Abstract
In this review, we provide a comprehensive overview of the impact of the COVID-19 pandemic on adult heart transplantation. We highlight the decline in the number of adult transplantations performed throughout the pandemic as a consequence of restrictions imposed on individual programs and hospitals. There were challenges to maintaining cardiac transplant activity at multiple levels, including organ donation in intensive care units, logistical difficulties with organ procurement, and rapidly changing resource considerations at health system and jurisdictional levels. We also review the impact of COVID-19 on cardiac transplant recipients. Despite the high rates of morbidity and mortality observed during the initial phases of the pandemic among heart transplant patients infected with COVID-19, the availability of effective vaccines, pre-exposure prophylaxis, and specific antiviral therapies have drastically improved outcomes over time. Vaccines have proven to be safe and effective in reducing infections and illness severity, but specific considerations in the immunocompromised solid organ transplant population apply, including the need for additional booster doses to achieve sufficient immunisation. We further outline the strong rationale for vaccination before transplantation wherever possible. Finally, the COVID-19 response created a number of barriers to safe and efficient post-transplantation care. Given the need for frequent evaluation and monitoring, especially in the first several months after cardiac transplantation, the pandemic provided the impetus to improve virtual care delivery and explore noninvasive rejection surveillance through gene expression profiling. We hope that lessons learned will allow us to prepare and pivot effectively during future pandemics and health care emergencies.
Résumé
Dans cette revue, nous présentons une vue d’ensemble de l’influence de la pandémie de COVID-19 sur la transplantation cardiaque chez l’adulte. Nous soulignons la diminution du nombre de transplantations adultes réalisées tout au long de la pandémie en raison des restrictions imposées aux programmes individuels et aux hôpitaux. Le maintien de l’activité de transplantation cardiaque s’est heurté à de multiples difficultés, notamment le don d’organes dans les unités de soins intensifs, les problèmes logistiques liés à l’obtention des organes et l’évolution rapide des ressources au niveau des systèmes de santé et des juridictions. Nous examinons également l’incidence de la COVID-19 sur les transplantés cardiaques. Malgré les taux élevés de morbidité et de mortalité observés au cours des phases initiales de la pandémie chez les patients ayant subi une transplantation cardiaque et infectés par la COVID-19, la disponibilité de vaccins efficaces, d’une prophylaxie préexposition et de thérapies antivirales spécifiques a permis d’améliorer considérablement les résultats au fil du temps. Les vaccins se sont révélés sûrs et efficaces pour réduire les infections et la gravité de la maladie, mais des considérations spécifiques s’appliquent à la population immunosupprimée ayant subi une transplantation d’organe solide, notamment la nécessité d’administrer des doses de rappel supplémentaires pour parvenir à une immunisation suffisante. Nous soulignons en outre les arguments solides en faveur de la vaccination avant la transplantation, dans la mesure du possible. Enfin, la réponse à la COVID-19 a créé un certain nombre d’obstacles à l’innocuité et à l’efficacité des soins post-transplantation. Étant donné la nécessité d’une évaluation et d’un suivi fréquents, en particulier au cours des premiers mois suivant une transplantation cardiaque, la pandémie a donné l’élan nécessaire pour améliorer la prestation de soins virtuels et explorer la surveillance non invasive du rejet par l’entremise du profil d’expression génique. Nous espérons que les enseignements tirés nous permettront de nous préparer et de réagir efficacement lors de futures pandémies et urgences sanitaires.
Maintaining safe and effective cardiac transplantation activity while ensuring the best possible outcome for patients requires coordinated participation from multiple parties, including individual transplant centres, donor hospitals, critical care units, jurisdictional organ donation organisations, and human health resources. The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 posed an immediate and severe threat to cardiac transplantation in Canada and across programs internationally. The rapid rise of COVID-19 infections and the commensurate impact on hospital resources generated significant anxiety among patients and health professionals engaged in organ donation and post-transplantation care. The response to COVID-19 also provided the impetus for necessary innovations in health care delivery, patient treatment, and infection-prevention measures. There is a paucity of literature addressing the implications of the COVID-19 experience on cardiac transplantation activity in any jurisdiction, so we sought to highlight the impact of the pandemic in the Canadian context, given our unique geographic and health system environment, and to contrast this with the international experience of transplantation in the COVID-19 era.
In this paper, we review the most significant challenges triggered by the pandemic as they relate to cardiac transplantation. We discuss the observed changes in overall transplantation activity across Canadian centres, as informed by survey data from individual cardiac transplant programs. Subsequently, we review the unique obstacles imposed by COVID-19 policies on organ donation, considering interrelated perspectives from the intensive care unit setting, the health care system, and organ procurement logistics in Canada and international jurisdictions. Finally, we discuss the impact of COVID-19 infection in cardiac transplant recipients, with a particular focus on patient outcomes, clinical therapeutics, vaccination and preventative therapies, and ultimately access to care considerations.
Overview of Cardiac Transplantation Activity in Canada During the Pandemic
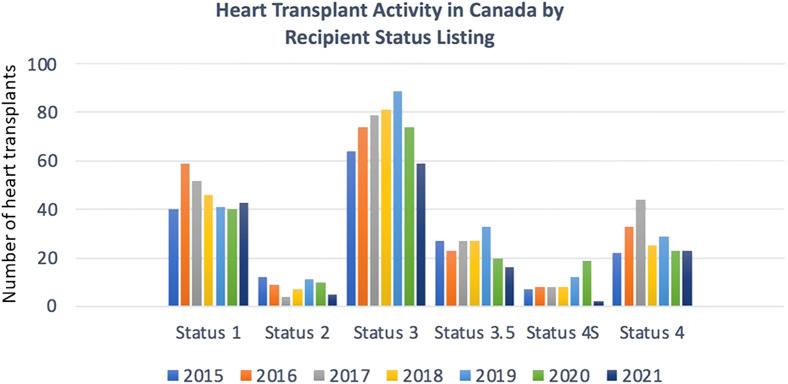
Historically, the annual number of heart transplantations performed in Canada has remained fairly stable year to year over the past 2 decades. There is a clear signal that the number of adult and paediatric heart transplantations declined overall during the COVID-19 period (Fig. 1 ).1 In 2020 and 2021, there were 162 adult and 26 paediatric and 126 adult and 16 paediatric transplantations, respectively, compared with a mean of 199 adult and 24 paediatric heart transplantations in the 5 years before. Of note, paediatric transplantation activity did not drop in parallel with adult transplantation activity during the initial phase of COVID-19 (2020), and reasons for this are unclear. It is possible that the pandemic had a delayed effect on paediatric organ donation rates, or that the pandemic disproportionately affected adult hospital resources more acutely. All transplantation programs ultimately observed reductions in volumes compared with historic averages. There were fewer transplants for recipients with greater severity of medical illness, as indicated by lower transplantation rates among those listed at higher status (status 3, 3.5, and the highly sensitised 4S population) (Fig. 2 ).1
Figure 1.
Heart transplantation activity in Canada. Data represent absolute numbers of adult and paediatric transplantations performed in Canada for the years 2015-2021.
