Abstract
Since microbes use carotenoids as an antioxidant for protection, dietary carotenoids could be associated with gut microbiota composition. We aimed to determine associations among reported carotenoid intake, plasma carotenoid concentrations, and fecal bacterial communities in pregnant women. Pregnant women (n=27) were enrolled in a 2-arm study designed to assess feasibility of biospecimen collection and delivery of a practical nutrition intervention. Plasma and fecal samples were collected and women were surveyed with a 24-hour dietary checklist and recalls. Plasma carotenoids were analyzed by HPLC using photodiode array detection. Fecal bacteria were analyzed by 16S rRNA DNA sequencing. Results presented are cross-sectional from the 36-week gestational study visit combined across both study arms due to lack of significant differences between intervention and usual care groups (n=23 women with complete data). Recent intake of carotenoid-containing foods included carrots, sweet potatoes, mangos, apricots, and/or bell peppers for 48% of women; oranges/orange juice (17%); egg (39%); tomato/tomato-based sauces (52%); fruits (83%); vegetables (65%). Average plasma carotenoid concentrations were 6.4ug/dL α-carotene (AC), 17.7ug/dL β-carotene (BC), 11.4ug/dL cryptoxanthin (CR), 39.0ug/dL trans-lycopene (TL), and 29.8ug/dL zeaxanthin and lutein (ZL). AC and BC concentrations were higher in women who recently consumed foods high in carotenoids. CR concentrations were higher in women who consumed oranges/orange juice. Microbiota α-diversity positively correlated with AC and BC. Microbiota β-diversity differed significantly across reported intake of carotenoid containing foods and plasma concentrations of AC. This may reflect an effect of high fiber or improved overall dietary quality, rather than a specific effect of carotenoids.
Keywords: carotenoids, plasma carotenoids, diet, pregnancy, bacteria, fecal, gut microbiota, gut microbiome, alpha diversity
1. Introduction
The extensive physiological needs of pregnancy require a nutrient-dense diet to support the health of both the mother and her baby during gestation and post-partum (Gernand, Schulze, Stewart, West, & Christian, 2016). Poor maternal diet quality during pregnancy is a significant predictor of obesity and cardiometabolic problems for the infant in later life (Fraser et al., 2010; Godfrey et al., 2017; Mamun et al., 2010). Conversely, a high-quality maternal diet can have positive long-term effects like improved child cognitive development (Borge, Aase, Brantsæter, & Biele, 2017). Overall dietary diversity, in terms of food choices, is a driving factor of microbial variation and stability (A. J. Johnson et al., 2019). A recent study in primates discovered that a high-fat diet consumed during pregnancy and postpartum was related to the reduction of commensal Campylobacter spp. in the juvenile gut, demonstrating that maternal diet may influence her child’s microbiome (Ma et al., 2014). In another study, germ-free mice were colonized with stool microbes from 2-week-old infants born to obese or normal-weight mothers. The mice colonized with the stool from obese mothers showed clinical signs of pediatric non-alcoholic fatty liver disease and decreased immune function (Soderborg et al., 2018). Combined, these studies suggest that the effect maternal characteristics have on child health outcomes may, in part, be facilitated by the gut microbiome.
Carotenoids are fat-soluble chemical compounds found in foods, some of which humans can metabolize into vitamin A. Carotenoids commonly found in the human diet include alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein, zeaxanthin, and lycopene (Russell & Paiva, 1999). Carotenoids are known for their antioxidant and anti-inflammatory properties (Eggersdorfer & Wyss, 2018), as well as their importance in immunity (Vaishnava & Hooper, 2011). Intake of certain carotenoids may be especially important for pregnant women, as their infants are susceptible to oxidative stress which can lead to retinopathy of prematurity (Giordano & Quadro, 2018; Zielińska, Wesołowska, Pawlus, & Hamułka, 2017). The importance of lutein and zeaxanthin in cognitive development in infants and adults alike stresses the value of adequate carotenoid intake during pregnancy (E. J. Johnson, 2014; Zielińska et al., 2017). Consumption of fruits and vegetables accounts for most carotenoid intake—so plasma carotenoids have been established as a biomarker for fruit and vegetable intake in pregnant women and other adults (W. K. Al-Delaimy et al., 2005; Brantsæter et al., 2007; Couillard, Lemieux, Vohl, Couture, & Lamarche, 2016).
Although humans must obtain carotenoids from the diet, some microbes in the environment have been shown to synthesize carotenoids for protection against oxidative damage (Saejung & Ampornpat, 2019; Vila, Hornero-Méndez, Azziz, Lareo, & Saravia, 2019). Therefore, it is plausible to consider that some of the biological effects that carotenoids have in humans may be related to the activities of the gut microbiota, but this has neither been adequately studied nor demonstrated within the literature (Lyu, Wu, Wang, Shen, & Lin, 2018). Despite most carotenoid absorption occurring within the upper gastrointestinal tract, recent research has demonstrated that some important lipid transporters involved in carotenoid uptake are located in the colon as well (Reboul, 2019). Considering the high proportion of carotenoids that reach the colon in humans (Rodríguez-Rodríguez et al., 2020), this further substantiates the plausibility of gut microbe-carotenoid interactions.
The objective of this study was to examine relationships between dietary carotenoid intake, plasma carotenoid concentrations, and the gut microbiota of pregnant women. We hypothesized that consumption of foods high in carotenoids during pregnancy would be positively associated with plasma carotenoid concentrations and alpha diversity of the fecal microbiota.
2. Materials and Methods
2.1. Subjects
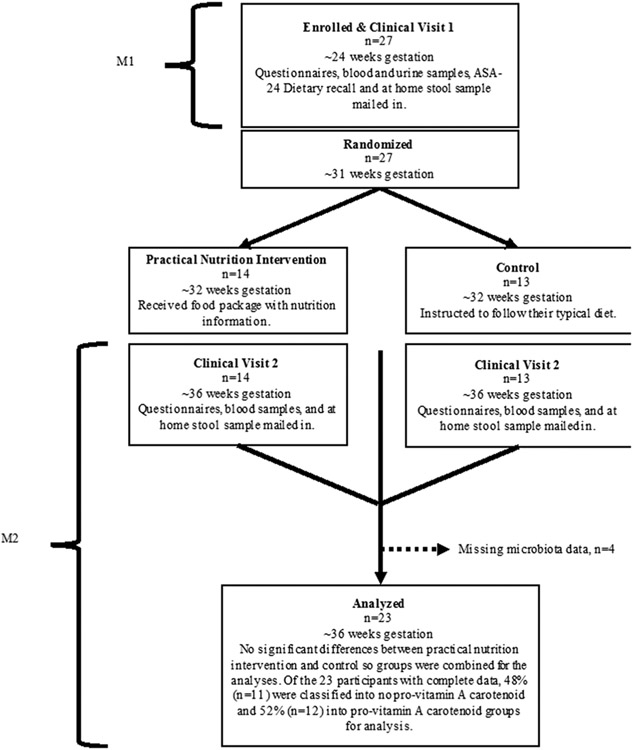
Employing a 2-arm, randomized, controlled feasibility trial, women (n=27) in mid-pregnancy were recruited from a prenatal care clinic serving a rural population in the northwest region of Michigan’s Lower Peninsula. To meet inclusion criteria, participants needed to be at least 18 years old, pregnant (22-30 weeks gestation), and have no contraindications to receiving a healthy food basket. Participants were then randomized to a practical nutrition intervention (n=13) or usual care group (n=14) and followed to 6 weeks postpartum. At 32 weeks pregnancy (M1 time point), the intervention group received a one-time delivery of a non-perishable healthy food basket (whole wheat cereal, oatmeal, dried fruit, canned beans) along with olive oil, vinegar, recipes for salad dressing and side dishes, and general nutrition information. From M1 until the baby was born, the intervention group received a weekly delivery of perishable foods, including: 3 large prepared salads, 2 quarts of soup including either legumes or whole grains (e.g., beans, barley), and 5 pieces of fresh fruit (e.g. apples, oranges). The usual care group did not receive any food packages or nutrition information. The purpose of the practical nutrition intervention was not to track exact nutrients consumed or deliver a set diet, but rather to test the feasibility of food delivery and assess participant satisfaction. Participants collected fecal samples at home and shipped them to the lab (described below). Fecal 16S rRNA DNA amplicons were only sequenced from participants with full fecal sample sets from both the woman (32 weeks gestation, pre-intervention (M1); 36 weeks gestation, mid-intervention (M2); and six weeks after child is born, post-intervention (M3)) and her infant. In total, four participants were excluded due to missing fecal samples, so 23 participants were used for this analysis of M2 samples (Figure 1). The Michigan State University Human Research Protection Program approved this study (IRB #16-1515).
