated ‘near’ (untrained sequences) and ‘far’ transfer (spontaneous writing). Effect sizes were quite substantial and induced after-effects on continued learning. In healthy subjects, it was also shown that atDCS improved retention of a force control task [33]. Though, this effect was not replicated on a sequencing task [31]. Earlier, we demonstrated that a 6-week intensive rehabilitation program without tDCS improved amplitudes of traditional writing skills, but these benefits were not maintained for writing on paper [1]. This underscores the importance of finding patient-friendly methods to boost motor learning transfer in PD.
In the current study, we used a more challenging learning paradigm than previously, requiring writing amplitudes of an increasing size while progressively withdrawing target lines, to be able to gauge short-term learning effects. The tDCS-related changes did not affect online practice but mostly modified learning after the passage of time [32, 33]. Since the sham group tended to worsen their writing at retention, the interpretation of our findings needs some further reflection. We have no explanation for the decrement in writing in the sham group, as baseline performance was comparable between groups. It may reflect that practising this rather difficult task without tDCS was quickly forgotten. It is, therefore, plausible that tDCS reversed this trend by increasing the susceptibility for sustained learning. The consistency of the tDCS effects involving several markers of consolidation, i.e., retention, transfer and continued learning, also underlines learning specificity. However, we did not check whether the quality of sleep was the same in each group and, therefore, were unable to fully exclude a possible sleep-related effect, although these proved absent in a study on healthy controls [32].
Interestingly, the TMS analysis showed that the tDCS + training group demonstrated inhibitory changes not found in the sham group. Previously, tDCS protocols were proposed to optimize the balance between excitatory and inhibitory signal transmission in the brain (i.e., homeostatic plasticity) [34]. Whether the increased inhibition levels found in this study were actually responsible for the gains in consolidation cannot be confirmed by our data. However, it has been increasingly demonstrated that the primary inhibitory neurotransmitter in the brain, Gamma-aminobutyric acid (GABA), has an impact on motor performance and learning in healthy ageing [35, 36]. In PD, it was revealed that alterations in inhibition were correlated with the severity of bradykinesia [37, 38] and associated with the long-term effects of gait training, primed with repetitive TMS [39]. All of these findings suggest a pivotal role of inhibitory processes in cortical LTP-like plasticity and could possibly explain the improved learning outcomes. Increased cortical plasticity and improved motor performance were also associated with normalized inhibition due to dopaminergic medication [11, 40]. Yet, transcranial alternating current stimulation was found to improve finger tapping by enhancing inhibitory GABAergic interneuronal activity [41] irrespective of medication state. Partly in line with the latter study, we also did not find baseline differences in SICI-levels between OFF and ON medication, but we did show better retention in ON than in OFF. Further investigation is warranted to determine whether there is a synergistic interplay between medication and tDCS.
To date, large variability has been reported in response to tDCS protocols [42] accredited to the participants’ clinical profiles and the cognitive decline in PD [43–45]. Using a stratified randomization procedure for age and disease stage, clinical assessments and writing performance were comparable between groups in this study. Still, disease duration was lower in the tDCS group, which could have affected motor learning ability and response to tDCS. Of note, it was shown previously that patients with increased disease severity tended to show greater tDCS-related improvements [46]. Nevertheless, the fact that we recruited patients with mild disease limits the generalizability of our work. Due to the TMS-eligibility criteria, the cortical excitability results were based on a small sample size. Since this analysis was considered exploratory, separate power calculations were not performed but are warranted for future excitability studies. Despite these drawbacks, we are encouraged by the positive results of tDCS on transfer and continued learning. For future work, we propose to investigate repeated stimulation sessions, longer follow-up periods and different task sets before the clinical implementation of tDCS as an adjunct to motor learning can be recommended in PD.
Conclusion
This study provides evidence for tDCS-mediated retention, transfer and continued learning ability of writing skills in PD, likely modulated by inhibitory processes. The present findings justify further work into the synergistic action of atDCS, motor training and dopaminergic therapy to boost activity-dependent plasticity in PD.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants for their commitment. Internal Funds of the KU Leuven (C14/17/115) and the Research Foundation Flanders (FWO) supported this study; SB is a doctoral researcher (Grant number 1167419N). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
SB: conceptualization, methodology, investigation, formal analysis, funding acquisition, writing—original draft. PH: conceptualization, writing—reviewing and editing. BV: investigation, writing—reviewing and editing. EN: conceptualization, writing—reviewing and editing. GV: supervision, writing—reviewing and editing. RM: supervision, writing—reviewing and editing. J-JOdX: conceptualization, methodology, investigation, supervision, funding acquisition, writing—reviewing and editing. AN: conceptualization, methodology, investigation, supervision, funding acquisition, writing—reviewing and editing.
