Abstract
An endophytic fungus strain DYSJ3 was isolated from a stem of Aphanamixis grandifolia Blume, which was identified as Aspergillus versicolor based on the morphological characteristics, internal transcribed spacer (ITS) and calmodulin gene sequences analyses. A. versicolor DYSJ3 exhibited strong antagonistic activity against Colletotrichum musae, C. gloeosporioides and Fusarium oxysporum f. sp. cubense with the inhibition rates of 61.9, 51.2 and 55.3% respectively. The antifungal metabolites mainly existed in the mycelium of A. versicolor DYSJ3, and its mycelial crude extract (CE) had broad-spectrum antifungal activities against plant pathogenic fungi. The CE had a good thermal stability, and the inhibition rate of 100 µg/mL CE against C. musae was above 70.0% after disposing at 120 °C for 1 h. Five secondary metabolites were isolated from the CE and identified as averufanin, ergosterol peroxide, versicolorin B, averythrin and sterigmatocystin. Activity evaluation showed versicolorin B exhibited inhibitory effects on the mycelial growth and conidial germination of C. musae, and sterigmatocystin had a weak inhibitory effect on the mycelial growth of C. musae.
Keywords: Aphanamixis grandifolia Blume, endophytic fungus, Aspergillus versicolor, Colletotrichum musae, antifungal metabolite
1. Introduction
Aphanamixis grandifolia Blume is an evergreen tree and mainly distributed across tropical and subtropical regions such as southern China, Malaysia, Indonesia and India [1]. Its leaves and roots have been used as traditional Chinese medicines to relieve limb numbness and joint pain, etc. [2]. Nowadays, studies on A. grandifolia are mainly focused on isolation and biological activities of phytochemicals. Numerous compounds, such as diterpenoids, triterpenoids, sesquiterpenoids, limonoids and phenylpropanoids, have been identified from A. grandifolia, and many of them exhibited antimicrobial and cytotoxic activities [3–5]. However, there is little research in the endophytes of A. grandifolia.
Endophytic fungi are a kind of microorganisms, which colonize within a living plant without causing obvious symptom [6]. It has been proved to produce abundant metabolites with various bioactive activities. Aspergillus versicolor is a common endophytic fungus, and it has been isolated from a variety of terrestrial plants, mangroves and marine algae, etc., such as Elaeocarpus decipiens [7], Anoectochilus roxburghii [8], Excoecaria agallocha [9], Sargassum thunbergii [10] and Hyrtios erectus [11]. A. versicolor is rich in secondary metabolites with antimicrobial, cytotoxic and antioxidative activities.
Colletotrichum musae is the main pathogen of banana anthracnose, which leads to huge losses during transportation and storage every year [12]. In this work, an endophytic fungus Aspergillus versicolor DYSJ3 was isolated from A. grandifolia, which exhibited broad-spectrum antifungal activity. Five compounds were isolated from the mycelium of A. versicolor DYSJ3, and their biological activities against C. musae were evaluated.
2. Materials and methods
2.1. Strains and media
Aspergillus versicolor DYSJ3 was isolated from a stem of Aphanamixis grandifolia, which was sampled in Danzhou of Hainan province, China. The pathogenic fungi used in this study included Colletotrichum musae, C. gloeosporioides, Fusarium oxysporum f. sp. cubense, F. oxysporum f. sp. cucumerinum, Botryosphaeria berengriana f. sp. piricola, F. graminearum Sehw. These fungi were provided by the School of Life and Pharmaceutical Sciences, Hainan University, Haikou, China. All fungal strains were maintained on potato dextrose agar (PDA: 200.0 g/L potato, 20.0 g/L glucose, 20.0 g/L agar) at 28 °C for seven days and kept at 4 °C.
2.2. Strain identification
Strain DYSJ3 was cultured on PDA and Czapek media (30.0 g/L sucrose, 3 g/L NaNO3, 1 g/L K2HPO4, 0.5 g/L MgSO4‧7H2O, 0.5 g/L KCl, 0.01 g/L FeSO4, 20 g/L agar) at 28 °C for two weeks, and the morphology of colony and spore were observed. Strain DYSJ3 was also inoculated on CYA and MEA incubating at 25 °C for seven days to determine its growth rate [13]. The genomic DNA of strain DYSJ3 was extracted using the method as described previously [14]. The partial internal transcribed spacer (ITS) sequence was obtained by PCR with ITS universal primers (ITS1: 5′-TCCGTAGGTGAACCTGCGG-3′, ITS4: 5′-TCCTCCGCTTATTGATATGC-3′) [15]. The partial calmodulin gene (CaM) sequence was amplified using universal primers (CMD5: 5′-CCGAGTACAAGGARGCCTTC-3′, CMD6: 5′-CCGATRGAGGTCATRACGTGG-3′) [16]. The sequence was analyzed in GenBank using Blastn. Multiple sequence alignment was performed by ClustalW, and the phylogenetic tree was constructed by the neighbor-joining method using MEGA 5.0 software.