Figure 1.
Flow Chart of Study Participants and Sampling
2.2. Sample Collection
Fecal samples were collected from women at M1, M2, and M3. Samples were sent to the lab by mail, and fecal aliquots were stored at −80°C upon reaching the lab. Average time from sample collection to receipt by the laboratory was 3.8 ± 1.9 days (median of 3.5 days). Biospecimens (blood and urine) were obtained at the time of routine prenatal glucose tolerance test (M2). These were immediately aliquoted and stored at −80°C.
2.3. Dietary Carotenoid Analysis
Due to gut microbial variation at any one time depending most on the previous 24 hours of dietary history (A. J. Johnson et al., 2019), participants were asked to record the foods that they consumed in the 24 hours immediately prior to collecting their fecal sample at about 36 weeks gestation (M2). They were instructed to write out what they ate open-endedly, and given a checklist to select specific foods. Using the open-ended responses in combination with the checklist, dietary carotenoid intake was considered positive among participants who reported consuming one or more foods determined as “high vitamin A” by the Food and Agriculture Organization’s Minimum Dietary Diversity for Women Index (FAO and FHI 360, 2016): carrots/sweet potatoes/mangos/apricots/bell peppers (CSMAB). If the participant did not report consumption of any of these foods, they were categorized into the no pro-vitamin A carotenoid group. Participants also completed several 24-hour recalls throughout the study period using the Automated Self-Administered 24-hour (ASA24) Dietary Assessment Tool, version 2016, developed by the National Cancer Institute, Bethesda, MD (Subar et al., 2012). The data collected from these 24-hour recalls were used to validate classification of carotenoid consumption groups through both the proximal dietary intake data (within two weeks of M2 fecal sample collection) and habitual intake data (throughout study period).
2.4. Plasma Carotenoid Analysis
The Minnesota CHEAR Exposure Assessment Hub performed carotenoid measurements on all M2 plasma samples. Five carotenoids were measured: alpha-carotene (AC), beta-carotene (BC), cryptoxanthin (CR), trans-lycopene (TL), and zeaxanthin & lutein (ZL). They were quantified by HPLC using photodiode array detection, using a previously published protocol (Bieri, Brown, & Smith, 1985; Craft, Brown, & Smith, 1988) with slight modification (Gross, Yu, Hannan, Prouty, & Jacobs, 2003). In this study, TL was measured since it is the major isomer constituting the lycopene profile in food (Clinton et al., 1996).
2.5. DNA Extraction and Amplification
Extraction of bacterial DNA was conducted using MoBio Powersoil DNA Isolation kit (Qiagen MoBio, Carlsabad, CA), and the specific processes of DNA extraction, V4 16S rRNA gene amplification, and sequencing were performed using a previously described protocol (Sugino, Paneth, & Comstock, 2019).
2.6. Processing and Analysis of Sequence Data
In mothur, processing of sequence reads occurred with the Illumina MiSeq SOP (Kozich, Westcott, Baxter, Highlander, & Schloss, 2013) using the High-Performance Computing Cluster at Michigan State University. Utilizing the SILVA reference taxonomy (v128) (Quast et al., 2013), operational taxonomic unit (OTU) taxonomies were specified by phylotype. Each sample was rarefied to 9000 reads 999 times, averaged, and rounded to the nearest integer prior to additional analysis. Adequate community coverage was confirmed with rarefaction curves.
2.7. Data Analysis
All comparisons of population characteristics were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Comparisons were using a chi-square test for categorical variables or ANOVA for continuous variables. The intervention and usual care groups from the original study design were compared for carotenoid intake using Fisher’s exact test to detect differences in reported intake within these food groups. Savage one-way analysis two-sided p > ∣Z∣ was used to test for significant differences between plasma carotenoid concentrations and reported intake of high-carotenoid-foods. Differences in 24-hour dietary recall between no pro-vitamin A carotenoid and pro-vitamin A carotenoid groups were determined with the Welch Two Sample t-test for parametric data and the Wilcoxon Mann-Whitney U test for nonparametric data. Normality of dietary intake data was confirmed with a Shapiro-Wilk test. Pearson correlation tests were used to determine associations between proximal carotenoid intake and the respective plasma concentration. Women’s pre-pregnancy BMI and M2 BMI were calculated using self-reported height and weight of the participants (Shin, Chung, Weatherspoon, & Song, 2014). Differences in BMI measures by reported high pro-vitamin A carotenoid food intake were tested using the Wilcoxon Mann-Whitney U test. Correlations between plasma carotenoid concentrations and BMI measures were tested using Spearman’s correlation coefficient. Alpha (within-sample) diversity (Chao1, inverse Simpson and Shannon indices) was calculated in R (R Core Team & Team, 2011) with the vegan package (Oksanen et al., 2015). Normality of the alpha diversity was confirmed using the Shapiro-Wilk test and ANOVA was used to test for differences by carotenoid intake. Correlations between plasma carotenoid concentrations and alpha diversity were tested using Pearson’s correlation coefficient. Sorensen (community composition) and Bray-Curtis (community structure) dissimilarities were calculated in R from the abundance data using the vegan package and plotted using principle coordinate analysis (PCoA). Permutational multivariate analysis of variation (PERMANOVA) was performed using the adonis function (vegan package in R) to test for significant differences in beta-diversity between carotenoid intake groups or across plasma carotenoid concentrations. Permutational analysis of multivariate dispersion (PERMDISP) (betadisper function in the vegan package) was used to test for differences in group dispersion. Individual taxa were compared between carotenoid intake groups using a negative binomial model in the MASS package (Venables & Ripley, 2002). Rarefied count data of taxa that composed ≥1% abundance (on average) were compared to plasma carotenoid concentrations using a negative binomial model in the MASS package. Post-hoc power analysis for the alpha diversity analyses was conducted through G*power version 3.1.9.2 (Faul, Erdfelder, Lang, & Buchner, 2007). The Benjamini-Hochberg method was used for false discovery rate correction. P-values less than 0.05 were considered significant.
3. Results and Discussion
The relationship between consumption of specific micronutrients, such as the B vitamins, and the gut microbiome have been examined (Das, Babaei, & Nielsen, 2019; Putnam & Goodman, 2020; Yoshii, Hosomi, Sawane, & Kunisawa, 2019). However, the association between the gut microbiome and other dietary microconstituents, such as carotenoids, is poorly understood. This is especially true in relation to pregnancy. Therefore, in this population of pregnant women, we investigated the relationship between consumption of carotenoid-rich foods, plasma concentrations of carotenoids, and the gut microbiota.
3.1. Subject Characteristics (Table 1)
Table 1.
Maternal characteristics at study enrollment
| Race/Ethnicity | N | % |
| Mixed Race | 2 | 9 |
| White | 21 | 91 |
| Hispanic/Latina | 2 | 10 |
| Did not respond | 1 | 5 |
| Education Level | ||
| High school diploma or equivalency | 3 | 13 |
| Some college or Associate’s degree | 9 | 39 |
| Bachelor’s degree or higher | 11 | 48 |
| Medicaid Health Insurance (yes) | 5 | 22 |
| Living with Baby’s Father (yes) | 23 | 100 |
| Ever Smoked | 8 | 35 |
| Body Mass Index (kg/m) | Mean ± SD | Range |
| Pre-Pregnancy | 27.4 ± 6.9 | 20.0, 41.6 |
| ~36 Weeks (M2) | 31.1 ± 6.5 | 24.4, 44.1 |
| Age (years) | 29.7 ± 4.3 | 22, 40 |
Of the participants, 9% (n=2) reported “mixed race” and 91% (n=21) reported “white” as their race. From those reporting a race of “white”, 10% (n=2) reported Hispanic/Latina ethnicity and 5% (n=1) did not respond. While 48% (n=11) of women had a bachelor’s degree or higher, 39% (n=9) attended some college or had an associate degree, and 13% (n=3) of women had a high school diploma or equivalent. All of the participants (N=23) reported that they lived with the baby’s father, while 22% (n=5) participants reported having Medicaid as their health insurance, and 35% (n=8) reported ever smoking. The average pre-pregnancy BMI of the participants was 27.4 (range 20.0-41.6) and the average M2 BMI was 31.1 (range 24.4 - 44.1). The mean age of the participants was 29.7 years old (range 22-40).
