Abstract
Disorders of cell number that result from an imbalance between the death of parenchymal cells and the proliferation or recruitment of maladaptive cells contributes to the pathogenesis of kidney disease. Acute kidney injury can result from an acute loss of kidney epithelial cells. In chronic kidney disease, loss of kidney epithelial cells leads to glomerulosclerosis and tubular atrophy, whereas interstitial inflammation and fibrosis result from an excess of leukocytes and myofibroblasts. Other conditions, such as acquired cystic disease and kidney cancer, are characterized by excess numbers of cyst wall and malignant cells, respectively. Cell death modalities act to clear unwanted cells, but disproportionate responses can contribute to the detrimental loss of kidney cells. Indeed, pathways of regulated cell death — including apoptosis and necrosis — have emerged as central events in the pathogenesis of various kidney diseases that may be amenable to therapeutic intervention. Modes of regulated necrosis, such as ferroptosis, necroptosis and pyroptosis may cause kidney injury directly or through the recruitment of immune cells and stimulation of inflammatory responses. Importantly, multiple layers of interconnections exist between different modalities of regulated cell death, including shared triggers, molecular components and protective mechanisms.
Subject terms: Acute kidney injury, Apoptosis
Pathways of regulated cell death may contribute to the pathogenesis of various kidney diseases. Here, the authors provide an overview of the relationship between necroptosis, pyroptosis, ferroptosis and apoptosis, the evidence supporting a role for these regulated pathways of necrosis in kidney disease, strategies for therapeutic targeting and research needs.
Key points
Regulated cell death through either apoptosis or necrosis contributes to parenchymal cell loss in acute and chronic kidney disease and may also modulate inflammation, fibrosis and the immune response.
Therapeutic strategies that modulate apoptosis may interfere with or promote regulated necrosis.
Available evidence in general supports a therapeutic benefit of targeting ferroptosis or necroptosis in preclinical models of kidney disease; however, the impact of targeting pyroptosis is less clear.
The diverse forms of regulated necrosis may be interrelated, share molecular pathways and cell defence mechanisms and coexist in the same kidney disease, affecting different cell types or the same cell type synchronously or sequentially.
Regulated necrosis may release damage-associated molecular patterns, which induce an inflammatory response that amplifies tissue injury (necroinflammation or immunogenic cell death).
Despite some promising preclinical data, no clinical studies have targeted regulated necrosis to prevent or treat human kidney disease.
Introduction
Kidney disease is one of the fastest growing global causes of death, with chronic kidney disease (CKD) projected to become the fifth global cause of death by 2040 (refs. 1,2). In addition to being a burden in its own right, CKD is intimately related to other forms of kidney disease3–8 (Fig. 1). For instance, the risk of acute kidney injury (AKI) is increased in persons with CKD, and AKI may itself cause or accelerate the progression of CKD9. CKD can also be complicated by the development of acquired cystic kidney disease or renal cell carcinoma7,8. Many of these conditions can be characterized as disorders of cell number. For example, CKD is characterized by the progressive loss of epithelial cells, such as podocytes and tubule cells, and of endothelial cells, which causes glomerulosclerosis, tubular atrophy and capillary rarefaction, respectively, as well as the proliferation and/or recruitment of maladaptive cells such as myofibroblasts and inflammatory cells.
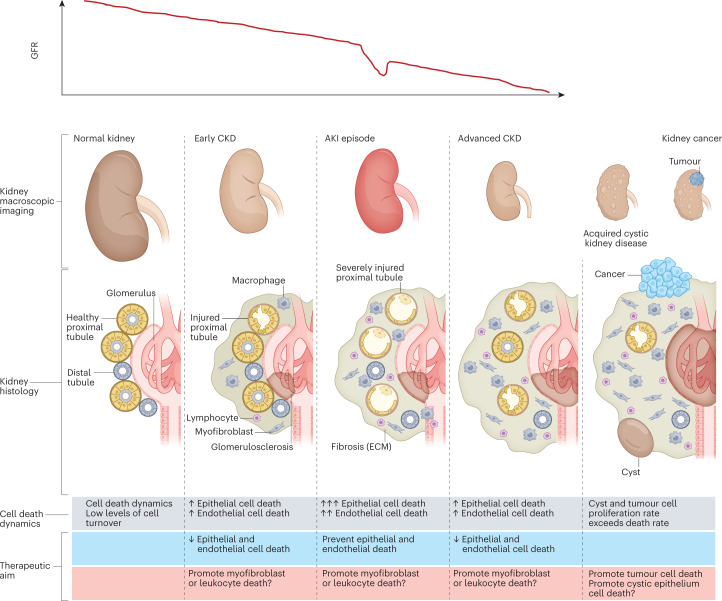
Fig. 1. The natural history of CKD and relationship to cell number and cell death.
The relationship between chronic kidney disease (CKD), acute kidney injury (AKI), acquired kidney cystic disease and kidney cancer can be conceptualized as interrelated processes, characterized by changes in cell number that are driven by the proliferation or death of key cell types. Differences in the dynamics of cell death and turnover represent potential therapeutic targets. AKI is more common in patients with prior CKD and may cause irreversible loss of kidney function or accelerate CKD progression. However, not all episodes of AKI are associated with prior CKD. Of note, AKI is not necessarily characterized by a loss of kidney epithelial cells, but cell death is common in severe episodes of AKI. Acquired kidney cystic disease is usually not clinically relevant although bleeding may occur3. However, acquired kidney cystic disease is a risk factor for renal cell carcinoma7,8. Both acquired kidney cystic disease and kidney cancer are associated with an increase in cell number. In kidney cysts, an excess number of epithelial cells allows cyst growth. Kidney cancer is characterized by an increased number of malignant epithelial cells; the goal of therapy in this context is to kill or extirpate these cells. Renal cell carcinoma may occur in individuals with or without prior CKD. However, CKD is a risk factor for renal cell carcinoma4. Of note, acquired cystic kidney disease renal cell carcinoma is considered a distinct renal neoplasm according to the International Society of Urological Pathology–WHO, and is associated with specific somatic mutations5. ECM, extracellular matrix; GFR, glomerular filtration rate.
Novel therapeutic approaches are needed to address the suboptimal outcomes in patients with kidney disease. Pathways that target cell death pathways and therefore alter cell number may therefore represent a therapeutic approach to prevent, treat or accelerate recovery from kidney disease by limiting the loss of kidney parenchymal cells and kidney inflammation. Two main forms of cell death are known: necrosis, in which rupture of the plasma membrane releases intracellular contents, and apoptosis, a form of regulated cell death in which dying cells are rapidly engulfed by adjacent cells before the plasma membrane ruptures and intracellular contents are released into the extracellular space10–13 (Fig. 2). Apoptosis is considered to be a non-immunogenic and non-inflammatory process that enables the orderly clearance of unwanted or damaged cells14,15. However, despite extensive efforts, therapeutic approaches to target apoptosis in AKI or CKD have not been successful. Contrary to the initial suggestions that necrosis is an unregulated process, several molecular programmes that result in regulated necrosis have been identified and offer new opportunities for therapeutic intervention16–18 (Box 1 and Supplementary Fig. 1). Evidence from preclinical models suggests that three main forms of regulated necrosis — ferroptosis, necroptosis and pyroptosis — may contribute to a range of kidney diseases (Supplementary Fig. 2). More limited evidence supports a role for mitochondrial permeability transition-regulated necrosis (MPT-RN), autosis and NETosis (derived from the term neutrophil extracellular traps (NETs)) in the pathogenesis of kidney disease18–28. However, much work is needed to understand the relative contribution of the diverse forms of regulated necrosis to human kidney disease, the extent to which the pathways are interconnected and the extent to which they can be targeted therapeutically. Here, we provide an overview of the relationship between necroptosis, pyroptosis, ferroptosis and apoptosis, the evidence supporting a role for these regulated pathways of necrosis in kidney disease, strategies for therapeutic targeting and research needs. Three other reviews in this issue of Nature Reviews Nephrology provide detailed overviews of the process of ferroptosis, necroptosis and pyroptosis in kidney disease29–31.
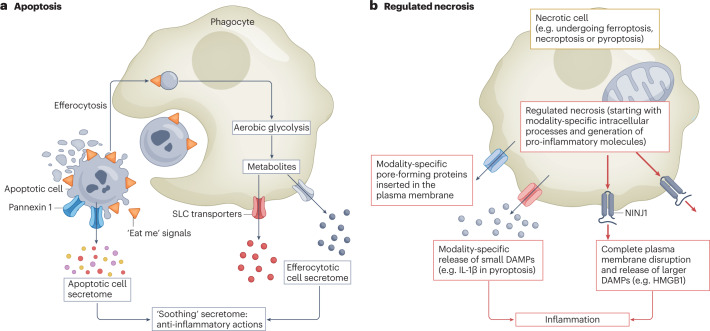
Fig. 2. Interaction between apoptosis, regulated necrosis and inflammation.
a, The process of apoptosis clears unwanted or damaged cells during development and under homeostatic conditions. Multiple steps within the process of apoptosis convey signals to dampen inflammation. Cells that are undergoing apoptosis display cell surface molecules (‘eat me’ signals) that promote their rapid engulfment (efferocytosis) and clearance by adjacent cells before the plasma membrane ruptures and the pro-inflammatory contents are released11. Additionally, caspase-mediated opening of pannexin 1 channels at the plasma membrane of the apoptotic cell facilitates the release of an apoptotic metabolite secretome that comprises AMP, GMP, creatine, spermidine, glycerol-3-phosphate, ATP, fumarate, succinate and other compounds that suppress inflammation in adjacent cells12. Furthermore, efferocytosis itself is associated with changes in phagocyte metabolism, including a switch to aerobic glycolysis and the release of glycolytic by-products such as lactate through solute carrier family (SLC) transporters to promote anti-inflammatory responses in adjacent cells13. Thus, overall the processes of apoptosis and efferocytosis are associated with a complex programme that promotes the ‘soothing’ of adjacent cells. b, By contrast, regulated necrosis can engage innate and adaptive immune responses at multiple steps through, for example, the lack of early engulfment, the generation of pro-inflammatory molecules (for example, IL-1β in pyroptosis) and the release of small damage-associated molecular patterns (DAMPs) through pore-forming proteins that are specific for each form of regulated necrosis. The final common pathway of these actions is disruption of the plasma membranes through the involvement of NINJ1, which allows the release of larger DAMPs.
Box 1 Key operational definitions of cell death terms.
Accidental cell death
Virtually instantaneous and uncontrollable form of cell death corresponding to the physical disassembly of the plasma membrane caused by extreme physical, chemical or mechanical cues.
Regulated cell death
Results from the activation of one or more signal transduction modules, and hence can be pharmacologically or genetically modulated (at least kinetically and to some extent). Variants of regulated cell death include programmed cell death (PCD), apoptosis and regulated necrosis.
Programmed cell death
Occurs in strictly physiological scenarios; that is, it does not relate to perturbations of homeostasis and hence does not occur in the context of failing adaptation to stress. PCD usually takes place by apoptosis
Apoptosis
Regulated cell death executed by enzymes termed caspases and characterized by specific morphological features (blebbing and cell and nuclear fragmentation, pyknosis), initial preservation of plasma membrane integrity and rapid engulfment by adjacent cells. It is considered not to promote inflammation
Intrinsic apoptosis: initiated by perturbations of the extracellular or intracellular microenvironment, demarcated by mitochondrial outer membrane permeabilization, and precipitated by executioner caspases, mainly caspase 3
Extrinsic apoptosis: initiated by perturbations of the extracellular microenvironment detected by plasma membrane receptors, propagated by caspase 8 and precipitated by executioner caspases, mainly caspase 3
Regulated necrosis
Regulated cell death characterized by an early loss of plasma membrane integrity and release to the extracellular space of intracellular contents that may be sensed as damage-associated molecular patterns (DAMPs) that activate innate and adaptive immune responses (that is, they may promote inflammation)
Autosis: a specific instance of autophagy-dependent cell death that critically relies on the plasma membrane Na+/K+-ATPase
Ferroptosis: initiated by oxidative perturbations of the intracellular microenvironment that is under constitutive control by glutathione peroxidase 4 (GPX4) and can be inhibited by iron chelators and lipophilic antioxidants. A key feature is peroxidation of membrane phospholipids
Necroptosis: triggered by perturbations of extracellular or intracellular homeostasis that critically depends on mixed lineage kinase domain-like protein (MLKL) and receptor-interacting serine/threonine-protein kinase 3 (RIPK3), and (at least in some settings) on the kinase activity of RIPK1
Pyroptosis: critically depends on the formation of plasma membrane pores by members of the gasdermin protein family, often (but not always) as a consequence of inflammatory caspase activation
NETotic cell death: Reactive oxygen species (ROS)-dependent modality restricted to cells of haematopoietic derivation and associated with the extrusion of neutrophil extracellular traps (NETs; networks of extracellular material, primarily composed of DNA from neutrophils, that bind pathogens but have also been implicated in triggering autoimmune responses in anti-neutrophil cytoplasmic autoantibody vasculitis and systemic lupus erythematosus)
Mitochondrial permeability transition-driven necrosis: triggered by perturbations of the intracellular microenvironment and relying on CYPD
Immunogenic cell death
Activates an adaptive immune response in immunocompetent hosts. This has been mainly linked to regulated necrosis.
Adapted from ref. 10, Springer Nature Limited.
Targeting cell death in kidney disease
Safety and efficacy are key requirements for the translation of any new therapy to the clinic, and depend on the extent to which the new therapy disrupts critical physiological homeostatic and adaptive processes and on the contexts in which they are used. Cell death can clear damaged or unwanted cells, and therefore has a key role in various processes, including: development, in which whole-organ structures may be transient; physiological tissue turnover; the elimination of cells that are infected, damaged or in the early stages of malignant transformation; and the elimination of cells that have accumulated in excess during repair processes32. Regulated cell death programmes probably evolved to protect complex organisms32, and these protective roles should be considered when developing approaches to target cell death therapeutically. Kidney cancer cells have developed mechanisms to evade cell death33, whereas inactivating genetic variants of the genes involved in cell death processes have long been known to contribute to the impaired deletion of self-reactive immune cells and the development of autoimmunity34,35. However, untimely, unwanted or excessive cell death can deplete important parenchymal cells and cause tissue injury. Moreover, certain forms of cell death can trigger inflammatory (necroinflammation) or autoimmune (immunogenic cell death) responses that can cause and/or amplify tissue injury10,36,37.