2.3. Determination of antagonistic activity
Antagonistic activity of strain DYSJ3 against pathogenic fungi was determined with the dual culture method. A mycelial plug (5 mm in diameter) of a pathogenic fungus was placed in the center of a PDA plate, and then strain DYSJ3 was inoculated into one side of the center with a distance of 20 mm. The plate inoculated the fungal mycelial plug only was used as a control. For the antagonistic activity of crude extract (CE) from the mycelia of strain DYSJ3, the CE was dissolved in acetone, and mixed with PDA medium at a final concentration of 500 µg/mL. The mycelial plugs of the pathogenic fungi were placed in the center of the PDA plates containing CE. After growing at 28 °C for seven days, the inhibition rates were calculated as follows:
Where R1 is the radius of fungal colony in control, and R2 is the radius of fungal colony in treatment group.
2.4. Preparation and activity evaluation of crude extract
Strain DYSJ3 was inoculated in potato dextrose broth (PDB) and cultured by orbital shaking at 28 °C, 180 rpm for seven days. Then mycelia pellets were obtained by filtration with gauze. After drying at 40 °C, the mycelia were mixed with 95% ethanol and broken using ultrasonic. The ethanol extract was concentrated by rotary evaporation, and further extracted with petroleum ether, ethyl acetate and n-butanol in turn. The crude extract (CE) was obtained using rotary evaporation. Antifungal activity of the CE against pathogenic fungi was determined by the previous method. As for thermal stability, 100 µg/mL CE was incubated at 50, 60, 70, 80, 90 and 120 °C for 1 h respectively, and their antagonistic activities against C. musae were determined.
2.5. Isolation and identification of compound
The CE was isolated using a silica gel column, eluting with petroleum ether, petroleum ether: ethyl acetate (200:1, 100:1), chloroform, chloroform: methanol (200:1, 100:1, 80:1, 60:1, 40:1, 20:1, 1:1) and methanol in turn. The fractions were examined by thin layer chromatography (TLC). After that, the fractions were further purified using a Sephadex LH-20 column eluting with chloroform and methanol (1:1) to obtain pure compounds. The molecular structure of the compound was determined by mass spectrometry (LCMS-IT-TOF) and NMR-spectra (1H-NMR, 13C-NMR).
2.6. Activity assessment of compound
The effect of compound on the mycelial growth of C. musae was assayed by the filter paper method. C. musae was inoculated on a PDA plate and cultured at 28 °C for four days, and then conidia were harvested by washing the plate with sterile distilled water and filtered by Miracloth. PDA was mixed with the conidia suspension (107 cfu/mL) at a ratio of 9:1 to obtain the tested plate. A filter paper dipped with a compound solution (500 µg/mL compound dissolved in acetone) was placed in the center of the tested plate, and the plate with the filter paper dipped with acetone was used as a control. The inhibition zone was observed after growing at 28 °C for four days. Regarding conidial germination, 20 µL of the conidial suspension (1 × 105 CFU/mL) mixed with a compound (final concentration was 100 µg/mL) was dropped on a glass slide, and kept at 25 °C for 8 h in a moist chamber. The conidial suspension without a compound was used as a negative control, and 100 µg/mL chlorothalonil (CTL) was as a positive agent. The conidia were observed by microscope.
