Abstract
Purpose:
Opioids and alcohol impact critical serotonin (5-HT) function in the developing placenta and fetus through the actions of immune proinflammatory factors. Yet, possible convergent effects of opioids and alcohol on human placental toll-like receptor 4 (TLR4) activation and subsequent 5-HT homeostasis remain entirely unknown. The purpose of this study was to examine the effect of prenatal exposure to opioids with or without prenatal alcohol exposure (PAE) on the expression of key placental immune and serotonin signaling factors in human placental tissue obtained from a well-characterized prospective cohort.
Methods:
Data were collected from a subset of participants enrolled in the prospective pre-birth Ethanol, Neurodevelopment, Infant, and Child Health (ENRICH-1) cohort. Women were recruited and classified into four study groups: 1) PAE (n = 20); 2) those taking medications for opioid use disorder (MOUD; n = 28), 3) concurrent PAE and MOUD (n = 20); and 4) controls (HC; n= 20) based on prospective, repeated self-report, and biomarker analysis. Placenta samples underwent tissue processing to identify mRNA for TLR4, nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), serotonin transporter (SERT), tryptophan hydroxylase (TPH1), indoleamine 2,3-Dioxygenase 1 (IDO) as well as protein concentrations of TLR4, IL-1β, TNF-α, SERT. To consider the association between study group and mRNA/protein expression of our targets, multivariable regression models were developed with inclusion of a priori selected covariates.
Results:
There was a significant negative association between PAE and SERT mRNA (β = −0.01; p < .01) and a positive association with TPH1 mRNA expression (β = 0.78; p < .05). In addition, there was a negative association between MOUD and TNF-α protein expression (β = −0.12; p < .05).
Conclusions:
This study provides the first evidence that PAE may inhibit SERT expression while simultaneously promoting increased TPH1 protein expression in human placenta. This may result in increased 5-HT in fetal circulation known to affect neurodevelopment. Our data suggest that opioids and alcohol may disturb the bidirectional, dynamic interaction between the placental immune and serotonin system. Given the implication for brain development and health across the life-span further investigation of these critical mechanisms in well-defined cohorts is required.
Keywords: medications for opioid use disorder (MOUD), prenatal alcohol exposure (PAE), placenta, immune, cytokines, serotonin
1. Introduction
Extensive evidence supports the adverse effects of opioid use during pregnancy on the health of women and infants (Piske et al., 2021; Tobon et al., 2019). The long-term consequences associated with the in utero exposure to medications for opioid use disorder [(MOUD), i.e., buprenorphine or methadone] remain uncertain given the influence of possible confounding factors, although some adverse effects have been reported (Bakhireva et al., 2019; Labella et al., 2021). Furthermore, 48% of pregnant women who reported nonmedical use of opioids in the past 30 days also reported concurrent alcohol use (Kozhimannil et al., 2017). In utero alcohol exposure is associated with a continuum of disabilities including structural, neurocognitive, and behavioral deficits collectively known as fetal alcohol spectrum disorder (FASD) (Wozniak et al., 2019). These adverse outcomes, may be mediated, in part, by impaired placental function. Specifically, prenatal opioid and alcohol exposures may perturb the bidirectional, dynamic interaction between the placental immune and serotonin system.
The placenta is a critical organ that facilitates the exchange of gases, nutrients, waste, and hormones between the mother and the developing fetus (Burton & Fowden, 2015). At the maternal-fetal interface, placental cells are bathed by maternal blood thereby allowing for direct transfer of maternal substances to the fetus (Burton & Fowden, 2015). Furthermore, teratogens such as alcohol and possibly opioids (Conradt et al., 2018), may exert indirect fetal effects via altered placental function. Critically, the placenta modulates fetal brain development through adaptive responses to changes in the maternal milieu (Nugent & Bale, 2015; St-Pierre et al., 2015), and such changes in placental function have been associated with altered infant neurobehavioral development (Green et al., 2015; Marsit et al., 2012). However, the impact that opioids and alcohol exert on placental function is relatively unknown.
Clinical evidence suggests that alcohol, and more recently opioids, directly impact fetal central nervous system (CNS) development (Radhakrishnan et al., 2021; Wozniak et al., 2019) Emerging clinical (Bodnar et al., 2020) and preclinical studies (Bodnar et al., 2016; Noor & Milligan, 2018; Sanchez et al., 2017) suggest in utero alcohol and/or opioid exposure alters neurological and neuroimmune function across life. Preclinical studies demonstrate that opioids, including buprenorphine and methadone, and alcohol activate the innate immune pattern recognition receptor (PRR), toll-like receptor 4 (TLR4), triggering the production of downstream proinflammatory factors thereby offering a framework to examine the consequences of these teratogens (Hutchinson et al., 2010; Pascual et al., 2017). It has been hypothesized that production of such immune factors, including those produced by placental cells (Edlow et al., 2019), may subsequently result in abnormal development of fetal microglia (Bilbo et al., 2018). Microglia are the brain’s resident immune cells and play a key role in healthy brain development and maintenance. In addition, because microglia are believed to be long-lived cells, Bilbo and colleagues (2018) postulate that changes in the structure or function of these cells have the potential to significantly alter neuroimmune function and behavior across the life span. While this work is predominantly preclinical, it provides compelling reasons for translation to clinical research with human populations.
Consequences of TLR4 activation, and the resultant proinflammatory factors, have recently been extended to include perturbing the expression and action of rate limiting transporters and enzymes of the serotonin (5-hydroxytryptamine; 5-HT) system (Williams et al., 2017). Serotonin derived from the placenta is the dominant source to the fetus during early brain development linking placental function to fetal neural development (Bonnin et al., 2011; Velasquez et al., 2013; Wu et al., 2016). The placenta synthesizes de novo 5-HT from maternal l-tryptophan via enzymatic activity of tryptophan hydroxylase (TPH1). Under normal physiological conditions, the idoleamine 2,3-dioxygenase (IDO) pathway competes with the metabolic pathway of tryptophan modulating 5-HT availability. Preclinical data provide evidence that inflammatory factors from immune TLR3 stimulation alter expression of placental TPH1 leading to changes in 5-HT homeostasis and ultimately disruption in neurodevelopment (Goeden et al., 2016). Furthermore, intrauterine inflammation from TLR4 activation by lipopolysaccharide (endotoxin) administration resulted in upregulation of IDO in rabbit placenta and fetal brain resulting in an abnormal shift of tryptophan metabolism in the brain (Williams et al., 2017).
Additionally, human placenta maintains 5-HT homeostasis through modulation of the uptake and metabolism of 5-HT from both maternal and fetal circulation. The organic cation transporter 3 (OCT3) is emerging as a possible player in removal of 5-HT from fetal circulation at term, while the 5-HT transporter (SERT) extracts 5-HT from the maternal circulation (Karahoda et al., 2020b). Once transported inside trophoblast cells, 5-HT is then subsequently degraded to an inactive metabolite by monoamine oxidase A (MAO-A) (Karahoda et al., 2020a). Preclinical models showed that TLR4 activation downregulates SERT activity and expression in human intestinal epithelial cells (Layunta et al., 2018; Mendoza et al., 2009). This suggests the possibility that opioids and alcohol may negatively affect critical 5-HT function in the developing placenta and fetus through the actions of proinflammatory immune signaling molecules.
In light of this background, we examined the effect of MOUD and PAE, both separately and in combination, on the expression of key placental immune factors indicative of TLR4 activation in addition to key 5-HT signaling factors in human placental tissue obtained from a well-characterized prospective cohort. We hypothesized that prenatal MOUD and/or alcohol use would engage the TLR4 pathway resulting in dysregulated immune cytokine expression and placental 5-HT system function. As both MOUD and PAE alone activate TLR4, we further hypothesized that this relationship would be magnified in placental tissue exposed to both substances.
2. Materials and Methods
2.1. Description of the parent ENRICH cohort
To accomplish our goal, we leveraged previously collected data and biological specimens from the well-characterized University of New Mexico (UNM) cohort, the Ethanol, Neurodevelopment, Infant, and Child Health (ENRICH-1) study. The ENRICH-1 protocol, and additional placenta analyses, have been approved by the UNM Human Research Protection Program, (UNM HRRC #12-390 and #19-222); all participants gave informed consent inclusive of placental tissue analysis. The ENRICH study methodology has been extensively described elsewhere (Bakhireva et al., 2015). Briefly, participants were recruited from UNM affiliated prenatal care clinics, including general obstetric clinics and a specialty clinic that provides prenatal and postpartum care to women with substance use disorders. Eligibility criteria for the parent cohort were: 1) at least 18 years old, 2) singleton pregnancy, 3) residing and planning to stay in the Albuquerque metropolitan area for two years, 4) ability to give informed consent in English. The following exclusion criteria were applied: 1) fetal diagnosis of structural anomaly; 2) greater than one urine drug test or more than monthly frequency (per self-report) use of cocaine, methamphetamines, or MDMA during the first trimester and no use in the second or third trimesters (ascertained via positive study urine drug screen, self-report, and/or medical record review). Women were recruited into four mutually exclusive groups 1) prenatal alcohol exposure (PAE), 2) receiving medications for opioid use disorder [(MOUD) buprenorphine and/or methadone] no alcohol use in pregnancy, 3) PAE and MOUD, and 4) no prenatal substance use (HC).
2.2. MOUD, Alcohol, and covariate measures
Eligibility for the alcohol-exposed groups (PAE, MOUD + PAE) included: 1) self-report of ≥ 3 drinks/week or > 2 binge drinking episodes (defined as ≥ 4 drinks/occasion) during the month around the last menstrual period (LMP); 2) alcohol use after the periconceptional period ascertained by prospective, repeated Timeline Follow-Back (TLFB) interviews and/or positive ethanol biomarkers. Participants in the opioid exposed groups (MOUD, MOUD + PAE) were required to be currently receiving MOUD with or without additional opioid use. Participants in the HC group were lifetime non-users of illicit drugs and tobacco, reported no alcohol use in pregnancy (confirmed by biomarkers), and no more than minimal alcohol use during the periconceptional period (≤ 2 standard drinks/week on average and no binge drinking episodes.
Alcohol, MOUD, and other substance use exposures in pregnancy were prospectively and repeatedly assessed by self-report (TLFB interviews) and medical record review occurring at baseline, throughout pregnancy, and at the time of delivery to confirm ongoing group eligibility. Self-report of alcohol, MOUD, other substance use exposure was confirmed by biomarker analysis at enrollment and time of delivery. Frequency and quantity of alcohol use was converted to absolute alcohol per day (AA/day) (Jacobson et al., 2002). Maternal serum, whole blood, and urine were collected at enrollment and at the time of delivery and analyzed at the U. S. Drug Testing Laboratory (Des Plaines, IL). Maternal blood biomarkers included phosphatidylethanol (PEth), gamma-glutamyltranspeptidase (GGT), and carbohydrate-deficient transferrin (% dCDT). Urine biomarkers included ethylglucuronide (uEtG), ethylsulfate (uEtS), urine drug screen (UDS)-7 (amphetamines, barbiturates, benzodiazepines, cocaine, opiates, PCP, cannabinoids/THC), and nicotine metabolites. Finally, alcohol exposure was confirmed by PEth in dried blood spots collected from the newborn during a routine medical screening.