Figure 2.
Heart transplantation activity by recipient listing status 2015-2021. Diminished transplantation activity was notable, particularly among higher-status (more urgent) patients, for 2020 and 2021 compared with the pre–COVID-19 era.
Several factors have been identified as contributing to this decline. We surveyed all adult and paediatric heart transplant programs in Canada with a response rate of 75% (12 of the 16 programs) (Supplemental Appendix S1). Two-thirds of respondent programs restricted heart transplantation activity at some point during the pandemic, and 50% cited cycles of repeated restrictions. These were primarily institution-based and program specific decisions that were managed by the clinical transplant programs. These decisions were most heavily influenced by intensive care unit capacity and staffing. Several other factors cited in the survey included local and provincial active COVID-19 case counts and trajectory, hospital outbreaks, and concerns for recipient safety on 2 fronts. Specific transplant patient safety concerns were related to the perception of increased mortality following COVID-19 infection after thoracic organ transplantation and of an absence of COVID-19–specific therapies throughout the initial phases of the pandemic. The majority of programs managed waitlists and transplantation activity on a case-by-case basis, often restricting heart transplantation to medically urgent outpatients and not considering stable outpatients or stable durable left ventricular assist device (LVAD) patients for transplantation. In addition, there were challenges with organ procurement owing to several issues, including donor hospitals restricting entrance to external medical teams, recipient hospital travel policy restrictions that included quarantine requirements, donor COVID-19 testing delays, and challenges with access to ground and air transportation. Finally, national data on eligible donors for heart transplants (neurologic determination of death [NDD] donors) suggest a slight decline in national NDD donors during the pandemic, although some jurisdictions observed increased donation (Fig. 3 ), suggesting donor availability was not a major factor in the decline in heart transplantation activity overall.1
Figure 3.
Neurologic determination of death (NDD) donors in Canada 2019 to present. Availability of NDD donors diminished slightly since 2019. It is unlikely this contributed significantly to an overall reduction in cardiac transplantation activity in this time period.
In addition to a decline in heart transplant volumes, the number of new listings for heart transplantation declined during the pandemic (Fig. 4 ).1 All programs continued to offer clinical services to assess patients for heart transplant candidacy through a combination virtual and in-person model, although many cited challenges in accessing certain diagnostic tests (eg, pulmonary function testing). The decline in new listings could be partially explained by the lower hospitalisation rates for cardiovascular disease (myocardial infarction and heart failure) that were widely observed during periods of intense public health restrictions, as well as a reduction in the proportion of patients identified as candidates for advanced heart failure therapies.2 , 3 Although national data on durable LVAD implantation rates are not available, respondents of the survey cite similar reasons for declines in number of durable LVAD implantations during the pandemic.
Figure 4.
New heart transplant listings in Canada, 2015-2021. In parallel with diminished cardiac transplantation activity in Canada since 2019 is an observed reduction in the number of patients listed for transplant since the onset of COVID-19.
Pandemic Impact on Organ Donation
ICU challenges
Some of the limitations observed in overall transplantation activity were related to ICU challenges4 and are worth further discussion. Given that all potential cardiac donor and recipient patients use ICU resources, it is understandable that COVID-19 put enormous strain on the ICU resources required to complete the organ donation process. The Canadian Institute for Health Informatics reported that from March 2020 to June 2021, there was an observed increase of approximately 3000 hospital admissions for respiratory conditions leading to 14,000 additional patients in ICUs compared with pre–COVID-19 data.5 The peak was observed during the Spring 2021 with an increased need for mechanical ventilation by up to 400%. Notably, Canada has one of the lowest ICU capacities among countries in the Organisation for Economic Cooperation and Development, with 12.9 ICU beds/100,000 population, compared with 25.8 for the USA and 33.9 for Germany.6 There is also significant regional variation across the country, with only, for example, 10.5 ICU beds/100,000 population in British Columbia and 21.8 in Newfoundland and Labrador.7
In this context, most provincial jurisdictions had to initiate prioritisation programs to relieve strain on hospitals and ICUs. Despite planned potential triage procedures, no such triage was ultimately activated in Canada.8 , 9 Most nonemergency surgical procedures had been postponed, and consequently organ donation surgeries were reduced in parallel with the pandemic evolution. As presented by Ibrahim et al.,10 it was thought to be reasonable and necessary to place a higher priority on allocating critical resources for treating infected patients with respiratory failure rather than transplant procedures and post-op care of newly transplanted patients.
During the COVID-19 pandemic, we also observed a change in the demographic of available donors in the ICU. Effectively, the various political and public health decisions to contain the pandemic across Canada resulted in an observed shift of ICU population. Initial lockdowns in the first months of the pandemic led to a 5% to 15% decline in trauma patients, leading to a proportional decrease in the availability of trauma donors. At the same time, a 35% increase in donor death by substance abuse was observed that further altered donor demographics.11 , 12
Health system challenges for organ donation and transplantation
Aside from the pandemic impact on the availability of suitable organ donors in Canada, there were also substantial challenges in the donation and procurement of organs because of multiple issues at a health systems and jurisdictional level. Table 1 outlines a number of specific challenges related to the procurement of donor hearts that had to be considered as a consequence of COVID-19 policies.
Table 1.
Organ donation considerations during the COVID-19 pandemic
| Transplantation area of concern | Issue | Comment |
|---|---|---|
| Donor declaration to potential candidacy |
|
|
| Donor |
|
|
| Donor testing |
|
|
| Critical care beds and resources |
|
|
| Donor organ retrieval |
|
|
CT, computed tomography; OPO, organ procurement organization; PCR, polymerase chain reaction.
Each jurisdiction or province in Canada had to develop a plan to try to safely continue cardiac transplantation through the pandemic, and the approach varied depending on regional factors. In Ontario, cardiac transplantation activity was significantly curtailed at the onset of the pandemic for 2 primary reasons: 1) to preserve hospital infrastructure and resources to allow treatment of COVID-19 patients, and 2) to avoid iatrogenic immunosuppression during a time when community or hospital exposure to the transplant recipient was a possibility. As a province with multiple adult heart transplant programs, the Ontario approach to modifying transplantation activity can serve as an example of jurisdictional policy setting in an effort to balance interests of critically sick cardiac patients with those of the general population and to respond to the dynamic nature of available resources. To safely proceed with heart transplantation, a number of conditions had to be considered, including:
-
•
Ensuring that risk of iatrogenic COVID exposure was minimised by developing local COVID-free pathways.
-
•
Ensuring a sustainable safe set of essential transplant-specific processes (personnel, diagnostic imaging, lab testing).
-
•
Ensuring the ability to do rapid donor and recipient pre-transplantation COVID screening.
-
•
Ensuring that processes were in place to protect procurement teams, including strongly encouraging the use of “local” procurement teams to mitigate risk.
-
•
Providing appropriate informed consent regarding individual patient risk and benefit.
-
•
Considering other factors that may affect outcome (eg, availability of personal protective equipment, availability of critical care medications, availability of extracorporeal membrane oxygenation or temporary LVAD if necessary, availability of care team personnel).
