Abstract
The Proterodiplostomidae Dubois, 1936 is a family of digeneans within the superfamily Diplostomoidea Poirier, 1886. Members of the family are distributed mostly in the tropics and subtropics, primarily in crocodilians, although some species are known from other reptiles. Despite their broad geographical distribution, the knowledge of proterodiplostomid diversity remains limited, mostly because a number of potential host species and regions of the world have not been sufficiently explored for these parasites. In this study, we use morphological and molecular data to describe four new genera (Afroproterodiplostomum gen. nov., Dungalabatrema gen. nov., Australiadiplostomum gen. nov. and Nattererodiplostomum gen. nov.) and five new species (Afroproterodiplostomum ingwenyae sp. nov., Australiadiplostomum blairi sp. nov., Dungalabatrema kostadinovae sp. nov., Dungalabatrema snyderi sp. nov. and Pseudoneodiplostomum angustus sp. nov.) of proterodiplostomids from crocodilians in Australia, South Africa and South America. Nattererodiplostomum gen. nov. has been established upon re-evaluation of the status of Proterodiplostomum medusae (Dubois, 1936) from caimans in Brazil using combined morphological and molecular evidence. Only a few previous studies provided DNA sequence data of proterodiplostomids. We generated partial 28S rDNA and cytochrome c subunit (cox1) mtDNA for three previously undescribed proterodiplostomids collected from Crocodylus spp. in Australia and South Africa. The newly generated 28S sequences were used to examine phylogenetic affinities of these taxa. All three newly sequenced proterodiplostomid species appeared in the phylogenetic tree in a strongly supported monophyletic clade comprising exclusively parasites of Crocodylus.
Keywords: Africa, Australia, crocodilians, Diplostomoidea, Proterodiplostomidae, South America
Introduction
The Diplostomoidea Poirier, 1886 is a diverse superfamily of digeneans that currently contains five families (the Bolbocephalodidae Strand, 1935, Cyathocotylidae Mühling, 1896, Diplostomidae Poirier, 1886, Proterodiplostomidae Dubois, 1936 and Strigeidae Railliet, 1919). Although these families, with the exception of the Bolbocephalodidae, include at least some digeneans known from crocodilian definitive hosts, the Proterodiplostomidae is the only family with the majority of its members parasitic in crocodilians (Achatz et al., 2019; Achatz, Brito, et al., 2021; Bisseru, 1956, 1957; Dubois, 1936, 1979; MacCallum, 1921; Niewiadomska, 2002; Tellez, 2013; Tkach et al., 2020).
Only a few studies have generated DNA sequences from proterodiplostomids (Achatz, Brito, et al., 2021; Hernández-Mena et al., 2017; Negrelli et al., 2020; Queiroz et al., 2020;Tkach et al., 2020), and only a single published study produced DNA sequences of proterodiplostomids from crocodilians (Tkach et al., 2020) with a few additional unpublished studies available in the GenBank. Tkach et al. (2020) published results of phylogenetic analyses based on the first substantial molecular dataset for the Proterodiplostomidae. Their phylogeny convincingly demonstrated the monophyly of the family. This was not the case, however, for the subfamilies of the Proterodiplostomidae recognized at the time, therefore Tkach et al. (2020) abandoned the use of subfamilies based on molecular phylogenetic data.
Herein, we describe five new species of proterodiplostomids collected from the Nile crocodile Crocodylus niloticus Laurenti in South Africa and the Australian freshwater crocodile Crocodylus johnstoni Krefft in Australia, using a combination of morphological and molecular data. We also establish four new proterodiplostomid genera from Africa, Australia and South America. Due to the substantial increase in the diversity at genus level and additional recent systematic changes in the group, we provide an amended key to assist in differentiation among proterodiplostomid genera. Due to exclusion of Proterodiplostomum medusae (Dubois, 1936) from Proterodiplostomum Dubois, 1936, an amended diagnosis of Proterodiplostomum is also provided. Newly generated and previously published partial sequences of the nuclear large ribosomal subunit (28S) gene of proterodiplostomids were used to study the phylogenetic interrelationships within the family.
Materials and Methods
Abbreviations of names of genera
Names of multiple genera discussed in this text begin with the same letters. In order to avoid confusion, we opt to use the following abbreviations to refer to genera: Af. – Afroproterodiplostomum gen. nov.; Al. – Alligator Cuvier; Au. – Australiadiplostomum gen. nov.; Ca. – Caiman Spix; Cr. – Crocodylus Laurenti; Cy. – Cystodiplostomum Dubois, 1936; He. – Herpetodiplostomum Dubois, 1936; Ht. – Heterodiplostomum Dubois, 1936; Ps. – Pseudoneodiplostomum Dubois, 1936; Pr. – Proterodiplostomum Dubois, 1936; Pe. – Pseudoneodiplostomoides Yamaguti, 1954; Pl. – Prolecithodiplostomum Dubois, 1936.
Sample collection and morphological study
Adult proterodiplostomids were obtained from the intestines of Cr. johnstoni from the Daly River near Oolloo Crossing, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E) in 2006 and Cr. niloticus from the Olifants River, Limpopo province (24°2’S, 31°13’E) and the Crocodile River, Mpumalanga province, South Africa (25°27’S, 31°58’E) in 2010 (Table 1). Live proterodiplostomids from Cr. johnstoni were rinsed in saline, heat-killed with hot water and fixed in 70% ethanol; the live proterodiplostomids from Cr. niloticus were killed with hot saline, fixed in 10% formalin, and transferred to 70% ethanol. The digeneans studied using light microscopy were stained with aqueous alum carmine following the protocol provided by Lutz et al. (2017). We also re-examined the morphology of the two Pseudoneodiplostomum cf. siamense specimens of Tkach et al. (2020). Stained proterodiplostomids were studied using a DIC-equipped Olympus BX51 compound microscope (Olympus Corp., Tokyo, Japan). Drawings were prepared using a Leica DMC 4500 microscope (Buffalo Grove, Illinois, U.S.A.) with the aid of a drawing tube. All measurements given in the text and tables are in micrometers. Type-series and morphological vouchers were deposited in the collection of the H. W. Manter Laboratory, University of Nebraska, Lincoln, Nebraska, U.S.A. and the Queensland Museum, Brisbane, Australia (QM).
Table 1.
Hosts, geographic origin, GenBank accession numbers and Harold W. Manter Laboratory (HWML) museum numbers of proterodiplostomids described in this study.
| Taxa | Host species | Geographic origin | Museum No. | Accession numbers |
|
|---|---|---|---|---|---|
| 28S | cox1 | ||||
| Afroproterodiplostomum ingwenyae sp. nov. | Crocodylus niloticus | South Africa | HWML 216796, 216797 | – | – |
| Australiadiplostomum blairi sp. nov. | Crocodylus johnstoni | Australia | QM G239753– G239754 | MT622365 * | MT603623 * |
| Dungalabatrema kostadinovae sp. nov. | Crocodylus johnstoni | Australia | QM G239755– G239762, HWML 216798 | OM569683 | OM568683, OM568684 |
| Dungalabatrema snyderi sp. nov. | Crocodylus johnstoni | Australia | QM G239763–G239775 HWML 216799 |
OM569684, OM569685 | OM568685– OM568688 |
| Pseudoneodiplostomum angustus sp. nov. | Crocodylus niloticus | South Africa | HWML 216800–216802 | OM569686 | – |
Sequences published by Tkach et al. (2020).
For comparative purposes we examined morphology the of the following proterodiplostomid specimens in the collection of the Natural History Museum, London (NHM): Pseudoneodiplostomum bifurcatum (Wedl, 1862) from Cr. niloticus collected in Zimbabwe (catalog number 1962.11.1.5-6, 1962.8.3.20-26), South Africa (1978.11.3.41-44) and Sudan (1962.4.27.4), Pseudoneodiplostomum sp. from Cr. niloticus in Ghana (1976.4.12.66-69, 1976.1.7.23), Pseudoneodiplostomum sp. from dwarf crocodile Osteolaemus tetraspis Cope in Nigeria (2006.12.6.58-61) and Pseudoneodiplostomum siamense (Poirier, 1886) from saltwater crocodile Crocodylus porosus Schneider in Papua New Guinea (1993.9.2-3).
The terms prosoma and opisthosoma are used to refer to the two distinct body parts/regions of proterodiplostomids. We also use the unified term “muscular pouch” to refer to the muscular structure surrounding one or more terminal parts of the reproductive system. The justification for our use of terminology is provided in detail by Tkach et al. (2020).
Molecular study
The genomic DNA of individual proterodiplostomids was isolated following the protocol provided by Tkach and Pawlowski (1999). We amplified fragments of 28S and mitochondrial cytochrome c oxidase subunit I (cox1) genes using polymerase chain reactions (PCR) from the four newly collected proterodiplostomid taxa. The PCR amplifications of 28S were performed using the forward primer digL2 (5’-AAG CAT ATC ACT AAG CGG-3’) and reverse primer 1500R (5’-GCT ATC CTG AGG GAA ACT TCG-3’) (Tkach et al., 2003). The fragment of the cox1 gene was amplified using forward primer Dipl_Cox_5’ (5’-ACK TTR GAW CAT AAG CG-3’) and reverse primer Dipl650R (5’-CCA AAR AAY CAR AAY AWR TGY TG-3’) (Achatz, Brito, et al., 2021). The PCRs were carried out in a total volume of 25 μl using One-Taq quick load PCR mix from New England Biolabs (Ipswich, Massachusetts, U.S.A.) according to the manufacturers’ instructions. Annealing temperatures of 53°C and 45°C were used for the ribosomal and mitochondrial amplifications, respectively.
Illustra ExoProStar PCR clean-up enzymatic kit from Cytiva (Marlborough, Massachusetts, U.S.A.) was used to purify PCR products and BrightDye Terminator Cycle Sequencing Kit (MCLAB, California, U.S.A.) was used to cycle-sequence purified PCR products. Sequencing reactions were cleaned using a BigDye Sequencing Clean Up Kit from MCLAB and subsequently run on an ABI 3130 automated capillary sequencer (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.A.). The PCR primers were used for sequencing reactions. In addition, internal forward primers DPL250F (5’-GGG TTG TTT GTG AAT GCA GCC C-3’) and DPL600F (5’- CGG AGT GGT CAC CAC GAC CG -3’) and internal reverse primers DPL350R (5’-GTT TAC CTC TGA GCG GTT TCA CG-3’), DPL700R (5’-CAG CTG ATT ACA CCC AAA G-3’), DPL1300R (5’-GCC TTT GGG TTT CGT AAC GCC-3’) and DPL1450R (5’-GAC GGG CCG GTG ATG CGC C-3’) were used for sequencing of 28S amplicons (Achatz et al., 2019; Achatz, Martens, et al., 2021). Contiguous sequences were assembled using Sequencher 4.2 software (GeneCodes Corp., Ann Arbor, Michigan, U.S.A.); newly generated sequences are deposited in GenBank (Table 1).
Despite our best efforts, we were unable to obtain high-quality sequence data of either locus from the new species and genus from Africa described in this work or cox1 from the new Pseudoneodiplostomum species.