2.3. Selection of subjects for placental study
The ENRICH-1 is a prospective cohort with four study visits: prenatal (Visit [V] 1), delivery/hospital stay (V2), 6-month infant evaluation (V3), and 20-month infant evaluation (V4). For the purposes of this study, data from V1 and V2 were used. Additional eligibility criteria for this study included banked placenta samples. Placenta samples were collected from more than 80% of participants who delivered. From these samples, we randomly selected 28 subjects in the MOUD group (to have equal representation of participants treated with methadone and buprenorphine), and 20 per group in 3 remaining groups resulting in a total sample size of 88.
2.4. Tissue collection for RNA and protein analysis
Placenta were collected immediately following delivery and placenta tissue samples were generally dissected within 2 hours. After removing the decidua basalis membrane, small samples one-half inch in diameter were taken from the maternal side of the placenta avoiding major vessels. Samples were rinsed in saline and blotted to remove excess blood and fluid. Samples were then placed in polypropylene tubes, frozen with liquid nitrogen, and stored in air-tight containers at −80 °C until analysis (Holbrook et al., 2019).
2.5. Total RNA isolation
Total RNA was extracted from placental tissue using methods previously described (Noor et al., 2019; Vanderwall et al., 2018) with minor modifications described here. Extraction was performed using the RNeasy Mini Kit (Qiagen; Cat #217004) per manufacturer’s instructions except where noted. Homogenization was performed using a cordless motor pestle system (VWR; cordless pestle motor: Cat#47747–370; 1.5 mL microtubes: Cat#47747–362; 1.5 mL pestle: Cat#47747–358; and 1.5 pestle and microtube combo: Cat#47747–366). Placenta tissue was transferred to microtubes prior to homogenization. Subsequently, 200 μl of Qiazol Lysis Reagen (Qiazol; Qiagen; Cat#79306) was added to the tube containing the tissue and the placenta tissue was chopped quickly using scissors for 1 minute. Placenta tissue was then homogenized with the motorized pestle for 1 minute.
Minor changes were incorporated for mRNA extraction using the RNeasy kit as follows. An initial homogenization in 200 μl of Qiazol occurred. The final volume was increased to 700 μl of Qiazol following homogenization. Samples were vortexed and incubated at room temperature (RT) for 7 minutes, followed by the addition of 140 μl of chloroform (Sigma-Aldrich; Cat#C2432). Samples were vigorously shaken by hand for 15s, incubated at RT for 4 minutes, hand-shaken vigorously for 10s, and then centrifuged in 4° C 12,000x g for 15 minutes. The aqueous layer was extracted and placed into a clean RNase/DNase/Protease free 1.5 ml tube and 1.5 x aqueous layer (450 μl) of 100% Ethanol (VWR, Cat#71006-012) was added to the tube, repeatedly pipetted 6 x to mix, moved to collection columns, and centrifuged in ~20° C at 9,000 x g for 30s.
This was followed by a wash of 700 μL of RWT (provided with Qiagen kit) and centrifuged (~20 °C at 9,000 × g, 30 s), washed 2 × with 500 μL RPE (provided with Qiagen kit) and centrifuged (~20 °C at 9,000 × g, 30 s) after each, and washed 2 × with 500 μL 80% EtOH (100% EtOH diluted with sterile RNase/DNase/Protease free water; Sigma-Aldrich; Cat#W4502), and centrifuged (~20 °C at 9,000 × g, 2 min) after each. Caps were cut from columns and samples were dried by centrifugation (~20 °C at 20,627 × g, 12 min), and placed into RNA collection tubes with 30 μL sterile water (provided with Qiagen kit) added directly to the column filter, and centrifuged (~20 °C at 20,627 × g, 1 min). The concentration and quality of the total RNA was assessed by NanoDrop (Thermo Scientific, MA, USA).
2.6. mRNA analysis by quantitative real-time PCR
Total RNA samples were diluted to a standardized RNA concentration of 190-210 ng/μl. Total RNA (1.2 μg) was used to synthesize cDNA. For reverse transcription (cDNA), SuperScript™ IV VILO™ cDNA Synthesis Kit (Invitrogen) was used per manufacturer’s instructions. Levels of mRNA transcripts were measured and analyzed, as previously described (Mellios et al., 2014; Vanderwall et al., 2018). The following dilution factor was applied to cDNA samples for assessment of transcripts of interest in given placenta tissue 1:2.5. The 1:200 dilutions of cDNA were used for assessment of the normalizer transcripts (18s RNA) for each of the tissue samples. Levels of gene expression of key downstream inflammatory factors indicative of TLR4 ligation, includingTLR4, NLRP3, IL-1β, TNF-α, as well as gene expression of the key transporter SERT, and rate limiting enzymes of the 5-HT pathway, TPH1, and IDO1 mRNAs, as well as the normalizer 18s rRNA (Rn18s), were assayed in triplicate via quantitative real-time PCR (qRT-PCR) with Taqman Gene Expression Assays (cat# 4351370, ThermoFisher Scientific). In cases of triplicates with standard deviation of more than 0.1, the average value of the two closest replicates was retained. All selected gene expression assays were identified by the manufacturer to be the “best coverage” assays, unless otherwise noted, and designed to exclude detection of genomic DNA. mRNA levels were analyzed with the formula: C=2^CTnormalizer/2^CTtarget, as previously described (Livak & Schmittgen, 2001; Mellios et al., 2014).
2.7. Determination of placental protein
Frozen placenta samples were homogenized in Pierce IP Lysis buffer with protease inhibitor (Thermo Cat#87788) while on ice in the following manner. 200 μl of lysis buffer with protease inhibitor was added to the tube containing the tissue and the placenta tissue was chopped quickly using scissors for 1 minute. Placenta tissue was then homogenized with the motorized pestle for 1 minute. The final volume was increased to 1000 μl of lysis buffer with protease inhibitor following homogenization. Samples were vortexed and affixed to a rotator at 5° C. This was repeated every 20 minutes for a total of 60 minutes. Samples were subsequently sonicated in a water bath sonicator for 10 minutes, in ice-cold water. Tissue samples were then centrifuged at 14,000 x g for 15 minutes to pellet cellular debris. Cellular lysates (supernatant) were collected in a new set of tubes and stored at − 80° C avoiding freeze/thaw cycles. Total protein concentrations were measured by Quickstart™ Bradford Protein Assay Kit (Biorad, CA, USA) according to manufacturer instructions. All total protein samples were then standardized to 1.2 mg/ml, saved in separate aliquots and stored at −80° C avoiding freeze/thaw cycles.
Upon activation, TLR4 will produce changes in a number of factors. The targets selected are the ones most well-known. Target protein levels were measured using commercially available ELISA kits for detecting human TLR4, IL-1β, TNF-α, and SERT (R&D Systems; Minneapolis, USA and Novus Biologicals, CO, USA). Standards and each experimental sample were run in duplicate on the 96-well ELISA plate. Equal volumes (75 ul or 50 ul) of standardized total protein aliquots were used in ELISA plate wells. All experiments and data analysis were performed according to manufacturer instructions. Average intra-assay coefficient of variation was 10% and inter-assay coefficient of variation was 9.8%.
2.8. Statistical analyses
We are aware of only one prior human study (Holbrook et al, 2019) which compared placenta proinflammatory proteins among PAE and non-PAE subjects. Means and standard deviations (SD) presented for IL-1β and TNF-α were used to conduct power calculations. Using the large effect size (Cohen’s d=0.82) reported for TNF-α, 19 subjects per group would be needed to achieve 80% power using a t-test for differences between two independent means with an alpha level of 0.05. However, a much smaller difference reported for IL-1β (Cohen’s d=0.39) would require 82 subjects per group. The sample size of 88 subjects available for the present analysis was expected to detect moderate-to-large effect size.
Descriptive statistics of means and SD were calculated for all interval-level variables and frequencies and percentages were calculated for categorical variables. Maternal socio-demographic and medical characteristics were compared across the four groups, using univariate measures appropriate to level of measurement, Chi-square test or Fisher’s exact test for categorical variables and one-way analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables. Raw data for mRNA and protein values are presented as mean +/− SD.
Associations between maternal primary exposures of interest (MOUD and PAE) and placental biomarkers (mRNA and protein expression) were examined using ANOVA followed by multivariable regression analysis. Main effects (MOUD and PAE as well as MOUD-by-PAE interaction) were included in the models along with covariates known to be associated with key dependent variables. A priori chosen covariates included maternal pre-pregnancy body mass index (BMI), maternal age, prenatal tobacco use, maternal perceived stress (PSS) at delivery, maternal viral hepatitis C infection, infant birth weight, type of delivery (vaginal vs. cesarean section), infant sex, gestational age at birth, and selective serotonin reuptake inhibitor (SSRI) exposure. Normality was examined with QQ-plots, Breusch-Pagan test and Shapiro-Wilk test. After careful examination of mRNA and protein model residual plots, Box-Cox procedures were performed on all variables. Values for mRNA and protein were natural log transformed with the exception of SERT mRNA, which required a square root transformation. Akaike’s Information Criteria (AIC), Bayesian Information Criteria (BIC), and adjusted R2 with backward and forward selection were used to identify the most parsimonious reduced model. One outlier (Cook’s d = 0.22) for NLRP3 mRNA was excluded from multivariable analysis. All analyses were performed in R.
3. Results
3.1. Socio-demographic and clinical characteristics
Participant socio-demographic and clinical characteristics by study group are shown in Table 1. The majority of participants identified as White (90%) and of Hispanic, Latino, or Spanish (68%) descent. Study groups did not significantly differ by maternal age, race or ethnicity, BMI, and type of delivery (p > 0.05). Significant differences in marital status, education level, employment status, insurance status, and perceived stress were noted among groups. No significant differences were observed in medical characteristics among the study groups except hepatitis C that was prevalent among patients in the MOUD (35.7%) and MOUD + PAE (35%) groups. Infants in both MOUD groups had lower birth weight, while there was no significant difference in the incidence of preterm birth among groups. There was a high prevalence of respiratory distress and neonatal infection requiring treatment in infants classified into the exposure groups.
Table 1.