3. Results
3.1. Identification of strain DYSJ3
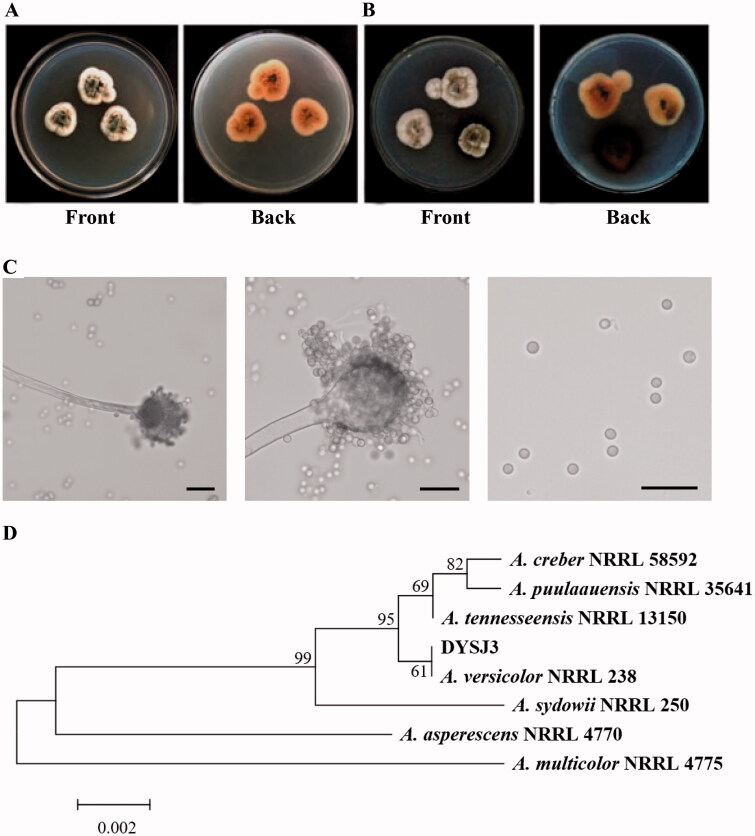
Strain DYSJ3 was isolated from a stem of A. grandifolia using the surface sterilization method. Strain DYSJ3 grows slowly on PDA and Czapek media, and its colony is irregularly round and compact (Figure 1(A, B). The color of colony is white at the beginning, and then the center turns to dark green, light yellow or reddish brown. On the CYA and MEA media, the average colony diameter of strain DYSJ3 attained 24.5 and 16.2 mm respectively at 7 d post-inoculation, which is consistent with the growth rate of A. versicolor type strain described previously [17]. The tip of conidiophore dilates into an apical sac and is nearly spherical. The apical sac bears small peduncles, which are monolayer or double-layer. The spore is colorless sphere and arranged in chain (Figure 1(C)). Then, we amplified the partial sequences of ITS and calmodulin gene, and phylogenetic analyses exhibit that strain DYSJ3 is both assigned to a branch with A. versicolor (Figure 1(D), Figure S1). Based on the above results, strain DYSJ3 is identified as A. versicolor.
Figure 1.
Identification of strain DYSJ3. (A) The colony morphology of strain DYSJ3 on PDA after culturing at 28 °C for five days. (B) The colony morphology of strain DYSJ3 on Czapek medium after culturing at 28 °C for five days. (C) The conidial head and spore morphology of strain DYSJ3. Bar = 10 μm. (D) Phylogenetic tree based on the ITS sequences of Aspergillus spp. and strain DYSJ3. The ITS sequences include A. creber (JQ301889), A. puulaauensis (JQ301893), A. tennesseensis (JQ301895), A. versicolor (EF652442), A. sydowii (EF652450), A. asperescens (EF652475) and A. multicolor (EF652477).
3.2. Antifungal activity of strain DYSJ3 and its crude extract
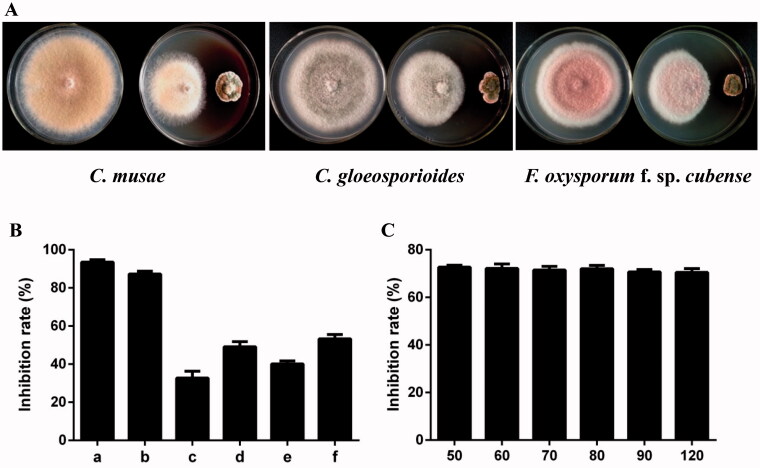
Antagonistic activity of strain DYSJ3 against three pathogenic fungi were determined by the dual culture method. As shown in Figure 2(A), strain DYSJ3 exhibits strong inhibitory effects on C. musae, C. gloeosporioides and F. oxysporum f. sp. cubense, and the inhibition rates are 61.9, 51.2 and 55.3% respectively. The CE from strain DYSJ3 shows broad-spectrum antifungal activity, and it shows higher antagonistic activity against C. musae and B. berengriana f. sp. Piricola than other fungi with the the inhibition rates of 93.5% and 87.3% respectively (Figure 2(B)). In addition, the CE has a good thermal stability, and the inhibition rate of 100 µg/mL CE against C. musae is above 70.0% after disposing at 120 °C for 1 h (Figure 2(C)).
Figure 2.
Antifungal activities of strain DYSJ3 and its crude extract. (A) Antagonistic activity of strain DYSJ3 against Colletotrichum musae, C. gloeosporioides and Fusarium oxysporum f. sp. cubense. (B) Antifungal activity of the CE. The tested pathogenic fungi include C. musae (a), Botryosphaeria berengriana f. sp. piricola (b), F. oxysporum f. sp. cucumerinum (c), C. gloeosporioides (d), F. oxysporum f. sp. cubense (e), F. graminearum Sehw (f). (C) Thermal stability of the CE. 100 μg/mL CE was incubated at 50, 60, 70, 80, 90 and 120 °C for 1 h respectively, and their antagonistic activities against C. musae were determined.
