Abstract
Background
Augmented renal clearance (ARC) is associated with lower blood plasma concentrations of renally excreted drugs; however, its time course is unknown. The current study aimed to determine the onset timing/duration of ARC, its risk factors, and its association with clinical outcomes by continuous monitoring of urinary creatinine clearance (CrCl) in critically ill patients.
Methods
Data were retrospectively obtained from the medical records of 2592 critically ill patients admitted to the intensive care unit (ICU) from January 2019 to June 2022 at a tertiary emergency hospital. Among these, patients with continuously measured urinary CrCl were selected and observed over time. We evaluated the onset timing and duration of ARC by plotting Kaplan–Meier curves. Furthermore, by multivariate analyses, factors associated with the onset and persistence of ARC were analyzed, and the association between the ARC time course and clinical outcomes was evaluated.
Results
The prevalence of ARC was 33.4% (245/734). ARC onset was within 3 days of admission in approximately half of the cases, and within 1 week in most of the other cases. In contrast, the persistence duration of ARC varied widely (median, 5 days), and lasted for more than a month in some cases. Multivariate analysis identified younger age, male sex, lower serum creatinine at admission, admission with central nervous system disease, no medical history, use of mechanically assisted ventilation, and vasopressor use as onset factors for ARC. Furthermore, factors associated with ARC persistence such as younger age and higher urinary CrCl on ARC day 1 were detected. The onset of ARC was significantly associated with reduced mortality, but persistent of ARC was significantly associated with fewer ICU-free days.
Conclusions
Despite the early onset of ARC, its duration varied widely and ARC persisted longer in younger patients with higher urinary CrCl. Since the duration of ARC was associated with fewer ICU-free days, it may be necessary to consider a long-term increased-dose regimen of renally excreted drugs beginning early in patients who are predicted to have a persistent ARC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-023-00660-9.
Keywords: Augmented renal clearance, Urinary creatinine clearance, Critical care, Intensive care unit
Background
Augmented renal clearance (ARC) is a phenomenon of increased renal excretion of circulating solutes, mostly defined by creatinine clearance (CrCl) > 130 mL/min/1.73 m2 [1, 2]. Since the first report by Udy et al. [3], ARC has gradually gained more attention; however, its importance has not yet been fully recognized in general clinical practice. Physicians and pharmacists are usually less likely to actively suspect ARC, because they often adjust (i.e., reduce) the dosage of renally excreted drugs to account for decreased renal function. However, ARC in critical care settings is not uncommon [4, 5]. This increased renal clearance above the normal range indicates a potential underdosing risk for many renally excreted drugs and contributes to treatment failure [6–8].
Previous ARC studies have reported its developmental mechanisms, prevalence, risk factors, and pharmacokinetics in renally excreted drugs [1–6]. Although there is a paucity of specific data on dosing regimens in patients with ARC, underdosing of antimicrobials is frequently reported and is an important issue in critically ill patients, where it is more likely to affect therapeutic outcomes [7, 9]. Furthermore, to evaluate renal function for drug administration in patients with ARC, direct measurement of urinary CrCl is recommended instead of alternative renal function estimation equations using serum creatinine (SCr), such as the Cockcroft–Gault (C–G) equation [10], modification of diet in renal disease (MDRD) equation [11], and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12], which are commonly used in clinical practice. Although urinary CrCl is inexpensive and practical, it requires labor to store urine. Therefore, few hospitals continuously and directly measure urinary CrCl in all critically ill patients, especially those without decreased renal function, contributing to the lack of recognition of ARC [13]. Despite the increased focus on ARC, a general lack of awareness about ARC and the need for continuous monitoring of urinary CrCl have impeded individualized drug design. Above all, the time course of ARC remains unknown, and the duration and risk factors for transient or persistent ARC symptoms are not clear [6, 14]. In patients with ARC, higher doses than the standard is required for renally excreted drugs; however, the specific duration of administration is unknown. This time-course study is essential, because high-dose regimens of renally excreted drugs for ARC may be excessive after ARC state has ended.
Considering the above, this study aimed to determine the time course of ARC (onset and duration) and to identify its risk factors by continuous direct measurement of urinary CrCl in critically ill patients. As an additional analysis, we examined the association between the time course of ARC and clinical outcomes.
