Abstract
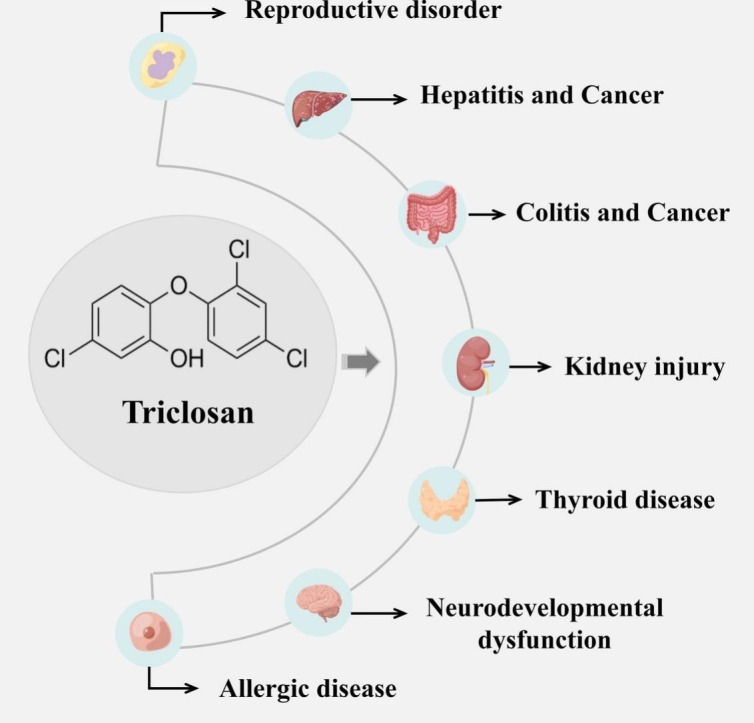
With the COVID-19 pandemic, the use of disinfectants has grown significantly around the world. Triclosan (TCS), namely 5-chloro-2-(2,4-dichlorophenoxy) phenol or 2,4,4′-trichloro-2′-hydroxydiphenyl ether, is a broad-spectrum, lipophilic, antibacterial agent that is extensively used in multifarious consumer products. Due to the widespread use and bioaccumulation, TCS is frequently detected in the environment and human biological samples. Accumulating evidence suggests that TCS is considered as a novel endocrine disruptor and may have potential unfavorable effects on human health, but studies on the toxic effect mediated by TCS exposure as well as its underlying mechanisms of action are relatively sparse. Therefore, in this review, we attempted to summarize the potential detrimental effects of TCS exposure on human reproductive health, liver function, intestinal homeostasis, kidney function, thyroid endocrine, and other tissue health, and further explore its mechanisms of action, thereby contributing to the better understanding of TCS characteristics and safety. Moreover, our work suggested the need to further investigate the biological effects of TCS exposure at the metabolic level in vivo.
Keywords: Triclosan, Human health effects, Reproductive disorders, Hepatotoxicity, Intestinal homeostasis, Kidney function
Graphical abstract
1. Introduction
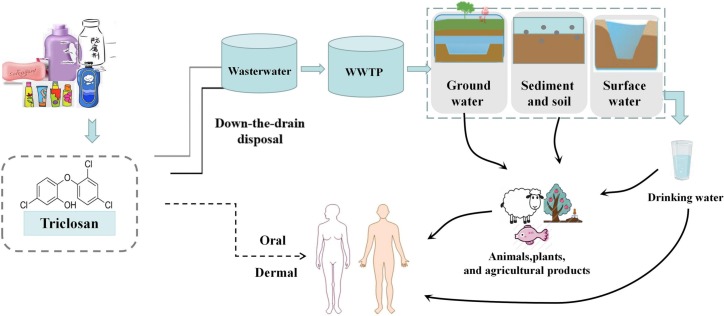
With the outbreak and epidemic of COVID-19, the use of disinfectants is expected to increase dramatically all over the world. Triclosan (TCS; 5-chloro-2-[2,4-dichloro-phenoxy] phenol) is a broad-spectrum, lipophilic, antibacterial agent. As a polychlorinated bisphenol compound, TCS has a distinct aromatic odor, not only easily soluble in water, but also well soluble in organic solvents, such as methanol, ethanol, and dimethyl sulfoxide (Alfhili and Lee, 2019). TCS has been extensively used in a variety of consumer products (Weatherly and Gosse, 2017), such as toothpastes, hand soaps, shampoos, cosmetics, and other consumer products, as well as in clinical settings (antiseptics, disinfectants) and medical devices (Montagnini et al., 2021). Currently, the detection rate of TCS in the environment is quite high and almost ubiquitous. TCS is a widespread environmental contaminant and has been determined in urine, blood, breast milk, amniotic fluid, and so on from the general population in different parts of the world (Etzel et al., 2017; Nasab et al., 2022; Weber et al., 2022). It is known that humans could be exposed to TCS through dermal contact and direct ingestion (Chen et al., 2019; Yueh and Tukey, 2016), whereas in the environment, TCS exposure occurs dominantly via the contaminated water, food, or animals (Milanović et al., 2021) (Fig. 1 ). Also, TCS has been detected to bioaccumulate in aquatic biota, such as algae (Mo et al., 2022), fish (Ku et al., 2014; Phillips et al., 2022), and marine mammals (Carey et al., 2016). Increasing literature has indicated that as a widely-used phenolic chemical, TCS has not only the endocrine-disrupting feature, but also the carcinogenic characteristic (Ashrap et al., 2017; Carey et al., 2016; Liu et al., 2022a; Yueh et al., 2014). In the research by Maksymowicz et al. (2022), TCS stimulated excessive proliferation of human ovarian cells by regulating the expression of genes involved in cell proliferation. Also, TCS was thought to potentially promote cancer metastasis through the epithelial-mesenchymal transition (EMT) process, in which cells lost their cell polarity, adhesion capacity, and thus initiated cancer progression and metastasis (Derouiche et al., 2017). In addition, mounting work has suggested that TCS exposure could contribute to other detrimental health effects, such as hepatic and renal toxicity (Ena et al., 2018; Tang et al., 2018), intestinal damage (Zhang et al., 2022a), thyroid function impairment (Milanović et al., 2021). However, the mechanisms by which TCS mediated negative health effects are still obscure (Gore et al., 2015).
Fig. 1.
A schematic model demonstrated the potential exposure routes of triclosan (TCS). TCS could be exposed to humans not only through the dermal contact and direct ingestion, but also via the contaminated water, food, plants, or animals.
At present, the reviews on TCS predominantly discuss epidemiological studies and environmental effects. Therefore, this paper reviews the literature on TCS exposure in humans and animals and cells, and further summarizes the deleterious effects and potential mechanisms of action of TCS accumulation in various tissues. A deeper and more comprehensive understanding of TCS safety has been gained from these insights.
2. Methodology
Literature search databases included PubMed, EMBASE, and Google Scholar up to January 2023. To find out relevant literature, search terms were consisted of “triclosan”, “human health effects”, “reproductive disorders”, “liver toxicity”, “intestinal homeostasis”, and “renal function”. From these searches, studies evaluating effects of TCS on health were screened out and identified. A total of 117 studies were met the inclusion criteria: (a) papers published since 2010, (b) available as full-text publications, (c) studies conducted on animals or humans, and (d) original or review articles.
3. Triclosan-induced toxic effects
3.1. Reproductive toxicity of triclosan
Growing research has suggested that the impairment of reproductive health is associated with environmental exposure to many chemicals, such as EDCs (Hipwell et al., 2019; Mínguez-Alarcón et al., 2019; Xu et al., 2022), which could influence reproductive health by regulating receptor binding, hormone biosynthesis, transport, metabolism, non-EATS pathways (Delbes et al., 2022), and so on. TCS is an emerging endocrine disruptor that could give rise to reproductive disorders in both men and women (Raj et al., 2021).
3.1.1. Effects of triclosan on male reproductive health
In mature male Wistar rats, a decline in the number of actively moving sperm and a reduction in testicular weight were observed after treatment with TCS (Yuan et al., 2022). Lan et al. (2015) assessed whether TCS exposure had adverse effects on sperm or reproductive organs in mammals, finding that TCS displayed a trend to accumulate in the epididymis of male Sprague Dawley (SD) rats and that rats treated with high doses (200 mg/kg) of TCS showed a significant decrease in daily sperm production (DSP), changes in sperm morphology, and an increase in the number of malformed sperm. Similar findings were reported in recent epidemiological studies, which pointed out that TCS exposure might negatively influence the semen quality (Jurewicz et al., 2018; Zhu et al., 2022). Exposure to low-level TCS brought about adverse effects on male semen quality, with an increases in sperm LIN and WOB observed in the third quartile of TCS exposure, implying a potential non-linear effect on male reproduction (Yuan et al., 2022). Moreover, low-level TCS exposure damaged sperm DNA and increased the percentage of sperm morphological abnormalities (Jurewicz et al., 2018). In a recent box of prospective studies, TCS exposure concentrations were divided into three tiers by determining urine samples from men, and the highest TCS exposure level was significantly associated with increased infertility, results demonstrating that the TCS exposure concentration was correlated positively with infertility, whereas negatively with fertility (Zhu et al., 2022). Furthermore, TCS also had the potential to influence hormone receptor expressions directly and indirectly or restrain testicular steroidogenesis, thereby perturbing male reproduction and fertility (Ha et al., 2018; Wang et al., 2018a; Yu et al., 2021). As expected, the deleterious effect of TCS on reproduction also frequently appeared in diverse animal models. A study on adult zebrafish revealed that TCS could disturb testicular development and spermatogenesis by interfering with sex hormone levels (Qiao et al., 2022). Likewise, our previous work uncovered the abnormal alteration in testicular tissue morphology and the suppression of testosterone biosynthesis in Leydig cells after TCS exposure (Duan et al., 2020). In addition, it is shown that TCS exposure may exert carcinogenic effects on reproductive organs (Derouiche et al., 2017; Lee et al., 2017). TCS stimulated human prostate cancer stromal cells to secrete vascular endothelial growth factor (VEGF), a factor that promotes tumor growth (Derouiche et al., 2017), and it could also advance prostate cancer metastasis through regulating epithelial mesenchymal transition (EMT) markers as well as related signaling pathways (Lee et al., 2017). From these studies, we have summarized the potential effects of TCS exposure on the male reproductive system (Table 1 ).