3.3. Isolation and identification of compound from CE
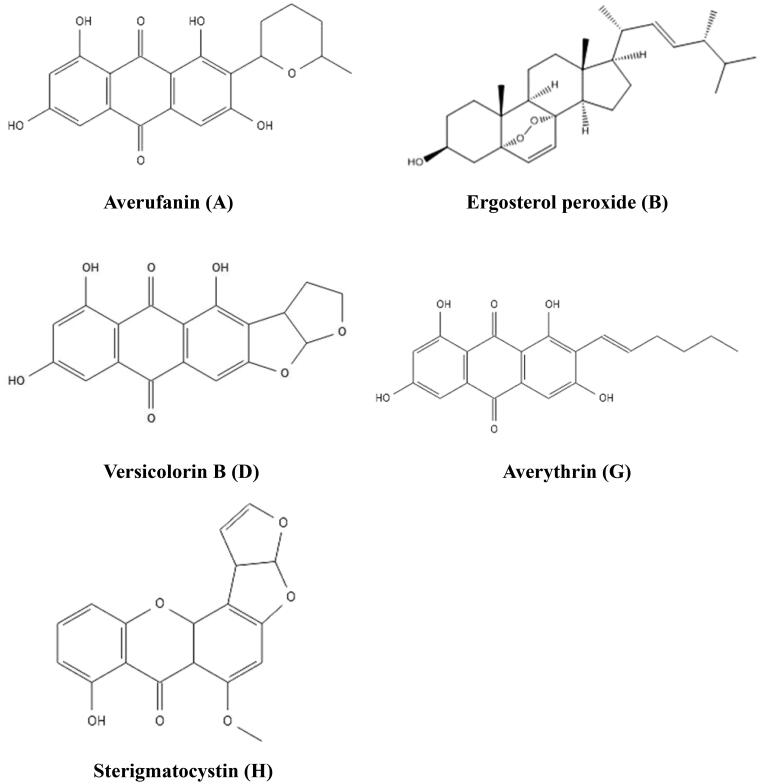
Strain DYSJ3 was inoculated into PDB for a large-scale culture, and 78 L fermentation broth was collected and 53 g CE was obtained. The CE was isolated and purified using a silica gel column and a Sephadex LH-20 column. Finally, five pure fractions were obtained and named as A, B, D, G and H. Based on the results of MS and NMR combined with referring to relevant references, the five compounds were identified as averufanin, ergosterol peroxide, versicolorin B, averythrin and sterigmatocystin respectively. The chemical structures of five compounds are shown in Figure 3.
Figure 3.
The suggested chemical structures of purified compounds.
The spectrum data are listed as follows:
Compound A (averufanin), orange yellow power, C20H18O7, 1H-NMR (500 MHz, CD3OD) δH: 7.24 (1H, d, J = 2.4 Hz, H-5), 7.12 (1H, brs, H-4), 6.63 (1H, d, J = 2.5 Hz, H-7), 5.15 (1H, dd, J = 2.2, 11.5 Hz, H-1′), 3.82 (1H, m, H-5′), 2.15 (2H, dd, J = 10.2, 11.5 Hz, H-2′), 1.77 (2H, m, H-3′), 1.77 (H, m, H-5′), 1.31 (3H, d, J = 6.2 Hz, H-CH3); 13C-NMR (125 MHz, CD3OD) δC: 163.9 (C-1), 120.4 (C-2), 161.5 (C-3), 109.0 (C-4), 134.9 (C-4a), 110.0 (C-5), 166.2 (C-6), 108.4 (C-7), 166.7 (C-8), 110.4 (C-8a), 190.6 (C-9), 109.7 (C-9a), 181.9 (C-10), 136.4 (C-10a), 76.8 (C-1′), 33.5 (C-2′), 23.9 (C-3′), 29.9 (C-4′), 76.7 (C-5′), 22.1 (C-5′-CH3). Averufanin was assigned compared with a literature [18].
Compound B (ergosterol peroxide), colorless crystal, C28H44O3, 1H-NMR (500 MHz, CDCl3) δH: 6.49 (1H, d, J = 8.51 Hz, H-7), 5.21 (1H, dd, J = 7.7, 15.1 Hz, H-23), 5.13 (1H, dd, J = 6.8, 15.3 Hz, H-22), 3.94 (1H, m, H-3), 1.07 (3H, s, H-19), 0.98 (3H, d, J = 6.61 Hz, H-21), 0.89 (3H, m, H-28), 0.87 (3H, s, H-18), 0.81 (3H, d, J = 4.8 Hz, H-26), 0.80 (3H, d, J = 6.8, H-27); 13C-NMR (125 MHz, CDCl3) δC: 34.8 (C-1), 30.2 (C-2), 66.5 (C-3), 37.0 (C-4), 82.3 (C-5), 135.6 (C-6), 130.8 (C-7), 79.6 (C-8), 51.2 (C-9), 37.0 (C-10), 23.5 (C-11), 39.5 (C-12), 44.7 (C-13), 51.8 (C-14), 20.8 (C-15), 28.8 (C-16), 56.3 (C-17), 13.0 (C-18), 18.3 (C-19), 39.9 (C-20), 20.9 (C-21), 135.3 (C-22), 132.4 (C-23), 42.9 (C-24), 33.2 (C-25), 20.1 (C-26), 19.8 (C-27), 17.7 (C-28). Ergosterol peroxide was assigned compared with a literature [19].