Methods
Data source
All data were retrospectively obtained from the medical records of 2592 critically ill patients admitted to the intensive care unit (ICU) from January 2019 to June 2022 at Hokkaido University Hospital (a tertiary care hospital). The urinary CrCl was measured at least once for nearly all of the initially screened patients. Of these, critically ill patients for whom urinary CrCl was continuously measured, were selected and observed over time. Patients were excluded if they were younger than 18 years, received renal replacement therapy, or had measurement deficiencies during the observation period (including discontinuous urinary CrCl measurements and short-term ICU stays within 3 days).
Renal function evaluation equation
Urinary CrCl was calculated over 6–24 h. The estimated CrCl value was calculated using the C–G equation [10]. The estimated glomerular filtration rate (GFR) was calculated using the MDRD [11] and CKD-EPI [12] equations. In this study, all renal function evaluation equations were represented on a per body surface area basis. We calculated body surface area using the Du-Bois formula [15].
Definitions and observations
Based on previous studies on non-achievement of target concentrations of renally excreted drugs, the cutoff for ARC was defined as urinary CrCl > 130 mL/min/1.73 m2 [1, 2, 6, 16]. In all critically ill patients included in the analysis, urinary CrCl levels were measured daily over time from ICU admission. In some non-ARC patients, we allowed measurement on the next day if urinary CrCl could not be measured but excluded patients if it could not be evaluated for more than 2 days. The maximum observation period for urinary CrCl until the onset of ARC was 30 days. The onset timing and duration for the first observed ARC were recorded.
Statistical analysis
Continuous data are presented as mean (standard deviation) or median (interquartile range). Categorical data are presented as counts (%).
The onset timing and duration of ARC were evaluated by plotting the cumulative incidence of ARC using Kaplan–Meier (KM) curves. The estimated time to ARC onset and duration of ARC were evaluated as the time from ICU admission to the onset of ARC and the time from the onset of ARC to the end of ARC, respectively. Furthermore, to identify independent factors associated with the onset and persistence of ARC, multivariate Cox regression models were used. The covariates used were based on the ARC consensus in the current literature [1, 2, 17–19] and data availability. The covariates used were age, sex, renal function, sequential organ failure assessment (SOFA) score, diagnosis at ICU admission, medical history, mechanically assisted ventilation, and vasopressor use (detailed description in Additional file 1: Table S1).
In addition, to evaluate the association between the time course of ARC and mortality a multivariate logistic model was used. In a similar fashion, a multivariate Cox regression model was used to evaluate the association between the time course of ARC and fewer ICU-free days. The covariates used were SOFA score at admission and subsequent degree of change, the ARC time course, plus factors selected from the aforementioned analyses based on the number of observed events and availability of data. The covariates used were SOFA score status (SOFA score at admission and subsequent degree of change), ARC status (presence of transient and persistent ARC with non-ARC as a reference), age, diagnosis at ICU admission, mechanically assisted ventilation, and vasopressor use (detailed description in Additional file 1: Table S2). Because no study has reported on the ARC time course to define the cutoff points for transient and persistent ARC, we established the median duration of ARC in KM curves. ICU-free days were defined as 28 days minus the number of days in the ICU (range: 0–28 days). For circumstances in which death occurred within 28 days and/or ICU stay was more than 28 days, ICU-free days were recorded as 0.
Two-sided p values < 0.05 were statistically significant. Statistical analyses were performed using JMP version 16.1 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
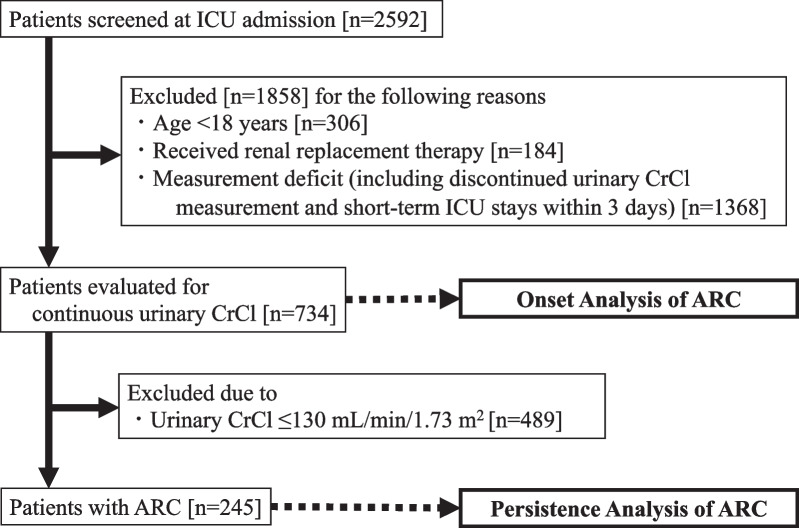
A total of 2592 patients were admitted to the ICU during the study period, of whom 734 met the inclusion criteria (Fig. 1). The prevalence of ARC in critically ill patients for whom urinary CrCl was consecutively measured was 33.4% (245/734). The baseline characteristics and laboratory data of the study population were typical of the profile of a mixed ICU cohort (Table 1) [13, 20, 21]. The median age was 70 years (55–79) and 58.2% of the patients were male. In patients with ARC, the median (interquartile range) urinary CrCl at ARC onset was 150.6 (137.2–176.9) mL/min/1.73 m2, but the median (interquartile range) C–G equation, MDRD equation, and CKD-EPI equation were 103.6 (86.3–133.5) mL/min/1.73 m2, 99.7 (84.1–130.8) mL/min/1.73 m2, and 88.7 (79.9–100.5) mL/min/1.73 m2, respectively, which were lower than urinary CrCl.