3.2. Dietary Intake & Plasma Carotenoid Measurements
There were no significant differences between the intervention group and the usual care group in reported consumption (yes/no) of high-pro-vitamin A carotenoid foods (Supplementary Table 1). Overall, 48% (n=11) reported consuming CSMAB (p=0.200), 17% (n=4) consumed oranges or orange juice (p=0.396), 39% (n=9) consumed egg (p=0.252), and 52% (n=12) consumed tomato or tomato-based sauces (p=0.320). Fruit and vegetable intake were also assessed and was not significantly different between the usual care and intervention group. Overall, 83% (n=19) consumed fruits (p=0.396), and 65% (n=15) consumed vegetables (p=0.315). Upon analysis of reported intakes of 100 dietary constituents (same as those analyzed between carotenoid consumption groups; Supplementary Table 2) collected from the proximal dietary recalls, there were no significant differences in reported consumption between the practical nutrition intervention and usual care groups (p-values not reported).
Of the 10 participants who received the food packages, 7 (70%) reported consuming “all” or “most” of the food package in the past week and 3 (30%) reported consuming “little” or “some”. However, there were no significant differences in plasma carotenoid concentrations between the 10 participants in the intervention group and the 13 participants in the usual care group. Furthermore, there was no significant overlap between intervention/usual care groups and consumers of pro-vitamin A carotenoids (Fisher’s exact; p=0.4136). This, along with the lack of significant differences in reported intakes of carotenoid-rich foods and other dietary constituents, validated combining the practical nutrition and intervention groups into a single study population for more robust analysis of the relationship between diet and gut microbial parameters.
Among all (N=23) participants, the total number that reported intake of at least one of the high-pro-vitamin A carotenoid foods (CSMAB) in the 24 hours prior to fecal sample collection was 11 (48%). Twelve (52%) participants did not report intake of a high pro-vitamin A carotenoid food. Of the participants, 78% (n=18) completed a proximal 24-hour recall (ASA24) within two weeks of M2 fecal sample collection. Throughout the study period, these 18 individuals completed an average of 5.3 ± 1.9 24-hour dietary recalls. Proximal dietary intakes of AC, BC, fiber, lycopene, tomatoes and tomato products, total red and orange vegetables, other vegetables, and total dark green, red and orange, starchy, and other vegetables (excluding legumes) were all consumed in significantly greater amounts amongst the pro-vitamin A carotenoid group (Table 2). Habitual dietary intake confirmed the significant differences in BC, fiber, tomatoes and tomato products, and total red and orange vegetables (Supplementary Table 2).
Table 2.
Selected dietary components from proximal 24-hour recalls exhibiting differences in reported consumption between no pro-vitamin A carotenoid and pro-vitamin A carotenoid groups.
| Dietary Component (Subar et al., 2012) | Mean +/− SE No Pro-vitamin A Carotenoid (n=9) |
Mean +/− SE Pro- vitamin A Carotenoid (n=9) |
P-Value | BH-Adjusted2 |
|---|---|---|---|---|
| Energy (kcal) | 2425.93 +/− 332.15 | 2189.3 +/− 117.55 | 0.931 | 0.991 |
| Fiber, total dietary (g) | 17.25 +/− 7.37 | 25.32 +/− 6.92 | 0.0291 | 0.991 |
| Carbohydrate (g) | 270.31 +/− 31.86 | 277.26 +/− 20.54 | 0.8571 | 1.000 |
| Total whole and refined grains (oz. eq.) | 6.27 +/− 0.77 | 7.34 +/− 0.75 | 0.3341 | 0.991 |
| Grains defined as whole grains and contain the entire grain kernel: the bran, germ, and endosperm (oz. eq.) | 1.3 +/− 0.44 | 1.31 +/− 0.27 | 0.9931 | 1.000 |
| Vitamin A, RAE (mcg_RAE) | 713.62 +/− 131.03 | 869.52 +/− 157.59 | 0.4581 | 0.991 |
| Retinol (mcg) | 622.15 +/− 137.73 | 531.49 +/− 89.95 | 0.5901 | 1.000 |
| Carotene, beta (mcg) | 945.38 +/− 281.94 | 3689.08 +/− 1142.91 | 0.019 | 0.991 |
| Carotene, alpha (mcg) | 189.99 +/− 93.59 | 684.17 +/− 237.67 | 0.040 | 0.991 |
| Cryptoxanthin, beta (mcg) | 90.19 +/− 26.02 | 54.99 +/− 17.98 | 0.340 | 0.991 |
| Lycopene (mcg) | 3297.50 +/− 2219.02 | 9335.26 +/− 3442.22 | 0.021 | 0.991 |
| Lutein + zeaxanthin (mcg) | 958.19 +/− 123.82 | 3433.88 +/− 1487.88 | 0.161 | 0.991 |
| Other red and orange vegetables, excluding tomatoes and tomato products (cup eq.) | 0.03 +/− 0.02 | 0.16 +/− 0.06 | 0.073 | 0.812 |
| Tomatoes and tomato products (cup eq.) | 0.18 +/− 0.13 | 0.45 +/− 0.09 | 0.029 | 0.487 |
| Total red and orange vegetables (tomatoes and tomato products + other red and orange vegetables) (cup eq.) | 0.21 +/− 0.13 | 0.61 +/− 0.1 | 0.006 | 0.487 |
| Total dark green, red and orange, starchy, and other vegetables; excludes legumes (cup eq.) | 1.01 +/− 0.24 | 2.01 +/− 0.36 | 0.0391 | 0.500 |
| Other vegetables (cup eq.) | 0.18 +/− 0.07 | 0.86 +/− 0.25 | 0.017 | 0.487 |
P-values computed with Welch Two Sample t-test for parametric data (Shapiro-Wilk; p>0.05); All others without superscript computed with Wilcoxon rank sum test for non-parametric data (Shapiro-Wilk; p≤0.05).
P-values adjusted using the Benjamini-Hochberg procedure.
Plasma concentrations of several carotenoids were significantly associated with reported consumption (yes/no) of foods known to contain those carotenoids (Supplementary Table 3). All of the participants exhibited plasma carotenoid concentrations reflective of adequate dietary pro-vitamin A carotenoid intake. Pregnant women who consumed foods high in pro-vitamin A carotenoids (CSMAB) had higher plasma concentrations of AC (overall 6.45±4.67ug/dL, consumed 9.48±4.65ug/dL, did not consume 3.68±2.51ug/dL; p=0.0017) and BC (overall 17.7±11.6ug/dL, consumed 23.2±11.6ug/dL, did not consume 12.7±9.26ug/dL; p=0.0465). Women who consumed oranges/orange juice had significantly higher concentrations of CR (overall 11.4±6.99ug/dL, consumed 19.0±11.0ug/dL, did not consume 9.84±4.91ug/dL; p=0.0245). ZL concentrations were significantly higher in pregnant women who consumed egg (overall 29.8±12.3ug/dl, consumed 36.4±15.4ug/dL, did not consume 25.6±7.81ug/dL; p=0.0174). TL concentrations (39.0±18.4ug/dL) did not differ by reported tomato intake, and total plasma carotenoid concentrations (104±39.8ug/dL) did not differ by consumption of any of these food groups. Correlations between proximal carotenoid intake from the 24-hour recalls and the respective plasma carotenoid concentrations were also found, with AC dietary intake positively correlating with AC plasma concentration (r=0.62, p=0.006) (Supplementary Figure 1A).
