ABSTRACT
The Corpus callosum (CC) is the largest commissural fibre tract, ensuring swift information transfer and integration in both cerebral hemispheres. Variations in morphometry exist. There is a paucity of data on CC dimensions in our subregion, and no standardized reference is available. The study aims to determine the CC dimensions among the adult population in southeast Nigeria. The result will provide reference ranges and form a benchmark for comparisons of CC-related pathologies. A retrospective study of CC morphometric dimensions in normal subjects who had cranial MRI over two years in Memfys Hospital, Enugu, Southeast Nigeria, using a 1.5T GE© 16 channel machine. The CC was segmentalized into seven subregions using the modified Witelson method with special computer software. All measurements were taken twice from the T1 mid-sagittal image, and the mean was used for computation. The results were analyzed using descriptive and inferential statistics. A total of 200 subjects were recruited for the study. The mean length and height of the CC were 75.58 ± 4.52 mm and 24.64 ± 3.40 mm, respectively. The width dimensions of the genu, body, rostrum and splenium were 10.88 ± 1.81 mm, 5.66 ± 1.32 mm, 3.65 ± 1.25 mm, and 10.02 ± 1.70 mm, respectively. No gender variations were noted among the different dimensions of CC (P = 0.90). The length and height of CC increase gradually with age and show a positive correlation. The width dimensions of the genu and splenium increase till middle age and subsequently decreases in line with brain atrophy (p = 0.0000& p = 0.004). Using Pearson’s correlation test, no correlation was noted in the dimensions of the body and rostrum of the corpus callosum when related to age and sex. (P = 0.92 & p = 0.66). Reference ranges of CC dimensions in our subregion were presented, and variations exist in its different morphometric dimensions which are affected by brain atrophy. Gender does not influence the dimensions in our subpopulations.
KEYWORDS: Corpus callosum, morphometric dimensions, subregion, reference ranges, Southeast Nigeria
1. Introduction
The Corpus callosum (CC) is the largest commissural fibre in the human brain that connects both cerebral hemispheres and ensures swift information transfer and activity integration across the midline, enabling humans to perform complex activities. [1–3] It was traditionally divided into the rostrum, genu, body, and splenium. Each of these parts has somatotropic connections with different parts of the cerebral hemispheres. Studies have shown variations in the dimensions with age, sex, racial origin, and various pathologies [3–5].
Witelson, in her study [2], noted that the CC is larger in the left-handed and ambidextrous, which is related to less cortical specialization and the need for information transfer across the midline. Others have reported a larger splenial dimension in women when compared to men [6,7]. They attributed this to superior sound and colour perception of women, which is controlled by the temporal and occipital lobes, respectively, and integrated across the midline by the splenial fibres; however, other researchers doubt the existence of this variation [8,9]. It is worth noting that the dimensions of CC is expected to reduce with aging in parallel with brain atrophy however, other authors could not demonstrate this finding [4,10].
Studies on information integration across the CC as it relates to the anatomical dimensions and diseases have interested neuroscientists and radiologists. A recent meta-analysis has shown reduced dimensions of CC in schizophrenia and bipolar disorders. [11]. They related this to the reduced white matter volume of the CC leading to failure of information integration across both hemispheres. Similarly, Khasawneh et al. [12], in a recent study, revealed a significant decrease in the size of CC in Alzheimer’s patients compared to control patients. Furthermore, Frazier et al [13], demonstrated that the CC dimension was least in the truncal area in patients with autism. This area connects both hemispheres’ premotor and supplementary motor areas and supports the aberrant connection hypothesis as the origin of motor dysfunction in autistic patients.
Earlier studies on CC dimensions were carried out on autopsy specimens; however, post-mortem changes that occur due to shrinkage and fixation agents affect the measured dimensions [2,7]. Use of high-resolution MRI, which gives excellent soft tissue differentiation and good spatial representation, has been the gold standard in morphometric studies of the CC [1,3,4,10,14]. There is no risk of exposure to ionizing radiation, and contrast agents are not required in CC studies, making it more acceptable to subjects. Though several MRI-based CC studies were done in developed countries, there is still a paucity of studies in sub-Saharan Africa, and to the best of the authors’ knowledge, none have been carried out among the southeastern population in Nigeria.