Maternal and infant characteristics by group
| Variable | Controls (n=20) |
MOUD (n=28) |
MOUD + PAE (n=20) |
PAE (n=20) |
p |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Maternal age (years) | 26.6 (4.0) | 27.3 (4.8) | 27.4 (5.7) | 28.5 (5.0 ) | 0.741 |
| Perceived Stress Score | 10.5 (6.0) | 15.5 (7.0) | 15.7 (5.2) | 16.3 (6.3) | 0.0211 |
| Gestational age at recruitment | 25.8 (9.4) | 22.1 (7.0) | 22.9 (7.2) | 23.6 (7.5) | 0.521 |
| n (%) | n (%) | n (%) | n (%) | ||
| Ethnicity (Hispanic/Latina) | 14 (70.0%) | 19 (67.9%) | 16 (80.0%) | 11 (55.0%) | 0.402 |
| Race: | 0.323 | ||||
| White | 19 (95.0%) | 26 (92.9%) | 19 (95.0%) | 16 (80.0%) | |
| Black or African American | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | |
| American Indian or other | 0 (0.0%) | 2 (7.1%) | 1 (5.0%) | 3 (15.0%) | |
| Marital status: | ≤0.0013 | ||||
| Single/ separated/ divorced | 3 (15.0%) | 10 (35.7%) | 6 (30.0%) | 0 (0.0%) | |
| Married/ cohabitating | 17 (85.0%) | 18 (64.3%) | 14 (70.0%) | 20 (100.0%) | |
| Education level: | ≤0.0013 | ||||
| High school or less | 7 (35.0%) | 16 (57.1%) | 12 (60.0%) | 4 (20.0%) | |
| Some college or vocational | 13 (65.0%) | 11 (39.3%) | 6 (30.0%) | 6 (30.0%) | |
| College degree or higher | 0 (0.0%) | 1 (3.6%) | 2 (10.0%) | 10 (50.0%) | |
| Currently employed: | 12 (60.0%) | 13 (46.4%) | 6 (30.0%) | 17 (85.0%) | 0.0033 |
| Health insurance: | <0.0012 | ||||
| Self-purchased/other/none | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | |
| Employer-based insurance | 8 (40.0%) | 1 (3.6%) | 1 (5.0%) | 9 (45.0%) | |
| Medicaid | 11 (55.0%) | 27 (96.4%) | 19 (95.0%) | 10 (50.0%) | |
| Maternal body mass index: | 0.213 | ||||
| < 30 | 13 (65.0%) | 24 (85.7%) | 19 (95.0%) | 17 (85.0%) | |
| ≥ 30 | 3 (15.0%) | 3 (10.7%) | 0 (0.0%) | 2 (10.0%) | |
| ≥ 35 | 4 (20.0%) | 1 (3.6%) | 1 (5.0%) | 1 (5.0%) | |
| Type of Delivery: | 0.623 | ||||
| Vaginal | 19 (95.0%) | 25 (89.3%) | 17 (85.0%) | 19 (95.0%) | |
| Cesarean section | 1 (5.0%) | 3 (10.7%) | 3 (15.0%) | 1 (5.0%) | |
| Co-morbidities: | |||||
| Hepatitis C | 0 (0.0%) | 10 (35.7%) | 7 (35.0%) | 0 (0.0%) | <.00013 |
| Hypertensive disorders | 1 (5.0%) | 8 (28.6%) | 2 (10.0%) | 3 (15.0%) | 0.153 |
| Gestational diabetes | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.623 |
| Infant Characteristics | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Gestational age at birth (weeks) | 38.8 (1.6) | 38.1 (1.5) | 38.5 (1.6) | 39.3 1.4) | |
| Birthweight (grams) | 3448.2 (636.9) | 2856.6 (403.9) | 2927.1 (522.9) | 3240.3 (504.9) | ≤0.0011 |
| n (%) | n (%) | n (%) | n (%) | ||
| Infant sex: male | 11 (55.0%) | 15 (53.6%) | 9 (45.0%) | 12 (60.0%) | 0.822 |
| APGAR score at 1 min <7 | 2 (10.0%) | 2 (7.1%) | 3 (15.0%) | 4 (20.0%) | 0.623 |
| APGAR score at 5 min <7 | 0 (0.0%) | 3 (10.71%) | 0 (0.0%) | 1 (5.0%) | 0.623 |
| Respiratory distress | 0 (0.0%) | 8 (28.6%) | 6 (30.0%) | 8 (40.0%) | ≤0.0013 |
| NOWS | -- | 13 (46.4%) | 5 (25%) | -- | <.00013 |
| Neonatal infection requiring treatment | 1 (5.0%) | 9 (32.1%) | 3 (15.0%) | 5 (25.0%) | 0.113 |
| Preterm delivery (<37 weeks) | 2 (10.0%) | 3 (10.7%) | 2 (10.0%) | 2 (10.0%) | 1.003 |
based on Kruskal-Wallis test;
based on Chi-square test;
based on Fisher’s exact test;
MOUD – medications for opioid use disorder; PAE – prenatal alcohol exposure; NOWS – neonatal opioid withdraw syndrome
Patterns of maternal substance use are presented in Table 2. In the MOUD group, 50% of patients were on methadone and 50% on buprenorphine, while in the MOUD + PAE group a higher proportion of patients were on buprenorphine (60%). Marijuana use was highly prevalent in the exposed groups (28.7% in the MOUD group, 60% in the MOUD + PAE group, and 50% in the PAE group). Tobacco use was also prevalent in both MOUD groups (55% in the MOUD + PAE group and 39.3% in the MOUD group). Alcohol use in the periconceptional period was high in the MOUD + PAE group (4.39 AA/day) and in the PAE group (0.82 AA/day) but the use substantially decreased in pregnancy (to 1.46 AA/day and 0.28 AA/day in the in MOUD + PAE and PAE groups, respectively). Per study eligibility criteria, alcohol use in the MOUD and Control groups was minimal in periconceptional period and 0 AA/day during pregnancy.
Table 2.
Alcohol and substance use co-exposures by group
| Controls (n=20) |
MOUD (n=28) |
MOUD + PAE (n=20) |
PAE (n=20) |
p | |
|---|---|---|---|---|---|
|
|
|||||
| n (%) | n (%) | n (%) | n (%) | ||
| MOUD | |||||
| Methadone only | -- | 14 (50.0%) | 6 (30.0%) | -- | <.00013 |
| Buprenorphine only | -- | 14 (50.0%) | 12 (60.0%) | -- | <.00013 |
| Other opioids | |||||
| Heroin | 0 (0.0%) | 9 (32.1%) | 9 (45.0%) | 0 (0.0%) | <.00013 |
| Misuse/use of prescription opioids | 2* (10.0%) | 3 (10.7%) | 9 (45.0%) | 2(10.0%) | .0133 |
| Marijuana | 0 (0.0%) | 8 (28.7%) | 12 (60.0%) | 10(50.0%) | <.00013 |
| Benzodiazepines | 0 (0.0%) | 4 (14.3%) | 7 (35.0%) | 0 (0.0%) | .00113 |
| Sedatives | 0 (0.0%) | 1 (3.57%) | 1 (5.00%) | 1 (5.0%) | 1.003 |
| SSRI | |||||
| Tobacco | 0 (0.0%) | 11 (39.3%) | 11 (55.0%) | 0 (0.0%) | <.00013 |
| Alcohol | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| AA/day around LMP (±2weeks) | 0.01 (0.0) | 0.0 (0.0) | 4.39 (7.1) | 0.82(0.7) | <.00011 |
| AA/day 30 days prior to enrollment | 0.0 (0.0) | 0.0 (0.0) | 0.001 (0.0) | 0.015(0.02) | <.00011 |
| AA/day 30 days prior to delivery | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.012(0.02) | <.00011 |
| AA/day across periconceptional period and pregnancy | 0.0 (0.0) | 0.0 (0.0) | 1.46 (2.3) | 0.28 (0.23) | <.00011 |
based on Kruskal-Wallis test,
based on Chi-square test,
based on Fisher’s exact test;
MOUD, medications for opioid use disorder; PAE – prenatal alcohol exposure; AA, absolute ounces of alcohol (1 standard drink equals approximately 0.5 AA); LMP, last menstrual period disorder; SSRI, selective serotonin reuptake inhibitor;
short-term use of opioid analgesics as prescribed
3.2. Maternal substance exposure alters placental mRNA expression
Raw mRNA expression data (prior to logit transformation) for all targets are presented in Figure 1. ANOVA showed a significant difference in NLRP3 mRNA expression among groups (p = 0.038). In addition, there was a significant difference in SERT mRNA expression among groups (p = 0.023).
Figure 1.
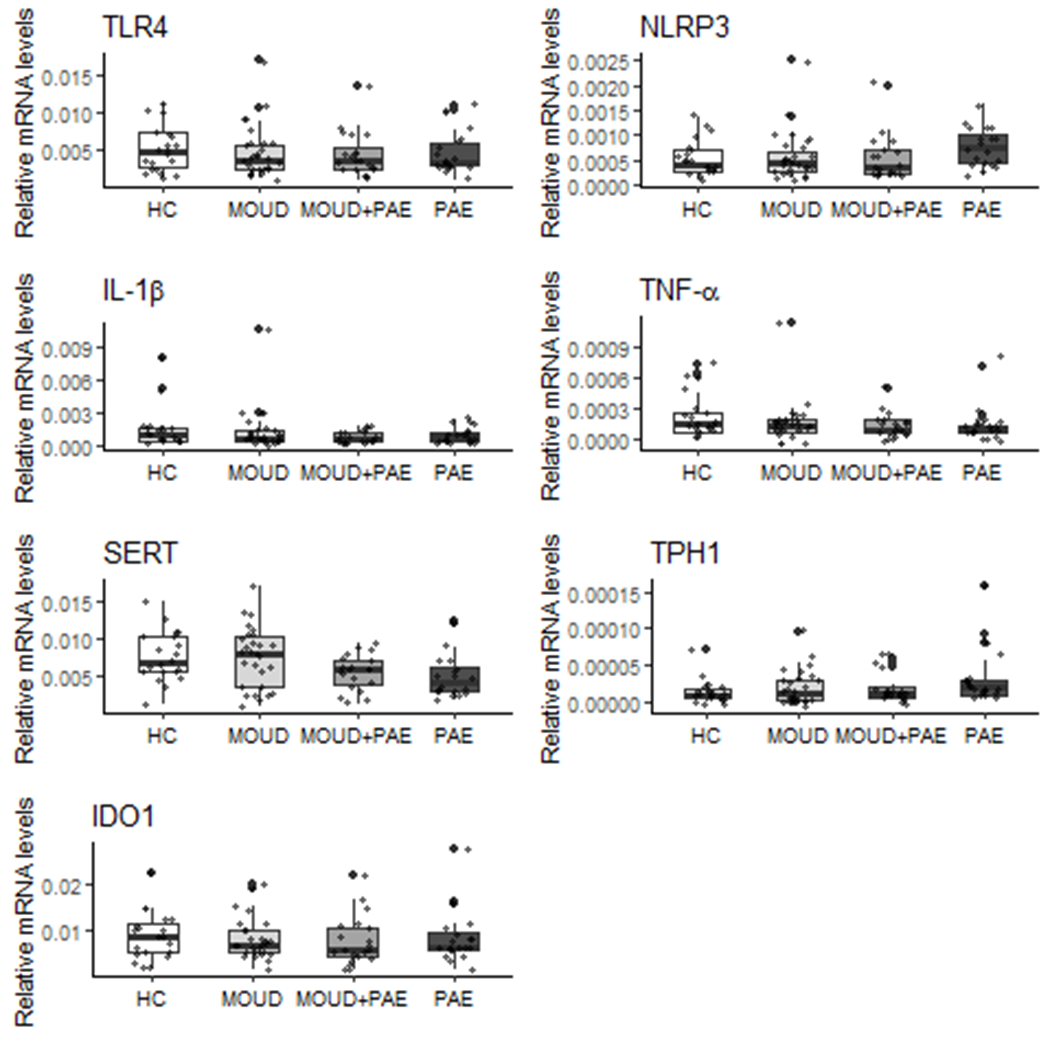
Effect of prenatal MOUD and Alcohol use on human placental mRNA expression. The equation C=2^Tnormalizer/2^Ttarget was used to quantify mRNA levels normalized to the housekeeping gene 18s. Raw data, prior to logit transformation, are presented as mean ± SD. Preliminary ANOVA analyses yielded significant main effects of group on NLRP3 and SERT mRNA despite appearance of outliers.
3.2.1. Effect of maternal MOUD and PAE on mRNA expression of placental immune factors indicative of TLR4 ligation
Results of the final multivariable regression models are shown in Table 3. Regression analysis demonstrated a trend for a positive association between PAE group and NLRP3 mRNA (β = 0.41; p < 0.1) and a negative association with the MOUD + PAE group (β = −0.54; p < 0.1). Additionally, there was a trend for a negative association between PAE and IL-1β mRNA (β = −0.34; p < 0.1), and TNF-α mRNA (β = −0.32; p < 0.1). Interestingly, there was a significant interaction between the study group and infant birthweight with respect to placental TNF-α mRNA expression (β = −0.0004; p < .05). The final model for IL-1β containing the study groups, infant birth weight, and the mode of delivery was statistically significant (p < 0.05) and explained 12% variance in the IL-1β expression.