Phylogenetic analyses
The three new sequences obtained and 28 previously published 28S sequences of proterodiplostomids were aligned using ClustalW as implemented in MEGA7 software (Kumar et al., 2016). The alignment was trimmed to the length of the shortest sequence included in the analysis (1,085 bp); 12 nucleotide positions with ambiguous homology were excluded from the analyses. Alaria mustelae Bosma, 1931 was used as the outgroup in the analysis based on the tree topology published by Tkach et al. (2020).
The phylogenetic analysis was conducted using Bayesian inference as implemented in MrBayes v3.2.6 software (Ronquist & Huelsenbeck, 2003). The general time-reversible model with estimates of invariant sites and gamma-distributed among-site variation (GTR + G + I) model was identified as the best-fitting nucleotide substitution model for all alignments using MEGA7 (Kumar et al., 2016). The phylogenetic analysis was performed using MrBayes software as follows: Markov chain Monte Carlo (MCMC) chains were run for 6,000,000 generations with sample frequency set at 1,000. The number of generations was determined to be sufficient because the standard deviation stabilized below 0.01. Log-likelihood scores were plotted and only the final 75% of trees were retained to produce the consensus trees.
Results
Molecular phylogeny
Overall, the 28S phylogeny was well-resolved and strongly supported, except for the most basal clades (Fig. 1). The branch topology and branch support values were similar to the results presented by Tkach et al. (2020) and Achatz, Brito, et al. (2021).
Fig. 1.
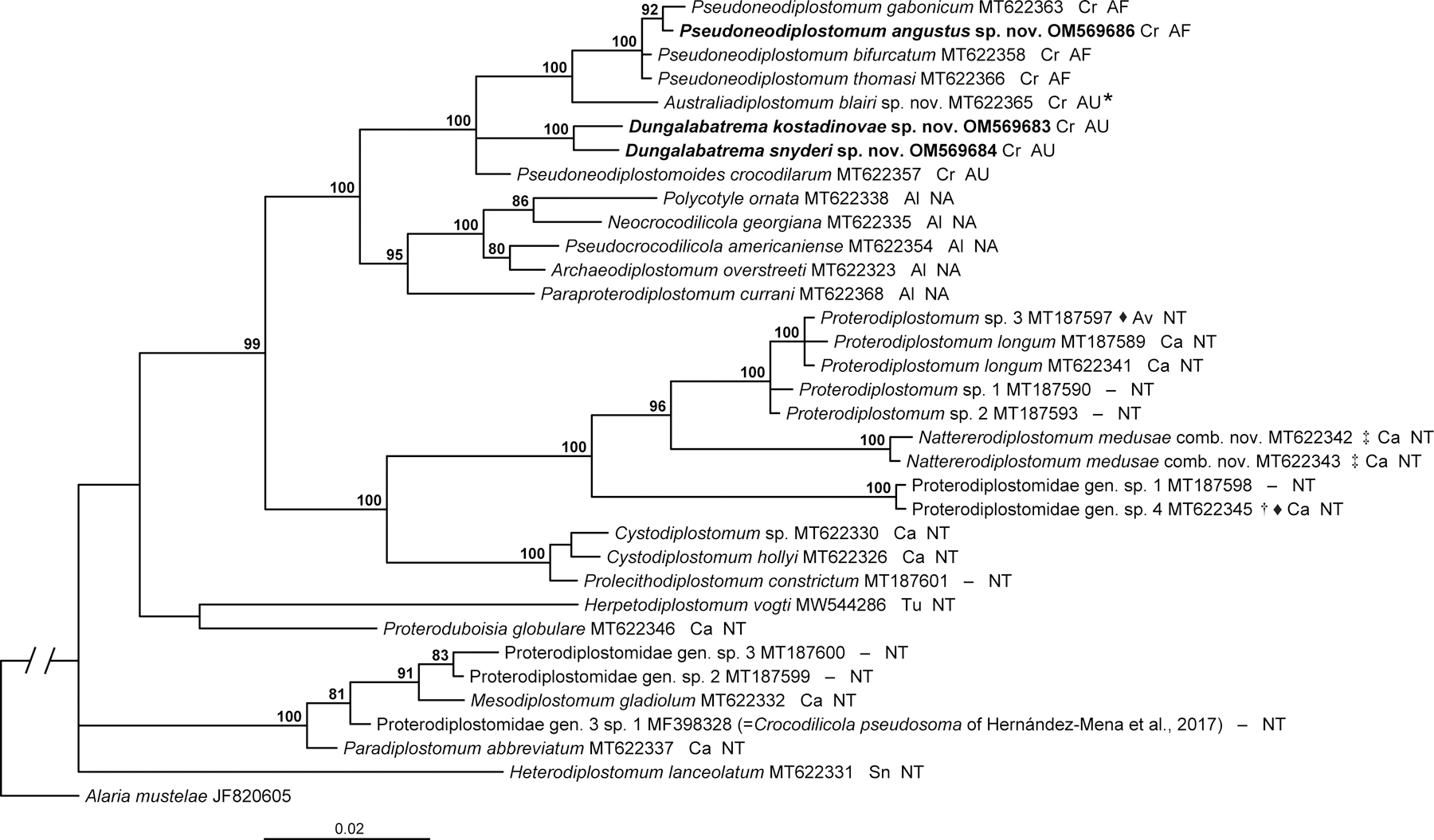
Phylogenetic interrelationships among 33 proterodiplostomids based on Bayesian Inference (BI) analysis of partial 28S rDNA gene sequences. Bayesian inference posterior probability values lower than 80% are not shown. The new sequences generated in this study are indicated in bold. The scale-bar indicates the number of substitutions per site. Definitive host groups and biogeographical realms are indicated next to species names. A dash indicates that definitive hosts are not known. Abbreviations for definitive host groups: Al, alligators (Alligator); Av, avians (birds); Ca, caimans; Cr, true crocodiles (Crocodylus); Sn, snakes; Tu, turtles. Abbreviations for biogeographical realms: AF, Afrotropical realm; AU, Australasian realm; NA, Nearctic realm; NT, Neotropical realm.* Formerly referred to as Pseudoneodiplostomum cf. siamense by Tkach et al. (2020). ‡ Formerly considered a member of Proterodiplostomum. † Formerly referred to as a member of Proterodiplostomum by Tkach et al. (2020). ♦ Specimen immature.
The basal polytomy in the phylogeny includes three clades. The first only contained Heterodiplostomum lanceolatum Dubois, 1936 (type-species) from the Neotropics. The second included a 100% supported clade including Paradiplostomum abbreviatum (Brandes, 1888) (type-species of the genus) and an 81% supported cluster of four other proterodiplostomids, all from the Neotropics. The latter cluster included a sequence from a misidentified Crocodilicola pseudostoma Willemoes-Suhm, 1870 of Hernández-Mena et al., 2017 (see Achatz, Brito, et al., 2021) + 91% supported clade of [Mesodiplostomum gladiolum Dubois, 1936 (type-species) + 83% supported grouping of two unknown proterodiplostomids].
The third clade in the basal polytomy was weakly supported and contained the majority of proterodiplostomid taxa (Fig. 1). It consisted of an unsupported clade of [Proteroduboisia globulare (Catto et Amato, 1994) (type-species) + Herpetodiplostomum vogti Achatz, Brito, Fecchio et Tkach, 2021], both from the Neotropics, and a 99% supported clade with all other proterodiplostomids. The latter clade included two 100% supported major subclades (Fig. 1).
The first of these major subclades included a 100% supported grouping of Prolecithodiplostomum spp. and Cystodiplostomum spp. + 100% supported clade of an unidentified proterodiplostomid genus and a 96% supported clade containing sequences of seven proterodiplostomids, all from the Neotropics. The 96% supported clade positioned Nattererodiplostomum medusae comb. nov. (previously Pr. medusae; see section 4.1) as a sister group to a 100% supported clade of Proterodiplostomum spp., which included the type-species, Proterodiplostomum longum (Brandes, 1888).
The second major subclade included a 95% supported clade containing the taxa from North America and a 100% supported clade containing the proterodiplostomids from Africa and Australia (Fig. 1). Within the 95% supported clade, Paraproterodiplostomum currani Tkach, Achatz et Pulis, 2020 (type-species) was positioned as a sister group to the 100% supported clade of [Archaeodiplostomum overstreeti Tkach, Achatz et Pulis, 2020 + Pseudocrocodilicola americaniense Byrd et Reiber, 1942 (80% supported)] and [Neocrocodilicola georgiana (Byrd et Reiber, 1942) + Polycotyle ornata Willemoes-Suhm, 1870 (86% supported)].
The 100% supported clade containing the proterodiplostomids from Africa and Australia included a basal polytomy with three clades (Fig. 1). The first clade only included Pseudoneodiplostomoides crocodilarum (Tubangui et Masiluñgan, 1936) from Australia. The second clade included both members of Dungalabatrema gen. nov. from Australia (100% supported). The third clade (100% supported) positioned Australiadiplostomum blairi sp. nov. (previously known as Pseudoneodiplostomum cf. siamense of Tkach et al. (2020); see section 3.2) from Australia as a sister group to the 100% supported clade of Pseudoneodiplostomum spp. from Africa (Fig. 1).
Descriptions of new taxa
Dungalabatrema gen. nov. Achatz, Chermak et Tkach
TYPE-SPECIES: Dungalabatrema kostadinovae sp. nov.
OTHER SPECIES: Dungalabatrema snyderi sp. nov.
ETYMOLOGY: The name of the new genus refers to the word for crocodile in one of the indigenous Australian languages, Larrakia (anglicized version is spelled ‘Dungalaba’).
DIAGNOSIS: Body distinctly bipartite; prosoma flattened, elongate, similar in length to cylindrical opisthosoma. Oral sucker and ventral sucker present; holdfast organ elliptical, with papillae on margin of median slit. Pseudosuckers absent. Pharynx moderately well-developed; ceca reaching near posterior margin of posterior testis or somewhat more posteriorly. Testes 2, tandem. Paraprostate short, surrounded by glandular muscular pouch. Ejaculatory duct joins proximal part of paraprostate within muscular pouch. Common male duct opens at apex of small genital cone. Ovary pretesticular; oötype intertesticular. Vitellarium distributed throughout prosoma and opisthosoma, anterior extent before or at level of ventral sucker, posterior extent reaching posterior testis. Metraterm opens into genital atrium separately from male duct near proximal part of genital cone. Genital atrium with subterminal opening, on dorsal side. Excretory pore terminal. In crocodilians. Australasia.
REMARKS
Dungalabatrema gen. nov. clearly belongs to the Proterodiplostomidae based on the presence of a paraprostate. This new genus can be distinguished from all other proterodiplostomid genera, except for Capsulodiplostomum Dwivedi, 1966, Herpetodiplostomum, Neocrocodilicola Tkach, Achatz et Pulis, 2020 and Pseudocrocodilicola Byrd et Reiber, 1942, by the presence of a muscular pouch surrounding the paraprostate.