Funding
Internal Funds of the KU Leuven (C14/17/115) and the Research Foundation Flanders (FWO) supported this study; SB is a doctoral researcher (Grant number 1167419N). All funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Conflicts of interest
The authors have no conflict of interest to report.
Ethical approval
The study was approved by the local Ethics Committee Research UZ/KU Leuven (S60893) and pre-registered in the Open Science Framework (OSF) (https://osf.io/gk5q8/)
References
- 1.Nackaerts E, et al. Relearning of writing skills in Parkinson's disease after intensive amplitude training. Mov Disord. 2016;31(8):1209–1216. doi: 10.1002/mds.26565. [DOI] [PubMed] [Google Scholar]
- 2.Paul SS, Dibble LE, Olivier GN, Walter C, Duff K, Schaefer SY. Dopamine replacement improves motor learning of an upper extremity task in people with Parkinson disease. Behav Brain Res. 2020;377:112213. doi: 10.1016/j.bbr.2019.112213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nackaerts E, et al. Handwriting training in Parkinson's disease: A trade-off between size, speed and fluency. PLoS ONE. 2017;12(12):e0190223. doi: 10.1371/journal.pone.0190223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu T, et al. Attention to automatic movements in Parkinson's disease: modified automatic mode in the striatum. Cereb Cortex. 2015;25(10):3330–3342. doi: 10.1093/cercor/bhu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim T, Kim H, Wright DL. Improving consolidation by applying anodal transcranial direct current stimulation at primary motor cortex during repetitive practice. Neurobiol Learn Mem. 2021;178:107365. doi: 10.1016/j.nlm.2020.107365. [DOI] [PubMed] [Google Scholar]
- 6.Simpson MW, Mak M. The effect of transcranial direct current stimulation on upper limb motor performance in Parkinson's disease: a systematic review. J Neurol. 2020;267(12):3479–3488. doi: 10.1007/s00415-019-09385-y. [DOI] [PubMed] [Google Scholar]
- 7.Broeder S, et al. tDCS-enhanced consolidation of writing skills and its associations with cortical excitability in Parkinson disease: a pilot study. Neurorehabil Neural Repair. 2019;33(12):1050–1060. doi: 10.1177/1545968319887684. [DOI] [PubMed] [Google Scholar]
- 8.Simpson MW, Mak M. Single session transcranial direct current stimulation to the primary motor cortex fails to enhance early motor sequence learning in Parkinson's disease. Behav Brain Res. 2022;418:113624. doi: 10.1016/j.bbr.2021.113624. [DOI] [PubMed] [Google Scholar]
- 9.Ziemann U, et al. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24(7):1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishore A, et al. Early, severe and bilateral loss of LTP and LTD-like plasticity in motor cortex (M1) in de novo Parkinson's disease. Clin Neurophysiol. 2012;123(4):822–828. doi: 10.1016/j.clinph.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Kojovic M, Bologna M, Kassavetis P, Murase N, Palomar FJ, Berardelli A, Rothwell JC, Edwards MJ, Bhatia KP. 2012, Functional reorganization of sensorimotor cortex in early Parkinson disease. Neurology. 2012;78:1441–1448. doi: 10.1212/WNL.0b013e318253d5dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakauer JW, et al. Motor learning. Compr Physiol. 2019;9(2):613–663. doi: 10.1002/cphy.c170043. [DOI] [PubMed] [Google Scholar]
- 13.Doyon J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Buch ER, et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol. 2017;128(4):589–603. doi: 10.1016/j.clinph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama S, et al. Cognitive control affects motor learning through local variations in GABA within the primary motor cortex. Sci Rep. 2021;11(1):18566. doi: 10.1038/s41598-021-97974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agboada D, et al. Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal transcranial direct current stimulation—better effects with intensified protocols? Brain Stimul. 2020;13(4):987–997. doi: 10.1016/j.brs.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Vaillancourt DE, et al. Dopamine overdose hypothesis: evidence and clinical implications. Mov Disord. 2013;14:1920–1929. doi: 10.1002/mds.25687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitsche MA, et al. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006;23(6):1651–1657. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- 19.Hughes AJ, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein M, Folstein S, McHugh P. “Mini-Mental State” a practical method for grading the cognitive state of patients for the clinician. J psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Poreh A, Shye S. Examination of the global and local features of the rey osterrieth complex figure using faceted smallest space analysis. Clin Neuropsychol. 2010;12(4):453–467. doi: 10.1076/clin.12.4.453.7240. [DOI] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;1971(9):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Schulz KF, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010 doi: 10.1016/j.ijsu.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broeder S, et al. Novel insights into the effects of levodopa on the up- and downstrokes of writing sequences. J Neural Transm. 2022;129:379–386. doi: 10.1007/s00702-022-02493-6. [DOI] [PubMed] [Google Scholar]
