Abstract
The objective of the study was to investigate whether adverse and benevolent childhood experiences were associated with trajectories of sleep quality throughout pregnancy. The study was conducted at obstetrics and gynecology clinics in the Rocky Mountain region of the USA. The participants of the study were pregnant individuals (N = 164). Sleep quality was measured with the Pittsburgh Sleep Quality Index at three gestational time points, and adverse childhood experiences (ACEs) and benevolent childhood experiences (BCEs) were assessed once. Multilevel models were conducted to examine the trajectory of sleep quality across gestation in relation to ACEs and BCEs. Sleep quality was similar in early to mid-pregnancy, with a worsening of sleep quality late in pregnancy, following a quadratic trajectory. Higher levels of ACEs predicted poorer prenatal sleep quality (b = 0.36, SE = 0.13, p = .004) throughout pregnancy, while higher levels of BCEs predicted better sleep quality (b = −0.60, SE = 0.17, p < .001) throughout pregnancy. Examination of ACEs subtypes revealed that childhood maltreatment predicted poor sleep quality (b = 0.66, SE = 0.18, p < .001), while childhood household dysfunction was not significantly associated (b = 0.33, SE = 0.21, p = .11). Associations remained after covarying for socioeconomic status and current stressful life events. Both adverse and benevolent childhood experiences predict sleep health during pregnancy. Prevention and intervention strategies targeting resilience and sleep quality during pregnancy should be implemented to promote prenatal health and well-being.
Keywords: Adverse childhood experiences, Benevolent childhood experiences, Sleep, Pregnancy, Resilience
Introduction
Poor sleep health, including short sleep duration, later or variable sleep timing, and poor sleep quality, is common among adults in the USA, with women experiencing poorer sleep quality than men (Ohayon et al., 2004). Further, poor sleep quality is more common during pregnancy as 78% of pregnant individuals experience decreased sleep quality as early as the first trimester (Lucena et al., 2018). The scant literature with repeated assessments of sleep quality during pregnancy suggests that sleep quality worsens across gestation (Lyu et al., 2020; Naud et al., 2010). Moreover, emerging research suggests that poor sleep quality during pregnancy is linked to harmful effects on health of both the pregnant individual and the offspring. For example, poor sleep quality predicts preterm birth (Okun et al., 2011), gestational diabetes (Cai et al., 2017), and poor prenatal health behaviors (Ulman et al., 2012). Sleep health during this critical life period may be affected by the various physiological and psychological changes associated with pregnancy or with current experiences. However, it also is likely that both adverse and positive life experiences that occur prior to pregnancy influence sleep quality during the prenatal period.
Adverse childhood experiences (ACEs) have pervasive implications for physical and mental health across the lifespan (Crandall et al., 2019; Felitti, 2009). Pregnancy may be a sensitive window when individuals are particularly vulnerable to the long-reaching effects of ACEs (Davis & Narayan, 2020). For instance, pregnant individuals who experienced childhood adversity may be haunted by recollections of unresolved trauma that are reawakened as they anticipate parenthood (Narayan et al., 2018; Slade & Cohen, 1996). Given the impact of ACEs on prenatal health, it is postulated that childhood adversity is implicated in prenatal sleep quality. Limited evidence suggests that childhood adversity is associated with poorer sleep quality among pregnant individuals. In a sample of 600 Peruvian pregnant individuals with one prenatal sleep assessment, childhood adversity predicted a 2.11-fold increased odds of poor sleep quality (Gelaye et al., 2015). Recently, a study of 250 pregnant individuals in the USA found a positive association between childhood adversity and poor sleep quality measured twice during pregnancy (Foss et al., 2021). These studies highlight the impact of early-life experiences on an important indicator of sleep health. However, research has not evaluated associations between ACEs and longitudinal and prospective assessment of prenatal sleep quality. Further, specific types of ACEs (childhood maltreatment vs. childhood household dysfunction) differentially affect adult health outcomes (Atzl et al., 2019; Merrick et al., 2020; Negriff, 2020), and may differentially affect prenatal sleep health (Brown et al., 2022). Thus, research is needed to examine relative effects of ACEs subtypes on sleep quality throughout pregnancy.
Despite known linkages between early-life adversities and maternal health (Atzl et al., 2019; Narayan et al., 2021), little is known about resilience processes that may promote positive functioning during the prenatal period. Resilience is a dynamic process whereby individuals display positive functioning despite experiencing adversity (Masten & Cicchetti, 2016; Wright et al., 2013). Studies suggest that benevolent childhood experiences (BCEs), such as having at least one trusted caregiver during childhood, are linked to more positive functioning during adulthood in individuals with and without childhood adversity (Crandall et al., 2019; Narayan et al, 2018, 2021). Higher levels of BCEs are associated with fewer post-traumatic stress disorder (PTSD) symptoms and stressful life events during pregnancy (Narayan et al., 2018), as well as less risky reproductive planning (Merrick et al., 2020), and fewer sleep difficulties in non-pregnant adults (Crandall et al., 2019). Although associations between BCEs and sleep quality during pregnancy remain to be tested, one study found that higher levels of BCEs predict fewer sleep difficulties in non-pregnant adults (Crandall et al., 2019). Taken together, these findings suggest that BCEs may be linked to sleep health in pregnant individuals.
The current study examined trajectories of prenatal sleep quality in relation to ACEs and BCEs. First, we characterized trajectories of sleep quality across gestation (hypothesis 1). We hypothesized that sleep quality would worsen during pregnancy. Next, we examined whether ACEs and BCEs separately predicted trajectories of prenatal sleep quality. We hypothesized that higher levels of ACEs would predict poorer sleep quality throughout gestation (hypotheses 2 and 3) and higher levels of BCEs would predict better sleep quality throughout pregnancy. We then explored whether ACEs and BCEs contributed independent predictive variance to sleep health throughout gestation when tested in the same model and whether BCEs moderated the association between ACEs and sleep quality (exploratory aim 4). Our final exploratory aim examined whether ACEs subtypes (childhood maltreatment and childhood household dysfunction) differentially predicted prenatal sleep quality (exploratory aim 5).
Method
Participants
Participants included 164 pregnant individuals from obstetrics and gynecology clinics in and around Denver, Colorado (see Davis et al., 2018 for more details). Recruitment was enriched with participants with elevated depression symptoms at enrollment based on routine obstetric screening. Initial inclusion criteria for enrollment included (a) age between 18 and 45 years, (b) singleton pregnancy, (c) gestational age (GA) less than 29 weeks at the time of enrollment, and (d) proficiency in English as all assessments were normed in the English language. Initial exclusion criteria at recruitment included (a) current illicit drug or methadone use, (b) major health conditions requiring invasive treatments (e.g., dialysis, blood transfusions, chemotherapy), (c) current or past symptoms of psychosis or mania based on the Structured Clinical Interview (SCID) for DSM-5, and (d) current participation in cognitive behavioral therapy or interpersonal therapy. Additionally, participants who experienced a miscarriage or fetal demise of the current pregnancy (n = 2) were not included in study analyses.