Significant gaps exist in the understanding of carotenoid metabolism, and carotenoid bioavailability is highly variable (Reboul, 2019). After a meal high in carotenoids, it has been shown that carotenoids in plasma chylomicrons increase 3-4 hours after ingestion and return to near baseline after 12 hours (though this varies with the proportion of fat in the meal) (Brown et al., 2004). Longitudinal endogenous carotenoid concentrations, however, are largely stable over time (Wael K. Al-Delaimy, Natarajan, Sun, Rock, & Pierce, 2008). Previously, three-day food records and dietary recalls within a three-week period have been shown to provide adequate dietary coverage to establish associations with plasma carotenoid concentrations (Pierce et al., 2006; Prasad et al., 2018). Additionally, results from the present study are consistent with other studies where reported dietary intake of carotenoids served as an accurate proxy for plasma carotenoid concentrations (W. K. Al-Delaimy et al., 2005; Tucker et al., 1999). Specifically, the women who reported consumption of foods high in certain carotenoids (CSMAB, orange juice, and egg) also had higher plasma concentrations of the associated carotenoid (AC, BC, CR, and ZL). The one food and plasma carotenoid pairing where this was not observed was TL and tomato intake, which may be due to the poor absorption of TL in the human body relative to other lycopene isomers (Rao & Agarwal, 2000; Stahl & Sies, 1992). Nonetheless, the plasma concentrations of most carotenoids suggest a confirmation of reported carotenoid intake. Collectively, this demonstrates that our method of diet data collection is a feasible way to measure women’s dietary intake and qualitatively estimate plasma carotenoid concentrations.
3.3. Associations with BMI
Reported Intake:
Those who reported no consumption of high-pro-vitamin A carotenoid foods had a significantly higher pre-pregnancy BMI (30.8 kg/m2) compared to those who reported consumption (23.6 kg/m2) (S=91 , p= 0.0106), and tended to have a higher M2 BMI (33.5 kg/m2) compared to those who did not (28.5 kg/m2) (S=103 , p= 0.0764).
Plasma:
Pre-pregnancy BMI was negatively associated with plasma concentrations of AC (ρ= −0.567, p=0.005), BC (ρ= −0.690, p=0.0003), CR (ρ = −0.504, p=0.014), TL (ρ = −0.453, p=0.030), and total plasma carotenoids (ρ= −0.625, p=0.001). BMI at M2 was negatively associated with AC (ρ = −0.418, p=0.047), BC (ρ = −0.469, p=0.024), and CR (ρ = −0.419, p=0.047), and tended to be negatively associated with total plasma carotenoids (ρ = −0.384, p=0.071). ZL plasma concentrations were not associated with BMI at either time point.
3.4. Alpha Diversity
Reported Intake:
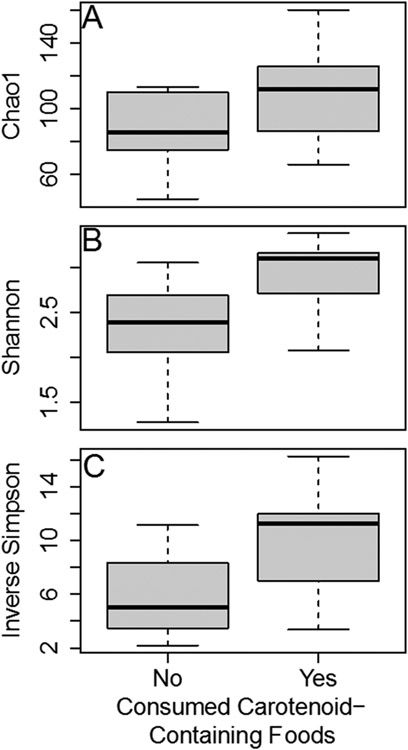
Comparing participants by reported intake of high-pro-vitamin A carotenoid foods, the fecal bacterial alpha-diversity of the women who reported consuming high-pro-vitamin A carotenoid-foods was significantly higher than those who did not consume such foods (Figure 2; Chao 1 (F=4.33, p=0.0498), Shannon (F=9.82, p=0.00502) and Inverse Simpson (F=7.75, p=0.0111)).
Figure 2.
Women (M2) who reported consuming pro-vitamin A carotenoid containing foods had a higher fecal bacterial alpha (within-sample) diversity as measured by (A) Chao 1 (p=0.0498), (B) Shannon (p=0.00502), and (C) Inverse Simpson (p=0.0111). Abbreviations: M2: 36 weeks gestation, mid-intervention.
Our study confirms the increase in microbial diversity that arises in conjunction with regular consumption of fruits and vegetables, which has been reported by others (Klimenko et al., 2018; Simpson & Campbell, 2015). Previously, it has been shown that vitamin A-sufficient children have higher alpha diversity than vitamin A-deficient children, indicated by significant differences in Shannon and Inverse Simpson scores but not Chao1 scores (Lv et al., 2016). Herein, all three measures of alpha diversity (Chao1, p=0.0498; Shannon, p=0.005; Inverse Simpson, p=0.011) were significantly positively associated with carotenoid consumption.
Plasma:
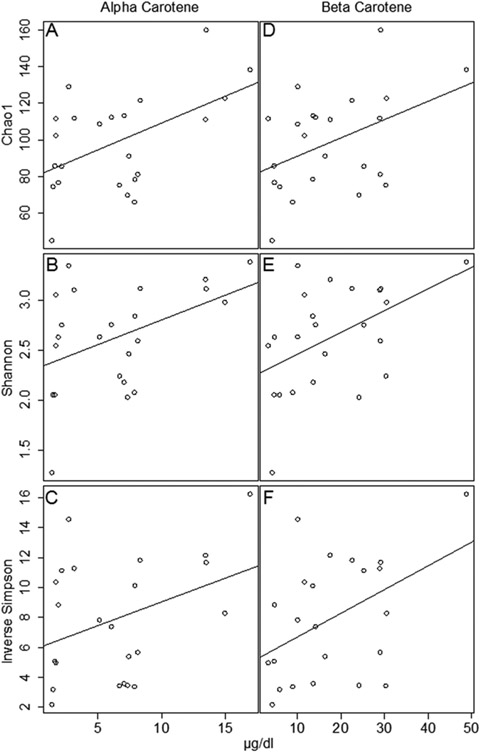
Overall, fecal bacterial alpha diversity was positively associated with participant plasma carotene concentrations (Figure 3). AC and alpha diversity were significantly positively associated as measured by Chao 1 (ρ=0.437, p=0.037), Shannon (ρ=0.492, p=0.017), and Inverse Simpson (ρ=0.426, p=0.043) (Figure 3A-C). BC tended to be positively associated with Chao 1 (ρ=0.401, p=0.059), and BC was significantly positively associated with Shannon (ρ=0.464, p=0.027) and Inverse Simpson alpha diversity (ρ=0.425, p=0.044) (Figure 3D-F). None of the fecal bacterial alpha diversity measures were associated with CR, TL, or ZL (data not shown). Differences in plasma carotenoid concentrations have long been known to be mediated by multiple factors such as diet, absorption, and metabolism, and we cannot be sure which of these affected the relationships observed. Experimental studies are needed to truly elucidate the nature of these carotenoids’ associations, or lack thereof, with gut microbial diversity.
Figure 3.
Fecal bacterial alpha diversity is positively associated with plasma alpha carotene concentrations as measured by (A) Chao 1 (p=0.0369), (B) Shannon (p=0.0170), and (C) Inverse Simpson (p=0.0430) and beta carotene concentrations as measured by (D) Chao 1 (p=0.0588), (E) Shannon (p=0.0268), and (F) Inverse Simpson (p=0.0445).
The power and effect sizes for all alpha diversity analyses are included (Supplementary Table 4).