The study aims to determine the CC dimensions in the sub-region and the sex and age-related variations in the study population. Comparisons between various CC morphometric dimensions will be made as it relates to other sub-population. This study will form the basis for possible comparison of CC dimensions in various pathologies and guide the Neuroradiologist in the sub-region in making a judgment about CC-related disorders.
2. Materials and methods
2.1. Patient selection
The retrospective study was carried out from January 2019 to January 2021 in Memfys hospital. Memfys is a major neurology and neurosurgical centre in the region and serves as a referral centre for patients requiring neuroimaging studies in Enugu, Nigeria. The subjects were pooled from individuals who routinely came to Memfys hospital for brain MRIs. The images were studied by two independent Neuroradiologists and found to be normal. Subjects with pathological findings were excluded, including but not limited to tumours, infections, trauma, hydrocephalus, demyelinating lesions, and congenital anomalies. Approval was obtained from the institutional ethics committee.
2.2. Imaging protocol
All examinations were done with a 1.5T GE healthcare 16-channel MRI scanner. Studies were performed using the standard head coil in the FSE T1WI, T2WI, and FLAIR in axial, coronal, and sagittal images. All measurements were taken from the T1WI sagittal image, and the set parameters were TR-744, TE-2.47, thickness 1.60 mm, and NEX 1.0. All images were performed by qualified radiographers who utilized the same protocol, and the images were stored in K-PACS workstation 1.5 archive and image retrieval systems.
2.3. Measurements
All measurements of the CC dimensions were taken in the mid-sagittal plane determined by the midpoints of anterior and posterior commissure, and also, the interhemispheric fissure with only traces of cortex visible on either side as described by the work of Allouh et al. and Pettey and Gee [8,15]. (Figure 1). The fronto-occipital diameter in the midsagittal plane was taken at the midline to determine the forebrain length (LM). The anterior-posterior diameter of the CC was taken from the most anterior to the most posterior point of the CC in the mid-sagittal plane (AB). In addition, the height was measured from the most superior to the most inferior point of the CC (Figure 2 & 3).
Figure 1.
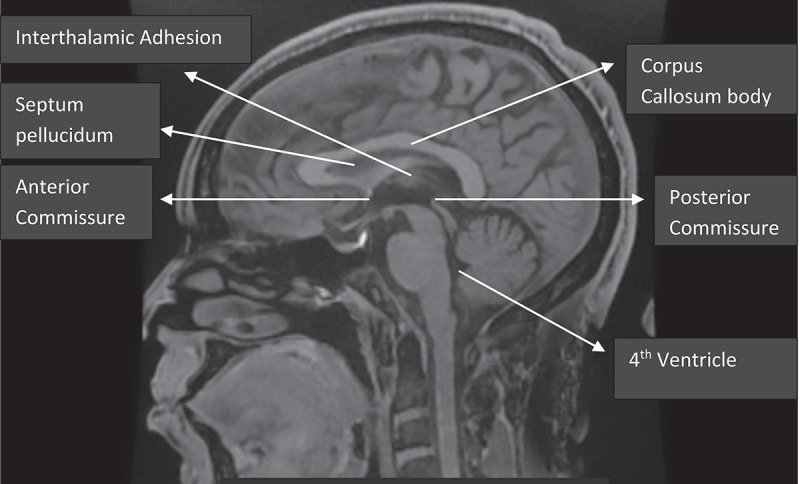
Mid-sagittal brain MRI showing the corpus callosum.
Figure 2.
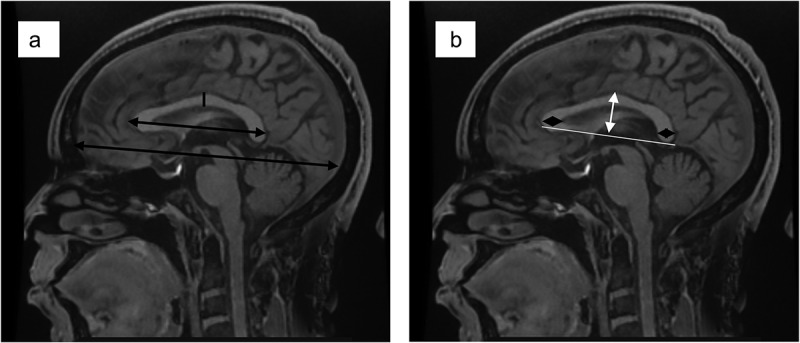
(a) the black double-ended arrows show the measurement of the anterior-posterior dimension of the corpus callosum and the fronto-occipital dimension of the brain respectively. (b). The white double-ended arrow shows the measurement of the height of the corpus callosum, while the black diamond-shaped arrows show the measurement of the thickness of the genu and splenium, respectively.