Compound D (versicolorin B), orange yellow power, C18H12O6, 1H-NMR (500 MHz, CD3OD) δH: 7.49 (1H, t, J = 7.17 Hz, H-6), 6.82 (1H, d, J = 7.2 Hz, H-5), 6.82 (1H, d, J = 2.6 Hz, H-4′), 6.74 (1H, d, J = 8.27 Hz, H-7), 6.50 (1H, s, H-2), 6.42 (1H, s, H-3′), 5.44 (1H, s, H-2′), 4.79 (1H, d, J = 6.94 Hz, H-1′), 3.98 (3H, s, H-CH3); 13C-NMR (125 MHz, CDCl3) δC: 163.4 (C-1), 56.9 (CH3O-1), 90.6 (C-2), 164.7 (C-3), 106.7 (C-4), 154.1 (C-4a), 106.0 (C-5), 135.8 (C-6), 111.4 (C-7), 109.1 (C-6), 162.4 (C-8), 109.1 (C-8a), 181.5 (C-9),155.1 (C-10a), 113.4 (C-1′), 48.2 (C-2′), 102.64 (C-3′), 145.5 (C-4′). Versicolorin B was assigned compared with a literature [20].
Compound G (averythrin), red powder, C16H16O3, 1H-NMR (500 MHz, CD3OD) δH: 7.35 (1H, s, H-4), 7.21 (1H, d, J = 2.4 Hz, H-5), 6.62 (1H, d, J = 2.4 Hz, H-7), 6.76 (1H, dt, J = 16.1, 1.4 Hz, H-1′), 7.03 (1H, dt, J = 16.1, 7.1 Hz, H-1′), 2.28 (1H, m, H-3′), 1.49 (1H, m, H-4′), 1.41 (1H, m, H-5′), 0.94 (1H, t, J = 7.3 Hz, H-6′); 13C-NMR (125 MHz, CD3OD) δC: 162.7 (C-1), 118.9 (C-2), 163.4 (C-3), 109.9 (C-4), 132.8 (C-4a), 109.3 (C-5), 163.7 (C-6), 109.5 (C-7), 166.1 (C-8), 108.9 (C-8a), 190.9 (C-9), 110.2 (C-9a), 181.8 (C-10), 136.5 (C-10a), 119.9 (C-1′), 139.5 (C-2′), 35.3 (C-3′), 32.4 (C-4′), 23.0 (C-5′), 14.2 (C-6′). Averythrin was assigned compared with a literature [21].
Compound H (sterigmatocystin), canary yellow crystal, C18H12O6, 1H-NMR (500 MHz, CD3OD) δH: 7.49 (1H, t, J = 7.17 Hz, H-6), 6.82 (1H, d, J = 7.2 Hz, H-5), 6.82 (1H, d, J = 2.6 Hz, H-4′), 6.74 (1H, d, J = 8.27 Hz, H-7), 6.50 (1H, s, H-2), 6.42 (1H, s, H-3′), 5.44 (1H, s, H-2′), 4.79 (1H, d, J = 6.94 Hz, H-1′), 3.98 (3H, s,H-CH3); 13C-NMR (125 MHz, CDCl3) δC: 163.4 (C-1), 56.9 (CH3O-1), 90.6 (C-2), 164.7 (C-3), 106.7 (C-4), 154.1 (C-4a), 106.0 (C-5), 135.8 (C-6), 111.4 (C-7), 109.1 (C-6), 162.4 (C-8), 109.1 (C-8a), 181.5 (C-9), 155.1 (C-10a), 113.4 (C-1′), 48.2 (C-2′), 102.64 (C-3′), 145.5 (C-4′). Sterigmatocystin was assigned compared with a literature [22].