Fig. 1.
Study flowchart. ICU intensive care unit, CrCl creatinine clearance, ARC augmented renal clearance
Table 1.
Patient characteristics (n = 734)
| Summary data | |
|---|---|
| Age, yearsa | 70 (55–79) |
| Male sexb | 427 (58.2%) |
| Weight, kga | 57.1 (47.8–66.9) |
| Body surface area, m2a | 1.60 (1.44–1.73) |
| Serum creatinine, mg/dLa | 1.04 (0.76–1.46) |
| Albumin, g/dLa | 3.2 (2.6–3.7) |
| Blood urea nitrogen, mg/dLa | 20 (15–33) |
| Admission diagnosisb | |
| Trauma | 95 (12.9%) |
| Central nervous system diseasec | 96 (13.1%) |
| Sepsis | 75 (10.2%) |
| Cardiovascular diseases | 278 (37.9%) |
| Digestive diseases | 41 (5.6%) |
| Infection (without sepsis) | 82 (11.2%) |
| Other | 103 (14%) |
| SOFA scorea | 5 (3–8) |
| ICU-free daysa | 21 (11–24) |
| Mortalityb | 103 (14%) |
aMedian (interquartile range)
bNumber (%)
cCentral nervous system disease refers to hospitalization for any of the following reasons: traumatic brain injury, intracerebral hemorrhage, subarachnoid hemorrhage, cerebral arteriovenous malformation, hydrocephalus, and status epilepticus
SOFA sequential organ failure assessment, ICU intensive care unit
Characteristics of ARC onset
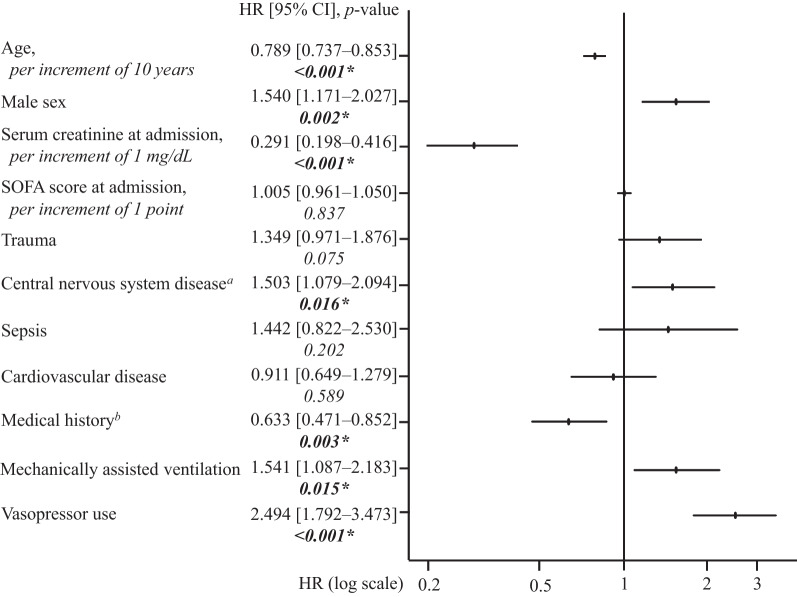
The median onset time of ARC was undefined, because the KM curve did not cross 50% given the small number of progression events (Fig. 2). In overall patients with ARC, the ARC onset occurred within 3 days of admission in approximately half of the cases, and within 1 week in most of the other cases. Multivariate Cox regression analysis identified younger age, male sex, lower SCr at admission, admission with central nervous system disease, no medical history, use of mechanically assisted ventilation, and vasopressor use as independent factors for development of ARC in a mixed ICU cohort (Fig. 3).