In developing a provincial protocol that incorporated the above health system requirements, the most objective and easily applied criteria were based on “percentage of critical care bed surge activity.” This was determined by operational leadership at individual centres and updated daily. To proceed responsibly, the cardiac transplant centres in Ontario required a “dynamic staged reduction” to meet ICU bed, staffing, and medical equipment needs for the preponderance of nontransplant patients. In addition, individual programs had to consider “inactivating” patients on the transplant list who were deemed to be a lower risk (ie, status 1 patients) or those with a durable LVAD who were being “bridged to transplant.” Across centres, all attempts were made to recognise the plight of highly sensitised patients who are inherently disadvantaged based on the limited availability of immunologically matched donors, and to proceed with transplantation if the appropriate organ became available. Ultimately, through a province-wide consensus process involving transplant cardiology, cardiovascular surgery, and critical care interests, a protocol was developed to guide cardiac transplantation activity in Ontario with the use of “COVID care tiers” (Table 2 ). Throughout the pandemic, each program’s COVID care tier status was updated on a weekly basis.
Table 2.
Modification of transplant activity in Ontario according to COVID-19 care tier
| COVID Care tier | ICU surge level | ICU surge % | Description of heart transplantation activity |
|---|---|---|---|
| 0 | Normal— Green |
< 100% | Usual activity (assuming Ontario institution is able to accommodate) |
| 1 | Minor— Yellow |
100%-110% | Status 4 and 4S Ontario/National programs; then to status 3.5 and 3 in Ontario
|
| 2 | Moderate— Orange |
111%-135% | Status 4 and 4S Ontario/National programs; then to status 3.5 and 3 in Ontario
|
| 3 | Severe— Red |
136%-175% | Status 4 only (if Ontario institution able to accommodate) |
| 4 | Massive | > 175% | No cardiac transplantation |
ICU, intensive care unit; LVAD, left ventricular assist device.
Organ procurement and logistical challenges
Among the many aspects of heart transplantation affected by the COVID-19 response in Canada, there were significant challenges affecting both the logistics and volume of organ procurements. As expected, this was not a phenomenon unique to our national environment, but was observed in most solid-organ transplant programs globally despite varying regional burden of COVID-19 cases.13, 14, 15, 16, 17, 18 Among contributors to this phenomenon were competing priorities for both resource and personnel allocation and availability, the dynamically changing landscape of public and health care–specific policies and restrictions, and efforts to maintain the safety and well-being of organ retrieval team members. Moreover, as an issue perhaps more pertinent to countries with an expansive geography like Canada, there were additional complications due to greater pre-pandemic reliance on distant organ procurement opportunities. These geographic considerations may have fostered divergent clinical procedures adopted across different regions and provinces in response to the pandemic, which was also noted in other jurisdictions.19
Among the many obstacles to procurement faced by Canadian heart transplant programs, the more pervasive ones were related to 1) travel and transportation factors, 2) personnel factors, and 3) donor site and other local factors. To varying degrees, these issues contributed to inefficiencies, delays, and on occasion even cancellation of organ retrievals. It is probable that the heart cohort may have been affected more negatively, owing to that allograft’s greater susceptibility to increases in ischemia time. Tables 3 and 4 , respectively, outline the more common issues faced by the retrieval teams as well as examples of mitigation strategies.
Table 3.
Challenges in organ procurement
| Travel/transportation factors | Personnel factors | Donor site factors |
|---|---|---|
| Travel restrictions complicating or limiting the team’s ability to procure organs interprovincially and internationally | “Sidelining” of team members owing to quarantine requirements after travel | Local hospital restrictions limiting entry and access to donor facilities |
| Limited space on flights and ground transportation for teams owing to distancing requirements | Staff shortage owing to reassignment, quarantine, illness, and fatigue | Diminished ICU and OR capacity |
| Availability of timely travel/transportation and lack of streamlined air flight/airport processes | Unpredictable “last-minute” changes in staffing | Variable OR pandemic protocols leading to delays and inefficiencies before and during the retrieval surgeries |
ICU, intensive care unit; OR, operating room.
Table 4.
Mitigating procurement measures during the pandemic
| Streamlined retrieval teams deployed for each procurement and use of multiple vehicles to abide by distancing requirements |
| Local procurement arrangements to obviate border crossing or long-distance travel |
| Up front clarification on donor sites’ hospital and OR restrictions and protocols |
| Consistent emphasis on personal protective measures |
| Facilitated access to rapid staff COVID testing before and after retrieval |
| Back-up on-call roster to ensure availability in case of staff “sidelining” |
| Monitoring staff well-being and providing accessible mental health resources |
OR, operating room.
Impact of the Pandemic on Cardiac Transplantation Activity Outside of Canada
The Canadian heart transplantation experience during the pandemic has been generally consistent with the impact observed in the United States. For example, an analysis from the United Network for Organ Sharing found a 37% reduction in new waitlisted patients, and a 26% reduction in organ retrievals and cardiac transplantations from March 15 to May 9, 2020 (early pandemic phase), compared with January 19 to March 15, 2020 (immediately preceding the pandemic).20 However, the reduction in heart transplantation activity across the United States was not universally observed, and the impact may have been more heterogeneous. One large-volume program actually reported an increase in heart transplantation activity in the early phase of the pandemic, likely related to greater availability of organs declined by competing centres.21 To our knowledge, comparable date from other countries has not been published.
Regarding critical care and donor management challenges outside of Canada, the international community has shared similar experiences. Many transplant programs were reluctant to bring stable transplant candidates into hospitals and ICUs where they might be at greater risk of being exposed to COVID-19.4 Another consequence of the early pandemic was a greater reluctance to use donors with circulatory death in whom postoperative transplantation recovery was anticipated to be prolonged.4 Additional obstacles observed during the pandemic were related to significant impairments to the standard donor evaluation. In the setting of reduced hospital diagnostic capacity, the ability to complete standard donor investigations, such as coronary angiography, was significantly limited. All of these ICU-related factors compounded challenges in organ donation and contributed to the observed reduction in solid organ transplantation during the COVID-19 period in most countries.13
Globally, efforts have been made to mitigate the negative impact of the pandemic with varying levels of success, with rates of procurement and transplantation unfortunately lagging in developing countries.22 These efforts are further complicated by the complex ethical questions that heart transplant programs had to face while navigating the response to COVID-19.10 , 23 Nonetheless, early and proactive planning and pandemic management as well as willingness to explore creative options have helped to manage many of the challenges in procurement and transplantation.24 , 25 An example of a successful approach to procurement was seen in Australia, a country with similar geographic procurement distances, where local heart and lung procurement teams were successfully created in South Australia in the absence of tertiary transplant facilities.26
At a health system level, some programs in the United States maintained capacity for transplantation by physically grouping waitlisted patients and those with advanced heart failure in dedicated hospital settings.27 Finally, a small number of centres in the United States procured hearts from COVID-19–positive donors and demonstrated acceptable short-term transplant outcomes.28
Impacts of COVID-19 for Individual Patients
Outcomes of COVID-19 infection in cardiac transplant recipients
Solid organ transplant (SOT) recipients are at risk of severe infection due to COVID-19 related to their comorbidities as well as their immunosuppressed state. Risk factors for severe illness among SOT recipients are similar to those in the general population, including age and comorbidities.29, 30, 31, 32 Since 2020, outcomes of infection with COVID-19 have evolved over the course of the pandemic with emerging variants of concern, introduction of immunisation, and access to early treatment.