Table 1.
Summary of male reproductive toxicity of triclosan.
| TCS exposure negative effects | Males |
|---|---|
| Semen quality | TCS exposure increased the percentage of morphologically abnormal sperm (Lan et al., 2015) and decreased the number of actively moving sperm (Delbes et al., 2022). Rats treated with high doses (200 mg/kg) of TCS showed a significant decrease in daily sperm production (DSP), changes in sperm morphology, and an increase in the number of malformed sperm (Lan et al., 2015) |
| Steroidogenesis | Inhibition of testosterone biosynthesis by suppressing steroidogenic enzyme activity through the regulation of various microRNA pathways (Ha et al., 2018). |
| Reproductive hormone levels | Long-term exposure to TCS interfered with sex hormone levels to impair reproduction of zebrafish. Testosterone levels were reduced, while E2 and Vtg were significantly elevated in zebrafish. TCS perturbed the testicular development and spermatogenesis (Duan et al., 2020). |
| Carcinogenic effect on reproductive organs | Studies have shown that TCS induced cell division in various tissues including the reproductive system, and TCS also promoted cancer progression by activating membrane ion channels (Maksymowicz et al., 2022). Moreover, TCS stimulated VEGF secretion through TRPAI channels and proliferated human prostate cancer cells to exert oncogenic effects (Lee et al., 2017). |
3.1.2. Effects of triclosan on female reproductive health and offspring
There is evidence that TCS exposure may interfere with ovarian functions as well as ovarian reserve (Jurewicz et al., 2019; Kim et al., 2014; Maksymowicz et al., 2022). The research performed in zebrafish revealed that high-dose TCS exposure resulted in ovarian oxidative damage and then accelerated ovarian cell apoptosis (Wang et al., 2020a). According to the work by Du et al. (2021), TCS exposure disordered energy metabolism by dominating glucose flow to perturb steroid hormone biosynthesis and hormone homeostasis, and then affected female reproductive development. In adult female mice, TCS exposure downregulated thyroid hormone levels, led to hyperprolactinemia, and then restrain hypothalamic kisspeptin expression, finally giving rise to reproductive endocrine disturbance and functional defects (Cao et al., 2018).
Furthermore, due to the increasing use of various daily products containing TCS, several epidemiological studies have pointed out a possible association between TCS and infertility and neonatal birth defects (Feng et al., 2016; Guo et al., 2021; Priyanka et al., 2020). Patti et al. (2021) assessed the relationship between TCS exposure during pregnancy and infant birth weight via the systematic evaluation and meta-analysis, and a significant negative association between TCS concentration and infant birth weight was observed in those with higher TCS exposure. Wang et al. (2018b) investigated the potential effects of antenatal TCS exposure on fetal reproductive hormones in cord blood and the underlying mechanisms related to placental steroidogenic enzymes, confirming that antenatal TCS exposure was associated with the testosterone concentration in cord blood in a dose-dependent manner and male infants were more sensitive and vulnerable to TCS exposure. Likewise, a significant association between prenatal and early TCS exposure and allergic diseases was manifested in children (Lee-Sarwar et al., 2018). In the medaka model, TCS-exposed embryos developed malformations in the early life stage and TCS in this range might alter the sex ratio of developing embryos (Song et al., 2020) (Table 2 ).
Table 2.
Summary of effects of triclosan on female reproductive health and offspring.
| TCS exposure negative effects | Females and offspring |
|---|---|
| Ovarian function | Higher doses of TCS led to ovarian oxidative damage and advanced reactive oxygen-dependent ovarian apoptosis (Guo et al., 2021). Urinary TCS concentration was also negatively correlated with sinus follicle count, negatively impacting ovarian reserve (Wang et al., 2020b). |
| Steroidogenesis | TCS could disorder energy metabolism and disrupt hormone homeostasis by dominating glucose flow to steroid hormone biosynthesis (Huang et al., 2020). |
| Reproductive hormone levels | Adult female mice exposed to TCS showed the lower serum luteinizing hormone (LH), follicle stimulating hormone (FSH), and progesterone, and gonadotropin releasing hormone (GnRH) mRNA levels (Cao et al., 2018). |
| Carcinogenic effect on reproductive organs | TCS facilitated ovarian cancer growth by regulating cell cycles and apoptosis-related genes through an ER-dependent pathway (Du et al., 2021). |
| Impact on offspring | Exposure to TCS during pregnancy and lactation could adversely influence the reproductive function and fertility in male rats of the F1 and F2 generations (Feng et al., 2016; Wang et al., 2018c), and lead to the decline of uterine weight and the occurrence of miscarriages (Patti et al., 2021). In addition, there was a significant association between prenatal TCS exposure and allergic disease in children (Lee et al., 2017). |
In conclusion, these studies have favorably demonstrated that TCS has the reproductive interference effect. In males, TCS may reduce testosterone levels by interacting with hormone receptors or by disrupting testicular steroid production. In addition, the bioaccumulation of TCS in reproductive organs may directly affect sperm synthesis and semen quality and have carcinogenic effects on reproductive organs. And in females, TCS exposure not only influences reproductive hormone levels, but also leads to the impairment of ovarian function and even has an impact on the next generation.
3.2. Gastrointestinal toxicity of triclosan
3.2.1. Hepatotoxicity of triclosan
Mounting evidence has indicated that the liver is a predominant organ of TCS metabolism and detoxification. Huang et al. (2020) employed GC-ECNI/MS to determine TCS levels in different human tissues and affirmed that the liver was the organ with the highest TCS concentration. Hepatotoxicity studies of TCS in different species have suggested that TCS exposure has harmful effects on the liver and may lead to the development of non-alcoholic fatty liver disease (NAFLD) (Li et al., 2023), hepatitis (Zhang et al., 2022b), and even liver cancer (Zhang et al., 2019). In mice, it was reported that TCS exposure triggered the disturbance of hepatic lipid metabolism and then promoted hepatic impairment (Zhang et al., 2022a). After 21 days of TCS exposure via lactation, the delivery of TCS contributed to the premature development of fatty liver in neonatal mice, which exhibited early onset liver endoplasmic reticulum (ER) stress and steatosis (Weber et al., 2022). In SD rats, perinatal TCS exposure gave rise to metabolic and gut microbiota disturbance in the offspring (Ma et al., 2020). Meanwhile, Yueh et al. (2020) explored the relationship between TCS and NAFLD and discovered that TCS could exacerbate the high-fat diet-induced metabolic disorder by impairing the regulation of fibroblast growth factor 21 (FGF21) expression. In addition, the prevalence of TCS in aquatic environments has made animal models of this habitat the subject of extensive studies of TCS toxicity. In peacock fish, TCS had the highest concentration in the liver (Escarrone et al., 2016). In a zebrafish model, TCS exposure resulted in the accumulation of lipid droplets in the liver by interfering with hepatic lipid metabolism (Wang et al., 2020b). Another study also revealed that the toxicity of TCS was closely associated with the induction of apoptosis in hepatocytes (Liu et al., 2019). Sun et al. (2021a) unveiled that chronic exposure to TCS in zebrafish triggered fatty liver and hepatitis, which were related to the expression of miR-30b and its targeted gene fat mass and obesity-associated protein (FTO). Likewise, Bao et al. (2021) estimated the toxic effects of TCS on mosquito fish and revealed that exposure to environmentally relevant concentrations of TCS could activate the nuclear factor E2-related factor 2 (Nrf2) protein expression in the liver of mosquito fish and cause oxidative stress to the exposed organisms. In an in vitro study, however, Sun et al. (2021b) showed that TCS suppressed fatty acid synthesis and human HepG2 cell growth by downregulating fatty acid synthase through multifarious miRNAs. Interestingly, another study uncovered that TCS weakened overall DNA methylation levels in HepG2 cells and accelerated hepatocarcinogenesis and progression (Ma et al., 2013). Thus, based on these ex vivo and in vivo studies, it is concluded that TCS exposure primarily contributes to disturbance in hepatic lipid metabolism, leading to the premature development of fatty liver, such as a study reporting that neonatal mice could increase the susceptibility to non-alcoholic fatty liver disease (NAFLD) through lactational exposure to TCS. However, there is a serious lack of studies in this direction that have been conducted on humans or human-derived tissues. This also informs us of the greater need for future longitudinal epidemiological studies to examine the correlation between TCS exposure concentrations and liver disease. The hepatotoxicity of TCS exposure has been summarized in Table 3 .
Table 3.