Fig. 2.
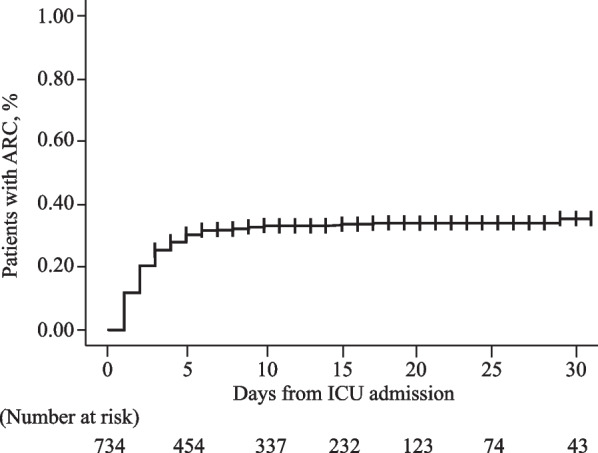
Cumulative incidence rate of ARC (n = 734). ARC augmented renal clearance, ICU intensive care unit
Fig. 3.
Factors associated with onset of ARC in a mixed ICU population (n = 734). aCentral nervous system disease refers to hospitalization for any of the following reasons: traumatic brain injury, intracerebral hemorrhage, subarachnoid hemorrhage, cerebral arteriovenous malformation, hydrocephalus, and status epilepticus. bMedical history of any of the following: chronic kidney disease, hypertension, diabetes, myocardial infarction, heart failure, chronic obstructive pulmonary disease, cirrhosis and liver failure. SOFA sequential organ failure assessment, HR Hazard ratio, CI confidence interval. *Significantly different (p value < 0.05)
Duration of ARC
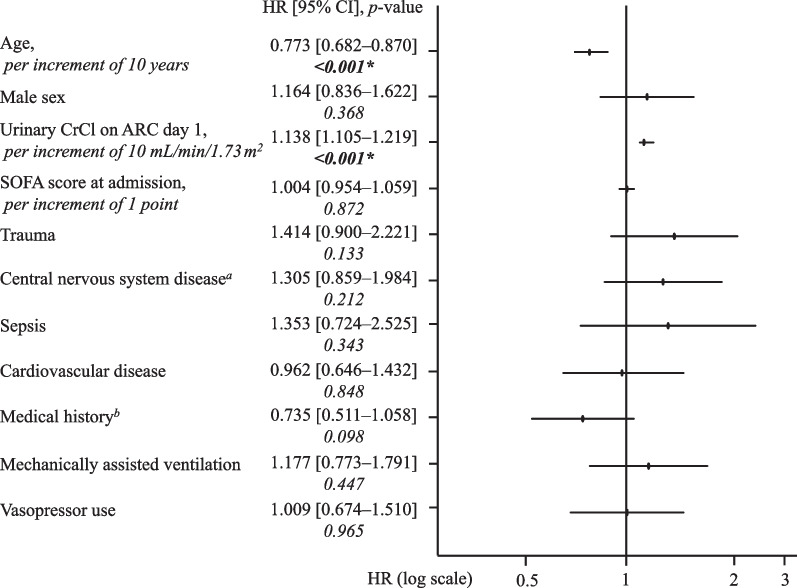
The median duration of ARC in KM curves was 5 days and ended within 3 weeks in many of those cases (Fig. 4). The duration of ARC had greater variability than the time of onset, with some cases persisting for more than a month. Multivariate Cox regression analysis identified younger age and higher urinary CrCl on ARC day 1 as independent factors for persistent ARC (Fig. 5).
Fig. 4.
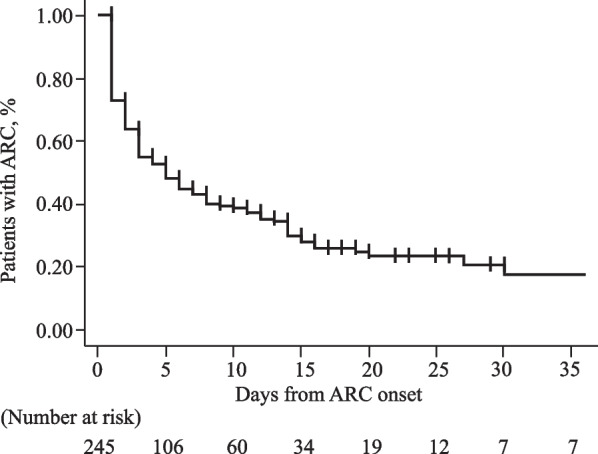
Cumulative persistence rate of ARC (n = 245). ARC augmented renal clearance
Fig. 5.