In the earlier phases of COVID-19, the literature reported a high incidence of hospitalisation in SOT recipients with COVID-19, with systematic reviews and meta-analyses reporting hospital admissions as high as 80% in SOT recipients,33 which was similar to rates observed in heart transplant recipients.30 Reassuringly, as time has progressed, advances have been made in management of infection as well as prevention through widespread vaccination, and outcomes in this patient population have improved, although SOT recipients remain at high risk for severe disease.34 , 35
The Omicron (B.1.1.529) variant was designated a variant of concern by the World Health Organisation on November 26, 2021. Since December 2021, the Omicron variants quickly became the dominant COVID-19 variant. In Ontario, data publicly available through Public Health Ontario reported hospitalisation or risk of death to be 65% lower (hazard ratio [HR] 0.35, 95% confidence interval [CI] 0.25-0.46) with Omicron cases from November 2021 to February 24, 2022, compared with matched Delta cases.36 Ontario data were consistent with those reported by other jurisdictions globally, supporting decreased hospitalisations, ICU admissions, and risk of death. For SOT recipients during a similar time period, Cochran et al.37 published single-centre data that identified 347 SOT recipients with a positive COVID-19 test from December 22, 2021, to February 9, 2022. Of this cohort, 90 SOT recipients (26%) required hospitalisation and 8 (2%) died. The authors compared this with historical data from 129 SOT recipients with a positive test from March to November 2020, of which 77 (59.7%) were hospitalised and total mortality was 9.7%.37 Similarly to the general population, risks of hospitalisation and death with Omicron in SOT recipients also are lower compared with previous variants.
Treatment of COVID-19 infection in SOT recipients
Therapies and management of COVID-19 infection have evolved since the emergence of the virus. Many of the originally used therapies tried in clinical management have been discontinued as evidence mounted through real-world use and rapidly initiated clinical trials. Current recommendations, supported by trials such as RECOVERY, ACTT-I and -II, and REMAP-CAP, include dexamethasone,38 remdesivir,39 and anti–interleukin (IL) 6 agents (tocilizumab or sarilumab)40 , 41 or JAK inhibitors (baricitinib)42 as the mainstays of treatment.
Until 2022, treatment guidelines focused on hospitalised patient,s and recommendations were stratified based on severity of illness from those requiring supplemental oxygen (moderately ill) to those requiring ventilatory or circulatory support (critically ill).43 Dexamethasone given as a 10-day course is recommended in all symptomatic hospitalised patients.44 Remdesivir is recommended for moderately ill patients,45 whereas for those who are critically ill or showing signs of systemic inflammation and disease progression, anti–IL-6 agents or JAK inhibitor is recommended.46 , 47 These therapeutic agents and recommendations apply to SOT recipients as well as the general population41 , 42 , 48 (Table 5 ).
Table 5.
Recommendations for treatment by severity of illness
| Severity of illness | Treatment options |
|---|---|
Mild (nonhospitalised or not requiring supplemental oxygen)
|
Nirmatrelvir (300 mg)/ritonavir (100 mg) (Paxlovid) × 5 days
Sotrovimab may still be offered in some jurisdictions, recognising reduced neutralisation to certain variants and after risk-benefit discussion with patient |
| Moderate (requiring new supplemental oxygen) | Dexamethasone, 6 mg PO/IV daily × 10 days (or until discharge) Remdesivir, 200 mg IV day 1, then 100 mg IV daily × 4 days |
| For patients with evidence of systemic inflammation and evidence of disease progression on dexamethasone | Tocilizumab, 400 mg IV Alternatives: sarilumab (400 mg IV) or baricitinib (4 mg PO/NG) daily × 14 days (or discharge) |
| Severe or critically ill (requiring ventilatory or circulatory support) | Dexamethasone, 6 mg PO/IV daily × 10 days (or until discharge) Tocilizumab, 400 mg IV Alternatives: sarilumab (400 mg IV) OR Baricitinib (4 mg PO NG) daily × 14 days (or discharge) |
In late 2021, attention shifted to early intervention and outpatient therapies as monoclonal antibodies became available to high-risk patients. Along with other transplant societies, the Canadian Society of Transplantation endorsed sotrovimab as first-line treatment for SOT recipients with mild to moderate infection with the goal of preventing hospital admission and severe illness.49 Several publications that review single-centre experience with sotrovimab in mild to moderate COVID-19 have shown tolerability and efficacy in SOT recipients.50 , 51 Data from a multicentre cohort study of 361 SOT recipients who received monoclonal antibody therapy early in the Omicron wave showed an overall hospitalisation rate of only 3%.52 Recognising limitations, including the need for intravenous administration of sotrovimab and the ability of centres to provide rapid access to care, the overall experience with sotrovimab in SOT recipients was positive. Despite this, recommendations for the use of sotrovimab changed53 in the spring of 2022 as subvariants, such as BA.2, became more prevalent and reduced neutralisation was shown. Early intervention has now shifted to antiviral therapies, although sotrovimab may still be available in some jurisdictions as a last-line agent.54
Because SOT recipients are classified as moderately to severely immunocompromised, they have been a priority group for access to early treatment with available antiviral therapies. The two currently available antiviral agents are nirmatrelvir/ritonavir (Paxlovid) and remdesivir. Nirmatrelvir/ritonavir is an oral antiviral consisting of nirmatrelvir, a SARS-CoV-2 main protease inhibitor, boosted by ritonavir, an HIV-1 protease inhibitor and CYP3A inhibitor that serves to increase nirmatrelvir levels. It was approved by Health Canada in January 2022 and remains the only available oral antiviral for early treatment of COVID-19 in Canada. Results from EPIC-HR study showed an 89% reduction in risk of hospitalisation or death compared with placebo in adult patients who were nonvaccinated and nonhospitalised with mild to moderate COVID-19 at high risk of progression to severe disease.55 Data from this trial were before the Omicron variants, but in vitro data on 50% inhibitory concentration have been reassuring.56 A gap in the literature currently exists regarding experience with nirmatrelvir/ritonavir use in SOT recipients. Najjar-Debbiny et al.57 included immunosuppressed patients when presenting real-world data during the initial phase of the Omicron wave and reported reduced risk of progression to severe COVID-19 or mortality with the use of Paxlovid in the first 5 days of infection.
