Abstract
Papiliotrema huenov was previously reported to be highly tolerant of a range of extremely toxic heavy metals. This study aimed to identify the potential of P. huenov to remove manganese and copper from aqueous solution. Physical conditions which affect removal of Mn(II) and Cu(II) were determined. Optimal temperature for adsorption of both metal ions was 30 °C, and optimal pH for maximum uptake of Mn(II) and Cu(II) were 5 and 6, respectively. Under these conditions, living cells of P. huenov accumulated up to 75.58% of 110 mg/L Mn(II) and 70.5% of 128 mg/L Cu(II) over 120 h, whereas, the removal efficiency of metal ions by dead cells over 1 h was 60.3% and 56.5%, respectively. These results indicate that living cells are more effective than dead biomass for bioremediation, but that greater time is required. The experimental data extends the potential use of P. huenov in biosorption and bioaccumulation of toxic heavy metals to copper and manganese, two of the most common industrial contaminants.
Keywords: Papiliotrema huenov, bioaccumulation, biosorption, manganese, copper
1. Introduction
Much of the environment, including water resources, has become heavily contaminated by a wide range of pollutants such as heavy metals [1]. Unlike organic waste, which can decompose without human assistance, heavy metals persist in the environment [2]. They accumulate in tissues throughout the food chain and are potentially lethal, at levels that exceed safe limits [2]. Manganese and copper are essential factors for many organisms and are incorporated into a number of metalloenzymes involved in hemoglobin formation, energy generation, carbohydrate metabolism, etc. [1,3]. However, several studies have linked excessive exposure to either copper or manganese to pathologies, such as Alzheimer’s disease and Wilson’s disease [4–6].
According to World Health Organization (WHO) guidelines [7] copper and manganese concentration should not exceed 2 mg and 0.1 mg per liter of drinking water, respectively. The presence of these metals at even low concentrations in the environment may be detrimental to species from microorganisms to man. Removing excess heavy metals from industrial wastewater before its release into the environment is vital to sustaining life.
Efforts to remove heavy metals such as manganese and copper from wastewater and soil are usually based on physicochemical processes such as ion-exchange, chemical precipitation, electro-flotation and membrane filtration [8]. These methods are effective, but often costly, produce a large amount of sludge that needs to be treated and, moreover perform ineffectively with low concentrations of metal ions [9]. An alternative way to treat heavy metal in water is bioremediation, which is a potentially inexpensive and effective method for heavy metal removal from wastewater using living organisms [10] or dried biomass [8,9]. Both plant and microbes can be used in bioremediation, however, microbes are preferred since they have different mechanisms to remove heavy metals [9] such as the mannoproteins of their cell walls are efficient at binding metal ions and altering the chemical characteristics of the cell wall can achieve more specific or more effective binding.
Using growing microorganisms for metal removal requires no separation of biomass during treatment, pre-preparation of dry biomass or storage and treatment of biomass prior to use. However, heavy metals are harmful to microorganisms since they form complexes with components of the cell membrane, causing loss of integrity, impaired function and consequently reduced bioremoval efficiency. Luckily, the problem of metal toxicity can be overcome by using metal-resistant microorganisms [11]. Some yeast strains, in particular, have shown the ability to live in high concentrations of heavy metals [12–14]. Advantages of using dry biomass to absorb metals in the solution include low maintenance and nutrient requirements, low cost and highly efficient metal removal from dilute solutions [8,15].
Yeasts are considered inexpensive biosorbents for effective removal of heavy metal and have a number of advantages such as ease of culture and scale up cultivation in inexpensive media to obtain a large biomass that is generally safe and easy to use.
Several studies have used yeast Saccharomyces cerevisiae as a biosorbent for removal of heavy metals [9,16]. For example, Fadel et al. screened eleven S. cerevisiae yeast strains for biosorption and bioaccumulation of Mn(II) from artificial aqueous solution [17]. Do Nascimento et al. used S. cerevisiae biomass for removal of Cu(II) [18]. Some studies used wild yeasts isolated from nature for bioremediation of heavy metals. Hernández Mata used Issatchenkia orientalis and Candida tropicalis for removal of Zn, Cu, Mn, and Fe [19]. More recently, Amorim used M. guilliermondii and M. caribbica as biosorbent to treat Mn(II) contaminated water [20].
Some studies have attempted to compare the use of living and nonliving yeast cells for the bioremoval of heavy metals from aqueous solution. Moreover, the performance of any biosorbent also depends on biomass characteristics, physico-chemical properties of the target metals and the micro-environment of contact solution (the initial pH of solution, temperature and interaction with other ions) [21]. Detailed comparisons of different microorganisms and of the use of living and non-living cells are essential to choosing the appropriate system for treating contaminated water.
Papiliotrema yeast species are wild yeast isolated from many habitats [22] and a limited number of studies, have shown promising results for the use of Papiliotrema yeast species in bioremediation [23]. Excitingly, in our previous study we showed that Papiliotrema huenov is resistant to numerous heavy metals including Ni, Pb, As and Cd [24], can live in a wide range of temperatures and grows well in inexpensive media, making it an extremely promising candidate for bioremediation. However, two key toxic metals, copper and manganese were not previously considered in detail.
The aim of this study was to investigate the possible use of P. huenov to remove Mn(II) and Cu(II) from aqueous solutions. Firstly, the tolerance of growing yeast cells to Mn(II) and Cu(II), and the metal uptake capacities of living cells was studied as a function of initial metal concentration. At the same time, SEM, and TEM analyses were carried out to reveal changes to cell surfaces and organelles after metal treatment. Secondly, the effects of the process parameters such as initial metal concentration, pH, temperature and inoculation dose of P. huenov strain on biosorption were investigated. The results demonstrated the effectiveness of P. huenov in removing Mn(II) and Cu(II) from aqueous solutions, by both bioaccumulation and biosorption.
2. Materials and methods
2.1. Heavy tolerance and bioaccumulation by P. huenov
Investigations into the effect of manganese and copper on P. huenov were based on cell dynamic development in liquid culture according to the method, previously described [24] with some modifications. Briefly, P. huenov from the collection at the institute of Biotechnology, Hue University was inoculated into fresh YPD medium agar (1% yeast extract, 1% peptone, 2% glucose, 2% agar). A single colony was inoculated into YPD medium and cultured overnight, following which 5 mL of culture was inoculated in 500 mL of liquid YD medium (1% yeast extract, 2% glucose) in Erlenmeyer flasks containing different concentrations of manganese (55, 110, 165, 275 and 385 mg/L), and copper (64, 128, 192, 320 and 448 mg/L) and incubated with shaking 150 rpm for 120 h at 30 °C. These concentration of Cu(II) and Mn(II) correspond to 1, 2, 3, 5 and 7 mM, doses that are widely used in microbial resistant screening and bioaccumulation experiments [18,25–28] and were used in our previous study.