Table 3.
Multiple regression analysis placental mRNA expression of key immune and serotonin signaling factors with selected covariates
| Predictors |
Placenta mRNA biomarkers
|
||||||
|---|---|---|---|---|---|---|---|
| TLR4 | NLRP3 | IL-1β | TNF-α | SERT | TPH1 | IDO1 | |
|
|
|||||||
| β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
|
| MOUD | −0.09 | 0.03 | −0.27 | −0.29 | 0.01 | −0.03 | −0.11 |
| (−0.35, 0.17) | (−0.36, 0.42) | (−0.67, 0.13) | (−0.65, 0.08) | (−0.003, 0.02) | (−0.65, 0.59) | (−0.36, 0.14) | |
| PAE | −0.03 | 0.41* | −0.34* | −0.32* | −0.01*** | 0.78** | −0.10 |
| (−0.29, 0.23) | (−0.01, 0.84) | (−0.71, 0.02) | (−0.65, 0.01) | (−0.02, −0.01) | (0.15, 1.41) | (−0.35, 0.16) | |
| MOUD x PAE | −0.54* | −0.76* | |||||
| (−1.12, 0.03) | (−1.61, 0.10) | ||||||
| Maternal BMI | 0.03* | ||||||
| (−0.004, 0.06) | |||||||
| Birthweight | −0.0003* | −0.0004** | |||||
| (−0.001, 0.0000) | (−0.001, −0.0000) | ||||||
| Model of delivery: | −0.70** | ||||||
| C-section vs vaginal | (−1.34, −0.06) | ||||||
| Maternal age | 0.001** | ||||||
| (0.0002, 0.002) | |||||||
| Infant sex: male | 0.27 | −0.01 | −0.23* | ||||
| (−0.08, 0.61) | (−0.02, 0.001) | (−0.48, 0.02) | |||||
| Hepatitis C | −0.02*** | 0.68** | |||||
| (−0.03, −0.004) | (0.07, 1.28) | ||||||
| Constant | −5.45*** | −7.74*** | −5.87*** | −8.50*** | 0.06*** | −11.57*** | −4.73*** |
| (−5.68, −5.22) | (−8.04, −7.44) | (−7.12, −4.62) | (−9.76, −7.23) | (0.03, 0.08) | (−12.02, −11.13) | (−5.00, −4.46) | |
|
| |||||||
| R2 | 0.01 | 0.07 | 0.12 | 0.12 | 0.24 | 0.12 | 0.05 |
| Adjusted R2 | −0.02 | 0.04 | 0.08 | 0.07 | 0.19 | 0.08 | 0.01 |
| F Statistic | 0.24 (df = 2; 85) |
2.15* (df = 3; 83) |
2.83** (df = 4; 83) |
2.21* (df = 5; 82) |
5.11*** (df = 5; 82) |
2.83** (df = 4; 83) |
1.39 (df = 3; 84) |
Note:
p<0.1
p<0.05
p<0.01;
values were natural log transformed with the exception of SERT mRNA, which required a square root transformation ^(0.5); Main effects (MOUD and PAE as well as MOUD-by-PAE interaction) as well as a priori covariates (maternal pre-pregnancy body mass index (BMI), maternal age, prenatal tobacco use, maternal perceived stress (PSS) at delivery, maternal viral hepatitis C infection, infant birth weight, type of delivery (vaginal vs. cesarean section), infant sex, gestational age at birth, SSRI exposure) were included in all models. Variables included in the final models shown were selected by Akaike information criteria, Bayesian information criteria, and adjusted R2 with backward and forward selection; MOUD – medications for opioid use disorder; PAE – prenatal alcohol exposure; SSRI – selective serotonin reuptake inhibitor
3.2.3. Effect of maternal MOUD and PAE on mRNA expression of key placental 5-HT signaling factors
Maternal substance use also had an effect on factors in the serotonin system. The R2 for the final SERT mRNA model containing study groups, maternal age, infant sex, and hepatitis infection was 0.24 (p < .01) which suggests this model explains 24% of variance in SERT mRNA. Importantly, there was a significant and independent effect of alcohol on SERT mRNA (β = −0.01; p < .01) when all other factors were held constant. In addition, PAE had a large significant and independent effect on TPH1 mRNA expression (β = 0.78; p < .05).
3.3. Maternal substance exposure alters placental protein expression
Raw data for all proteins (prior to logit transformation) are presented in Figure 2. Final multivariable regression models are shown in Table 4. There was a significant negative association between MOUD and TNF-α protein expression (β = −0.12; p < .05). The multivariable models with the study groups and covariates were significant for TNF-α (R2 = 0.12; p < 0.05) and IL-1β (R2 = 0.15; p < 0.05). When all factors were held constant, a significant group by infant sex interaction as well as group by birthweight interaction with respect to IL-1β protein expression (β = −0.26; p < 0 .01) were observed. Final models for TLR4 and SERT were not significant across exposure groups.
Figure 2.
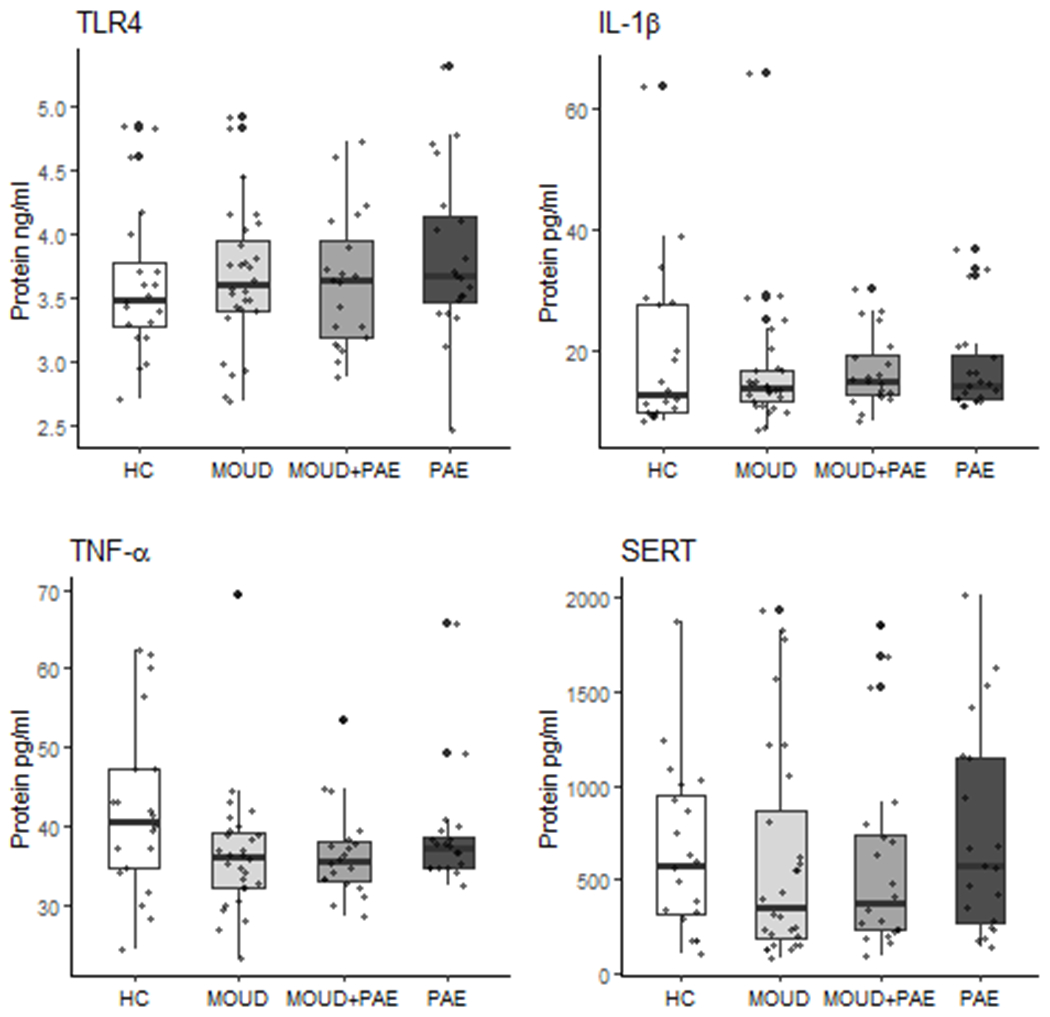
Effect of prenatal MOUD and Alcohol use on human placental protein expression. Raw data, prior to logit transformation, are presented as mean ± SD. Preliminary ANOVA analyses revealed no significant differences among groups
Table 4.
Multiple regression analysis of placental protein expression of key immune and serotonin signaling factors with select covariates
| Predictors |
Placental protein biomarkers
|
|||
|---|---|---|---|---|
| TLR4 | IL-1β | TNF-α | SERT | |
|
|
||||
| β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
|
| MOUD | −0.02 | 0.14 | −0.12** | −0.24 |
| (−0.09, 0.05) | (−0.08, 0.37) | (−0.21, −0.02) | (−0.60, 0.11) | |
| PAE | 0.02 | 0.04 | −0.04 | 0.08 |
| (−0.05, 0.08) | (−0.15, 0.23) | (−0.13, 0.04) | (−0.28, 0.44) | |
| Birthweight | 0.0002* | |||
| (−0.0000, 0.0004) | ||||
| Infant sex: male | −0.05 | −0.29*** | ||
| (−0.11, 0.02) | (−0.48, −0.09) | |||
| PSS | −0.01 | |||
| (−0.03, 0.003) | ||||
| Hepatitis C | −0.20 | |||
| (−0.46, 0.05) | ||||
| Gestational age | 0.03* | |||
| (0.0003, 0.06) | ||||
| Tobacco | 0.09 | |||
| (−0.02, 0.20) | ||||
| Constant | 1.32*** | 2.39*** | 2.61*** | 6.23*** |
| (1.25, 1.39) | (1.71, 3.06) | (1.53, 3.68) | (5.91, 6.55) | |
|
| ||||
| R2 | 0.03 | 0.15 | 0.12 | 0.02 |
| Adjusted R2 | −0.003 | 0.09 | 0.08 | 0.001 |
| F Statistic | 0.91 (df = 3; 84) | 2.38** (df = 6; 80) | 2.83** (df = 4; 83) | 1.03 (df = 2; 85) |
Note:
p<0.1
p<0.05
p<0.01;
values were natural log transformed; Main effects (MOUD and PAE as well as MOUD-by-PAE interaction) as well as a priori covariates (maternal pre-pregnancy body mass index (BMI), maternal age, prenatal tobacco use, maternal perceived stress (PSS) at delivery, maternal viral hepatisis C infection, infant birth weight, type of delivery (vaginal vs. cesarean section), infant sex, gestational age at birth, SSRI exposure) were included in all models. Variables included in the final models shown were selected by Akaike information criteria, Bayesian information criteria, and adjusted R2 with backward and forward selection; MOUD – medications for opioid use disorder; PAE – prenatal alcohol exposure; PSS – Perceived Stress Score
3.4. Effect of covariates on placental genes
Several a priori chosen covariates were also noted to have significant associations with placental biomarkers (Table 3; Table 4). As noted above, mode of delivery (β = −0.70; p < .05) was significantly associated with IL-1β mRNA while infant male sex (β = −0.29; p < .01) was significantly associated with IL-1β protein expression. Infant birthweight (β = −0.0004; p < .05) was also significantly associated placental TNF-α mRNA expression. Maternal age (β = 0.001; p < .05) and maternal viral hepatitis C (β = −0.02; p < .01) were statistically significantly associated with SERT mRNA. Similarly, maternal viral hepatitis C (β = 0.68; p < .05) was significantly associated with TPH1 mRNA. Overall, these covariates did not greatly influence the outcomes as indicated by the changes from R2 to adjusted R2 (Table 3; Table 4).
