Abstract
BACKGROUND
Antiobesity medication may be useful for the treatment of pediatric obesity, yet few safe and effective options exist. We evaluated phentermine/topiramate (PHEN/TPM) for weight management in adolescents with obesity.
METHODS
This 56-week, randomized, double-blind trial enrolled adolescents 12 to less than 17 years of age with obesity. Participants were randomly assigned 1:1:2 to receive either placebo (n=56), mid-dose PHEN/TPM (7.5 mg/46 mg; n=54), or top-dose PHEN/TPM (15 mg/92 mg; n=113), respectively. All participants received lifestyle therapy. The primary end point was mean percent change in body-mass index (BMI) from randomization to week 56.
RESULTS
Participants had a mean (±SD) age of 14.0±1.4 years and a mean (±SD) BMI of 37.8±7.1 kg/m2; 54.3% were female. The primary end point of percent change in BMI at week 56 showed differences from placebo of −10.44 percentage points (95% CI, −13.89 to −6.99; P<0.001) and −8.11 percentage points (95% CI, −11.92 to −4.31; P<0.001) for the top and mid doses of PHEN/TPM, respectively. Differences from placebo in percent change in triglycerides nominally favored PHEN/TPM (mid dose, −21%; 95% CI, −40 to −2; and top dose, −21%; 95% CI, −38 to −4), as did differences in percent change in high-density lipoprotein cholesterol (HDL-C) (mid dose, 10%; 95% CI, 3 to 18; and top dose, 9%; 95% CI, 2 to 15). The incidence of participants reporting at least one adverse event was 51.8%, 37.0%, and 52.2% in the placebo, mid-dose, and top-dose groups, respectively. Serious adverse events were reported for two participants in the top-dose group.
CONCLUSIONS
PHEN/TPM at both the mid and top doses offered a statistically significant reduction in BMI and favorably impacted triglyceride and HDL-C levels in adolescents with obesity. (Funded by VIVUS LLC, with project support provided by Covance LLC; ClinicalTrials.gov number, NCT03922945.)
Introduction
Lifestyle therapy is the foundation of treatment for pediatric obesity but often fails as a singular approach owing to the complexity of the disease with multiple influences including genetics, environment, social factors, and biological forces that drive human energy regulation.1-3 Indeed, many adolescents with obesity do not achieve or maintain clinically meaningful weight reduction with lifestyle therapy alone.4-6 Orlistat and liraglutide reduce body-mass index (BMI; weight in kilograms divided by the square of the height in meters) beyond lifestyle therapy in adolescents by approximately 3% and 4.5%, respectively, and are the only antiobesity medications currently approved by the U.S. Food and Drug Administration (FDA) for chronic use in adolescents7,8; only liraglutide is approved by the European Medicines Agency. Thus, more options are needed for adolescents with obesity.3,9
A fixed dose combination of immediate-release phentermine (PHEN) and extended-release topiramate (TPM) was approved in July 2012 by the FDA as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with overweight or obesity. A mean placebo-subtracted weight loss of 9.8% to 11.0% was achieved after 1 year of treatment in adults with obesity randomly assigned to top-dose PHEN/TPM (15 mg/92 mg), whereas those randomly assigned to mid-dose PHEN/TPM achieved a weight loss of 7.5% (7.5 mg/46 mg).10,11 Notably, the weight loss was sustained over 2 years with continued treatment, providing evidence of durability.12 In addition to weight loss, participants randomly assigned to PHEN/TPM experienced significant reductions in blood pressure, improved glycemic parameters, increased high-density lipoprotein cholesterol (HDL-C), and reduced triglycerides.10,12 In adolescents with obesity, an 8-week pharmacokinetic and pharmacodynamic trial of PHEN/TPM demonstrated exposure comparable to that observed in adults and a tolerable safety profile.13
We conducted a randomized, double-blind, placebo-controlled clinical trial that evaluated the efficacy and safety of two doses of PHEN/TPM (7.5 mg/46 mg and 15 mg/92 mg) administered orally once daily for 56 weeks, as an adjunct to lifestyle therapy, for weight management in adolescents with obesity. We hypothesized that both doses of PHEN/TPM would lead to a greater percent reduction in BMI compared with placebo.
Methods
TRIAL OVERSIGHT
This trial was conducted from May 2, 2019, to April 16, 2021, at 26 sites in the United States. The trial protocol and statistical analysis plan are available in the Supplementary Appendix. The trial was designed by representatives from the trial sponsor (VIVUS LLC, Campbell, CA) with input from clinical and research professionals in obesity medicine. Covance LLC (Princeton, NJ), a contract research organization, performed project management, clinical monitoring, data management, statistical analysis, and clinical study report preparation. The trial sponsor performed medical monitoring and safety reporting. Safety of the participants and evaluation of the benefit–risk balance was overseen by an independent external data monitoring committee (members listed on Supplementary Appendix, page 4). All authors interpreted the data.
Written informed consent was obtained from parents/legally acceptable representatives and informed assent was obtained from participants before initiation of any trial-related procedures, including determining suitability for participation. The study was conducted in accordance with the Declaration of Helsinki and its most recent update and with the International Council for Harmonization E6 (R2) Good Clinical Practices guideline. The study was also conducted in accordance with local legal and regulatory requirements, and with standard operating procedures in place.