Table 1 shows participants’ demographic characteristics. They were, on average, 30 years old (SDage = 5.36) with household annual income of $60,000. Fifty-two percent obtained less than a bachelor’s degree, 34% lived at or below 200% of the federal poverty line, and 80% lived with a partner at the time of enrollment. Participants identified as 51% non-Latinx White, 26% Latinx/Hispanic, 12% as African American/Black, 6% Asian American/Asian, 1% American Indian/Alaska Native, 1% Native Hawaiian/Pacific Islander, and 3% multiracial.1
Table 1.
Participant Characteristics (N = 164)
| Mean (SD)/Percentage | |
|---|---|
|
| |
| Age | 30.31 (5.36) |
| Race and ethnicity | |
| Non-Latinx White | 51% |
| Hispanic/Latinx | 26% |
| Black/African American/Haitian | 12% |
| Asian/Asian American | 6% |
| More than one race | 3% |
| American Indian/Alaska Native | 1% |
| Native Hawaiian/Pacific Islander | 1% |
| Education | |
| Less than high school | 4% |
| High school or higher | 36% |
| Associate degree | 12% |
| College degree | 26% |
| Graduate degree | 22% |
| Income | $60,000 ($56,734)1,* |
| Income-to-needs ratio (INR) | 2.97 (3.18)1,* |
| At or below the 200% poverty line | 34% |
| Cohabitation status | |
| Living with partner | 80% |
| Not living with partner | 20% |
| Parity | |
| Nulliparous | 42% |
| Multiparous | 58% |
| Obstetric complications | |
| None | 31% |
| One | 38% |
| Two or more | 31% |
| Adverse childhood experiences (ACEs) | 2.21 (2.12) |
| Benevolent childhood experiences (BCEs) | 8.87 (1.68) |
| Stressful life events during pregnancy (LEC-5) | 1.11 (1.65) |
| Sleep quality early in pregnancy (PSQI) | 6.61 (3.57) |
| Sleep quality mid-pregnancy (PSQI) | 6.77 (3.58) |
| Sleep quality late in pregnancy (PSQI) | 7.59 (3.74) |
Notes:
Median income reported
An outlier for income (i.e., SD ≥ 5 above the mean) was converted to the value 3 SDs above the mean, preserving its rank as the highest value.
Participants were compensated at each time point in which data was collected. This study was approved by the University of Denver and the Colorado Multiple Institutional Review Board (DU IRB and COMIRB). All participants provided written and informed consent. This study was preregistered and can be accessed at https://osf.io/mc86y/. Participants who completed prenatal assessment of sleep quality and ACEs or BCEs at preregistration submission were included.
Measures
Adverse Childhood Experiences (ACEs)
ACEs were measured using the Adverse Childhood Experiences Scale (Felitti et al., 1998), a 10-item retrospective self-report questionnaire of adverse experiences encountered from birth to 18 years of age. Participants indicated whether they experienced each of 10 types of childhood adversity. The 10 items were summed for the composite ACEs score with higher scores indicating more adversity. Additionally, the ACEs scale contains two subscales: childhood maltreatment (physical, emotional, and sexual abuse, physical and emotional neglect) and childhood household dysfunction (parental separation or divorce, caregiver substance use, mental health, domestic violence, and incarceration). The ACEs scale shows moderate test–retest reliability (r = 0.65; Dube et al., 2004). All participants completed the ACEs scale.
Benevolent Childhood Experiences (BCEs)
BCEs were measured using the Benevolent Childhood Experiences (BCEs) Scale (Narayan et al., 2018), a 10-item self-report questionnaire of positive experiences from birth to 18 years of age. Parallel to the ACEs scale, participants endorsed the occurrence of each type of positive experience, including relational trust and security (at least one trusted caregiver, teacher, friend, or adult) and positive, predictable life quality (enjoyment of school, predictable meals, and bedtime). Items were summed; higher scores indicated higher BCEs. Prior work with diverse perinatal populations has validated the scale, which has good test–retest reliability (r = 0.80; Narayan et al., 2018). Eighteen percent of the participants had missing BCEs data due to delayed administration of instrument after data collection began.
Sleep Quality
Prenatal sleep quality was measured using the Pittsburg Sleep Quality Index (PSQI; Buysse et al., 1989). The PSQI is a 19-item self-report questionnaire composed of seven sleep subscales (sleep latency, sleep duration, sleep disturbances, sleep medication, sleep subjective quality, sleep efficiency, and daytime dysfunction), which are equally weighed on a 0–3 scale. The subscale scores are summed, yielding an overall subjective sleep quality score ranging from 0 to 21. Higher scores indicate poor sleep quality (Buysse et al., 1989). The PSQI shows good convergent and discriminant validity in pregnancy (Zhong et al., 2015). The overall PSQI score was used as the primary outcome in this study. The PSQI was assessed longitudinally at 17 (M = 17.3 weeks, SD = 4.6; 0% missing data), 29 (M = 28.6 weeks, SD = 3.9; 0% missing data), and 35 (MGA = 35.3 weeks, SDGA = 1.7; 15% missing data) gestational weeks. Acceptable internal consistency across time points was observed in this sample (αs = 0.74 to 0.75).
Stressful Life Events During Pregnancy
Life stress during pregnancy was measured using the Life Events Checklist (LEC-5; Weathers et al., 2013), a 17-item self-report questionnaire that assesses individuals’ exposure to traumatic events. Participants were asked to respond about any traumatic events, including but not limited to natural disasters, assault, and life-threatening health conditions. Participants were also asked if, during their current pregnancy, (1) the event happened to them, (2) the event happened during their job, (3) the event happened to someone close to them, (4) they were unsure if it applied to them, or (5) it was not applicable to them. Items were then recoded into 1, 2, 3 = 1, and 4, 5 = 0. Items were added together to yield a sum score (0 to 17), with higher scores indicating greater stress exposure during pregnancy. The LEC-5 possesses good test–retest reliability (r = 0.82; Gray et al., 2004). Seven percent of the participants had missing prenatal stressful life events data.
Sociodemographic Characteristics
Pregnant individuals reported age, race, ethnicity, marital and cohabitation status, educational attainment, and household income via a structured interview. Additionally, the income-to-needs ratio (INR) for each participant was calculated by dividing reported annual household income by the federal poverty threshold associated with the number of individuals living in the same household, as specified by the United States Census Bureau (2020). One outlier for income (i.e., SD ≥ 5 above the mean) was converted to the value 3 SDs above the mean, preserving its rank as the highest value. All participants provided marital and cohabitation status, race, ethnicity, age, and educational attainment. Seven percent of participants has missing INR data.