The topology of the terminal reproductive ducts in members of Dungalabatrema gen. nov. can be used to differentiate the genus from the remaining four genera, since it has a pouch surrounding the paraprostate. In the new genus, the ejaculatory duct joins the proximal part of paraprostate within the muscular pouch. The metraterm also opens into the genital atrium separately from the male duct near the proximal part of the genital cone; the metraterm does not join or enter the muscular pouch. In contrast, the muscular pouch of Capsulodiplostomum encloses the paraprostate, ejaculatory duct and uterus. In Herpetodiplostomum, the ejaculatory duct opens into the genital atrium separately from the paraprostate, while in Neocrocodilicola and Pseudocrocodilicola the ejaculatory duct, paraprostate and metraterm unite to form a hermaphroditic duct. Furthermore, the new genus appears as quite distant from Herpetodiplostomum, Neocrocodilicola and Pseudocrocodilicola in the molecular phylogeny (Fig. 1); no DNA sequence data is currently available for Capsulodiplostomum.
Dungalabatrema kostadinovae sp. nov. Achatz, Chermak et Tkach
HOLOTYPE: QM G239755, labeled ex. Crocodylus johnstoni, small intestine, Ooloo Crossing, Daly River, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E), 24 May 2006, coll. V. Tkach. PARATYPES: QM G239756 - 239762 (lot of 7 slides), labels identical to the holotype; HWML 216798 (lot of 2 slides), labels identical to the holotype.
TYPE LOCALITY: Daly River near Oolloo Crossing, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E).
TYPE HOST: Crocodylus johnstoni Krefft (Crocodilia: Crocodylidae).
SITE IN HOST: Small intestine.
REPRESENTATIVE DNA SEQUENCES: OM569683 (28S), OM568683, OM568684 (cox1).
ETYMOLOGY: The species is named after Dr. Aneta Kostadinova in recognition of her numerous, significant contributions to helminthology, including the systematics of diplostomoideans.
ZOOBANK REGISTRATION: urn:lsid:zoobank.org:act:05CFD841-953C-43CE-BD44-8F9417AB38AD.
Description (Figs 2–4):
Figures 2–6.
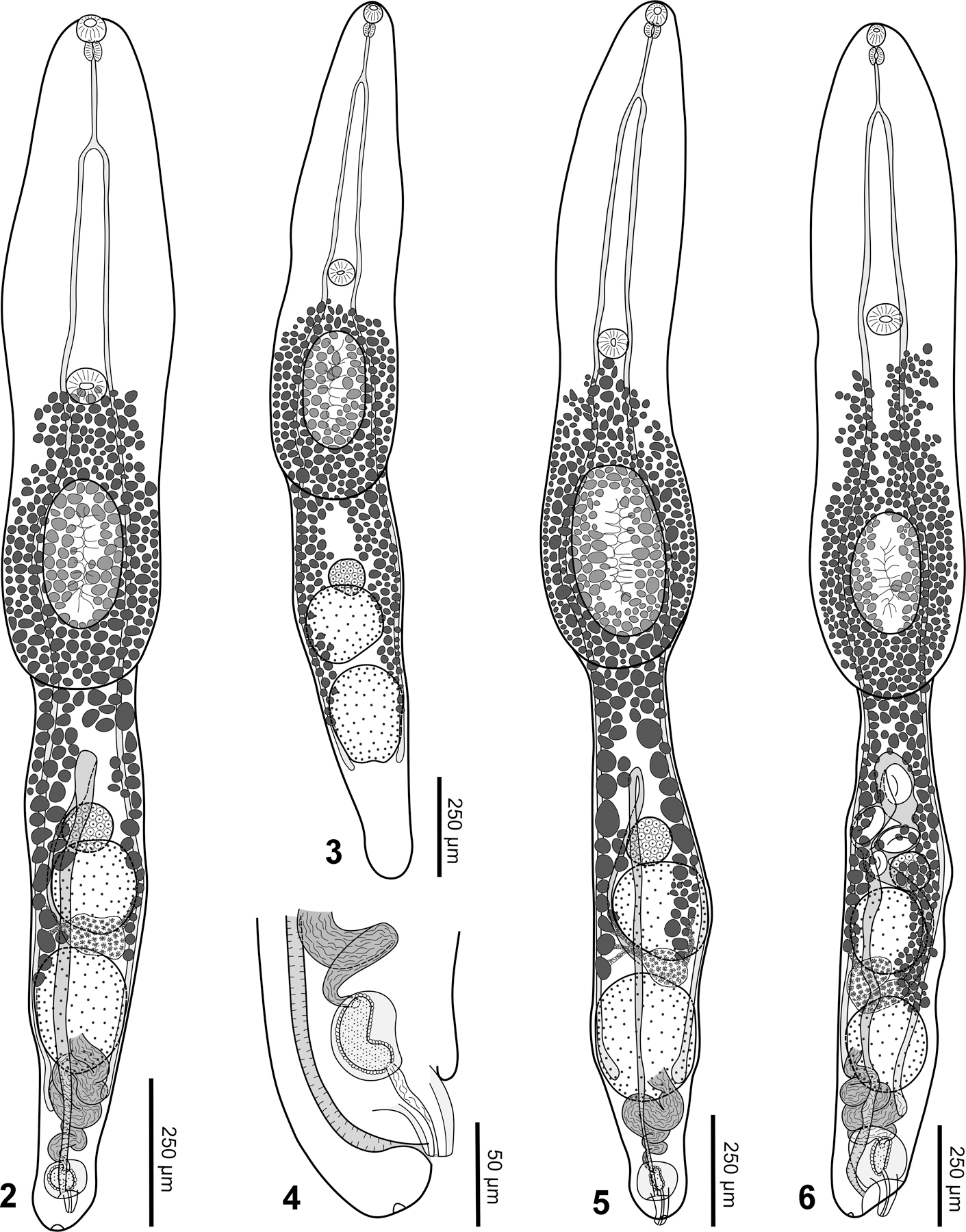
Two new species of Dungalabatrema gen. nov. 2. Dungalabatrema kostadinovae sp. nov., ventral view of the holotype. 3. Dungalabatrema kostadinovae sp. nov., ventral view of a paratype, uterus and terminal reproductive structures not shown. 4. Dungalabatrema kostadinovae sp. nov., lateral view of posteror portion of opisthosoma of a paratype. 5. Dungalabatrema snyderi sp. nov., ventral view of holotype. 6. Dungalabatrema snyderi sp. nov., ventral view of a paratype.
Based on 10 adult specimens; measurements of holotype given in text; measurements of entire series provided in Table 2. Body 2,132 long, consisting of distinct prosoma and opisthosoma; prosoma elongate, 1,169 × 296, width fairly consisent across prosoma; opisthosoma elongate, subcylindrical, 963 × 199. Prosoma:opisthosoma length ratio 1.2. Forebody 31% of body length. Oral sucker subterminal, 46 × 49. Pseudosuckers absent. Ventral sucker 63 × 73, somewhat larger than oral sucker, located near mid-length of prosoma; oral:ventral sucker width ratio 0.7. Holdfast organ posterior to ventral sucker, distance between ventral sucker and holdfast organ 12% of prosoma length; oval with ventral muscular portion, papillate, 246 × 123. Proteolytic gland not well observed, obscured by vitellarium, located near posterior margin of holdfast organ. Prepharynx not observed. Pharynx oval, 37 × 30. Esophagus 151 long, much longer than pharynx. Cecal bifurcation in anterior fifth of prosoma. Ceca slender, extending to near posterior margin of posterior testis.
Table 2.
Morphometric characters of the new proterodiplostomids. Ranges are followed by mean values in parentheses. Australiadiplostomum blairi sp. nov. is not in the table as its inclusion would be repetitive of the description.
| Species | Dungalabatrema kostadinovae sp. nov. | Dungalabatrema snyderi sp. nov. | Afroproterodiplostomum ingwenyae sp. nov. | Pseudoneodiplostomum angustus sp. nov. |
|---|---|---|---|---|
| Host | Cr. johnstoni | Cr. johnstoni | Cr. niloticus | Cr. niloticus |
| Locality | Australia | Australia | South Africa | South Africa |
| Number of specimens | n = 10 | n = 15 | n = 3 | n = 7 |
| Body length | 1,956–2,191 (2,123) | 1,671–3,512 (2,656) | 3,268* | 2,200–2,587 (2,381) |
| Prosoma length | 1,099–1,230 (1,161) | 1,092–1,968 (1,501) | 1,698* | 1,492–1,784 (1,604) |
| Prosoma width | 284–321 (301) | 170–563 (377) | 706–787 (747) | 251–499 (381) |
| Opisthosoma length | 804–1,032 (961) | 578–1,544 (1,152) | 1,252–1,570 (1,406) | 517–803 (713) |
| Opisthosoma width | 190–274 (224) | 130–398 (269) | 558–595 (577) | 244–364 (322) |
| Prosoma:opisthosoma length ratio | 1.1–1.4 (1.2) | 1.0–1.9 (1.4) | 1.1* | 1.9–2.2 (2.1) |
| Forebody (% of body length) | 27–32% (30%) | 23–31% (27%) | 27%* | 32–38% (35%) |
| Oral sucker length | 42–48 (46) | 37–64 (48) | 75* | 38–49 (45) |
| Oral sucker width | 38–49 (45) | 36–56 (45) | 72* | 41–46 (44) |
| Ventral sucker length | 58–68 (64) | 61–120 (87) | 103–126 (114) | 76–98 (85) |
| Ventral sucker width | 63–74 (70) | 61–125 (88) | 124–137 (131) | 74–105 (91) |
| Oral sucker:ventral sucker width ratio | 0.5–0.8 (0.6) | 0.4–0.7 (0.5) | 0.5* | 0.4–0.5 (0.5) |
| Holdfast organ length | 246–332 (272) | 195–534 (344) | 521–688 (592) | 229–490 (378) |
| Holdfast organ width | 123–173 (156) | 112–290 (192) | 296–353 (325) | 110–248 (185) |
| Distance between ventral sucker and holdfast organ (% of prosoma length) | 10–15% (13%) | 14–27% (20%) | 5%* | 7–15% (11%) |
| Pharynx length | 34–43 (39) | 31–61 (45) | 64* | 36–56 (47) |
| Pharynx width | 25–32 (28) | 26–41 (32) | 57* | 26–30 (28) |
| Esophagus length | 76–151 (111) | 95–175 (113) | 167* | 102–126 (114) |
| Anterior testis length | 137–187 (168) | 97–243 (185) | 252–342 (295) | 161–214 (193) |
| Anterior testis width | 136–188 (153) | 195–278 (196) | 419–441 (430) | 195–277 (237) |
| Posterior testis length | 190–266 (221) | 108–310 (221) | 275–357 (329) | 166–264 (208) |
| Posterior testis width | 135–183 (158) | 118–288 (202) | 427–495 (461) | 178–298 (244) |
| Ovary length | 60–89 (81) | 55–164 (106) | 137–174 (162) | 67–104 (88) |
| Ovary width | 61–90 (81) | 54–139 (102) | 173–218 (196) | 87–131 (104) |
| Egg length | 95–110 (101) | 93–101 (98) | 101–128 (115) | 111–122 (116) |
| Egg width | 65–68 (67) | 65–74 (68) | 59–69 (65) | 62–72 (66) |
| Anterior vitellarium free zone (% of prosoma length) | 55–65% (60%) | 50–65% (57%) | 50%* | 48–61% (56%) |
| Posterior vitellarium free zone (% of opisthosoma length) | 39–68% (47%) | 38–67% (49%) | 40–50% (44%) | 55–83% (67%) |
Only a single complete specimen was available for measurement.