Figure 3.
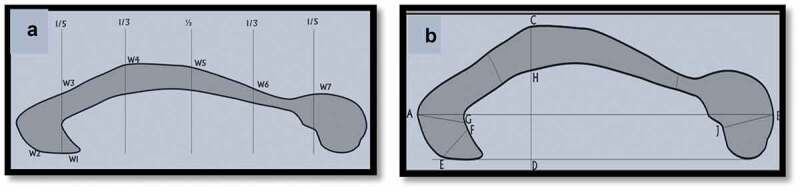
Artistic representation of the corpus callosum showing the various parts.
Furthermore, the CC was segmentalized using the special computer measurement software into seven subregions guided by the method proposed by Witleson [2] and with some modifications as used in many other morphometric corpus callosa studies [1,3]. Five perpendicular lines were placed across the maximum length of the CC at 1/5th, 1/3rd 1/2 distance from the outer limits of both the genu and splenium. This divides the CC into seven subregions: rostrum, genu, anterior body, mid-body, posterior body, isthmus, and splenium. (Figure 3a) The maximum diameter (width) of the rostrum (EF), genu (AG), midbody (CH), and splenium
(JB) were measured using the same software (Figure 3b). The mean of two measurements of each dimension was taken to ensure the accuracy and reliability of the values obtained.
Figure 3a shows segments of the corpus callosum using the modified Witelson’s method [1–3]. Five perpendicular lines were drawn from the maximum length of the CC at 1/5, 1/3, and ½ distance from the outer limit of the genu and splenium. W1-W7 represents the rostrum, genu, anterior body, midbody, posterior body, isthmus, and splenium respectively. Figure 3b shows the dimensions measured in the study – AB-length, CD-height, EF-rostrum, AG-genu, CH-midbody, and JB-splenium.
2.4. Statistical methods
Data analysis was done using SPSS version 22. A one-way ANOVA test was used to determine the significance of the difference in the mean values of the measured CC dimensions among different age groups. Pearson’s correlation was used to determine the relationship between age and corpus callosum dimensions. We also utilized the independent t-test comparing the mean values of the measured dimension of the CC and their variations with the sex of the subjects
3. Results
About 5520 images were analyzed over the study period, and 200 met the inclusion criteria and were included in the study. The mean age of the patients was 43.57 ± 19.02 years, and a male preponderance with a ratio of 1.25 :1.
The mean fronto-occipital diameter of the brain in the midsagittal plane was 161.40 ± 6.0 mm for males and 157.34 ± 6.1 mm for females and was not statistically significant between the two groups (p = 0.0691).
The Mean anteroposterior length of the corpus callosum was 75.58 ± 4.52 mm, and the height was 24.63 ± 3.40 mm. The mean diameters of the genu, body, rostrum and splenium were 10.88 ± 1.80 mm, 5.63 ± 1.32 mm, 3.65 ± 1.25 mm, and 10.92 ± 1.70 mm, respectively.
Table 1 shows an analysis of variance for comparing the means of corpus callosum dimensions across different age groups.
Table 1.
Analysis of variance for comparing the means of the dimensions of the corpus callosum across different age groups.