Similar concentrations of these metals, added to YD broth but without yeast cells, were used as controls. Cell growth was measured by monitoring optical density at 600 nm (Optizen POP UV-VIS Spectrophotometer, K LAB, Daejeon, Korea) every 2 h. Biomass was determined as dry culture (in grams) per liter of broth according to [29] with slight modifications. Briefly, 5 mL of culture was collected by centrifugation at 5000 rpm for 5 min, at room temperature, followed by removal of the supernatant. The remaining pellet was washed with 5 mL of distilled water and centrifuged to collect precipitate cells, then washed cells with 1 mL distilled water and transferred to a preweighed 1.5 mL eppendorf tube. After centrifugation all supernatants completely discarded and the pellet was rotated in an oven at 90 °C for 12 h, after which the Eppendorf tube was weighed again to determine cell biomass, all measurements were performed in triplicate. The biomass yield of culture was calculated by multiplying the biomass to the culture volume. The standard curve was built for 1, 2, 3, 4 and 5 L of cultures and used for further experiments.
Cell biomass was collected at time intervals by centrifugation at 10,000 rpm for 5 min to remove metal-bound yeast cells. The supernatants were analyzed by atomic adsorption spectrometry (Shimadzu 6880, Shimadzu, Kyoto, Japan) to determine the equilibrium manganese or copper concentration. Maximum uptake (qmax, mg/g) is the amount of bioaccumulated Mn(II) and Cu(II) per unit of dry weight of living biomass at the end of 120 h. The average absorption capacity was identified according to [30] the following equation:
| (1) |
where Ci and Cf are initial and final concentrations of manganese or copper, respectively.
2.2. Scanning electron microscopy (SEM)
SEM was used to investigate the morphological differences between yeast cells treated with Mn(II) or Cu(II) and untreated cells to investigate changes in cell surfaces and to estimate the adsorption of these heavy metals. The analytical condition parameters were as follows: secondary electron mode (SE), 20,000X magnification, electron beam voltage of 2.0 kV, working distance of 3.9 mm, and temperature of 20 °C. Yeast samples were examined after cell fixation and vacuum freeze drying.
2.3. Transmission electron microscopy (TEM)
Sample preparation was carried out as previously described [31] with some modifications. After transfer to flat specimen carriers containing 1% lecithin in chloroform, treated and untreated yeast cells were frozen in an EM PACT2 high-pressure freezer (Leica, Leica Camera, Wetzlar, Germany). Samples were then freeze-substituted in an automatic FS machine (Leica) in 100% acetone/2% osmium tetroxide as follows: 96 h at −90 °C, increased 5 °C per hour for 14 h, 24 h at −20 °C, increased 3 °C per hour for 8 h, then 18 h at 4 °C. Substituted samples were washed 3 times (15 min each time) at room temperature in 100% acetone, infiltrated in 1:2 (v:v), 1:1 (v:v) and 2:1 (v:v) Epon/acetone mixtures for 1 h each and embedded in pure Epon. A Reichert-Jung Ultracut E ultramicrotome was used to cut ultra-thin sections, which were stained with uranyl acetate and lead citrate. A JEM-1011 transmission electron microscope (JEOL, Tokyo, Japan) operating at 80 kV was used to examine colony sections. Fine structure measurements were performed using a Veleta camera and iTEM 5.1 software (Olympus Soft Imaging Solution GmbH, Olympus, Münster, Germany).
2.4. Biosorption experiments
2.4.1. Preparation of biomass
Papiliotrema huenov cells were grown in a 1000 mL Erlenmeyer flask containing 200 mL of YD medium (pH 6.5) in a shaking incubator at 150 rpm and 30 °C for 48 h. The yeast cells were harvested in stationary phase by centrifugation at 10,000 rpm for 10 min. The cells were washed three times with deionized water and oven-dried at 80 °C for 48 h (dry weight).
2.4.2. Stock solution preparation
Solutions of Mn(II) and Cu(II) with concentrations ranging from 1 mM to 7 mM were prepared from MnCl2·4H2O and CuSO4.5H2O. The pH of each test solution was determined and adjusted to desired value by adding the appropriate volume of 1 M HCl or 1 M NaOH before the addition of biomass.
2.4.3. Effect of initial pH and yeast biomass dose on biosorption
The initial concentration of heavy metals commonly used in biosorption experiments were in the range 10–100 mg/L [15,18,32–34]. However, there have been reports of copper/manganese concentrations from 150 mg/L [26,35], even up to 500 mg/L [36] or 1000 mg/L [37]. The dose of the initial concentration of metals may be dependent on the characteristics of the biosorbent as well as the purpose of the research application [38]. We planned to identify materials that could be applied to the treatment of Cu(II)/Mn(II) in traditional industrial villages in Vietnam such as a copper casting village in Hue city of Vietnam with a very high concentration of copper in its wastewater. A range of metal concentrations (50–500 mg/L) was investigated in our study. It was found that an initial concentration of copper and manganese of 110 and 128 mg/L (respectively) was optimal for P.Huenov biomass (data not shown).
To assess the effect of pH on biological absorption, 2 g/L of yeast biomass was exposed to 100 mL of solution containing 110 mg/L Mn(II) or 128 mg/L Cu(II) in flasks at pH values ranging from 2 to 7 and shaken at 150 rpm for 180 min at 30 °C.
The effect of P. huenov biomass dose from 0.5 to 5 g/L was also determined in aqueous solution at optimal pH.
2.4.4. Effect of temperature on biosorption
To evaluate the effect of temperature on biosorption, 2 g/L (optimal pH above) yeast biomass was suspended in 110 mg/L of Mn2+ or 128 mg/L of Cu2+ solution. These studies were carried out in a shaking water bath at 20, 30, 40 and 50 °C with exposure times ranging from 0 to 180 min. After incubation, the mixtures were centrifuged at 6000 rpm for 10 min. The residual metal content was analyzed by atomic adsorption spectroscopy. Finally, metal uptake was calculated as follows (2):
| (2) |
where Co and Ct are the initial and final metal ion concentrations (mg/L), respectively, V is the volume of the solution (in mL), and m is yeast biomass dry weight (g).
2.4.5. Biosorption models
Biosorption isotherms are specific constant values, describing the surface characteristics and affinity of biosorbents, and can be utilized to compare the biosorptive capacity of biomass for heavy metals [39]. In this study, the Freundlich (1906) and Langmuir (1916) isotherm models were applied to analyze the biosorption isotherms of Mn(II) and Cu(II).