Testes 2, tandem, entire; anterior testis 166 × 152, posterior testis 214 × 162. Seminal vesicle post-testicular, compact, coiled, ventral to posterior testis, continuing as ejaculatory duct before connecting to proximal part of paraprostate. Paraprostate short, surrounded by somewhat larger glandular muscular pouch. Common male efferent duct opening at apex of small genital cone within genital atrium. Genital cone likely protrusible.
Ovary primarily pretesticular, ventral to anterior testis, oval or subspherical 83 × 90. Oötype, Mehlis’ gland and uterine seminal receptacle intertesticular. Vitelline follicles extend anteriorly to level of, or slightly posterior to, ventral sucker, and posteriorly to level of posterior testis; vitellarium ventral and lateral to gonads. Vitelline reservoir intertesticular. Uterus ventral to gonads, extending anteriorly from ovary before turning and extending posteriorly. Metraterm opening into genital atrium near proximal end of genital cone; genital atrium opening subterminal on dorsal side. Uterus contains no eggs in holotype, up to 3 eggs in paratypes (95–110 × 65–68). Excretory vesicle not well-observed. Excretory pore terminal.
Dungalabatrema snyderi sp. nov. Achatz, Chermak et Tkach
HOLOTYPE: QM G239763, labeled ex. Crocodylus johnstoni, small intestine, Ooloo Crossing, Daly River, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E), 24 May 2006, coll. V. Tkach. PARATYPES: QM G239764 - 239773 (lot of 10 slides), labels identical to the holotype; HWML 216799 (lot of 2 slides), labels identical to the holotype. HOLOGENOPHORE: QM G239774 - 239775 (lot of 2 slides), labels identical to the holotype.
TYPE LOCALITY: Daly River near Oolloo Crossing, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E).
TYPE HOST: Crocodylus johnstoni Krefft (Crocodilia: Crocodylidae).
SITE IN HOST: Small intestine.
TYPE MATERIAL: The type series consists of 15 adult specimens deposited in the HWML.
REPRESENTATIVE DNA SEQUENCES: OM569684, OM569685 (28S), OM568685–OM568688 (cox1).
ETYMOLOGY: The species is named after Dr. Scott Snyder in recognition of his contributions to helminthology and especially to our knowledge of helminths of Australian reptiles, as well as for being instrumental in collecting specimens from Australian crocodiles.
ZOOBANK REGISTRATION: urn:lsid:zoobank.org:act:47952873-14E5-478E-B7DB-CCFFDD8760D0.
Description (Figs 5, 6):
Based on 15 adult specimens; measurements of holotype given in text; measurements of entire series given in Table 2. Body 2,432 long, consisting of distinct prosoma and opisthosoma; prosoma elongate, slipper-shaped (with slight constriction in middle) in some specimens, 1,379 × 314, widest at level of holdfast organ; opisthosoma elongate, subcylindrical, 1,053 × 232. Prosoma:opisthosoma length ratio 1.3. Forebody 29% of body length. Oral sucker subterminal, 41 × 43. Pseudosuckers absent. Ventral sucker 66 × 65, located near mid-length of prosoma, somewhat larger than oral sucker; oral:ventral sucker width ratio 0.7. Holdfast organ oval with ventral muscular portion, papillate, 308 × 162, situated posterior to ventral sucker; distance between ventral sucker and holdfast organ 16% of prosoma length. Proteolytic gland not well observed, obscured by vitellarium, located near posterior margin of holdfast organ. Prepharynx not observed. Pharynx oval, 53 × 39. Esophagus 100 long, much longer than pharynx. Cecal bifurcation in anterior fifth of prosoma. Ceca slender, extending to near posterior margin of posterior testis.
Testes 2, tandem, entire; anterior testis 191 × 184, posterior testis 256 × 200. Seminal vesicle post-testicular, compact, coiled, ventral to posterior testis, continuing as ejaculatory duct before connecting to proximal part of paraprostate. Paraprostate short, surrounded by much larger glandular muscular pouch. Wall of muscular pouch fairly thick. Common male efferent duct opening at apex of small genital cone within genital atrium. Genital cone likely protrusible.
Ovary subspherical, 102 × 92, antero-ventral to anterior testis. Oötype, Mehlis’ gland and uterine seminal receptacle intertesticular. Vitelline follicles extend anteriorly beyond holdfast organ, up to level of ventral sucker, and posteriorly to level of posterior testis; vitellarium ventral and lateral to gonads. Vitelline reservoir intertesticular. Uterus ventral to gonads, extending anteriorly from ovary before turning and extending posteriorly. Metraterm opening into genital atrium near proximal end of genital cone; genital atrium opening subterminal on dorsal side. Uterus contains no eggs in holotype, up to 4 eggs in paratypes (93–101 × 65–74). Excretory vesicle not well-observed. Excretory pore terminal.
REMARKS: The two members of Dungalabatrema gen. nov. are morphologically similar; therefore, fully mature, properly fixed specimens must be used for differentiation between these species. The two species can be differentiated based on the size/thickness of the glandular muscular pouch (smaller/thinner in D. kostadinovae sp. nov. vs. larger/thicker in Dungalabatrema snyderi sp. nov.) and the average distance between the ventral sucker and holdfast organ (ventral sucker and holdfast organ separated by 10–15% (mean 13%) of prosoma length in D. kostadinovae sp. nov. vs. ventral sucker and holdfast organ separated by 14–27% (mean 20%) of prosoma length in D. snyderi sp. nov.) (Table 2). Despite the morphological similarities, the two species differ by 0.5% in the partial sequences of 28S and 8% in the partial sequences of cox1 which is significant, especially considering that the two species occur in the same area and may be found in the same host individual.
Australiadiplostomum gen. nov. Achatz, Chermak et Tkach
TYPE-SPECIES: Australiadiplostomum blairi sp. nov.
ETYMOLOGY: The name of the new genus reflects the continent from which the digeneans were collected.
DIAGNOSIS: Body distinctly bipartite; prosoma flattened, elongate, similar in length to cylindrical opisthosoma. Oral sucker and ventral sucker present; holdfast organ elliptical, with papillae on margin of median slit. Pseudosuckers absent. Pharynx present; ceca reaching near level of genital atrium. Testes 2, tandem. Paraprostate short. Ejaculatory duct joins paraprostate near the midpoint of paraprostate. Common male duct opens at apex of small genital cone. Ovary pretesticular; oötype intertesticular. Vitellarium distributed throughout prosoma and opisthosoma, anterior extent at level of ventral sucker, posterior extent reaching posterior testis. Metraterm opens into genital atrium separately from male duct. Genital atrium with well-developed glandular papilla, with subterminal opening on dorsal side. Excretory pore terminal. In crocodilians. Australasia.
REMARKS: Australiadiplostomum gen. nov. clearly belongs within the Proterodiplostomidae due to the presence of a paraprostate. The new genus can be distinguished from the majority of other proterodiplostomid genera based on the topology of the terminal reproductive ducts. In the new genus, the ejaculatory duct joins the paraprostate and the common male efferent duct opens into the genital atrium separately from the metraterm. The only other genera that share this topology of the terminal reproductive ducts are Dungalabatrema gen. nov., Paraproterodiplostomum Tkach, Achatz et Pulis, 2020, Pseudoneodiplostomoides and Pseudoneodiplostomum. However, Australiadiplostomum gen. nov. posseses a well-developed glandular papilla in the genital atrium that is absent in all four of these genera. In addition, Australiadiplostomum gen. nov. lacks the muscular pouch that is present in Dungalabatrema gen. nov. The new genus also lacks the characteristic invaginations of the genital atrium that are present in Pseudoneodiplostomoides. The paraprostate of Australiadiplostomum gen. nov. is not well-developed, while the paraprostate of Paraproterodiplostomum is strongly developed. The molecular phylogeny (Fig. 1) also positions Australiadiplostomum gen. nov. seperately from Paraproterodiplostomum.
We have examined specimens from a farmed Cr. porosus collected in Papua New Guinea deposited in the NHM under accession number 1993.9.2-3 and identified as Ps. siamense. Despite the poor quality of the specimens, it was clear that it is morphologically consistent with the diagnosis of Australiadiplostomum gen. nov., although we cannot exclude that it may represent a different species. The state of the specimens did not allow for a more definitive conclusion.
Australiadiplostomum blairi sp. nov. Achatz, Chermak et Tkach
HOLOTYPE: QM G239753, labeled ex. Crocodylus johnstoni, small intestine, Ooloo Crossing, Daly River, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E), 24 May 2006, coll. V. Tkach.
PARATYPE: QM G239754 (lot of 1 slide), labels identical to the holotype.
SYNONYM: Pseudoneodiplostomum cf. siamense sensu Tkach et al. (2020)
TYPE LOCALITY: Daly River near Oolloo Crossing, Northern Territory, Australia (14°00.31΄S, 131°14.46΄E).
TYPE HOST: Crocodylus johnstoni Krefft (Crocodilia: Crocodylidae).
SITE IN HOST: Small intestine.
REPRESENTATIVE DNA SEQUENCE: MT622365 (28S), MT603623 (cox1)
ETYMOLOTY: The species is named in honor of Dr. David Blair, in recognition of his numerous, multi-faceted contributions to trematodology, including studies of digeneans in crocodiles.
ZOOBANK REGISTRATION: urn:lsid:zoobank.org:act:1F1F4B15-47FE-4FEA-A294-EADEE6F8A934.
Description (Figs 7–9):
Figures 7–13.