Summary of liver toxicity of triclosan.
| Models | Hepatotoxic effects | References |
|---|---|---|
| C57BL/6 mice | TCS-exposed mice showed hepatic hypertrophy, increase in liver lipid levels, and upregulation of fatty acid oxidation and inflammation-related genes. | Huang et al., 2020 |
| C57BL/6 mice | TCS aggravated high-fat diet-induced metabolic disorders by disrupting the regulation of FGF21 expression. | Yueh et al., 2020 |
| Sprague Dawley (SD) rats | More upregulated genes in carbohydrate and lipid metabolic pathways were observed in aged rats as TCS exposure contributed to metabolic disturbance and these effects would accumulate over time. | Ma et al., 2020 |
| C57/B6 mice | TCS transmission through lactation resulted in adipogenesis, ER stress, PPARα activation, immune inflammation in the neonatal liver, and nonalcoholic fatty liver disease in neonatal C57/B6 mice. | Weber et al., 2022 |
| Zebrafish | TCS regulated fto-mediated m6 methylation by inhibiting miR-30b expression, which led to the disturbance of lipid metabolism and elevation of TG and TC levels in zebrafish. | Sun et al., 2021a |
| Zebrafish | Severe hepatocyte atrophy and necrosis in the liver tissue of zebrafish larvae after TCS exposure, with a marked increase in the gap between the liver plates. | Liu et al., 2019 |
| Mosquito fish | TCS exposure perturbed the antioxidant system in mosquito fish liver tissues, and a number of oxidative stress-related biomarkers were displayed in a concentration-dependent (e.g. NQO1 mRNA, CAT mRNA) and/or time-dependent (e.g. GSH content) manner. | Bao et al., 2021 |
| HepG2 cells | TCS treatment significantly restrained DNA methylation levels and suppressed DNA methyltransferase 1 activity in HepG2 cells. | Ma et al., 2013 |
3.2.2. Intestinal toxicity of triclosan
Triclosan is frequently used as an antibacterial agent in a variety of consumer products such as toothpaste and soap. Surprisingly, one study reported that TCS-containing soaps failed to provide any additional skin disinfection benefits in comparison to TCS-free soaps (Kim et al., 2015). Several recent studies displayed that low-dose TCS exposure could not only aggravate colonic inflammation and exacerbate colitis-related colon tumorigenesis (Liu et al., 2022a; Sanidad et al., 2019; Yang et al., 2019), but also interfere with the gut microbiota in mouse models (Hu et al., 2016; Yang et al., 2018), indicating that TCS might detrimentally affect gut health. Gaulke et al. (2016) exposed adult zebrafish to food containing TCS for four to seven days and then analyzed their microbial communities using 16S rRNA amplicon sequencing, data exhibiting that TCS exposure disturbed the composition and ecological dynamics of microbial communities in the gut of zebrafish and then facilitated the instability and reorganization of microbial communities. The findings were in line with that of the study by Zang et al. (2019), which observed that TCS exposure perturbed the intestinal mucosal immune system and intestinal microflora of adult zebrafish. In other fish, low-level TCS exposure also interfered with microbial nitrogen metabolism in the fish gut (Narrowe et al., 2015). Furthermore, in mouse models, short-term exposure to TCS at relatively low doses brought about low-grade colonic inflammation in normal C57BL/6 mice, whereas in dextran sulfate sodium (DSS)-induced C57BL/6 mice, TCS treatment exacerbated colitis in mice as well as colonic tumourigenesis and shortened the colonic length (Sanidad et al., 2022; Yang et al., 2018). At the cellular level, Wang et al. (2018c) reported that TCS mediated autophagy in macrophages and non-phagocytic cells in vitro, killing E. faecalis, Lactobacillus and E. coli, disrupting the balance of the intestinal flora, and having a carcinogenic characteristic. In a previous epidemiological study, however, Adgent and Rogan (2015) examined the association between TCS and enterolactone, and the cross-sectional survey found that TCS exposure was not correlated with the production of enterolactone. Nevertheless, the use of enterolactone, an intestinal metabolite, as a marker of gut flora function is novel but crude, and only provides limited information about overall gut health. In summary, these studies have convincingly indicated that TCS exposure may exacerbate the development of colitis and colon tumors, alter the diversity of the intestinal microbiota, and lead to disorders of the intestinal flora. Therefore, the effects of TCS exposure on the human microbiota deserve further exploration. The intestinal toxicity of TCS exposure has been summarized in Table 4 .
Table 4.
Summary of intestinal toxicity of triclosan.
| Models | Enterotoxic effects | References |
|---|---|---|
| C57BL/6 mice | TCS exacerbated colitis through a gut microbiota-dependent mechanism by which gut microbial GUS proteins mediated the colonic reactivation of TCS from its inactive glucosinolate metabolites and drove the intestinal toxicity of TCS in the process. | Zhang et al., 2022b |
| C57BL/6 mice | TCS exposure triggered low-grade colonic inflammation, increased colitis, and exacerbated colitis-associated colon cancer in mice, and it adversely influenced colonic inflammation and associated colon tumorigenesis primarily by modulating intestinal microbiota and TLR4 signaling. | Yang et al., 2018 |
| Sprague Dawley (SD) rats | Low-dose TCS treatment disordered the intestinal microbiota in adolescent rats and led to an increase in the abundance of Spirochetes. | Hu et al., 2016 |
| C57/B6 mice Balb/c mice |
TCS exposure changed the diversity and composition of the intestinal microbiota in Balb/c mice. TCS treatment upregulated levels of sexual cytokines in the DSS-induced mouse model, decreased Occludin levels, and exacerbated the extent of intestinal mucosal and crypt damage, inflammatory cell infiltration, and glandular cell heterotype. | Liu et al., 2022c |
| Zebrafish | TCS exposure led to the disorder of zebrafish intestinal metabolism, as well as abnormalities in the intestinal mucosal immune system, while TCS treatment resulted in upregulation of pro-inflammatory genes (IL-1β, TNF-α) and elevated MDA concentrations. | Zang et al., 2019 |
| Zebrafish | TCS exposure impaired the structure and ecodynamics of the adult zebrafish gastrointestinal microbiome. | Gaulke et al., 2016 |
| Pimephales promelas | The Pimephales promelas gut microbiome was rapidly and significantly altered after exposure to low levels of environmentally relevant TCS, but recovered from this short-term perturbation in a fairly short period of time. | Narrowe et al., 2015 |
| Raw264.7/ HeLa cells | TCS mediated autophagy of HeLa and Raw264.7 cells probably via the AMPK/ULK1 and JNK/ERK/p38 pathways in a dose-dependent fashion. | Wang et al., 2018c |
3.3. Nephrotoxicity of triclosan
The kidney is critical in the elimination of toxins, yet there is evidence that exposure to environmental pollutants may lead to early kidney injury, chronic kidney disease (CKD), and end-stage renal disease (ESRD) (Ena et al., 2018; Lim and Yoon, 2019; Zheng et al., 2017). A previous epidemiological investigation revealed that TCS exposure was positively associated with urinary β2-microglobulin expression which is a marker of early kidney injury (Lim and Yoon, 2019). In a rat model study, Ena et al. (2018) investigated the toxic effect of subchronic TCS exposure on male rat kidneys and demonstrated that high-dose TCS exposure induced histological changes as evidenced by the reduced Bowman's space, tubular lumen occlusion, and tubular epithelial cell degeneration. Moreover, Huang et al. (2023) discovered that TCS treatment gave rise to renal lipid accumulation and disruption of fatty acid metabolism in mice, finally leading to renal impairment of male C57BL/6 mice. In addition, several studies have proposed the pro-oxidant effect of TCS (Teplova et al., 2017), while oxidative stress could also lead to decreased renal function through inflammation, glomerular filtration barrier damage, and fibrosis (Ratliff et al., 2016). In human HRGEC cells, TCS treatment brought about oxidative stress and apoptosis, and then mediated kidney damage (Ma et al., 2022). Through these studies, it is concluded that TCS exposure dominantly disrupts the glomerular filtration barrier and causes impairment of renal function. However, systematic studies on the effect of TCS on the kidney are still largely lacking.
3.4. The toxicity of triclosan on other organs
Apart from those harmful effects of TCS mentioned above, several literature has referred to other deleterious health effects mediated by TCS exposure in the past few years, such as the thyroid function damage (Zhang et al., 2018), neurodevelopmental toxicity (Wang et al., 2021), immune dysfunction (Bera et al., 2020; Zhao et al., 2022), and cytotoxicity (Querido et al., 2022). In cohort studies, TCS exposure was significantly correlated with the allergic disease in preschool children (Lin et al., 2022; Spanier et al., 2014), and the estrogenic effect of TCS might affect the prognosis of female breast cancer (Ilozumba et al., 2022). Moreover, it was reported that TCS exposure triggered neurotoxicity (Wang et al., 2022). Diao et al. (2022) recently elucidated that TCS exerted neurodevelopmental toxicity by upregulating miR-144 expression and causing abnormal regulation of neurologically related genes. Also, neurobehavioral toxicity was observed in the offspring of mice treated subcutaneously with TCS (Tran et al., 2020). Zhang et al. (2018) investigated the potential effect of TCS exposure on the thyroid function in SD rats and noted that TCS decreased thyroid hormone levels including total thyroxine (TT4), free thyroxine (FT4), total triiodothyronine (TT3), and free triiodothyronine (FT3) by restraining thyroid peroxidase (TPO). In a zebrafish model, Tang et al. (2022) discovered that exposure to TCS postponed the hatching of zebrafish embryos and downregulated TT4 and FT3 levels in zebrafish larvae and FT4 levels in adult zebrafish. Meanwhile, a recent epidemiological study pointed out a significant inverse association between maternal urinary TCS and cord blood FT3 as well as maternal blood FT4 and TSH concentrations in late pregnancy (Wang et al., 2017a). Furthermore, a latest research discovered that TCS induced immunotoxicity in zebrafish by regulating post-miR-19a and socs3b expressions, as well as the expression of II-6 and STAT3 (Zhao et al., 2022). Querido et al. (2022) revealed that self-sterilizing coatings containing TCS manifested low levels of cyto- and genotoxicity. Thus, these effects discussed in this section, together with relevant in vitro and in vivo studies, provide support for the epidemiological findings and suggest that the safety of TCS as a bacteriostatic agent remains highly controversial.