Although nirmatrelvir/ritonavir is currently recommended in many jurisdictions for early intervention in high-risk patient groups, there is concern about its significant interaction with calcineurin inhibitors, mammalian target of rapamycin inhibitors, and other drugs commonly used in transplantation.58 , 59 Therefore, if this therapy is considered as treatment of COVID-19 in SOT, rigourous strategies to mitigate these interactions and perform therapeutic drug monitoring of transplant medications should be in place.48 , 60 Strong consideration should also be given to avoiding nirmatrelvir/ritonavir altogether in SOT recipients.61
Remdesivir, already used for hospitalised patients, is another option available for early intervention and outpatient treatment. Data presented in the PINETREE trial showing that if given within the first 7 days of symptom onset, a 3-day course of remdesivir reduces risk of hospitalisation or death by 87% compared with placebo in patients at high risk for COVID-19 progression.62 Solera et al.63 published a prospective cohort study on SOT recipients with COVID-19 infection during the Omicron BA.2 wave. The authors similarly observed that 3 doses of remdesivir given within 7 days of symptom onset significantly reduced the risk of hospitalisation (HR 0.12, 95% CI 13.6-31.4); none of the patients receiving remdesivir in the study required ICU admission or died. Thus, remdesivir should be considered as an early therapy with the caveat that access to 3 days of intravenous administration is needed.
Over time, treatment of COVID-19 infection has become increasingly standardised, and access to early intervention has been shown to improve outcomes, including in high-risk SOT recipients, although further studies in this patient population are needed.
Prevention of COVID-19 infection in transplant recipients
Vaccination
During the initial vaccine rollout in 2021, 2 types of COVID-19 vaccines were available in Canada: adenoviral vector vaccines (ChAdOx1-S; AstraZeneca) and mRNA vaccines (Pfizer, Moderna). The adenoviral vector vaccine had significant thrombotic adverse events and was eventually discontinued in Canada. A protein-based adjuvant vaccine (Nuvaxovid; Novavax) is also now available but lacks data in the transplant setting. Thus, the vast majority of vaccines administered as well as the research performed has been with mRNA vaccines. An analysis from the United States showed that vaccine effectiveness of 2 doses was approximately 59%, with a subsequent high rate of breakthrough infections.64 A third dose of COVID-19 vaccine was implemented in the transplant population as part of the primary vaccination series based on a randomised trial.65 In a study of COVID-19 outcomes for transplant patients during the Omicron BA.1 wave, it was shown that receipt of 3 or more doses of vaccine was an independent factor in reducing disease severity. However, breakthrough infections continue to occur because of waning immunity and the immune escape by new variants; therefore, booster vaccine doses have been recommended for the cardiac transplant population.65, 66, 67
Ideally, the COVID-19 vaccine series should be completed a minimum of 2 weeks before transplantation. In the post-transplantation setting, it is recommended to delay the first/next dose at least 1 month after transplantation (with a consideration to expand this period up to 3 months in case of the use of T- or B-cell depleting therapy). Patients receiving mycophenolate-based immunosuppression tend to have reduced immune responses to mRNA vaccination.68 Some studies have suggested an association between vaccination and the development of new or increasing donor-specific antibodies, but without a significant impact on graft function.69 A recent systematic review and meta-analysis that reviewed the rate of rejection after COVID-19 infection or vaccination found a very small number of cases, including only 1 heart transplant recipient, suggesting that this should not be a reason to avoid vaccines.70
In case of mRNA and adjuvant recombinant protein vaccines, it is suggested that the primary series should be completed with the same vaccine, if possible. For booster doses, different vaccines can be used, especially in those that previously received ChAdOx1-S, because mRNA “heterologous” booster vaccines demonstrate a lower rate of breakthrough infection than homologous boosters.71
Pre-exposure prophylaxis
Although monoclonal antibodies have been used as treatment for mild COVID-19, one preparation (Evusheld; AstraZeneca) is available for pre-exposure prophylaxis.72 Evusheld is a combination of 2 fully human SARS-CoV-2–neutralizing monoclonal antibodies (tixagevimab and cilgavimab) and was approved in Canada for COVID-19 prevention in immunocompromised populations. Its approval is based on the results of a double-blind randomised clinical trial (PROVENT) that compared with placebo, Evusheld reduced COVID-19 infections by 77%. This was predominantly during the Delta variant wave and in unvaccinated high-risk populations (≥ 60 years old, obese, chronic obstructive pulmonary disease, immunocompromised, history of severe or serious adverse reaction to any U.S. Food and Drug Administration [FDA]–licensed vaccine). The effect lasted the duration of the study follow-up (9 months).73 Real-world studies in transplant cohorts show that Evusheld is effective.74 , 75 However, there is significant variability in the concentration of Evusheld required to neutralize Omicron variants, leading to uncertainty whether Evusheld will continue to be effective as new variants emerge.76 To increase effectiveness, the FDA has recommended that a double dose of Evusheld be given every 6 months.
Mandatory COVID-19 vaccination in waitlisted patients
With the availability of safe and effective vaccines for COVID-19, most Canadian heart transplant programs mandated immunisation before listing for transplant by late 2021 (while allowing for individual exemptions on a case-by-case basis). The rationale for this is based on several factors including the following:
-
•
Pre-transplantation vaccination for prospective heart transplant recipients lowers risk for the individual patient and their families. SOT recipients, including heart transplant recipients, are at increased risk of poor outcomes with COVID-19 infection compared with the general population.28
-
•
Pre-transplantation vaccination lowers risk for close contacts that may include other immunosuppressed transplant recipients during frequent contacts with the health care system.
-
•
Pre-transplantation vaccination reduces the risk of recipients becoming critically sick and requiring ICU care with severe COVID-19 infection, thereby facilitating greater ICU capacity.
-
•
Vaccines are more immunogenic in immunocompetent patients, therefore likely affording greater immunity if administered before transplantation.77 , 78
-
•
The Canadian Cardiac Transplant Network’s Eligibility Criteria for Heart Transplant requires patients to demonstrate commitment to healthy behaviours and adherence to therapeutic plans to optimise allograft outcomes at the individual level. This rationale is similar to the requirement for smoking cessation and other self-care behaviours before listing for a transplant.79
-
•
The availability of donor hearts is insufficient to meet the demand in Canada, and there is an ethical obligation to ensure that organs are used in recipients with the greatest chance for a favourable post-transplantation outcome. The obligations also extend to the donor, the donor’s family, and other patients on heart transplant waitlists.
Recognising that vaccine efficacy wanes over time and newer variants of COVID-19 are more resistant to immunisation with first-generation mRNA vaccines, the currently available vaccines do, however, remain protective against severe COVID-19 infection.64 , 65 At this time, adult heart transplant programs in Canada continue to require pre-transplantation COVID-19 vaccination before transplant eligibility.