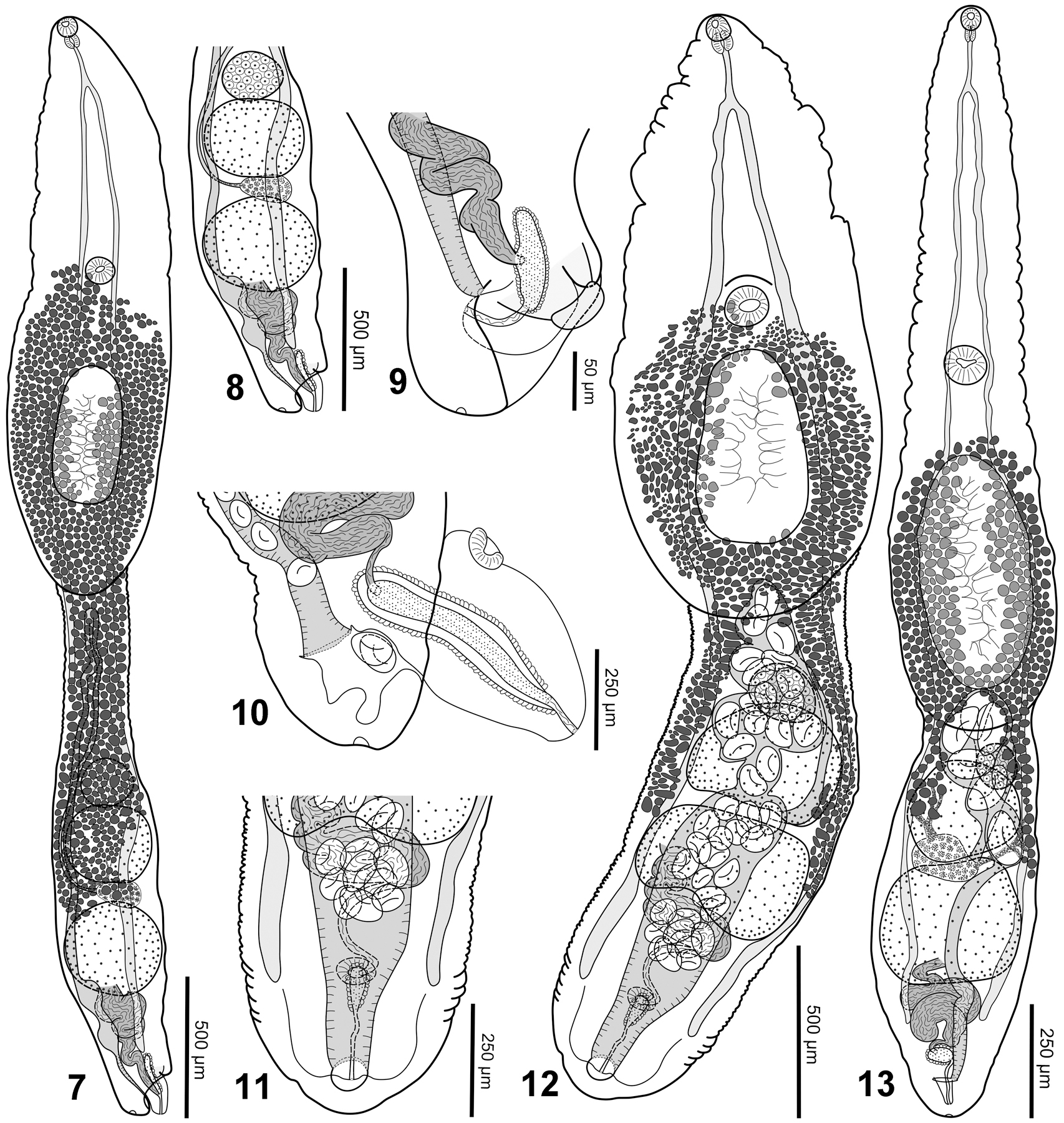
Australiadiplostomum blairi sp. nov., Afroproterodiplostomum ingwenyae sp. nov. and Pseudoneodiplostomum angustus sp. nov. 7. Australiadiplostomum blairi sp. nov., ventral view of the holotype (opisthosoma is somewhat twisted laterally). 8. Australiadiplostomum blairi sp. nov., posterior portion of opisthosoma of the holotype, vitellarium not shown. 9. Australiadiplostomum blairi sp. nov., posterior portion of opisthosoma of the paratype, lateral view. 10. Afroproterodiplostomum ingwenyae sp. nov. lateral view posterior portion of opisthosoma of a paratype. 11. Afroproterodiplostomum ingwenyae sp. nov., ventral view posterior portion of opisthosoma of the holotype. 12. Afroproterodiplostomum ingwenyae sp. nov., ventral view of the holotype. 13. Pseudoneodiplostomum angustus sp. nov., ventral view of the holotype.
Based on 2 adult specimens; measurements of the holotype are provided first with the measurements of the paratype in parentheses. Width measurements of gonads in paratype not provided as the opisthosoma is lateral. Body 3,443 (2,983) long, consisting of distinct prosoma and opisthosoma; prosoma elongate, 1,795 × 509 (1,418 × 506), widest at level of holdfast organ; opisthosoma elongate, subcylindrical, 1,648 × 342 (1,565 × 316). Prosoma:opisthosoma length ratio 1.1 (0.9). Forebody 24% (21%) of body length. Oral sucker subterminal, 57 × 62 (52 × 53). Pseudosuckers absent. Ventral sucker 87 × 88 (84 × 88), somewhat larger than oral sucker, located near mid-length of prosoma; oral:ventral sucker width ratio 0.7 (0.6). Holdfast organ posterior to ventral sucker, oval with ventral muscular portion, papillate, 459 (364) × 252; distance between ventral sucker and holdfast organ 14% (18%) of prosoma length. Proteolytic gland not well observed, obscured by vitellarium, located near posterior margin of holdfast organ. Prepharynx not observed. Pharynx oval, 55 × 47 (53 × 43). Esophagus 133 (87) long, longer than pharynx. Cecal bifurcation in anterior fifth of prosoma. Ceca slender, extending to near level of genital atrium.
Testes 2, tandem, entire; anterior testis 243 (240) × 275, posterior testis 291 (260) × 296. Seminal vesicle post-testicular, compact, with few coils, continuing as ejaculatory duct before joining paraprostate near the midpoint of paraprostate. Paraprostate short. Common male efferent duct opening at apex of small genital cone. Genital cone positioned within genital atrium, likely protrusible.
Ovary primarily pretesticular, subspherical 156 (155) × 159. Oötype, Mehlis’ gland and uterine seminal receptacle intertesticular. Vitelline follicles extend anteriorly beyond holdfast organ, up to level of ventral sucker, and posteriorly to level of posterior testis; vitellarium ventral and lateral to gonads. Vitelline reservoir intertesticular. Uterus ventral to gonads, extending anteriorly from ovary to near prosoma-opisthosoma junction before turning and extending posteriorly. Metraterm opening into genital atrium separate from genital cone; genital atrium contains a well-developed glandular papilla, opening subterminal on dorsal side. Papilla likely eversible. Uterus contains two eggs in holotype (92–99 × 54–64). Excretory vesicle not well-observed. Excretory pore terminal.
Afroproterodiplostomum gen. nov. Achatz, Chermak, Junker et Tkach
TYPE-SPECIES: Afroproterodiplostomum ingwenyae sp. nov.
ETYMOLOGY: The name of the new genus reflects the continent from which the proterodiplostomids were collected.
DIAGNOSIS: Body distinctly bipartite; prosoma flattened, elongate, length similar to cylindrical opisthosoma. Oral sucker and ventral sucker present; holdfast organ elliptical, with papillae on margin of median slit. Pseudosuckers absent. Pharynx present; ceca reaching near level of genital atrium. Testes 2, tandem. Paraprostate well-developed, claviform, primarily positioned within large genital cone. Ejaculatory duct joins proximal part of paraprostate; common male efferent duct opens at apex of genital cone. Genital cone with muscular sucker-like structure on dorsal, proximal surface. Ovary pretesticular; oötype intertesticular. Vitellarium distributed throughout prosoma and opisthosoma, anterior extent at level of ventral sucker, posterior extent reaching posterior testis. Metraterm opens into genital atrium separately from male duct. Genital atrium with subterminal opening, on dorsal side. Excretory pore terminal. In crocodilians. Afrotropics.
REMARKS: Despite the lack of DNA sequences from Afroproterodiplostomum gen. nov., this new genus clearly belongs to the Proterodiplostomidae based on the presence of a paraprostate organ. The new genus can be readily distinguished from other proterodiplostomid genera based on the presence of a muscular sucker-like structure on the genital cone. The only other genus with a musculur sucker-like structure associated with the reproductive structures is Proterodiplostomum; however, the muscular sucker-like structure of Proterodiplostomum is positioned on the wall of the genital atrium. In addition, the ejaculatory duct in Afroproterodiplostomum gen. nov. joins the proximal part of the paraprostate, while in Proterodiplostomum the ejaculatory duct does not join the paraprostate. It is worth noting that the ejaculatory duct may join the efferent duct of the paraprostate in Proterodiplostomum.
Afroproterodiplostomum ingwenyae sp. nov. Achatz, Chermak, Junker et Tkach
HOLOTYPE: HWML 216796, labeled ex. Crocodylus niloticus, small intestine, Olifants River, Limpopo province, South Africa (24°2’S, 31°13’E), 13 July 2010, coll. K. Junker.
PARATYPES: HWML 216797 (lot of 2 slides), labels identical to the holotype.
TYPE LOCALITY: Olifants River, Limpopo province, South Africa (24°2’S, 31°13’E).
TYPE HOST: Crocodylus niloticus Laurenti (Crocodilia: Crocodylidae).
SITE IN HOST: Small intestine.
ETYMOLOGY: The species epithet refers to the isiZulu word for crocodile (‘ingwenya’).
ZOOBANK REGISTRATION: urn:lsid:zoobank.org:act:1501394B-CCAC-4CE5-A8BB-4EC9C36DFF07.
Description (Figs 10–12):
Based on 3 adult specimens; measurements of holotype given in text; measurements of entire series given in Table 2. Body 3,268 long, consisting of distinct prosoma and opisthosoma; prosoma foliate, 1,698 × 595, widest at level of holdfast organ; opisthosoma subcylindrical, 1,570 × 595. Prosoma:opisthosoma length ratio 1.1. Forebody 26% of body length. Oral sucker subterminal, 75 × 72. Pseudosuckers absent. Ventral sucker 113 × 137, somewhat larger than oral sucker, located near mid-length of prosoma; oral:ventral sucker width ratio 0.5. Holdfast organ 567 × 353, posterior to ventral sucker, oval with ventral muscular portion, papillate; distance between ventral sucker and holdfast organ 5% of prosoma length. Proteolytic gland not well observed, obscured by vitellarium, located near posterior margin of holdfast organ. Prepharynx not observed. Pharynx subspherical, 64 × 57. Esophagus 167 long, much longer than pharynx. Cecal bifurcation in anterior fifth of prosoma. Ceca slender, extending to near level of genital atrium.
Testes 2, tandem, entire, 342 × 441, posterior testis 354 × 495. Seminal vesicle post-testicular, compact, continuing as ejaculatory duct before joining proximate part of paraprostate. Paraprostate well-developed, primarily positioned within large genital cone. Common male efferent duct opening at apex of genital cone. Genital cone positioned within genital atrium, protrusible, with muscular sucker-like structure on dorsal, proximal surface.
Ovary pretesticular, transversely oval, obscured by eggs in holotype, 174 × 218. Oötype, Mehlis’ gland and uterine seminal receptacle intertesticular. Vitelline follicles extend anteriorly to the level of ventral sucker and posteriorly to the level of the posterior testis; vitellarium ventral and lateral to gonads. Vitelline reservoir intertesticular. Uterus ventral to gonads, extending anteriorly from ovary to near the prosoma-opisthosoma junction before turning and extending posteriorly. Metraterm opening into genital atrium separate from genital cone. Genital atrium opening subterminal on dorsal side. Uterus contains 44 eggs in holotype (101–128 × 59–69). Excretory vesicle not well-observed. Excretory pore terminal.