Cell death and more specifically, regulated necrosis may represent a therapeutic target in kidney diseases, including AKI and CKD of various aetiologies, either by preventing unwanted cell death or by promoting the death of specific cell types (Fig. 1).
Preventing cell death
Therapeutic approaches that target cell death in the context of AKI and CKD should prevent aberrant kidney epithelial and endothelial cell death as well as the inflammatory and fibrotic processes that result from certain cell death modalities. However, interventions that prevent the death of parenchymal kidney cells should not interfere with the clearance of irreversibly damaged or premalignant cells38 (Supplementary Fig. 3). Of note, some evidence suggests that papillary carcinoma may be triggered by the ischaemic necrosis of proximal tubule cells8,39. In addition, clear cell carcinoma may be triggered by metabolic overload of the remnant nephron’s proximal tubules in CKD, highlighting that therapies aimed at preventing tubular cell death in CKD should not interfere with antitumour surveillance mechanisms that kill cells in the early stages of tumorigenesis8. Thus, interventions that aim to prevent cell death should ideally differentially affect different cell types, to avoid adverse consequences40.
Promoting cell death
Approaches to promote cell death underlie the mainstay of current anticancer therapies and are also used clinically to eliminate autoimmune lymphoid cells. A better understanding of the specific molecules that promote the survival of kidney cancer cells could enable specific targeting of these molecules and increase the safety of interventions. Therapeutic approaches to the promotion of cell death may also be considered to clear unwanted cells that actively contribute to kidney inflammation and fibrosis. For example, apoptosis of myofibroblast populations contributed to renal recovery following ischaemia–reperfusion injury-associated AKI (IRI-AKI)41, whereas specific clearing of macrophages successfully halted disease progression in a model of experimental crescentic glomerulonephritis42. Ideally, such unwanted cells should be cleared by promoting apoptosis, given the potential adverse pro-inflammatory consequences of regulated necrosis that might amplify kidney injury by invoking a more intense inflammatory response or by triggering autoimmunity. Kidney cancer may be an exception, since promoting regulated necrosis-induced immunogenicity towards malignant cells may be beneficial33. In other contexts, the need to both promote and prevent cell death should be balanced. For example, efforts to prevent the death of tubule cells induced by the nephrotoxic effects of cisplatin should not interfere with the ability of cisplatin to induce the death of tumour cells.
Apoptosis
Caspase-dependent apoptosis accounts for approximately 90% of cell turnover under homeostatic conditions43. Its homeostatic role is closely linked to its non-inflammatory nature11–13 (Fig. 2). From the early 1990s to the early 2010s, the kidney and cell death literature was dominated by reports of the contribution of apoptosis to the pathogenesis of AKI and CKD15,44. However, apoptosis may also contribute to kidney repair by clearing excess myofibroblasts and tubule cells following their compensatory proliferation after injury41,45.
Apoptosis has specific morphological features that can be assessed histologically (Supplementary Table 1). However, the identification of morphologically apoptotic cells in tissue sections is burdensome and hampered by their short half-life46, and was soon replaced by easier-to-quantify histological assessment of DNA breaks as identified by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)47,48 or evidence of caspase 3 activation49. However, TUNEL might stain necrotic cells50 and caspase 3 activation is now known to contribute to pyroptosis through the cleavage of gasdermins51. Thus, older studies of apoptosis in kidney disease may not have clearly differentiated apoptosis from regulated necrosis.
The molecular regulation of apoptosis has largely been characterized15 (Fig. 3a,b). However, and as discussed below, we now know that multiple connections exist between apoptosis and cascades of regulated necrosis (Figs. 3,4). For example, some caspases can cleave gasdermins and gasdermin amino-terminal (NT) fragments to regulate pyroptosis30,51. In addition, TNF superfamily cytokines that usually promote apoptosis by recruiting death receptor multiprotein complexes might also activate receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and thereby trigger necroptosis in the context of caspase 8 inhibition (Fig. 3c,d). Moreover, compounds that induce apoptosis, such as BH3-mimetic drugs or inhibitors of anti-apoptotic BCL-2 family proteins, cooperate with inducers of ferroptosis in causing cell death52. In this regard, the tumour-suppressive transcription factor p53 promotes apoptosis by upregulating mediators of apoptosis, such as BAX, and downregulating anti-apoptotic molecules such as BCL-2 (refs. 53,54), but also sensitizes cells to ferroptosis by downregulating SLC7A11, which encodes a subunit of the system xc− cystine/glutamate antiporter, and consequently decreasing cystine uptake55 (Fig. 3e). Nephrotoxic drugs, such as cisplatin, might also induce apoptosis and ferroptosis in tubule cells, and targeting a single molecule, such as dipeptidase 1 (DPEP1), may protect from both56.
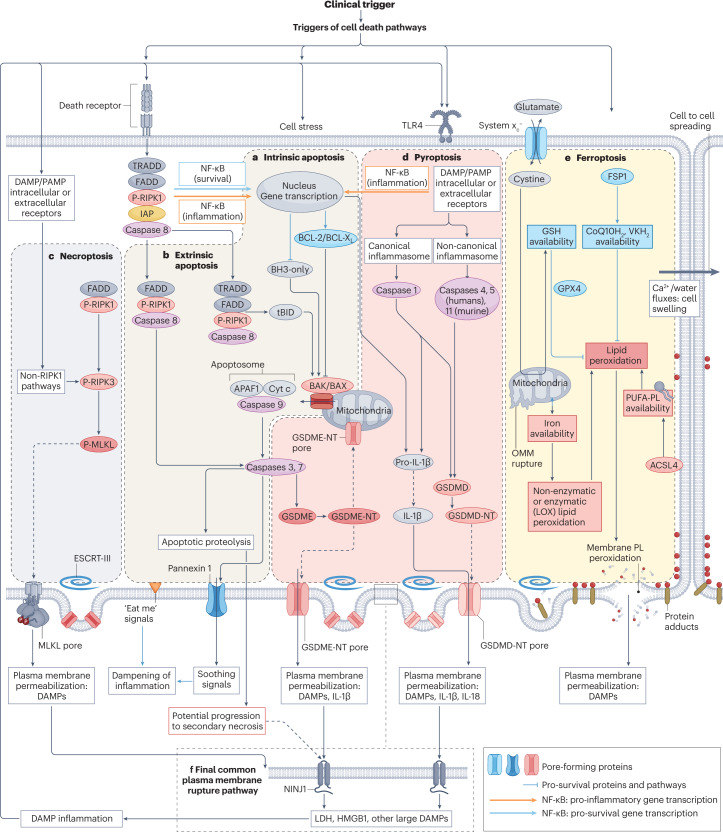
Fig. 3. Key molecular processes and interactions between cell death pathways.
Two main pathways for apoptosis are well characterized. a, In the intrinsic pathway, stressors or the lack of survival signals decrease the balance between pro-survival BCL-2 family proteins and pro-apoptotic BH3-only proteins, favouring the BAK/BAX-dependent mitochondrial outer membrane permeabilization, which results in the release of pro-apoptotic mitochondrial proteins, including cytochrome c, and leads to the formation of a multiprotein structure called the apoptosome that incorporates APAF1 and procaspase 9. This series of events results in activation of caspase 9 — an initiator caspase that subsequently activates executioner caspases such as caspase 3 and caspase 7 that contribute decisively to the dismantling of cell structures. b, In the extrinsic apoptosis pathway, activation of death receptors of the tumour necrosis factor receptor (TNFR) superfamily leads to activation of the initiator caspase 8, which in turn activates executioner caspases and may also process the BH3-only protein BID to tBID, which activates the mitochondrial apoptotic pathway. However, there are alternative outcomes for TNFR activation by TNF, depending on the recruitment of specific proteins to multiprotein complexes. The presence of inhibitor of apoptosis (IAP) proteins activates NF-κB to increase the transcription of anti-apoptotic proteins, although NF-κB can also induce a pro-inflammatory response. In absence of IAPs, caspase 8 is activated. Inhibition of caspase 8 may trigger necroptosis (see below). Apoptotic cells express “eat-me” signals on their surface, which promotes their rapid engulfment (efferocytosis) by adjacent cells. In addition, they secrete anti-inflammatory molecules (soothing signals). However, extensive apoptosis or decreased clearance of apoptotic cells may lead to secondary necrosis, in which the cell membrane is permeabilized through engagement of NINJ1 and damage-associated molecular patterns (DAMPs) released. c, Necroptosis. The core features of necroptosis are receptor-interacting serine/threonine-protein kinase 3 (RIPK3) activation by phosphorylation and the subsequent phosphorylation of mixed lineage kinase domain-like protein (MLKL). Phosphorylated MLKL oligomerizes to form pores that disrupt the plasma membrane, allowing the release of cell contents. Canonical RIPK3 activation is mediated by its interaction with the RIP homotypic interaction motif-containing proteins RIPK1, TIR domain-containing adapter molecule 1 (TICAM1) or Z-DNA-binding protein 1 (ZBP1). Binding of RIPK3 to RIPK1 occurs in response to activation of TNFRs, whereas binding of RIPK3 to TICAM1 is induced by activation of Toll-like receptors (TLRs), and binding to ZBP1 occurs in response to the presence of Z-RNA as a result of viral infection. Non-canonical RIPK3 activation occurs in response to activation of the cell membrane Na+/H+ exchanger 1 and increased intracellular pH (not shown). d, Pyroptosis. The key feature of pyroptosis is the enzymatic processing of gasdermins to amino-terminal (NT) fragments that oligomerize to assemble a plasma membrane pore, allowing the release of intracellular contents and DAMPs. The best-characterized model involves the NLRP3 (canonical) inflammasome-mediated activation of caspase 1 in macrophages, which processes pro-IL-1β to IL-1β, pro-IL-18 to IL-18 and gasdermin D (GSDMD) to GSDMD-NT. However, other inflammasomes and enzymes may process gasdermins in other cell systems and IL-1β may not be a major component of pyroptosis in cell types with low IL-1β gene expression. Additionally, gasdermins may permeabilize mitochondria, which, together with the potential for caspase 3 to cleave gasdermin E (GSDME) and caspase 8 to cleave GSDMD, provides links to apoptosis. e, Ferroptosis. The central event in ferroptosis is the peroxidation of plasma membrane phospholipids (PL) in an iron-dependent manner. The sensitivity of cells to ferroptosis will depend on iron availability (noting that mitochondria may serve to store iron), and on the capacity for enzymatic (for example, mediated by lipoxygenases (LOX)) or non-enzymatic lipid peroxidation as well as on the presence of cell defences against lipid peroxidation, including adequate glutathione (GSH) stores which are maintained through the entry of cystine through the system xc− cystine/glutamate antiporter. The enzyme glutathione peroxidase 4 (GPX4) requires GSH for its antioxidant function. Additional antioxidant systems include ferroptosis suppressor protein 1 (FSP1), which maintains vitamin K (VK) and coenzyme Q10 (CoQ10; also known as ubiquinone) in a reduced state (VKH2 and CoQ10H2, respectively). The main consequence of ferroptosis is peroxidation of membrane PL, shown as red dots in the figure, that results in membrane protein adducts and membrane rupture. Following some triggers, ferroptosis may spread from cell to cell in a wave-like pattern via a process that involves volume shifts and calcium fluxes, as has been described in kidney tubules. f, Shared features between the different cell death modalities include the ability of the repair machinery, endosomal sorting complexes required for transport III (ESCRT-III), to repair membranes and the need for NINJ1 in the final step of plasma membrane fragmentation, which leads to the release of the larger DAMPs and proteins. However, NINJ1 is not required for plasma membrane rupture during ferroptosis. Of note, although all molecular pathways in the figure are represented in the same cell to emphasize the interconnections between pathways, not all cell types have the intracellular machinery or microenvironment required for all forms of regulated cell death to proceed. For pyroptosis, the increased transcription, processing and release of IL-1β have been mainly characterized in macrophages, whereas epithelial cells have a more limited capacity to release this interleukin. ACSL, long-chain fatty acid–CoA ligase 4; OMM, outer mitochondrial membrane; PAMP, pathogen-associated molecular pattern; PUFA, polyunsaturated fatty acid; PUFA-PL, PUFA-containing phospholipids.
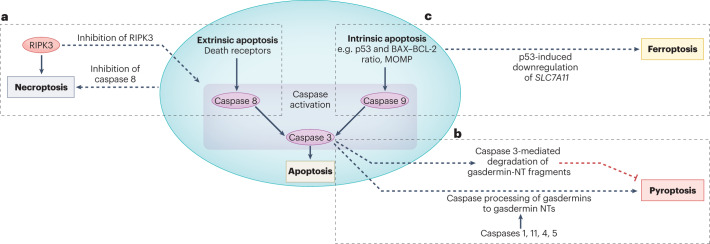
Fig. 4. Examples of interactions between apoptosis and regulated necrosis.
The molecular pathways that lead to activation of extrinsic or intrinsic apoptosis may also modulate or trigger different forms of regulated necrosis. Thus, interventions aimed at inhibiting apoptosis may trigger or modulate modalities of regulated necrosis, potentially affecting their safety and efficacy. a, Following the activation of death receptors, such as TNF receptor, inhibition of caspase 8 promotes necroptosis over apoptosis. Conversely, inhibitors of receptor-interacting serine/threonine-protein kinase 3 (RIPK)3 or certain kinase-dead RIPK3 mutants will prevent necroptosis but result in the assembly of multimeric protein complexes that activate caspase 8 and induce apoptotic cell death. b, Caspases are among the enzymes than can cleave gasdermins to generate amino-terminal (NT) fragments that oligomerize at the plasma membrane to form protein pores and trigger pyroptosis. In pyroptosis, canonical activation of the NLRP3 inflammasome results in activation of caspase 1, whereas non-canonical inflammasomes will activate murine caspase 11 (caspase 4 and caspase 5 in humans). In apoptosis, the executioner caspase 3 is activated by either caspase 8 (extrinsic apoptosis pathway) or caspase 9 (intrinsic apoptosis pathway). Caspases that are activated during apoptosis, such as caspase 8 and caspase 3 may also cleave gasdermins to gasdermin-NT fragments, as exemplified here for caspase 3. Additionally, caspase 3 may degrade NT fragments from gasdermins B and D, potentially protecting from pyroptosis. A more detailed representation of the interaction of caspases with gasdermins and gasdermin NTs is shown in Supplementary Fig. 4b. c, Upstream regulators of apoptosis may also modulate ferroptosis. As an example, the tumour-suppressive transcription factor p53 promotes apoptosis by upregulating mediators of apoptosis, such as BAX, and downregulating anti-apoptotic molecules such as BCL-2 (refs. 53,54), but also sensitizes cells to ferroptosis by downregulating SLC7A11, which encodes a subunit of the system xc− cystine–glutamate antiporter, and consequently decreasing cystine uptake55. MOMP, mitochondrial outer membrane permeabilization.