Prenatal Obstetric Factors
Gestational dating, parity, and prenatal obstetric complications were assessed via medical records. An obstetric complications score was computed based on the occurrence of pregnancy-related complications (prenatal infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, vaginal bleeding, placenta previa, or anemia), and summed into a total index (Hobel, 1982). This approach has been validated in past prenatal studies (Howland et al., 2020). Thirty-eight percent of our sample had one of the obstetric complications and 31 had two or more. The association between number of obstetric complications and sleep quality was evaluated using a one-way analysis of variance (ANOVA) comparing no complications, one complication, and two or more complications. Sleep quality scores (PSQI at early pregnancy) did not differ as a function of obstetric complications, F(2,142) = 0.46, p = 0.63. Eleven percent of participants had missing obstetric complication and parity data.
Analytical Approach
Preliminary Analyses
Preliminary analyses evaluated the association between childhood experiences, sleep quality across gestation, and covariates identified based on previous literature (Miller-Graff & Cheng, 2017; Osnes et al., 2019). Variables associated significantly with prenatal sleep quality were selected as covariates. Bivariate correlations were used to evaluate INR, parental age, and number of prenatal stressful life events as potential covariates. Further, multiple one-way analysis of variance (ANOVA) tests were conducted to test educational attainment, and obstetric complications as potential covariates. INR and educational attainment emerged as the factors significantly correlated with sleep quality at least once during pregnancy (see Table 2 for all correlations). However, because of shared variance between prenatal INR and educational attainment, INR was selected as the key covariate. We included experiences of prenatal stressful life events in sensitivity analyses (even though prenatal stressors did not meet traditional covariate criteria; see Table 2) to test whether childhood experiences were associated with sleep quality above and beyond prenatal stressful experiences. Additional sensitivity analyses were performed to test whether childhood experiences were associated with prenatal sleep after covarying obstetric complications. Correlations and ANOVAs were conducted using IBM SPSS, Version 25 (IBM Corp., 2017).
Table 2.
Bivariate Correlations Across Study Variables and Continuous Covariates
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1. ACEs | |||||||||
| 2. BCEs | −.40** | ||||||||
| 3. Prenatal LEC-5 | −.002 | .17 | |||||||
| 4. T1 PSQI | .21** | −.37** | −.09 | ||||||
| 5. T2 PSQI | .26** | −.31** | −.01 | .72** | |||||
| 6. T3 PSQI | .20* | −.27** | −.02 | .69** | .80* | ||||
| 7. Parental Age | −.11 | .14 | .13 | .02 | .12 | .12 | |||
| 8. INR | −.25** | .24** | .23** | −.23** | −.11 | −.12 | .37** | ||
| 9. Cohabitation | .03 | −.03 | .02 | −.12 | .01 | .06 | −.13 | .39** | |
| 10. Parity | −.004 | −.02 | −.10 | .08 | .11 | .04 | .30** | −.22** | .02 |
Notes:
p < .05
p < .01
ACEs = Adverse Childhood Experiences, BCEs = Benevolent Childhood Experiences, LEC-5 = Life Event Checklist, PSQI = Pittsburg Sleep Quality Index, T1 = Time point 1, T2 = Time point 2, T3 = Time point 3, INR = Income-to-needs ratio.
Primary Analyses
Multilevel modeling (MLM) was used to estimate the association between childhood experiences and trajectories of prenatal sleep quality using HLM 8 software (Raudenbush & Congdon, 2021). MLM assumes the data collected is nested within persons, allowing for variability to be modeled at multiple hierarchical levels. At level 1, prenatal sleep quality was regressed on linear and quadratic indices of gestational weeks. Given that prenatal sleep quality was collected at three gestational intervals, we were limited in our ability to test quadratic effects as random effects (Hoffman, 2015). Thus, the intercept and linear slope were tested as random parameters, whereas quadratic growth was tested as a fixed parameter. At level 2, the time-invariant variables included predictors (i.e., ACEs and BCEs) and covariates. We conducted Little’s (1988) missing at random test and findings were nonsignificant χ2 (112) = 101.1, p = 0.76, suggesting that data were missing at random. Given the low level of missing data in this study, full information maximum likelihood (FIML) was used to address missing data. FIML is a relatively accurate and unbiased method for dealing with missing data within nested, hierarchical models (Black, 2011). Using FIML results in a more accurate imputation of data while improving statistical power within MLM models.
We fit multilevel models to find the best-fitting trajectory of sleep quality across gestational weeks and to account for within-participant correlation across measurements of sleep quality. Linear and quadratic growth curves were included in our models to test for changes in sleep quality over the course of gestation (hypothesis 1). Next, ACEs and BCEs were separately added as predictors of the trajectory of sleep quality across gestation (hypotheses 2 and 3). Hypothesis testing was performed to determine if either ACEs or BCEs were associated with sleep quality. Sensitivity analyses were then performed to determine if associations persisted after inclusion of INR and stressful life events during pregnancy as covariates. Additional sensitivity analyses were performed to test obstetric complications as a covariate (see Supplement 2). The associations between childhood experiences and prenatal sleep quality were tested at the intercept, linear slope, and quadratic growth curve.
Exploratory Analyses
Using the models described above, exploratory analyses were conducted first adding both ACEs and BCEs to the model to determine if ACEs and BCEs contributed unique variance to sleep quality when modeled together. Second, the interaction term (ACEs × BCEs) was added to determine whether higher levels of BCEs would buffer the association between ACEs and sleep quality across gestation (exploratory aim 4).
Next, the two subscales of ACEs (childhood maltreatment and childhood household dysfunction) were examined to determine the differential associations of subtypes of childhood adversity in relation to prenatal sleep quality (exploratory aim 5).
Results
On average, participants experienced between two and three ACEs (M = 2.21, SD = 2.12; see Table 1), which is consistent with existing prenatal literature (Miller-Graff & Cheng, 2017). Forty-four percent of the sample (n = 72) endorsed all 10 BCEs, with an average between eight and nine BCEs (M = 8.87, SD = 1.68), also similar to other prenatal samples (Narayan et al., 2018). Higher ACEs were moderately associated with lower BCEs (r = −0.40, p < 0.001). Similar to previous studies (Lucena et al., 2018; Naud et al., 2010), 66% of participants were classified as “poor sleepers” (PSQI score ≥ 5; Tomfohr-Madsen et al., 2015) at the first assessment. The percentage of individuals considered “poor sleepers” increased from 66% at the first to 77% at the last assessment.