- 25.Kang SY, et al. Characteristics of the sequence effect in Parkinson's disease. Mov Disord. 2010;25(13):2148–2155. doi: 10.1002/mds.23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauwels L, Swinnen SP, Beets IA. Contextual interference in complex bimanual skill learning leads to better skill persistence. PLoS ONE. 2014;9(6):e100906. doi: 10.1371/journal.pone.0100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Waelvelde H, et al. SOS: a screening instrument to identify children with handwriting impairments. Phys Occup Ther Pediatr. 2012;32(3):306–319. doi: 10.3109/01942638.2012.678971. [DOI] [PubMed] [Google Scholar]
- 28.Thair H, et al. Transcranial direct current stimulation (tDCS): a Beginner's guide for design and implementation. Front Neurosci. 2017;11:641. doi: 10.3389/fnins.2017.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broeder S, et al. The effects of dual tasking on handwriting in patients with Parkinson's disease. Neuroscience. 2014;263:193–202. doi: 10.1016/j.neuroscience.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Saucedo Marquez CM, et al. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci. 2013;7:333. doi: 10.3389/fnhum.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis J, et al. Time- but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cereb Cortex. 2015;25(1):109–117. doi: 10.1093/cercor/bht208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis J, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;3(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai Z, Ma C, Jin X. Homeostatic activity regulation as a mechanism underlying the effect of brain stimulation. Bioelectron Med. 2019;5:16. doi: 10.1186/s42234-019-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maes C, et al. GABA levels are differentially associated with bimanual motor performance in older as compared to young adults. Neuroimage. 2021;231:117871. doi: 10.1016/j.neuroimage.2021.117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuypers K, et al. Age-related GABAergic differences in the primary sensorimotor cortex: a multimodal approach combining PET, MRS and TMS. Neuroimage. 2021;226:117536. doi: 10.1016/j.neuroimage.2020.117536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ammann C, et al. Cortical disinhibition in Parkinson's disease. Brain. 2020;143(11):3408–3421. doi: 10.1093/brain/awaa274. [DOI] [PubMed] [Google Scholar]
- 38.Leodori G, et al. Motor cortical network excitability in Parkinson's disease. Mov Disord. 2022;37:734–744. doi: 10.1002/mds.28914. [DOI] [PubMed] [Google Scholar]
- 39.Chung CL, Mak MK, Hallett M. Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann Neurol. 2020;88(5):933–945. doi: 10.1002/ana.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni Z, et al. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neuroloy. 2013;80(19):1746–1753. doi: 10.1212/WNL.0b013e3182919029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerra A, et al. Driving motor cortex oscillations modulates bradykinesia in Parkinson's disease. Brain. 2022;145(1):224–236. doi: 10.1093/brain/awab257. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, et al. Transcranial direct current stimulation for Parkinson's disease: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13:746797. doi: 10.3389/fnagi.2021.746797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Firouzi M, et al. Transcranial direct-current stimulation enhances implicit motor sequence learning in persons with Parkinson's disease with mild cognitive impairment. J Neuropsychol. 2021;15(3):363–378. doi: 10.1111/jnp.12231. [DOI] [PubMed] [Google Scholar]
- 44.Broeder S, et al. Does transcranial direct current stimulation during writing alleviate upper limb freezing in people with Parkinson’s disease? A pilot study. Hum Mov Sci. 2019;65(17):S0167–9457. doi: 10.1016/j.humov.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Manor B, et al. Multitarget transcranial electrical stimulation for freezing of gait: a randomized controlled trial. Mov Disord. 2021;36(11):2693–2698. doi: 10.1002/mds.28759. [DOI] [PubMed] [Google Scholar]
- 46.Kaski D, et al. Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clin Rehabil. 2014;28(11):1115–1124. doi: 10.1177/0269215514534277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.