4. Potential mechanisms of triclosan-induced toxic effects
4.1. Triclosan and reproductive function disruption
Epidemiological studies have suggested that TCS exposure may influence semen quality (sperm alignment, viability, morphology, CASA parameters) and damage sperm DNA (Jurewicz et al., 2018). The biological mechanism by which TCS affects semen quality is currently uncertain. However, in vitro studies in zebrafish demonstrated that TCS affected the testicular development and perturbed spermatogenesis by depressing the hormone biosynthesis of male zebrafish, such as testosterone (T), estradiol (E2), and vitellogenin (Vtg) (Qiao et al., 2022). Arginine is the main component of sperm proteins and has an essential role in promoting sperm production and providing energy for sperm motility (Ma et al., 2019). Exposure to TCS gave rise to a significant decrease in arginine synthesis and expression of retinoic acids (cyp3a65 and ugt1a1), ultimately leading to a reduction in sperm count (Qiao et al., 2022). Meanwhile, in female zebrafish, high concentrations of TCS were suspected to cause a significant decline in zebrafish spawning capacity and to downregulate Vtg levels in female zebrafish through estrogenic effects, thereby lowering mature oocyte and egg production (Qiao et al., 2022). The study performed in zebrafish reported that after exposure to TCS for 42 days, an accumulation in the malondialdehyde (MDA) content in the ovaries of the high-dose TCS group, an elevation in similar to metallothionein-B (sMT—B) and Bax gene expression, and a decline in Bcl-2/Bax ratio were observed, which eventually contributed to oxidative damage to the ovary, caused mitochondrial disorders, and accelerated ovarian apoptosis (Wang et al., 2020a). Moreover, Lin et al. (2017) evaluated the relationship between TCS-induced toxic processes and microRNA expression in a zebrafish model, concluding that TCS exposure led to the upregulation of mature miR-125b that was concomitant with consistent changes in pri-mir-125b-1 and pri-mir-125b-3 among its 3 pri-mir-125bs. It is known that steroidogenesis could be regulated by microRNAs through diverse pathways, such as modulating the expression of their target mRNAs and related molecules involved in spermatogenesis (Duan et al., 2020). Our previous research noted that TCS restrained testicular steroidogenesis through the miR-6321/JNK/Nur77 cascade response in male SD rats given TCS daily for 31 days by gavage (Ha et al., 2018). We also found that in male SD rats and Leydig cell lines, TCS induced miR-142-5p overexpression in testicular tissues of rats, then directly suppressed the Jak1/Stat1 pathway, and increased Dax1 expression, eventually leading to the inhibition of steroidogenic protein P450c17 and subsequent repression of testosterone biosynthesis (Duan et al., 2020). Additionally, other studies have indicated that TCS could interference with reproductive health through oxidative stress, lipid metabolism disorder, apoptosis, and other related signaling pathways (Kim et al., 2014; Zang et al., 2019). A gestational mouse model study, displayed that TCS influenced placental development through the peroxisome proliferator-activated receptor-γ (PPARγ) pathway and PPARγ activation or overexpression antagonized the TCS-induced increase in inflammatory gene expression (Li et al., 2021). Reports by Qiao et al. (2022) speculated that TCS might alter sex hormone levels by interfering with the lipid metabolism of ovarian cells, finally leading to reproductive dysfunction. In contrast, in in vitro studies, TCS stimulated ovarian cancer growth by regulating cell cycles and apoptosis-related genes through an ER-dependent pathway (Kim et al., 2014). Furthermore, Huo et al. (2022) reported that TCS modulated the miR-218-1-3p/SLC35C1 axis to regulate the proliferation, migration, invasion and inflammatory response of trophoblast cells in vitro, providing new insights into the prevention of spontaneous abortion.
Collectively, TCS could interfere with reproductive health through oxidative stress (Basini et al., 2022), hormonal dysregulation (Arismendi et al., 2022; Basini et al., 2021; Dong et al., 2022), germ cell autophagy and apoptosis (Liu et al., 2022b), steroidogenesis suppression (Yawer et al., 2020), and mitochondrial dysfunction (Du et al., 2021) (Table 5 ), although the specific mechanisms of action of TCS in infertility are still not fully elucidated yet. For example, does TCS exposure affect spermatogenesis by the disturbing blood-testis barrier, or affect semen quality by inducing apoptosis or pyroptosis of spermatogonia and spermatocytes? These are all areas for further research and discussion in the future.
Table 5.
Summary of potential mechanisms of TCS-induced reproductive disorders.
| References | Models | Mechanisms | Target genes/proteins/biomarkers | Responses |
|---|---|---|---|---|
| Qiao et al., 2022 | Zebrafish(male) | Hormone synthesis | E2 VTG |
Upregulated by TCS Upregulated by TCS |
| Arismendi et al., 2022 | Sprague Dawley rats | Hormonal imbalance | P4 T |
Downregulated by TCS Downregulated by TCS |
| Li et al., 2021 | HTR-8/SVneo cells JEG-3 cells |
PPARγ pathway Inflammation Cell migration |
PPARγ IL-6 TNF-α IL-1β P65 MMP-2 MMP-9 |
Downregulated by TCS Upregulated by TCS Upregulated by TCS Upregulated by TCS Upregulated by TCS Downregulated by TCS Downregulated by TCS |
| Liu et al., 2022c | Zebrafish |
Oxidative stress Apoptosis |
ROS SOD P53 Caspase-8 Caspase-3 Caspase-9 |
Upregulated by TCS Upregulated by TCS Upregulated by TCS Upregulated by TCS Upregulated by TCS Upregulated by TCS |
| Dong et al., 2022 | ICR mice | Receptivity of uterus | Cldn7 Crb3 ERα |
Upregulated by TCS Upregulated by TCS Downregulated by TCS |
| Huo et al., 2022 | JEG3 cells HTR-8 cells |
Cell proliferation, migration, invasion | c-Jun miR-218-1-3p SLC35C1 |
Upregulated by TCS Upregulated by TCS Downregulated by TCS |
| Du et al., 2021 | KGN cells Primary rGCs cells |
Steroidogenesis Mitochondrial damage |
P4 HSD3B2 E2 HSD17B1 ATP PKM2 GLUT1 |
Upregulated by TCS Upregulated by TCS Upregulated by TCS Upregulated by TCS Downregulated by TCS Upregulated by TCS Upregulated by TCS |
| Duan et al., 2020 | Sprague Dawley rats | Testosterone synthesis | miR-142-5p P450c17 Sp1 DAX1 |
Upregulated by TCS Downregulated by TCS Downregulated by TCS Upregulated by TCS |
| Yawer et al., 2020 | TM3 Leydig cells | Testicular cell signaling | Cx43 p-Erk1/2 p38/MAPK |
Downregulated by TCS Upregulated by TCS Upregulated by TCS |
| Wang et al., 2020a | Zebrafish |
Oxidative stress Apoptosis |
SOD CAT SMT-B MT-2 Bax Bcl-2 |
Downregulated by TCS Downregulated by TCS Downregulated by TCS Upregulated by TCS Upregulated by TCS Downregulated by TCS |
| Ha et al., 2018 | Sprague Dawley rats | Steroidogenesis | miR-6321 P-JNK P-c-Jun Nur77 |
Upregulated by TCS Downregulated by TCS Downregulated by TCS Downregulated by TCS |
| Basini et al., 2022 | Swine Granulosa Cells | Steroidogenesis Oxidative stress |
E2 P4 NO SOD O2− |
Downregulated by TCS Downregulated by TCS Downregulated by TCS Upregulated by TCS Downregulated by TCS |
| Basini et al., 2021 | Swine Luteal Cells | Hormonal imbalance Oxidative stress |
ATP P4 NO SOD |
Downregulated by TCS Downregulated by TCS Upregulated by TCS Downregulated by TCS |
4.2. Triclosan and hepatotoxicity
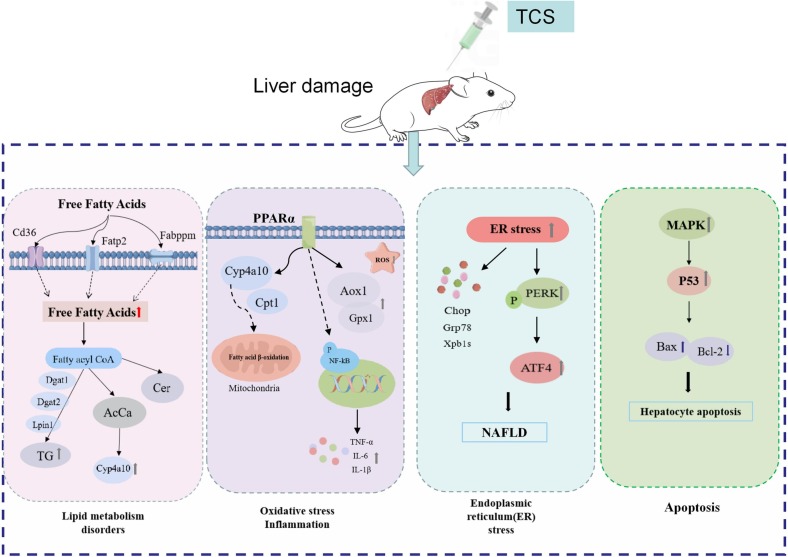
The liver plays a crucial role in metabolism. Accumulating studies have demonstrated that long-term exposure to TCS might have unfavorable health effects on the liver, and it could perturb liver metabolic function, aggravate liver fibrosis, accelerate hepatocellular carcinoma development through lipid metabolism disorder (Huang et al., 2020; Weber et al., 2022), induce oxidative stress (Wang et al., 2017b), facilitate mitochondrial dysfunction (Belosludtsev et al., 2018; Pereira-Maróstica et al., 2022; Tenkov et al., 2022), and modulate hepatocyte apoptosis (Liu et al., 2019). Free fatty acids (FFAs) are an important source of lipid synthesis and a recent research exhibited that both FFAs levels and fatty acid uptake related gene expressions were significantly elevated in mice exposed to TCS, including fatty acid translocase (Fat, also known as Cd36), fatty acid transport proteins 2 and 5 (Fatp2 and Fatp5) (Huang et al., 2020). The accumulation of hepatic triacylglycerols (TG) might be associated with increased effectiveness of FFAs and upregulation of TG biosynthesis-related genes, including phosphatidic acid phosphate hydrolase (Lpin1) and diacylglycerol o-acyltransferases 1 and 2 (Dgat1 and Dgat2). In contrast, hepatic TG accumulation was usually considered benign to hepatic steatosis (Paul et al., 2022). Here, it was also noted that TCS was able to activate peroxisome proliferator-activated receptor α (PPARα) without altering PPARα levels, whereas it induced the mRNA expression of PPARα-targeted genes, such as Cyp4a10 and Cpt1. As Cyp4a10 plays an important role in microsomal fatty acid (FA) β-oxidation, all these alterations gave rise to mitochondrial dysfunction and exacerbated liver impairment. The findings were supported by other study, which reported that TCS induced PPARα targets, cytochrome P450 4A (CYP4a) and acyl-coenzyme A oxidase 1 (ACOX1), and then contributed to the increase in liver weight and hepatocyte peroxisome production (Tang et al., 2018). In addition, increased FA oxidation and the risk of reactive oxygen species (ROS) production with increased acyl carnitine (AcCa) levels in the liver of TCS-exposed mice had been implicated (Huang et al., 2020). Several studies have indicated that TCS could induce oxidative stress to promote the hepatocellular carcinoma development and ROS production, acting as a liver tumor promoter (Yueh et al., 2014). Yueh et al. and Wang et al. administered TCS treatment in the diet and reported that TCS exposure resulted in upregulated expression of key antioxidant enzymes (HO-1, Gsta1) and oxidative stress-related genes such as Gpx1 and Aox1 in the liver of C57B/L mice. In a zebrafish model, Liu et al. (2019) displayed that TCS exposure accumulated MDA content and activated the MAPK/p53 signaling pathway to induce hepatic oxidative stress. TCS treatment brought about the accumulation of 8-OHdG in human HepG2 cells, sustaining the notion that TCS exposure contributed to oxidative DNA damage (Ma et al., 2013). In addition, a previous work manifested that increased inflammation was also considered to be the most common and relevant mechanism of liver damage (Todoric et al., 2020). In contrast, ceramide (Cer), a member of the sphingolipid family of lipids, is not only an essential component of the cell membrane but also has cell signaling properties (Han, 2016). Cer could also regulate the expression of pro-inflammatory genes via activating the nuclear factor kappa light chain enhancer (NF-κB) in activated B cells, and then promote a sustained and robust feedback mechanism (Pan et al., 2021). A study by Huang et al. (2020) demonstrated a significant increase in Cer levels in TCS-exposed mice and an increase in inflammatory cytokine (TNF-α, IL-1β and IL-6) mRNA expression was observed. In other in vivo studies, TCS stimulated ER stress in primary hepatocytes, activated transcription factor 4 (ATF4) through protein kinase R-like kinase (PERK) activation, finally contributing to the progression of simple steatosis to NASH (Weber et al., 2022).