Factors associated with persistence of ARC (n = 245). aCentral nervous system disease refers to hospitalization for any of the following reasons: traumatic brain injury, intracerebral hemorrhage, subarachnoid hemorrhage, cerebral arteriovenous malformation, hydrocephalus, and status epilepticus. bMedical history of any of the following: chronic kidney disease, hypertension, diabetes, myocardial infarction, heart failure, chronic obstructive pulmonary disease, cirrhosis and liver failure. ARC augmented renal clearance, SOFA sequential organ failure assessment, HR Hazard ratio, CI confidence interval. *Significantly different (p value < 0.05)
Relationship between time course of ARC and clinical outcomes
The factors associated with mortality or ICU-free days in a mixed ICU cohort are shown in Additional file 1: Tables S3–S4. The cutoff point for classifying the duration of ARC persistence was set as 5 days, which was the median time in KM curves. The onset of ARC was significantly associated with reduced mortality. In contrast, persistent ARC was significantly associated with fewer ICU-free days.
Discussion
Although it has been suggested that renal excretory drug doses may need to be adjusted upward in patients with ARC, the specific duration of ARC is unknown [7]; thus, the duration must be clarified to prevent excessive drug exposure. To our knowledge, this is the first study to capture in detail the time course (onset timing and duration) of ARC and its risk factors.
There are few reports on the ARC onset timing, but basically the phenomenon of ARC appears relatively early. Patients with traumatic brain injury have been reported to have an early onset of ARC, with many patients showing markedly elevated renal function parameters in the first 2 days after getting injured [22, 23]. Udy et al. similarly highlighted the occurrence of ARC during the first week after ICU admission [24]. In contrast, in a study of critically ill patients with COVID-19, the onset of ARC was delayed (mean, 28 days) [25]. This delay in the mean onset timing of ARC may be due to the inclusion of the patients without ARC in the analysis. Among the patients with ARC in this study, the onset of ARC occurred early after ICU admission.
The median duration of ARC in KM curves was 5 days, but there was a larger variation than in the time of onset, with ARC lasting for more than a month in some cases. Although no specific duration of ARC has been reported, multivariate analysis suggests that younger patients and those with higher urinary CrCl on ARC day 1 appear to have more persistent ARC. These persistent factors of ARC indicate greater renal functional reserve (RFR). RFR describes the capacity to increase GFR under certain physiological conditions (e.g., pregnancy and solitary kidney) or pathological stimuli (e.g., hypertension, nephrotic syndrome, and polycystic kidney disease) [26]. This hyperfiltration state may occur in patients who are critically ill, young, traumatized, heavily infused, or have excessive cardiac output, all of which are risk factors for ARC [6, 19, 24, 27]. Thus, it is suggested that it may be necessary to consider a long-term increased-dose regimen of renally excreted drugs in patients with ARC and without depressed potential RFR.
Our ARC cohort study revealed that the onset of ARC was significantly associated with a reduction in mortality, but ARC persistence was significantly associated with fewer ICU-free days. This seemingly contradictory result may reflect the conflicting clinical feature of favorable ARC prognosis and the resistance to renal excretion drug therapy [8, 20, 21, 28, 29]. In general, acute kidney injury in the ICU is associated with increased mortality [30]. In contrast, risk factors for ARC are indicators of good renal function. Therefore, ARC itself is associated with a good prognosis [20, 21, 29]. However, previous reports have not examined the persistence of ARC in detail. Theoretically, in patients with ARC, lower blood plasma concentrations of renally excreted drugs will be frequently observed. In other words, the longer the duration of the ARC, the lower the exposure to the drug and the higher the likelihood of clinical failure. Considering the aforementioned report and the results of this study, it is suggested that although the association between ARC and mortality may be mitigated by good prognostic risk factors for ARC onset, persistent ARC may be associated with clinical failures, such as prolonged patient treatment periods. The impact of antimicrobial underdosing on drug resistance and the biological impact of anticonvulsant and anticoagulant underdosing require further investigation in patients with persistent ARC [31–33].