3.5. Beta Diversity
Reported Intake:
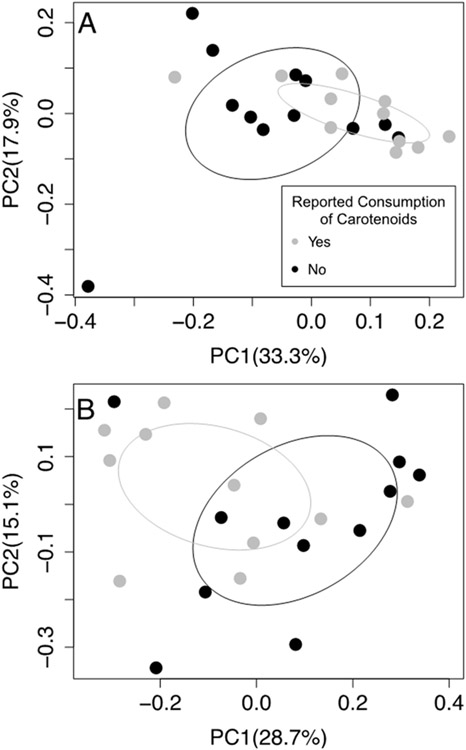
The fecal bacterial community composition (beta diversity) in pregnant women (M2, 36 weeks gestation) who reported intake was significantly different than those who did not report intake (Figure 4; Sorenson (F=2.10, p=0.0493), Bray-Curtis (F=1.96, p=0.0421)). Those who reported a higher intake of carotenoids were often characterized by higher abundances of Ruminococcaceae_UCG 002 (Supplementary Figure 2; R2 =0.52). This finding is valuable in shedding light on possible dietary factors that may shape the Ruminococcus enterotype. While it is well established that the Bacteroides enterotype is associated with protein and animal fat, and the Prevotella enterotype with fiber and carbohydrate intake, dietary associations with the Ruminococcus enterotype are not as well-defined (Arumugam et al., 2011; Wu et al., 2011). The current literature suggests that Ruminococcus is variably related to both plant and animal consumption due its associations with resistant-starch (Abell, Cooke, Bennett, Conlon, & McOrist, 2008; Ze, Duncan, Louis, & Flint, 2012), but also with low fiber diets (Whisner, Maldonado, Dente, Krajmalnik-Brown, & Bruening, 2018) and trimethylamine n-oxide (a microbial metabolite associated with animal proteins) (De Filippis et al., 2016; Tomova et al., 2019). Evidently, Ruminococcus has complex and species-specific relations with diet that are difficult to characterize, but our study suggests that in terms of dietary microconstituents, carotenoids may be a piece of the puzzle.
Figure 4.
Fecal bacterial community composition (beta (across sample) diversity) in women (M2) was significantly associated with reported intake of pro-vitamin A carotenoid containing foods as measured by the (A) Sorenson metric (p=0.0493) and the (B) Bray-Curtis metric (p=0.0421). Reported consumption is based on the 24 hours immediately prior to collecting participant fecal sample. Abbreviations: M2: 36 weeks gestation, mid-intervention.
Plasma:
Sorensen beta diversity was significantly associated with plasma AC concentrations (F=2.03, p=0.0439) and tended to be associated with plasma BC concentrations (F=1.74, p=0.089) (Supplementary Figure 3A-B). Bray-Curtis beta diversity tended to be associated with TL (F= 1.82, p=0.061), but was not significantly related with any other plasma carotenoids (Supplementary Figure 3C). Neither Sorenson nor Bray-Curtis beta diversity were significantly related to plasma CR or ZL.
3.6. Individual Taxa
Reported Intake:
When classifying participant microbiota communities by reported consumption of carotenoids, significant differences in the genera and phyla were observed (Table 3). Genus level mean percent abundance among those reporting high-pro-vitamin A carotenoid food consumption (Akkermansia [4.1 ± 2.8], Prevotella [1.4 ± 3.8], and Acidaminococcus [0.7 ± 2.1]) were all significantly lower (p<0.001) than individuals who reported no high-pro-vitamin A carotenoid food consumption (Akkermansia [5.9 ± 6.8], Prevotella [2.0 ± 5.9], Acidaminococcus [2.6 ± 6.7]). At the phylum level, mean percent abundance of Verrucomicrobia was also significantly lower (p<0.001) among individuals who reported high-pro-vitamin A carotenoid food consumption (4.1 ± 2.8) in comparison to the individuals who did not (5.9 ± 6.8).
Table 3.
Genera and phyla in the maternal microbiota that differ between women who consumed carotenoid containing foods and those who did not consume such foods.
| Genus | Reported Pro-Vitamin A Carotenoid Food Consumption |
No Report of Pro-Vitamin A Carotenoid Food Consumption |
P- value2 |
|---|---|---|---|
| Akkermansia | 4.1 ± 2.81 | 5.9 ± 6.8 | <0.001 |
| Prevotella 9 | 1.4 ± 3.8 | 2.0 ± 5.9 | <0.001 |
| Acidaminococcus | 0.7 ± 2.1 | 2.6 ± 6.7 | <0.001 |
| Phylum | |||
| Verrucomicrobia | 4.1 ± 2.8 | 5.9 ± 6.8 | <0.001 |
Values reported as mean % abundance ± SD; negative binomial regression on the bacterial count data.
P-values adjusted using the Benjamini-Hochberg procedure.
One study utilizing food frequency questionnaires administered to pregnant mothers in their second trimester found that reported retinol intake was positively associated with the Preoteobacteria phylum in maternal fecal samples collected four days after delivery (Mandal et al., 2016), but we found no such relation among our participants in the third trimester of pregnancy. In mice, Akkermansia, both in fecal and cecal samples, has been shown to be highly responsive to fucoxanthin (a xanthophyll carotenoid) supplementation (Guo et al., 2019). The mice eating a normal chow diet supplemented with fucoxanthin exhibited significant decreases in fecal Akkermansia levels, and the high-carotenoid consumers in our study also exhibited significant reductions in Akkermansia.
Plasma concentrations:
Plasma concentrations of carotenoids were also significantly related to fecal bacterial genera (Table 4). AC concentrations were negatively related to relative abundance of Akkermansia (p<0.0001) and positively related to Phascolarctobacterium (p<0.001). BC was positively related to relative abundance of Ruminococcaceae UCG002 (p<0.001). TL was negatively related to Akkermansia (p<0.05), Escherichia Shigella (p<0.01), Phascolarctobacterium, Ruminococcaceae UCG002, and Prevotella 9 (p<0.001), but positively associated with Ruminococcus 2 (p<0.001). CR concentrations were positively related to Phascolarctobacterium (p<0.001) and negatively related to Prevotella 9 (p<0.001). Lastly, ZL was positively related to Akkermansia and Phascolarctobacterium (p<0.001), and negatively related to Prevotella 9 (p<0.001).
Table 4.
Relationships between specific genera in the maternal microbiota and plasma carotenoids.
| Plasma Carotenoid1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AC | BC | TL | CR | ZL | ||||||
| Genus | Slope2 | p-value3 | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value |
| Bacteroides | −0.0307 | 0.801 | −0.0145 | 0.710 | 0.0106 | 0.436 | −0.00936 | 0.921 | −0.00141 | 0.981 |
| unclassified Lachnospiraceae | 0.0395 | 0.739 | 0.0257 | 0.462 | −0.00808 | 0.714 | −0.00605 | 0.921 | −0.00416 | 0.981 |
| Faecalibacterium | 0.0858 | 0.381 | 0.0252 | 0.467 | 0.00421 | 0.853 | 0.00319 | 0.921 | −0.000468 | 0.981 |
| Roseburia | −0.0529 | 0.739 | 0.000409 | 1.000 | 0.0108 | 0.624 | −0.0315 | 0.724 | −0.00283 | 0.981 |
| Blautia | −0.0216 | 0.801 | 0.00734 | 1.000 | −0.00167 | 0.923 | −0.0343 | 0.724 | −0.00619 | 0.981 |
| Bifidobacterium | −0.0512 | 0.739 | −0.00264 | 1.000 | −0.0106 | 0.798 | −0.0408 | 0.724 | 0.0265 | 0.981 |
| Subdoligranulum | 0.0407 | 0.801 | 0.0252 | 0.894 | 0.0212 | 0.510 | 0.0519 | 0.724 | 0.0257 | 0.981 |
| Alistipes | 0.00259 | 0.957 | −0.00631 | 1.000 | −0.00144 | 0.923 | 0.0253 | 0.839 | −0.000445 | 0.981 |
| Parabacteroides | −0.0618 | 0.739 | −0.0260 | 0.467 | 0.00663 | 0.798 | −0.00642 | 0.921 | −0.00515 | 0.981 |
| Lachnospiraceae_ge | −0.0308 | 0.801 | −0.000905 | 1.000 | 0.00520 | 0.798 | −0.0272 | 0.773 | −0.0112 | 0.981 |
| Ruminococcaceae_UCG002 | 0.0500 | 0.801 | 0.0180 | <0.001 | −0.0164 | <0.001 | 0.0281 | 0.921 | −0.00254 | 0.548 |
| Escherichia Shigella | −0.0102 | 0.951 | −0.0282 | 0.808 | −0.0752 | <0.01 | 0.0143 | 0.921 | −0.0177 | 0.981 |
| Akkermansia | −0.01494 | <0.001 | −0.00137 | 0.462 | −0.00149 | <0.05 | 0.0015 | 0.724 | 0.0179 | <0.001 |
| Phascolarctobacterium | 0.0138 | <0.001 | −0.00237 | 0.192 | −0.00547 | <0.001 | 0.0182 | <0.001 | 0.0262 | <0.001 |
| Ruminococcus_2 | −0.0130 | 0.951 | −0.00303 | 1.000 | 0.0198 | <0.001 | −0.0203 | 0.921 | 0.0235 | 0.981 |
| Prevotella_9 | 0.0586 | 0.924 | −0.00259 | 1.000 | −0.171 | <0.001 | −0.115 | <0.001 | −0.127 | <0.001 |
Abbreviations: α-carotene (AC), β-carotene (BC), cryptoxanthin (CR), trans-lycopene (TL), zeaxanthin and lutein (ZL).