4. Discussion
To our knowledge, this is the first report to demonstrate a significant and negative association between PAE and placental SERT mRNA as well as a significant positive association between PAE and TPH1 mRNA expression. We also found a significant effect of MOUD on placental TNF-α protein expression. Additionally, results indicate that PAE may exert an inhibitory effect on the expression of TNF-α mRNA and IL-1β mRNA in human placental tissue. Other findings include a significant negative association between infant male sex and placental IL-1β protein expression as well as infant birthweight and placental IL-1β expression.
4.1. Placental serotonin
A number of transporters, receptors, and rate-limiting enzymes tightly regulate 5-HT, a crucial placental neurotransmitter. It is reported to play a crucial role in fetal neurodevelopment including neuronal cell division, differentiation, migration, and synaptogenesis, as well as development and organization of neurons in the sensory cortex (Garbarino et al., 2019). The serotonin transporter (SERT) is responsible for the high-affinity uptake of 5-HT from maternal circulation into cells where it is degraded to an inactive metabolite by MAO-A thereby contributing to 5-HT homeostasis within the system (Karahoda et al., 2020b). To our knowledge, these findings are the first report of a significant and independent association between PAE and decreased SERT mRNA expression in human placental tissue.
Clinical studies provide evidence of the importance of placental serotonergic signaling in fetal neurodevelopment. In human placental tissue, increased methylation of the gene that encodes the G-coupled, 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) has been associated with infant alteration in motor control, specifically poorer quality of movement, in neonates (Paquette et al., 2013). Higher placental levels of SERT mRNA were associated with maternal report of increased infant regulatory behavioral challenges (Räikkönen et al., 2015). Similarly, alteration of 5-HT signaling through maternal use of SSRIs, which block the uptake of 5-HT into cells (Ornoy & Koren, 2019), has been associated with neuro-structural and -behavioral effects that may be long-lasting (Rotem-Kohavi & Oberlander, 2017). These include microstructural brain changes visible with magnetic resonance imaging in exposed neonates (Jha et al., 2016), increased risk of Postnatal Adaption Syndrome (Levinson-Castiel et al., 2006), alterations in pain and stress response during the first two months of life (Oberlander et al., 2002, 2005), and speech and language disorders (Brown et al., 2016). These effects also appear to be long-lasting as evidenced by report of increased rates of depression in SSRI exposed adolescents (Malm et al., 2016). These findings are consistent with preclinical studies in which excess 5-HT during CNS development was correlated with changes in cortical interneuron migration (Riccio et al., 2009) and decreased serotonergic axon growth (Goeden et al., 2016) in mice. Furthermore, PAE was associated with inhibition of 5-HT neurons which persisted into the young adulthood (Sari & Zhou, 2004). Together, these clinical and preclinical studies demonstrate perturbation of the delicate 5-HT balance during pregnancy may mediate the development of critical brain structures leading to alterations in neurobehavior that may persist in to later life.
Under normal conditions, human placenta regulates the uptake and metabolism of 5-HT from both maternal and fetal circulation via rate limiting transporters and enzymes working in concert. Novel preclinical evidence demonstrates a potential role for OCT3 in the extraction of 5-HT from fetal circulation while SERT extracts 5-HT from the maternal circulation. This suggests at term gestation, OCT3 to a greater role, and SERT possibly to a lesser role, protect the fetus from elevated levels of 5-HT through uptake from the fetal and maternal circulation into trophoblast cells where it is subsequently degraded thus ensuring minimal exposure of the fetus to maternal 5-HT (Karahoda et al., 2020b). In fact, at term, protein levels of SERT and MAO-A are upregulated thus increasing the capacity of the placenta to clear circulating 5-HT (Karahoda et al., 2020a). Serotonin homeostasis in the maternal-placental-fetal axis is tightly regulated and any disturbances in the system may have the potential to disrupt neural programming. Importantly, our results suggest that alcohol may inhibit this important protective mechanism by impairing placental clearance of 5-HT through downregulation of SERT mRNA in the placenta and thus possibly influencing fetal neurodevelopment.
The current report also demonstrates for the first time that PAE was associated with a significant increase in TPH1 mRNA expression in human placental tissue. While there are no clinical studies related to this phenomenon, preclinical evidence demonstrated that early in gestation, the fetus is dependent upon the conversion of maternal tryptophan to 5-HT via TPH1 activity (Bonnin & Levitt, 2011). However, from mid-gestation onward, the fetus is able to independently synthesize 5-HT from maternal tryptophan, and diminished placental TPH1 activity is thought to be a compensatory response (Karahoda et al., 2020b; Sano et al., 2016). Preclinical evidence has demonstrated that consequences associated with increased TPH1 gene expression in mouse placenta include increased 5-HT concentration in the fetal brain as well as alterations in 5-HT neurons (Goeden et al., 2016). Therefore, it would seem reasonable that increased TPH1 mRNA in human placenta may also lead to increased 5-HT in fetal circulation creating a further risk for fetal dysregulated neurodevelopment.
Taken together, our data suggest alcohol has an effect on serotonin pathways in the placenta, during late gestational stages (and possibly earlier), including SERT and the TPH1 enzymatic pathway. The combination of decreased SERT mRNA and increased TPH1 mRNA expression in the placenta may result in increased circulating 5-HT levels in the maternal-placental-fetal axis that affect downstream fetal neurodevelopmental processes. This may ultimately contribute to the phenotype associated with prenatal alcohol exposure and FASD. As this is the first report of such an effect, further interrogation of the multiple factors in this pathway are warranted.
4.2. Placental TLR4 pathway
Abundant TLR4 expression is present in placental trophoblasts cells (Hauguel-de Mouzon & Guerre-Millo, 2006; Holmlund et al., 2002; Kumazaki et al., 2004). Evidence from preclinical studies reveal that upon ligand binding, TLR4 initiates the sequential activation of several intracellular signaling molecules including nuclear factor-kappa B (NF-κB) inducing mRNA transcription of potent proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β. However, mature IL-1β production from its precursor pro-IL-1β requires downstream NLRP3 and Caspase-1 activation (Eidson et al., 2017; Jacobsen et al., 2014; Lacagnina et al., 2018). The ability of human trophoblast cells to secrete IL-1β through the NLRP3 inflammasome has been confirmed (Tamura et al., 2017).
Our results show novel expression patterns of TLR4 downstream proinflammatory cytokines in human placental tissue. The current paradigm surrounding the immunological stages of pregnancy posits three distinct stages. The first trimester is associated with inflammation, the second trimester involves a switch to an anti-inflammatory state that is required for growth, and during the third trimester there is a reversal to the requisite pro-inflammatory state needed for initiation of labor (Mor et al., 2017). Challenges to this immune environment may lead to adverse outcomes for the mother and the developing fetus.
Our findings indicated that PAE was associated with a positive trend in NLRP3 mRNA expression. Furthermore, our data indicated that both PAE and MOUD may inhibit mRNA transcripts of proinflammatory cytokines downstream of TLR4 and NLRP3. Specifically, we found PAE was associated with decreased TNF-α and IL-1β mRNA expression and MOUD was independently and significantly associated with decreased TNF-α protein expression in human placenta. There are relatively few studies examining the effect of PAE on human placenta (Holbrook et al., 2019). While mRNA production for IL-1β must occur prior to the action of NLRP3, which then converts pro-IL-1β to mature IL-1β, little is known about what stimulates NLRP3 mRNA production and how that stimulation may feedback onto NF-κB, which is the transcription factor responsible for mRNA IL-1β and TNFα.
Our findings that alcohol use during pregnancy decreased IL-1β and TNFα mRNA levels while elevations in NLRP3 mRNA were observed, suggests that other factors not yet understood are influencing the mRNA regulation and maturation of these cytokines. One possibility might include the complex interplay among maternal characteristics including psychosocial stress and resilience factors, such as socioeconomic status or social support. These factors are known to influence stress biology (Monk et al., 2019; Verner et al., 2021), including stress factors such as glucocorticoids, which have been shown to alter proinflammatory cytokine production (Xu et al., 2005). Given this, initial analyses were adjusted for a priori covariates (i.e., maternal pre-pregnancy BMI, maternal age, prenatal tobacco use, maternal perceived stress, maternal viral hepatitis C infection, infant birth weight, type of delivery, infant sex, gestational age at birth, SSRI exposure). Additionally, given that the R&D ELISA detects both immature pro-IL-1β and mature, releasable IL-1β, it is possible that the predicted elevations in IL-1β protein release were masked by inclusion of pro-IL-1β quantification.
Clinical studies examining the effect of MOUD on the innate immune response are more abundant. Data from these studies demonstrated methadone therapy was associated with inconsistent effects on proinflammatory cytokines including no effect of methadone therapy on plasma TNF-α levels in individuals with OUD (Lu et al., 2019) and significantly lower levels of plasma TNF-α levels in individuals with OUD two weeks after start of therapy compared to pre-therapy (Salarian et al., 2018). Interestingly, buprenorphine inhibited levels of TNF-α mRNA and protein in macrophages isolated from human umbilical cord blood (Sun et al., 2017). This finding is particularly relevant because while human cord blood and placenta are different tissues they are both fetal in nature. Therefore, prenatal MOUD may have a similar effect on TNF-α in human placental tissue. This is consistent with our novel finding that MOUD was independently and significantly associated with decreased TNF-α protein expression in human placenta. Preclinical data support opioids, including buprenorphine and methadone (Hutchinson et al., 2010a; Hutchinson et al., 2010b), bind TLR4 on cell lines transfected with human TLR4. Separate lines of evidence suggest that opioids, such as morphine, induce the production of downstream proinflammatory factors including IL-1β and TNF-α from CNS glia (Ellis et al., 2016; Grace et al., 2016). However, the effect of opioids on immune signaling in placental cells has not been characterized. Given our findings, it is possible that factors not yet explored, such as non-coding microRNAs (miRNAs) or other epigenetic factors could play an important role. The placenta has been shown to express miRNAs, which are critical posttranscriptional regulators of gene expression (Tsamou et al., 2021). Of note, miRNAs have been shown to target the TLR4 signaling response and regulate TLR-induced cytokines (He et al. 2014; O’Neill et al., 2011). This represents an important avenue of investigation for future studies.
4.3. Intersection of placental serotonin and TLR4 pathway
The alterations in placental cytokine milieu noted above may also influence the tightly controlled fetal serotonin system. When activated by an opioid, TLR4 initiates activation of NF-κB with subsequent expression of downstream NLRP3 and pro-inflammatory cytokines (Eidson et al., 2017; Jacobsen et al., 2014; Lacagnina et al., 2018; Mulla et al., 2009; Trotta et al., 2014). Similarly, chronic alcohol use enhances TLR4-mediated immune activation (Crews et al., 2013). Moreover, both opioids (Zhang et al., 2020) and alcohol can induce endogenous immune molecules (ie., High mobility group box 1 [HMGB1]) with the capacity to activate TLR4 and induce a positive feed-forward loop of TLR4-NLRP3-mediated immune actions (Coleman et al., 2018).