The role of apoptosis in kidney disease is supported by studies in humans, demonstrating activation of pro-apoptotic pathways in kidney tissue, and by preclinical data, which indicate that interference with bona fide pro-apoptotic proteins is protective15. In an example that reached early clinical development, targeting of p53 protected cultured tubule epithelial cells from apoptosis and mice from cisplatin-induced AKI and IRI-AKI57,58. However, a phase III trial of teprasiran — a small interfering RNA that targets p53 — in >1,000 high-risk patients undergoing cardiac surgery was terminated for lack of efficacy in reducing major adverse kidney-related events59 despite promising phase II results60.
Our improved understanding of shared molecular mechanisms between apoptosis and pathways of regulated necrosis requires a re-evaluation of the established literature on apoptosis and the kidney Unintended modulation of regulated necrosis mechanisms by interventions that aim to inhibit apoptosis may compromise their safety and efficacy; improved understanding of the interconnectivity of molecular events may optimize therapeutic approaches that target apoptosis.
Regulated necrosis
Several forms of regulated necrosis exist — each characterized by specific molecular programmes and response to inhibitors.
Necroptosis
Necroptosis is a kinase-mediated form of regulated necrosis that is characterized by the oligomerization and phosphorylation of RIPK3 and phosphorylation of the pseudokinase mixed lineage kinase domain-like protein (MLKL) (Fig. 3c). Phosphorylation of MLKL promotes its oligomerization and translocation to the plasma membrane where it executes necroptosis by inducing calcium influx, exposing phosphatidyl serine on the outer leaflet of the plasma membrane and disrupting the plasma membrane61,62.
The best-characterized trigger for necroptosis is TNF receptor activation, which leads to the formation of RIPK1–RIPK3 complexes under conditions that prevent caspase 8 activation (Supplementary Fig. 4a). However, additional canonical and non-canonical pathways for RIPK3 activation exist that do not require RIPK163,64 (Fig. 3c); moreover, RIPK3 can promote inflammatory responses independent of necroptosis65,66. In kidney cells, inhibition of caspases prevents apoptosis, but may lead to RIPK1-mediated necroptosis, as observed in a model of cytokine-induced tubule cell death18,67.
Inhibitors of necroptosis are termed necrostatins and — together with genetically modified mice — have been instrumental in characterizing the role of necroptosis in disease. However, early necrostatins were non-specific and, hence, caution is warranted when interpreting findings from studies that have used these tools. For example, necrostatin 1 (Nec1) — the first necroptosis inhibitor described — inhibits both RIPK1 and indoleamine-2,3-dioxygenase (IDO), which catabolizes tryptophan into kynurenine and modulates innate and adaptive immunity, and other enzymes68,69. Moreover, Nec1 may also inhibit ferroptosis induced by glutathione peroxidase 4 (GPX4) deficiency in fibroblasts in a manner that is independent of IDO and RIPK1 inhibition70. A more recently developed compound, Nec1f, simultaneously inhibits RIPK1 and some forms of ferroptosis in tubular cells, but has not been compared with Nec1, and its mechanism of ferroptosis inhibition is poorly understood71. Nec1s is a more selective and stable inhibitor of RIPK1 than Nec1, and does not inhibit ferroptosis69,70.
Further complicating the study of necroptosis, specific RIPK3 inhibitors, such as GSK’872, also promote apoptosis by interacting with RIPK3 to activate caspase 8 via the recruitment of RIPK1, resulting in the assembly of a caspase 8–FADD–cFLIP complex independent of the effects on MLKL72 (Supplementary Fig. 4a). This pathway is also induced by certain kinase-dead mutants of RIPK3 (ref. 72). Such crosstalk between components of necroptotic, apoptotic and ferroptotic pathways complicates assessment of the role of necroptosis in kidney injury in vivo as well as approaches for its therapeutic targeting.
Pyroptosis
Pyroptosis is a pro-inflammatory form of regulated necrosis that involves the formation of cell membrane pores by oligomers of the N-terminal fragment of gasdermins (gasdermin-NT) following gasdermin cleavage73,74 (Fig. 3d). Pyroptosis contributes to the innate immune response and can be triggered by inflammasome-dependent and inflammasome-independent pathways. The best-characterized pyroptosis pathway occurs in macrophages, and is triggered by engagement of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns with the NLRP3 inflammasome, leading to activation of caspase 1. Caspase 1 consequently cleaves gasdermin D (GSDMD) to generate GSDMD-NTs that oligomerize and insert into the plasma membrane to cause membrane permeabilization75,76. Inflammasome and caspase 1 activation also increases processing and secretion of pro-inflammatory cytokines such as IL-1β and IL-18. Of note, the release of IL-1β through GSDMD-NT-induced pores is a key feature of macrophages undergoing pyroptosis but can also occur in viable macrophages, indicating that the role of GSDMD-NT pores in cell death and IL-1β release can be dissociated77. The mechanisms underlying this dissociation are unclear, but may involve the actions of defence mechanisms against membrane disruption by pore-forming proteins (such as the endosomal sorting complexes required for transport III (ESCRT-III) machinery, as described below), dynamic regulation of pore opening by local phosphoinositide circuitry, blockade of downstream NINJ1 membrane clustering, or other regulatory mechanisms78–80. Gasdermins can also be processed to pore-forming NT fragments by caspases other than caspase 1, including endogenous or microbial proteases (Supplementary Fig. 4b,c). For example, inflammasome-activated caspase 11 (or its human homologues caspase 4 and caspase 5) can also process GSDMD to generate GSDMD-NT75,76 whereas caspase 3 processes gasdermin E (GSDME) to generate GSDME-NT in epithelial cells to trigger pyroptosis81. Neutrophil pyroptosis also contributes to the process of NETosis and can be triggered by non-caspase proteolytic enzymes82,83 (Supplementary Fig. 4c). Although gasdermins are expressed in the kidney, the contribution of pyroptosis to kidney disease is unclear despite beneficial effects of targeting the NLRP3 inflammasome84, since inflammasome activation and the release of IL-1β are not necessarily indicative of pyroptotic cell death85.
Ferroptosis
Ferroptosis is characterized by the iron-dependent peroxidation of membrane phospholipids and the subsequent formation of protein adducts and disruption of the plasma membrane86,87 (Fig. 3e). Ferroptosis can be triggered by a loss of the reduction capacity of cells. Thus, cell sensitivity to ferroptosis is critically dependent on the availability of iron and on the inactivation or suboptimal function of antioxidant defences. Additionally, increased availability of lipid substrates through an increased activity of the enzyme long-chain fatty acid–CoA ligase 4 (ACSL4) favours ferroptosis. The process of lipid peroxidation can involve enzymatic pathways (for example, involving iron-containing lipoxygenases (LOXs)) or involve non-enzymatic mechanisms. The peroxidation of membrane phospholipids contributes to disruption of the outer mitochondrial membrane disruption and rupture of the plasma membrane by as-yet poorly characterized mechanisms that may include the induction of protein adducts31,70.
The entry of cystine into cells through the system xc− cystine–glutamate antiporter enables the repletion of glutathione (GSH), which is required for GPX4 to catalyse the reduction of double-oxygenated phospholipids into mono-oxygenated phospholipids70. In parallel, ferroptosis suppressor protein 1 (FSP1) maintains vitamin K (VK) and coenzyme Q10 (CoQ10; also known as ubiquinone) in a reduced state (VKH2 and CoQ10H2, respectively), further enabling the reduction of phospholipids. Loss of FSP1 or GPX4 function sensitizes kidney tubule cells to ferroptosis, with one study demonstrating that acquired systemic loss of GPX4 or FSP1 sensitizes mice to AKI71.
One interesting feature of ferroptosis is that it can rapidly propagate from cell to cell, in a wave-like manner88. Such synchronized necrosis has been observed in the renal tubules of models of preclinical IRI and oxalate crystal-induced AKI23. Synchronization of tubule cell death is not observed for necroptosis or pyroptosis, which may explain differences in the extent and distribution of kidney tubule involvement in models of AKI. However, synchronized ferroptosis may be stimulus-dependent, as was observed following a decrease in GSH availability achieved through inhibition of the system xc− or in the context of excess iron, but not following inhibition of GPX4 (ref. 88). Propagation of the ferroptosis-inducing signal occurs upstream of cell rupture and involves calcium fluxes and the spreading of a cell swelling effect in a lipid peroxide-dependent and iron-dependent manner88. In zebrafish, sublethal lipid peroxidation may also spread from cell to cell89.
Ferroptosis may be modulated by the availability of iron or antioxidants (for example, GSH, VKH2 and CoQ10H2), by inhibiting enzymes that synthesize or peroxidize phospholipids or by peroxyl radical scavengers. The radical trapping antioxidants ferrostatin 1 and liproxstatin 1 have been widely used to inhibit ferroptosis, but ferroptosis may also be inhibited by Nec1, Nec1f and other non-specific compounds31.
Interconnections between cell death pathways
Multiple layers of interconnections exist between different modalities of regulated cell death. These include molecular events that are shared between different cell death modalities, including shared triggers, final execution events and cell protective mechanisms (Figs. 3,4 and Supplementary Fig. 4), but also the possibility that one form of cell death may induce a different form of cell death in the same or other cells (Fig. 5).
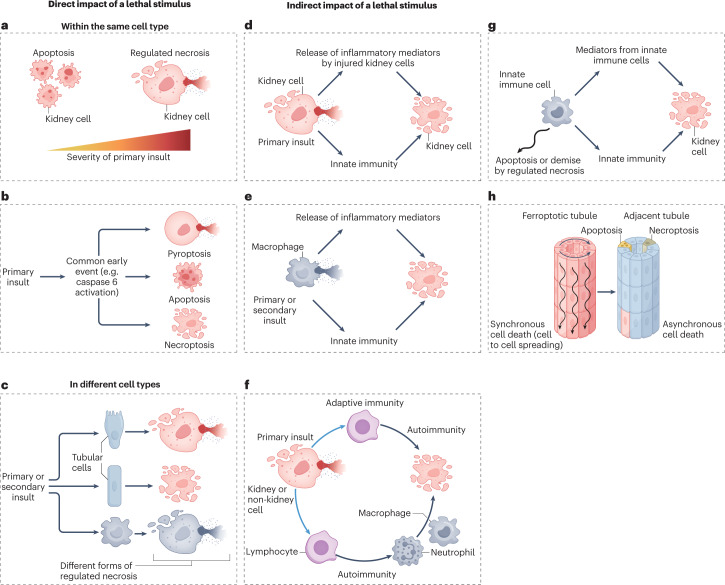
Fig. 5. Interactions between different types of regulated necrosis: conceptual models that may explain the involvement of multiple forms of cell death in the same kidney disease.
The interconnectivity of different modes of cell death suggests that multiple modes of cell death probably concurrently or sequentially coexist within the diseased kidney. The mechanisms underlying the initiation of different cell death pathways are complex and may involve various processes. Primary insults are triggers of injury (for example, cisplatin or ischaemia–reperfusion) and secondary insults are the mediators released in response to cell stress or cell death, such as TNF superfamily cytokines. a, The severity of insult may affect the activation of different cell death pathways. Of note, necrosis is not necessarily associated with a greater severity of insult. c, A primary insult might activate several cell death programmes in the same cell that are mutually exclusive so that inhibition of one will result in death from the other. PANoptosis triggered by caspase 6 activation is one example of such an event, and the interaction between extrinsic apoptosis and necroptosis illustrated in Fig. 4a is another example. c, Primary or secondary insults may differentially impact the mode of cell death in different cell types. d, A primary insult (the cause of the kidney disease) may result in the release of inflammatory mediators by injured kidney cells (secondary insults) and the release of danger-associated molecular signals that trigger an innate immune response that, in turn, amplifies cell death. e, Regulated necrosis of innate immune cells might result in the release of inflammatory mediators and the activation of an innate immune response that, in turn, causes a different form of cell death in kidney epithelial cells. f, A primary insult might result in immunogenic cell death, and the resultant adaptive immune response might further cause cell death and tissue injury via antibodies or cytotoxic T cells. g, Cell death might clear innate immune cells and limit the inflammatory response. In this scenario, inhibition of cell death may result in persistent or amplified inflammation that triggers the death of kidney epithelial cells. h, Ferroptosis might induce synchronized renal tubule cell death following the intercellular propagation of cell death in a wave-like pattern, potentially affecting whole nephrons23. By contrast, apoptosis and necroptosis occur in isolated cells. Inflammatory cytokines released by cells recruited in response to ferroptosis may, in turn, induce apoptosis and/or necroptosis of individual tubule cells located in nearby tubules.
Moreover, the same stimulus may induce different forms of regulated cell death, depending on the intensity of the stimulus (Fig. 5a), or on the presence of co-stimulatory factors. Outside the kidney, it has been well characterized that some stimuli (for example, viruses) can simultaneously trigger pyroptosis, apoptosis and necroptosis in a process termed PANoptosis in innate immune cells, through the generation of PANoptosome complexes that integrate components of the individual cell death pathways in a multiprotein complex that includes caspases, RIPK1, RIPK3 and ZBP1 (refs. 90,91) (Fig. 5b). Caspase 6 regulates ZBP1-mediated NLRP3 inflammasome activation and PANoptosis in a protease-independent manner91. However, although increased caspase 6 levels or activity have been observed in preclinical models of AKI or CKD, the role of caspase 6 in kidney disease has not been characterized92,93. When several cell death programmes are activated concurrently, inhibition of just one molecular pathway will not prevent cell death but rather may change the modality of cell death, as observed for different apoptosis pathways94. For example, MLKL deficiency in cultured fibroblasts prevented necroptosis but favoured the occurrence of ferroptosis induced by cystine deprivation, whereas deficiency of the pro-ferroptotic protein ACSL4 prevented ferroptosis but favoured the occurrence of necroptosis induced by TNF and pan-caspase inhibition95. Similar interconnections have been described between apoptosis and pyroptosis, and between extrinsic apoptosis and necroptosis, including in the context of cytokine-induced tubular cell death18,30,51,67,96 (Fig. 4a,b). In the latter case, inhibition of multiple caspases by the pan-caspase inhibitor zVAD-fmk shifted the mode of cell death from apoptosis to pro-inflammatory necroptosis and increased the number of dead cells67,96, illustrating the potential dangers of interfering with cell death pathways.