In summary, in addition to the disorder of lipid metabolism, oxidative stress, inflammation, endoplasmic reticulum stress, and mitochondrial dysfunction discussed above (Fig. 2 ), TCS-mediated hepatotoxicity might be correlated with the disturbance of bile acid metabolism (Song et al., 2022) and immune dysfunction (Liu et al., 2019). However, the deleterious effects of TCS exposure on the liver in mammals in vivo have been explored rarely, especially underlying molecular mechanisms. Therefore, we could combine metabolomics and lipidomics to reveal the metabolic mechanism of TCS-induced hepatocyte toxicity in the future. In addition, the role of gut microbiota on TCS-induced liver injury is still unknown, which should also be the focus of our future research.
Fig. 2.
A schematic model displayed the underlying mechanisms of TCS-mediated hepatic damage. TCS exposure impaired liver function predominantly via the lipid metabolism, oxidative stress, inflammation, endoplasmic reticulum stress, and hepatocyte apoptosis.
4.3. Triclosan and intestinal injury
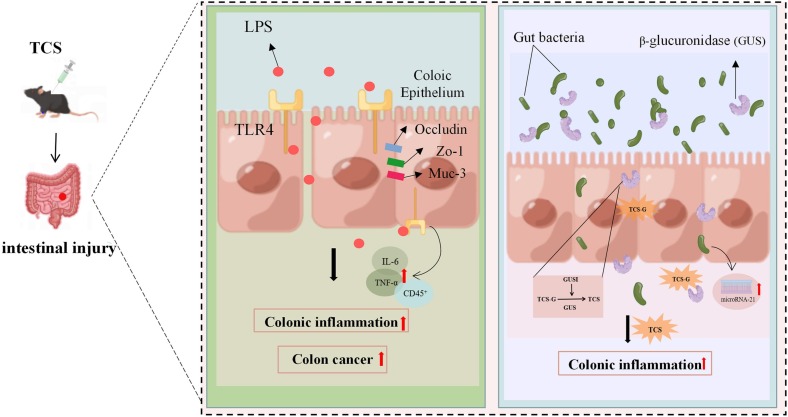
Previous studies displayed that TCS exposure facilitated colonic inflammation and aggravated the severity of colitis and colon cancer (Yang et al., 2018), but detailed mechanisms by which TCS led to colonic inflammation and associated colon tumourigenesis are currently unknown. A latest cohort study detected the unconjugated OH-TCS metabolite in human feces of TCS-exposed subjects. Aromatic hydroxylation is the predominant oxidative metabolic process of TCS and is associated with its toxicological effects in host tissues (Zhang et al., 2023). In a study by Yang et al. (2019), TCS was exposed to C57BL/6 mice via diet for three weeks and the results showed that TCS triggered colonic inflammation in mice by disturbing the intestinal microbiota and activating the Toll-like receptor 4 (TLR4) signaling. TLR4 recognizes lipopolysaccharides that are common components of many Gram-negative and some Gram-positive bacteria and play a critical role in intestinal bacterial-host interactions (Abreu, 2010). Another study also noted that TCS treatment altered the expression of colonic proteins related with the maintenance of intestinal permeability, including ocludin, tight junction protein-1 (ZO-1), and mucin-3 (MUC3) (Yang et al., 2018). The study by Zhao et al. (2022) revealed that TCS exposure resulted in colitis in mice, mainly because specific gut microbial β-glucuronidase (GUS) homologs converted TCS to TCS-G. In zebrafish models, long-term exposure to high concentrations of TCS disordered the composition of the microbiota in the gastrointestinal tract and lessened its diversity (Tang et al., 2021). Furthermore, former studies proposed that the gut microbiome was necessary for the pro-inflammatory effect of TCS (Gao et al., 2017; Sanidad et al., 2022), and intestinal barrier dysfunction and intestinal microbiota disorder were associated with miR-21 (Johnston et al., 2018). Taken together, these investigations above suggest that the detrimental effect of TCS on gut health may be related to the gut microbiome (Fig. 3 ), providing new mechanistic insights into TCS exposure and related diseases. In the future, we should pay more attention to the type and abundance of intestinal flora influenced by TCS exposure and then explore the biological significance.
Fig. 3.
A schematic model revealed that the possible mechanisms of TCS-caused intestinal injury. TCS treatment perturbed gut health through the induction of inflammation and the disorder of gut microbiome.
5. Conclusion and prospect
Triclosan is a synthetic antimicrobial agent with a long history. In recent years, TCS has been considered as a novel endocrine disruptor and long-term exposure to TCS could have unfavorable effects on human health. Several in vivo and in vitro studies have suggested a possible association between TCS exposure and multiple adverse health outcomes, such as the impairment of reproductive function, disturbance in hepatic lipid metabolism, kidney damage, colitis, and so on. However, there is a relative lack of mechanism-based human studies in terms of number and scope, and most cohorts and in vitro studies have predominantly evaluated the toxic effects induced by TCS exposure, without in-depth dissection of the specific mechanisms of action and cellular targets of TCS. For example, recent studies have suggested that TCS could influence reproductive health and liver function by interfering with lipid metabolism (Huang et al., 2020; Zang et al., 2019), but these studies have not delved into the relevant microRNA gene expression that affects fatty acid synthesis and metabolism. In addition, a growing number of studies have indicated that TCS exposure disturbs intestinal flora homeostasis and has a carcinogenic potential (Wang et al., 2018c), but there are limited studies on how TCS perturbs intestinal flora homeostasis, and there is a serious lack of cohort studies based on population data. Consequently, there is a great need for further work to focus on the underlying mechanisms of action of TCS exposure and characterize its toxic effects in animal and cell models. With the current explosion of COVID-19, it is foreseeable that antimicrobial agents will be used in large quantities, and the safety of TCS is worth further discussing and verifying.
CRediT authorship contribution statement
Xuhui Chen: Methodology, Data collection, Writing - original draft.
Li Mou, Jiayuan Qu, and Liling Wu: Literature search, Data collection.
Changjiang Liu: Conceptualization, Funding acquisition, Writing - review & editing.
Declaration of competing interest
None.
Acknowledgements
This study was supported by the Chongqing Science and Technology Bureau, China (2019cstc-jbky-01702).
Editor: Daqiang Yin
Data availability
No data was used for the research described in the article.