As shown in Table 2, high ACEs and low BCEs correlated with poor prenatal sleep quality at all time points. Stressful life events during pregnancy were not significantly correlated with sleep quality at any time point.
Primary Analyses
Trajectories of Sleep Quality Across Gestation
Of the linear and quadratic growth curves analyzed, deviance scores indicated that a quadratic growth curve yielded a better fit for the trajectories of prenatal sleep quality (Δχ2(1) = 2222.22–2211.81.04 = 10.41, p = 0.002). Descriptively, PSQI scores were similar early and mid-pregnancy with a worsening of sleep quality late in pregnancy (see Table 1 and Fig. 1). The random effects within the intercept and the slope were significant indicating the presence of between-subjects variance in sleep quality scores across gestation (all ps < 0.01; see Table 3). Random effects were thus retained in our final model.
Fig. 1.
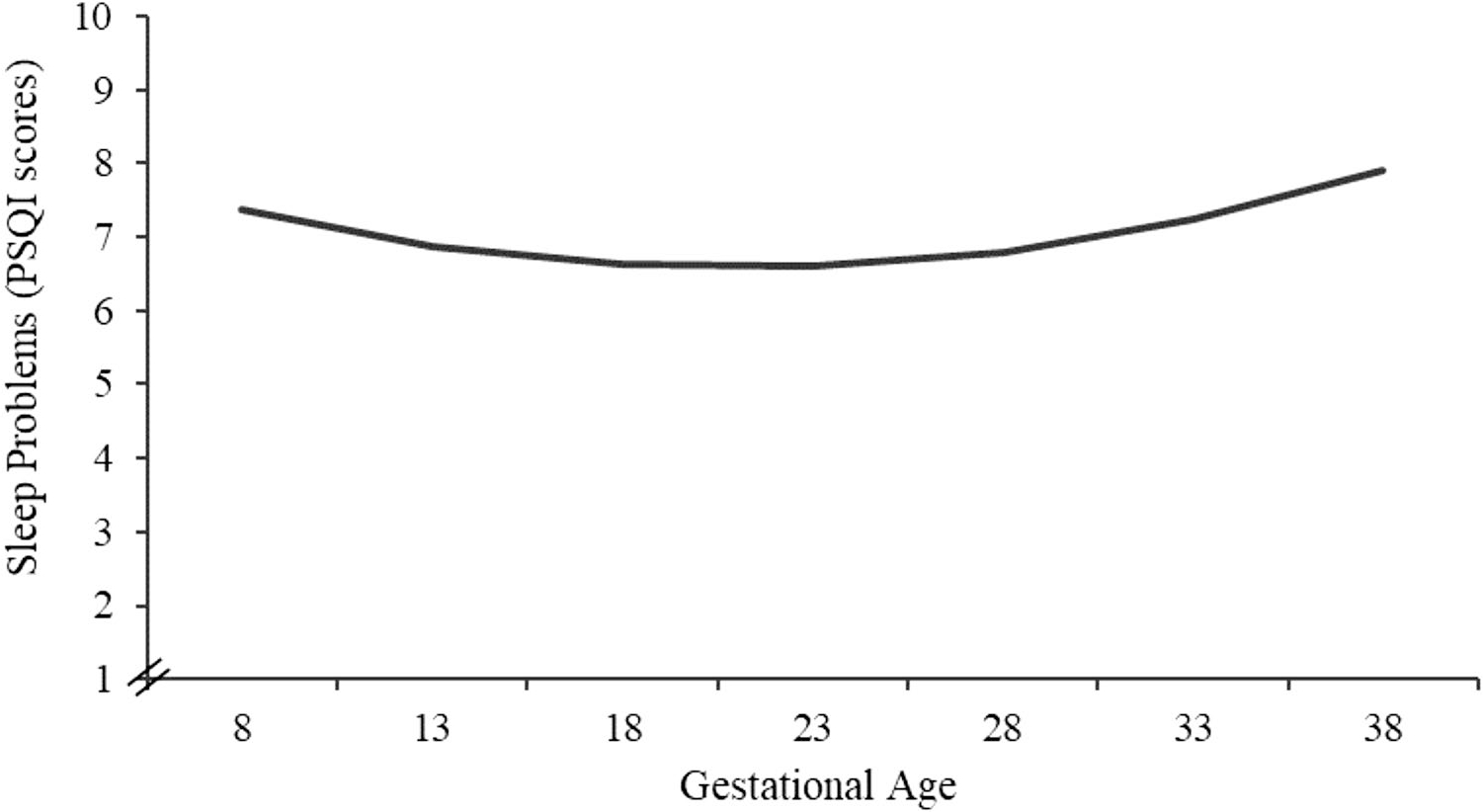
Prenatal sleep quality (PSQI) across gestation is characterized by a quadratic growth. Figure displays predicted data based on beta coefficients
Table 3.
Hierarchical Linear Models of Sleep Quality Across Gestation & Predictors
| Model 1 | ||||
|
| ||||
| Linear Slope | Quadratic Growth | |||
|
|
||||
| Fixed Effects | Intercept Centered at 8 Weeks (b0) | 6.22 | 7.27 | |
| Linear Slope (b1) | 0.034** | −0.12** | ||
| Quadratic Growth (b2) | -- | 0.0047** | ||
| Random Effects a | Error (σ2e) | 2.75 | 2.59 | |
| Intercept (σ2b0) | 10.93*** | 11.42*** | ||
| Slope (σ2b1) | 0.009*** | 0.009*** | ||
|
| ||||
| Model 2b | ||||
|
| ||||
| ACEs | ACEs & Covariates | |||
|
|
||||
| Fixed Effects | Intercept Centered at 8 Weeks (b0) | 7.27 | 7.28 | |
| ACEs (b01) | 0.36 | 0.34* | ||
| Prenatal Stressful Life Events (b02) | -- | −0.065 | ||
| INR (b03) | -- | −0.15⸸ | ||
| Linear Slope (b1) | −0.12** | −0.12* | ||
| ACEs (b11) | 0.003 | -- | ||
| Quadratic Growth (b2) | 0.005** | 0.005** | ||
| ACEs (b21) | −0.0002 | -- | ||
| Random Effects a | Error (σ2e) | 2.59 | 2.69 | |
| Intercept (σ2b0) | 13.28*** | 9.15*** | ||
| Slope (σ2b1) | 0.009*** | 0.009*** | ||
|
| ||||
| Model 3b | ||||
|
| ||||
| BCEs | BCEs & Covariates | |||
|
|
||||
| Fixed Effects | Intercept Centered at 8 Weeks (b0) | 8.85 | 8.48 | |
| BCEs (b01) | −0.85** | −0.59*** | ||
| Prenatal Stressful Life Events (b02) | -- | −0.01 | ||
| INR (b03) | -- | −0.13 | ||
| Linear Slope (b1) | −0.15* | −0.15** | ||
| BCEs (b11) | 0.006 | -- | ||
| Quadratic Growth (b2) | 0.005* | 0.005*** | ||
| BCEs (b21) | 0.0002 | -- | ||
| Random Effects a | Error (σ2e) | 2.64 | 2.70 | |
| Intercept (σ2b0) | 9.69*** | 8.58*** | ||
| Slope (σ2b1) | 0.008*** | 0.009*** | ||
|
| ||||
| Model 4 | ||||
|
| ||||
| ACEs & BCEs | ACEs × BCEs Interaction | |||
|
|
||||
| Fixed Effects | Intercept Centered at 8 Weeks (b0) | 8.43 | 8.38 | |
| ACEs (b01) | 0.29* | 0.28⸸ | ||
| BCEs (b02) | −0.55** | −0.53** | ||
| Interaction (b03) | -- | −0.03 | ||
| Linear Slope (b1) | −0.14** | −0.14** | ||
| Quadratic Growth (b2) | 0.005*** | 0.005*** | ||
| Random Effects a | Error (σ2e) | 2.65 | 2.65 | |
| Intercept (σ2b0) | 9.36*** | 9.37*** | ||
| Slope (σ2b1) | 0.009*** | 0.009*** | ||
Notes:
p < .08
p < .05
p < .01
p < .001
Intercept and linear slope were tested as random parameters, whereas quadratic growth was tested as a fixed parameter.