The Freundlich isotherm is based on sorption on heterogeneous surface and active sites. The Freundlich model (3) is presented below:
| (3) |
where q = heavy metal adsorbed by the biosorbent (mg/g); Ce = the equilibrium concentration of metal (mg/L) in the solution; Kf is a Freundlich constant indicating the binding capacity and 1/n is an empirical parameter indicating the biosorption intensity, which varies with the heterogeneity of the biosorbent. An efficient absorption process yields a Freundlich constant n between 1 and 10. A high value of n implies a stronger interaction between the adsorbent cell surface and divalent metals.
The parameters can be determined through linearization of Equation (4) as follows:
| (4) |
The Langmuir isotherm is used to examine the absorption of gases on a solid surface, and sorption is considered a chemical phenomenon. This isotherm has been successfully applied to many pollutant biosorption processes and is the most widely used isotherm for the biosorption of a solute from a liquid solution. The classical Langmuir model is described by the following Equation (5):
| (5) |
where q = heavy metal adsorbed on the biosorbent (mg/g); Ce = final concentration of metal (mg/L) in the solution; qmax = maximum possible amount of metal uptake; Kd = equilibrium constant related to the affinity of the binding sites for the metals. The Langmuir model equation was linearized (6) to obtain the qmax value:
| (6) |
2.5. Statistical analysis
All experiments were carried out in triplicate and results expressed as the mean ± standard deviation (SD). The resulting data were subjected to one-way analysis of variance (ANOVA) followed by Student’s t-test to estimate t-value, p-value and confidence levels and results were considered statistically significant when p < 0.05.
3. Results and discussion
3.1. Bioaccumulation of Mn(II) and Cu(II) by P. huenov
3.1.1. Effect of initial metal ion concentration on growth and metal ion bioaccumulation
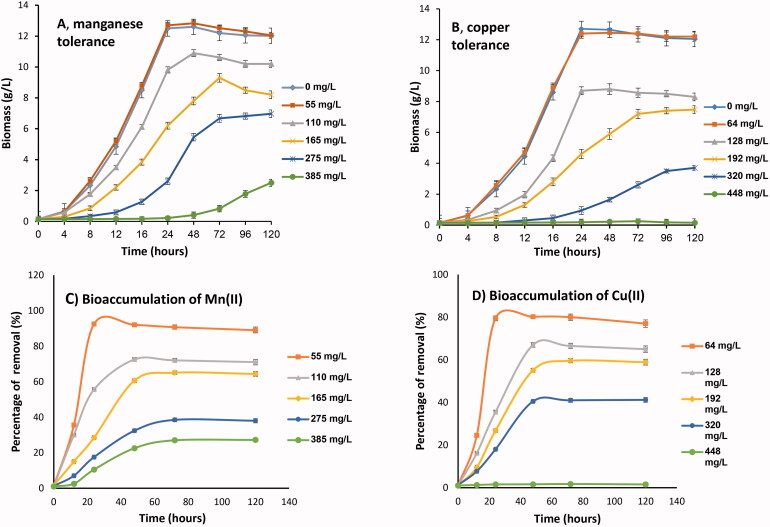
In previous findings, we showed that P. huenov can tolerate high levels of some heavy metals such as Ni, Pd, and Cd [24]. In this study, we observed that yeast cells can grow even better at low concentrations of manganese (55 mg/L), or copper (64 mg/L), since biomass (g/L) or cell density (OD) of cultures treated with these metals (12.55–12.8 g/L) was higher than that of controls (12.5 g/L) (Figure 1(A,B); Table 1). Cells grew quickly, and reached stationary phase after 48 h. The obtained results are in agreement with previous findings that, at low concentrations, manganese and copper may promote microbial cell growth [12,40,41]. However, P. huenov cell growth was inhibited by manganese or copper levels above these concentrations (Figure 1(A,B); Table 1).
Figure 1.
Growth of Papiliotrema huenov, and bioaccumulation at different initial concentrations of manganese, and copper. (A,C) Manganese tolerance and uptake; (B,D) copper tolerance and uptake (cells were incubated at 30 °C, shaking 150 rpm, at optimal pH). Bars represent standard deviation (SD).
Table 1.
Effect of initial Mn(II), Cu(II) concentrations on the yeast growth rate, bioaccumulation, and removal efficiency (at 30 °C, incubation period 120 h).
| Initial concentration (Ci) mg/L |
Growth rate (µ) |
Biomass (g/L) |
qmax (mg/g) |
% removal of metal |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mn(II) | Cu(II) | Mn(II) | Cu(II) | Mn(II) | Cu(II) | Mn(II) | Cu(II) | Mn(II) | Cu(II) |
| 0 | 0 | 3.54 | 3.54 | 12.5 | 12.5 | 0 | 0 | 0 | 0 |
| 55 | 64 | 3.6 | 3.5 | 12.8 | 12.55 | 9.85 | 9.6 | 96.5 | 89.42 |
| 110 | 128 | 2.75 | 2.4 | 10.6 | 8.75 | 16.61 | 15.7 | 75.58 | 70.51 |
| 165 | 192 | 2.04 | 1.85 | 8.2 | 7.4 | 20.5 | 22.6 | 64.6 | 58.6 |
| 275 | 320 | 1.63 | 1.03 | 6.75 | 3.65 | 29.3 | 30.3 | 38.5 | 19.27 |
| 385 | 448 | 0.46 | 0 | 2.4 | 0 | 32.4 | 0 | 27.2 | 0 |
Manganese in the range 110–275 mg/L delayed log-phase growth by about 4 − 12 h with stationary phase being, reached after 72 h and yielding much less biomass (≤10.6 g/L) than the control (12.5 g/L) as shown in Table 1 and Figure 1(A). Although, cells can survive in up to 385 mg/L of Mn(II) ions, the results obviously indicated that addition of Mn(II) led to a significant decrease in cell growth, and subsequently reduced metal uptake (Figure 1(C)). Lower initial Mn(II) ion concentrations favored greater uptake efficiency, Mn(II) concentrations exceeding 55 mg/L, resulted in a decrease in removal efficiency from 96.5% to 27.2% of total Mn(II) within 120 h (Table 1). Mn(II) uptake increased from 9.85 to 32.4 mg/g with increasing Mn(II) concentration (55–385 mg/L).
It was also clear that increasing Cu(II) ion concentration caused a decrease in cell growth and bioaccumulation efficiency. Dynamic growth of P. huenov cells was delayed in the range 128–320 mg/L of copper by 12–20 h, and cell yield decreased from 70% (8.75 g/L) to 29.2% (3.65 g/L) when grown with 128–320 mg/L Cu(II) compared to control (12.5 g/L, without copper) as shown in Figure 1(B) and Table 1. Subsequently, living P. huenov cells took up less than 71% (70.5–19.27%) of total Cu(II) within 120 h. In addition, neither yeast cell growth nor Cu(II) uptake occurred at 448 mg/L of initial Cu(II) concentration (Table 1; Figure 1(B,D)).