References
- Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Adgent M.A., Rogan W.J. Triclosan and prescription antibiotic exposures and enterolactone production in adults. Environ. Res. 2015;142:66–71. doi: 10.1016/j.envres.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfhili M.A., Lee M.H. Triclosan: an update on biochemical and molecular mechanisms. Oxidative Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1607304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arismendi D., Alanis C., Richter P., Paredes A.H. Effect of triclosan exposure on ovarian hormones, trace elements and growth in female rats. Chemosphere. 2022;307 doi: 10.1016/j.chemosphere.2022.135964. [DOI] [PubMed] [Google Scholar]
- Ashrap P., Zheng G., Wan Y., Li T., Hu W., et al. Discovery of a widespread metabolic pathway within and among phenolic xenobiotics. Proc. Natl. Acad. Sci. U. S. A. 2017;114:6062–6067. doi: 10.1073/pnas.1700558114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., He C., Ku P., Xie M., Lin J., et al. Effects of triclosan on the RedoximiRs/Sirtuin/Nrf2/ARE signaling pathway in mosquitofish (Gambusia affinis) Aquat. Toxicol. 2021;230 doi: 10.1016/j.aquatox.2020.105679. [DOI] [PubMed] [Google Scholar]
- Basini G., Bussolati S., Bertini S., Quintavalla F., Grasselli F. Evaluation of triclosan effects on cultured swine luteal cells. Animals (Basel) 2021;11:606. doi: 10.3390/ani11030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basini G., Grasselli F., Quintavalla F., Bussolati S., Andreoli V., et al. Redox status, estrogen and progesterone production by swine granulosa cells are impaired by triclosan. Animals (Basel) 2022;12:3559. doi: 10.3390/ani12243559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosludtsev K.N., Belosludtseva N.V., Tenkov K.S., Penkov N.V., Agafonov A.V., et al. Study of the mechanism of permeabilization of lecithin liposomes and rat liver mitochondria by the antimicrobial drug triclosan. Biochim. Biophys. Acta Biomembr. 2018;1860:264–271. doi: 10.1016/j.bbamem.2017.09.018. [DOI] [PubMed] [Google Scholar]
- Bera K.K., Kumar S., Paul T., Prasad K.P., Shukla S.P., et al. Triclosan induces immunosuppression and reduces survivability of striped catfish Pangasianodon hypophthalmus during the challenge to a fish pathogenic bacterium Edwardsiella tarda. Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109575. [DOI] [PubMed] [Google Scholar]
- Cao X., Hua X., Xiong J.W., Zhu W.T., Zhang J., et al. Impact of triclosan on female reproduction through reducing thyroid hormones to suppress hypothalamic kisspeptin neurons in mice. Front. Mol. Neurosci. 2018;11:6. doi: 10.3389/fnmol.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D.E., Zitomer D.H., Hristova K.R., Kappell A.D., McNamara P.J. Triclocarban influences antibiotic resistance and alters anaerobic digester microbial community structure. Environ. Sci. Technol. 2016;50:126–134. doi: 10.1021/acs.est.5b03080. [DOI] [PubMed] [Google Scholar]
- Chen J., Meng X.Z., Bergman A., Halden R.U. Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 2019;365:502–510. doi: 10.1016/j.jhazmat.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Delbes G., Blázquez M., Fernandino J.I., Grigorova P., Hales B.F., et al. Effects of endocrine disrupting chemicals on gonad development: mechanistic insights from fish and mammals. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112040. [DOI] [PubMed] [Google Scholar]
- Derouiche S., Mariot P., Warnier M., Vancauwenberghe E., Bidaux G., et al. Activation of TRPA1 channel by antibacterial agent triclosan induces VEGF secretion in human prostate cancer stromal cells. Cancer Prev. Res. (Phila.) 2017;10:177–187. doi: 10.1158/1940-6207.CAPR-16-0257. [DOI] [PubMed] [Google Scholar]
- Diao W., Qian Q., Sheng G., He A., Yan J., et al. Triclosan targets miR-144 abnormal expression to induce neurodevelopmental toxicity mediated by activating PKC/MAPK signaling pathway. J. Hazard. Mater. 2022;431 doi: 10.1016/j.jhazmat.2022.128560. [DOI] [PubMed] [Google Scholar]
- Dong G., Sun R., Zhang R., Qin Y., Lu C., et al. Preimplantation triclosan exposure alters uterine receptivity through affecting tight junction protein. Biol. Reprod. 2022;107:349–357. doi: 10.1093/biolre/ioac092. [DOI] [PubMed] [Google Scholar]
- Du Y., Wang B., Cai Z., Zhang H., Wang B., et al. The triclosan-induced shift from aerobic to anaerobic metabolism link to increased steroidogenesis in human ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2021;220 doi: 10.1016/j.ecoenv.2021.112389. [DOI] [PubMed] [Google Scholar]
- Duan P., Huang X., Ha M., Li L., Liu C. miR-142-5p/DAX1-dependent regulation of P450c17 contributes to triclosan-mediated testosterone suppression. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2020.137280. [DOI] [PubMed] [Google Scholar]
- Ena L., Lim J.S., Son J.Y., Park Y.J., Lee Y.H., et al. Evaluation of subchronic exposure to triclosan on hepatorenal and reproductive toxicities in prepubertal male rats. J. Toxic. Environ. Health A. 2018;81:421–431. doi: 10.1080/15287394.2018.1451188. [DOI] [PubMed] [Google Scholar]
- Escarrone A.L., Caldas S.S., Primel E.G., Martins S.E., Nery L.E. Uptake, tissue distribution and depuration of triclosan in the guppy Poecilia vivipara acclimated to freshwater. Sci. Total Environ. 2016;560–561:218–224. doi: 10.1016/j.scitotenv.2016.04.039. [DOI] [PubMed] [Google Scholar]
- Etzel T.M., Calafat A.M., Ye X., Chen A., Lanphear B.P., et al. Urinary triclosan concentrations during pregnancy and birth outcomes. Environ. Res. 2017;156:505–511. doi: 10.1016/j.envres.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Zhang P., Zhang Z., Shi J., Jiao Z., et al. Endocrine disrupting eEffects of triclosan on the placenta in pregnant rats. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Tu P., Bian X., Chi L., Ru H., et al. Profound perturbation induced by triclosan exposure in mouse gut microbiome: a less resilient microbial community with elevated antibiotic and metal resistomes. BMC Pharmacol. Toxicol. 2017;18:46. doi: 10.1186/s40360-017-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulke C.A., Barton C.L., Proffitt S., Tanguay R.L., Sharpton T.J. Triclosan exposure is associated with rapid restructuring of the microbiome in adult zebrafish. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., et al. EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015;36:E1–e150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Wu C., Zhang J., Li W., Lv S., et al. Prenatal exposure to multiple phenolic compounds, fetal reproductive hormones, and the second to fourth digit ratio of children aged 10 years in a prospective birth cohort. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.127877. [DOI] [PubMed] [Google Scholar]
- Ha M., Zhang P., Li L., Liu C. Triclosan suppresses testicular steroidogenesis via the miR-6321/JNK/ Nur77 cascade. Cell. Physiol. Biochem. 2018;50:2029–2045. doi: 10.1159/000495049. [DOI] [PubMed] [Google Scholar]
- Han X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016;12:668–679. doi: 10.1038/nrendo.2016.98. [DOI] [PubMed] [Google Scholar]
- Hipwell A.E., Kahn L.G., Factor-Litvak P., Porucznik C.A., Siegel E.L., et al. Exposure to non-persistent chemicals in consumer products and fecundability: a systematic review. Hum. Reprod. Update. 2019;25:51–71. doi: 10.1093/humupd/dmy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Raikhel V., Gopalakrishnan K., Fernandez-Hernandez H., Lambertini L., et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome. 2016;4:26. doi: 10.1186/s40168-016-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Cao G., Deng C., Chen Y., Wang T., et al. Adverse effects of triclosan on kidney in mice: implication of lipid metabolism disorders. J. Environ. Sci. (China) 2023;124:481–490. doi: 10.1016/j.jes.2021.11.032. [DOI] [PubMed] [Google Scholar]
- Huang W., Xie P., Cai Z. Lipid metabolism disorders contribute to hepatotoxicity of triclosan in mice. J. Hazard. Mater. 2020;384 doi: 10.1016/j.jhazmat.2019.121310. [DOI] [PubMed] [Google Scholar]
- Huo W., Wang Y., Chen T., Cao T., Zhang Y., et al. Triclosan activates c-Jun/miR-218-1-3p/SLC35C1 signaling to regulate cell viability, migration, invasion and inflammatory response of trophoblast cells in vitro. BMC Pregnancy Childbirth. 2022;22:470. doi: 10.1186/s12884-022-04791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilozumba M.N., Shelver W.L., Hong C.C., Ambrosone C.B., Cheng T.D. Urinary concentrations of triclosan, bisphenol a, and brominated flame retardants and the association of triclosan with demographic characteristics and body fatness among women with newly diagnosed breast cancer. Int. J. Environ. Res. Public Health. 2022;19:4681. doi: 10.3390/ijerph19084681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D.G.W., Williams M.A., Thaiss C.A., Cabrera-Rubio R., Raverdeau M., et al. Loss of MicroRNA-21 influences the gut microbiota, causing reduced susceptibility in a murine model of colitis. J. Crohns Colitis. 2018;12:835–848. doi: 10.1093/ecco-jcc/jjy038. [DOI] [PubMed] [Google Scholar]
- Jurewicz J., Radwan M., Wielgomas B., Kałużny P., Klimowska A., et al. Environmental levels of triclosan and male fertility. Environ. Sci. Pollut. Res. Int. 2018;25:5484–5490. doi: 10.1007/s11356-017-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J., Wielgomas B., Radwan M., Karwacka A., Klimowska A., et al. Triclosan exposure and ovarian reserve. Reprod. Toxicol. 2019;89:168–172. doi: 10.1016/j.reprotox.2019.07.086. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Yi B.R., Go R.E., Hwang K.A., Nam K.H., et al. Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ. Toxicol. Pharmacol. 2014;37:1264–1274. doi: 10.1016/j.etap.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Kim S.A., Moon H., Lee K., Rhee M.S. Bactericidal effects of triclosan in soap both in vitro and in vivo. J. Antimicrob. Chemother. 2015;70:3345–3352. doi: 10.1093/jac/dkv275. [DOI] [PubMed] [Google Scholar]