The ESCRT-III machinery facilitates membrane repair following the engagement of regulated necrosis pathways, and therefore represents a survival mechanism62,97,98. ESCRT-III protects cells from necroptosis, ferroptosis and pyroptosis by trapping sections of membrane that have been damaged or in which protein pore complexes have oligomerized, and shedding them into intracellular or extracellular vesicles97. As a consequence, knockdown of ESCRT-III sensitizes cells to regulated necrosis. Charged multivesicular body protein 1A (CHMP1A) is a component of ESCRT-III that protects cells from ferroptosis and was identified in a genome-wide association study as a risk gene for kidney disease56.
NINJ1-mediated plasma membrane disruption seems to be a final common pathway shared by pyroptosis, necroptosis and secondary forms of necrosis when apoptotic cells are not rapidly cleared, that triggers cell lysis and the release of large DAMPs99. The protective effects of glycine against cell membrane rupture may be mediated by inhibition of NINJ1-mediated membrane clustering80. However, NINJ1 is not required for ferroptotic cell lysis100 (Fig. 3f). Beyond NINJ1, other stimuli might trigger different forms of cell death in different cell types (Fig. 5c). The precise cell death pathways involved may depend on the nature or severity of the insult, the existence of environmental or genetic modulators of gene expression, the sensitivity of cells to potentially lethal stimuli, the specific cell types involved and the strain or species studied.
A different form of interaction takes place when a specific modality of cell death triggers cell death in other cells or cell types (Fig. 5d–f). For example, the regulated necrosis of tubule cells might release pro-inflammatory mediators and recruit innate immune cells, such as macrophages, that in turn, release cytokines that amplify tubular injury, for example, through cytokine-induced necroptosis (Fig. 5d). The release of cytokines might similarly occur following the death of innate immune cells, such as macrophages, by pyroptosis (Fig. 5e). Regulated necrosis can also be immunogenic and stimulate an adaptive immune response to trigger autoimmunity (or alloimmunity in the case of kidney grafts); these immune cells may subsequently induce a different form of regulated death in their target cells (Fig. 5f). The immune response may not necessarily be directed against kidney cell antigens. For example, both necroptosis and ferroptosis may result in NETosis82,83,101,102, and NETosis is thought to contribute to autoimmunity in anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis — a disease that is associated with kidney cell death (Fig. 5f). Finally, the death of inflammatory cells might limit inflammation and tissue injury during repair. In this context, inhibition of immune cell death might contribute to persistent inflammation or autoimmunity (Fig. 5g).
Overall, our understanding of the interconnections between different modalities of regulated cell death implies that the impact of targeting regulated necrosis pathways may differ according to the stage of disease or between different diseases according to the primary insult and the presence of secondary mediators of injury. The available evidence also implies that different forms of regulated cell death may coexist simultaneously in the same or different cell types, or be activated sequentially even in the same individual. In this regard, interventions that target secondary or minor forms of cell death rather than the predominant form of cell death or main cellular target of the disease might still provide benefit. For example, therapeutic benefit was achieved by targeting autosis — a form of regulated necrosis that was only observed in pericytes — in a preclinical model of IRI-AKI20. Finally, interventions that effectively inhibit a key modality of cell death might become ineffective if cells switch to a different form of death. As an example from the AKI literature, ferroptosis might propagate from cell to cell within the same tubule23, and secondarily recruit an inflammatory response that may trigger necroptosis or other forms of cell death in adjacent tubules96,103 (Figs. 5h,6), suggesting that the timing of interventions may be critical96,103–105. All of these possibilities must be considered when developing interventions to target cell death.
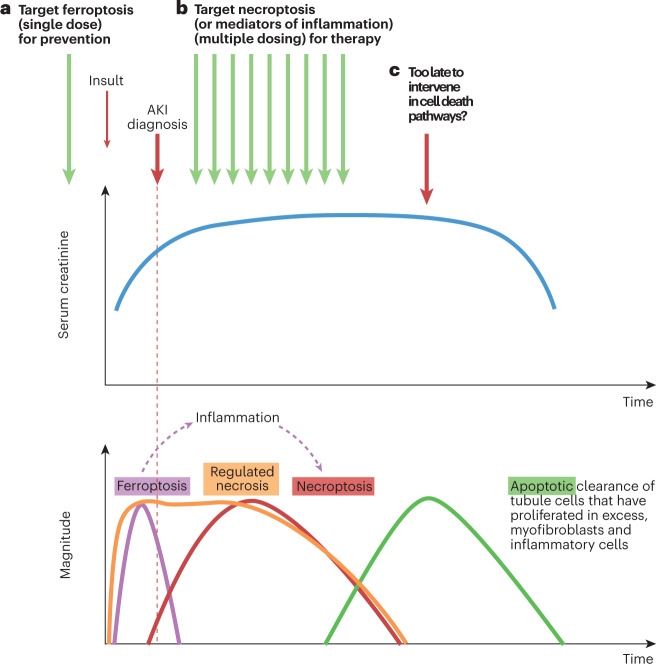
Fig. 6. Interactions between different forms of cell death and their impact on the design of therapeutic approaches for different contexts of use in AKI.
a, In a hypothetical scenario in which an insult causes an initial wave of cell death (for example, an early wave of ferroptosis-induced tubule cell death), the specific form of cell death may be targeted using a preventive approach; for example, with prophylactic administration of an inhibitor. This preventive intervention may avoid the initial wave of cell death and all subsequent consequences, including the development of acute kidney injury (AKI). b, If unchecked, the initial wave of regulated necrosis might result in the release of damage-associated molecular patterns (DAMPs) that triggers inflammation and a second wave of inflammatory cytokine-mediated regulated necrosis, such as necroptosis. In this scenario, a single preventive administration of a cytokine or necroptosis inhibitor will not prevent AKI, as it will not prevent the original wave of cell death caused by ferroptosis. However, necroptosis or other forms of inflammation-related cell death may be amenable to treatment, for example, through initiation of therapy after injury has occurred. This scenario represents the usual clinical situation, in which interventions are prescribed after diagnosis of AKI. Therapy would be expected to be maintained over several days, since DAMPs will be continuously released by ongoing cell death. Successful treatment will not prevent AKI but will accelerate recovery by interfering with the amplification loop of inflammation-related cell death. Biomarkers of regulated necrosis will be needed to assess whether cell death is still ongoing and if so, what is the predominant active mode of kidney cell death. c, In advanced stages of AKI, apoptotic cell death acts to clear excess myofibroblasts and leukocytes as well as excess tubule cells; at this stage, interference with apoptosis might be deleterious while targeting other forms of cell death might be futile. This conceptual representation is based on observations in a mouse model of AKI induced by a folic acid overdose96,103,104 and on descriptions of morphological apoptosis during the recovery phase of human AKI105. The role of ferroptosis in the early stages of this preclinical model and of the contribution of necroptosis to longer term outcomes has been validated in several studies117,143. However, validation of this conceptual representation for forms of human AKI requires further studies and clinical trials.
Regulated necrosis in kidney disease
Although evidence from interventional preclinical studies suggests a role for regulated necrosis in the pathogenesis of both AKI and CKD17–19,21,23,27–31,70,71,96,101,103,106–152 (Supplementary Tables 2,4), evidence from human interventional studies is lacking. In the future, the clinical relevance of preclinical observations will be informed by the results of clinical trials.
Acute kidney injury
Morphological evidence of tubular cell apoptosis, necrosis and loss of tubule cells from undetermined causes has been observed in human AKI153. In general, inhibition of necroptosis and ferroptosis has been demonstrated to be protective in various models of AKI, including preclinical models of IRI-AKI, nephrotoxin-induced AKI, cytokine storm-associated AKI and crystal-induced AKI, whereas the role of pyroptosis in AKI is disputed127,128,154. However, only a handful of laboratories have addressed the relative contribution of the diverse forms of cell death under similar experimental conditions.
Ischaemia–reperfusion injury-induced AKI
IRI-AKI is characterized by morphological evidence of tubule cell apoptosis and caspase activation155. Protection is typically afforded by the inhibition of pro-apoptotic proteins such as BAX and BAK156, whereas the effect of caspase inhibition is controversial17,40,157. In one study, administration of the pan-caspase inhibitor zVAD-fmk at the time of reperfusion prevented the early onset of IRI-AKI (at 24 h)157; however, other studies found no benefit of zVAD-fmk at 48 h after reperfusion17,157, and found it to be ineffective when administered 2 h after reperfusion157. Opposing effects of caspase 3 deficiency have also been observed in the acute and chronic stages of IRI-AKI, with increasing tubule injury scores and serum creatinine levels in the early stages AKI and increasing chronic capillary rarefaction and renal fibrosis in longer term follow-up after IRI, illustrating the potential differential impact of cell death pathway interference on different cells types and on short-term versus long-term outcomes40.
Evidence from interventional studies indicates that necroptosis, MPT-RN and ferroptosis may contribute to the pathogenesis of IRI-AKI18,23. In mouse models of IRI-AKI, inhibition of necroptosis through pretreatment with Nec1 or genetic deletion of RIPK3 afforded partial protection from tubule injury and increases in serum urea and creatinine levels17,18. Moreover, mice with deletion of both the mitochondrial protein CYPD and RIPK3 demonstrated greater protection from IRI-AKI than mice with deletion of CYPD or RIPK3 alone18. However, contradictory results also exist. For example, a randomized, controlled trial in 154 patients undergoing coronary artery bypass grafting surgery with extracorporeal circulation found that prophylactic cyclosporine did not reduce the risk of AKI158, arguing against a pathophysiological role of MPT-RN in IRI-AKI. However, cyclosporine is nephrotoxic and may not therefore be a suitable agent to administer to patients at risk of AKI. Moreover, some studies have found that MLKL deficiency is not as protective as RIPK3 deficiency or catalytically inactive RIPK1 in preventing IRI-AKI159, although other studies have found RIPK3 or MLKL deficiency to be equally protective160. Surprisingly, mice with deletion of both Ripk3 and Mlkl were not protected from IRI-AKI, illustrating the complexities of cell death pathways160.
Ferrostatin 1 and the more stable ferrostatin 1 analogue SRS 16-86 protected mice from severe IRI-AKI, reducing tubule injury and improving renal function23. Combined inhibition of necroptosis, MPT-RN and ferroptosis offered complete protection from severe IRI-AKI, being more effective than any monotherapy tested23. Nec1f, which, as discussed above, targets both RIPK1 and ferroptosis, also improved survival in a mouse model of IRI-AKI71.
Evidence linking pyroptosis and, more specifically, specific mediators of pyroptosis to IRI-AKI is contradictory. Some studies have found that deletion of caspase 11 or GSDME protects mice from IRI-AKI127,130, supporting a pathogenic role for pyroptosis in IRI-AKI. By contrast, others have found that Gsdmd-knockout mice develop more severe IRI-AKI, which is attenuated by co-deletion of MLKL128, suggesting that necroptosis might be amplified if pyroptosis is blocked. That study also found that Gsdme-knockout mice were hypersensitive to IRI-AKI128, again contradicting earlier work130. The reasons for these discrepant findings are not clear, however, and further work is needed to understand these issues.
Although available evidence suggests that necroptosis, MPT-RN, ferroptosis and potentially pyroptosis may contribute to IRI-AKI, whether individual pathways contribute to the loss of specific cell types and the factors that result in the activation of these pathways, are currently unclear. Moreover, the existence of discrepant results suggests that adverse outcomes may be a consequence of strategies that target specific pathways of regulated necrosis and at this point, the extent to which human studies will reproduce the preclinical data is unclear.
Toxin-induced AKI
Nephrotoxic agents induce tubule cell death, with nephrotoxic AKI being the most common contributor to hospital-acquired AKI. Nephrotoxicity is the dose-limiting effect of the most widely studied nephrotoxic drug, the antineoplastic agent, cisplatin161,162. Apoptosis, necroptosis, MPT-RN, ferroptosis and pyroptosis may contribute to cisplatin-induced AKI. However, the literature is not uniform. In human tubule cells, low cisplatin concentrations induce apoptosis, whereas higher concentrations induce necrosis163. In cultured primary proximal tubular cells, combined targeting of necroptosis (with the non-specific inhibitor Nec1) and caspases (with zVAD-fmk) provided better protection against cisplatin-induced death than individual treatments109,164. However, in another study that used human proximal tubule HK-2 cells, ferrostatin 1 provided weak protection against cisplatin-induced death, whereas Nec1 was not at all protective120. In mouse models of cisplatin-induced AKI, inhibition of necroptosis through administration of Nec1 or genetic deletion of RIPK3 or MLKL prevented injury but did not reduce cell death as assessed by TUNEL staining18,109. Of note, dual inhibition of apoptosis and necroptosis pathways increased survival18,109. A role for MPT-RN and ferroptosis in cisplatin nephrotoxicity is supported by the greater survival of Ppid-knockout mice compared with that of wild-type mice18, and the finding that a single prophylactic dose of ferrostatin 1 improved renal function and decreased kidney injury in mice, 72 h after administration of cisplatin120. However, similarly to IRI-AKI, the role of pyroptosis in cisplatin-induced AKI is less clear. Some studies have found that deletion of caspase 11, GSDMD or GSDME protects mice from cisplatin AKI127,130, whereas in at least one study, deletion of GSDMD led to exacerbation of cisplatin-AKI128.