3.4. Activity evaluation of compound
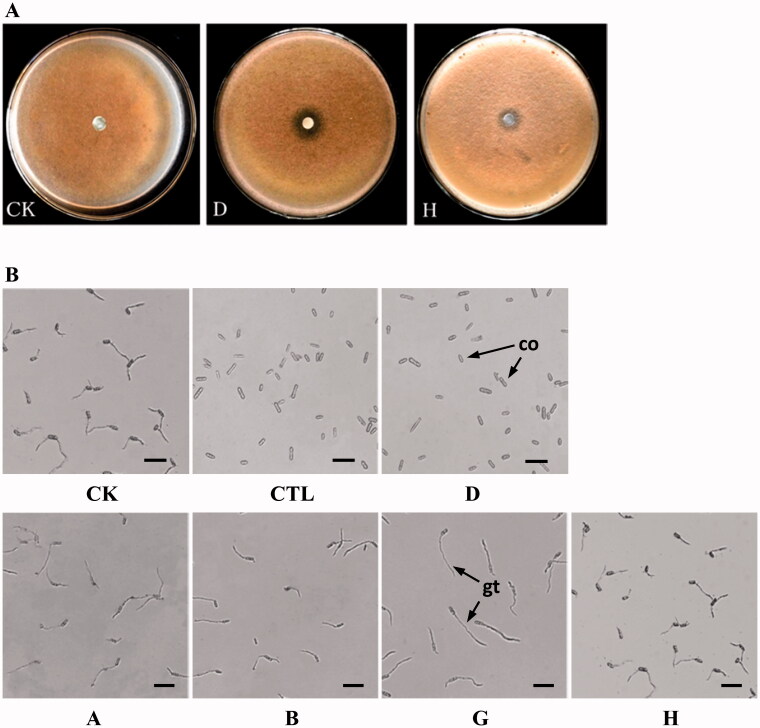
The inhibitory effects of five compounds on the mycelia growth of C. musae were determined by the filter paper method. As shown in Figure 4(A), compound D exhibits the strongest antagonistic activity against the mycelia growth of C. musae, and the diameter of the inhibition zone is 14.8 mm. Compound H has a weak inhibitory effect on mycelia growth, while no effect is found in the other three compounds. As for conidial germination, 100 µg/mL of compound D can completely inhibit the spore germination of C. musae as comparable to CTL (Figure 4(B)). The other four compounds have no obvious effects on the conidial germination of C. musae.
Figure 4.
Activity evaluations of five compounds against Colletotrichum musae. (A) Inhibitory effects of compound D and H on the mycelial growth of C. musae. (B) Inhibitory effects of five compounds on the conidial germination of C. musae. co: conidium, gt: germ tube, bar = 20 μm.
4. Discussion
A. grandifolia is a traditional Chinese medical plant, which is used to cure limb numbness, inconvenient flexion and cold, etc. A. grandifolia is rich in bioactive metabolites, and lots of compounds have been isolated and identified from its stem, fruit and leaf [23]. Endophytic fungi living in medicinal plants are likely to have gene recombination or mutual interference with the host in the process of long-term co-evolution. It may not only synthesize physiologically active substances that are the same or similar to their hosts, but also produce some active compounds with a unique structure and a novel skeleton. However, there is little research related to the endophytic fungi from A. grandifolia. In this study, an endophytic fungus strain DYSJ3 was isolated and identified as A. versicolor based on the morphology, growth characteristics and ITS sequence analysis.
A. versicolor is a common fungus widely distributed in air, soil, plant debris and marine environments [24]. Previous studies showed A. versicolor and its metabolites exhibited various bioactive activities, especially antimicrobial activity. Three compounds isolated from an endophytic fungus A. versicolor of Sargassum thunbergii showed obvious antagonistic activity against Escherichia coli and Staphyloccocus aureus [10]. A. versicolor Im6-50 isolated from a ridge soil of potato field could inhibit potato powdery scab caused by Spongospora subterranea f. sp. subterranea [25]. A. versicolor from a leaf of Elaeocarpus decipiens Hemsl could produce aspergoterpenins A–D, which exhibited antimicrobial activities against Erwinia carotovora sub sp. Carotovora [7]. Diorcinol and 4-methoxycarbonyldiorcinol identified from an endophytic fungus A. versicolor OUCMDZ-2738 of Enteromorpha prolifera were proved to have good inhibitory effects on Pseudomonas aeruginosa [26]. In our work, A. versicolor DYSJ3 was found to have good antagonistic activity against banana anthracnose fungus C. musae for the first time, and it also exhibited broad-spectrum antifungal activity against other plant pathogenic fungi.
The inhibitory effect of the fermentation filtrate from strain DYSJ3 on C. musae was also determined using the Oxford cup method [27], whereas no obvious activity was detected. Therefore, the antifungal metabolites were mainly existed in the mycelium of strain DYSJ3. As for the extraction of CE from the mycelium, we used three organic solvents as extractants, and only the CE from ethyl acetate had antifungal activity against C. musae. Among six pathogenic fungi, the CE exhibited better antagonistic activity against C. musae and B. berengriana f. sp. piricola than the others, and displayed relatively lower activity against F. oxysporum f. sp. cucumerinum and F. oxysporum f. sp. cubense. We speculated that the antifungal metabolites against Fusarium spp. were not easy to be extracted by ethyl acetate. The CE also had a good thermal stability, which could withstand a temperature of 120 °C for 1 h at least.