Pseudoneodiplostomum Dubois, 1936
Pseudoneodiplostomum angustus sp. nov. Achatz, Chermak, Junker et Tkach
HOLOTYPE: HWML 216800, labeled ex. Crocodylus niloticus, small intestine, Crocodile River, Mpumalanga province, South Africa (25°27’S, 31°58’E), 12 July 2010, coll. K. Junker. PARATYPES: HWML 216801 (lot of 4 slides), labels identical to the holotype. HOLOGENOPHORE: HWML 216802 (lot of 1 slide), label identical to the holotype.
TYPE LOCALITY: Crocodile River, Mpumalanga province, South Africa (25°27’S, 31°58’E).
TYPE HOST: Crocodylus niloticus Laurenti (Crocodilia: Crocodylidae).
SITE IN HOST: Small intestine.
REPRESENTATIVE DNA SEQUENCE: OM569686 (28S)
ETYMOLOGY: the specific epithet refers to the narrow body of the new species, particularly the prosoma, which is unique among African Pseudoneodiplostomum spp.
ZOOBANK REGISTRATION: urn:lsid:zoobank.org:act:B0478530-511C-4B25-A36C-5F97A24E3595.
Description (Fig. 13):
Based on 7 adult specimens; measurements of holotype given in text; measurements of entire series given in Table 2. Body 2,321 long, consisting of distinct prosoma and opisthosoma; prosoma elongated, narrows anterior to holdfast organ, 1,529 × 347 long, with maximum width at level of holdfast organ; opisthosoma 792 × 343, cylindrical, much shorter than prosoma. Prosoma:opisthosoma length ratio 1.9. Forebody 32% of body length. Oral sucker subterminal, 38 × 41. Pseudosuckers absent. Ventral sucker larger than oral sucker, 81 × 89, located near midpoint of prosoma; oral:ventral sucker width ratio 0.5. Holdfast organ posterior to ventral sucker; oval with ventral muscular portion, papillate, 229 × 110; distance between ventral sucker and holdfast organ 10% of prosoma length. Proteolytic gland not-well observed, located near posterior margin of holdfast organ. Prepharynx not observed. Pharynx oval, 36 × 26. Esophagus 102, longer than pharynx. Cecal bifurcation in anterior quarter of prosoma. Ceca slender, extending to near posterior end of opisthosoma.
Testes 2, tandem, entire, occupy most of opisthosoma; anterior testis 214 × 246, posterior testis 250 × 298. Seminal vesicle post-testicular, compact, coiled, ventral to posterior testis, continuing as ejaculatory duct before connecting to side of paraprostate. Paraprostate weakly developed. Common male efferent duct exiting posterior margin of paraprostate and opens at apex of a small genital cone within the genital atrium separately from metraterm. Genital cone likely protrusible.
Ovary pretesticular, oval or subspherical, obscured by eggs in holotype. Oötype, Mehlis’ gland and uterine seminal receptacle intertesticular. Vitelline follicles distributed from level near anterior margin of holdfast organ to typically near anterior margin of posterior testis; vitellarium ventral and lateral to gonads. Vitelline reservoir intertesticular. Uterus ventral to gonads. Metraterm opening into genital atrium near proximal end of genital cone; genital atrium subterminal, dorsal. Eggs 111–122 × 62–72; uterus contains six eggs in holotype, up to 21 eggs in paratypes. Excretory vesicle not well-observed. Excretory pore terminal.
REMARKS: Pseudoneodiplostomum angustus sp. nov. belongs to Pseudoneodiplostomum based on the organisation of the terminal reproductive ducts and evidence from the molecular phylogenetic analysis (Fig. 1). Based on the existing knowledge of proterodiplostomid taxonomy and distribution, combined with the data on the current and recent distributions of the crocodilian hosts, it is extremely unlikely that a species of Pseudoneodiplostomum may be distributed across multiple biogeographic realms. Therefore, we differentiate the new species only from the four other congeners known from the Afrotropics. It is worth noting that thevoucher specimens of Ps. bifurcatum from the NHM collection corresponded to the original description of Ps. bifurcatum.
The new species can be most obviously distinguished from Ps. bifurcatum, Pseudoneodiplostomum gabonicum Dubois, 1948, Pseudoneodiplostomum niloticum Saad, 2006 and Pseudoneodiplostomum thomasi (Dollfus, 1935) based on the shape of prosoma and prosoma:opisthosoma length ratio. The prosoma of the new species is elongated and is about twice as long as the opisthosoma; the prosoma of the three other species is elongate and is of equal length or shorter than the opisthosoma.
In addition, Ps. angustus sp. nov. can be differentiated from Ps. gabonicum and Ps. thomasi by the anterior extent of the vitellarium. The vitellarium in the new species may only minimally extend past the level of the holdfast organ while in Ps. gabonicum and Ps. thomasi it reaches at least the level of the ventral sucker. The new species and Ps. niloticum have a similar body length (2,220–2,587 in the new species vs 1,980–2,340 in Ps. niloticum), but organs of the new species are noticeable smaller. For instance, the suckers width (oral: 41–46; ventral: 74–105) and holdfast organ size (229–490 × 110–248) of Ps. angustus sp. nov. are smaller than the sucker widths (oral: 50–70; ventral: 100–120) and holdfast organ size (560–680 × 240–350) of Ps. niloticum. Pseudoneodiplostomum angustus sp. nov. and Ps. bifurcatum can also be distingushed based on the size of genital cone (small in Ps. angustus sp. nov. vs. large in Ps. bifurcatum). The new species differs from the other Pseudoneodiplostomum spp. from the Afrotropics by 0.1–0.2% in the 28S sequences, except for Ps. niloticum which lacks DNA sequence data.
Discussion
Remarks on the diversity of proterodiplostomids
The crocodilian hosts of most proterodiplostomids belong to an extremely ancient lineage of tetrapods that existed well before the breakup of ancient supercontinents and continental drift that ultimately resulted in the formation of modern continents and major islands (Brochu, 1997, 2003; Oaks, 2011). Despite the significant loss of crocodilian diversity since the Mesozoic era, their proterodiplostomid parasites remained diverse and that diversity is likely underestimated. With the addition of 5 new species described in the present study, there are 44 currently accepted proterodiplostomid species, 33 of which parasitize crocodilian definitive hosts. Spectacled caiman Caiman crocodilus Linnaeus and yacare caiman Caiman yacare Daudin have the richest proterodiplostomid fauna with each reported as a host of 10 species of proterodiplostomids. Based on the present study, Crocodylus niloticus is the third richest host species with 8 currently-recognized proterodiplostomid parasites (Saad, 2006; Tellez et al., 2013; Tkach et 2020; present data). Our molecular phylogenetic analysis and morphological study allowed the description of Dungalabatrema gen. nov. and its two new species from Australia. Prior to this study, the only proterodiplostomid species known from Australia were Pe. crocodilarum and the previously misidentified Pseudoneodiplostomum cf. siamense of Tkach et al. (2020). Our re-examination of Pseudoneodiplostomum cf. siamense of Tkach et al. (2020) provided evidence that necessitated the erection of Australiadiplostomum gen. nov. for these specimens as well as their description as a distinct species (Au. blairi sp. nov.).
Afroproterodiplostomum gen. nov. is only the third genus of proterodiplostomids found in crocodilians in the Afrotropics, whereas Ps. angustus sp. nov. and Af. ingwenyae sp. nov. are the sixth and seventh proterodiplostomid species described from Africa. We hypothesize that there may be further unknown diversity of proterodiplostomids from the Afrotropics and Australasia. For instance, the very broadly distributed saltwater crocodile Cr. porosus may have historically carried its proterodiplostomids across its distribution area, thus facilitating diversification of its specific proterodiplostomid fauna and exchange with other crocodilian species. This may lead to speciation through evolutionary host switching with subsequent radiation in a new host. Likewise, Cr. niloticus is broadly distributed throughout most of Africa. It would not be surprising if the proterodiplostomid fauna of Cr. niloticus varied throughout the continent. At the same time, practically nothing is currently known about the proterodiplostomid fauna of the majority of crocodilian species, e. g., the American crocodile Crocodylus acutus Cuvier, Cuban crocodile Crocodylus rhombifer Cuvier, Chinese alligator Alligator sinensis (Fauvel), New Guinea crocodile Crocodylus novaeguineae (Schmidt), Philippine crocodile Crocodylus mindorensis Schmidt, West African crocodile Crocodylus suchus Geoffroy, to name a few. Notably, the specimens of Pseudoneodiplostomum sp. from Cr. niloticus in Ghana (1976.4.12.66–69, 1976.1.7.23) and Pseudoneodiplostomum sp. from dwarf crocodile Osteolaemus tetraspis Cope in Nigeria deposited in the NHM collection and examined as part of this study, almost certainly represent new species. However, the poor condition of these specimens did not allow for quality descriptions.
The analysis of 28S seqeunces (Fig. 1) positioned Pl. constrictum (type-species), Cystodiplostomum hollyi Dubois, 1936 and an unknown Cystodiplostomum sp. within a 100% supported clade. Within this clade, the two Cystodiplostomum spp. formed an unsupported grouping. Based on our analysis, Cystodiplostomum sp. may belong to a separate, currently undescribed genus. Unfortunately, no morphological vouchers of Cystodiplostomum sp. of Tkach et al. (2020) are available. However, the topology of the terminal reproductive ducts of Prolecithodiplostomum spp. and Cy. hollyi are identical (Tkach et al., 2020). The genera are separated based on the presence of a sucker-like dorsal invagination of the body near the midpoint of the opisthosoma. This sucker-like structure is not clearly associated with any terminal reproductive ducts or the genital atrium in Cy. hollyi (Dubois, 1936). Tkach et al. (2020) convincingly demonstrated that the topology of the terminal reproductive ducts and associated structures are the most reliable features to differentiate among proterodiplostomid genera. The new genera described in the present study further demonstrate the utility of these features for genus-level differentiation. In our opinion, the phylogenetic results (Fig. 1) and the identical topology of reproductive ducts suggest that Cystodiplostomum should be considered a junior synonym of Prolecithodiplostomum. However, we do not take here a formal nomenclatural action on the matter because we do not have well fixed, accurately identified and sequenced specimens of Prolecithodiplostomum.
Proterodiplostomum was previously the most speciose genus of proterodiplostomids in the Neotropics. After the most recent revision of the genus by Tkach et al. (2020), it currently contains only three species: Pr. longum (type-species), Proterodiplostomum tumidulum Dubois, 1936 and Pr. medusae. Proterodiplostomum longum and Pr. tumidulum were described with a sucker-like muscular structure in the genital atrium. Proterodiplostomum medusae lacks such a structure, but has distinct muscular bundles in the wall of the genital atrium (see Fig. 5J, K of Tkach et al., 2020). Dubois (1936) originally did not provide a generic placement for Pr. medusae based on the morphology of his material and named it Diplostome medusae. The molecular phylogeny further illustrates differences between Pr. medusae and Pr. longum. Proterodiplostomum medusae was positioned as a long-branch sister group to the 100% supported clade containing all other Proterodiplostomum spp. The branch length of the Pr. medusae clade indicates significant differences from Proterodiplostomum spp. (including Pr. longum); it is much greater than branch lengths of the majority of other proterodiplostomids (Fig. 1). Based on the absence of a sucker-like muscular structure in the genital atrium and evidence from the molecular phylogeny (Fig. 1), we establish Nattererodiplostomum gen. nov. for Pr. medusae. A diagnosis of Nattererodiplostomum gen. nov. is provided below as well as an amended diagnosis of Proterodiplostomum.