Of more clinical interest than preclinical cisplatin nephrotoxicity are endogenous toxins, such as haem proteins (for example, haemoglobin and myoglobin, which are released following intravascular haemolysis or rhabdomyolysis, respectively) that may cause AKI, particularly in environments with inadequate access to kidney replacement therapy. Some evidence suggests that ferroptosis contributes to rhabdomyolysis-associated AKI118. Despite the activation of apoptosis and necroptosis pathways during rhabdomyolysis, only ferrostatin 1 reduced oxidative stress, cell death and preserved kidney function, both in vivo and in vitro, whereas the pan-caspase inhibitor zVAD and RIPK3 deficiency were not protective107. Inhibition of ferroptosis also has protective effects on organs outside the kidneys; for example, by reducing neuron lipid peroxidation and cell death after haemorrhagic stroke165. Additionally, inhibition of ferroptosis with three different molecules (ferrostatin 1, liproxstatin 1 and UAMC-3203) reduced both single (including the kidney) and multiple organ dysfunction, and prevented death in a mouse model of multiple organ dysfunction induced by intravascular haemolysis166.
Thus, a body of evidence suggests the involvement of different specific forms of regulated necrosis in diverse models of nephrotoxicity, but the results are not always concordant. Such divergency may be expected, given different experimental conditions and the possibility that toxin doses may activate different molecular pathways. However, the heterogeneity in preclinical findings limits their clinical translation, given the lack of tools to identity the predominant form(s) of regulated necrosis under clinical conditions. Prevention of haem-induced AKI (that is, with myoglobin or haemoglobin) may offer a better opportunity for clinical development than prevention of cisplatin-induced AKI, unless the interventions can be locally targeted to tubule cells. The observed beneficial effect of targeting ferroptosis fits well with our understanding of the tubule iron overload that occurs with haem nephropathy.
Cytokine storm-associated AKI
A cytokine storm is a life-threatening systemic inflammatory syndrome, characterized by elevated levels of circulating cytokines and immune-cell hyperactivation in response to diverse triggers, including inflammatory disorders, such as endotoxaemia, sepsis and COVID-19, and can lead to the development of AKI167,168. Approaches to targeting bacterial sepsis (for example, as induced in animal models by caecal ligation and puncture (CLP)) and endotoxaemia (for example, as induced in animals by administration of lipopolysaccharide (LPS)) differ in that interventions for bacterial sepsis must not interfere with the antibacterial capabilities of the immune system, whereas experimental endotoxaemia is sterile. Targeting of ferroptosis (through administration of Fer1), necroptosis (through pharmacological or genetic inhibition of RIPK3) and pyroptosis (through deletion of caspase 11, caspase 1 or GSDMD) has demonstrated kidney-protective effects in mouse models of sepsis and endotoxaemia114,115,148,169. However, the impact of Mlkl deletion is disputed in CLP with at least one study demonstrating a lack of kidney protection in Mlkl-knockout mice114. Combined deletion of Ripk3 and Gsdmd or of Mlkl and Gsdmd provided better protection against CLP-induced AKI and death than deletion of either gene alone169. Moreover, combined deletion of Ripk3 and Gsdmd also improved survival upon challenge with TNF or LPS than deletion of either gene alone169. Disulfiram — a drug that is used to treat alcohol addiction — inhibited GSDMD-induced pore formation, pyroptosis and cytokine release in cultured cells and LPS-induced septic death in mice, while allowing processing of IL-1β and GSDMD170. Thus, available evidence in general supports pathogenic roles for ferroptosis, necroptosis and pyroptosis in cytokine storm-induced AKI, suggesting these pathways could represent potential therapeutic targets.
Crystal-induced AKI
Crystal nephropathies can trigger three different pathways of regulated necrosis: necroptosis, ferroptosis and MPT-RN, and cause AKI and the development of CKD. Clear evidence also suggests that the NLRP3 inflammasome and IL-1β contribute to oxalate-induced kidney inflammation and AKI84, although — as described below — the dependency of this process on pyroptosis has not been demonstrated.
Prophylactic inhibition of necroptosis, MPT-RN or ferroptosis offered protection from calcium oxalate-induced nephropathy 24 h after injury19,23,108. In another study, dual blockade of necroptosis and MPT-RN was more effective than monotherapy, but the effect of ferrostatin 1 was not tested19. Calcium oxalate may activate the inflammasome in kidney immune cells directly84 or indirectly through DAMPs, such as ATP, released from tubule cells that undergo necrosis following internalization of calcium oxalate84,171. Inhibition of necroptotic tubular cell death prevented inflammasome activation in immune cells84,108,171. The dual necroptosis–ferroptosis inhibitor (Nec1f) was also protective71, whereas the inhibition of apoptosis was not protective108.
The role of pyroptosis in calcium oxalate-induced AKI has not been well characterized. In one study, neither GSDMD nor MLKL deficiency prevented monosodium urate crystal-induced cell death and IL-1β release in macrophages or in vivo172. However, Gsdmd-knockout mice had more severe kidney injury at 20 days (that is, during the subacute phase) than wild-type mice128. By contrast, other studies have shown that inhibition of the NLRP3 inflammasome protects mice against crystal-induced chronic nephrocalcinosis and fibrosis173,174.
Folic acid-induced AKI (FA-AKI) is thought to be dependent on crystalluria104 and to involve a sequence of events characterized by an initial wave of ferroptosis within the first 24 h that triggers TNF-like weak inducer of apoptosis (TWEAK)-mediated inflammation and necroptosis. Targeting of TWEAK or necroptosis shortened the duration of AKI, and led to improved kidney function and histology 96 h after exposure to folic acid in mice. However, in one study, specific targeting of bone marrow-derived RIPK3 induced an early decrease in kidney inflammation independent of effects on tubule cell death and did not improve kidney function 48 h after administration of folic acid65, suggesting that RIPK3 might have a dual role in FA-AKI by promoting both early inflammation through its function in innate immune cells and later tubule cell necroptosis in response to inflammatory cytokines through its function in tubular cells. FA-AKI has also been used as a model to explore the effect of ferroptosis on the lipidome103,117,175.
Chronic kidney disease
Information regarding the role of regulated necrosis in preclinical models of CKD is limited. CKD is a complex entity — it can occur as a consequence of a variety of aetiologies and has variable pathophysiology, which can involve an adaptive response to the loss of functioning nephrons, genetic defects and ongoing toxic, metabolic, mechanical or immune insults. Interfering with cell death pathways may therefore differentially affect processes such as nephron loss, inflammation, autoimmunity, fibrosis and cystogenesis. Although limited, some available data support roles for necroptosis, ferroptosis and pyroptosis on diverse readouts associated with CKD.
For example, inhibition of necroptosis with the non-specific inhibitor, Nec1, preserved renal function in rats with subtotal nephrectomy111, whereas deletion of Ripk3 improved kidney function in mice with adenine-induced CKD116. Nec1 also decreased kidney inflammation and fibrosis in a model of unilateral ureteral obstruction (UUO)-induced CKD110 and similarly, Ripk3 knockout reduced kidney fibrosis in mouse models of UUO, adenine-induced CKD and diabetes116,144. However, the effect of Ripk3 deletion on fibrosis might be independent of necroptosis, since Mlkl-knockout mice were not protected from UUO-induced kidney fibrosis116, and Nec1 has targets other than RIPK1.
Evidence also suggests a pathogenic role for ferroptosis and pyroptosis in the transition of AKI to CKD and in the development of kidney fibrosis. Administration of the antioxidant, liproxstatin 1, or the caspase 1 inhibitor, belnacasan (also known as VX-765), which is used to target pyroptosis, ameliorated the maladaptive kidney response and development of fibrosis 14 days after severe IRI-AKI176. Liproxstatin 1 also reduced kidney fibrosis induced by UUO124. Deletion of Gsdme also decreased inflammation and kidney fibrosis after UUO or subtotal nephrectomy and preserved kidney function in the latter135,177. Deletion of Gsdmd also provided renal protection against UUO-induced kidney fibrosis through effects on neutrophil function178. Disulfiram protected mice from albuminuric nephropathy induced by the presence of APOL1 risk alleles152, which are thought to underly the high incidence of the so-called hypertensive nephropathy in African Americans179.
TWEAK has pro-proliferative and pro-inflammatory effects as well as an ability to induce cell death180–182, which probably contributes to the ability of this cytokine to promote cystogenesis183. Of interest, administration of Nec1 aggravated disease in a murine autosomal dominant polycystic kidney disease model with selective deletion of Pkd1 in the kidney, potentially by decreasing the loss of cyst wall cells184. Similarly, administration of Fer1 decreased cyst growth and preserved kidney function in a mouse model of polycystic kidney disease with systemic deletion of Pkd1, whereas the induction of ferroptosis by erastin further inhibited cystine uptake and aggravated disease123.
Although it is difficult to dissect the impact of regulated necrosis on autoimmunity, inflammation and kidney cell death in autoimmune disease, regulated necrosis may lead to CKD through the induction of autoimmunity. NETosis is thought to have a key role in the development of autoimmunity against nuclear and neutrophil antigens in systemic lupus erythematosus and ANCA vasculitis21. Targeting necroptosis in myeloid cells reduced the formation of NETs and glomerular injury in experimental ANCA vasculitis101 and administration of the RIPK3 inhibitor, GSK872, inhibited the development of lupus nephritis in MRL/lpr mice113. However, MRL/lpr mice demonstrate defects in caspase 8-dependent apoptotic clearance of lymphocytes due to defective FAS signalling and thus, RIPK3 inhibitors might promote caspase 8-dependent apoptosis in this model72. The role of pyroptosis in the context of autoimmune kidney disease is unclear. The inhibitor of caspases 1, 4, 5 and 11, Ac-FLTD-CMK, prevented the development of lupus nephritis in MRL/lpr mice134 — an effect that was attributed to reduced pyroptosis. However, studies that claim an impact of caspase or NLRP3 targeting on disease outcomes through the modulation of pyroptosis should be confirmed by demonstrating that targeting of gasdermins is also beneficial. In this regard, deletion of Gsdmd increased mortality, kidney inflammation, albuminuria and autoantibody production and impaired kidney function in a model of imiquimod-induced lupus nephritis185. By contrast, the opposite outcome (that is, kidney protection) was achieved following the administration of disulfiram in pristane-induced autoimmune immune complex nephritis186. Protection from both autoimmunity and kidney disease was also described in Gsdme-knockout mice in the pristane model187.
Thus, the impact of regulated necrosis in preclinical models of CKD seems to be more complex than in preclinical models of AKI, potentially owing to the presence of differences in cell death dynamics, pathophysiology and disease readouts, and the potential consequences of cell death processes over a prolonged period of time. Of note, preclinical models are not able to mimic the full complexity of human CKD, and better models are likely to be required to better delineate the contribution of cell death pathways to disease pathogenesis.
Towards clinical implementation
The clinical implementation of therapeutic approaches for targeting regulated necrosis rests on the availability of biomarkers that can provide readouts of drug efficacy and the availability of drugs that can be rapidly brought through clinical development with minimal safety concerns.
In search of biomarkers
Contrary to apoptosis, no morphological features that are visible by optical microscopy are sufficiently specific for necroptosis, pyroptosis or ferroptosis to enable their quantification in kidney biopsy samples. This limitation impairs our ability to characterize the occurrence and dynamics of diverse forms of regulated necrosis in clinical kidney disease. Moreover, biomarkers are needed to enable the non-invasive monitoring of specific forms of regulated necrosis and their response to therapy. Although phosphorylation of MLKL (at Ser358) has been proposed as a histological indicator of necroptotic activity188, it does not indicate that necroptosis has actually occurred, since cells have defence mechanisms that expel phosphorylated MLKL to maintain cell viability49,62. Similarly, although the expression of ferroptosis activator ACSL4 correlates negatively with estimated glomerular filtration rate in patients with CKD56, the presence of ACSL4 does not necessarily indicate that ferroptosis has occurred, since ACSL4 is involved in lipid biosynthesis but not in lipid peroxidation. Moreover, although lipid peroxidation is a hallmark of ferroptosis and can be detected by using BODIPY or probing for malondialdehyde, not all lipid peroxidation results in ferroptosis. In this regard, BODIPY actually detects the lipid oxidation that occurs in non-canonical pyroptosis, which takes place after membrane permeabilization and therefore represents a part of the process that does not contribute to cell killing189. Similarly, 4-hydroxynonenal (4-HNE) is a lipid peroxidation end product that forms adducts with proteins and has long been used as a marker of oxidative stress in tissue sections, but may also not represent ferroptotic cell death190. Work is ongoing to develop ferroptosis-specific lipidomics panels that can be assessed non-invasively in urine. For example, phospholipid oxidation products (that is, oxygenated polyunsaturated phosphatidylethanolamines) were increased in cell pellets from urine samples obtained from patients with AKI who did not recover117.
Drug repurposing
Drug repurposing is the safest way to fast-track a drug for a new clinical indication, although care is needed as many therapeutics are nephrotoxic and may therefore have adverse effects in the context of kidney disease. Of note, a screen of candidate drugs to prevent maladaptive repair in proximal tubule cells identified pyroptosis and ferroptosis as key driver pathways suitable for drug targeting176.
Multiple studies have found that cilastatin — a DPEP1 inhibitor that is used clinically to prevent DPEP1-mediated degradation of imipenem — protects the kidney against nephrotoxicity induced by drugs such as cisplatin, gentamicin and vancomycin191–193, or haem proteins194 and against IRI-AKI195 and FA-AKI56. The mechanism of this kidney-protective effect is unclear and may be multifactorial, since DPEP1 has various functions, including roles in neutrophil sequestration by endothelial cells and is an enzyme and regulator of transport and signalling in tubule epithelial cells. Overexpression of DPEP1 is associated with kidney disease in humans, whereas Dpep1 knockdown in mice increased total and free GSH levels in kidneys and protected against cisplatin-induced tubule cell apoptosis and ferroptosis with no effect on necroptosis or pyroptosis56. Consistent with a role for DPEP1 in decreasing antioxidant defences and in ferroptosis, cilastatin reduced lipid peroxidation in cisplatin-induced AKI191. Moreover, the sensitization of cells to ferroptosis in response to dexamethasone through glucocorticoid receptor-induced DPEP1 expression and GSH depletion was prevented by cilastatin or genetic inactivation of Dpep1 (ref. 196). Additionally, cilastatin has been reported to decrease the uptake of several nephrotoxins by tubule cells, potentially through DPEP1-mediated interference with cholesterol lipid raft dynamics (which by itself may also decrease signalling through death receptors) and interference with, or inhibition of megalin191,192,194,197. Thus, although the molecular mechanism of kidney protection is unclear, different laboratories have demonstrated kidney protection by cilastatin in various models of AKI56,191–193,197–203. As a consequence of these findings, cilastatin is now undergoing clinical development as a kidney-protective molecule203.