PARTICIPANTS
Eligible participants were 12 to less than 17 years of age, with a BMI in the 95th percentile or greater for age and sex, a Tanner stage greater than 1, a stable body weight, and a documented history of insufficient weight loss with lifestyle modification. Major exclusion criteria included treatment with antiobesity medications, history of bariatric surgery or eating disorders, stimulant use, type 1 diabetes, congenital heart disease, obesity of a known genetic or endocrine origin, elevated blood pressure, history of bipolar disorder or psychosis, major depressive disorder, current depression of moderate or greater severity, or presence or history of suicidal behavior or ideation with intent to act. The full list of inclusion and exclusion criteria is provided on Supplementary Appendix, page 5.
TRIAL DESIGN, PROCEDURES, AND END POINTS
This was a multicenter, randomized, double-blind, placebo-controlled, clinical trial designed to evaluate the safety and efficacy of PHEN/TPM over a 56-week treatment period in adolescents with obesity. Participants were randomly assigned in a 1:1:2 ratio to receive placebo, mid-dose PHEN/ TPM (7.5 mg/46 mg), or top-dose PHEN/TPM (15 mg/92 mg) taken orally once daily in the morning. Randomization was stratified by age group (12 to 14 vs. 15 to 16 years) and sex. Details of the dose titration schedule are provided on Supplementary Appendix, page 7. Participants who were unable to tolerate the assigned dose were switched to a reduced dose level or could take a drug holiday, typically limited to less than 2 weeks. If intolerance persisted after down-titration and/or a drug holiday and reinstitution of treatment, participants were removed from study treatment and encouraged to remain in the trial for follow-up assessments according to the protocol.
All participants, regardless of group assignment, were instructed to follow a mild hypocaloric diet modification program representing a 500-kilocalorie/day deficit and to implement a family-based lifestyle modification program for adolescents, as tolerated, throughout the study period. The lifestyle program included physical activity, behavior change, and family support. The same lifestyle modification program was implemented across all sites at routine study visits by a study coordinator or dietician and included training of both participants and their parents/guardians. Typically, between 5 and 15 minutes of visit time was dedicated to lifestyle training, with early study visits (baseline through week 12) toward the high end of this range, and visits later in the study toward the low end.
The primary end point was mean percent change in BMI from randomization to week 56. Secondary end points included changes from randomization to week 56 in the percentage of participants achieving a 5%, 10%, and 15% or greater BMI reduction as well as waist circumference, fasting insulin and whole-body insulin sensitivity index (WBISI), percent change in triglycerides and HDL-C, and blood pressure. The WBISI is also known as the Matsuda Index and provides an estimate of insulin sensitivity derived from the standard 2-hour oral glucose tolerance test. WBISI scores range widely but are typically between 0 and 10, with lower scores indicating greater insulin resistance and higher scores indicating greater insulin sensitivity; normal ranges have not yet been established in youth.
Exploratory end points included changes from randomization to week 56 in Impact of Weight on Quality of Life-Kids (IWQOL-Kids) questionnaire scores and glycemic and lipid markers. IWQOL-Kids assesses age-appropriate weight-related quality of life addressing physical comfort, body esteem, social life, and family relations. Scores range from 0 (worst quality of life) to 100 (best quality of life); normal ranges have not yet been established in youth.
Safety end points included the incidence of adverse events and serious adverse events, vital signs, laboratory parameters, electrocardiogram results, physical examinations, cognitive function tests using the Cambridge Neuropsychological Test Automated Battery (CANTAB), presence and severity of depression using the Patient Health Questionnaire (PHQ)-9 Modified for Teens, suicidal/ideation using the Columbia Suicide Severity Rating Scale (C-SSRS), bone age (x-ray of the hand and wrist), effect on bone mineral density, and bone mineral content using dual energy x-ray absorptiometry. The CANTAB assesses memory, attention, and reaction time. Scored domains included “Total Errors Adjusted” (0 to 137, with lower scores deemed better), “First Attempt Memory Score” (0 to 27, with higher scores deemed better), “Percent Correct Immediate” (0 to 100, with higher scores deemed better), “Percent Correct Delayed” (0 to 100, with higher scores deemed better), and “Forward Span Length” (2 to 9, with higher scores deemed better). Normal ranges for CANTAB have not yet been established in youth. PHQ-9 is a nine-item questionnaire with scores ranging from 0 to 27. PHQ-9 scores indicate the following: 0 to 4, no depression; 5 to 9, mild depression; 10 to 14, moderate depression, 15 to 19, moderately severe depression, and 20 to 27, severe depression. The C-SSRS consists of five items rating the presence of suicidal ideation ranging from wishing to be dead to active ideation with a specific plan and intent, and five items rating the presence of suicidal behavior ranging from preparatory acts or behavior to actual attempt. We conservatively estimated a treatment difference between the mid dose and placebo of approximately two units of BMI. Enrollment of 200 participants (50 to placebo, 50 to mid-dose PHEN/TPM, and 100 to top-dose PHEN/TPM) would allow at least 80% power to detect a statistically significant difference between the mid-dose and placebo and at least 90% power between the top-dose and placebo.