- Ku P., Wu X., Nie X., Ou R., Wang L., et al. Effects of triclosan on the detoxification system in the yellow catfish (Pelteobagrus fulvidraco): expressions of CYP and GST genes and corresponding enzyme activity in phase I, II and antioxidant system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014;166:105–114. doi: 10.1016/j.cbpc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Lan Z., Hyung K.T., Shun B.K., Hui C.X., Sik K.H. Triclosan exhibits a tendency to accumulate in the epididymis and shows sperm toxicity in male Sprague-dawley rats. Environ. Toxicol. 2015;30:83–91. doi: 10.1002/tox.21897. [DOI] [PubMed] [Google Scholar]
- Lee-Sarwar K., Hauser R., Calafat A.M., Ye X., O'Connor G.T., et al. Prenatal and early-life triclosan and paraben exposure and allergic outcomes. J. Allergy Clin. Immunol. 2018;142:269–278. doi: 10.1016/j.jaci.2017.09.029. e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.M., Hwang K.A., Choi K.C. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol. Cell. Endocrinol. 2017;457:103–113. doi: 10.1016/j.mce.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Li J., Quan X., Zhang Y., Yu T., Lei S., et al. PPARγ regulates triclosan induced placental dysfunction. Cells. 2021;11:86. doi: 10.3390/cells11010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang J.D., Xiao H., He S., He T.T., et al. Triclocarban and triclosan exacerbate high-fat diet-induced hepatic lipid accumulation at environmental related levels: the potential roles of estrogen-related receptors pathways. Sci. Total Environ. 2023;858 doi: 10.1016/j.scitotenv.2022.160079. [DOI] [PubMed] [Google Scholar]
- Lim S., Yoon J.H. Exposure to environmental pollutants and a marker of early kidney injury in the general population: results of a nationally representative cross-sectional study based on the korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci. Total Environ. 2019;681:175–182. doi: 10.1016/j.scitotenv.2019.04.081. [DOI] [PubMed] [Google Scholar]
- Lin J., Wang C., Liu J., Dahlgren R.A., Ai W., et al. Up-stream mechanisms for up-regulation of miR-125b from triclosan exposure to zebrafish (Danio rerio) Aquat. Toxicol. 2017;193:256–267. doi: 10.1016/j.aquatox.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Lin M.H., Chiu S.Y., Ho W.C., Chi K.H., Liu T.Y., et al. Effect of triclosan on the pathogenesis of allergic diseases among children. J. Expo. Sci. Environ. Epidemiol. 2022;32:60–68. doi: 10.1038/s41370-021-00304-w. [DOI] [PubMed] [Google Scholar]
- Liu F., Zhang Y., Wang F. Environmental relevant concentrations of triclosan affected developmental toxicity, oxidative stress, and apoptosis in zebrafish embryos. Environ. Toxicol. 2022;37:848–857. doi: 10.1002/tox.23448. [DOI] [PubMed] [Google Scholar]
- Liu J., Tao Y., Haikun W., Lanfang Y., Jingyi L., et al. Triclosan exposure induced disturbance of gut microbiota and exaggerated experimental colitis in mice. BMC Gastroenterol. 2022;22:469. doi: 10.1186/s12876-022-02527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Cui H., Huang Y., Zhou Y., Hu J., et al. Enzyme-mediated reactions of phenolic pollutants and endogenous metabolites as an overlooked metabolic disruption pathway. Environ. Sci. Technol. 2022;56:3634–3644. doi: 10.1021/acs.est.1c08141. [DOI] [PubMed] [Google Scholar]
- Liu M., Ai W., Sun L., Fang F., Wang X., et al. Triclosan-induced liver injury in zebrafish (Danio rerio) via regulating MAPK/p53 signaling pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;222:108–117. doi: 10.1016/j.cbpc.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Ma H., Zheng L., Li Y., Pan S., Hu J., et al. Triclosan reduces the levels of global DNA methylation in HepG2 cells. Chemosphere. 2013;90:1023–1029. doi: 10.1016/j.chemosphere.2012.07.063. [DOI] [PubMed] [Google Scholar]
- Ma P., Zhang Z., Zhou X., Luo J., Lu H., et al. Characterizing semen abnormality male infertility using non-targeted blood plasma metabolomics. PLoS One. 2019;14 doi: 10.1371/journal.pone.0219179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Chen C., Wang J.B., Cheng J.L., Shen S., et al. Triclosan-induced oxidative stress injury and apoptosis by regulating the PI3K/Akt/Caspase-3 signaling pathway in human tenal glomerular endothelial cells. Biomed. Environ. Sci. 2022;35:547–551. doi: 10.3967/bes2022.073. [DOI] [PubMed] [Google Scholar]
- Ma Y., Guo Y., Ye H., Zhang J., Ke Y. Perinatal triclosan exposure in the rat induces long-term disturbances in metabolism and gut microbiota in adulthood and old age. Environ. Res. 2020;182 doi: 10.1016/j.envres.2019.109004. [DOI] [PubMed] [Google Scholar]
- Maksymowicz M., Ręka G., Machowiec P., Piecewicz-Szczęsna H. Impact of triclosan on female and male reproductive system and its consequences on fertility: a literature review. J Family Reprod Health. 2022;16:33–42. doi: 10.18502/jfrh.v16i1.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanović M., Đurić L., Milošević N., Milić N. Comprehensive insight into triclosan-from widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. Int. 2021:1–22. doi: 10.1007/s11356-021-17273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L., Messerlian C., Bellavia A., Gaskins A.J., Chiu Y.H., et al. Urinary concentrations of bisphenol a, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ. Int. 2019;126:355–362. doi: 10.1016/j.envint.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J., Qi Q., Hao Y., Lei Y., Guo J. Transcriptional response of a green alga (Raphidocelis subcapitata) exposed to triclosan: photosynthetic systems and DNA repair. J. Environ. Sci. (China) 2022;111:400–411. doi: 10.1016/j.jes.2021.04.023. [DOI] [PubMed] [Google Scholar]
- Montagnini B.G., Forcato S., Pernoncine K.V., Monteiro M.C., Pereira M.R.F., et al. Developmental and reproductive outcomes in male rats exposed to triclosan: two-generation study. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.738980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narrowe A.B., Albuthi-Lantz M., Smith E.P., Bower K.J., Roane T.M., et al. Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. 2015;3:6. doi: 10.1186/s40168-015-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasab H., Rajabi S., Mirzaee M., Hashemi M. Association of urinary triclosan, methyl triclosan, triclocarban, and 2,4-dichlorophenol levels with anthropometric and demographic parameters in children and adolescents in 2020 (case study: Kerman, Iran) Environ. Sci. Pollut. Res. Int. 2022;29:30754–30763. doi: 10.1007/s11356-021-18466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Qin C., Han X. Lipid metabolism and lipidomics applications in cancer research. Adv. Exp. Med. Biol. 2021;1316:1–24. doi: 10.1007/978-981-33-6785-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti M.A., Henderson N.B., Gajjar P., Eliot M., Jackson-Browne M., et al. Gestational triclosan exposure and infant birth weight: a systematic review and meta-analysis. Environ. Int. 2021;157 doi: 10.1016/j.envint.2021.106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Lewinska M., Andersen J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Maróstica H.V., Bracht L., Comar J.F., Peralta R.M., Bracht A., et al. The rapid transformation of triclosan in the liver reduces its effectiveness as inhibitor of hepatic energy metabolism. Toxicol. Appl. Pharmacol. 2022;442 doi: 10.1016/j.taap.2022.115987. [DOI] [PubMed] [Google Scholar]
- Phillips J., Haimbaugh A.S., Akemann C., Shields J.N., Wu C.C., et al. Developmental phenotypic and transcriptomic effects of exposure to nanomolar levels of 4-nonylphenol, triclosan, and triclocarban in zebrafish (Danio rerio) Toxics. 2022;10:53. doi: 10.3390/toxics10020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka T.A., Maske P., Mote C., Dighe V. Gestational and lactational exposure to triclosan causes impaired fertility of F1 male offspring and developmental defects in F2 generation. Environ. Pollut. 2020;257 doi: 10.1016/j.envpol.2019.113617. [DOI] [PubMed] [Google Scholar]
- Qiao Y., He J., Han P., Qu J., Wang X., et al. Long-term exposure to environmental relevant triclosan induces reproductive toxicity on adult zebrafish and its potential mechanism. Sci. Total Environ. 2022;826 doi: 10.1016/j.scitotenv.2022.154026. [DOI] [PubMed] [Google Scholar]
- Querido M.M., Rosário F., Bessa M.J., Mendes F., Teixeira J.C., et al. In vitro cyto- and genotoxicity assessment of antibacterial paints with triclosan and isoborneol. Toxics. 2022;10:58. doi: 10.3390/toxics10020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj S., Singh S.S., Singh S.P., Singh P. Evaluation of triclosan-induced reproductive impairments in the accessory reproductive organs and sperm indices in the mice. Acta Histochem. 2021;123 doi: 10.1016/j.acthis.2021.151744. [DOI] [PubMed] [Google Scholar]
- Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanidad K.Z., Wang G., Panigrahy A., Zhang G. Triclosan and triclocarban as potential risk factors of colitis and colon cancer: roles of gut microbiota involved. Sci. Total Environ. 2022;842 doi: 10.1016/j.scitotenv.2022.156776. [DOI] [PubMed] [Google Scholar]
- Sanidad K.Z., Xiao H., Zhang G. Triclosan, a common antimicrobial ingredient, on gut microbiota and gut health. Gut Microbes. 2019;10:434–437. doi: 10.1080/19490976.2018.1546521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Wang X., Bhandari R.K. Developmental abnormalities and epigenetic alterations in medaka (Oryzias latipes) embryos induced by triclosan exposure. Chemosphere. 2020;261 doi: 10.1016/j.chemosphere.2020.127613. [DOI] [PubMed] [Google Scholar]