A. versicolor can produce a variety of bioactive metabolites, such as anthraquinones [28], alkaloids [29], polyketide [30], diphenyl ethers [31], lactones [32], peptides [33] and terpenoids [7]. The metabolites were isolated from the CE of A. versicolor DYSJ3, and five known compounds were identified. Compounds A, D and G were identified as averufanin, versicolorin B and averythrin respectively, which all belong to anthraquinone derivatives. Compounds B and H were identified as ergosterol peroxide and sterigmatocystin respectively. There were several reports related to antimicrobial activities of five compounds. Averufanin was proved to have antibacterial activity against Staphylococcus aureus and Enterococcus faecium with the MIC values of 4.6 and 9.3 µg/mL respectively [34]. It was reported that versicolorin B, averythrin and sterigmatocystin from a rhizospheric Aspergillus sp. YIM PH30001 showed antifungal activity against the root rot fungus Fusarium solani, and the three compounds also had inhibitory activities against Bacillus subtilis [35]. In addition, versicolorin B from a marine-derived fungus A. versicolor MF180151 exhibited moderate antibacterial activity against Staphylococcus aureus and methicillin-resistant S. aureus [36]. In this study, versicolorin B was found to have obvious inhibitory effects on the mycelial growth and conidial germination of C. musae, and sterigmatocystin exhibited slight antagonistic activity against the mycelial growth. The other three compounds showed no antifungal activity toward C. musae. In conclusion, an endophytic fungus A. versicolor DYSJ3 was isolated from A. grandifolia, which exhibited good antagonistic activity against C. musae, C. gloeosporioides and F. oxysporum f. sp. cubense. The antifungal metabolites mainly exist in the mycelium of A. versicolor DYSJ3, and its CE shows broad-spectrum inhibitory activity against plant pathogenic fungi and has a good thermal stability. Five compounds were isolated from the CE, in which versicolorin B exhibited good inhibitory effects on the mycelial growth and conidial germination of C. musae.
Supplementary Material
Funding Statement
This work was financially supported by the Natural Science Foundation of Hainan Province [2019RC074] and the National Natural Science Foundation of China [31860480].
Disclosure statement
All of the authors declare that they have no conflict of interest.
References
- 1.Zhang R, He H, Di Y, et al. Chemical constituents from Aphanamixis grandifolia. Fitoterapia. 2014;92:100–104. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Wang J, Gu Y, et al. Diverse prieurianin-type limonoid derivatives from the fruits of Aphanamixis grandifolia and their absolute configuration determination. Tetrahedron. 2014;70(37):6594–6606. [Google Scholar]
- 3.Astulla A, Hirasawa Y, Rahman A, et al. Melidianolic acid a and B, new antimalarial acyclic diterpenes from Aphanamixis grandifolia. Nat Prod Commun. 2011;6:323–326. [PubMed] [Google Scholar]
- 4.Yang SP, Chen HD, Liao SG, et al. Aphanamolide A, a new limonoid from Aphanamixis polystachya. Org Lett. 2011;13(1):150–153. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Q, Guan B, Ren J, et al. Aphanamgrandiol A, a new triterpenoid with a unique carbon skeleton from Aphanamixis grandifolia. Fitoterapia. 2013;86:217–221. [DOI] [PubMed] [Google Scholar]
- 6.Clay K, Schardl C.. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat. 2002;160(Suppl S4):S99–S127. [DOI] [PubMed] [Google Scholar]
- 7.Guo ZY, Tan MH, Liu CX, et al. Aspergoterpenins a(-)D: four new antimicrobial bisabolane sesquiterpenoid derivatives from an endophytic fungus Aspergillus versicolor. Molecules. 2018;23:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng M, Liu Y, Huang Y, et al. New bioactive secondary metabolites from the Anoectochilus roxburghii endophytic fungus Aspergillus versicolor. Fitoterapia. 2020;143:104532. [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Liu Y, Li T, et al. 3-Arylisoindolinone and sesquiterpene derivatives from the mangrove endophytic fungi Aspergillus versicolor SYSU-SKS025. Fitoterapia. 2018;124:177–181. [DOI] [PubMed] [Google Scholar]
- 10.Miao FP, Li XD, Liu XH, et al. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar Drugs. 2012;10(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Gendy M, Yahya S, Hamed AR, et al. Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from Red Sea sponge Hyrtios erectus. Appl Biochem Biotechnol. 2018;185(3):755–777. [DOI] [PubMed] [Google Scholar]
- 12.Williamson SM, Guzmán M, Marin DH, et al. Evaluation of Pseudomonas syringae strain ESC-11 for biocontrol of crown rot and anthracnose of banana. Biol Control. 2008;46(3):279–286. [Google Scholar]