STATISTICAL ANALYSIS
Descriptive summary statistics were used to describe continuous variables (number of observations, mean [±SD], minimum, median, and maximum) and categorical variables (frequency counts and percentages). Data in summary tables are presented by treatment group, assessment, and visit (where applicable). Efficacy end points were analyzed using the full analysis set, which included all randomly assigned participants per the intention-to-treat (ITT) principle. Safety end points were analyzed using the safety analysis set, which included all randomly assigned participants exposed to one or more doses of randomly assigned treatment. The primary end point and continuous secondary efficacy end points were assessed using a mixed model for repeated-measures procedure with factors of treatment, visit, treatment-by-visit interaction, baseline value, and age and sex stratification. Categorical secondary efficacy end points were evaluated using the Cochran-Mantel Haenszel test controlling for age and sex stratification. For the primary end point, missing data were handled with the multiple-imputation method under the assumption that participants with missing data at week 56 had responded as if treated with placebo for the entire treatment period. To control overall type 1 error across secondary end points, a Hochberg multiplicity adjustment was first applied to key secondary end points of waist circumference and proportions of participants with a 5%, 10%, and 15% or greater BMI reduction. Only when comparisons of both the mid and top doses with placebo were significant at a P value of 0.05 or less was a similar procedure applied to other secondary end points evaluating obesity-related comorbidities. Once a P value greater than 0.05 was encountered, only point estimates and 95% confidence intervals (CIs) were provided. The CIs were not adjusted for multiple comparisons and should not be used to infer definitive treatment effects.
Details of the statistical methodology can be found in the statistical analysis plan on Supplementary Protocol, page 134.
Results
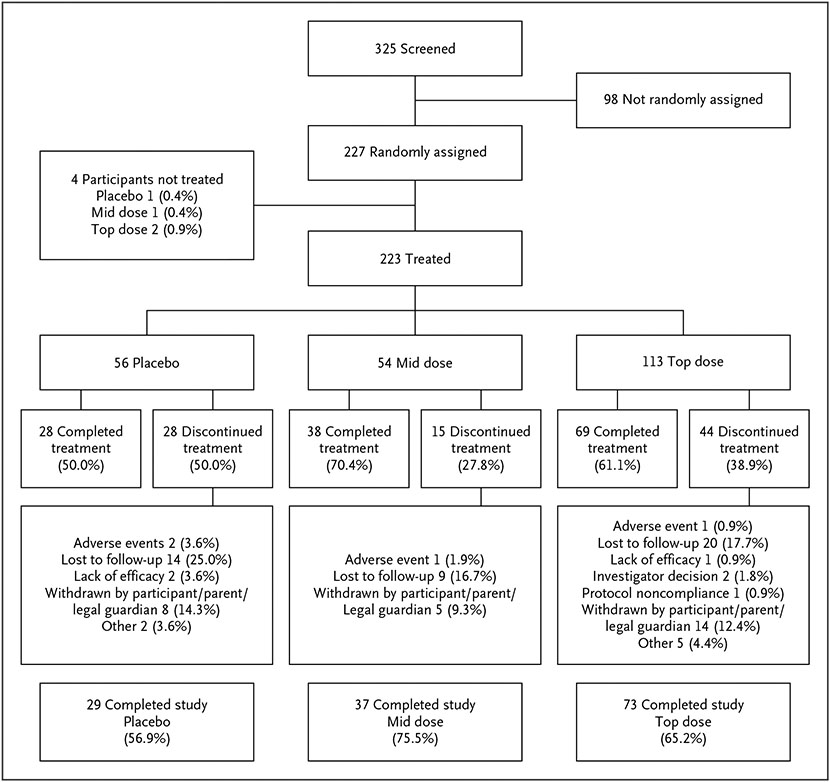
TRIAL POPULATION
Of 325 participants screened, 227 were randomly assigned to treatment and 223 received at least one dose of treatment; 56 received placebo, 54 mid-dose PHEN/TPM, and 113 top-dose PHEN/TPM (Fig. 1, CONSORT diagram). The study was completed by 29 (56.9%), 37 (75.5%), and 73 (65.2%) participants receiving placebo, mid-dose PHEN/TPM, and top-dose PHEN/TPM, respectively. At baseline, there were no notable differences among the three treatment groups (Table 1) or differences between participants who completed the trial versus those who did not complete the trial (Table S2). Most participants were female (121 [54.3%]), and White (149 [66.8%]). The mean (±SD) age was 14.0–1.4 years, with 136 participants (61.0%) in the 12- to 14-year stratum and 87 (39.0%) in the 15- to 16-year stratum. Mean (±SD) baseline weight, BMI, and BMI as a percentage of the 95th percentile were 106.1–23.7 kg, 37.8–7.1 kg/m2, and 142.4–26.8%, respectively. Overall, participants in the trial were generally representative of the adolescent population with obesity in the United States in terms of sex (relatively equal percentage of girls and boys enrolled) and race and ethnicity (enrollment: African American, approximately 27%; Hispanic/Latino, approximately 32%) (Table S1).
Figure 1. CONSORT Diagram.
The mid and top doses are 7.5 mg/46 mg and 15 mg/92 mg of phentermine/topiramate, respectively. For randomly assigned and not treated, the denominator for the percentage in each treatment group is the number of total participants randomly assigned to treatment. For discontinued, the percentage is calculated from participants in the safety population in each treatment group. For primary reason discontinued from study treatment, the percentage is calculated from participants in the intent-to-treat population in each treatment group.
Table 1.