Vitamin K may also prevent ferroptosis, at least in some clinical scenarios. Vitamin K antagonists, such as the oral anticoagulant warfarin, inhibits the vitamin K epoxide reductase (VKOR), which normally reduces vitamin K epoxide to vitamin K hydroquinone — a radical-trapping antioxidant and inhibitor of (phospho)lipid peroxidation204. The ability of vitamin K to reverse warfarin toxicity may be explained by the ability of FSP1 to reduce vitamin K to vitamin K hydroquinone, since VKOR remains inhibited. In 2022, vitamin K1 and a form of vitamin K2 — menaquinone 4 (MK-4) — were shown to inhibit ferroptosis in cultured cells and improve outcomes in a mouse model of IRI-AKI204. By contrast, the vitamin K antagonist phenprocoumon, which is in clinical use in Germany, exacerbated outcomes204,205. This knowledge may drive the reinterpretation of the reported adverse effects of vitamin K antagonists in kidney disease, which include the development of haematuria-associated AKI during periods of warfarin overdosing206–211, vascular calcification212 and an increased risk of CKD progression213,214. Patients on chronic oral vitamin K antagonist anticoagulants are frequently co-prescribed proton pump inhibitors (PPIs) to decrease the risk of gastrointestinal bleeding whereas haematuria — which may result from anticoagulant overdosing — may lead to an overload of iron in tubule epithelial cells206–208,210. Interestingly, PPIs have also been associated with acute tubulointerstitial nephritis, an increased risk of incident and progressive CKD215 and with AKI in patients treated with immune checkpoint inhibitors, possibly as a consequence of unsuppressed autoimmunity211. The induction of an immunogenic form of cell death — such as ferroptosis — could underlie a cause–effect relationship between PPIs, vitamin K antagonists and nephrotoxicity. Such a link is biologically plausible given the ability of vitamin K to prevent ferroptosis and the association between haematuria and iron overload. However, findings from experimental models and systems are contradictory. For example, omeprazole induced tubular cell death in healthy mice in vivo216 and another PPI, lansoprazole, increased the severity of experimental cisplatin-induced AKI217. By contrast, one in vitro study found that omeprazole could scavenge lipid peroxyl radicals, and demonstrated short-term (48 h) anti-ferroptotic effects in cultured cells following cystine deprivation; however, omeprazole was toxic at higher doses218. Furthermore, another in vitro study found that the induction of tubule cell death by omeprazole was exacerbated by iron overload, but was not prevented by ferrostatin 1 (ref. 216), questioning the contribution of ferroptosis to this process.
Other compounds that are currently in clinical use or readily available, including indole-3-carbinol, rifampicin, promethazine, carvedilol, oestradiol and T3, also have anti-ferroptotic effects on cultured tubule cells218. In vivo, promethazine and rifampicin reduced the severity of cisplatin-induced AKI218.
The anti-epileptic drug primidone inhibits RIPK1. Administration of primidone 5 days prior to surgery protected mice from IRI-AKI151. However, primidone overdose might cause crystalluria and AKI in humans219, and long-term nephrotoxic effects (including kidney cysts, chronic nephropathy and kidney adenoma) were observed in rats exposed to high doses of primidone in toxicological studies220. This pattern of nephrotoxicity (that is, the development of cysts and adenoma) might be compatible with its role in the inhibition of kidney cell death. Another anti-epileptic drug, phenytoin, which shares a hydantoin scaffold with Nec1, attenuated RIPK1 kinase activity and protected mice from IRI-AKI188.
The potential of agents that modulate iron availability to prevent ferroptosis is also being explored. Specifically, iron binders that decrease iron availability protect cells from ferroptosis221. However, this strategy may be risky, especially in the context of proximal tubule cell ferroptosis. For example, the dose-limiting adverse effect of the iron binder deferasirox is proximal tubule cell injury that leads to Fanconi syndrome and/or AKI222. Mechanistically, deferasirox induces necrosis in a manner that is dependent on the depletion of iron and is prevented by the anti-apoptotic protein BCL-XL but is not prevented by necroptosis inhibitors223. By contrast, preconditioning with iron sucrose (FeS) protected mice against selected forms of AKI via a mechanism associated with upregulation of hepcidin224,225. A FeS formulation (‘RBT-3’) is under clinical development for AKI224.
Safety considerations
Safety aspects were usually not addressed in early animal studies; however, they are a key consideration for clinical translation. As discussed above, some agents that target components of regulated necrosis pathways are nephrotoxic and may therefore have limited utility in patients with kidney disease. The existence of preclinical findings showing that targeting cell death can actually exacerbate kidney injury40,128,184 highlights these safety concerns. Interference with cell death processes has potential to induce devastating adverse effects in organs or systems beyond the kidney, for example, by inducing malignancy or autoimmunity through the impaired clearance of premalignant or autoimmune cells, respectively. In support of this possibility, suppressed regulated necrosis has been observed in malignant cells33. A further cause for concern in the context of oncology is the potential nephrotoxicity of therapies that aim to promote regulated necrosis in malignancy. For example, whole-body inactivation of Gpx4 in mice triggered kidney failure and death within 10–15 days70; however, agents are being developed to degrade GPX4 and induce ferroptosis for the treatment of malignancy226.
Conclusions
Available preclinical evidence suggests that regulated cell death may contribute to changes in cell number and to inflammatory and autoimmune responses driving AKI and potentially CKD. However, discrepancies exist in the outcomes of interventions designed to target different pathways of regulated necrosis in experimental studies, and gaps remain in our understanding of the processes involved. An important first step in addressing these knowledge gaps is to identify non-invasive biomarkers of different forms of regulated necrosis that are consistent across animal models and human disease. Such tools will facilitate the development and translation of therapeutics — a process that is complicated and, beyond a requirement for a better understanding of underlying disease mechanisms, requires identification of the optimal intervention, target and patient population. Timing of the intervention is particularly relevant, since the main modality of regulated necrosis may evolve over time and different molecular or cell targets may be relevant for prevention or treatment. The identification of molecular or histological markers of ongoing necroptosis, ferroptosis or pyroptosis in clinical samples will enable real-time monitoring and allow adjustments to the timing and type of intervention as well as and evaluation of the patient response to therapy.
Given the potential for interaction between different forms of cell death (Fig. 5), pan-cell death inhibitors that act on two or three pathways may increase the likelihood of success71,95,166,227,228. However, safety concerns may be exacerbated by such an approach. Such interventions may not only modulate the process in the intended cell targets (for example, tubule epithelial cells in AKI) but also modulate cell death in unintended targets, such as inflammatory cells, fibroblasts and malignant cells. Overall, therapeutic modulation of regulated necrosis is promising based on preclinical studies of preventive and treatment approaches in AKI and CKD, but only clinical trials will confirm its clinical relevance.
Supplementary information
Acknowledgements
The authors’ research is funded by Instituto de Salud Carlos III (ISCIII)-Fondo de Investigacion Sanitaria (FIS)-Fondo Europeo de Desarrollo Regional FEDER (grants PI18/01366, PI21/00251, PI19/00588, P21/01453, PI18/01133, PI19/00815, PI22/00469, PI22/00050), European Research Area PerMed JTC2018 (KIDNEY ATTACK AC18/00064) and the RICORS programme to RICORS2040 (RD21/0005/0001), and also by Comunidad de Madrid en Biomedicina (grant P2022/BMD-7223, CIFRA_COR-CM). A.B.S and M.D.S.-N. were supported by the MICINN Ramon y Cajal programme.
Author contributions
A.B.S. and A.O. wrote the article. All the authors researched data for the article, contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Nephrology thanks Daniel Muruve and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
A.O. has received grants from Sanofi and consultancy, speaker fees or travel support from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Idorsia, Chiesi, Otsuka, Novo-Nordisk and Vifor Fresenius Medical Care Renal Pharma, and is the Director of the Catedra Mundipharma-UAM for diabetic kidney disease and the Catedra Astrazeneca-UAM for chronic kidney disease and electrolytes. A.O. also has stock in Telara Farma. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41581-023-00694-0.
References
- 1.Foreman KJ, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz A. RICORS2040: the need for collaborative research in chronic kidney disease. Clin. Kidney J. 2021;15:372–387. doi: 10.1093/ckj/sfab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman, A. B., Rahbari-Oskoui, F. F & Bennett, W. M. Acquired cystic disease of the kidney in adults. UpToDatehttps://www.uptodate.com/contents/acquired-cystic-disease-of-the-kidney-in-adults (2023).
- 4.Denton MD, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int. 2002;61:2201–2209. doi: 10.1046/j.1523-1755.2002.00374.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, et al. Acquired cystic kidney disease-associated renal cell carcinoma (ACKD-RCC) harbor recurrent mutations in KMT2C and TSC2 genes. Am. J. Surg. Pathol. 2020;44:1479–1486. doi: 10.1097/PAS.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013;3:1–150. [Google Scholar]
- 7.Maremonti F, Meyer C, Linkermann A. Mechanisms and models of kidney tubular necrosis and nephron loss. J. Am. Soc. Nephrol. 2022;33:472–486. doi: 10.1681/ASN.2021101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peired AJ, Lazzeri E, Guzzi F, Anders HJ, Romagnani P. From kidney injury to kidney cancer. Kidney Int. 2021;100:55–66. doi: 10.1016/j.kint.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina CB, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580:130–135. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morioka S, et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563:714–718. doi: 10.1038/s41586-018-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanz AB, Santamaría B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 2008;19:1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 16.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol. Dial. Transpl. 2012;27:3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 17.Linkermann A, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 18.Linkermann A, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulay SR, et al. Mitochondria permeability transition versus necroptosis in oxalate-induced AKI. J. Am. Soc. Nephrol. 2019;30:1857–1869. doi: 10.1681/ASN.2018121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández Á, et al. Interaction between the autophagy protein beclin 1 and Na+,K+-ATPase during starvation, exercise, and ischemia. JCI Insight. 2020;5:e133282. doi: 10.1172/jci.insight.133282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 2016;12:402–413. doi: 10.1038/nrneph.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lood C, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linkermann A, et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl Acad. Sci. USA. 2013;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 27.Kumar SV, et al. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J. Am. Soc. Nephrol. 2015;26:2399–2413. doi: 10.1681/ASN.2014070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng F, et al. Neutrophil extracellular traps induce glomerular endothelial cell dysfunction and pyroptosis in diabetic kidney disease. Diabetes. 2022;71:2739–2750. doi: 10.2337/db22-0153. [DOI] [PubMed] [Google Scholar]
- 29.Kolbrink B, von Samson-Himmelstjerna FA, Murphy JM, Krautwald S. Role of necroptosis in kidney health and disease. Nat. Rev. Nephrol. 2023 doi: 10.1038/s41581-022-00658-w. [DOI] [PubMed] [Google Scholar]
- 30.Elias EE, Lyons B, Muruve DA. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol. 2023 doi: 10.1038/s41581-022-00662-0. [DOI] [PubMed] [Google Scholar]
- 31.Bayir H, et al. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat. Rev. Nephrol. 2023 doi: 10.1038/s41581-023-00689-x. [DOI] [PubMed] [Google Scholar]
- 32.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020;21:678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 33.Ganini C, et al. No time to die: how kidney cancer evades cell death. Int. J. Mol. Sci. 2022;23:6198. doi: 10.3390/ijms23116198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 36.von Mässenhausen A, Tonnus W, Linkermann A. Cell death pathways drive necroinflammation during acute kidney injury. Nephron. 2018;140:144–147. doi: 10.1159/000490807. [DOI] [PubMed] [Google Scholar]
- 37.Mulay SR, Linkermann A, Anders HJ. Necroinflammation in kidney disease. J. Am. Soc. Nephrol. 2016;27:27–39. doi: 10.1681/ASN.2015040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. Detection of PKD1 and PKD2 somatic variants in autosomal dominant polycystic kidney cyst epithelial cells by whole-genome sequencing. J. Am. Soc. Nephrol. 2021;32:3114–3129. doi: 10.1681/ASN.2021050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peired AJ, et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci. Transl Med. 2020;12:eaaw6003. doi: 10.1126/scitranslmed.aaw6003. [DOI] [PubMed] [Google Scholar]
- 40.Yang B, et al. Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2018;29:1900–1916. doi: 10.1681/ASN.2017050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou YH, et al. Methylation in pericytes after acute injury promotes chronic kidney disease. J. Clin. Invest. 2020;130:4845–4857. doi: 10.1172/JCI135773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffield JS, et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am. J. Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savill J. Apoptosis and the kidney. J. Am. Soc. Nephrol. 1994;5:12–21. doi: 10.1681/ASN.V5112. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu A, Yamanaka N. Apoptosis and cell desquamation in repair process of ischemic tubular necrosis. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993;64:171–180. doi: 10.1007/BF02915110. [DOI] [PubMed] [Google Scholar]
- 46.González-Cuadrado S, et al. Agonistic anti-Fas antibodies induce glomerular cell apoptosis in mice in vivo. Kidney Int. 1997;51:1739–1746. doi: 10.1038/ki.1997.239. [DOI] [PubMed] [Google Scholar]
- 47.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito H, Kasagi N, Shomori K, Osaki M, Adachi H. Apoptosis in the human allografted kidney. Analysis by terminal deoxynucleotidyl transferase-mediated DUTP-botin nick end labeling. Transplantation. 1995;60:794–798. doi: 10.1097/00007890-199510270-00006. [DOI] [PubMed] [Google Scholar]
- 49.Tonnus W, et al. The pathological features of regulated necrosis. J. Pathol. 2019;247:697–707. doi: 10.1002/path.5248. [DOI] [PubMed] [Google Scholar]
- 50.Vanden Berghe T, et al. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods. 2013;61:117–129. doi: 10.1016/j.ymeth.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Rogers C, et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019;10:1689. doi: 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moujalled D, et al. BH3 mimetic drugs cooperate with temozolomide, JQ1 and inducers of ferroptosis in killing glioblastoma multiforme cells. Cell Death Differ. 2022;29:1335–1348. doi: 10.1038/s41418-022-00977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 54.Miyashita T, Harigai M, Hanada M, Reed JC. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 55.Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan Y, et al. A single genetic locus controls both expression of DPEP1/CHMP1A and kidney disease development via ferroptosis. Nat. Commun. 2021;12:5078. doi: 10.1038/s41467-021-25377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molitoris BA, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dagher PC. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int. 2004;66:506–509. doi: 10.1111/j.1523-1755.2004.761_7.x. [DOI] [PubMed] [Google Scholar]
- 59.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03510897 (2021).