These TLR4 downstream immune factors are known to influence transporters and enzymes that regulate 5-HT homeostasis. For example, in cell culture and mouse synaptosomes, treatment with Il-1β and TNF-α resulted in increased 5-HT uptake (Zhu et al., 2006). In contrast to this, exposure of the human enterocyte cell line Caco-2 to the TLR4 ligand LPS resulted in inhibition of SERT dependent 5-HT uptake (Mendoza et al., 2009). Notably, in human placental choriocarcinoma cells, IL-1β has been shown to upregulate SERT gene expression (Kekuda et al., 2000). These disparate findings across models highlight the limitations of translation of findings from in vitro cell cultures and animal models (Rothbauer et al., 2017) to humans. Thus, further robust exploration of these pathways in human placental tissues is needed. In addition, further studies are needed that examine the implications of dysregulation in the placental 5-HT pathway for neurodevelopmental processes in children.
When examining our findings in the context of this work it is also important to consider the dynamic balance between pro- and anti-inflammatory cytokines. The inflammatory response is regulated by the production of ant-inflammatory cytokines (Petrovsky, 2001) that, in preclinical studies, have been shown to decrease SERT activity (Baganz & Blakely, 2013). Thus, given our results show a possible decrease in the placental proinflammatory cytokines IL-1β and TNF-α, there may be an associated increase in anti-inflammatory cytokines, such as IL-10, that are contributing to our finding of decreased expression of SERT mRNA. Therefore, in future studies, we will interrogate this dynamic relationship in placental tissue in order to further inform our understanding of the regulatory crosstalk between immune factors and 5-HT signaling in human placenta. Furthermore, as discussed above, miRNAs are small non-coding RNAs that target expression of proteins affecting the magnitude of the response (O’Neill et al., 2011). Novel evidence form a mouse model suggests that miRNAs are important regulators of gene expression in the placenta (Strawn et al., 2021) thus representing another important avenue of investigation. Finally, emerging clinical evidence revealed maternal serum C-reactive protein concentrations were associated with expression of genes involved in placental 5-HT function (Karahoda et al., 2021) suggesting a possible role for maternal inflammatory factors in placental 5-HT homeostasis.
Although the long-term effects of prenatal exposure to methadone or buprenorphine remain in question, emerging evidence suggests an association with adverse outcomes including microstructural changes in white matter at birth of infants prenatally exposed to methadone (Monnelly et al., 2018) and decreased volumes of basal ganglia, thalamus, and cerebellar white matter in opioid exposed (including MOUD) school age children compared to unexposed children (Sirnes et al., 2017). These findings are in line with a recent systematic review and meta-analysis that reported prenatal opioid exposure (including methadone and buprenorphine) was negatively associated with cognitive development in children age 0 to 6 years and physical development from age 6 months into adolescence (Yeoh et al., 2019). And, while the immediate and long-lasting effects of PAE have long been appreciated, the underlying mechanisms remain unclear (Lees et al., 2020). Furthermore, polysubstance use is common in women receiving treatment for OUD (Bakhireva et al., 2018), yet there are few investigations of the effects of the co-exposure of MOUD and alcohol on the developing fetus and newborn. Therefore, future studies are needed to examine the complex interplay among prenatal substance exposure, placenta/fetal programming factors, and NOWS severity. Although mechanistic underpinnings remain in question, our novel findings represent a pathway through which prenatal exposure to MOUD and alcohol may dysregulate the fetal serotonin system and program fetal neurodevelopment.
4.4. Interaction between infant characteristics and placental immune factors
While not our primary outcome of interest, our data showed male sex was significantly associated with decreased expression of IL-1β protein in human placenta relative to females when other factors were held constant. It is becoming increasingly apparent that males and females adapt differently to in utero stressors. Trophoblast cells, one of the main components of the placenta, arise from the outer layer of the blastocyst and differentiate into villous and extravillous trophoblastic cells (Staud & Karahoda, 2018). As the predominant tissue specific cell of the placenta, the trophoblast, arises from the embryo and shares the biological sex of the embryo. This likely accounts for sex as a biological difference in placental function and therefore infant outcomes (Di Renzo et al., 2015; Rosenfeld, 2015). According to the viability-vulnerability theory, male fetuses and infants experience greater morbidity and mortality compared to females when exposed to early adversity in utero (Sandman et al., 2013). Females are believed to have greater adaptive abilities and exhibit more subtle effects such as increased risk for anxiety and affective disorders (Sandman et al., 2013). While speculative, these differences may lie in differential immune responses in male versus female placenta. Considering the potential influence of such differences on developmental trajectories, future consideration of sex-dependent differences is necessary to inform future treatment guidelines for pregnant women and exposed infants.
4.5. Limitations and implications for future research
As with all research, we acknowledge potential limitations to our work. First, human clinical research is inherently complex with noted difficulties in parsing out potential confounding factors. The data used in this study are from a well-characterized prospective cohort, with information about prenatal exposures via repeated assessment including both self-report and biomarkers. Thus, a broad range of maternal and perinatal factors were assessed and factors known to be associated with immune activation and serotonin system function were controlled for in statistical analysis. However, the literature remains unclear for the effect of certain co-exposures, such as cannabis use, on immune function during human pregnancy (Dong et al., 2019). In addition, current evidence supports that risky alcohol consumption among women of higher socioeconomic status might be more prevalent (McKetta & Keys, 2020). While our findings are in line with these data, we acknowledge the effect of potential residual socio-economic confounders. Therefore, these represent important considerations for future research.
Second, we acknowledge that given the available sample size of 88 for this analysis, the study was powered to detect only moderate-to-large effect sizes. The limited sample size also did not allow for stratification by the type of maternal MOUD. Given that, buprenorphine is a partial μ-opioid receptor agonist and κ-opioid receptor antagonist while methadone is a full μ-opioid receptor agonist (Tran et al., 2017), the effect of these medications on proinflammatory and anti-inflammatory factors, both individually and in networks, may vary and require further investigation. Similarly, while our report identified important sex differences in the effects of alcohol and opioids on placenta targets, these associations should be examined in larger studies.
While we demonstrated that PAE is associated with alterations in the placental 5-HT system, this system involves complex interactions among rate limiting transporters and enzymes. Late in gestation, the serotonin system is thought to provide protection for the fetus from excess 5-HT (Karahoda et al., 2020b). Therefore, other transporters and enzymes involved in the uptake and degradation of 5-HT are of particular interest. While SERT is located in the apical, maternal facing, membrane of the placenta (Prasad et al., 1996), OCT3 is abundant in the basal membrane (Sata et al., 2005). Importantly, 5-HT is a demonstrated substrate of OCT3 (Koepsell et al., 2007). Karhoda et al. (2020b) provided the first evidence that placental OCT3 may play a potential role in the reuptake of 5-HT from fetal circulation. As both SERT and OCT3 are affected by maternal exposures, continued examination of alterations in these protective mechanisms is warranted to inform potential targets for preventive treatments.
It is important to note that a better understanding of the response of these key placental immune factors and factors in the 5-HT system to MOUD and/or alcohol may also inform clinical practice. For example, in response to these substances, endogenous molecules known as damage-associated molecular patterns (DAMPs) may be released and initiate a sterile inflammatory response including activation of inflammatory signaling pathways. Specifically, DAMPs act as ligands to TLR4, ultimately resulting in production of proinflammatory factors (Fleshner et al., 2016). Emerging preclinical evidence suggests that blockade of TLR4 signaling may mitigate the subsequent downstream cascade of inflammatory factors representing a novel therapeutic target (Robertson et al., 2018). For example, while (−)-naloxone is a widely-known mu-opioid receptor antagonist, its enantiomer, (+)-naloxone has more recently been discovered to specifically block TLR4 signaling thus preventing the release of downstream inflammatory molecules in mouse placental tissue (Chin et al., 2019). Thus, a robust understanding of these pathways in human placenta tissue may offer the potential to inform evidence-based prevention and treatment approaches for pregnant women living with substance use disorders.
5. Conclusions
To our knowledge, our study provides the first evidence that PAE may inhibit the important protective mechanism the serotonin transporter performs in human placenta while simultaneously promoting increased TPH1 protein expression. Together, this may result in increased 5-HT in fetal circulation, which may significantly influence fetal neurodevelopment. Furthermore, it appears that MOUD and alcohol disturb the bidirectional, dynamic interaction between the placental immune and serotonin system. Given the implication for preventative and therapeutic targets for exposed mothers and infants, further investigation of these critical mechanisms in well-defined cohorts of maternal-infant dyads has significant implications for improving perinatal outcomes.
Highlights:
PAE may inhibit the important protective mechanism of the serotonin transporter while simultaneously promoting increased TPH1 protein expression in human placenta.
Prenatal opioid and alcohol exposures may affect expression of cytokines in human placenta.
Prenatal opioid and alcohol exposures may perturb the bidirectional, dynamic interaction between the placental immune and serotonin system.
Financial support:
This work was supported by an award from the National Center for Advancing Translational Science, National Institutes of Health under grant number UL1TR001449; CTSC006-7 PI Ruyak; and Sigma Theta Tau/Western Institute of Nursing Research; PI Ruyak; and the National Institutes of Health (R01AA025967; PI Milligan; R01 AA021771; PI Bakhireva).