Demographics and Baseline Characteristics.*
| Characteristic | Placebo (n=56) |
Mid-Dose PHEN/TPM (n=54) |
Top-Dose PHEN/TPM (n=113) |
Overall (N=223) |
|---|---|---|---|---|
| Age at screening — yr | 14.0±1.41 | 14.1±1.28 | 13.9±1.36 | 14.0±1.35 |
| Sex — no. (%) | ||||
| Female | 30 (53.6) | 28 (51.9) | 63 (55.8) | 121 (54.3) |
| Male | 26 (46.4) | 26 (48.1) | 50 (44.2) | 102 (45.7) |
| Tanner stage — no. (%)† | ||||
| 2 | 7 (12.5) | 6 (11.1) | 21 (18.6) | 34 (15.3) |
| 3 | 16 (28.6) | 10 (18.5) | 16 (14.2) | 42 (18.8) |
| 4 | 14 (25.0) | 20 (37.0) | 37 (32.7) | 71 (31.8) |
| 5 | 19 (33.9) | 18 (33.3) | 38 (33.6) | 75 (33.6) |
| Race — no. (%) | ||||
| White | 42 (75.0) | 36 (66.7) | 71 (62.8) | 149 (66.8) |
| Black or African American | 10 (17.9) | 14 (25.9) | 36 (31.9) | 60 (26.9) |
| Other | 4 (7.1) | 4 (7.4) | 4 (3.5) | 12 (5.4) |
| American Indian or Alaska Native | 0 | 0 | 1 (0.9) | 1 (0.4) |
| Asian | 0 | 0 | 1 (0.9) | 1 (0.4) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 |
| Ethnicity — no. (%) | ||||
| Hispanic or Latino | 13 (23.2) | 25 (46.3) | 34 (30.1) | 72 (32.3) |
| Not Hispanic or Latino | 42 (75.0) | 28 (51.9) | 79 (69.9) | 149 (66.8) |
| Not stated | 1 (1.8) | 1 (1.9) | 0 | 2 (0.9) |
| Weight — kg | 102.2±21.8 | 105.2±22.4 | 108.5±25.0 | 106.1±23.7 |
| Height — cm | 167.2±7.6 | 168.6±8.0 | 166.3±7.8 | 167.1±7.8 |
| BMI‡ | 36.4±6.4 | 36.9±6.8 | 39.0±7.4 | 37.8±7.1 |
| BMI % of 95th percentile | 136.6±23.1 | 138.7±25.2 | 147.0±28.6 | 142.4±26.8 |
| Waist circumference — cm | 111.1±14.0 | 111.9±15.5 | 116.5±16.8 | 114.0±15.9 |
| Fasting insulin — μIU/ml | 33.2±40.0 | 26.9±19.3 | 26.6±22.8 | 28.4±27.7 |
| WBISI (Matsuda) — mmol/l§ | 2.5±1.7 | 3.0±2.5 | 2.7±2.1 | 2.7±2.1 |
| Triglycerides — mg/dl¶ | 118.3±46.1 | 120.1±61.6 | 112.2±63.2 | 115.7±58.8 |
| HDL-C — mg/dl | 47.2±9.7 | 47.2±8.9 | 46.7±10.1 | 46.9±9.7 |
| Systolic blood pressure — mm Hg | 117.7±10.4 | 121.4±9.2 | 117.4±10.2 | 118.5±10.1 |
| Diastolic blood pressure — mm Hg | 71.7±8.3 | 75.8±6.7 | 72.9±7.3 | 73.3±7.5 |
| Heart rate — beats per min | 76.8±9.9 | 78.6±9.6 | 76.2±9.6 | 76.9±9.7 |
| IWQOL total score∥ | 87.1±10.2 | 85.1±13.1 | 82.7±14.2 | 84.4±13.1 |
Values shown are mean (±SD) unless otherwise noted. The mid and top doses are 7.5 mg/46 mg and 15 mg/92 mg of phentermine/topiramate (PHEN/TPM), respectively. Denominators for percentages are based on the number of participants with nonmissing data in each treatment group for the relevant variable. BMI denotes body-mass index, HDL-C high-density lipoprotein cholesterol, IWQOL Impact of Weight on Quality of Life-Kids, and WBISI whole-body insulin sensitivity index.
One participant in the top-dose group with Tanner stage 1 was randomly assigned to treatment. A protocol deviation was reported, and the participant was subsequently withdrawn from the trial.
BMI is weight in kilograms divided by the square of the height in meters.
The WBISI provides an estimate of insulin sensitivity derived from the standard 2-hour oral glucose tolerance test. Scores range widely but are typically between 0 and 10, with lower scores indicating greater insulin resistance and higher scores indicating greater insulin sensitivity; normal ranges have not yet been established in youth.
To convert values for triglycerides to millimoles per liter, multiply by 0.01129; for HDL-C, multiply by 0.02586.
The IWQOL assesses age-appropriate weight-related quality of life addressing physical comfort, body esteem, social life, and family relations. Scores range from zero (worst quality of life) to 100 (best quality of life); normal ranges have not yet been established in youth.