| Age in Years |
||||||||
|---|---|---|---|---|---|---|---|---|
| Dimensions | 18–27 (n=32) | 28–37 (n=33) | 38–47 (n=46) | 48–57 (n=27) | 58–67 (n=31) | >67 (n=31) | F | P |
| LENGTH(AB) | 74.12±4.86 | 74.78±3.89 | 75.25±5.19 | 75.17±3.65 | 76.77±4.99 | 77.39+4.56 | 2.288 | 0.048* |
| GENU(AG) | 10.71±1.87 | 11.45±1.49b | 11.59±1.73 | 11.45±1.94 | 10.25±1.98 | 9.84+1.84 | 5.454 | 0.000* |
| BODY(CH) | 5.59+1.37 | 6.10+1.24 | 5.76+1.22 | 5.67+1.09 | 5.31+1.49 | 5.39+1.52 | 1.486 | 0.196 |
| ROSTRUM(EF) | 3.58±1.22 | 4.03±1.13 | 3.81±1.35 | 3.48±0.96 | 3.41±1.22 | 3.61±1.62 | 1.074 | 0.376 |
| HEIGHT(CD) | 23.55±2.64 | 25.03±4.26 | 23.12±3.80 | 24.07±2.30 | 25.24+3.87 | 26.82+3.51 | 5.050 | 0.000* |
| SPLENIUM(JB) | 11.22+1.70 | 11.01+1.51 | 11.38+1.83 | 11.42+1.62 | 9.94+1.56 | 10.56+1.98 | 3.609 | 0.004* |
3.1. Variations of the dimensions of the corpus callosum across different age groups
The length and height of the CC increase significantly with age (p = 0.048 & p = 0.000 respectively). In contrast, no statistical significance was noted in the dimensions of the body and rostrum across different age groups. Both the genu and splenial diameters increase to middle age and subsequently declines(Table 1)
Using Pearson’s correlation analysis to determine the relationship between age and dimensions of the CC, demonstrates a statistically signifcant but weak correlation between lenght and height of CC in relation to age. On the contrary, the genu, body, and splenial dimensions were weakly negatively correlated and also shows statistical significance. (Table 2).
Table 2.
Pearson correlation test of the relationship between the actual ages and corpus callosum dimensions.
| Dimensions | Frequency | Pearson correlation | P-value |
|---|---|---|---|
| Length (AB) | 200 | 0.229** | 0.001 |
| GENU(AG) | 200 | −0.236** | 0.001 |
| BODY (CH) | 200 | −0.149* | 0.036 |
| ROSTRUM(EF) | 200 | −0.106 | 0.134 |
| HEIGHT(CD) | 2002 | 0.250** | 0.000 |
| SPLENIUM(JB) | 200 | −0.212** | 0.003 |
** Correlation is significant at the 0.01 level (2-tailed). * Correlation is significant at the 0.05 level (2-tailed).
3.2. Variation of dimensions of the corpus callosum with gender
The mean dimensions of the length, height, rostrum, and splenium were marginally larger in males but did not reach statistical significance. Similarly, the genu and body of the CC were larger in females, but both measurements were not statisticaly significant. (Table 3)
Table 3.
Comparing the means of the dimensions of the corpus callosum across different sex groups.
| Dimensions | SEX | N | Mean | t-value | P-value |
|---|---|---|---|---|---|
| LENGTH(AB) | MALE | 111 | 75.94+4.96 | 1.311 | 0.191 |
| FEMALE | 89 | 75.06+4.30 | |||
| GENU(AG) | MALE | 111 | 10.88+1.92 | −0.410 | 0.682 |
| FEMALE | 89 | 10.99+1.89 | |||
| BODY(CH) | MALE | 111 | 5.64+1.39 | −0.091 | 0.927 |
| FEMALE | 89 | 5.66+1.26 | |||
| ROSTRUM(EF) | MALE | 111 | 3.71+1.34 | 0.439 | 0.661 |
| FEMALE | 89 | 3.63+1.21 | |||
| HEIGHT(CD) | MALE | 111 | 24.77+4.03 | 0.977 | 0.330 |
| FEMALE | 89 | 24.25+3.23 | |||
| SPLENIUM(JB) | MALE | 111 | 11.01+1.74 | 0.554 | 0.580 |
| FEMALE | 89 | 10.87+1.83 |
Table 4 Compares the Corpus callosum dimension of southeastern Nigerians and other populations
Table 4.
Shows the comparison of the corpus callosum dimension across the different populations.