Abbreviations:
- 5-HT
5-hydroxytryptamine; serotonin
- MOUD
medications for opioid use disorder
- PAE
prenatal alcohol exposure
- TLR4
toll-like receptor 4
- NLRP3
nucleotide-binding oligomerization domain (NOD)-like receptor protein 3
- IL-1β
interleukin (IL)-1β
- TNF-α
tumour necrosis factor α
- SERT
serotonin transporter
- TPH1
tryptophan hydroxylase
- IDO1
indoleamine 2,3-Dioxygenase 1
Footnotes
Declarations of interest: none
References
- Baganz NL, & Blakely RD (2013). A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chemical Neuroscience, 4(1), 48–63. 10.1021/cn300186b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Holbrook BD, Shrestha S, Leyva Y, Ashley M, Cano S, Lowe J, Stephen JM, & Leeman L (2019). Association between prenatal opioid exposure, neonatal opioid withdrawal syndrome, and neurodevelopmental and behavioral outcomes at 5–8 months of age. Early Human Development, 128, 69–76. 10.1016/j.earlhumdev.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Lowe JR, Gutierrez HL, & Stephen JM (2015). Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) prospective cohort: Study design considerations. Advances in Pediatric Research, 2(2015). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4610372/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Shrestha S, Garrison L, Leeman L, Rayburn WF, & Stephen JM (2018). Prevalence of alcohol use in pregnant women with substance use disorder. Drug and Alcohol Dependence, 187, 305–310. 10.1016/j.drugalcdep.2018.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, & Tran PK (2018). Beyond infection—Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Experimental Neurology, 299, 241–251. 10.1016/j.expneurol.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar TS, Hill LA, & Weinberg J (2016). Evidence for an immune signature of prenatal alcohol exposure in female rats. Brain, Behavior, and Immunity, 58, 130–141. 10.1016/j.bbi.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar TS, Raineki C, Wertelecki W, Yevtushok L, Plotka L, Granovska I, Zymak-Zakutnya N, Pashtepa A, Wells A, Honerkamp-Smith G, Coles CD, Kable JA, Chambers CD, Weinberg J, & and the CIFASD. (2020). Immune network dysregulation associated with child neurodevelopmental delay: Modulatory role of prenatal alcohol exposure. Journal of Neuroinflammation, 17(1), 39. 10.1186/s12974-020-1717-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, & Levitt P (2011). A transient placental source of serotonin for the fetal forebrain. Nature, 472(7343), 347–350. 10.1038/nature09972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, & Levitt P (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience, 197, 1–7. 10.1016/j.neuroscience.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Gyllenberg D, Malm H, McKeague IW, Hinkka-Yli-Salomäki S, Artama M, Gissler M, Cheslack-Postava K, Weissman MM, Gingrich JA, & Sourander A (2016). Association of selective serotonin reuptake inhibitor exposure during pregnancy with speech, scholastic, and motor disorders in offspring. JAMA Psychiatry, 73(11), 1163. 10.1001/jamapsychiatry.2016.2594 [DOI] [PubMed] [Google Scholar]
- Burton GJ, & Fowden AL (2015). The placenta: A multifaceted, transient organ. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1663), 20140066. 10.1098/rstb.2014.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin PY, Dorian C, Sharkey DJ, Hutchinson MR, Rice KC, Moldenhauer LM, & Robertson SA (2019). Toll-like receptor-4 antagonist (+)-naloxone confers sexually dimorphic protection from inflammation-induced fetal programming in mice. Endocrinology, 160(11), 2646–2662. 10.1210/en.2019-00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Zou J, Qin L, & Crews FT (2018). HMGB1/IL-1β complexes regulate neuroimmune responses in alcoholism. Brain, Behavior, and Immunity, 72, 61–77. 10.1016/j.bbi.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Crowell SE, & Lester BM (2018). Early life stress and environmental influences on the neurodevelopment of children with prenatal opioid exposure. Neurobiology of Stress, 9, 48–54. 10.1016/j.ynstr.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, & Zou J (2013). High mobility group box 1/toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry, 73(7), 602–612. 10.1016/j.biopsych.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo GC, Picchiassi E, Coata G, Clerici G, & Brillo E (2015). Is there a sex of the placenta? Journal of Pediatric and Neonatal Individualized Medicine, 4(2), e040246. 10.7363/040246 [DOI] [Google Scholar]
- Dong C, Chen J, Harrington A, Vinod KY, Hegde ML, & Hegde VL (2019). Cannabinoid exposure during pregnancy and its impact on immune function. Cellular and Molecular Life Sciences, 76(4), 729–743. 10.1007/s00018-018-2955-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow AG, Glass RM, Smith CJ, Tran PK, James K, & Bilbo S (2019). Placental macrophages: A window into fetal microglial function in maternal obesity. International Journal of Developmental Neuroscience, 77(1), 60–68. 10.1016/j.ijdevneu.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Inoue K, Young LJ, Tansey MG, & Murphy AZ (2017). Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology, 42(3), 661–670. 10.1038/npp.2016.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, Ayala M, Hutchinson MR, Falci S, Rice KC, Maier SF, & Watkins LR (2016). Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain, Behavior, and Immunity, 58, 348–356. 10.1016/j.bbi.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Frank M, & Maier SF (2016). Danger signals and inflammasomes: Stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 10.1038/npp.2016.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino VR, Gilman TL, Daws LC, & Gould GG (2019). Extreme enhancement or depletion of serotonin transporter function and serotonin availability in autism spectrum disorder. Pharmacological Research, 140, 85–99. 10.1016/j.phrs.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano S, Petroff MG, Warren BD, Jasti S, Linscheid C, Ward A, Kramer A, Dobrinskikh E, Sheiko MA, Gale M, Golden-Mason L, Winn VD, & Rosen HR (2015). Hepatitis C virus sensing by human trophoblasts induces innate immune responses and recruitment of maternal nk cells: Potential implications for limiting vertical transmission. The Journal of Immunology, 195(8), 3737–3747. 10.4049/jimmunol.1500409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N, Velasquez J, Arnold KA, Chan Y, Lund BT, Anderson GM, & Bonnin A (2016). Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. Journal of Neuroscience, 36(22), 6041–6049. 10.1523/JNEUROSCI.2534-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, & Watkins LR (2016). Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences, 113(24), E3441–E3450. 10.1073/pnas.1602070113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Kappil M, Lambertini L, Armstrong DA, Guerin DJ, Sharp AJ, Lester BM, Chen J, & Marsit CJ (2015). Expression of imprinted genes in placenta is associated with infant neurobehavioral development. Epigenetics, 10(9), 834–841. 10.1080/15592294.2015.1073880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, & Guerre-Millo M (2006). The placenta cytokine network and inflammatory signals. Placenta, 27(8), 794–798. 10.1016/j.placenta.2005.08.009 [DOI] [PubMed] [Google Scholar]
- He X, Jing Z, & Cheng G (2014). MicroRNAs: New regulators of Toll-Like Receptor signaling pathways. BioMed Research International, 2014, 945169. 10.1155/2014/945169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook BD, Davies S, Cano S, Shrestha S, Jantzie LL, Rayburn WF, Bakhireva LN, & Savage DD (2019). The association between prenatal alcohol exposure and protein expression in human placenta. Birth Defects Research, 0(0). 10.1002/bdr2.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, EkstrÖm ES, & Scheynius A (2002). Expression and regulation of the pattern recognition receptors toll-like receptor-2 and toll-like receptor-4 in the human placenta. Immunology, 107(1), 145–151. 10.1046/j.1365-2567.2002.01491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, & Watkins LR (2010a). Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience, 167(3), 880–893. 10.1016/j.neuroscience.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, … Watkins LR (2010b). Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain, Behavior, and Immunity, 24(1), 83–95. 10.1016/j.bbi.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JHW, Watkins LR, & Hutchinson MR (2014). Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. International Review of Neurobiology, 118, 129–163. 10.1016/B978-0-12-801284-0.00006-3 [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, & Jacobson JL (2002). Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics, 109(5), 815–825. 10.1542/peds.109.5.815 [DOI] [PubMed] [Google Scholar]
- Jha SC, Meltzer-Brody S, Steiner RJ, Cornea E, Woolson S, Ahn M, Verde AR, Hamer RM, Zhu H, Styner M, Gilmore JH, & Knickmeyer RC (2016). Antenatal depression, treatment with selective serotonin reuptake inhibitors, and neonatal brain structure: A propensity-matched cohort study. Psychiatry Research: Neuroimaging, 253, 43–53. 10.1016/j.pscychresns.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahoda R, Abad C, Horackova H, Kastner P, Zaugg J, Cerveny L, Kucera R, Albrecht C, & Staud F (2020a). Dynamics of tryptophan metabolic pathways in human placenta and placental-derived cells: effect of gestation age and trophoblast differentiation. Frontiers in Cell and Developmental Biology, 8. 10.3389/fcell.2020.574034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahoda R, Horackova H, Kastner P, Matthios A, Cerveny L, Kucera R, Kacerovsky M, Tebbens JD, Bonnin A, Abad C, & Staud F (2020b). Serotonin homeostasis in the materno-foetal interface at term: Role of transporters (SERT/SLC6A4 and OCT3/SLC22A3) and monoamine oxidase A (MAO-A) in uptake and degradation of serotonin by human and rat term placenta. Acta Physiologica, 229(4), e13478. 10.1111/apha.13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahoda R, Robles M, Marushka J, Stranik J, Abad C, Horackova H, Tebbens JD, Vaillancourt C, Kacerovsky M, & Staud F (2021). Prenatal inflammation as a link between placental expression signature of tryptophan metabolism and preterm birth. Human Molecular Genetics, ddab169. 10.1093/hmg/ddab169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R, Leibach FH, Furesz TC, Smith CH, & Ganapathy V (2000). Polarized distribution of interleukin-1 receptors and their role in regulation of serotonin transporter in placenta. Journal of Pharmacology and Experimental Therapeutics, 292(3), 1032–1041. [PubMed] [Google Scholar]
- Ko JY, Haight SC, Schillie SF, Bohm MK, & Dietz PM (2019). National trends in hepatitis c infection by opioid use disorder status among pregnant women at delivery hospitalization—United States, 2000-2015. MMWR. Morbidity and Mortality Weekly Report, 68(39), 833–838. 10.15585/mmwr.mm6839a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, & Volk C (2007). Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical Research, 24(7), 1227–1251. 10.1007/s11095-007-9254-z [DOI] [PubMed] [Google Scholar]
- Kozhimannil KB, Graves AJ, Levy R, & Patrick SW (2017). Nonmedical use of prescription opioids among pregnant U.S. women. Women’s Health Issues, 27(3), 308–315. 10.1016/j.whi.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki K, Nakayama M, Yanagihara I, Suehara N, & Wada Y (2004). Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Human Pathology, 35(1), 47–54. 10.1016/j.humpath.2003.08.027 [DOI] [PubMed] [Google Scholar]
- Labella MH, Eiden RD, Tabachnick AR, Sellers T, & Dozier M (2021). Infant neurodevelopmental outcomes of prenatal opioid exposure and polysubstance use. Neurotoxicology and Teratology, 86, 107000. 10.1016/j.ntt.2021.107000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Watkins LR, & Grace PM (2018). Toll-like receptors and their role in persistent pain. Pharmacology & Therapeutics, 184, 145–158. 10.1016/j.pharmthera.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layunta E, Latorre E, Forcén R, Grasa L, Plaza MA, Arias M, Alcalde AI, & Mesonero JE (2018). NOD1 downregulates intestinal serotonin transporter and interacts with other pattern recognition receptors. Journal of Cellular Physiology, 233(5), 4183–4193. 10.1002/jcp.26229 [DOI] [PubMed] [Google Scholar]