Impact of the Pandemic on Access to Post-transplantation Care
Post–heart transplantation care has evolved over the COVID-19 period. Preventive measures have been emphasised owing to the immunocompromised state of heart transplant patients and risk of contracting severe COVID-19 infection.80 Major cardiology and transplant societies, including the Canadian Cardiovascular Society, European Society of Cardiology, Heart Failure Society of America, International Society for Heart and Lung Transplantation, and American Society for Transplantation, published recommendations emphasising the need to balance in-person patient visits against risk of acquiring infection while ensuring continuity of care and preservation of care capacity.81, 82, 83, 84, 85 Key recommendations for general heart failure patients were extrapolated to the heart transplant population, including avoiding routine and nonurgent hospital visits and adopting remote monitoring and telemedicine.80
Provincial reimbursement for virtual visits was limited before COVID-19, but important changes to billing codes facilitated the transition to virtual care. This allowed for a win-win situation for physicians and transplant patients, especially during government and hospital mandated cancellations of in-person clinics.85 Reasons for mandatory clinic cancellations have included restructuring and reprioritisation of services as well as patients’ hesitancy to seek in-person medical care out of fear of viral exposure.86 Furthermore, the excess of COVID-19 inpatient cases limited capacity for non–COVID-19 patients. For example, in new post-transplantation patients, there were pressures to avoid prolonged postoperative care owing to the need to free up ICU capacity for critically sick COVID-19 patients.87 As noted, in some areas, heart transplantation surgeries were halted altogether or restricted to urgent high priority status–listed inpatients.83 There are reports of programs opting to implant durable LVADs as bridges to transplant in anticipation of increased transplant waiting times during the pandemic, despite LVADs being more susceptible to infection.83 More recently, with declining active COVID-19 cases, there has been a scaling back of virtual care billing codes, normalisation of policies, and a rebound surge of in-person workload.86
In the early post-transplantation period, frequent in-person clinic reviews and cardiac allograft surveillance testing with endomyocardial biopsies and echocardiography are routine practice.87 Beyond 6 to 12 months after transplantation, clinical follow-up is usually increased to every 3 to 6 months. Some Canadian programs successfully leveraged access to alternative options to endomyocardial biopsy for rejection surveillance, specifically, blood-based tests including AlloMap gene expression profiling and AlloSure donor-derived cell-free DNA. For example, Amadio et al.88 studied 90 patients more than 6 months after heart transplantation who had their routine biopsies replaced by AlloMap and AlloSure testing; 42% of their patients were able to reduce immunosuppression, and this approach led to reduced anxiety and exposure for patients during the pandemic.87 Unfortunately, these tests are not widely available in Canada.87 Notably, AlloMap has not been validated in the early post-transplantation period when rejection risk is higher, nor for antibody-mediated rejection.89
The COVID-19 experience has had a lasting impact on the access and triaging of post-transplantation care. Furthermore, with the development of new therapy for both prevention and treatment of COVID-19, there has been an increased demand for access to cardiac transplant specialists, especially for guidance on drug-drug interactions with immunotherapy and to identify those who are at greater risk and require prioritisation for therapy.83 Understanding the pandemic experience will allow for improved preparedness to maintain high-quality post-transplantation care across programs in the setting of future challenges.
Conclusion
There were multiple and interrelated challenges imposed by the COVID-19 response that conspired to reduce overall transplantation activity in Canada and internationally and cause distress for both patients and providers. There were adverse impacts at multiple levels, including individual transplant programs, donor hospitals and ICUs, jurisdictional organ donation organisations, prospective transplant candidates, and post–heart transplantation patients. Transplant patients have also suffered severe outcomes from COVID-19 infection and remain vulnerable. The availability of effective vaccines, antiviral agents, population-level immunity and emerging innovative care models have improved the capacity to provide appropriate and timely post-transplantation care in the face of an ongoing pandemic response.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
Dr Kumar has received research grant support from Roche and GSK and consultancy fees from Roche, GSK, Merck, and Exevir. The other authors have no conflicts of interest to disclose.
Footnotes
See page 861 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2023.03.014.
Supplementary Material
References
- 1.Canadian Blood Services Organ donation and transplantation data—preliminary results. https://profedu.blood.ca/en/organs-and-tissues/covid-19-update/national-covid-19-impact-data Available at:
- 2.d’Amato A., Severino P., Saglietto A., et al. Heart failure hospitalisation rate reduction during COVID-19 pandemic [abstract] J Am Coll Cardiol. 2021;77(18_Supp_1):791. [Google Scholar]
- 3.Bhatt A., Moscone A., McElrath E., et al. Fewer hospitalisations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76:280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky B.J., Chiang T.P.Y., Werbel W.A., et al. Early impact of COVID-19 on transplant centre practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canadian Institute for Health Information. COVID-19’s impact on hospital services. https://www.cihi.ca/en/covid-19-resources/impact-of-covid-19-on-canadas-health-care-systems/hospital-services Available at:
- 6.Organisation for Economic Co-operation and Development. Intensive care beds capacity. https://www.oecd.org/coronavirus/en/data-insights/intensive-care-beds-capacity Available at:
- 7.Canadian Institute for Health Information Care in Canadian ICUs—data tables. https://www.cihi.ca/en/care-in-canadian-icus-data-tables Available at:
- 8.Brindle M., Doherty G., Lillemoe K., Gawande A. Approaching surgical triage during the COVID-19 pandemic. Ann Surg. 2020;272:e40–e42. doi: 10.1097/SLA.0000000000003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiseman S.M., Crump R.T., Sutherland J.M. Surgical wait list management in Canada during a pandemic: many challenges ahead. Can J Surg. 2020;63:e226–e228. doi: 10.1503/cjs.006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim B., Dawson R., Chandler J.A., et al. The COVID-19 pandemic and organ donation and transplantation: ethical issues. BMC Med Ethics. 2021;22:142. doi: 10.1186/s12910-021-00711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelico R., Trapani S., Manzia T.M., et al. The COVID-19 outbreak in Italy: Initial implications for organ transplantation programs. Am J Transplant. 2020;20:1780–1784. doi: 10.1111/ajt.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed O., Brockmeier D., Lee K., Chapman W.C., Doyle M.B.M. Organ donation during the COVID-19 pandemic. Am J Transplant. 2020;20:3081–3088. doi: 10.1111/ajt.16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loupy A., Aubert O., Reese P.P., Bastien O., Bayer F. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395(10237):e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimaz S., Panahi L., Pouy S. Organ procurement and transplantation during the COVID-19 pandemic in Iran. Korean J Transplant. 2022;36:79–80. doi: 10.4285/kjt.21.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czerwinski J., Antoszkiewicz K., Woderska-Jasinska A., et al. The current status of organ donation and transplantation in Poland. Poltransplant activity. Transplant Proc. 2022;54:837–847. doi: 10.1016/j.transproceed.2022.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Merola J., Schilsky M.L., Mulligan D.C. The impact of COVID-19 on organ donation, procurement, and liver transplantation in the United States. Hepatol Commun. 2021;5:5–11. doi: 10.1002/hep4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudgins J.J., Boyer A.J., Orr K.D., et al. The impact and implications of the COVID-19 pandemic on organ procurement outside of an epicentre. Prog Transplant. 2021;31:171–173. doi: 10.1177/15269248211002808. [DOI] [PubMed] [Google Scholar]