The results indicate that P. huenov was tolerant of both manganese and copper. These findings were in accordance with other reports studied in yeasts [12] and filamentous fungi [26], and the organism was more resistant to heavy metals than Candida tropicalis [13], but more sensitive than yeast Meyerozyma guilliermondii and M. caribbica that can live in up to 32 mM of Mn(II) [20].
Both manganese and copper are essential elements, but an over-dose of either one clearly inhibited yeast cell growth and damaged yeast cells. Consequently, higher metal removal efficiency by yeast cells was observed at lower metal ion concentrations for both manganese and copper, while higher metal concentrations caused cell toxicity and decreased bioaccumulation of metal ions. Similar results were observed in other reports. For example, the copper removal efficiency of Pichia stipitis decreased from 89% to 61% when initial copper concentration was increased from 10 to 100 mg/L [25]. That of Candida spp. reduced from 81% to 4.9% when the initial copper concentration increased from 97.6 mg/L to 1518 mg/L [42]. The maximum uptake of Mn(II) by the manganese resistant Bacillus cereus HM-5 decreased from 99% to 67% when the initial Mn(II) concentration increased from 600 to 800 mg/L [43].
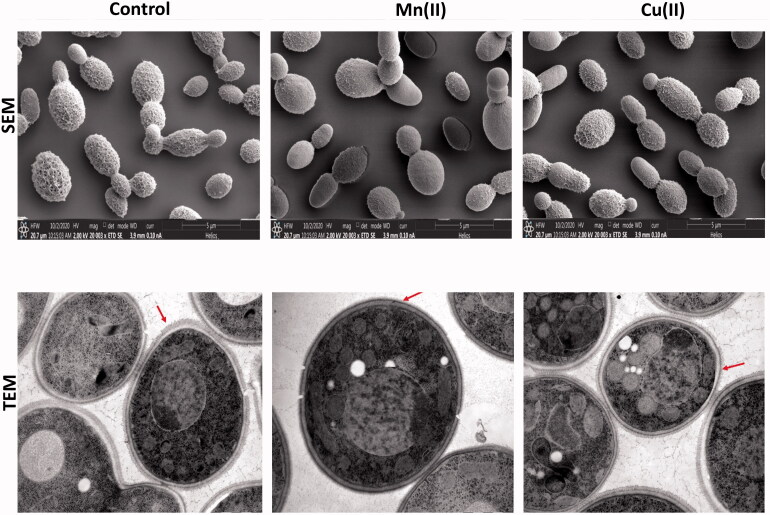
3.1.2. Electron microscopy analysis
Electron microscopy (SEM and TEM) analysis clearly revealed a relationship between bioaccumulation efficiency and the effect of metal ions on the cell wall surface and organelle structures such as the vacuole. There were no apparent differences among cells that were grown without heavy metals or at low concentration, 55 mg/L of Mn(II) or 64 mg/L of Cu(II) as shown in Figure S1. Similar results were observed in R. mucilaginosa yeast cells treated with 50 mg/L (equivalent to 0.91 mM Mn2+) and those that were not treated [33]. In contrast, S. cerevisiae cells grown in medium with 1 mM of Cu2+ (64 mg/L) exhibited defects in size, shape and adhesion compared to control cells [41].
Papiliotrema huenov cells exhibited a rough, complexly folding capsule (Figure S1) leading to an increased contact surface, consequently which may contribute to the interaction with metals, and thus promote biosorption and bioaccumulation [44–46].
After treatment with a high concentration (385 mg/L) of Mn(II), cell-surface morphology changed. Capsule complexity decreased (as indicated by the red arrow in Figure 2), and cells appeared to be damaged by heavy metal ions. In particular, cells exposed to manganese seemed to have larger vacuole compared to untreated cells (Figure 2), which may permit yeast cells to tolerate and accumulate high concentration of manganese. Cells treated with high concentrations of copper underwent significant changes in the shape and size of vacuoles (Figure 2), and in the length and complexity of the capsular (Figure 2) compared to untreated cells. It has been reported that heavy metals affect enzymes of yeast vacuole [47]. To date, there is no direct evidence describing the effects of heavy metals on yeast vacuolar volume. However, it has suggested that vacuolar volume may be altered by some toxins. For example, that of Pseudokirchneriella subcapitata increased upon treatment with Cd or Zn [48], while that of Chlamydomonas acidophila doubled in volume upon exposure to Cd [49]. In agreement with these findings, the observation in this study indicated that high concentrations of either Mn(II) or Cu(II) may induce changes in vacuolar volume, and capsular structure of P. huenov cells.
Figure 2.
Electron microscopy microgragh of Papiliotrema huenov cells treated with 385 mg/L Mn(II), and 320 mg/L Cu(II) (cells were incubated at 30 °C, shaking 150 rpm, at optimal pH).
These changes in cellular structures of treated cells confirmed the bioaccumulation efficiency, supporting the bioaccumulation pattern of these metals, which reached maximum after an incubation time of 24–48 h, followed by a slight decrease in uptake of Mn(II) or Cu(II) (Figure 1(C,D)). This observation indicated that passive absorption (with rapid binding of the metal to the yeast cell surface) was followed by slow active import of Mn(II) or Cu(II) ions, and destroyed the cellular structure of living cells leading to partial release of metal ions. Similar results were observed in other studies [25,49] in which the localization of Cu(II) or Mn(II) on the cell wall surface layer was confirmed. In addition, it has been determined that amines, phosphates and carboxyl groups confer the major negative charge of yeast cells, enhancing their ability to bind heavy-metal cations [50].
3.1.3. Effect of pH and temperature on bioaccumulation of manganese and copper
pH and temperature are two factors that may directly affect bioaccumulation since they influence not only the growth ability of cells, but also adsorption of metal ions to the cells [43].
Initially, the manganese and copper bioaccumulation efficiency of P. huenov was dependent on the pH of the medium. As the results in Table 2 demonstrate, the specific rate of growth and bioaccumulation increased in line with pH. As pH rose from 3 to 5 removal efficiency of Cu(II) increased from 32.38 to 71.6%, and drastically decreased (60.43%) at a pH of 6. A similar correlation was observed, between pH and Mn(II) removal, as pH increased from 3 to 6, removal efficiency also increased, reaching a maximum of 75.41% at pH 6 (Table 2). Although higher pH boosted cell growth from 6.52 g/L to 9.63 g/L, accumulation started to decrease at pH 6 for Cu(II), and above pH 7 for Mn(II) (data not shown).
Table 2.