Values reported as slope of the negative binomial regression on the bacterial count data (e.g. For every 1 unit increase in Akkermansia count abundance, there is an expected 0.0148 log decrease of plasma AC).
All p-values adjusted using the Benjamini-Hochberg procedure.
Light gray shading underscores significant negative relations, whereas darker gray shading underscores significant positive relations.
In our study, Akkermansia was shown to be negatively correlated with AC, a pro-vitamin A carotenoid. However, in 2-year old girls, plasma retinol concentrations were significantly associated with increases in the Verrucomicrobia phylum and Akkermansia genus (Huda et al., 2019). Our study adds to the current body of evidence suggesting that carotenoids are significantly associated with Akkermansia, a mucin-degrading gut microbe. We hypothesize that the observed correlations between Akkermansia and carotenoid intake arise from regulatory and antioxidant functions that carotenoids play in maintaining gut mucosal integrity and function (Baralic et al., 2015; Biesalski, 2016; Lyu et al., 2018; Naguib, 2000). Other notable plasma carotenoid correlations with genera abundances include the increases in Ruminococcaceae_UCG 002 in association with BC and TL. This finding again stresses the importance of exploration into the Ruminococcus enterotype and its possible associations with carotenoids, especially considering the previously-demonstrated enrichment of atherosclerotic patients in the Ruminococcus enterotype with concurrent reductions in the phytoene dehydrogenase genetic pathway which is significantly associated with plasma BC concentrations (Karlsson et al., 2012). Similar to Akkermansia, some species within Ruminococcaceae, such as Ruminococcus gnavus, are mucin-degraders, which may explain the observed associations between Ruminococcus and certain carotenoids in our study as well as vitamin A deficiency in a previous study conducted in mice (Hibberd et al., 2017).
3.7. Dietary Fiber vs. Carotenoid Effects
Elucidating the effects of a specific micronutrient on the individual, let alone the gut microbiome, while balancing the complex interactions from overall dietary intake remains an intrinsic challenge of nutritional research (Weaver & Miller, 2017). Due to the association between carotenoid-containing foods and dietary factors such as fiber, it is unclear whether the alpha and beta diversity differences observed between pro-vitamin A carotenoid groups are due to causative effects of the carotenoids themselves. This is especially the case since the pro-vitamin A carotenoid group reported a significantly higher fiber intake than those recording no consumption (Table 2). Dietary fiber can inhibit carotenoid absorption by interfering with micelle formation and interfacing with enterocytes (Palafox-Carlos, Ayala-Zavala, & González-Aguilar, 2011; Riedl, Linseisen, Hoffmann, & Wolfram, 1999; van het Hof, West, Weststrate, & Hautvast, 2000) and is also associated with increased gut microbial richness (Makki, Deehan, Walter, & Bäckhed, 2018; Tap et al., 2015). These interactions obfuscate our ability to differentiate between carotenoid versus dietary effects. Nonetheless, several studies examining vitamin A deficiency find important changes in the gut microbiome (Hibberd et al., 2017; Huda et al., 2019; Lv et al., 2016), suggesting a potential role for pro-vitamin A carotenoids irrespective of diet. Furthermore, the agreement of dietary and plasma-level data suggests that some of the observed taxa associations, such as Akkermansia, arose from the carotenoids themselves due to the roles of carotenoids and Akkermansia in maintaining the integrity of the gut mucosa. The potential mechanisms by which pro-vitamin A carotenoids influence the gut microbiome are unclear, but some research in mice has hinted towards immunoregulation, mediated by vitamin A and commensal gut microbial interactions, having downstream effects that help prevent dysbiosis and maintain gut barrier function (Grizotte-Lake et al., 2018; Iyer & Vaishnava, 2019; Sirisinha, 2015; Spencer et al., 2014).
3.8. Strengths and Limitations
There are several limitations to this study. First, a small sample size (N=23) of women during late pregnancy (36-weeks gestation) restricts the generalizability of the results. In addition, volunteers were self-selected to be in this study, which was advertised as a program where participants would receive and eat a healthy food package. As a result, many of these self-selected participants were already eating healthy diets in comparison with the typical American diet. Despite the range of BMIs amongst the women, the observed correlations between BMI and dietary/plasma carotenoids are likely reflective of chronic dietary patterns (Kornatowski & Comstock, 2018), although further examination with long-term dietary tracking is needed to truly ascertain these relationships. Another possible limitation is that genetic polymorphisms in the metabolism of carotenoids were not measured, and such polymorphisms have been shown to affect plasma carotenoid concentrations (Moran, Erdman, & Clinton, 2013), which could unknowingly and unintentionally affect these results. However, considering the strong, repeatedly-demonstrated associations between dietary intake and plasma carotenoids (W. K. Al-Delaimy et al., 2005) and that dietary fruits/vegetables account for ~90% of carotenoid intake (Maiani et al., 2009), diet is likely to be the principle determinant of plasma carotenoid concentrations. Future studies would do well to account for genetic variants between participants in addition to dietary information to better elucidate these relationships. We do not account for the dilution of blood during pregnancy, but this has no significant impact on our results since we only report cross-sectional associations at a common gestational age. A strength of this study is that reported recent intake of high-carotenoid foods was significantly related to plasma carotenoid concentrations, adding to the current body of evidence. Utilizing both dietary and plasma carotenoid markers provided a multifocal perspective to our study which increases the relevance to the average consumer, as plasma-level information is often inaccessible. Additionally, the use of 24-hour dietary recalls to substantiate classification of carotenoid consumer groups provided both a proximal and habitual view of the dietary patterns of the participants.
4. Conclusion
Overall, this study provides a first analysis of the relationship between dietary or plasma carotenoids and the gut microbiota in pregnant women. Higher dietary and plasma carotenoids were associated with a more diverse gut microbial composition (alpha diversity) in this population. Gut microbial communities were significantly different between participants who reported intake of pro-vitamin A carotenoids versus those who did not (beta diversity). These results may reflect an effect of high fiber or improved overall dietary quality, rather than a specific effect of carotenoids, on the microbiota. High alpha diversity is generally accepted to be a positive characteristic of gut communities. It is possible that a diverse microbiota could be one mechanism by which carotenoids promote health. Several taxa were significantly associated with dietary and plasma carotenoids. The associations with Akkermansia and Ruminococcus specifically merit further research due to the potential interplay of carotenoids with the gut mucosa. In the future, research examining the underlying mechanisms responsible for the observed associations between carotenoids and the gut microbiome may be useful in identifying the functional aspects of carotenoids in the gut microenvironment. A larger, targeted study which focuses on a carotenoid-only intervention, will be needed to determine if diets high in carotenoids influence the microbiota independently of diets high in other fruits or vegetables.