- Lees B, Mewton L, Jacobus J, Valadez EA, Stapinski LA, Teesson M, Tapert SF, & Squeglia LM (2020). Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study. American Journal of Psychiatry, 177(11), 1060–1072. 10.1176/appi.ajp.2020.20010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson-Castiel R, Merlob P, Linder N, Sirota L, & Klinger G (2006). Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Archives of Pediatrics & Adolescent Medicine, 160(2), 173–176. 10.1001/archpedi.160.2.173 [DOI] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.), 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, Weissman M, Wickramaratne P, Artama M, Gingrich JA, & Sourander A (2016). Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: A national register-based study. Journal of the American Academy of Child and Adolescent Psychiatry, 55(5), 359–366. 10.1016/j.jaac.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit CJ, Maccani MA, Padbury JF, & Lester BM (2012). Placental 11-Beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLOS ONE, 7(3), e33794. 10.1371/journal.pone.0033794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetta SC, & Keyes KM (2020). Trends in U.S. women’s binge drinking in middle adulthood by socioeconomic status, 2006-2018. Drug and Alcohol Dependence, 212, 108026. 10.1016/j.drugalcdep.2020.108026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Woodson J, Garcia RI, Crawford B, Sharma J, Sheridan SD, Haggarty SJ, & Sur M (2014). B2-Adrenergic receptor agonist ameliorates phenotypes and corrects microRNA-mediated IGF1 deficits in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America, 111(27), 9947–9952. 10.1073/pnas.1309426111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza C, Matheus N, Iceta R, Mesonero JE, & Alcalde AI (2009). Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immunity, 15(4), 243–250. 10.1177/1753425909104781 [DOI] [PubMed] [Google Scholar]
- Monk C, Lugo-Candelas C, & Trumpff C (2019). Prenatal developmental origins of future psychopathology: mechanisms and pathways. Annual Review of Clinical Psychology, 15(1), 317–344. 10.1146/annurev-clinpsy-050718-095539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, Semple SI, Bastin ME, & Boardman JP (2018). Prenatal methadone exposure is associated with altered neonatal brain development. NeuroImage: Clinical, 18, 9–14. 10.1016/j.nicl.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Aldo P, & Alvero AB (2017). The unique immunological and microbial aspects of pregnancy. Nature Reviews Immunology, 17(8), 469–482. 10.1038/nri.2017.64 [DOI] [PubMed] [Google Scholar]
- Mulla MJ, Yu AG, Cardenas I, Guller S, Panda B, & Abrahams VM (2009). ORIGINAL ARTICLE: Regulation of Nod1 and Nod2 in first trimester trophoblast cells. American Journal of Reproductive Immunology, 61(4), 294–302. 10.1111/j.1600-0897.2009.00694.x [DOI] [PubMed] [Google Scholar]
- Noor S, & Milligan ED (2018). Lifelong impacts of moderate prenatal alcohol exposure on neuroimmune function. Frontiers in Immunology, 9. 10.3389/fimmu.2018.01107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor S, Sun MS, Vanderwall AG, Havard MA, Sanchez JE, Harris NW, Nysus MV, Norenberg JP, West HT, Wagner CR, Jantzie LL, Mellios N, & Milligan ED (2019). LFA-1 antagonist (BIRT377) similarly reverses peripheral neuropathic pain in male and female mice with underlying sex divergent peripheral immune proinflammatory phenotypes. Neuroimmunology and Neuroinflammation, 6. 10.20517/2347-8659.2019.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Eckstein Grunau R, Fitzgerald C, Ellwood A-L, Misri S, Rurak D, & Riggs KW (2002). Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatric Research, 51(4), 443–453. 10.1203/00006450-200204000-00008 [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, & Riggs W (2005). Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics, 115(2), 411–425. 10.1542/peds.2004-0420 [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Sheedy FJ, & McCoy CE (2011). MicroRNAs: The fine-tuners of Toll-like receptor signaling. Nature Reviews. Immunology, 11(3), 163–175. 10.1038/nri2957 [DOI] [PubMed] [Google Scholar]
- Ornoy A, & Koren G (2019). SSRIs and SNRIs (SRI) in pregnancy: Effects on the course of pregnancy and the offspring: How far are we from having all the answers? International Journal of Molecular Sciences, 20(10), 2370. 10.3390/ijms20102370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lesseur C, Armstrong DA, Koestler DC, Appleton AA, Lester BM, & Marsit CJ (2013). Placental HTR2A methylation is associated with infant neurobehavioral outcomes. Epigenetics, 8(8), 796–801. 10.4161/epi.25358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Montagud-Romero S, Forteza J, Rodríguez-Arias M, Miñarro J, & Guerri C (2017). TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. Journal of Neuroinflammation, 14, 145. 10.1186/s12974-017-0918-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N (2001). Towards a unified model of neuroendocrine–immune interaction. Immunology & Cell Biology, 79(4), 350–357. 10.1046/j.1440-1711.2001.01029.x [DOI] [PubMed] [Google Scholar]
- Piske M, Homayra F, Min JE, Zhou H, Marchand C, Mead A, Ng J, Woolner M, & Nosyk B (2021). Opioid use disorder and perinatal outcomes. Pediatrics. 10.1542/peds.2021-050279 [DOI] [PubMed] [Google Scholar]
- Prasad PD, Hoffmans BJ, Moe AJ, Smith CH, Leibach FH, & Ganapathy V (1996). Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells. Placenta, 17(4), 201–207. 10.1016/S0143-4004(96)90039-9 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Grecco G, Stolze K, Atwood B, Jennings SG, Lien IZ, Saykin AJ, & Sadhasivam S (2021). Neuroimaging in infants with prenatal opioid exposure: Current evidence, recent developments and targets for future research. Journal of Neuroradiology, 48(2), 112–120. 10.1016/j.neurad.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räikkönen K, Pesonen A-K, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, Villa P, Laivuori H, Hämäläinen E, Seckl JR, & Reynolds RM (2015). Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychological Medicine, 45(15), 3217–3226. 10.1017/S003329171500121X [DOI] [PubMed] [Google Scholar]
- Riccio O, Potter G, Walzer C, Vallet P, Szabó G, Vutskits L, Kiss JZ, & Dayer AG (2009). Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Molecular Psychiatry, 14(3), 280–290. 10.1038/mp.2008.89 [DOI] [PubMed] [Google Scholar]
- Robertson SA, Wahid HH, Chin PY, Hutchinson MR, Moldenhauer LM, & Keelan JA (2018). Toll-like receptor-4: A new target for preterm labour pharmacotherapies? Current Pharmaceutical Design, 24(9), 960–973. 10.2174/1381612824666180130122450 [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS (2015). Sex-specific placental responses in fetal development. Endocrinology, 156(10), 3422–3434. 10.1210/en.2015-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem-Kohavi N, & Oberlander TF (2017). Variations in neurodevelopmental outcomes in children with prenatal SSRI antidepressant exposure. Birth Defects Research, 109(12), 909–923. 10.1002/bdr2.1076 [DOI] [PubMed] [Google Scholar]
- Rothbauer M, Patel N, Gondola H, Siwetz M, Huppertz B, & Ertl P (2017). A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Scientific Reports, 7(1), 5892. 10.1038/s41598-017-06364-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JJ, Noor S, Davies S, Savage D, & Milligan ED (2017). Prenatal alcohol exposure is a risk factor for adult neuropathic pain via aberrant neuroimmune function. Journal of Neuroinflammation, 14(1), 254. 10.1186/s12974-017-1030-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, & Davis EP (2013). Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. Journal of Psychosomatic Research, 75(4), 327–335. 10.1016/j.jpsychores.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Ferchaud-Roucher V, Kaeffer B, Poupeau G, Castellano B, & Darmaun D (2016). Maternal and fetal tryptophan metabolism in gestating rats: Effects of intrauterine growth restriction. Amino Acids, 48(1), 281–290. 10.1007/s00726-015-2072-4 [DOI] [PubMed] [Google Scholar]
- Sari Y, & Zhou FC (2004). Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcoholism: Clinical and Experimental Research, 28(6), 941–948. 10.1097/01.ALC.0000128228.08472.39 [DOI] [PubMed] [Google Scholar]
- Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, Uchiumi T, Kuwano M, Nagata H, Tsukimori K, Nakano H, & Sawada Y (2005). Functional analysis of organic cation transporter 3 expressed in human placenta. Journal of Pharmacology and Experimental Therapeutics, 315(2), 888–895. [DOI] [PubMed] [Google Scholar]
- Sirnes E, Oltedal L, Bartsch H, Eide GE, Elgen IB, & Aukland SM (2017). Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Human Development, 106–107, 33–39. 10.1016/j.earlhumdev.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Staud F, & Karahoda R (2018). Trophoblast: The central unit of fetal growth, protection and programming. The International Journal of Biochemistry & Cell Biology, 105, 35–40. 10.1016/j.biocel.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Strawn M, Samal A, Sarker MB, Dhakal P, & Behura SK (2021). Relevance of microRNAs to the regulation of the brain-placental axis in mice. Placenta, 112, 123–131. 10.1016/j.placenta.2021.07.293 [DOI] [PubMed] [Google Scholar]
- Sun J, Guo W, & Du X (2017). Buprenorphine differentially affects M1- and M2-polarized macrophages from human umbilical cord blood. European Cytokine Network, 28(2), 85–92. 10.1684/ecn.2017.0392 [DOI] [PubMed] [Google Scholar]
- Tamura K, Ishikawa G, Yoshie M, Ohneda W, Nakai A, Takeshita T, & Tachikawa E (2017). Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1β secretion in human trophoblasts. Journal of Pharmacological Sciences, 135(2), 89–95. 10.1016/j.jphs.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Tobon AL, Habecker E, & Forray A (2019). Opioid use in pregnancy. Current Psychiatry Reports, 21(12), 118. 10.1007/s11920-019-1110-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Griffin BL, Stone RH, Vest KM, & Todd TJ (2017). Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 37(7), 824–839. 10.1002/phar.1958 [DOI] [PubMed] [Google Scholar]
- Trotta T, Porro C, Calvello R, & Panaro MA (2014). Biological role of Toll-like receptor-4 in the brain. Journal of Neuroimmunology, 268(1), 1–12. 10.1016/j.jneuroim.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Tsamou M, Vrijens K, Wang C, Winckelmans E, Neven KY, Madhloum N, de Kok TM, & Nawrot TS (2021). Genome-wide microRNA expression analysis in human placenta reveals sex-specific patterns: An ENVIRONAGE birth cohort study. Epigenetics, 16(4), 373–388. 10.1080/15592294.2020.1803467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwall AG, Noor S, Sun MS, Sanchez JE, Yang XO, Jantzie LL, Mellios N, & Milligan ED (2018). Effects of spinal non-viral interleukin-10 gene therapy formulated with d-mannose in neuropathic interleukin-10 deficient mice: Behavioral characterization, mRNA and protein analysis in pain relevant tissues. Brain, Behavior, and Immunity, 69, 91–112. 10.1016/j.bbi.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez JC, Goeden N, & Bonnin A (2013). Placental serotonin: Implications for the developmental effects of SSRIs and maternal depression. Frontiers in Cellular Neuroscience, 7. 10.3389/fncel.2013.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner G, Epel E, Lahti-Pulkkinen M, Kajantie E, Buss C, Lin J, Blackburn E, Räikkönen K, Wadhwa PD, & Entringer S (2021). Maternal psychological resilience during pregnancy and newborn telomere length: A prospective study. American Journal of Psychiatry, 178(2), 183–192. 10.1176/appi.ajp.2020.19101003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Zhang Z, Nance E, Drewes JL, Lesniak WG, Singh S, Chugani DC, Rangaramanujam K, Graham DR, & Kannan S (2017). Maternal inflammation results in altered tryptophan metabolism in rabbit placenta and fetal brain. Developmental Neuroscience, 39(5), 399–412. 10.1159/000471509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Riley EP, & Charness ME (2019). Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. The Lancet Neurology, 18(8), 760–770. 10.1016/S1474-4422(19)30150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-H, Choi S, & Levitt P (2016). Differential patterning of genes involved in serotonin metabolism and transport in extra-embryonic tissues of the mouse. Placenta, 42, 74–83. 10.1016/j.placenta.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Makris A, Thornton C, & Hennessy A (2005). Glucocorticoids inhibit placental cytokines from cultured normal and preeclamptic placental explants. Placenta, 26(8–9), 654–660. 10.1016/j.placenta.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Yeoh SL, Eastwood J, Wright IM, Morton R, Melhuish E, Ward M, & Oei JL (2019). Cognitive and motor outcomes of children with prenatal opioid exposure: A systematic review and meta-analysis. JAMA Network Open, 2(7), e197025–e197025. 10.1001/jamanetworkopen.2019.7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yang M, Chen C, Liu L, Wei X, & Zeng S (2020). Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Frontiers in Immunology, 11. 10.3389/fimmu.2020.01455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C-B, Blakely RD, & Hewlett WA (2006). The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology, 31(10), 2121–2131. 10.1038/sj.npp.1301029 [DOI] [PubMed] [Google Scholar]