| Studies/Country | Number of respondents | Mean/median age (years) | Forebrain Length (mm) |
CC length (mm) | CC height (mm) | Genu width (mm) |
Body width (mm) | Splenium width (mm) |
|---|---|---|---|---|---|---|---|---|
| Index study 2021 (Nigeria) | 200 | 43.57±19.02 | 159.37±6.1 | 75.94±4.9 | 24.77±4.0 | 10.88±1.9 | 5.64±1.4 | 11.01±1.7 |
| Allouh et al [8] 2020 (Jordan) | 100 | ¶ | 157.4 ±6.55 | 68.45±4.1 | — | 10.85±1.4 | 6.15±0.8 | 16.65±2.4 |
| Arda et al10
2019 (Turkey) |
436 | 47.05±19.8 | – | 68.0±4.9 | 24.7±3.1 | 10.7±1.8 | 5.81±1.1 | 13.6±0.9 |
| Takeda et al [16] 2003 (Japan) |
205 | 59.25±19.2 | – | 69.7±4.2 | 25.8±2.8 | —- | 5.58±1. | 9.94±1.6 |
| Junle et al [4] 2008 (China) |
286 | 40–49¥ | – | 70.74±4.4 | 24.59±2.7 | 11.68±1.4 | 6.33±0.9 | 11.53±1.3 |
| Krishna et al [14] 2020(India) | 420 | 31–40¥ | — | 69.59±5.59 | —- | 10.47±1.76 | 5.36±0.98 | 10.03±1.58 |
¶- young adults(20–45 years) ¥- median age group
4. Discussion
The corpus callosum (CC) is the largest inter-hemispheric white matter tract in the human brain and is characterized by variations in morphology among individuals of different gender and race [8,10,14,16,17]. This MRI-based morphometric study of the CC was carried out in southeastern Nigeria. We analyzed these variations and provided normative anatomical reference data to assist in future diagnostic and disease-related investigations and possibly may be useful in forensic medicine. The mean length of the CC as found in our study (75.94 mm), while similar to that found in the Western population (73.70 mm) by Prendergast et al [1], was above the mean length found among Middle Eastern Arabs (68.45 mm) [8], Japanese (69.70 mm) [16], Indian (69.59 mm) [14] and Chinese (70.74 mm) [4]. In contrast, the height of the CC was similar between the index study and other studies in Asia and the Middle east [4,10,16].
We further observed that the length and height of the CC in our population increase significantly with age and shows positive correlation. Takeda et al. [16] noted a similar increase in corpus callosum length and maximum height with age. They postulated that age-related expansion of the lateral ventricle resulted in arching and elongation of the CC, and this was related with the increased Evans index in the older population [16,18]. Other studies in the middle east, Europe, and Asia also showed a similar increase in length and height of CC with ageing [8,14,17].
Allouh and colleagues [8] demonstrated racial differences in the length and thickness of the CC between the Arabs and the Turkish people. Housseni et al [17] similarly highlighted that even among the same Arabs in northern Iran, variations exist between different ethnic groups. They noted that in all dimensions measured, the Turkmen tribe had larger sizes of the CC segments than the Fars tribe, with the length and splenial dimension showing statistical significance. This similar inter-ethnic variation can be demonstrated in Nigeria since this study was carried out in southeastern Nigeria, predominately occupied by the Igbo ethnic group. Conducting similar studies among other ethnic groups within the country may reveal similar variations, help evaluate patients with different pathologies, and provide valuable data for comparison with other regions worldwide.
It is general knowledge that males have a larger average body size than women, which correlates with brain size, callosal area, and forebrain diameter, as noted in the literature [3,5,19]. In this study, the forebrain size assessed by the mean fronto-occipital diameter was greater in males than females (161.40 vs 157.34 mm respectively) as expected; however, no statistically significant difference was found between both. In the review of 43 studies in a meta analysis by Driesen and Raz [5] comparing the area of the CC in men and women, they found that the absolute CC and splenium area were larger in males however, when they adjusted the CC area to brain size, they noted that the female brains were larger.
Furthermore, we observed variations in the dimensions of the various components of the CC as Age increases. The diameter of the genu and splenium increased to middle age and subsequently declined, which was statistically significant. This is unlike what we noted in the width of the rostrum and body of the CC, which did not differ significantly with age. The gradual reduction in the width of the splenium and genu with age may be related to the overall brain atrophy, which may be more marked in the genu and splenial area that contains the major bulk of CC fibres connecting the frontal and occipital lobes, respectively. Fling et al. [20], in their study, associated age-related decline of the genu with poorer working memory and psychomotor performance in subjects greater than 65 years. Similar variation in the dimension of the CC was reported by Krishna et al. [14]. In their study, they noted that the mean thickness of the genu, isthmus and splenium peaked in young adults and subsequently declined.