ACEs and BCEs were initially tested under intercept, linear slope, and quadratic growth. However, they were only retained in final models at levels in which they were statistically significant (e.g., intercept).
Adverse Childhood Experiences
Higher ACEs predicted higher PSQI scores, indicating poorer sleep quality. Associations were present at the intercept, the earliest assessment (b = 0.58, p = 0.01), and persisted throughout pregnancy (see Supplement 1 for models centered at 20 and 30 weeks). ACEs were not associated with changes in sleep quality and did not significantly account for the linear (b = −0.02, p = 0.29) or quadratic (all bs = 0.0007, p = 0.32) growth of sleep quality. Thus, ACEs were retained in the final model at the intercept. Associations between ACEs and sleep quality remained statistically significant when stressful life events during pregnancy and INR were covaried (see Table 3 and Fig. 2a). These associations remained when covarying obstetric complications (see Supplement 2a).
Fig. 2.
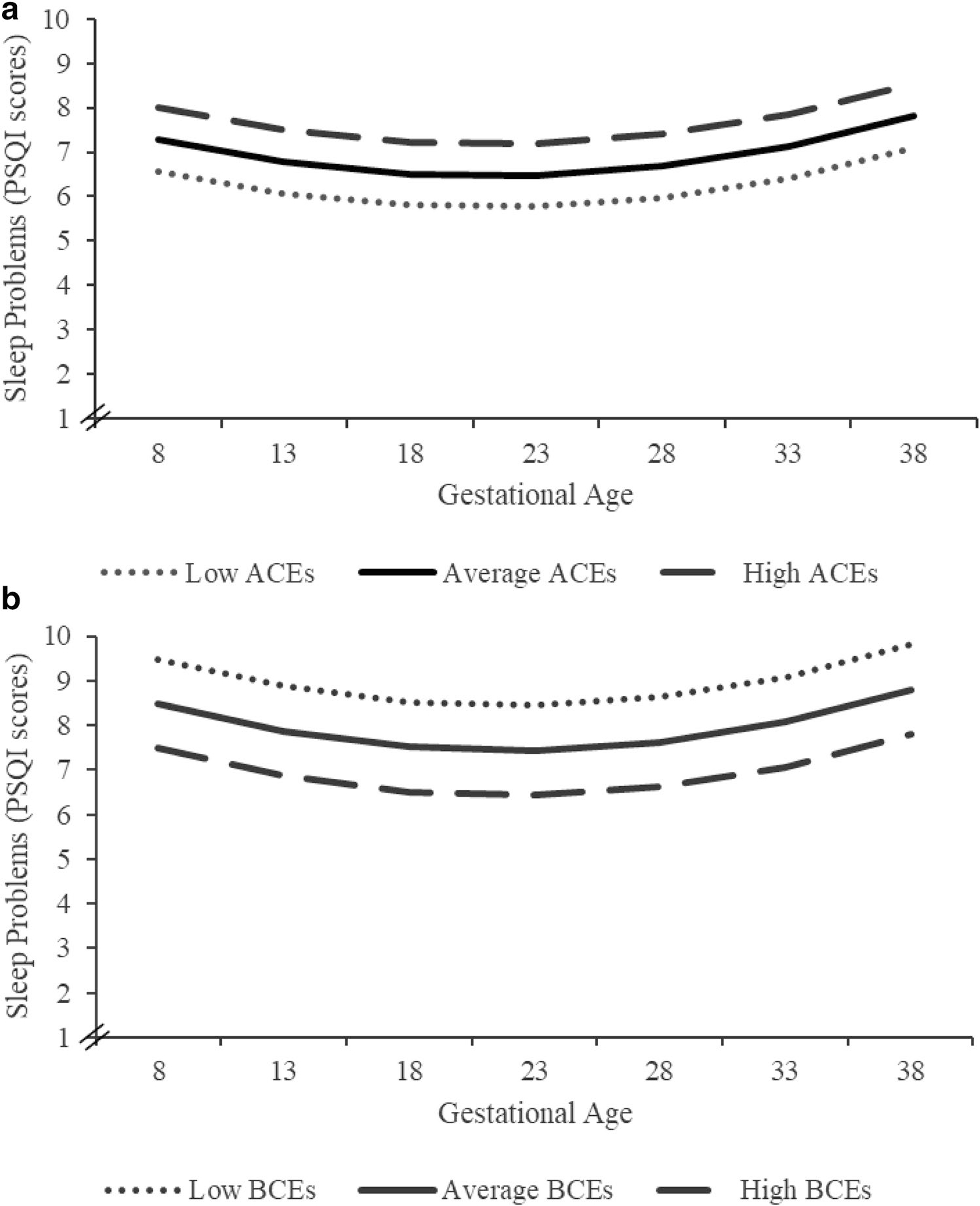
Sleep quality across gestation and childhood experiences. High ACEs predict poorer sleep quality in relation to lower ACEs. High BCEs predict better sleep quality in relation to lower BCEs. Data were analyzed continuously; for visualization purposes, Fig. 2 displays predicted data based on beta coefficients, mean, and standard deviation of ACEs or BCEs. Average ACEs and BCEs = means of sample, high and low ACEs and BCEs = ± 1 SD respectively. Approximate values: high ACEs = 4, average ACEs = 2, low ACEs = 0; high BCEs = 10, average BCEs = 9, low BCEs = 7
Benevolent Childhood Experiences
Higher numbers of BCEs predicted lower PSQI scores, indicating better sleep quality. Associations were present at the intercept (b = −0.91, p < 0.001) and persisted throughout pregnancy (see Supplement 2 for models centered at 20 and 30 weeks). BCEs did not significantly account for the linear (b = 0.01, p = 0.62) or quadratic (b = −0.00007, p = 0.93) growth of sleep quality, and thus, BCEs were retained in our final model only at the intercept. The relation between BCEs and sleep quality remained significant when stressful life events and INR were covaried (see Table 3 and Fig. 2b). These associations remained when covarying obstetric complications (see Supplement 2b).