The choice of renal function assessment formula has a significant influence on ARC diagnosis. In various studies, the most common criterion for ARC is CrCl > 130 mL/min/1.73 m2, which recommends urinary CrCl to assess its renal function [2, 4, 34]. In contrast, in patients with ARC, the renal function estimation equation (C–G equation, MDRD equation, CKD-EPI equation) does not correlate with urinary CrCl and is estimated lower than it [28, 35]. Similarly in the present study, the alternative renal function estimation equation was estimated to be lower than urinary CrCl. In the future, it will be necessary to develop a new prediction formula that modifies the current renal function estimation equations by capturing the characteristics of renal function over time in patients with ARC, aiming to improve the ability to estimate renal function.
Despite the inability of alternative renal function estimators correctly evaluate ARC using SCr, direct measurement of urinary CrCl is often not performed continuously, especially in patients without decreased renal function because of the effort required to store urine. Therefore, a prediction score of ARC with high accuracy is necessary. Currently, several scoring systems are available for predicting ARC; however, the adaptability of these scores to critically ill patients in mixed ICUs is limited. The ARC score reported by Udy et al. [36] selected three factors (age ≤ 50 years, trauma, and SOFA score ≤ 4); however, in our study, sepsis, trauma, and SOFA score were not selected as a persistent factor for ARC. The ARCTIC score reported by Barletta et al. [14] (SCr < 0.7 mg/dL, male sex, age < 56 years, age 56–75 years) is more user-friendly but applies only to a small population of patients suffering from trauma. In addition, these scores are intended to detect ARC, but they do not consider its persistence. Renal function is dynamic, especially in patients with ARC. The Gijsen et al. score [18] (days from ICU admission, age, sex, SCr, trauma, and cardiac surgery) may be a more useful adjunct tool for predicting ARC persistence, because it predicts daily ARC in a heterogeneous population of critically ill patients.
This study had some limitations. First, our results were not validated prospectively. In the critical care setting, we were able to obtain statistically adequate numbers of patients with ARC, and considering the high heterogeneity of its target patients, the results should be interpreted with caution. Second, the study design may have introduced a selection bias in ARC incidence, because it excluded patients with short-term ICU stays (< 3 days) and patients with measurement deficits (1368 patients). However, in a random-effects meta-analysis, the prevalence (95% confidence interval) of ARC in mixed ICU was 36% (31–41%), which was not different from the prevalence in the present study (245/734 [33.4%]) [4]. Moreover, the objective of this study was to determine the time course of ARC as evaluated by direct measurement of urinary CrCl, and we were able to obtain the sample size necessary for this purpose. Third, we observed the onset of ARC from the time of ICU admission. We could not rule out the possibility that ARC may have developed before that time. In this study, the cumulative incidence of ARCs on the first day of ICU admission was approximately 10%. Previous ARC studies also appear to be replete with reports of ARC occurring from initial observation [24, 29]. Since CrCl generally has a maximum range of about 120 mL/min/1.73 m2 [26, 37], patients with ARC are expected to develop ARC after the event occurs (i.e., after ICU admission). Thus, although some patients had ARC at the time of ICU admission, it appears reasonable that ARC would develop relatively early, because the cumulative incidence of ARC is almost maximal within the first week after ICU admission. Fourth, the persistence of ARC was associated with fewer ICU-free days; however, the specific treatment was not examined. In our cohort study, we adjusted for confounding factors (such as severity and initial diagnosis) as much as possible; however, details of therapeutic drugs were not examined, and our findings must be interpreted with caution. Fifth, in a recent study, direct measurement of 6-h urinary CrCl tended to overestimate patients with ARC compared to GFR using iohexol [38]. Nevertheless, the invasive and labor-intensive aspects of daily measurement of GFR with iohexol to capture the time course of ARC are impractical. Direct measurement of urinary CrCl at defined urine collection intervals is an inexpensive and simple method to improve the accuracy of the dynamic assessment of patients with ARC [7, 35, 39]. Finally, this study examined the persistence of first-onset ARC but not recurrent ARC. In fact, some patients in our study exhibited ARC again after ARC paused initially (unpublished data), which may influence the pharmacokinetics of subsequent renally excreted drugs. Therefore, further elucidation of the ARC time course requires an appropriate definition of ARC and proper monitoring of renal function, which should be evaluated in a multicenter prospective study.
Conclusions
Despite the early onset of ARC, its duration varied widely and ARC persisted longer in younger patients with higher urinary CrCl. Since the duration of ARC was associated with fewer ICU-free days, it may be necessary to consider a long-term increased-dose regimen of renally excreted drugs beginning early in patients who are predicted to have a persistent ARC. The results of this retrospective cohort study will support physicians and pharmacists in determining the drug dosing regimens for patients with ARC.