- 13.Samson RA, Visagie CM, Houbraken J, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talbot NJ, Salch YP, Ma M, et al. Karyotypic variation within clonal lineages of the rice blast fungus, Magnaporthe grisea. Appl Environ Microbiol. 1993;59(2):585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Shinsky TJ, White TJ, eds). Academic Press Inc, 1990. New York:315–322. [Google Scholar]
- 16.Hong SB, Go SJ, Shin HD, et al. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia. 2005;97(6):1316–1329. [DOI] [PubMed] [Google Scholar]
- 17.Jurjevic Z, Peterson SW, Horn BW.. Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus. 2012;3(1):59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai K, Ohte S, Ohshiro T, et al. Selective inhibition of acyl-CoA:cholesterol acyltransferase 2 isozyme by flavasperone and sterigmatocystin from Aspergillus species. J Antibiot. 2008;61(9):568–572. [DOI] [PubMed] [Google Scholar]
- 19.Krzyczkowski W, Malinowska E, Suchocki P, et al. Isolation and quantitative determination of ergosterol peroxide in various edible mushroom species. Food Chem. 2009;113(1):351–355. [Google Scholar]
- 20.Hamasaki T, Hatsuda Y, Terasaima N, et al. Studies on the metabolites of Aspergillus versicolor (vuillemin) tiraboschi. Part V. Isolation and structures of three new metabolites, versicolorins A, B and C. Agric Biol Chem. 1967;31:11–17. [Google Scholar]
- 21.Birkinshaw JH, Roberts JC, Roffey P.. Studies in mycological chemistry. Part XIX. “product B” (averantin) [1,3,6,8-tetrahydroxy-2-(1-hydroxyhexyl)anthraquinone], a pigment from Aspergillus versicolor (vuillemin) tiraboschi. J Chem Soc Perkin Trans. 1966;9:855–857. [DOI] [PubMed] [Google Scholar]
- 22.Lee YM, Li H, Hong J, et al. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch Pharm Res. 2010;33(2):231–235. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Yuan C, Cao M, et al. New acyclic diterpenoids from the fruits of Aphanamixis grandifolia and structure revision of nemoralisin B. Phytochem Lett. 2014;8:81–85. [Google Scholar]
- 24.Ye Y, Xia C, Yang J, et al. Isocoumarins from the fermentation products of an endophytic fungus of Aspergillus versicolor. Phytochem Lett. 2014;10:215–218. [Google Scholar]
- 25.Nakayama T. Biocontrol of powdery scab of potato by seed tuber application of an antagonistic fungus, Aspergillus versicolor, isolated from potato roots. J Gen Plant Pathol. 2017;83(4):253–263. [Google Scholar]
- 26.Liu W, Wang L, Wang B, et al. Diketopiperazine and diphenylether derivatives from marine algae-derived Aspergillus versicolor OUCMDZ-2738 by epigenetic activation. Mar Drugs. 2018;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Zhang K, Ke Z, et al. Optimisation of medium and culture conditions for the production of antifungal substances to Colletotrichum musae by Trametes elegans SR06. Biocontrol Sci Techn. 2016;26(11):1538–1551. [Google Scholar]
- 28.Wang W, Chen R, Luo Z, et al. Antimicrobial activity and molecular docking studies of a novel anthraquinone from a marine-derived fungus Aspergillus versicolor. Nat Prod Res. 2018;32(5):558–563. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Miao MM, Du G, et al. Aspergillines A-E, highly oxygenated hexacyclic indole-tetrahydrofuran-tetramic acid derivatives from Aspergillus versicolor. Org Lett. 2014;16(19):5016–5019. [DOI] [PubMed] [Google Scholar]
- 30.Salendra L, Luo X, Lin X, et al. Versispiroketal A, an unusual tetracyclic bridged spiroketal from the sponge-associated fungus Aspergillus versicolor SCSIO 41013. Org Biomol Chem. 2019;17(8):2182–2186. [DOI] [PubMed] [Google Scholar]
- 31.Li XB, Zhou YH, Zhu RX, et al. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem Biodivers. 2015;12(4):575–592. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Sun M, Hao H, et al. Avertoxins A-D, prenyl asteltoxin derivatives from Aspergillus versicolor Y10, an endophytic fungus of Huperzia serrata. J Nat Prod. 2015;78(12):3067–3070. [DOI] [PubMed] [Google Scholar]
- 33.Hou XM, Zhang YH, Hai Y, et al. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar Drugs. 2017;15:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozkaya FC, Ebrahim W, El-Neketi M, et al. Induction of new metabolites from sponge-associated fungus Aspergillus carneus by OSMAC approach. Fitoterapia. 2018;131:9–14. [DOI] [PubMed] [Google Scholar]
- 35.Liu K, Zheng Y, Miao C, et al. The antifungal metabolites obtained from the rhizospheric Aspergillus sp. YIM PH30001 against pathogenic fungi of Panax notoginseng. Nat Prod Res. 2014;28(24):2334–2337. [DOI] [PubMed] [Google Scholar]
- 36.Hu J, Li Z, Gao J, et al. New diketopiperazines from a marine-derived fungus strain Aspergillus versicolor MF180151. Mar Drugs. 2019;17:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.