Exploratory Analyses
ACEs and BCEs: Main Effects and Moderation
When ACEs and BCEs were modeled simultaneously, BCEs (b = −0.56, p = 0.001) and ACEs (b = 0.30, p = 0.04; see Table 3) accounted for unique variance in prenatal sleep quality. The interaction between ACEs and BCEs, however, was not significantly associated with sleep quality (b = −0.03, p = 0.68; see Table 3).
Childhood Maltreatment and Childhood Household Dysfunction
Consideration of the two subscales of ACEs revealed that higher levels of childhood maltreatment significantly predicted poorer prenatal sleep quality (b = 0.66, p < 0.001). Conversely, higher levels of childhood household dysfunction did not significantly predict sleep quality (b = 0.33, p = 0.11).
Discussion
This study provides novel evidence that childhood adversity and benevolent childhood experiences are associated with sleep health during pregnancy. Overall, prenatal sleep quality follows a quadratic trajectory with PSQI scores at similar ranges during early and mid-gestation and worsening late in pregnancy. This study provides empirical data exploring the changes in sleep quality across gestation in relation to early-life adverse and benevolent experiences. Specifically, ACEs predicted poorer sleep quality and BCEs predicted better sleep quality across gestation even after covarying current life stressors. These findings are consistent with the hypothesis that both positive and negative childhood experiences influence sleep health during the prenatal period above and beyond current life experiences. Prenatal sleep quality is associated with the health of the pregnant individual (Lyu et al., 2020; Whitaker et al., 2021) and birth outcomes (Okun et al., 2011), and thus, understanding factors that impact prenatal sleep has important implications for intergenerational health.
Our findings are consistent with the emerging literature examining ACEs and sleep quality in non-pregnant and pregnant individuals (Crandall et al., 2019; Menke et al., 2019). Our research extends the existing body of knowledge by including three time points of sleep quality across gestation, enabling us to extrapolate trajectories of sleep quality. Further, this study provides novel evidence suggesting that ACEs subtypes, childhood maltreatment, and childhood household dysfunction exert differential effects on prenatal sleep quality. More specifically, childhood maltreatment more strongly predicted poorer sleep quality, relative to childhood household dysfunction, which was not significantly associated. Existing literature examining the differential effects of ACEs subtypes also shows childhood maltreatment robustly predicts prenatal well-being (Atzl et al., 2019; Merrick et al., 2020). The differential effects of childhood maltreatment versus childhood household dysfunction may be due to the biological embedding of childhood maltreatment on physiological processes that impact sleep quality during pregnancy.
In contrast to ACEs, BCEs were associated with improved sleep quality throughout gestation, even after covarying household income and stressful life events during pregnancy. BCEs had not previously been associated with prenatal sleep. However, this finding is consistent with a study of non-pregnant adults indicating that positive childhood experiences promote sleep health and supports the hypothesis that individuals who report higher numbers of positive childhood resources and relationships may sleep better during pregnancy (Crandall et al., 2019). Sleep health practitioners may benefit from these findings by assessing BCEs and using them to further understand how a pregnant individual’s childhood experiences might be reflected in their prenatal sleep health. These findings further highlight the importance of leveraging positive childhood experiences during the sensitive window of pregnancy as a target for promoting health and wellness among pregnant individuals.
Consistent with the existing literature, ACEs and BCEs were only moderately correlated in our study, and BCEs independently predicted prenatal outcomes (Cárdenas et al., 2022; Narayan et al., 2018). Adverse and benevolent childhood experiences may be independent experiences, and the presence of one does not prevent or exclude the presence of the other (Narayan et al., 2021). Our finding that BCEs predict sleep health independent of ACEs is consistent with literature documenting that BCEs are associated with mental health during pregnancy, independent of ACEs (Cárdenas et al., 2022; Narayan et al., 2018). Moreover, we found that ACEs and BCEs did not interact to predict prenatal sleep quality; that is, BCEs did not buffer against the effects of ACEs on poorer sleep quality. These findings are consistent with previous research that has largely shown that BCEs do not necessarily protect against (interact with) ACEs, but rather, have promotive (direct effects) on most positive outcomes (Doom et al., 2021; Narayan et al., 2018). However, it may be the case that BCEs interact with ACEs to predict some outcomes but not others (Crandall et al., 2019), so the interplay of BCEs and ACEs remains a viable area for future research.
Current findings suggest that ACEs and BCEs are processes that may each contribute to sleep health in pregnancy. Additionally, these findings suggest that the presence of BCEs may promote sleep health in pregnant individuals, regardless of their level of childhood adversity. This finding underscores the need for providers to assess not only ACEs in healthcare settings, but also BCEs or they will otherwise miss an alternative promotive pathway contributing to sleep health during pregnancy. Assessing BCEs may thus provide a more complex and holistic assessment of childhood experiences and their potential predictive effects on prenatal sleep health. Given that BCEs may be leveraged to promote resilience (Narayan et al., 2018), understanding links to sleep health underscores an important opportunity to improve prenatal well-being.
The present study contained several strengths and limitations. A key strength is the use of repeated assessments of sleep quality throughout gestation in a diverse group of pregnant individuals. However, sleep quality was assessed via self-report; therefore, future studies should incorporate objective measures of sleep quality and other indices of sleep health by using actigraphy or polysomnography. A key strength included the longitudinal modeling of sleep throughout gestation. However, we were limited in our ability to test both linear and quadratic effects as random effects and, thus, we were unable to interpret individual variance in the quadratic curves of our model. To resolve this, future studies could include at least five repeated assessments of prenatal sleep quality (Hoffman, 2015). Another strength was the inclusion of both adverse and benevolent childhood experiences. It is the case that the reporting of childhood experiences is subject to retrospective recall; it is therefore possible that our findings reflect biased recall and pregnant individuals who sleep better report a higher number of favorable childhood experiences. It is noteworthy though that sleep was not associated with proximal factors such as life events in pregnancy. Further, both ACEs and BCEs have maintained validity when compared to prospective recall and other assessments of positive childhood experiences, respectively (Baldwin et al., 2019; Narayan et al., 2020). Future studies would, nevertheless, benefit from prospective assessments of adverse and positive childhood experiences in relation to prenatal sleep health. Additionally, sleep health was not assessed prior to conception, and thus, future research could test links between ACEs, BCEs, and sleep quality from preconception through pregnancy.