PRIMARY EFFICACY AND SECONDARY OUTCOMES: BMI AND WAIST CIRCUMFERENCE
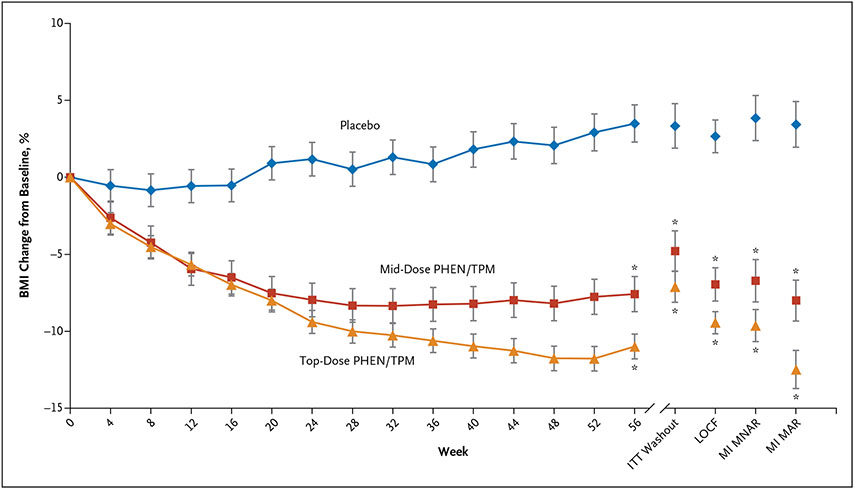
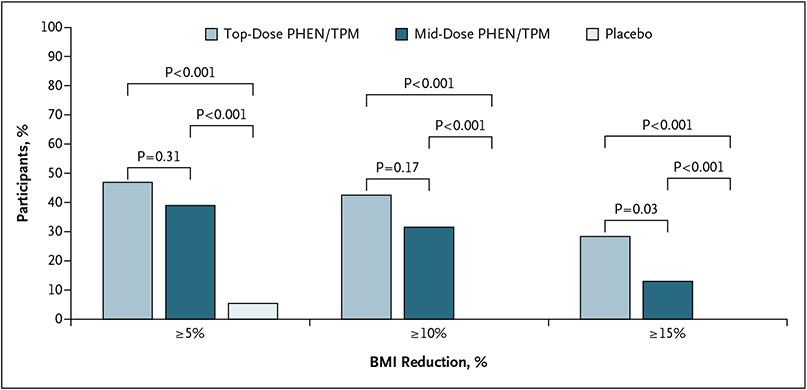
BMI and waist circumference outcomes are shown in Table 2. The treatment difference between the top dose and placebo for percent change in BMI at week 56 was statistically significant (LS mean difference, −10.44 percentage points; 95% CI, −13.89 to −6.99; P<0.001) (Fig. 2). The treatment difference between mid dose and placebo for percent change in BMI at week 56 was also statistically significant (LS mean difference, −8.11%; 95% CI, −11.92 to −4.31; P<0.001) (Fig. 2). The difference in LS mean percent change in BMI between the top dose and mid dose at week 56 was not statistically significant (LS mean difference, −2.33%; 95% CI, −5.27 to 0.62; P=0.12). Figure 3 shows the percentage of participants receiving placebo, mid dose, and top dose who achieved a BMI reduction of 5% or greater (3 [5.4%], 21 [38.9%], and 53 [46.9%], respectively), 10% or greater (0 [0.0%], 17 [31.5%], and 48 [42.5%], respectively), and 15% or greater (0 [0.0%], 7 [13.0%], and 32 [28.3%], respectively) for the ITT population. Treatment with both the mid and top doses resulted in higher proportions of participants with a 5% or greater BMI reduction than placebo (mid dose: RR, 4.6; 95% CI, 1.7 to 12.7; P<0.001; and top dose: RR, 5.6; 95% CI, 2.2 to 14.4; P<0.001), 10% or greater BMI reduction than placebo (mid dose: RR, 9.3; 95% CI, 2.3 to 38.0; P<0.001; and top dose: RR, 12.2; 95% CI, 3.1 to 48.3; P<0.001), and a 15% or greater BMI reduction than placebo (mid dose: RR, 4.3; 95% CI, 1.0 to 19.3; P = 0.008; and top dose: RR, 8.1; 95% CI, 2.0 to 32.7; P<0.001). The treatment differences between top dose and placebo and mid dose and placebo for mean change in waist circumference at week 56 were −9.58 cm (95% CI, −12.83 to −6.33; P<0.001) and −7.72 cm (95% CI, −11.43 to −4.02; P<0.001), respectively.
Table 2.