Effect of pH on the yeast growth rate, maximum bioaccumulation of Mn(II), Cu(II) and removal efficiency (at 30 °C, incubation period 120 h).
| pH | Initial concentration (Ci) mg/L |
Growth rate (µ) |
Biomass (g/L) |
qmax (mg/g) |
% removal of metal |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn(II) | Cu(II) | Mn(II) | Cu(II) | Mn(II) | Cu(II) | Mn(II) | Cu(II) | Mn(II) | Cu(II) | |
| 3 | 92.3 | 97.45 | 1.72 | 1.69 | 6.9 | 6.52 | 28.5 | 29.55 | 31.5 | 32.38 |
| 4 | 96.5 | 116.37 | 2.34 | 1.97 | 9.4 | 7.45 | 23.1 | 21.86 | 54.82 | 58.25 |
| 5 | 102.1 | 128.2 | 2.55 | 2.29 | 9.75 | 8.94 | 19.46 | 15.61 | 66.45 | 71.6 |
| 6 | 109.5 | 105.62 | 2.6 | 2.41 | 10.2 | 9.63 | 16.47 | 19.4 | 75.41 | 60.43 |
It is well known that pH is a critical factor for organisms that influences bioaccumulation of heavy metals. When pH was too low, microorganisms experience high stress levels (being unable to reach a density higher than 6.5 g/L), and there were high levels of free metal ions. At alkaline pH the ions precipitated as insoluble hydroxides or oxides [39]. Thus, metal ion availability is dependent on the pH of the medium. The optimal pH for maximum bioaccumulation of Cu(II) and Mn(II) by P. huenov yeast cells were 5 and 6, respectively. pH 5 was also optimal for bioaccumulation of Cu(II) and Pb(II) by Penicillium spp [51] and by Rhizopus arrhizus and Aspergillus niger [52], while a pH of 6 was determined as optimal pH for Cr(III) and Zn(II) removal by Aspergillus versicolor [53].
Papiliotrema huenov grew at temperatures ranging from 20 to 35 °C, and the optimum temperature was 25 °C. However, optimal removal of metal ions was observed at 30 °C with removal of 75.5% of Mn(II), and 70.82% of Cu(II) in the presence of 110 mg/L of manganese and of 128 mg/L of copper over 120 h by 10.05 and 9.56 g/L of biomass, respectively (Table 3). With further increases in temperature, both growth and percentage metal removal decreased. These results were in agreement with other reports about Cu(II), and Mn(II) bioaccumulation in microorganisms [17,20,25,32] wherein, maximum accumulation occurred at 25–30 °C.
Table 3.
Effect of temperature on bioaccumulation of Mn(II) and Cu(II) (concentration of metal ions used at 110 mg/L of Mn(II) and 128 mg/L of Cu(II)).
| Temperature (°C) | Growth control (g/L) | Growth with Cu(II) | Growth with Mn(II) | % removal of Cu(II) | % removal of Mn(II) |
|---|---|---|---|---|---|
| 20 | 8.03 | 6.1 | 6.24 | 50.43 | 56.43 |
| 25 | 13.07 | 10.4 | 10.72 | 60.65 | 68.65 |
| 30 | 12.45 | 9.56 | 10.05 | 70.82 | 75.5 |
| 35 | 5.14 | 3.47 | 3.35 | 55.36 | 62.36 |
In general, at the optimal pH and temperature the maximum uptake capacity exhibited by the yeast P. huenov was 15.7 mg/g Cu(II) and 16.61 mg/g Mn(II). Similar results were observed in bioaccumulation of Cu(II) by Pichia stipitis yeast [25] and Aspergillus niger [45], which were capable of taking up 15.85 and 15.6 mg/g Cu(II), respectively. Copper uptake by yeast P. huenov was higher than that of Candida spp. isolated from wastewater sediment (up to 11.5 mg/g) and Rhizopus arrhizus (10.76 mg/g). The maximum copper uptake by four yeast strains in Donmez and Aksu studies were 9.05, 11.25, 1.27 and 14.79 mg/g for S. cerevisiae, K. marxianus, S. pombe and Candida spp, respectively [54].
Ruas et al. reported that living R. mucilaginosa yeast can remove 10.6% of an initial 50 mg/L Mn(II) during 7 days of incubation [33]. Live Mn- resistant S. cerevisiae mutants were also reported to have a maximum Mn(II) uptake more than four-fold that of the parental strain [55]. Previous reports also determined the maximum uptake capacities of some heavy metals by other living yeasts. For example, 30 mg/g Cr(III) by S. cerevisiae [56] and 9.1 mg/g by adapted P. stipites [25]. Recently, Das et al. reported that C. tropicalis was capable of bioaccumulating 49.6% of Pb(II) and 55.5% of Cd(II) [57]. Khan et al. used Pichia hampshirensis 4Aer to remove up to 28 mM/g Cd(II) in large scale experiments [58].
A wide range of living microbial biomass has been tested for Mn(II) and Cu(II) bioaccumulation [59,60]. Comparing the maximum Mn(II) and Cu(II) uptake capacities of growing P. huenov yeast used in this study with those obtained in the literature mentioned above indicates that P. huenov is effective for this purpose.
3.2. Biosorption experiments
3.2.1. Effect of pH on biosorption
pH is one of the most important factors influencing biosorption since it is associated with the competition of hydrogen ions and heavy metal ions for active sites on the surface of biosorbent [39]. At low pH, the active sites of biosorbent surfaces became positively charged which, together with hydrogen ions, reduces the uptake of metal cations [30]. As shown in Figure 3(A), the uptake capacities of Mn and Cu ions showed a similar trend. At pH <2 the absorption capacity was dramatically reduced to only 8.8 and 7.5 mg/g Cu(II) and Mn(II), respectively. As the pH increases, biosorbent surfaces become more negatively charged, leading to increased binding of cations at active sites and increased uptake. However, when pH increased to 7, biosorption of these ions decreased due to precipitation of cations [30,39].
Figure 3.
Biosorption experiments: effect of initial solution pH (A), biosorbent dose (B) and temperature (C,D) on biosorption capacity and removal efficiency of Cu(II) and Mn(II) by non-living biomass of Papiliotrema huenov (initial ion concentration: 110, and 128 mg/L of Mn, and Cu respectively).
As for Mn(II), biosorption capability increased dramatically as the pH increased from 4 to 6, and reached a maximum value of 28.5 mg/g at pH 6 (Figure 3(A)). Similar patterns have been identified in other studies by Hasan et al. [50] and Vijayaraghavan et al. who observed that optimal pH was 6 while maximum biosorption of manganese was 13.31 mg/g and 59.4 mg/g, respectively [61]. In other studies, manganese biosorption by S. cerevisiae F-25 [17], A. niger [32] and Pseudomonas aeruginosa AT18 [34] exhibited higher biosorption at pH 7 with maximum biosorption capacities of 22.5 mg/g, 14.58 and 38.2 mg/g, respectively.