Supplementary Information
Additional file 1: Table S1. Detailed description of the covariates used for multivariate Cox hazards analysis to identify factors associated with the onset or persistence of ARC. Table S2. Detailed description of the covariates used for multivariate analysis to identify factors associated with mortality or fewer ICU-free days. Table S3. Independent factors affecting mortality, as determined by a multiple logistic regression analysis. Table S4. Independent factors affecting lesser ICU-free days, as determined by a multiple Cox regression analysis.
Acknowledgements
Not applicable.
Abbreviations
- ARC
Augmented renal clearance
- CrCl
Creatinine clearance
- SCr
Serum creatinine
- C–G
Cockcroft–Gault
- MDRD
Modification of diet in renal disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- ICU
Intensive care unit
- GFR
Glomerular filtration rate
- SOFA
Sequential organ failure assessment
- RFR
Renal function reserve
Author contributions
RM conceived and designed the study. MH, SI, MS, and YT designed the study. RM, MH, and YT obtained epidemiological data. RM, MH, and SI performed the statistical analyses. RM and MH wrote the manuscript. SI, MS, and YT contributed equally to the study. All the authors have read and approved the final version of the manuscript.
Funding
None.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of Hokkaido University Hospital (No. 022-0077) and was conducted in accordance with the Declaration of Helsinki and STROBE statement. Given the retrospective nature of the study, the requirement for informed consent from the subjects was waived by the committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39:346–354. doi: 10.1002/phar.2231. [DOI] [PubMed] [Google Scholar]
- 2.Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís MÁ. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet. 2018;57:1107–1121. doi: 10.1007/s40262-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 3.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49:1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Hefny F, Stuart A, Kung JY, Mahmoud SH. Prevalence and risk factors of augmented renal clearance: a systematic review and meta-analysis. Pharmaceutics. 2022;14:445. doi: 10.3390/pharmaceutics14020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baptista JP, Martins PJ, Marques M, Pimentel JM. Prevalence and risk factors for augmented renal clearance in a population of critically ill patients. J Intensive Care Med. 2020;35:1044–1052. doi: 10.1177/0885066618809688. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y, Wang Y, Ma Y, Wang P, Zhong J, Chu Y. Augmented renal clearance: what have we known and what will we do? Front Pharmacol. 2021;12:723–731. doi: 10.3389/fphar.2021.723731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva CM, Baptista JP, Santos I, Martins P. Recommended antibiotic dosage regimens in critically ill patients with augmented renal clearance: a systematic review. Int J Antimicrob Agents. 2022;59:1065–1069. doi: 10.1016/j.ijantimicag.2022.106569. [DOI] [PubMed] [Google Scholar]
- 8.Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28:695–700. doi: 10.1016/j.jcrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Terawaki H, Nakayama M, Asahi K, Kakamu T, Hayakawa T, Iseki K, et al. Comparison of predictive value for first cardiovascular event between Japanese GFR equation and coefficient-modified CKD-EPI equation. Clin Exp Nephrol. 2015;19:387–394. doi: 10.1007/s10157-014-0997-7. [DOI] [PubMed] [Google Scholar]
- 13.Tomasa-Irriguible TM, Sabater-Riera J, Pérez-Carrasco M, Ortiz-Ballujera P, Díaz-Buendía Y, Navas-Pérez A, et al. Augmented renal clearance. An unnoticed relevant event. Sci Prog. 2021;104:368504211018580. doi: 10.1177/00368504211018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barletta JF, Mangram AJ, Byrne M, Sucher JF, Hollingworth AK, Ali-Osman FR, et al. Identifying augmented renal clearance in trauma patients: validation of the augmented renal clearance in trauma intensive care scoring system. J Trauma Acute Care Surg. 2017;82:665–671. doi: 10.1097/TA.0000000000001387. [DOI] [PubMed] [Google Scholar]
- 15.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5:303–311. [PubMed] [Google Scholar]
- 16.Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017;9:36. doi: 10.3390/pharmaceutics9030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobbs AL, Shea KM, Roberts KM, Daley MJ. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy. 2015;35:1063–1075. doi: 10.1002/phar.1653. [DOI] [PubMed] [Google Scholar]
- 18.Gijsen M, Huang CY, Flechet M, Van Daele R, Declercq P, Debaveye Y, et al. Development and external validation of an online clinical prediction model for augmented renal clearance in adult mixed critically ill patients: the augmented renal clearance predictor. Crit Care Med. 2020;48:e1260–e1268. doi: 10.1097/CCM.0000000000004667. [DOI] [PubMed] [Google Scholar]