Supplementary Material
Practical Application:
Little is known about the association between the gut microbiome and specific dietary microconstituents, such as carotenoids, especially during pregnancy. This research demonstrates that a carotenoid-rich diet during pregnancy supports a diverse microbiota, which could be one mechanism by which carotenoids promote health.
Acknowledgements
KYS was supported by a graduate research assistantship from the Michigan State University Department of Food Science and Human Nutrition. ENH was supported in part by the MSU Professorial Assistantship program. This research was financially supported by start-up funds provided to SSC and JMK by Michigan State University. Carotenoid analyses were provided by the NIH funded Children’s Health Exposure Analysis Resource (CHEAR) Pilot and Feasibility (P&F) Program (CHEAR P&F 2018-PF05), which was funded by NIH 1U2CES026533. This work was supported in part through computational resources and services provided by the Institute for Cyber-Enabled Research at Michigan State University.
Nomenclature
- AC
α-carotene
- BC
β-carotene
- CSMAB
high-carotenoid foods (Carrots/sweet potatoes/mangos/apricots/bell peppers)
- CR
Cryptoxanthin
- M1
32 weeks gestation, pre-intervention
- M2
36 weeks gestation, mid-intervention
- M3
Six weeks after child is born, post-intervention
- OTU
Operational taxonomic unit
- PCoA
Principal coordinates analysis
- TL
trans-lycopene
- ZL
Zeaxanthin and lutein
Footnotes
Conflicts of Interest
KMS, ENH, KYS, KRV, LAP, RK, MDG, JMK, and SSC report no conflicts of interest.
References
- Abell GCJ, Cooke CM, Bennett CN, Conlon MA, & McOrist AL (2008). Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiology Ecology, 66(3), 505–515. 10.1111/j.1574-6941.2008.00527.x [DOI] [PubMed] [Google Scholar]
- Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, … Riboli E (2005). Plasma carotenoids as biomarkers of intake of fruits and vegetables: Individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). European Journal of Clinical Nutrition. 10.1038/sj.ejcn.1602252 [DOI] [PubMed] [Google Scholar]
- Al-Delaimy Wael K., Natarajan L, Sun X, Rock CL, & Pierce J (2008). Reliability of plasma carotenoid biomarkers and its relation to study power. Epidemiology, 19(2), 338–344. 10.1097/EDE.0b013e3181635dc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, … Bork P (2011). Enterotypes of the human gut microbiome. Nature, 473(7346), 174–180. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralic I, Andjelkovic M, Djordjevic B, Dikic N, Radivojevic N, Suzin-Zivkovic V, … Pejic S (2015). Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evidence-Based Complementary and Alternative Medicine, 2015, 783761. 10.1155/2015/783761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri JG, Brown ED, & Smith JC (1985). Determination of individual carotenoids in human plasma by high performance liquid chromatography. Journal of Liquid Chromatography, 8(3), 473–484. 10.1080/01483918508067094 [DOI] [Google Scholar]
- Biesalski HK (2016). Nutrition meets the microbiome: micronutrients and the microbiota. Annals of the New York Academy of Sciences, 1372(1), 53–64. 10.1111/nyas.13145 [DOI] [PubMed] [Google Scholar]
- Borge TC, Aase H, Brantsæter AL, & Biele G (2017). The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: A systematic review and meta-analysis. BMJ Open. 10.1136/bmjopen-2017-016777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, Rasmussen SE, Alexander J, Samuelsen SO, & Meltzer HM (2007). Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutrition. 10.1017/S1368980007339037 [DOI] [PubMed] [Google Scholar]
- Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, & White WS (2004). Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. American Journal of Clinical Nutrition, 80(2), 396–403. 10.1093/ajcn/80.2.396 [DOI] [PubMed] [Google Scholar]
- Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, & Erdman JW (1996). cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 5(10), 823–833. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8896894 [PubMed] [Google Scholar]
- Couillard C, Lemieux S, Vohl MC, Couture P, & Lamarche B (2016). Carotenoids as biomarkers of fruit and vegetable intake in men and women. British Journal of Nutrition. 10.1017/S0007114516003056 [DOI] [PubMed] [Google Scholar]
- Craft NE, Brown ED, & Smith JC (1988). Effects of storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clinical Chemistry, 34(1), 44–48. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3338183 [PubMed] [Google Scholar]
- Das P, Babaei P, & Nielsen J (2019). Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genomics, 20(1), 208. 10.1186/s12864-019-5591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, … Ercolini D (2016). High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut, 65(11), 1812–1821. 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- Eggersdorfer M, & Wyss A (2018). Carotenoids in human nutrition and health. Archives of Biochemistry and Biophysics. 10.1016/j.abb.2018.06.001 [DOI] [PubMed] [Google Scholar]
- FAO and FHI 360. (2016). Minimum Dietary Diversity for Women: A Guide for Measurement.
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). GPOWER: A general power analysis program. Behavior Research Methods. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Fraser A, Tilling K, MacDonald-Wallis C, Sattar N, Brion MJ, Benfield L, … Lawlor DA (2010). Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 10.1161/CIRCULATIONAHA.109.906081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Schulze KJ, Stewart CP, West KP, & Christian P (2016). Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nature Reviews Endocrinology. 10.1038/nrendo.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano E, & Quadro L (2018). Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Archives of Biochemistry and Biophysics. 10.1016/j.abb.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, & Broekman BFP (2017). Influence of maternal obesity on the long-term health of offspring. The Lancet Diabetes and Endocrinology. 10.1016/S2213-8587(16)30107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizotte-Lake M, Zhong G, Duncan K, Kirkwood J, Iyer N, Smolenski I, … Vaishnava S (2018). Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity, 49(6), 1103–1115.e6. 10.1016/j.immuni.2018.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Yu X, Hannan P, Prouty C, & Jacobs DR (2003). Lipid standardization of serum fat-soluble antioxidant concentrations: The YALTA study. American Journal of Clinical Nutrition. 10.1093/ajcn/77.2.458 [DOI] [PubMed] [Google Scholar]
- Guo B, Yang B, Pang X, Chen T, Chen F, & Cheng KW (2019). Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food and Function, 10(9), 5644–5655. 10.1039/c9fo01018a [DOI] [PubMed] [Google Scholar]
- Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW, … Gordon JI (2017). The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Science Translational Medicine, 9(390). 10.1126/scitranslmed.aal4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda MN, Ahmad SM, Kalanetra KM, Taft DH, Alam MJ, Khanam A, … Stephensen CB (2019). Neonatal Vitamin A Supplementation and Vitamin A Status Are Associated with Gut Microbiome Composition in Bangladeshi Infants in Early Infancy and at 2 Years of Age. The Journal of Nutrition, 149(6), 1075–1088. 10.1093/jn/nxz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer N, & Vaishnava S (2019). Vitamin A at the interface of host– commensal–pathogen interactions. PLoS Pathogens, 15(6). 10.1371/journal.ppat.1007750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, … Knights D (2019). Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host and Microbe. 10.1016/j.chom.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Johnson EJ (2014). Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutrition Reviews, 72(9), 605–612. 10.1111/nure.12133 [DOI] [PubMed] [Google Scholar]
- Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, … Nielsen J (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature Communications, 3(1), 1–8. 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimenko NS, Tyakht AV, Popenko AS, Vasiliev AS, Altukhov IA, Ischenko DS, … Alexeev DG (2018). Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients, 10(5), 576. 10.3390/nu10050576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornatowski BM, & Comstock SS (2018). Dietary diversity is inversely correlated with pre-pregnancy body mass index among women in a Michigan pregnancy cohort. PeerJ, 6, e5526. 10.7717/peerj.5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, & Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Applied and Environmental Microbiology, 79(17), 5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Wang Y, Yang T, Zhan X, Li Z, Hu H, … Chen J (2016). Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. Journal of Clinical Biochemistry and Nutrition. 10.3164/jcbn.15-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Wu L, Wang F, Shen X, & Lin D (2018, April 1). Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Experimental Biology and Medicine, Vol. 243, pp. 613–620. 10.1177/1535370218763760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, … Aagaard KM (2014). High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nature Communications. 10.1038/ncomms4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiani G, Castón MJP, Catasta G, Toti E, Cambrodón IG, Bysted A, … Schlemmer U (2009, September 1). Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Molecular Nutrition and Food Research, Vol. 53, pp. 194–218. 10.1002/mnfr.200800053 [DOI] [PubMed] [Google Scholar]