Nattererodiplostomum gen. nov. Achatz et Tkach
TYPE-SPECIES: Nattererodiplostomum medusae comb. nov.
ETYMOLOGY: The new genus is named in honor of Johann Natterer, the Austrian naturalist who collected the type-specimens of N. medusae comb. nov. in Brazil (Dubois, 1936).
DIAGNOSIS: Body distinctly bipartite; prosoma flattened, spatulate, shorter than cylindrical opisthosoma. Oral sucker and ventral sucker moderately developed; holdfast organ elliptical, with papillae on margin of median slit. Pseudosuckers absent. Pharynx moderately well-developed; ceca reaching near level of genital atrium. Testes 2, tandem. Paraprostate well-developed, tubular, reaching close to posterior testis. Ejaculatory duct and efferent duct of paraprostate open together at apex of genital cone. Ovary pretesticular; oötype intertesticular. Vitellarium distributed throughout prosoma and opisthosoma, anterior extent before or after ventral sucker, posterior extent reaching near level of genital atrium. Metraterm opens into genital atrium near base of genital cone, separately from male ducts. Distinct bundles of muscles present in wall of genital atrium. Genital atrium with subterminal opening, on dorsal side. Excretory pore terminal. In crocodilians. Neotropics.
REMARKS: Nattererodiplostomum gen. nov. can be differentiated from all proterodiplostomid genera based on the combination of dense muscle bundles in the wall of the genital atrium that do not form a sucker-like structure, and the topology of the terminal reproductive ducts. This includes the metraterm opening into the genital atrium separately from the ejaculatory duct and paraprostate (see Fig. 5 in Tkach et al. (2020)).
Proterodiplostomum Dubois, 1936
TYPE-SPECIES: Proterodiplostomum longum (Brandes, 1888)
OTHER SPECIES: Proterodiplostomum tumidulum Dubois, 1936.
DIAGNOSIS (after Tkach et al., 2020, amended): Body distinctly bipartite; prosoma flattened, spatulate, typically much shorter than cylindrical opisthosoma. Oral sucker and ventral sucker present; holdfast organ elliptical, elongate, with papillae on margin of median slit. Pseudosuckers absent. Pharynx present; ceca reaching near level of genital atrium. Testes 2, tandem. Paraprostate well-developed, tubular, reaching close to posterior testis. Ejaculatory duct and efferent duct of the paraprostate open together at apex of genital cone. Ovary pretesticular; oötype intertesticular. Vitellarium distributed throughout prosoma and opisthosoma, anterior extent before or after ventral sucker, posterior extent reaching about level of paraprostate. Metraterm opens into genital atrium near base of genital cone, separately from male ducts. Muscular sucker-like structure in wall of genital atrium. Genital atrium with subterminal opening on dorsal side. Excretory pore terminal. In crocodilians. Neotropics.
Based on our phylogenetic analysis (Fig. 1), Proterodiplostomidae gen. sp. 1 (GenBank MT187598) from peacock bass Cichla monoculus Agassiz in Brazil is likely congeneric with Proterodiplostomidae gen. sp. 4 from Ca. yacare in Brazil (Fig. 1) previously published by Tkach et al. (2020) as Proterodiplostomum sp. (GenBank MT622345). The two forms are clearly distinct species-level lineageas as they demonstrate 9.3% difference in partial sequences of cox1 (GenBank MT603611 vs. MT185367). The specimens of Proterodiplostomidae gen. sp. 4 studied by Tkach et al. (2020) were immature and only tentatively placed into Proterodiplostomum. Our phylogenetic results, especially the long branch lengths, strongly suggest that the two species in this clade of Proterodiplostomidae gen. represent yet another new genus waiting for formal description.
Considering the recent synonimzations of genera (Achatz, Brito, et al., 2021) and the erection of four new genera in the present work, we provide an amended key to the genera of the Proterodiplostomidae after Tkach et al. (2020).
Amended key to the genera of the Proterodiplostomidae
| 1a. Paraprostate absent | 2 |
| 1b. Paraprostate present | 3 |
| 2a. Ejaculatory duct and metraterm merge to form hermaphroditic duct near apex of genital cone. Hermaphroditic duct not enclosed in a muscular pouch. Pseudosuckers absent. In crocodilians. Neotropics | Mesodiplostomum |
| 2b. Ejaculatory duct and metraterm merge to form hermaphroditic duct enclosed in a muscular pouch. Pseudosuckers present. In snakes. Palaearctic and Orient | Proalarioides |
| 3a. Paraprostate surrounded by muscular pouch. Paraprostate duct eversible. Ejaculatory duct and metraterm open side by side. In snakes. Neotropics | Heterodiplostomum |
| 3b. Paraprostate not surrounded by muscular pouch; alternatively, muscular pouch surrounds other terminal elements of reproductive system in addition to paraprostate | 4 |
| 4a. Entire paraprostate, ejaculatory duct, and metraterm enclosed in muscular pouch. Ejaculatory duct and metraterm open separately. In crocodilians. India | Capsulodiplostomum |
| 4b. Paraprostate, ejaculatory duct, and metraterm not enclosed together in muscular pouch. Ejaculatory duct and metraterm open separately or have a common opening | 5 |
| 5a. Vitellarium confined to opisthosoma. In crocodilians. Neotropics | Massoprostatum |
| 5b. Vitellarium distributed differently | 6 |
| 6a. Holdfast organ relatively massive, typically occupying about half of prosoma | 7 |
| 6b. Holdfast organ not as massive, typically occupying about 25–30% of prosoma | 8 |
| 7a. Ejaculatory duct joins distal part of paraprostate. Two muscular pits, occasionally sucker-like, present in wall of genital atrium. In crocodilians. Australasia | Pseudoneodiplostomoides |
| 7b. Ejaculatory duct and paraprostate do not join/unite. Muscular pits in wall of genital atrium absent. In snakes. Neotropics | Ophiodiplostomum |
| 8a. Metraterm, ejaculatory duct and paraprostate join to form a common duct or all three share common opening | 9 |
| 8b. Metraterm, ejaculatory duct and paraprostate do not form common duct. Ejaculatory duct and paraprostate or ejaculatory duct and metraterm may join or share common opening | 13 |
| 9a. Opisthosoma with longitudinal row of sucker-like structures on dorsal side. In crocodilians. Nearctic | Polycotyle |
| 9b. Opisthosoma without dorsal sucker-like structures | 10 |
| 10a. Terminal part of paraprostate, ejaculatory duct and metraterm enclosed in muscular pouch | 11 |
| 10b. Paraprostate, ejaculatory duct, and metraterm not enclosed in muscular pouch | 12 |
| 11a. Ejaculatory duct typically joins paraprostate near its midpoint. In crocodilians. Nearctic | Pseudocrocodilicola |
| 11b. Ejaculatory duct joins paraprostate near its proximal end. In crocodilians. Nearctic. | Neocrocodilicola |
| 12a.Vitelline follicles confined to area around holdfast organ. Separation between prosoma and opisthosoma indistinct. In crocodilians. Nearctic and Neotropics | Crocodilicola |
| 12b. Vitelline follicles distributed in both prosoma and opisthosoma, extending well beyond area around holdfast organ. Separation between prosoma and opisthosoma distinct. In crocodilians. Nearctic | Archaeodiplostomum |
| 13a. Paraprostate opens separately from ejaculatory duct and metraterm. Ejaculatory duct and metraterm may join or share common opening | 14 |
| 13b. Metraterm opens separately from ejaculatory duct and paraprostate. Ejaculatory duct and paraprostate may join or share common opening | 17 |
| 14a. Genital cone present | 15 |
| 14b. Genital cone absent | 16 |
| 15a. Genital cone massive, equal to about 1/4 of total body length. In crocodilians. Neotropics | Paradiplostomum |
| 15b. Genital cone much smaller, not more than 1/8 of total body length. In crocodilians and chelonians. Neotropics | Herpetodiplostomum |
| 16a. Thick-walled, sucker-like dorsal invagination of body present near midpoint of opisthosoma or slightly more posterior. In crocodilians. Neotropics | Cystodiplostomum |
| 16b. No thick-walled, sucker-like dorsal invagination of body present. In crocodilians. Neotropics | Prolecithodiplostomum |
| 17a. Either sucker-like muscular structure or distinct musclular bundles in the wall of the genital atrium present | 18 |
| 17b. Genital atrium without sucker-like structure or distinct muscular bundles | 19 |
| 18a. Sucker-like muscular structure in the wall of the genital atrium present. In crocodilians. Neotropics | Proterodiplostomum |
| 18b. Distinct muscle bundles in the wall of the genital atrium present. In crocodilians. Neotropics | Nattererodiplostomum gen. nov. |
| 19a. Genital cone with sucker-like muscular structure. In crocodilians. Afrotropics | Afroproterodiplostomum gen. nov. |
| 19b. Genital cone without sucker-like muscular structure | 20 |
| 20a. Genital atrium with well-developed glandular papilla. In crocodilians. Australasia | Australiadiplostomum gen. nov. |
| 20b. Genital atrium without a well-developed glandular papilla. Smaller papillae may be present in genital atrium | 21 |
| 21a. Ejaculatory duct does not join paraprostate. Ejaculatory duct and paraprostate share common opening. In crocodilians and snakes. Neotropics | Proteroduboisia |
| 21b. Ejaculatory duct joins paraprostate | 22 |
| 22a. Glandular muscular pouch surrounding paraprostate and terminal portion of ejaculatory duct. In crocodilians. Australasia | Dungalabatrema gen. nov. |
| 22b. Glandular muscular pouch surrounding paraprostate absent | 23 |
| 23a. Ejaculatory duct joins paraprostate near its distal end. Paraprostate well-developed. In crocodilians. Nearctic | Paraproterodiplostomum |
| 23b. Ejaculatory duct joins proximal half of paraprostate. Paraprostate weakly developed. In crocodilians. Africa, Australasia and Neotropics | Pseudoneodiplostomum |
Host associations and biogeography
Similar to the results of Tkach et al. (2020) and Achatz, Brito, et al. (2021), the proterodiplostomids from Neotropical caimans (Alligatoridae Gray, Caimaninae Brochu) were placed into three separate clades among the most basal lineages in our phylogeny (Fig. 1). Proteroduboisia globulare (from a caiman) and He. vogti (from a Neotropical turtle (Chelidae Gray: Chelinae Gray)) were placed together in an unsupported clade. The other proterodiplostomid from a non-crocodilian host, Ht. lanceolatum, from a Neotropical snake (Colubridae Oppel; Dipsadinae Bonaparte) did not form a clade with any other proterodiplostomids. At the same time, the proterodiplostomids of alligators (Alligatoridae Gray: Alligatorinae Gray) from the Nearctic and crocodiles (Crocodylidae Cuvier; Crocodylinae Cuvier) from the Afrotropics and Australasia were both monophyletic and strongly supported (Fig. 1). Our analysis includes eight proterodiplostomids from crocodiles compared to the five proterodiplostomids from crocodiles sequenced by Tkach et al. (2020). The results of the present study further support close co-evolutionary relationships between Crocodylus spp. and their proterodiplostomids.