Estimated Mean Change from Baseline in Primary and Secondary End Points at Week 56.*
| End Point | Placebo (n=56) |
Mid-Dose PHEN/TPM (n=54) |
Top-Dose PHEN/TPM (n=113) |
Mid Dose vs. Placebo (95% CI) |
Top Dose vs. Placebo (95% CI) |
|---|---|---|---|---|---|
| Percent BMI, percentage point difference† | 3.34±1.44 | −4.78±1.30 | −7.11±1.01 | −8.11 (−11.92 to −4.31) | −10.44 (−13.89 to −6.99) |
| BMI | 1.20±0.46 | −2.53±0.44 | −4.15±0.31 | −3.73 (−4.97 to −2.50) | −5.35 (−6.44 to −4.27) |
| BMI % of the 95th percentile | 4.97±1.74 | −9.41±1.65 | −15.28±1.16 | −14.38 (−19.07 to −9.69) | −20.25 (−24.37 to −16.13) |
| Weight, kg | 6.57±1.28 | −5.49±1.23 | −9.23±0.86 | −12.06 (−15.55 to −8.58) | −15.80 (−18.82 to −12.77) |
| Waist circumference, cm | 0.31±1.39 | −7.42±1.29 | −9.27±0.91 | −7.72 (−11.43 to −4.02) | −9.58 (−12.83 to −6.33) |
| WBISI (Matsuda), mmol/l‡ | 1.06±0.62 | 1.25±0.54 | 1.85±0.38 | 0.19 (−1.45 to 1.82) | 0.80 (−0.64 to 2.24) |
| HDL-C, percentage point difference | −3.65±2.85 | 6.65±2.45 | 5.10±1.76 | 10.30 (2.91 to 17.70) | 8.75 (2.15 to 15.35) |
| Triglycerides, percentage point difference | 8.35±7.37 | −12.79±6.30 | −12.37±4.49 | −21.14 (−40.24 to −2.05) | −20.72 (−37.71 to −3.72) |
| Systolic blood pressure, mm Hg | 2.80±1.62 | −0.97±1.50 | 1.00±1.04 | −3.76 (−8.09 to 0.56) | −1.80 (−5.58 to 1.97) |
| Diastolic blood pressure, mm Hg | 3.11±1.33 | −0.87±1.22 | 0.14±0.85 | −3.99 (−7.53 to −0.45) | −2.97 (−6.07 to 0.12) |
| IWQOL total score§ | 2.94±1.89 | 4.31±1.70 | 3.11±1.20 | 1.37 (−3.64 to 6.39) | 0.17 (−4.25 to 4.60) |
| Heart rate, beats per min | 1.46±1.72 | −1.31±1.57 | 5.27±1.10 | −2.77 (−7.32 to 1.79) | 3.82 (−0.17 to 7.81) |
Values are means (±SE) unless indicated otherwise. The mid and top doses are 7.5 mg/46 mg and 15 mg/92 mg of phentermine/topiramate (PHEN/TPM), respectively. Denominators for percentages are based on the number of participants with nonmissing data in each treatment group for the relevant variable. BMI denotes body-mass index, CI confidence interval, HDL-C high-density lipoprotein cholesterol, IWQOL Impact of Weight on Quality of Life-Kids, and WBISI whole-body insulin sensitivity index.
Percent BMI was the only variable for which washout imputation was used. The CIs for secondary outcomes were not adjusted and inferences drawn from the intervals may not be reproducible. BMI is weight in kilograms divided by the square of the height in meters.
The WBISI provides an estimate of insulin sensitivity derived from the standard 2-hour oral glucose tolerance test. Scores range widely but are typically between 0 and 10, with lower scores indicating greater insulin resistance and higher scores indicating greater insulin sensitivity; normal ranges have not yet been established in youth.
The IWQOL assesses age-appropriate weight-related quality of life addressing physical comfort, body esteem, social life, and family relations. Scores range from zero (worst quality of life) to 100 (best quality of life); normal ranges have not yet been established in youth.
Figure 2. Percent Body-Mass Index Change Over Time.
Plot of least square means (±SE) of percentage change in body-mass index (BMI) using observed data from baseline to week 56 by treatment group (with the primary analysis, modified intent-to-treat [ITT], and other imputation models). The mid and top doses are 7.5 mg/46 mg and 15 mg/92 mg of phentermine/topiramate (PHEN/TPM), respectively. LOCF denotes last observation carried forward, MAR missing at random, MI multiple imputation, and MNAR, missing not at random. *P<0.001 versus placebo.
Figure 3. Percentage of Participants Achieving Various Body-Mass Index Reduction Benchmarks.
Percentage of participants achieving a reduction of 5%, 10%, and 15% or greater in body-mass index (BMI) from baseline to week 56 by treatment group, intent-to-treat (ITT) population. The mid and top doses are 7.5 mg/46 mg and 15 mg/92 mg of phentermine/ topiramate (PHEN/TPM), respectively.
CARDIOMETABOLIC RISK FACTORS AND HEALTH-RELATED QUALITY OF LIFE
Cardiometabolic risk factor and health-related quality of life outcomes are shown in Table 2. LS mean treatment differences between top dose and placebo for triglycerides and HDL-C were −20.72% (95% CI, −37.71 to −3.72) and 8.75% (95% CI, 2.15 to 15.35), respectively. LS mean treatment differences between mid dose and placebo for triglycerides and HDL-C were −21.14% (95% CI, −40.24 to −2.05) and 10.30% (95% CI, 2.91 to 17.70), respectively. There were no statistically significant treatment differences for change at week 56 in fasting insulin, WBISI, IWQOL-Kids questionnaire scores, glycemic markers, or total or low-density lipoprotein cholesterol among any of the groups. Changes at week 56 for systolic blood pressure did not show significant differences between groups.
SAFETY
A summary of the adverse events is shown in Table 3. The incidence of participants reporting at least one adverse event was 51.8%, 37.0%, and 52.2% in the placebo, mid-dose, and top-dose groups, respectively. Adverse events leading to dose reduction occurred in three participants: one (1.9%) in the mid-dose group and two (1.8%) in the top-dose group. Adverse events leading to discontinuation of study treatment occurred in three participants: two (3.6%) in the placebo group and one (0.9%) in the top-dose group. One participant (2.0%) in the placebo group, one participant (2.0%) in the mid-dose group, and two participants (1.8%) in the top-dose group withdrew from the study due to treatment-emergent adverse events.
Table 3.