In contrast to other reports in literature [4,5,14,16,21], Arda and his colleague [10] reported a statistically significant increase in splenial length with increasing age and demonstrated a positive correlation (correlation coefficient of 0.11). However, no statistically significant relationship was found when they assessed the splenial index (callosal height/splenial length), which they believed was a more reliable marker. In addition, in that same study, the genu and all portions of the CC body decreased with age in consonant with other literature findings [4,16,21].
The sexual dimorphism of the CC has been a thing of debate in the literature [1,7,8,10,21,22]. De Lacoste-Utamsing and Holloway [7] pointed out that the splenium is the major site of sex difference in the CC as they described the splenial length longer and ‘more bulbous’ in females. They speculated that the possible peculiar cognitive and visuospatial ability in females might be related to their larger splenium with more neuronal connection; however, this theory is entirely out of favour in modern literature and was difficult to reproduce in other studies [5,21]. In this index study, the splenium was larger in males than in females, and there were no significant differences between them. Similarly, we found no statistically significant difference between the mean dimensions of the other parts of the CC related to gender, although the length, height, and rostrum were larger in males while the genu and body were larger in females.
Autism, a neurodevelopmental condition characterized by communication and social interaction challenges, repetitive behaviours, and abnormal responses to sensory stimuli, has been attributed to cortical underconnectivity and reduced corpus callosum size [13]. Prigge et al. [23] found that autistic patients have smaller total areas of the CC, genu, splenium, and isthmus, which persist into adulthood, and most importantly, the isthmus which connects the superior temporal and parietal regions of the two hemispheres lack the expected increase from childhood to adolescence. They also related the smaller callosal area to greater social impairment, lower intelligence quotient, and slower processing speed. Similarly, a lower total area of the CC and the dimensions of the anterior genu and body, isthmus, and posterior splenium were observed in schizophrenic patients compared to their aged-matched cohorts [24]. In addition, like autistic patients, the age-related increase in the size of the CC seen in normal patients was absent in people with schizophrenia [11,24].
Although this study demonstrated variations in CC dimension as it relates to age and gender, we only measured the width using the standardized method described by Witelson [2]. nonetheless, assessment of the total callosal area and the individual dimensional areas would have given better representation. In addition, we did a simple morphometric analysis and did not relate these dimensions to the disease process; nevertheless, we believe that the findings in this study will form a framework for other studies to assess how these dimensions relate to disease among patients in our sub-region.
5. Conclusion
This study showed variation in the morphometric dimensions of the CC among subjects in relation to age and gender among populations in southeastern Nigeria. The length and height of the CC significantly increase with age while the genu and splenium increases to middle age and subsequently declines. In addition, no relationship was found in the dimensions of rostrum and body in relation to age. Our study did not show significant sexual dimorphism. Furthermore, in comparing our data to other regions of the world, there exist inter-racial and regional variations and this may in CC dimensions. Further studies on this may be useful in understanding various disease processes related to the CC.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Prendergast DM, Ardekani B, Ikuta T, et al. Age and sex effects on corpus callosum morphology across the lifespan. Hum Brain Mapp. 2015. Jul ;36(7):2691–7. DOI: 10.1002/hbm.22800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229(4714). DOI: 10.1126/science.4023705 [DOI] [PubMed] [Google Scholar]
- [3].Mourgela S, Anagnostopoulou S, Sakellaropoulos A, et al. An MRI study of sex- and age-related differences in the dimensions of the corpus callosum and brain. Neuroanatomy. 2007;6:63–65. www.neuroanatomy.org [Google Scholar]
- [4].Junle Y, Youmin G, Yanjun G, et al. A MRI quantitative study of corpus callosum in normal adults. J Med Coll PLA. 2008;23(6):346–351. [Google Scholar]