- Song Y., Zhang C., Lei H., Qin M., Chen G., et al. Characterization of triclosan-induced hepatotoxicity and triclocarban-triggered enterotoxicity in mice by multiple omics screening. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.156570. [DOI] [PubMed] [Google Scholar]
- Spanier A.J., Fausnight T., Camacho T.F., Braun J.M. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 2014;35:475–481. doi: 10.2500/aap.2014.35.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Zhao T., Long K., Wu M., Zhang Z. Triclosan down-regulates fatty acid synthase through microRNAs in HepG2 cells. Eur. J. Pharmacol. 2021;907 doi: 10.1016/j.ejphar.2021.174261. [DOI] [PubMed] [Google Scholar]
- Sun L., Gao M., Qian Q., Guo Z., Zhu P., et al. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m(6)A methylation level to cause lipid metabolism disorder in zebrafish. Sci. Total Environ. 2021;770 doi: 10.1016/j.scitotenv.2021.145285. [DOI] [PubMed] [Google Scholar]
- Tang N., Fan P., Chen L., Yu X., Wang W., et al. The effect of early life exposure to triclosan on thyroid follicles and hormone levels in zebrafish. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.850231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Fan P., Yu X., Ma R., Tao Y., et al. Effects of long-term triclosan exposure on microbiota in zebrafish. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., M M.V., Wu Y., Beland F.A., Olson G.R. Role of peroxisome proliferator-activated receptor alpha (PPARα) and PPARα-mediated species differences in triclosan-induced liver toxicity. Arch. Toxicol. 2018;92:3391–3402. doi: 10.1007/s00204-018-2308-7. [DOI] [PubMed] [Google Scholar]
- Tenkov K.S., Dubinin M.V., Vedernikov A.A., Chelyadnikova Y.A., Belosludtsev K.N. An in vivo study of the toxic effects of triclosan on Xenopus laevis (Daudin, 1802) frog: assessment of viability, tissue damage and mitochondrial dysfunction. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022;259 doi: 10.1016/j.cbpc.2022.109401. [DOI] [PubMed] [Google Scholar]
- Teplova V.V., Belosludtsev K.N., Kruglov A.G. Mechanism of triclosan toxicity: mitochondrial dysfunction including complex II inhibition, superoxide release and uncoupling of oxidative phosphorylation. Toxicol. Lett. 2017;275:108–117. doi: 10.1016/j.toxlet.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Todoric J., Di Caro G., Reibe S., Henstridge D.C., Green C.R., et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020;2:1034–1045. doi: 10.1038/s42255-020-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D.N., Jung E.M., Yoo Y.M., Lee J.H., Jeung E.B. Perinatal exposure to triclosan results in abnormal brain development and behavior in mice. Int. J. Mol. Sci. 2020;21:4009. doi: 10.3390/ijms21114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chen L., Zhao S., Hu Y., Zhou Y., et al. Impacts of prenatal triclosan exposure on fetal reproductive hormones and its potential mechanism. Environ. Int. 2018;111:279–286. doi: 10.1016/j.envint.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Wang C., Huang W., Lin J., Fang F., Wang X., et al. Triclosan-induced liver and brain injury in zebrafish (Danio rerio) via abnormal expression of miR-125 regulated by PKCα/Nrf2/p53 signaling pathways. Chemosphere. 2020;241 doi: 10.1016/j.chemosphere.2019.125086. [DOI] [PubMed] [Google Scholar]
- Wang C., Yu Z., Shi X., Tang X., Wang Y., et al. Triclosan enhances the clearing of pathogenic intracellular salmonella or candida albicans but disturbs the intestinal microbiota through mTOR-independent autophagy. Front. Cell. Infect. Microbiol. 2018;8:49. doi: 10.3389/fcimb.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang X., Huang H., Wang H. Triclosan regulates alternative splicing events of nerve-related genes through RNA-binding protein CELF2 to induce zebrafish neurotoxicity. Hazard. Mater. 2021;413 doi: 10.1016/j.jhazmat.2021.125414. [DOI] [PubMed] [Google Scholar]
- Wang F., Liu F., Chen W., Xu R., Wang W. Effects of triclosan (TCS) on hormonal balance and genes of hypothalamus-pituitary-gonad axis of juvenile male Yellow River carp (Cyprinus carpio) Chemosphere. 2018;193:695–701. doi: 10.1016/j.chemosphere.2017.11.088. [DOI] [PubMed] [Google Scholar]
- Wang F., Zheng F., Liu F. Effects of triclosan on antioxidant- and apoptosis-related genes expression in the gill and ovary of zebrafish. Exp. Anim. 2020;69:199–206. doi: 10.1538/expanim.19-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ouyang F., Feng L., Wang X., Liu Z., et al. Maternal urinary triclosan concentration in relation to maternal and neonatal thyroid hormone levels: a prospective study. Environ. Health Perspect. 2017;125 doi: 10.1289/EHP500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Song J., Wang X., Qian Q., Wang H. Study on the toxic-mechanism of triclosan chronic exposure to zebrafish (Danio rerio) based on gut-brain axis. Sci. Total Environ. 2022;844 doi: 10.1016/j.scitotenv.2022.156936. [DOI] [PubMed] [Google Scholar]
- Wang Z., Li X., Klaunig J.E. Investigation of the mechanism of triclosan induced mouse liver tumors. Regul. Toxicol. Pharmacol. 2017;86:137–147. doi: 10.1016/j.yrtph.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Weatherly L.M., Gosse J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017;20:447–469. doi: 10.1080/10937404.2017.1399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A.A., Yang X., Mennillo E., Ding J., Watrous J.D., et al. Lactational delivery of triclosan promotes non-alcoholic fatty liver disease in newborn mice. Nat. Commun. 2022;13:4346. doi: 10.1038/s41467-022-31947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Jia Y., Sun Z., Su J., Liu Q.S., et al. Environmental pollution, a hidden culprit for health issues. Eco-Environment Health. 2022;1:31–45. doi: 10.1016/j.eehl.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang W., Romano K.A., Gu M., Sanidad K.Z., et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang W., Zhang G. Consumer antimicrobials on gut microbiota and gut health. DNA Cell Biol. 2019;38:7–9. doi: 10.1089/dna.2018.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawer A., Sychrová E., Labohá P., Raška J., Jambor T., et al. Endocrine-disrupting chemicals rapidly affect intercellular signaling in leydig cells. Toxicol. Appl. Pharmacol. 2020;404 doi: 10.1016/j.taap.2020.115177. [DOI] [PubMed] [Google Scholar]
- Yu J., Wu Y., Li H., Zhou H., Shen C., et al. BMI1 drives steroidogenesis through epigenetically repressing the p38 MAPK pathway. Front. Cell. Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.665089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G., Ma Y., Zeng Y., Pan H., Liu P., et al. Associations between low-dose triclosan exposure and semen quality in a chinese population. Environ. Pollut. 2022;299 doi: 10.1016/j.envpol.2022.118926. [DOI] [PubMed] [Google Scholar]
- Yueh M.F., He F., Chen C., Vu C., Tripathi A., et al. Triclosan leads to dysregulation of the metabolic regulator FGF21 exacerbating high fat diet-induced nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. U. S. A. 2020;117:31259–31266. doi: 10.1073/pnas.2017129117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh M.F., Taniguchi K., Chen S., Evans R.M., Hammock B.D., et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc. Natl. Acad. Sci. U. S. A. 2014;111:17200–17205. doi: 10.1073/pnas.1419119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh M.F., Tukey R.H. Triclosan: a widespread environmental toxicant with many biological effects. Annu. Rev. Pharmacol. Toxicol. 2016;56:251–272. doi: 10.1146/annurev-pharmtox-010715-103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L., Ma Y., Huang W., Ling Y., Sun L., et al. Dietary lactobacillus plantarum ST-III alleviates the toxic effects of triclosan on zebrafish (Danio rerio) via gut microbiota modulation. Fish Shellfish Immunol. 2019;84:1157–1169. doi: 10.1016/j.fsi.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang H., Sanidad K.Z., Zhang J., Wang G., Zhang R., et al. Microbiota-mediated reactivation of triclosan oxidative metabolites in colon tissues. J. Hazard. Mater. 2023;445 doi: 10.1016/j.jhazmat.2022.130509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Shao X., Zhao H., Li X., Wei J., et al. Integration of metabolomics and lipidomics reveals metabolic mechanisms of triclosan-induced toxicity in human hepatocytes. Environ. Sci. Technol. 2019;53:5406–5415. doi: 10.1021/acs.est.8b07281. [DOI] [PubMed] [Google Scholar]
- Zhang J., Walker M.E., Sanidad K.Z., Zhang H., Liang Y., et al. Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract. Nat. Commun. 2022;13:136. doi: 10.1038/s41467-021-27762-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Yang M., Zeng L., Liu C. P38/TRHr-dependent regulation of TPO in thyroid cells contributes to the hypothyroidism of triclosan-treated rats. Cell. Physiol. Biochem. 2018;45:1303–1315. doi: 10.1159/000487558. [DOI] [PubMed] [Google Scholar]
- Zhang P., Zheng L., Duan Y., Gao Y., Gao H., et al. Gut microbiota exaggerates triclosan-induced liver injury via gut-liver axis. J. Hazard. Mater. 2022;421 doi: 10.1016/j.jhazmat.2021.126707. [DOI] [PubMed] [Google Scholar]
- Zhao C., Xie R., Qian Q., Yan J., Wang H., et al. Triclosan induced zebrafish immunotoxicity by targeting miR-19a and its gene socs3b to activate IL-6/STAT3 signaling pathway. Sci. Total Environ. 2022;815 doi: 10.1016/j.scitotenv.2022.152916. [DOI] [PubMed] [Google Scholar]
- Zheng L.Y., Sanders A.P., Saland J.M., Wright R.O., Arora M. Environmental exposures and pediatric kidney function and disease: a systematic review. Environ. Res. 2017;158:625–648. doi: 10.1016/j.envres.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Xie C., Zhao S., Zhang D., Zhang H. Environmental exposure to triclosan and male fecundity: a prospective study in China. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.814927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.