Adverse Events Reported by System Organ Class and Preferred Term with an Incidence of 3% of Participants or Greater in All Treatment Groups, Safety Population.*
| System Organ Class and Preferred Term | Placebo (n=56) |
Mid-Dose PHEN/TPM (n=54) |
Top-Dose PHEN/TPM (n=113) |
Overall (N=223) |
|---|---|---|---|---|
| Any treatment-emergent adverse event | 29 (51.8) | 20 (37.0) | 59 (52.2) | 108 (48.4) |
| Infections and infestations | 15 (26.8) | 9 (16.7) | 25 (22.1) | 49 (22.0) |
| Covid-19 | 4 (7.1) | 2 (3.7) | 4 (3.5) | 10 (4.5) |
| Upper respiratory tract infection | 4 (7.1) | 1 (1.9) | 4 (3.5) | 9 (4.0) |
| Nasopharyngitis | 3 (5.4) | 1 (1.9) | 2 (1.8) | 6 (2.7) |
| Gastroenteritis viral | 2 (3.6) | 1 (1.9) | 1 (0.9) | 4 (1.8) |
| Influenza | 0 | 2 (3.7) | 2 (1.8) | 4 (1.8) |
| Nervous system disorders | 7 (12.5) | 5 (9.3) | 16 (14.2) | 28 (12.6) |
| Headache | 5 (8.9) | 4 (7.4) | 5 (4.4) | 14 (6.3) |
| Dizziness | 0 | 1 (1.9) | 4 (3.5) | 5 (2.2) |
| Gastrointestinal disorders | 8 (14.3) | 7 (13.0) | 12 (10.6) | 27 (12.1) |
| Abdominal pain | 2 (3.6) | 0 | 0 | 2 (0.9) |
| Nausea | 2 (3.6) | 2 (3.7) | 5 (4.4) | 9 (4.0) |
| Respiratory, thoracic and mediastinal disorders | 7 (12.5) | 4 (7.4) | 13 (11.5) | 24 (10.8) |
| Nasal congestion | 4 (7.1) | 3 (5.6) | 3 (2.7) | 10 (4.5) |
| Oropharyngeal pain | 2 (3.6) | 0 | 3 (2.7) | 5 (2.2) |
| General disorders and administration site conditions | 3 (5.4) | 2 (3.7) | 13 (11.5) | 18 (8.1) |
| Pyrexia | 1 (1.8) | 1 (1.9) | 5 (4.4) | 7 (3.1) |
| Injury, poisoning, and procedural complications | 5 (8.9) | 5 (9.3) | 7 (6.2) | 17 (7.6) |
| Ligament sprain | 0 | 2 (3.7) | 2 (1.8) | 4 (1.8) |
| Psychiatric disorders | 1 (1.8) | 4 (7.4) | 10 (8.8) | 15 (6.7) |
| Depression | 0 | 1 (1.9) | 5 (4.4) | 6 (2.7) |
| Musculoskeletal and connective tissue disorders | 1 (1.8) | 4 (7.4) | 10 (8.8) | 15 (6.7) |
| Arthralgia | 0 | 1 (1.9) | 4 (3.5) | 5 (2.2) |
| Back pain | 2 (3.6) | 0 | 2 (1.8) | 4 (1.8) |
| Vascular disorders | 2 (3.6) | 0 | 2 (1.8) | 4 (1.8) |
| Hypertension | 2 (3.6) | 0 | 2 (1.8) | 4 (1.8) |
Data are no. (%). The mid and top doses are 7.5 mg/46 mg and 15 mg/92 mg of phentermine/topiramate (PHEN/TPM), respectively. The denominator for percentages is the number of participants in the safety population. The MedDRA Dictionary (version 23.1) was used for coding. If a participant experienced more than one episode of an adverse event, the participant was counted only once within a preferred term. If a participant experienced more than one adverse event within a system organ class, the participant was counted once for each preferred term and once for the system organ class.
Overall, three serious adverse events (bile duct stone, depression, and suicidal ideation) were reported in two participants who were both in the top-dose group (Supplementary Appendix, page 10). One participant was hospitalized for a bile duct stone within 1 week of completing the study. The other participant experienced depression and suicidal ideation, which were initially considered related to the study drug by the site investigator and the study drug was discontinued. However, the participant experienced several recurrences of these events over a period of approximately 105 days after study drug discontinuation, and all of these recurrences were considered by the investigator to be unrelated to the study drug. In addition, although these events occurred in a participant randomly assigned to the top-dose group, the events occurred during the first month of treatment before the participant was scheduled to titrate up to their randomized dose level. Consequently, this participant was never exposed to a dose higher than the mid dose during study participation (i.e., in effect, the events occurred while taking the mid dose).
Events related to psychiatric disorders (system organ class) occurred in four participants (7.4%) in the mid-dose group and 10 participants (8.8%) in the top-dose group compared with one participant (1.8%) in the placebo group (Supplementary Appendix, page 11). There were no clinically relevant differences observed across groups with respect to mental health questionnaire (PHQ-9 and C-SSRS) results. Results of the CANTAB assessments (cognition) demonstrated no apparent differences among groups. No adverse events related to drug abuse, dependence, or withdrawal were reported. There were no apparent differences among groups in bone age or bone health assessments.