- 60.Thielmann M, et al. Teprasiran, a small interfering RNA, for the prevention of acute kidney injury in high-risk patients undergoing cardiac surgery: a randomized clinical study. Circulation. 2021;144:1133–1144. doi: 10.1161/CIRCULATIONAHA.120.053029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014;14:759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 62.Gong YN, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic. Cell. 2017;169:286–300.e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, Fan W, Guo J, Wang X. Osmotic stress activates RIPK3/MLKL-mediated necroptosis by increasing cytosolic pH through a plasma membrane Na+/H+ exchanger. Sci. Signal. 2022;15:eabn5881. doi: 10.1126/scisignal.abn5881. [DOI] [PubMed] [Google Scholar]
- 64.Ortiz A, Sanz AB. A pathway of osmotic stress-induced necroptosis. Nat. Rev. Nephrol. 2022;18:609–610. doi: 10.1038/s41581-022-00607-7. [DOI] [PubMed] [Google Scholar]
- 65.Martin-Sanchez D, et al. Bone marrow-derived RIPK3 mediates kidney inflammation in acute kidney injury. J. Am. Soc. Nephrol. 2022;33:357–373. doi: 10.1681/ASN.2021030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guerrero-Mauvecin J, et al. RIPK3 and kidney disease. Nefrologia. 2022 doi: 10.1016/j.nefro.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Justo P, et al. Cytokine cooperation in renal tubular cell injury: the role of TWEAK. Kidney Int. 2006;70:1750–1758. doi: 10.1038/sj.ki.5001866. [DOI] [PubMed] [Google Scholar]
- 68.Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20:185–187. doi: 10.1038/cdd.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi N, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedmann Angeli JP, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tonnus W, et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 2021;12:4402. doi: 10.1038/s41467-021-24712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mandal P, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandenabeele P, Bultynck G, Savvides SN. Pore-forming proteins as drivers of membrane permeabilization in cell death pathways. Nat. Rev. Mol. Cell Biol. 2022 doi: 10.1038/s41580-022-00564-w. [DOI] [PubMed] [Google Scholar]
- 74.Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374:1076–1080. doi: 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- 75.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 76.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 77.Evavold CL, et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao A, Kagan JC. Gasdermin pore forming activities that promote inflammation from living and dead cells. J. Mol. Biol. 2022;434:167427. doi: 10.1016/j.jmb.2021.167427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santa Cruz Garcia AB, Schnur KP, Malik AB, Mo GCH. Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 2022;13:52. doi: 10.1038/s41467-021-27692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borges JP, et al. Glycine inhibits NINJ1 membrane clustering to suppress plasma membrane rupture in cell death. eLife. 2022;11:e78609. doi: 10.7554/eLife.78609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rogers C, et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen KW, et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018;3:eaar6676. doi: 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- 83.Sollberger G, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018;3:eaar6689. doi: 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- 84.Mulay SR, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J. Clin. Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou B, Abbott DW. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 2021;35:108998. doi: 10.1016/j.celrep.2021.108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin-Sanchez D, et al. Ferroptosis and kidney disease. Nefrologia. 2020;40:384–394. doi: 10.1016/j.nefro.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Riegman M, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 2020;22:1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katikaneni A, et al. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat. Cell Biol. 2020;22:1049–1055. doi: 10.1038/s41556-020-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pandian N, Kanneganti TD. PANoptosis: a unique innate immune inflammatory cell death modality. J. Immunol. 2022;209:1625–1633. doi: 10.4049/jimmunol.2200508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng M, Karki R, Vogel P, Kanneganti TD. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181:674–687.e13. doi: 10.1016/j.cell.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee MH, Choi EN, Jeon YJ, Jung SC. Possible role of transforming growth factor-β1 and vascular endothelial growth factor in Fabry disease nephropathy. Int. J. Mol. Med. 2012;30:1275–1280. doi: 10.3892/ijmm.2012.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh AB, Kaushal V, Megyesi JK, Shah SV, Kaushal GP. Cloning and expression of rat caspase-6 and its localization in renal ischemia/reperfusion injury. Kidney Int. 2002;62:106–115. doi: 10.1046/j.1523-1755.2002.00427.x. [DOI] [PubMed] [Google Scholar]
- 94.Ortiz A, Justo P, Sanz A, Lorz C, Egido J. Targeting apoptosis in acute tubular injury. Biochem. Pharmacol. 2003;66:1589–1594. doi: 10.1016/S0006-2952(03)00515-X. [DOI] [PubMed] [Google Scholar]
- 95.Müller T, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol. Life Sci. 2017;74:3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin-Sanchez D, et al. TWEAK and RIPK1 mediate a second wave of cell death during AKI. Proc. Natl Acad. Sci. USA. 2018;115:4182–4187. doi: 10.1073/pnas.1716578115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pedrera L, et al. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021;28:1644–1657. doi: 10.1038/s41418-020-00691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rühl S, et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956–960. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- 99.Kayagaki N, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131–136. doi: 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- 100.Hirata Y, et al. Lipid peroxidation increases membrane tension, Piezo1 gating and cation permeability to execute ferroptosis. Preprint at bioRxivhttps://biorxiv.org/content/10.1101/2022.10.31.514557v1 (2022). [DOI] [PubMed]
- 101.Schreiber A, et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl Acad. Sci. USA. 2017;114:E9618–E9625. doi: 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chauhan D, et al. GSDMD drives canonical inflammasome-induced neutrophil pyroptosis and is dispensable for NETosis. EMBO Rep. 2022;23:e54277. doi: 10.15252/embr.202154277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin-Sanchez D, et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 2017;28:218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Metz-Kurschel U, et al. Folate nephropathy occurring during cytotoxic chemotherapy with high-dose folinic acid and 5-fluorouracil. Ren. Fail. 1990;12:93–97. doi: 10.3109/08860229009087124. [DOI] [PubMed] [Google Scholar]
- 105.Olsen S, et al. Primary acute renal failure (“acute tubular necrosis”) in the transplanted kidney: morphology and pathogenesis. Medicine. 1989;68:173–187. doi: 10.1097/00005792-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Linkermann A, et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J. Am. Soc. Nephrol. 2013;24:1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Homsi E, Andreazzi DD, Faria JB, Janino P. TNF-α-mediated cardiorenal injury after rhabdomyolysis in rats. Am. J. Physiol. Ren. Physiol. 2015;308:F1259–F1267. doi: 10.1152/ajprenal.00311.2014. [DOI] [PubMed] [Google Scholar]
- 108.Mulay SR, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat. Commun. 2016;7:10274. doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y, et al. A role for tubular necroptosis in cisplatin-induced AKI. J. Am. Soc. Nephrol. 2015;26:2647–2658. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao X, et al. Inhibition of necroptosis attenuates kidney inflammation and interstitial fibrosis induced by unilateral ureteral obstruction. Am. J. Nephrol. 2017;46:131–138. doi: 10.1159/000478746. [DOI] [PubMed] [Google Scholar]
- 111.Zhu Y, Cui H, Xia Y, Gan H. RIPK3-mediated necroptosis and apoptosis contributes to renal tubular cell progressive loss and chronic kidney disease progression in rats. PLoS ONE. 2016;11:e0156729. doi: 10.1371/journal.pone.0156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu BF, et al. Protective effect of inhibiting necroptosis on gentamicin-induced nephrotoxicity. FASEB J. 2022;36:e22487. doi: 10.1096/fj.202200163R. [DOI] [PubMed] [Google Scholar]
- 113.Guo C, et al. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J. Autoimmun. 2019;103:102286. doi: 10.1016/j.jaut.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sureshbabu A, et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight. 2018;3:e98411. doi: 10.1172/jci.insight.98411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang S, et al. RIPK3 mediates renal tubular epithelial cell apoptosis in endotoxin-induced acute kidney injury. Mol. Med. Rep. 2019;20:1613–1620. doi: 10.3892/mmr.2019.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Imamura M, et al. RIPK3 promotes kidney fibrosis via AKT-dependent ATP citrate lyase. JCI Insight. 2018;3:e98411. doi: 10.1172/jci.insight.94979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wenzel SE, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628–641.e6. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guerrero-Hue M, et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019;33:8961–8975. doi: 10.1096/fj.201900077R. [DOI] [PubMed] [Google Scholar]
- 119.He Z, et al. Role of ferroptosis induced by a high concentration of calcium oxalate in the formation and development of urolithiasis. Int. J. Mol. Med. 2021;47:289–301. doi: 10.3892/ijmm.2020.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng F, Sharma I, Dai Y, Yang M, Kanwar YS. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Invest. 2019;129:5033–5049. doi: 10.1172/JCI129903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim DH, et al. Farnesoid X receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes. Redox Biol. 2022;54:102382. doi: 10.1016/j.redox.2022.102382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiu W, et al. Melatonin suppresses ferroptosis via activation of the Nrf2/HO-1 signaling pathway in the mouse model of sepsis-induced acute kidney injury. Int. Immunopharmacol. 2022;112:109162. doi: 10.1016/j.intimp.2022.109162. [DOI] [PubMed] [Google Scholar]
- 123.Zhang X, et al. Ferroptosis promotes cyst growth in autosomal dominant polycystic kidney disease mouse models. J. Am. Soc. Nephrol. 2021;32:2759–2776. doi: 10.1681/ASN.2021040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang B, et al. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021;12:843. doi: 10.1038/s41419-021-04137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye Z, et al. Caspase-11 mediates pyroptosis of tubular epithelial cells and septic acute kidney injury. Kidney Blood Press. Res. 2019;44:465–478. doi: 10.1159/000499685. [DOI] [PubMed] [Google Scholar]
- 126.Yang JR, et al. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am. J. Physiol. Ren. Physiol. 2014;306:F75–F84. doi: 10.1152/ajprenal.00117.2013. [DOI] [PubMed] [Google Scholar]
- 127.Miao N, et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019;96:1105–1120. doi: 10.1016/j.kint.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 128.Tonnus W, et al. Gasdermin D-deficient mice are hypersensitive to acute kidney injury. Cell Death Dis. 2022;13:792. doi: 10.1038/s41419-022-05230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, et al. Activation of GSDMD contributes to acute kidney injury induced by cisplatin. Am. J. Physiol. Ren. Physiol. 2020;318:F96–F106. doi: 10.1152/ajprenal.00351.2019. [DOI] [PubMed] [Google Scholar]
- 130.Xia W, et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12:139. doi: 10.1038/s41419-021-03431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Z, et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. 2018;9:983. doi: 10.1038/s41419-018-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lv D, et al. Treatment of membranous nephropathy by disulfiram through inhibition of podocyte pyroptosis. Kidney Dis. 2022;8:308–318. doi: 10.1159/000524164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang H, et al. Complement induces podocyte pyroptosis in membranous nephropathy by mediating mitochondrial dysfunction. Cell Death Dis. 2022;13:281. doi: 10.1038/s41419-022-04737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cao H, et al. Novel effects of combination therapy through inhibition of caspase-1/gasdermin D induced-pyroptosis in lupus nephritis. Front. Immunol. 2021;12:720877. doi: 10.3389/fimmu.2021.720877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y, et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 2021;28:2333–2350. doi: 10.1038/s41418-021-00755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doll S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Y, et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur. J. Pharmacol. 2020;888:173574. doi: 10.1016/j.ejphar.2020.173574. [DOI] [PubMed] [Google Scholar]
- 138.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988;34:474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- 139.Zager RA. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J. Clin. Invest. 1992;90:711–719. doi: 10.1172/JCI115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J, et al. Ferroptosis, a new target for treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced CKD rat model. Cell Death Discov. 2022;8:127. doi: 10.1038/s41420-022-00931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Avunduk MC, Yurdakul T, Erdemli E, Yavuz A. Prevention of renal damage by alpha tocopherol in ischemia and reperfusion models of rats. Urol. Res. 2003;31:280–285. doi: 10.1007/s00240-003-0329-y. [DOI] [PubMed] [Google Scholar]
- 142.Abdel-Daim MM, et al. The ameliorative effects of ceftriaxone and vitamin E against cisplatin-induced nephrotoxicity. Environ. Sci. Pollut. Res. Int. 2019;26:15248–15254. doi: 10.1007/s11356-019-04801-2. [DOI] [PubMed] [Google Scholar]
- 143.Shi Y, et al. RIPK3 blockade attenuates kidney fibrosis in a folic acid model of renal injury. FASEB J. 2020;34:10286–10298. doi: 10.1096/fj.201902544RR. [DOI] [PubMed] [Google Scholar]
- 144.Shi Y, et al. RIPK3 blockade attenuates tubulointerstitial fibrosis in a mouse model of diabetic nephropathy. Sci. Rep. 2020;10:10458. doi: 10.1038/s41598-020-67054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Piao SG, et al. Inhibition of RIP1-RIP3-mediated necroptosis attenuates renal fibrosis via Wnt3α/β-catenin/GSK-3β signaling in unilateral ureteral obstruction. PLoS ONE. 2022;17:e0274116. doi: 10.1371/journal.pone.0274116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu CH, et al. RIPK3 inhibitor-AZD5423 alleviates acute kidney injury by inhibiting necroptosis and inflammation. Int. Immunopharmacol. 2022;112:109262. doi: 10.1016/j.intimp.2022.109262. [DOI] [PubMed] [Google Scholar]
- 147.Wang JN, et al. RIPK1 inhibitor Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via attenuating necroptosis, inflammation and oxidative stress. Clin. Sci. 2019;133:1609–1627. doi: 10.1042/CS20190599. [DOI] [PubMed] [Google Scholar]