Several future directions can be considered based on the study’s findings. This study identifies early-life experiences that may contribute to sleep quality throughout pregnancy, suggesting that supportive resources during childhood may have lasting impacts on perinatal health and offspring outcomes. However, the extent and nature of early-life experiences and their relation to prenatal sleep quality would benefit from further investigation (Brown et al., 2022). Childhood experiences have been shown to predict prenatal processes including brain responses to emotional stimuli (Fuligni et al., 2021) and cortisol concentrations (Swales et al., 2018). Existing literature has posited these biological markers as potential mechanisms underlying the association between childhood experiences and sleep health later in life (Fuligni et al., 2021; van Dalfsen & Markus, 2018). Thus, future research examining the biophysiological mechanisms linking childhood experiences and sleep quality during pregnancy is needed. Our findings also inform future research aimed to examine prenatal sleep quality as a process implicated in the intergenerational transmission of risk and resilience. Childhood adversity can have intergenerational repercussions (Madigan et al., 2017; Narayan et al., 2020), and sleep quality during key sensitive periods (e.g., pregnancy) has been theorized to mediate the relation between childhood adversity and later health outcomes (Fuligni et al., 2021). As such, future studies should examine the mediating role of sleep health during pregnancy when assessing childhood experiences and prenatal and infant outcomes. Uncovering the intergenerational effects of prenatal sleep health may in turn inform the development of effective and efficient preventive interventions.
Supplementary Material
Acknowledgements
The authors wish to thank the participants in the study for sharing and trusting their unique life experiences multiple times during pregnancy and beyond. We also thank the dedicated Care Project team for all of their efforts to complete this project.
Funding
This work is supported by the National Institutes of Health R01MH109662; R01HL155744; and diversity training supplement 3R01HL155744-01S1.
Footnotes
Conflict of Interest The authors declare no competing interests.
Participants selected categories of race/ethnicity with which they identified. We recognize the ways in which such imposed categorization can minimize the complexities of human experience and, advertently or inadvertently, cause harm. These categorizations should be used with caution within academic realms and while interacting with participants and the public.
Research Involving Human Participants and/or Animals This study was accepted by the University of Denver and Colorado Multiple Institutional Review Boards (DU IRB; COMIRB).
Informed Consent Informed and written consent was collected from all participants in this study.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s42844-022-00070-0.
References
- Atzl VM, Narayan AJ, Rivera LM, & Lieberman AF (2019). Adverse childhood experiences and prenatal mental health: Type of ACEs and age of maltreatment onset. Journal of Family Psychology, 33(3), 304. [DOI] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A (2019). Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry, 76(6), 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A (2011). Missing data techniques for multilevel modeling: Implications of model misspecification. Journal of Applied Statistics, 38(9), 1845–1865. [Google Scholar]
- Brown SM, Rodriguez K, Smith A, Ricker A, & Williamson AA (2022). Associations between childhood maltreatment and behavioral sleep disturbances across the lifespan: A systematic review. Sleep Medicine Reviews, 64. 10.1016/j.smrv.2022.101621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D, Reynolds C, Monk T, Berman S, & Kupfer D (1989). The Pittsburg sleep quality index: A new instruments for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cai S, Tan S, Gluckman P, Godfrey K, Saw S, Teoh O, Chong Y, Meaney M, Kramer M, Gooley J, & Gusto study group. (2017). Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep, 40(2). 10.1093/sleep/zsw058 [DOI] [PubMed] [Google Scholar]
- Cárdenas EF, Kujawa A, & Humphreys KL (2022). Benevolent childhood experiences and childhood maltreatment history: Examining their roles in depressive symptoms across the peripartum period. Adversity and Resilience Science, 3, 169–179. 10.1007/s42844-022-00062-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall A, Miller J, Cheung A, Novilla L, Glade R, Novilla M, Magnusson B, Leavitt B, Barnes M, & Hanson C (2019). ACEs and counter-ACEs: How positive and negative childhood experiences influence adult health. Child Abuse & Neglect, 96, 104089. 10.1016/j.chiabu.2019.104089 [DOI] [PubMed] [Google Scholar]
- Davis EP, & Narayan AJ (2020). Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Development and Psychopathology, 32, 1625–1639. 10.1017/S0954579420001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Hankin B, Swales D, & Hoffman C (2018). An experimental test of the fetal programming hypothesis: Can we reduce child oncogenic vulnerability to psychopathology by decreasing maternal depression? Developmental Psychopathology, 30(3), 787–806. 10.1017/S0954579418000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Seok D, Narayan AJ, & Fox KR (2021). Adverse and benevolent childhood experience predict mental health during the COVID-19 pandemic. Adversity and Resilience Science, 2(3), 193–204. 10.1007/s42844-021-00038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S, Williamson D, Thompson T, Felitti V, & Anda R (2004). Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse & Neglect, 28(7), 729–737. 10.1016/j.chiabu.2003.08.009 [DOI] [PubMed] [Google Scholar]
- Felitti V (2009). Adverse childhood experiences and adult health. Academic Pediatrics, 9(3), 131–132. 10.1016/j.acap.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Felitti V, Anda R, Nordenberg D, Williamson D, Spitz A, Edwards V, Koss M, & Marks J (1998). Relationship of childhood abuse and household dysfunction to many of the leading cause of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Foss S, Gustafsson H, Berry O, Hipwell A, Werner E, Peterson B, & Monk C (2021). Associations between childhood maltreatment, poor sleep, and prenatal distress in pregnancy adolescents. Development and Psychopathology, 1–10. 10.1017/S0954579420002163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni A, Chiang J, & Tottenham N (2021). Sleep disturbance and the long-term impact of early adversity. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2021.03.021 [DOI] [PubMed] [Google Scholar]
- Gelaye B, Kajeepeta S, Zhong Q, Borba C, Rondon M, Sánchez S, Henderson D, & Williams M (2015). Childhood abuse is associated with stress-related sleep disturbance and poor sleep quality in pregnancy. Sleep Medicine, 16, 1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Litz B, Hsu J, & Lombardo T (2004). Psychometric properties of the life events checklist. Assessment, 11(4), 330–341. 10.1177/1073191104269954 [DOI] [PubMed] [Google Scholar]