Cu(II) absorption increased steadily as the pH increased and achieved a maximum value of 26.6 mg/g, and at pH 5 (Figure 3(A)). Similar results were obtained by other researchers, whose experiments used yeast biomass [25,62] or alternative biomass [21,26]. Maximum pH for optimum copper uptake was determined at 4 for A. niger [63], 5.2 for Candida krusei [64] and 6 for Pichia pastoris [65].
In general, maximum biosorption of copper and manganese was observed at pH 5 and 6, respectively. Thus, the remaining biosorption experiments were performed at these pH values.
3.2.2. Effect of biosorbent dose on biosorption
The dose of biosorbent is a significant factor affecting the sorption ability and heavy metal removal efficiency [39,66]. Biosorption of either Mn(II) or Cu(II) with varying amounts of yeast biomass in the range of 0.5–3 g/L is shown in Figure 3(B). Uptake of Mn(II) and Cu(II) reached maxima of 41.65 mg/g and 39.3 mg/g respectively at 0.5 g/L of biomass (Figure 3(B)), while the percentage removal of Mn(II) and Cu(II) from solution was 47.5% and 41.1% respectively. The percentage removal of manganese and copper from solution increased in line with the increase in biomass concentration, and a dose of 3 g/L led to 57.75% and 43.81% reduction in sorption ability to 23.9 and 19.3 mg/g respectively. However, the sorption ability of P. huenov biomass decreased with increasing concentration of yeast cells, possibly because metal uptake is more effective when the inter-cellular distance is large enough to allow optimal electrostatic interaction between cells; an important factor for biosorption [39]. This trend is in agreement with the results of other authors [26,30]. The optimal dose of biosorbent for biosorption of 110 g/L of Mn(II), and 128 mg/L of Cu(II) from solution, was 2 g/L, leading to percentage removal of 60.3% and 56.5%, with a sorption capacity of 27.1 and 26.4 mg/g respectively and this dose was utilized in further experiments.
3.2.3. Effect of temperature on biosorption
Temperature is one of the most influential factors in the kinetics of biological absorption [30,39]. We investigated the effect of temperature in the 20–50 °C range. As shown in Figure 3(C,D), the sorption capacities of Mn and Cu at 20 °C were 21.52 mg/g, and 23.2 mg/g and increased to 27.79 mg/g and 26.6 mg/g, respectively, at 30 °C over 60 min.
At temperatures over 30 °C, the sorption capacities of these metals decreased from 24.7 and 24.4 mg/g at 40 °C to 17.1 and 18.5 mg/g at 50 °C for Mn and Cu, respectively. The results indicate that within the temperature range investigated, the biological uptake of Mn(II) and Cu(II) by biomass of P. huenov was a thermal reaction; an increase in temperature resulted in accelerated ion diffusion. This was in agreement with the results of other authors, which determined that increasing temperature from 20 to 40 °C resulted in an increase in the maximum biosorption of Ni(II) from 11.48 to 15.64 mg/g and of Zn(II) from 15.9 to 19.21 mg/g using Y. lipolytica biomass [67]. However, C. krusei biosorption deceased when temperature increased to 50 °C, probably due to denaturation of the enzymes, reducing the uptake of copper(II) [64]. In this study, maximum removal of manganese and copper was achieved at 30 °C for the two types of P. huenov biomass (dead and live). Similar results were also observed with yeast Rhodotorula mucilaginosa [68], Pichia stipites [25] and S. cerevisiae [17].
3.2.4. Mn and Cu biosorption isotherms and kinetics
3.2.4.1. Biosorption isotherm models
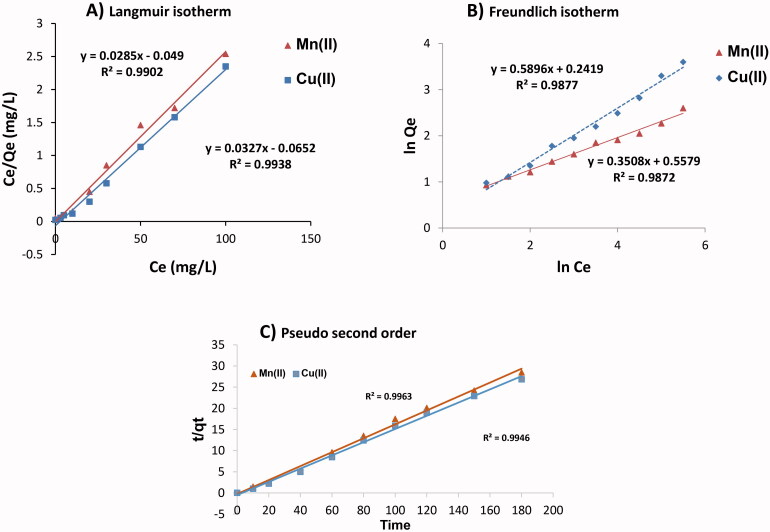
To understand the performance of Mn and Cu biosorption by P. huenov, the Langmuir and Freundlich sorption equations were used to analyze the Mn(II) and Cu(II) adsorption process (presented in Table 4; Figure 4(A,B)). The adsorption calculations revealed that Mn(II) and Cu(II) biosorption fitted the Langmuir model (R2 > 0.99) better than the Freundlich model with an R2 < 0.988. Based on Langmuir isotherm, the predicted maximum uptake capacities (qmax) of Mn and Cu were 35.02 mg/g and 30.7 mg/g respectively.
Table 4.
Langmuir and Freundlich model for the biosorption of Mn(II) and Cu(II) on Papiliotrema huenov biomass.
| Metal ion | Langmuir |
Freundlich |
||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R 2 | KF (L/mg) | 1/n | R 2 | |
| Mn(II) | 35.02 | 0.58 | 0.9902 | 1.27 | 0.589 | 0.9872 |
| Cu(II) | 30.7 | 0.5 | 0.9938 | 1.75 | 0.35 | 0.9877 |
Figure 4.
Isothermal and kinetics model of metal biosorption: linearized Langmuir isotherm (A), Freundlich isotherm (B) and, Pseudo-second order C.
Similar biosorption results were observed in other reports of Sahan et al. [69] and Omar et al. [70] using Trametes versicolor, Ulva lactuca as biosobents. The maximum sorption capacities in present study are much higher than other studies of Fadel et al. [17], Dutta et al. [71], Parvathi et al. [32] and Chang et al. [72] using S. cerevisiae, A. niger and Pseudomonas aeruginosa PU21; the corresponding values were 22.5 mg/g (Mn), 17.24 mg/g (Cu), 19.34 mg/g (Cu) and 18.95 mg/g (Mn). But lower compared to the results of Amirnia et al. [73] using S. cerevisiae and Hasan et al. [50] who used Bacillus species with the maximum sorption capacity were 42.55 mg/g (Cu), 43.5 mg/g (Mn), respectively.