All three species newly sequenced in the present study were collected from Crocodylus spp. in Africa and Australia. All of them appeared in the phylogenetic tree within a 100% supported clade containing exclusively parasites of Crocodylus, with Ps. angustus n. sp. clustering with its congeners, while Dungalabatrema spp. formed an independent 100% supported clade (Fig. 1).
Interestingly, the proterodiplostomids of Cr. johnstoni collected in Australia did not form a monophyletic clade separate from proterodiplostomids of Cr. niloticus collected in Africa; the taxa from Australia were distributed across three clades within a polytomy, one of which included the strongly supported clade of Pseudoneodiplostomum spp. from Africa (Fig. 1). This topology suggests that African crocodilians may have received their ancestral proterodiplostomid fauna from Australasian crocodilians. Oaks (2011) suggested a disperal of Crocodylus spp. out of Australasia into the Afrotropics, which is supported by the current phylogenetic data on their proterodiplostomids.
Our analysis only included Pseudoneodiplostomum spp. from the Afrotropics (Fig. 1). However, three Pseudoneodiplostomum spp. are known from other biogeographic realms, namely Ps. siamense and described Pseudoneodiplostomum dolfusi Dubois, 1948 from the Siamese crocodile Crocodylus siamensis Schneider in the Indomalayan realm and Pseudoneodiplostomum groschafti Moravec, 2001 described from Cr. rhombifer in the Neotropics (Dubois, 1936, 1948; Moravec, 2001). Delfino et al. (2020) provided evidence which suggests that Crocodylus likely dispersed from the Afrotropics into the Neotropics. This could explain the introduction of Pseudoneodiplostomum in the New World. Obtaining sequence data from not yet sequenced members of Pseudoneodiplostomum will likely provide clarification for this and other questions of their historical biogeography and taxonomy.
Conclusions
Thorough morphological study of our specimens from three continents, combined with available molecular data, revealed five new species and required the establishment of four new genera. Our morphological study has yielded additional support for the use of the terminal reproductive ducts and associated structures in proterodiplostomid systematics, especially for differentiation among genera. This work, along with recently published studies (Achatz, Brito et al., 2021; Tkach et al., 2020), makes it clear that the proterodiplostomid diversity is significantly greater than previously known, particularly in Afrotropical and Australasian crocodilians. Currently, DNA sequences from Afrotropical crocodilians are only available for proterodiplostomids collected from a single species in South Africa, and no DNA sequence data is available for proterodiplostomids collected from the Indomalayan realm. Future studies should focus on obtaining and sequencing well-fixed adult proterodiplostomids from currently understudied hosts.
Acknowledgments
We thank Dr. Scott Snyder for organizing and assisting with the collection of helminths from Australian freshwater crocodiles. We are grateful to Dr. Danny Govender (SANParks, South Africa), Prof. Joop Boomker (University of Pretoria, South Africa) as well as Mr. Frans R. Masubelle and Mr. Daniel M. Chipana (ARC-Onderstepoort Veterinary Institute, South Africa) for facilitating and assisting in parasite collections from Nile crocodiles.
Funding
This work was supported by the National Science Foundation (grant number DEB-0515492), (grant number DEB-1120734) for VVT. TJA was supported by the University of North Dakota (Joe K. Neel Memorial Award, Esther Wadsworth Hall Wheeler Award, Student Research Stipend and Summer Doctoral Fellowship) and Annual Midwestern Conference of Parasitologists (AMCOP Student Research Grant). TPC was supported by the National Institute of General Medical Sciences of the National Institutes of Health (Institutional Development Award (IDeA) grant number P20GM103442) to the University of North Dakota School of Medicine & Health Sciences. Examination of proterodiplostomid specimens deposited in the collections of the Natural History Museum, London, received support from the SYNTHESYS+ Project http://www.synthesys.info/ financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 823827, to VVT.
Footnotes
ZOOBANK REGISTRATION: urn:lsid:zoobank.org:pub:C30F977D-4A0B-4D8C-8BAF-0D6C48B5396B.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Achatz TJ, Brito ES, Fecchio A, & Tkach VV (2021). Description and phylogenetic position of a new species of Herpetodiplostomum from Phyrnops geoffroanus in Brazil and a re-evaluation Cheloniodiplostomum. Journal of Parasitology, 107(3), 455–462. 10.1645/21-18 [DOI] [PubMed] [Google Scholar]
- Achatz TJ, Martens JR, Kostadinova A, Pulis EE, Orlofske SA, Bell JA, Fecchio A, Oyarzún-Ruiz P, Syrota YY & Tkach VV Molecular phylogeny of Diplostomum, Tylodelphys, Austrodiplostomum and Paralaria (Digenea: Diplostomidae) necessitates systematic changes and reveals a history of evolutionary host switching events. International Journal for Parasitology, in press. 10.1016/j.ijpara.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz TJ, Pulis EE, Junker K, Binh TT, Snyder SD, & Tkach VV (2019). Molecular phylogeny of the Cyathocotylidae (Digenea, Diplostomoidea) necessitates systematic changes and reveals a history of host and environment switches. Zoologica Scripta, 48(4), 545–556. 10.1111/zsc.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseru B (1956). On three new species of strigeid trematodes from an African crocodile and the erection of a new family, Neostrigeidae. Journal of Helminthology, 30(4), 217–232. 10.1017/S0022149X00033198 [DOI] [Google Scholar]
- Bisseru B (1957). On two new trematodes (Proterodiplostomidae) from an African crocodile, and a list of strigeid parasites from Africa. Journal of Helminthology, 31(1–2), 85–102. 10.1017/S0022149X00033320 [DOI] [PubMed] [Google Scholar]
- Brochu CA (1997). Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis. Systematic Biology, 46(3), 479–522. 10.1093/sysbio/46.3.479 [DOI] [PubMed] [Google Scholar]
- Brochu CA (2003). Phylogenetic approaches toward crocodylian history. Annual Review of Earth and Planetary Sciences, 31, 357–397. 10.1146/annurev.earth.31.100901.141308 [DOI] [Google Scholar]
- Delfino M, Iurino DA, Mercurio B Piras P, Rook L, & Sardella R (2020). Old African fossils provide new evidence for the origin of the American crocodiles. Scientific Reports, 10(1), Article 11127. 10.1038/s41598-020-68482-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois G (1936). Les diplostomes de reptiles (Trematoda: Proterodiplostomidae nov. fam.) du Musée de Vienne. Bulletin de la Société Neuchâteloise des Sciences Naturelles, 61, 5–80. [Google Scholar]
- Dubois G (1948). Sur Trois Diplostomes de Crocodiliens (Trematoda: Strigeida). Annales de Parasitologie, 23, 5–13. [PubMed] [Google Scholar]
- Dubois G (1979). Révision et nouvelle clé de déterminations des Diplostomes de Reptiles. Bulletin de la Société Neuchâteloise des Sciences Naturelles, 102, 39–48. [Google Scholar]
- Hernández-Mena DI, García-Varela M, & Pérez-Ponce de León G (2017). Filling the gaps in the classification of the Digenea Carus, 1863: systematic position of the Proterodiplostomidae Dubois, 1936 within the superfamily Diplostomoidea Poirier, 1886, inferred from nuclear and mitochondrial DNA sequences. Systematic parasitology, 94, 833–848. 10.1007/s11230-017-9745-1 [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, & Tamura K (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz HL, Tkach VV, & Weckstein JD (2017). Methods for specimen-based studies of avian symbionts. In Webster M (Ed.), The Role of Collections in Ornithology: The extended specimen. Studies in Avian Biology (pp. 127–183). CRC Press. [Google Scholar]
- MacCallum GA (1921). Studies in helminthology. Zoopathologica, New York Zoological Society 1, 141–204. [Google Scholar]
- Moravec F (2001). Some helminth parasites from Morelet’s crocodile, Crocodylus moreletii, from Yucatan, Mexico. Folia Parasitologica, 48, 47–62. [DOI] [PubMed] [Google Scholar]
- Negrelli DC, Vieira DHMD, Abdallah VD, & Azevedo RK 2020. Molecular characterization of the progenetic metacercariae Crocodilicola pseudostoma parasitizing Rhamdia quelen (Siluriformes, Heptapteridae) in Brazil. Anais da Academia Brasileira de Ciências, 92(2), Article e20181388. 10.1590/0001-3765202020181388 [DOI] [PubMed] [Google Scholar]
- Niewiadomska K (2002). Family Proterodiplostomidae Dubois, 1936. In Gibson DI, Jones A, & Bray RA (Eds.), Keys to the Trematoda, Volume 1 (pp. 215–229). CAB International and The Natural History Museum. [Google Scholar]
- Oaks JR (2011). A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution, 65(11), 3285–3297. 10.1111/j.1558-5646.2011.01373.x [DOI] [PubMed] [Google Scholar]
- Queiroz MS, López-Hernández D, Locke SA, Pinto HA, & Anjos LA, (2020). Metacercariae of Heterodiplostomum lanceolatum (Trematoda: Proterodiplostomidae) found in Leptodactylus podicipinus (Anura: Leptodactylidae) from Brazil: a morphological, molecular and ecological study. Journal of Helminthology, 94, Article E66. 10.1017/S0022149X19000646 [DOI] [PubMed] [Google Scholar]
- Ronquist F, & Huelsenbeck JP (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12), 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Saad AI (2006). Digenetic trematodes infecting the Nile crocodile Crocodylus niloticus Laurentia, 1768 from Lake Nasser, Egypt, with the description of two new species of the Proterodiplostomidae Dubois, 1936. Journal of the Egyptian-German Society of Zoology, 50D, 13–35. [Google Scholar]
- Tellez M (2013). A Checklist of Host-Parasite Interactions of the Order Crocodylia University of California Press. pp. 1–376. [Google Scholar]
- Tkach VV, Achatz TJ, Pulis EE, Junker K, Snder SD, Bell JA, Halajan A, & Melo FTV, (2020). Phylogeny and systematics of the Proterodiplostomidae Dubois, 1936 (Digenea: Diplostomoidea) reflect the complex evolutionary history of the ancient digenean group. Systematic Parasitology, 97, 409–439. 10.1007/s11230-020-09928-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM, & Swiderski Z (2003). Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Systematic Parasitology, 56, 1–15. 10.1023/A:1025546001611 [DOI] [PubMed] [Google Scholar]
- Tkach VV, & Pawlowski J (1999). A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitologica, 44(2), 147–148. [Google Scholar]