- [5].Driesen NR, Raz A. The influence of sex, age, and handedness on corpus callosum morphology: a meta-analysis. Psychobiology. 1995;23(3):240–247. DOI: 10.3758/BF03332028 [DOI] [Google Scholar]
- [6].Holloway RL, Anderson PJ, Defendini R, et al. Sexual dimorphism of the human corpus callosum from three independent samples: relative size of the corpus callosum. Am J Phys Anthropol. [Internet] 1993. [Cited 2022 Jul 30];92(4):481–498. Available from: https://pubmed.ncbi.nlm.nih.gov/8296877/ [DOI] [PubMed] [Google Scholar]
- [7].De Lacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. [Internet] 1982. [Cited 2022 Jul 30];216(4553):1431–1432. Available from: https://pubmed.ncbi.nlm.nih.gov/7089533/ [DOI] [PubMed] [Google Scholar]
- [8].Allouh MZ, Al Barbarawi MM, Ali HA, et al. Morphometric analysis of the corpus callosum according to age and sex in Middle Eastern Arabs: racial comparisons and clinical correlations to autism spectrum disorder. Front Syst Neurosci. 2020. Jun 23;14:30. DOI: 10.3389/fnsys.2020.00030. PMID: 32655379; PMCID: PMC7324941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci Biobehav Rev. 1997;21(5):581–601. [DOI] [PubMed] [Google Scholar]
- [10].Arda KN, Akay S. The relationship between corpus callosum morphometric measurements and age/gender characteristics: a comprehensive mr imaging study. J Clin Imaging Sci. 2019;9(33):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao G, Lau WKW, Wang C, et al. A comparative multimodal meta-analysis of anisotropy and volume abnormalities in white matter in people suffering from bipolar disorder or schizophrenia. Schizophr Bull. 2022. Jan 21;48(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khasawneh RR, Abu-El-Rub E, Alzubi A, et al. Corpus callosum anatomical changes in Alzheimer patients and the effect of acetylcholinesterase inhibitors on corpus callosum morphometry. PLoS ONE. 2022;17(7 July):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frazier TW, Keshavan MS, Minshew NJ, et al. A two-year longitudinal MRI study of the corpus callosum in autism. J Autism Dev Disord. [Internet] 2012. NovJul [Cited 2022 30];42(11):2312–2322. Available from: https://pubmed.ncbi.nlm.nih.gov/22350341/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krishna VBM, Sekhar KC, Pravallika D, et al. Corpus callosal morphometry in mid-sagittal plane MRI in patients of different age groups: a retrospective study. Int J Anat Radiol Surg. 2022; DOI: 10.7860/IJARS/2022/51289.2780 [DOI] [Google Scholar]
- [15].Pettey DJ, Gee JC. Sexual dimorphism in the corpus callosum: a characterization of local size variations and a classification driven approach to morphometry. Neuroimage. 2002;17(3):1504–1511. [DOI] [PubMed] [Google Scholar]
- [16].Takeda S, Hirashima Y, Ikeda H, et al. Determination of indices of the corpus callosum associated with normal aging in Japanese individuals. Neuroradiology. 2003. Aug 1;45(8):513–518. [DOI] [PubMed] [Google Scholar]
- [17].Hosseini HN, Mohammadi MR, Aarabi M, et al. Ethnicity influences corpus callosum dimensions. Neurol Res Int. 2018;2018:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sullivan EV, Pfefferbaum A, Adalsteinsson E, et al. Differential rates of regional brain change in callosal and ventricular size: a 4-year longitudinal MRI study of elderly men. Cereb Cortex. [Internet] 2002. Apr 1 [Cited 2022 Jul 30];12(4):438–445. Available from: https://europepmc.org/article/MED/11884358. [DOI] [PubMed] [Google Scholar]
- [19].Karakaş P, Koç Z, Koç F, et al. Morphometric MRI evaluation of corpus callosum and ventricles in normal adults. Neurol Res. 2011. Dec;33(10):1044–1049. [DOI] [PubMed] [Google Scholar]
- [20].Fling BW, Chapekis M, Reuter-Lorenz PA, et al. Age differences in callosal contributions to cognitive processes. Neuropsychologia. [Internet] 2011. Jul; 49(9):2564–2569. [Cited 2022 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/21601582/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bishop KM, Wahlsten Bishop D. Sex differences in the human corpus callosum: myth or reality? Available from: http://www.elsevier.com [DOI] [PubMed]
- [22].Guz W, Pazdan D, Stachyra S, et al. Analysis of corpus callosum size depending on age and sex. Folia Morphol. 2019;78(1):24–32. [DOI] [PubMed] [Google Scholar]
- [23].Prigge MBD, Lange N, Bigler ED, et al. Corpus callosum area in children and adults with autism. Res Autism Spectr Disord. 2013. Feb;7(2):221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keshavan MS, Diwadkar VA, Harenski K, et al. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72(6):757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]