KL is the Langmuir constant with respect to adsorption capacity in the Langmuir model. When the value of KL is high, the affinity of metal ions for the biological adsorbent increases. The KF in the Freundlich model indicates adsorption levels. Consistent with the Langmuir model, when the KF value is low, adsorption of heavy metals is low, whereas the higher the KF value, the stronger the adsorption capacity [74]. In addition, 1/n is a constant reflecting the adsorption intensity of biosorbent, this value in our experiment was observed to be 0.33 and 0.51 for Mn(II) and Cu(II) respectively, which are within the 0.1 to 1.0 range that indicates optimum Mn(II) and Cu(II) ion biosorption on P. huenov biomass. Therefore, Langmuir and Freundlich are both able to accurately describe the adsorption process. Mn(II) and Cu(II) absorption is mainly carried out by single-layer adsorption to P. huenov, and a greater range of adsorption mechanisms needs to be further investigated.
The capacity of P. huenov to absorb Mn(II) and Cu(II) was significantly stronger than that of other biomass, making it a promising choice for use in removing heavy metals from solution. Moreover, our results indicated the superior metal removal efficiency of living cells compared to dead cells, which is consistent with other studies [28,75]. This is thought to be due to metal sequestration on the cell wall (biosorption), which is then absorbed into living cells (bioaccumulation), as opposed to biosorption alone for dead cells. It should be noted that chromium and lead removal by P. simplicissimum [28] exceeded 80% and lead tolerance was extremely high. However, the species removed 60.9% of copper at an initial concentration of 100 mg/L while P. huenov achieved 70.5% removal with an initial copper concentration of 128 mg/L. Given the high copper levels in wastewater from Vietnamese copper casting villages, this increased bioaccumulation could be crucial. Bioaccumulation using live cells takes longer than biosorption. In our study, bioaccumulation took up to 5 days while that of Chen et al. [28] took up to 11 days. Bioaccumulation also requires more biomass to absorb metals than biosorption using dead cells (60–120 min) with 2 g dry weight.
3.2.4.2. Adsorption kinetics
To investigate the kinetic mechanism that control the adsorption of manganese and copper on P. huenov biomass a pseudo-first-order model (7) and a pseudo-second-order model (8) were used according to previous studies [39,76]
| (7) |
| (8) |
where K1 and K2 are the equilibrium rate constants of pseudo-first and second-order adsorption kinetics, qt (mg/g) the amount of adsorbate on adsorbent at time (t:min), and qe the equilibrium uptake (mg/g). Equation 7 describes the dependence of log(qe – qt) on contact time (t), while equation 8 relates t/qt to t. The plots showing (qe – qt) against time as a pseudo-first-order model have not been included since the coefficient of determination for this model is relatively low (R2 = 0.893 for Cu(II) and R2 = 0.885 for Mn(II) biosorption as seen in Table 5).
Table 5.
Kinetic parameters for the biosorption of Mn(II) and Cu(II) on Papiliotrema huenov biomass.
| Metal ion | qeexp (mg/g) | Pseudo-first order |
Pseudo-second order |
|||||
|---|---|---|---|---|---|---|---|---|
| K1 (1/min) | qe (mg/g) | R 2 | K2 (g/mg·min) | qe (mg/g) | h (mg/g·min) | R 2 | ||
| Cu(II) | 26.54 | 0.071 | 8.95 | 0.893 | 0.022 | 26.36 | 8.13 | 0.9946 |
| Mn(II) | 27.75 | 0.059 | 6.77 | 0.885 | 0.018 | 27.59 | 8.95 | 0.9963 |
Figure 4(C) shows manganese and copper removal with the use of P. huenov biomass through the pseudo-second order modeling at 30 °C and pH 5.5 with 110 and 128 mg/L of Mn(II) and Cu(II) respectively. The correlation coefficients of the pseudo-second order model were very high (>0.994, Table 5) and much higher than the pseudo-first order model. Moreover, the theoretical equilibrium uptake (qe) values calculated from the pseudo-second-order kinetic model were in close agreement with the experimental values as shown in Table 5. The results obtained above demonstrated that the kinetics of copper and manganese biosorption by yeast P. huenov progressed in line with the pseudo-second order model. Similar results concerning the kinetics of copper and manganese were obtained using different yeast biomass as biosorbent, such as S. cerevisiae [17,77], Rhodotorula mucilaginosa [68] and C. krusei [64]; or using alternative fungal biomass such as Aspergillus sp. TU-GM14 [78], and Penicillium camemberti [79]. However, Amorim et al. demonstrated that manganese biosorption by yeast M. caribbica and M. guilliermondii biomass fit the pseudo-first order model [20]. Many of these studies achieved high rates of metal removal but showed that metal biosorption was highly dependent on technical aspects such as grinding to produce nano biomass, immobilization in alginate beads or on Detarium microcarpum matrix, bead size, use of a batch system or fixed column set-up, bed depth, and the initial concentrations of biomass and manganese ions [78,79]. They may also require the use of hazardous materials such as glutaraldehyde and formaldehyde.
4. Conclusion
Industrial wastewater is heavily contaminated with heavy metals such as copper and manganese. These can be a serious threat to human health as well as life on planet Earth. Metal tolerant microbes can act like micro-filters or factories capable of removing those harmful elements [58]. In this study, we characterized a metal tolerant yeast, and also explored its potential application in the removal of Mn(II) and Cu(II). Many yeast species have been used for heavy metal removal including Candida tropicalis, Pichia fermentans, Rhodotorula rubra, Pichia kudriavzevii, Candida guilliermondii, Saccharomyces cerevisiae, Rhodotorula calyptogenae [80–83]. It should be borne in mind that several of these species are opportunistic pathogens in humans. Even S. cerevisiae can infect immunocompromized individuals and large-scale bioremediation would involve the presence of high concentrations of these potentially dangerous microbes [84,85].