- Hobel CJ (1982). Identification of the patient at risk. In Bolognese RJ, Schwartz RH, & Schneider J (Eds.), Perinatal medicine: Management of the high risk fetus and neonate (pp. 3–28). Baltimore: Williams & Wilkins. [Google Scholar]
- Hoffman L (2015). Longitudinal analysis: Describing within-person changes over time. Routledge. 10.4324/9781315744094 [DOI] [Google Scholar]
- Howland MA, Sandman C, Davis EP, & Glynn LM (2020). Prenatal maternal psychological distress and fetal developmental trajectories: Associations with infant temperament. Development and Psychopathology, 32, 1685–1695. 10.1017/S095457942000142X [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2017). Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83, 1198–1202. 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- Lucena L, Frange C, Tufik S, & Hachul H (2018). Sleeping for two: The importance of good sleep during pregnancy. Women and Birth, 31, 142–143. 10.1016/j.wombi.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Lyu J, Zhu Q, Tong S, Su X, Li S, & Hua J (2020). Trajectories of sleep quality and associations with excessive gestational weight gain during pregnancy. Sleep and Biological Rhythms, 18, 249–257. [Google Scholar]
- Madigan S, Wade M, Plamodon A, Maguire J, & Jenkins J (2017). Maternal adverse childhood experience and infant health: Biomedical and psychosocial risks as intermediary mechanisms. The Journal of Pediatrics. 10.1016/j.jpeds.2017.04.052 [DOI] [PubMed] [Google Scholar]
- Masten A, & Cicchetti D (2016). Resilience in development: Progress and transformation. Developmental Psychopathology, 4, 271–333. 10.1002/9781119125556.devpsy406 [DOI] [Google Scholar]
- Menke R, Swanson L, Erickson N, Reglan G, Thompson S, Harris K, Rosenblum K, Lopez J, Muzik M, & WIMH Group. (2019). Childhood adversity and sleep are associated with symptoms severity in perinatal women presenting for psychiatric care. Archives of Women’s Mental Health, 22(4), 457–465 10.1007/s00737-018-0914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick J, Narayan A, Atzl V, Harris W, & Lieberman A (2020). Type versus timing of adverse and benevolent childhood experiences for pregnancy women’s psychological and reproductive health. Children and Youth Services Review, 114. 10.1016/j.childyouth.2020.105056 [DOI] [Google Scholar]
- Miller-Graff L, & Cheng P (2017). Consequences of violence across the lifespan: Mental health and sleep quality in pregnant women. Psychological Trauma: Theory, Research, Practice, and Policy, 9(5), 587–595. 10.1037/tra0000252 [DOI] [PubMed] [Google Scholar]
- Narayan A, Rivera L, Bernstein R, Harris W, & Lieberman A (2018). Positive childhood experiences predict less psychopathology and stress in pregnant women with childhood adversity: A pilot study of the benevolent childhood experiences (BCEs) scale. Child Abuse & Neglect, 78, 19–30. 10.1016/j.chiabu.2017.09.022 [DOI] [PubMed] [Google Scholar]
- Narayan AJ, Atzl VM, Merrick JS, Harris WW, & Lieberman AF (2020). Developmental origins of ghosts and angels in the nursery: Adverse and benevolent childhood experiences. Adversity and Resilience Science, 1, 121–134. [Google Scholar]
- Narayan AJ, Lieberman AF, & Masten AS (2021). Intergenerational transmission and prevention of adverse childhood experiences (ACEs). Clinical psychology review, 85, 101997. 10.1016/j.cpr.2021.101997 [DOI] [PubMed] [Google Scholar]
- Naud K, Ouellet A, Brown C, Pasquier J, & Moutquin M (2010). Is sleep disturbed in pregnancy? Journal of Obstetrics and Gynecology Canada, 32(1), 28–34. 10.1016/S1701-2163(16)34400-0 [DOI] [PubMed] [Google Scholar]
- Negriff S (2020). ACEs are not equal: Examining the relative impact of household dysfunction versus childhood maltreatment on mental health in adolescence. Social Science & Medicine, 245, 112696. 10.1016/j.socscimed.2019.112696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M, Carskadon M, Guilleminault C, & Vitiello M (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep, 27(1), 1255–1273. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- Okun M, Schetter CD, & Glynn L (2011). Poor sleep quality is associated with preterm birth. Sleep, 34(11), 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osnes R, Roaldest J, Follestad T, & Eberhard-Gran M (2019). Insomnia late in pregnancy is associate with perinatal anxiety: A longitudinal cohort study. Journal of Affective Disorders, 248, 155–165. 10.1016/j.jad.2019.01.027 [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Congdon RT (2021). HLM 8: Hierarchical linear and nonlinear modeling. Chapel Hill, NC: Scientific Software International, Inc. [Google Scholar]
- Slade A, & Cohen L (1996). The process of parenting and the remembrance of things past. Psychology, 17(3), 217–238. [DOI] [Google Scholar]
- Swales DA, Stout-Oswald SA, Glynn LM, Sandman C, Wing D, & Davis EP (2018). Exposure to traumatic events in childhood predicts cortisol production among high risk pregnant women. Biological Psychology, 139, 186–192. 10.1016/j.biopsycho.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr-Madsen L, Buliga E, Letorneau N, Campbell T, & Giesbrecht G (2015). Trajectories of sleep quality and associations with mood during the perinatal period. Sleep, 38(8), 1237–1245. 10.5665/sleep.4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulman T, Holle A, Torgersen L, Stoltenberg C, Reichborn-Kjennerud T, & Bulik C (2012). Sleep disturbances and binge eating disorder symptoms during and after pregnancy. Sleep, 35(10), 1403–1411. 10.5665/sleep.2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. (2020). How the Census Bureau measures poverty. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html
- van Dalfsen J, & Markus CR (2018). The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: A systematic review. Sleep Medicine Review, 39, 187–194. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013). The life events checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD. https://www.ptsd.va.gov. Accessed December 2021 [Google Scholar]
- Whitaker K, Zhang D, Kline C, Catov J, & Gibbs B (2021). Associations of sleep with sedentary behavior and physical activity patterns across pregnancy trimesters. Women’s Health Issues. in Press. 10.1016/j.whi.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MO, Masten AS, Narayan AJ (2013). Resilience Processes in Development: Four Waves of Research on Positive Adaptation in the Context of Adversity. In: Goldstein S, Brooks R (eds.), Handbook of Resilience in Children. Springer. 10.1007/978-1-4614-3661-4_2 [DOI] [Google Scholar]
- Zhong QY, Gelaye B, Sánchez SE, & Williams MA (2015). Psychometric Properties of the Pittsburgh Sleep Quality Index (PSQI) in a Cohort of Peruvian Pregnant Women. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 11(8), 869–877. 10.5664/jcsm.4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