Discussion
PHEN/TPM at both the mid and top doses, as an adjunct to lifestyle therapy, led to a superior reduction in the percent change in BMI versus placebo in adolescents with obesity. Although consensus has been established in adults that a loss of 3% to 5% of body weight is considered clinically meaningful,14 a similar threshold of BMI reduction in children and adolescents has yet to be identified and agreed upon. In the context of the current study, it is relevant to note that the degree of BMI reduction in both the mid- and top-dose groups of PHEN/TPM was sufficient to elicit improvements in certain cardiometabolic risk factors, suggesting that the treatment effect was in fact clinically significant. Moreover, a systematic review15 conducted in support of the Endocrine Society pediatric obesity treatment clinical practice guideline4 concluded that a BMI reduction of at least 1.6 kg/m2 could be considered clinically meaningful. The mean placebo-subtracted BMI reduction with the mid dose of PHEN/TPM exceeded this threshold by more than twofold and the top dose of PHEN/TPM by more than threefold. From a categorical perspective, a much higher proportion of participants assigned to PHEN/TPM compared with placebo were able to reduce their BMI below thresholds of 5%, 10%, or 15%. Mean reductions in BMI with PHEN/TPM observed in this trial were higher than mean reductions in BMI reported in lifestyle therapy trials,5,6 tertiary care pediatric weight management programs in the United States,16 and the adolescent clinical trials of orlistat and liraglutide.7,8
Regarding notable secondary outcomes in the current trial, weight loss with PHEN/TPM at both the mid and top doses reduced waist circumference compared with placebo. In addition, weight loss with both the mid and top doses of PHEN/TPM was associated with reduced triglycerides and increased HDL-C. Although these changes fell short of statistical significance after adjustment for multiple secondary end points, the magnitude of these effects was similar to that previously observed in adults.10-12 The finding of improvements in the lipid profile are particularly important, since PHEN/TPM is currently the only antiobesity medication for adolescents that has now demonstrated a degree of weight loss associated with favorable changes in cardiometabolic risk factors.7,8
In general, the safety profile of PHEN/TPM was consistent with the findings from adult trials.10-12 The overall incidence of any adverse event was relatively similar across the placebo, mid-dose, and top-dose groups. Few participants withdrew from the study due to adverse events. Only three serious adverse events (bile duct stone, depression, and suicidal ideation) were reported in two participants in the top-dose group, although one of those participants had been exposed to only the mid dose. Importantly, PHEN/TPM had no apparent effect on growth, development, mental health, or cognition.
Strengths of this trial included the relatively large sample size, the randomized and placebo-controlled trial design, and the diversity of the sample population. The primary limitation was the degree of attrition, which requires context. This trial was conducted during the Covid-19 pandemic, which led to public health emergency declarations in the United States and resulting stay-at-home orders and restrictions. Due to local regulations impacting study sites, not all planned assessments could be performed, including the final 56-week assessment. Despite additional efforts to retain participants by performing remote visits and direct shipping of study medications, we suspect that hesitancy to attend in-person visits may at least partly explain the higher than expected lost to follow-up rate. From an analysis perspective, we utilized conservative multiple imputation techniques, specified a priori in the statistical analysis plan, which minimized the potential of overestimating the treatment effect. Finally, weight loss trajectories in the context of obesity interventions may have been negatively altered in youth during the Covid-19 pandemic,17 which may have influenced BMI outcomes across all groups in the current trial, including those of the placebo group receiving lifestyle therapy only.
In conclusion, PHEN/TPM at both the mid and top doses, as an adjunct to lifestyle therapy, offered a statistically significant reduction in BMI and waist circumference as well as favorably impacted levels of triglycerides and HDL-C in adolescents with obesity. The safety profile was similar to that observed in adults.
Supplementary Material
Disclosures
This trial was funded by VIVUS LLC, with project support provided by Covance LLC.
We thank the participants and their families, the site personnel who assisted with the trial, and the data monitoring committee.
Footnotes
Author disclosures and other supplementary materials are available at evidence.nejm.org.
A data sharing statement provided by the authors is available at evidence.nejm.org.
References
- 1.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–1604. DOI: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 2.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol 2011;301:R581–R600. DOI: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardel MI, Atkinson MA, Taveras EM, Holm JC, Kelly AS. Obesity treatment among adolescents: a review of current evidence and future directions. JAMA Pediatr 2020;174:609–617. DOI: 10.1001/jamapediatrics.2020.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017;102:709–757. DOI: 10.1210/jc.2016-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 2017;317:2417–2426. DOI: 10.1001/jama.2017.6803. [DOI] [PubMed] [Google Scholar]
- 6.Ells LJ, Rees K, Brown T, et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int J Obes 2018;42:1823–1833. DOI: 10.1038/s41366-018-0230-y. [DOI] [PubMed] [Google Scholar]
- 7.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 2005;293:2873–2883. DOI: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 8.Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 2020;382:2117–2128. DOI: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava G, Fox CK, Kelly AS, et al. Clinical considerations regarding the use of obesity pharmacotherapy in adolescents with obesity. Obesity (Silver Spring) 2019;27:190–204. DOI: 10.1002/oby.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–1352. DOI: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 11.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 2012;20:330–342. DOI: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012; 95:297–308. DOI: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsia DS, Gosselin NH, Williams J, et al. A randomized, double-blind, placebo-controlled, pharmacokinetic and pharmacodynamic study of a fixed-dose combination of phentermine/topiramate in adolescents with obesity. Diabetes Obes Metab 2020;22:480–491. DOI: 10.1111/dom.13910. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(Suppl 2):S102–S138. DOI: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajjo T, Almasri J, Al Nofal A, et al. The association of weight loss and cardiometabolic outcomes in obese children: systematic review and meta-regression. J Clin Endocrinol Metab 2017;102:758–762. DOI: 10.1210/jc.2016-2575. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, King EC, Christison AL, et al. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr 2019;208:57–65.e4. DOI: 10.1016/j.jpeds.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 17.Appelhans BM, French SA, Martin MA, Lui K, Janssen I. Attenuated efficacy of pediatric obesity treatment during the COVID-19 pandemic. Obesity (Silver Spring) 2022;30:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.