- 148.Yang M, et al. Caspase-1-inhibitor AC-YVAD-CMK inhibits pyroptosis and ameliorates acute kidney injury in a model of sepsis. Biomed. Res. Int. 2021;2021:6636621. doi: 10.1155/2021/6636621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen X, Wang H, Weng C, Jiang H, Chen J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021;12:186. doi: 10.1038/s41419-021-03458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martin-Sanchez D, et al. Targeting of regulated necrosis in kidney disease. Nefrologia. 2018;38:125–135. doi: 10.1016/j.nefro.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 151.Riebeling T, et al. Primidone blocks RIPK1-driven cell death and inflammation. Cell Death Differ. 2021;28:1610–1626. doi: 10.1038/s41418-020-00690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu J, et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J. Clin. Invest. 2021;131:e136329. doi: 10.1172/JCI136329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Olsen TS, Olsen HS, Hansen HE. Tubular ultrastructure in acute renal failure in man: epithelial necrosis and regeneration. Virchows Arch. A Pathol. Anat. Histopathol. 1985;406:75–89. doi: 10.1007/BF00710559. [DOI] [PubMed] [Google Scholar]
- 154.Tonnus W, Linkermann A. Gasdermin D and pyroptosis in acute kidney injury. Kidney Int. 2019;96:1061–1063. doi: 10.1016/j.kint.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 155.Schumer M, et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am. J. Pathol. 1992;140:831–838. [PMC free article] [PubMed] [Google Scholar]
- 156.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013;84:138–148. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Daemen MA, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J. Clin. Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ederoth P, et al. Cyclosporine before coronary artery bypass grafting does not prevent postoperative decreases in renal function: a randomized clinical trial. Anesthesiology. 2018;128:710–717. doi: 10.1097/ALN.0000000000002104. [DOI] [PubMed] [Google Scholar]
- 159.Newton K, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moerke C, Bleibaum F, Kunzendorf U, Krautwald S. Combined knockout of RIPK3 and MLKL reveals unexpected outcome in tissue injury and inflammation. Front. Cell Dev. Biol. 2019;7:19. doi: 10.3389/fcell.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tang C, Livingston MJ, Safirstein R, Dong Z. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat. Rev. Nephrol. 2023;19:53–72. doi: 10.1038/s41581-022-00631-7. [DOI] [PubMed] [Google Scholar]
- 162.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin. Nephrol. 2003;23:460–464. doi: 10.1016/S0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 163.Sancho-Martínez SM, Piedrafita FJ, Cannata-Andía JB, López-Novoa JM, López-Hernández FJ. Necrotic concentrations of cisplatin activate the apoptotic machinery but inhibit effector caspases and interfere with the execution of apoptosis. Toxicol. Sci. 2011;122:73–85. doi: 10.1093/toxsci/kfr098. [DOI] [PubMed] [Google Scholar]
- 164.Tristão VR, et al. Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis. 2016;21:51–59. doi: 10.1007/s10495-015-1190-5. [DOI] [PubMed] [Google Scholar]
- 165.Zille M, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48:1033–1043. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Coillie S, et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat. Commun. 2022;13:1046. doi: 10.1038/s41467-022-28718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fajgenbaum DC, June CH. Cytokine storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gaestel M, Nebreda AR, Yaffe MB. Cytokine storm. N. Engl. J. Med. 2021;384:e59. doi: 10.1056/NEJMc2036236. [DOI] [PubMed] [Google Scholar]
- 169.Chen H, et al. RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ. 2020;27:2568–2585. doi: 10.1038/s41418-020-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu JJ, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020;21:736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 2005;68:1543–1553. doi: 10.1111/j.1523-1755.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 172.Rashidi M, et al. The pyroptotic cell death effector gasdermin D is activated by gout-associated uric acid crystals but is dispensable for cell death and IL-1β release. J. Immunol. 2019;203:736–748. doi: 10.4049/jimmunol.1900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Anders HJ, et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 2018;93:656–669. doi: 10.1016/j.kint.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 174.Ludwig-Portugall I, et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016;90:525–539. doi: 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 175.Martín-Saiz L, et al. Ferrostatin-1 modulates dysregulated kidney lipids in acute kidney injury. J. Pathol. 2022;257:285–299. doi: 10.1002/path.5882. [DOI] [PubMed] [Google Scholar]
- 176.Balzer MS, et al. Single-cell analysis highlights differences in druggable pathways underlying adaptive or fibrotic kidney regeneration. Nat. Commun. 2022;13:4018. doi: 10.1038/s41467-022-31772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu M, et al. Gasdermin E deletion attenuates ureteral obstruction- and 5/6 nephrectomy-induced renal fibrosis and kidney dysfunction. Front. Cell Dev. Biol. 2021;9:754134. doi: 10.3389/fcell.2021.754134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Y, et al. GSDMD-dependent neutrophil extracellular traps promote macrophage-to-myofibroblast transition and renal fibrosis in obstructive nephropathy. Cell Death Dis. 2022;13:693. doi: 10.1038/s41419-022-05138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sanz AB, et al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J. Am. Soc. Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ucero AC, et al. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim. Biophys. Acta. 2013;1832:1744–1755. doi: 10.1016/j.bbadis.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 182.Sanz AB, et al. Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J. Cell Mol. Med. 2009;13:3329–3342. doi: 10.1111/j.1582-4934.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cordido A, et al. TWEAK signaling pathway blockade slows cyst growth and disease progression in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2021;32:1913–1932. doi: 10.1681/ASN.2020071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang B, et al. Interleukin-1 receptor activation aggravates autosomal dominant polycystic kidney disease by modulating regulated necrosis. Am. J. Physiol. Ren. Physiol. 2019;317:F221–F228. doi: 10.1152/ajprenal.00104.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang X, et al. Effects of gasdermin D in modulating murine lupus and its associated organ damage. Arthritis Rheumatol. 2020;72:2118–2129. doi: 10.1002/art.41444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhuang L, et al. Disulfiram alleviates pristane-induced lupus via inhibiting GSDMD-mediated pyroptosis. Cell Death Discov. 2022;8:379. doi: 10.1038/s41420-022-01167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Luo G, et al. Blocking GSDME-mediated pyroptosis in renal tubular epithelial cells alleviates disease activity in lupus mice. Cell Death Discov. 2022;8:113. doi: 10.1038/s41420-022-00848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.von Mässenhausen A, et al. Phenytoin inhibits necroptosis. Cell Death Dis. 2018;9:359. doi: 10.1038/s41419-018-0394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wiernicki B, et al. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020;11:922. doi: 10.1038/s41419-020-03118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang Y, et al. 4-hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J. Mol. Cell Cardiol. 2010;49:576–586. doi: 10.1016/j.yjmcc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 191.Humanes B, et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. 2012;82:652–663. doi: 10.1038/ki.2012.199. [DOI] [PubMed] [Google Scholar]
- 192.Jado JC, et al. Nephroprotective effect of cilastatin against gentamicin-induced renal injury in vitro and in vivo without altering its bactericidal efficiency. Antioxidants. 2020;9:821. doi: 10.3390/antiox9090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Humanes B, et al. Protective effects of cilastatin against vancomycin-induced nephrotoxicity. Biomed. Res. Int. 2015;2015:704382. doi: 10.1155/2015/704382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Matsushita K, et al. Cilastatin ameliorates rhabdomyolysis-induced AKI in mice. J. Am. Soc. Nephrol. 2021;32:2579–2594. doi: 10.1681/ASN.2020030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hong YA, et al. Cilastatin preconditioning attenuates renal Ischemia-reperfusion injury via hypoxia inducible factor-1α activation. Int. J. Mol. Sci. 2020;21:3583. doi: 10.3390/ijms21103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.von Mässenhausen A, et al. Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor-induced dipeptidase-1 expression and glutathione depletion. Sci. Adv. 2022;8:eabl8920. doi: 10.1126/sciadv.abl8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hori Y, et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J. Am. Soc. Nephrol. 2017;28:1783–1791. doi: 10.1681/ASN.2016060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Choudhury SR, et al. Dipeptidase-1 is an adhesion receptor for neutrophil recruitment in lungs and liver. Cell. 2019;178:1205–1221.e17. doi: 10.1016/j.cell.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 199.Lau A, et al. Dipeptidase-1 governs renal inflammation during ischemia reperfusion injury. Sci. Adv. 2022;8:eabm0142. doi: 10.1126/sciadv.abm0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pérez M, et al. Inhibition of brush border dipeptidase with cilastatin reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. Nephrol. Dial. Transpl. 2004;19:2445–2455. doi: 10.1093/ndt/gfh397. [DOI] [PubMed] [Google Scholar]
- 201.Moreno-Gordaliza E, et al. Lipid imaging for visualizing cilastatin amelioration of cisplatin-induced nephrotoxicity. J. Lipid Res. 2018;59:1561–1574. doi: 10.1194/jlr.M080465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tejedor A, et al. Cilastatin protection against cyclosporin A-induced nephrotoxicity: clinical evidence. Curr. Med. Res. Opin. 2007;23:505–513. doi: 10.1185/030079906X167633. [DOI] [PubMed] [Google Scholar]
- 203.González-Nicolás M, González-Guerrero C, Pérez-Fernández VA, Lázaro A. Cilastatin: a potential treatment strategy against COVID-19 that may decrease viral replication and protect from the cytokine storm. Clin. Kidney J. 2020;13:903–905. doi: 10.1093/ckj/sfaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mishima E, et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. 2022;608:778–783. doi: 10.1038/s41586-022-05022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kolbrink B, et al. Vitamin K1 inhibits ferroptosis and counteracts a detrimental effect of phenprocoumon in experimental acute kidney injury. Cell Mol. Life Sci. 2022;79:387. doi: 10.1007/s00018-022-04416-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brodsky SV, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am. J. Kidney Dis. 2009;54:1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 207.Brodsky SV, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Moreno JA, et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin. J. Am. Soc. Nephrol. 2012;7:175–184. doi: 10.2215/CJN.01970211. [DOI] [PubMed] [Google Scholar]
- 209.Martín Cleary C, et al. Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury. Nephrol. Dial. Transpl. 2010;25:4103–4106. doi: 10.1093/ndt/gfq493. [DOI] [PubMed] [Google Scholar]
- 210.Gutiérrez E, et al. Oxidative stress, macrophage infiltration and CD163 expression are determinants of long-term renal outcome in macrohematuria-induced acute kidney injury of IgA nephropathy. Nephron Clin. Pract. 2012;121:c42–c53. doi: 10.1159/000342385. [DOI] [PubMed] [Google Scholar]
- 211.Gérard AO, et al. Risk factors associated with immune checkpoint inhibitor-induced acute kidney injury compared with other immune-related adverse events: a case-control study. Clin. Kidney J. 2022;15:1881–1887. doi: 10.1093/ckj/sfac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Poterucha TJ, Goldhaber SZ. Warfarin and vascular calcification. Am. J. Med. 2016;129:635.e1–635.e4. doi: 10.1016/j.amjmed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 213.Wei FF, et al. Vitamin K dependent protection of renal function in multi-ethnic population studies. EBioMedicine. 2016;4:162–169. doi: 10.1016/j.ebiom.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yao X, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J. Am. Coll. Cardiol. 2017;70:2621–2632. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 215.Al-Aly Z, Maddukuri G, Xie Y. Proton pump inhibitors and the kidney: implications of current evidence for clinical practice and when and how to deprescribe. Am. J. Kidney Dis. 2020;75:497–507. doi: 10.1053/j.ajkd.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 216.Fontecha-Barriuso M, et al. Molecular pathways driving omeprazole nephrotoxicity. Redox Biol. 2020;32:101464. doi: 10.1016/j.redox.2020.101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ye L, et al. Lansoprazole promotes cisplatin-induced acute kidney injury via enhancing tubular necroptosis. J. Cell Mol. Med. 2021;25:2703–2713. doi: 10.1111/jcmm.16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mishima E, et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 2020;31:280–296. doi: 10.1681/ASN.2019060570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Raizman JE, Colbourne PD, LeGatt DF, Chow D, Rudolph L. Glistening urine sample from a patient with delirium. Clin. Chem. 2017;63:1306–1307. doi: 10.1373/clinchem.2017.272377. [DOI] [PubMed] [Google Scholar]
- 220.National Toxicology Program. NTP toxicology and carcinogenesis studies of primidone (CAS no. 125-33-7) in F344/N rats and B6C3F1 mice (feed studies) Natl Toxicol. Program. Tech. Rep. Ser. 2000;476:1–290. [PubMed] [Google Scholar]
- 221.Fan X, et al. Hemopexin accumulates in kidneys and worsens acute kidney injury by causing hemoglobin deposition and exacerbation of iron toxicity in proximal tubules. Kidney Int. 2022;102:1320–1330. doi: 10.1016/j.kint.2022.07.024. [DOI] [PubMed] [Google Scholar]
- 222.Díaz-García JD, et al. Deferasirox nephrotoxicity – the knowns and unknowns. Nat. Rev. Nephrol. 2014;10:574–586. doi: 10.1038/nrneph.2014.121. [DOI] [PubMed] [Google Scholar]
- 223.Martin-Sanchez D, et al. Deferasirox-induced iron depletion promotes BclxL downregulation and death of proximal tubular cells. Sci. Rep. 2017;7:41510. doi: 10.1038/srep41510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zager RA, Johnson ACM, Therapeutics R. Iron sucrose (‘RBT-3’) activates the hepatic and renal HAMP1 gene, evoking renal hepcidin loading and resistance to cisplatin nephrotoxicity. Nephrol. Dial. Transpl. 2021;36:465–474. doi: 10.1093/ndt/gfaa348. [DOI] [PubMed] [Google Scholar]
- 225.Scindia Y, et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J. Am. Soc. Nephrol. 2015;26:2800–2814. doi: 10.1681/ASN.2014101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang C, et al. Dual degradation mechanism of GPX4 degrader in induction of ferroptosis exerting anti-resistant tumor effect. Eur. J. Med. Chem. 2022;247:115072. doi: 10.1016/j.ejmech.2022.115072. [DOI] [PubMed] [Google Scholar]
- 227.Delehouzé C, et al. Nigratine as dual inhibitor of necroptosis and ferroptosis regulated cell death. Sci. Rep. 2022;12:5118. doi: 10.1038/s41598-022-09019-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dodo K, et al. Development of a water-soluble indolylmaleimide derivative IM-93 showing dual inhibition of ferroptosis and NETosis. ACS Med. Chem. Lett. 2019;10:1272–1278. doi: 10.1021/acsmedchemlett.9b00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.