- Makki K, Deehan EC, Walter J, & Bäckhed F (2018). The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host and Microbe. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Mamun AA, Kinarivala M, O’Callaghan MJ, Williams GM, Najman JM, & Callaway LK (2010). Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: Evidence from 21 y postpartum follow-up. American Journal of Clinical Nutrition. 10.3945/ajcn.2009.28950 [DOI] [PubMed] [Google Scholar]
- Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, … Eggesbø M (2016). Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome, 4(1), 55. 10.1186/s40168-016-0200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NE, Erdman JW, & Clinton SK (2013). Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Archives of Biochemistry and Biophysics. 10.1016/j.abb.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib YMA (2000). Antioxidant activities of astaxanthin and related carotenoids. Journal of Agricultural and Food Chemistry, 48(4), 1150–1154. 10.1021/jf991106k [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, … Wagner H (2015). Vegan: community ecology. R Package Version 2.2-1. 10.1029/2006JF000545 [DOI] [Google Scholar]
- Palafox-Carlos H, Ayala-Zavala JF, & González-Aguilar GA (2011). The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. Journal of Food Science. 10.1111/j.1750-3841.2010.01957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Sun S, Al-Delaimy W, Flatt SW, Kealey S, … Caan BJ (2006). Increases in plasma carotenoid concentrations in response to a major dietary change in the women’s healthy eating and living study. Cancer Epidemiology Biomarkers and Prevention, 15(10), 1886–1892. 10.1158/1055-9965.EPI-05-0928 [DOI] [PubMed] [Google Scholar]
- Prasad M, Takkinen HM, Uusitalo L, Tapanainen H, Ovaskainen ML, Alfthan G, … Virtanen SM (2018). Carotenoid intake and serum concentration in young finnish children and their relation with fruit and vegetable consumption. Nutrients, 10(10). 10.3390/nu10101533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam EE, & Goodman AL (2020). B vitamin acquisition by gut commensal bacteria. PLOS Pathogens, 16(1), e1008208. 10.1371/journal.ppat.1008208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, … Glöckner FO (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research, 41(D1). 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, & Team, R. (2011). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. [Google Scholar]
- Rao AV, & Agarwal S (2000). Role of Antioxidant Lycopene in Cancer and Heart Disease. Journal of the American College of Nutrition, 19(5), 563–569. 10.1080/07315724.2000.10718953 [DOI] [PubMed] [Google Scholar]
- Reboul E (2019). Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients, 11(4). 10.3390/nu11040838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Linseisen J, Hoffmann J, & Wolfram G (1999). Some Dietary Fibers Reduce the Absorption of Carotenoids in Women. The Journal of Nutrition. 10.1093/jn/129.12.2170 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez E, Beltrán-de-Miguel B, Samaniego-Aguilar KX, Sánchez-Prieto M, Estévez-Santiago R, & Olmedilla-Alonso B (2020). Extraction and Analysis by HPLC-DAD of Carotenoids in Human Faeces from Spanish Adults. Antioxidants, 9(6), 484. 10.3390/antiox9060484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RM, & Paiva SAR (1999). β-Carotene and Other Carotenoids as Antioxidants. Journal of the American College of Nutrition, 18(5), 426–433. 10.1080/07315724.1999.10718880 [DOI] [PubMed] [Google Scholar]
- Saejung C, & Ampornpat W (2019). Production and Nutritional Performance of Carotenoid-Producing Photosynthetic Bacterium Rhodopseudomonas faecalis PA2 Grown in Domestic Wastewater Intended for Animal Feed Production. Waste and Biomass Valorization. 10.1007/s12649-017-0070-3 [DOI] [Google Scholar]
- Shin D, Chung H, Weatherspoon L, & Song WO (2014). Validity of Prepregnancy Weight Status Estimated from Self-reported Height and Weight. Maternal and Child Health Journal, 18(7), 1667–1674. 10.1007/s10995-013-1407-6 [DOI] [PubMed] [Google Scholar]
- Simpson HL, & Campbell BJ (2015). Review article: dietary fibre-microbiota interactions. Alimentary Pharmacology & Therapeutics, 42(2), 158–179. 10.1111/apt.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisinha S (2015, June 1). The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pacific Journal of Allergy and Immunology, Vol. 33, pp. 71–90. Allergy and Immunology Society of Thailand. [PubMed] [Google Scholar]
- Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, … Friedman JE (2018). The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nature Communications. 10.1038/s41467-018-06929-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, … Belkaid Y (2014). Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science, 343(6169), 432–437. 10.1126/science.1247606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W, & Sies H (1992). Uptake of lycopene and its geometrical isomers is greater from heat- processed than from unprocessed tomato juice in humans. Journal of Nutrition. 10.1093/jn/122.11.2161 [DOI] [PubMed] [Google Scholar]
- Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, … Potischman N (2012). The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. Journal of the Academy of Nutrition and Dietetics, 112(8), 1134–1137. 10.1016/j.jand.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino KY, Paneth N, & Comstock SS (2019). Michigan cohorts to determine associations of maternal pre-pregnancy body mass index with pregnancy and infant gastrointestinal microbial communities: Late pregnancy and early infancy. PLOS ONE, 14(3), e0213733. 10.1371/journal.pone.0213733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tap J, Furet JP, Bensaada M, Philippe C, Roth H, Rabot S, … Leclerc M (2015). Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environmental Microbiology. 10.1111/1462-2920.13006 [DOI] [PubMed] [Google Scholar]
- Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, & Kahleova H (2019, April 17). The effects of vegetarian and vegan diets on gut microbiota. Frontiers in Nutrition, Vol. 6, p. 47. 10.3389/fnut.2019.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Chen H, Vogel S, Wilson PWF, Schaefer EJ, & Lammi-Keefe CJ (1999). Carotenoid Intakes, Assessed by Dietary Questionnaire, Are Associated with Plasma Carotenoid Concentrations in an Elderly Population. The Journal of Nutrition. 10.1093/jn/129.2.438 [DOI] [PubMed] [Google Scholar]
- Vaishnava S, & Hooper LV (2011). Eat your carrots! T cells are RARing to Go. Immunity. 10.1016/j.immuni.2011.03.007 [DOI] [PubMed] [Google Scholar]
- van het Hof KH, West CE, Weststrate JA, & Hautvast JGAJ (2000). Dietary Factors That Affect the Bioavailability of Carotenoids. The Journal of Nutrition. 10.1093/jn/130.3.503 [DOI] [PubMed] [Google Scholar]
- Venables WN, & Ripley BD (2002). Modern Applied Statistics with S-Plus. In World. 10.2307/2685660 [DOI] [Google Scholar]
- Vila E, Hornero-Méndez D, Azziz G, Lareo C, & Saravia V (2019). Carotenoids from heterotrophic bacteria isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnology Reports. 10.1016/j.btre.2019.e00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, & Miller JW (2017). Challenges in conducting clinical nutrition research. Nutrition Reviews, 75(7), 491–499. 10.1093/nutrit/nux026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisner CM, Maldonado J, Dente B, Krajmalnik-Brown R, & Bruening M (2018). Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: A cross-sectional study. BMC Microbiology, 18(1), 210. 10.1186/s12866-018-1362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, … Lewis JD (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science, 334(6052), 105–108. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii K, Hosomi K, Sawane K, & Kunisawa J (2019, April 17). Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Frontiers in Nutrition, Vol. 6, p. 48. 10.3389/fnut.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ze X, Duncan SH, Louis P, & Flint HJ (2012). Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME Journal, 6(8), 1535–1543. 10.1038/ismej.2012.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska MA, Wesołowska A, Pawlus B, & Hamułka J (2017). Health effects of carotenoids during pregnancy and lactation. Nutrients. 10.3390/nu9080838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.