- 18.Goff R.R., Wilk A.R., Toll A.E., McBride M.A., Klassen D.K. Navigating the COVID-19 pandemic: initial impacts and responses of the Organ Procurement and Transplantation Network in the United States. Am J Transplant. 2021;21:2100–2112. doi: 10.1111/ajt.16411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyarsky B.J., Ruck J.M., Chiang T.P., et al. Evolving impact of COVID-19 on transplant center practices and policies in the United States. Clin Transplant. 2020;34:e14086. doi: 10.1111/ctr.14086. [DOI] [PubMed] [Google Scholar]
- 20.DeFilippis E.M., Sinnenberg L., Reza N., et al. Trends in the US heart transplant waitlist activity and volume during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Cardiol. 2020;5:1048–1052. doi: 10.1001/jamacardio.2020.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balsara K.R., Rahaman Z., Sandhaus E., et al. Prioritising heart transplantation during the COVID-19 pandemic. J Cardiac Surg. 2021;36:3217–3221. doi: 10.1111/jocs.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kute V.B., Tullius S.G., Rane H., et al. Global impact of the COVID-19 pandemic on solid organ transplant. Transplant Proc. 2022;54:1412–1416. doi: 10.1016/j.transproceed.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire A.L., Aulisio M.P., Davis F.D., et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. Am J Bioeth. 2020;20:15–27. doi: 10.1080/15265161.2020.1764138. [DOI] [PubMed] [Google Scholar]
- 24.Hugo C., Strassburg C., Stecher M., Rahmel A. Stable and safe organ procurement and transplantation during SARS-CoV-2 pandemic in Germany. Transpl Int. 2020;33:1335–1336. doi: 10.1111/tri.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman A.L., Delli Carpini K.W., Ezzell C., Irving H. There are no best practices in a pandemic: organ donation within the COVID-19 epicentre. Am J Transplant. 2020;20:3089–3093. doi: 10.1111/ajt.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimpel D.J., Crouch G., Szpytma M., et al. Development of an organ procurement team in South Australia in response to COVID-19. Aust N Z J Surg. 2022;92:1863–1866. doi: 10.1111/ans.17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh MN, Ravichandran AK, Seasor E, Salerno CT. Clinical distancing of hospitalized patients with advanced heart failure and cardiac transplantation during COVID-19. J Heart Lung Transplant 2020;39:730. [DOI] [PMC free article] [PubMed]
- 28.Kates O.S., Haydel B.M., Florman S.S., et al. COVID-19 in solid organ transplant: a multi-centre cohort study. Clin Infect Dis. 2021;73:e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullan C.W., Komlo C., Clark K.A.A., et al. Survival after heart transplantation from SARS-CoV-2 positive donors. JACC Heart Fail. 2022;10:874–876. doi: 10.1016/j.jchf.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sofka S., Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genuardi M.V., Moss N., Najjar S.S., et al. Coronavirus disease 2019 in heart transplant recipients: risk factors, immunosuppression, and outcomes. J Heart Lung Transplant. 2021;40:926–935. doi: 10.1016/j.healun.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz-Arocutipa C., Carvallo-Castañeda D., Luis-Ybañez O., et al. COVID-19 in heart transplant recipients during February-August 2020: a systematic review. Clin Transplant. 2021;35:e14390. doi: 10.1111/ctr.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raja M.A., Mendoza M.A., Villavicencio A., et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev. 2021;35:100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radcliffe C., Palacios C.F., Azar M.M., Cohen E., Malinis M. Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge. Am J Transplant. 2022;22:2458–2463. doi: 10.1111/ajt.17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saharia K.K., Anjan S., Streit J., et al. Clinical characteristics of COVID-19 in solid organ transplant recipients following COVID-19 vaccination: a multicentre case series. Transpl Infect Dis. 2022;24:e13774. doi: 10.1111/tid.13774. [DOI] [PubMed] [Google Scholar]
- 36.Ulloa A.C., Buchan S.A., Daneman N., Brown K.A. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286–1288. doi: 10.1001/jama.2022.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cochran W., Shah P., Barker L., et al. COVID-19 clinical outcomes in solid organ transplant recipients during the omicron surge. Transplantation. 2022;106:e346. doi: 10.1097/TP.0000000000004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.RECOVERY Collaborative Group. Dexamethasone in hospitalised patients with COVID-19—preliminary report. Available at: https://www.nejm.org/doi/suppl/10.1056/NEJMoa2021436/suppl_file/nejmoa2021436_preliminary.pdf.
- 39.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 40.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with Covid-19 (RECOVERY): results of a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariette X., Hermine O., Tharaux P.L., et al. Effectiveness of tocilizumab in patients hospitalized with Covid-19: a follow-up of the CORIMUNO-TOCI-1 randomised clinical trial. JAMA Intern Med. 2021;181:1241–1243. doi: 10.1001/jamainternmed.2021.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institutes of Health Therapeutic management of hospitalized adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults--therapeutic-management/ Available at:
- 44.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris A.M., Juni P., Odutayo A., et al. Remdesivir for hospitalized patients with COVID-19. May 6, 2021. https://covid19-sciencetable.ca/sciencebrief/remdesivir-for-hospitalized-patients-with-covid-19/ Available at:
- 46.Morris A.M., Stall N.M., Bobos P., et al. Tocilizumab for hospitalised patients with COVID-19. March 1, 2021. https://covid19-sciencetable.ca/sciencebrief/tocilizumab-for-hospitalized-patients-with-covid-19/ Available at:
- 47.Hempel A.K., Jevtic S.D., Vandersluis S., et al. Baricitinib for hospitalised patients with COVID-19. January 8, 2022. https://covid19-sciencetable.ca/sciencebrief/baricitinib-for-hospitalized-patients-with-covid-19/ Available at:
- 48.Avery R.K. Update on COVID-19 therapeutics for solid organ transplant recipients, including the omicron surge. Transplantation. 2022;106:1528–1537. doi: 10.1097/TP.0000000000004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canadian Society of Transplantation CST statement on the use of monoclonal antibodies against SARS-CoV-2 for prophylaxis and update on early outpatient therapy for COVID-19 in SOT recipients. January 12, 2022. https://www.cst-transplant.ca/_Library/Coronavirus/CST_statement_on_the_use_of_Monoclonal_Antibodies_against_COVID-19_v_1_2022.pdf Available at:
- 50.Dhand A., Okumura K., Wolfe K., et al. Sotrovimab for treatment of Covid-19 in solid organ transplant recipients. Transplantation. 2022;106:e336–e337. doi: 10.1097/TP.0000000000004136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinchera B., Buonomo A.R., Scotto R., et al. Sotrovimab in solid organ transplant patients with early, mild/moderate SARS-CoV-2 infection: a single-centre experience. Transplantation. 2022;106:e343–e345. doi: 10.1097/TP.0000000000004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yetmar Z.A., Beam E., O’Horo J.C., et al. Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the omicron epoch. Transpl Infect Dis. 2022;24:e13901. doi: 10.1111/tid.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Food and Drug Administration. FDA updates Sotrovimab emergency use authorization. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization April 5, 2022. Available at:
- 54.British Columbia COVID-19 Therapeutics Committee British Columbia Centre for Disease Control. Antimicrobial and immunomodulatory therapy in adult patients with COVID-19. http://www.bccdc.ca/health-professionals/clinical-resources/covid-19-care/treatments Available at:
- 55.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalised adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takashita E., Yamayoshi S., Fukushi S., et al. Efficacy of antiviral agents against the omicron subvariant BA.2.75. N Engl J Med. 2022;387:1236–1238. doi: 10.1056/NEJMc2209952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Najjar-Debbiny R., Gronich N., Weber G., et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76:e342–e349. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fishbane S., Hirsch J.S., Nair V. Special considerations for paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am J Kidney Dis. 2022;79:480–482. doi: 10.1053/j.ajkd.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stawiarski K., Avery R., Pharmd S.S., Umapathi P., Md R.A. Risks of paxlovid in a heart transplant recipient. J Heart Lung Transplant. 2023;42:30–32. doi: 10.1016/j.healun.2022.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salerno D.M., Jennings D.L., Lange N.W., et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of Covid-19 in solid organ transplant recipients. Am J Transplant. 2022;22:2083–2088. doi: 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.American Society of Transplantation AST statement on oral antiviral therapy for COVID-19 for organ transplant recipients. https://www.myast.org/sites/default/files/AST%20Statement%20on%20Oral%20Antiviral%20Therapy%20for%20COVID%20Jan%204%20%282%29.pdf Available at:
- 62.Gottlieb R.L., Vaca C.E., Paredes R., et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solera J.T., Árbol B.G., Bahinskaya I., et al. Short-course early outpatient remdesivir prevents severe disease due to Covid-19 in Organ transplant recipients during the omicron BA.2 wave. Am J Transplant. 2023;23:78–83. doi: 10.1111/ajt.17199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Embi P.J., Levy M.E., Naleway A.L., et al. Effectiveness of 2-dose vaccination with mRNA Covid-19 vaccines against Covid-19–associated hospitalisations among immunocompromised adults—nine states. MMWR Morb Mortal Wkly Rep. 2021;70:1553–1559. doi: 10.15585/mmwr.mm7044e3. January-September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall V.G., Ferreira V.H., Ku T., et al. Randomised trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peled Y., Afek A., Kreiss Y., et al. Kinetics of cellular and humoral responses to third BNT162B2 COVID-19 vaccine over six months in heart transplant recipients - implications for the omicron variant. J Heart Lung Transplant. 2022;41:1417–1425. doi: 10.1016/j.healun.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solera J.T., Árbol B.G., Alshahrani A., et al. Impact of vaccination and early monoclonal antibody therapy on Covid-19 outcomes in organ transplant recipients during the omicron wave. Clin Infect Dis. 2022;75:2193–2200. doi: 10.1093/cid/ciac324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bae S., Alejo J.L., Chiang T.P.Y., et al. mTOR inhibitors, mycophenolates, and other immunosuppression regimens on antibody response to SARS-CoV-2 mRNA vaccines in solid organ transplant recipients. Am J Transplant. 2022;22:3137–3142. doi: 10.1111/ajt.17158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCune T.R., Bray R.A., Baran D.A., et al. Development of donor-specific antibodies after SARS-CoV-2 vaccination in kidney and heart transplant recipients. Transpl Immunol. 2022;75:101722. doi: 10.1016/j.trim.2022.101722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alhumaid S., Rabaan A.A., Dhama K., et al. Solid organ rejection following SARS-CoV-2 vaccination or COVID-19 infection: a systematic review and meta-analysis. Vaccines. 2022;10:1289. doi: 10.3390/vaccines10081289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayr F.B., Talisa V.B., Shaikh O., Yende S., Butt A.A. Effectiveness of homologous or heterologous COVID-19 boosters in veterans. N Engl J Med. 2022;386:1375–1377. doi: 10.1056/NEJMc2200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iketani S., Liu L., Guo Y., et al. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nature. 2022;604(7906):553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levin M.J., Ustianowski A., de Wit S., et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen Y., Flahault A., Chavarot N., et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022;28:1654.e1-4. doi: 10.1016/j.cmi.2022.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al Jurdi A., Morena L., Cote M., et al. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant. 2022;22:3130–3136. doi: 10.1111/ajt.17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karaba AH, Kim JD, Chiang TP-Y, et al. Omicron BA.1 and BA.2 neutralizing activity following pre-exposure prophylaxis with tixagevimab plus cilgavimab in vaccinated solid organ transplant recipients. May 26, 2022. medRxiv 2022.05.24.22275467.
- 77.Aslam S., Ison M.G. SARS-CoV-2 vaccination in heart transplantation: what we do and do not know. J Heart Lung Transplant. 2022;41:158–160. doi: 10.1016/j.healun.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peled Y., Ram E., Lavee J., et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2022;41:148–157. doi: 10.1016/j.healun.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canadian Cardia Transplant Network Cardiac transplantation: eligibility and listing criteria in Canada. 2012. https://ccs.ca/app/uploads/2020/12/CCTN_Cardiac_Transplantation_Eligibility_and_Listing_Criteria_in_Canada_2012.pdf Available at:
- 80.Task Force for the Management of Covid-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the Covid-19 pandemic: part 2—care pathways, treatment, and follow-up. Eur Heart J. 2022;43:1059–1103. doi: 10.1093/eurheartj/ehab697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roifman I., Arora R., Bewick D., et al. Cardiovascular care delivery during the second wave of Covid-19 in Canada. Can J Cardiol. 2021;37:790–793. doi: 10.1016/j.cjca.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorodeski E.Z., Goyal P., Cox Z.L., et al. Virtual visits for care of patients with heart failure in the era of Covid-19: a statement from the Heart Failure Society of America. J Card Fail. 2020;26:448–456. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.International Society of Heart and Lung Transplantation Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf Available at:
- 84.American Society of Transplantation Covid-19: FAQs for organ transplantation. August 19, 2022. https://www.myast.org/faqs-organ-transplantation Available at:
- 85.Bader F., Manla Y., Atallah B., Starling R.C. Heart failure and Covid-19. Heart Fail Rev. 2021;26:1–10. doi: 10.1007/s10741-020-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fersia O., Bryant S., Nicholson R., et al. The impact of the Covid-19 pandemic on cardiology services. Open Heart. 2020;7:e001359. doi: 10.1136/openhrt-2020-001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chih S., McDonald M., Dipchand A., et al. Canadian Cardiovascular Society/Canadian Cardiac Transplant Network position statement on heart transplantation: patient eligibility, selection, and post-transplantation care. Can J Cardiol. 2020;36:335–356. doi: 10.1016/j.cjca.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 88.Amadio J.M., Rodenas-Alesina E., Superina S., et al. Sparing the prod: providing an alternative to endomyocardial biopsies with noninvasive surveillance after heart transplantation during Covid-19. CJC Open. 2022;4:479–487. doi: 10.1016/j.cjco.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhuo D.X., Ginder K., Hardin E.A. Markers of immune function in heart transplantation: implications for immunosuppression and screening for rejection. Curr Heart Fail Rep. 2021;18:33–40. doi: 10.1007/s11897-020-00499-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.