- 19.Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol. 2015;10:382–389. doi: 10.2215/CJN.03080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udy AA, Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomized, placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017;49:624–630. doi: 10.1016/j.ijantimicag.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Kawano Y, Maruyama J, Hokama R, Koie M, Nagashima R, Hoshino K, et al. Outcomes in patients with infections and augmented renal clearance: a multicenter retrospective study. PLoS ONE. 2018;13:e0208742. doi: 10.1371/journal.pone.0208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campassi ML, Repetto FG, Banegas Litardo DM, Castor R, Gómez G, Tiseyra B, et al. Incidence and determinants of augmented renal clearance in traumatic brain injury: a prospective observational study. J Crit Care. 2022;70:154065. doi: 10.1016/j.jcrc.2022.154065. [DOI] [PubMed] [Google Scholar]
- 23.Udy A, Boots R, Senthuran S, Stuart J, Deans R, Lassig-Smith M, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111:1505–1510. doi: 10.1213/ANE.0b013e3181f7107d. [DOI] [PubMed] [Google Scholar]
- 24.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 2014;42:520–527. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 25.Beunders R, van de Wijgert IH, van den Berg M, van der Hoeven JG, Abdo WF, Pickkers P. Late augmented renal clearance in patients with COVID-19 in the intensive care unit. A prospective observational study. J Crit Care. 2021;64:7–9. doi: 10.1016/j.jcrc.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:94–100. doi: 10.1159/000363721. [DOI] [PubMed] [Google Scholar]
- 27.Ronco C, Bellomo R, Kellum J. Understanding renal functional reserve. Intensive Care Med. 2017;43:917–920. doi: 10.1007/s00134-017-4691-6. [DOI] [PubMed] [Google Scholar]
- 28.Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45:385–392. doi: 10.1016/j.ijantimicag.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Egea A, Dupuis C, de Montmollin E, Wicky PH, Patrier J, Jaquet P, et al. Augmented renal clearance in the ICU: estimation, incidence, risk factors and consequences-a retrospective observational study. Ann Intensive Care. 2022;12:88. doi: 10.1186/s13613-022-01058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 31.Olofsson SK, Cars O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin Infect Dis. 2007;45:S129–S136. doi: 10.1086/519256. [DOI] [PubMed] [Google Scholar]
- 32.Bilbao-Meseguer I, Barrasa H, Rodríguez-Gascón A, Asín-Prieto E, Maynar J, Sánchez-Izquierdo JÁ, et al. Optimization of levetiracetam dosing regimen in critically ill patients with augmented renal clearance: a Monte Carlo simulation study. J Intensive Care. 2022;10:21. doi: 10.1186/s40560-022-00611-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobesh PP, Trujillo TC. Coagulopathy, venous thromboembolism, and anticoagulation in patients with COVID-19. Pharmacotherapy. 2020;40:1130–1151. doi: 10.1002/phar.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikami R, Imai S, Hayakawa M, Sugawara M, Takekuma Y. Clinical applicability of urinary creatinine clearance for determining the initial dose of vancomycin in critically ill patients. J Infect Chemother. 2022;28:199–205. doi: 10.1016/j.jiac.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011;15:R139. doi: 10.1186/cc10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013;17:R35. doi: 10.1186/cc12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37:49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 38.Collet M, Hijazi D, Sevrain P, Barthélémy R, Labeyrie MA, Prié D, et al. Evaluation of glomerular filtration rate using iohexol plasma clearance in critically ill patients with augmented renal creatinine clearance: a single-centre retrospective study. Eur J Anaesthesiol. 2021;38:652–658. doi: 10.1097/EJA.0000000000001501. [DOI] [PubMed] [Google Scholar]
- 39.Carlier M, Dumoulin A, Janssen A, Picavet S, Vanthuyne S, Van Eynde R, et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 2015;41:427–435. doi: 10.1007/s00134-014-3641-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Detailed description of the covariates used for multivariate Cox hazards analysis to identify factors associated with the onset or persistence of ARC. Table S2. Detailed description of the covariates used for multivariate analysis to identify factors associated with mortality or fewer ICU-free days. Table S3. Independent factors affecting mortality, as determined by a multiple logistic regression analysis. Table S4. Independent factors affecting lesser ICU-free days, as determined by a multiple Cox regression analysis.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.