This detailed examination of the Ni, Pb, As and Cd-tolerant yeast P. huenov extends its range of heavy metal tolerance (and removal) to include copper and manganese: two very common heavy metals in the wastewater from many common industrial processes. The species was capable of reducing heavy metals both in bioaccumulation and biosorption. The species has not been shown to be pathogenic in humans, reducing any risk associated with its use. The bioaccumulation characteristics of yeast P. huenov, which was highly tolerant of heavy metals and efficiently removed up to 96.5% of Mn(II) and 89.4% Cu(II) at initial metal concentrations 55 and 64 mg/L of Mn(II) and Cu(II), respectively. The initial pH greatly affected Mn(II) and Cu(II) uptake and maximum adsorption was observed at pH 6.0 and 5.0, respectively. Ideal temperature for maximum uptake capacities was determined at 30 °C for both bioaccumulation and biosorption. In particular, P. huenov biomass was ideal for removing Mn(II) and Cu(II) from aqueous solution in a short time with a high capacity of biosorption 27.79 mg/g and 26.6 mg/g over 60 min, respectively. There was good correlation between the experimental Mn(II) and Cu(II) removal efficiency (%) and the values predicted by the Langmuir isotherm model.
The results indicated that using P. huenov yeast cell for bioaccumulation of Mn(II) and Cu(II) was a viable option for removal of Mn(II) and Cu(II) ions and that the live cells were significantly more effective than using dead biosorbent. Live cells achieved 75.58% and 70.5% removal compared with 60.3% and 56.5% for dead biomass, beginning with initial metal concentrations of 110 mg/L Mn(II) and 128 mg/L Cu(II).
The results can be used to strategize future applications of P. huenov for heavy metal treatment in wastewater.
Supplementary Material
Acknowledgements
The authors thank Dr. Nguyen Thanh Dong from UniCRE, Czech Republic for advice and correct data, Dr. Derek Wilkinson from Charles University, Czech Republic for proofreading of the manuscript.
Funding Statement
This work was supported by Hue University under the Core Research Program, Grant No. [NCM.DHH2019.01] and Grant No. [NCM.DHH2020.13].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Tchounwou PB, Yedjou CG, Patlolla AK, et al. . Heavy metal toxicity and the environment. Mol Clin Environ Toxicol. 2012;101:133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaishankar M, Tseten T, Anbalagan N, et al. . Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern BR. Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health A. 2010;73(2):114–127. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Yang X.. The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev. 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neal SL, Zheng W.. Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep. 2015;2(3):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer GJ. The risks of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer's disease. J Am Coll Nutr. 2009;28(3):238–242. [DOI] [PubMed] [Google Scholar]
- 7.Gupte A, Mumper RJ.. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35(1):32–46. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Chen C.. Biosorbents for heavy metals removal and their future. Biotechnol Adv. 2009;27(2):195–226. [DOI] [PubMed] [Google Scholar]
- 9.Soares EV, Soares HMVM.. Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: a review. Environ Sci Pollut Res Int. 2012;19(4):1066–1083. [DOI] [PubMed] [Google Scholar]
- 10.Mishra A, Malik A.. Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol. 2013;43(11):1162–1222. [Google Scholar]
- 11.Igiri BE, Okoduwa SIR, Idoko GO, et al. . Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J Toxicol. 2018;2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadkertiová R, Molnárová J, Lux A, et al. . Yeasts associated with an abandoned mining area in pernek and their tolerance to different chemical elements. Folia Microbiol. 2016;61(3):199–207. [DOI] [PubMed] [Google Scholar]
- 13.Rehman A, Farooq H, Shakoori AR.. Copper tolerant yeast, Candida tropicalis, isolated from industrial effluents: its potential use in wastewater treatment. Pak J Zool. 2007;39:405. [Google Scholar]
- 14.Rehman A, Anjum MS, Hasnain S.. Cadmium biosorption by yeast, Candida tropicalis CBL-1, isolated from industrial wastewater. J Gen Appl Microbiol. 2010;56(5):359–368. [DOI] [PubMed] [Google Scholar]
- 15.Farhan SN, Khadom AA.. Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int J Ind Chem. 2015;6(2):119–130. [Google Scholar]
- 16.Massoud R, Hadiani MR, Hamzehlou P, et al. . Bioremediation of heavy metals in food industry: application of Saccharomyces cerevisiae. Electron J Biotechnol. 2019;37:56–60. [Google Scholar]
- 17.Fadel M, Hassanein NM, Elshafei MM, et al. . Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. HBRC J. 2017;13(1):106–113. [Google Scholar]
- 18.do Nascimento JM, de Oliveira JD, Rizzo ACL, et al. . Biosorption Cu (II) by the yeast Saccharomyces cerevisiae. Biotechnol Reports. 2019;21:e00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández Mata KM, Monge Amaya O, Certucha Barragán MT, et al. . Metallic biosorption using yeasts in continuous systems. Int J Photoenergy. 2013;2013:1–4. [Google Scholar]
- 20.Amorim SS, Ruas FAD, Barboza NR, et al. . Manganese (Mn2+) tolerance and biosorption by Meyerozyma guilliermondii and Meyerozyma caribbica strains. J Environ Chem Eng. 2018;6(4):4538–4545. [Google Scholar]
- 21.Chen XC, Wang YP, Lin Q, et al. Biosorption of copper(II) and zinc(II) from aqueous solution by Pseudomonas putida CZ1. Colloids Surf B Biointerfaces. 2005;46(2):101–107. [DOI] [PubMed] [Google Scholar]
- 22.Surussawadee J, Khunnamwong P, Srisuk N, et al. . Papiliotrema siamense fa, sp. nov., a yeast species isolated from plant leaves. Int J Syst Evol Microbiol. 2014;64(Pt 9):3058–3062. [DOI] [PubMed] [Google Scholar]
- 23.Hung C-S, Barlow DE, Varaljay VA, et al. . The biodegradation of polyester and polyester polyurethane coatings using Papiliotrema laurentii. Int Biodeterior Biodegradation. 2019;139:34–43. [Google Scholar]
- 24.Nguyen KCT, Nguyen PV, Truong HTH.. Heavy metal tolerance of novel papiliotrema yeast isolated from Vietnamese mangosteen. Mycobiology. 2020;48(4):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilmazer P, Saracoglu N.. Bioaccumulation and biosorption of copper (II) and chromium (III) from aqueous solutions by Pichia stipitis yeast. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 2009;84:604–610. [Google Scholar]
- 26.Iskandar NL, Zainudin NAIM, Tan SG.. Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci. 2011;23(5):824–830. [DOI] [PubMed] [Google Scholar]
- 27.Anand P, Isar J, Saran S, et al. . Bioaccumulation of copper by trichoderma viride. Bioresour Technol. 2006;97(8):1018–1025. [DOI] [PubMed] [Google Scholar]
- 28.Chen SH, Cheow YL, Ng SL, et al. . Bioaccumulation and biosorption activities of indoor Metal-Tolerant Penicillium simplicissimum for removal of toxic metals. Int J Environ Res. 2020;14(2):235–242. [Google Scholar]
- 29.Zamani J, Pournia P, Seirafi HA. A novel feeding method in commercial Baker's yeast production. J Appl Microbiol. 2008;105(3):674–680. [DOI] [PubMed] [Google Scholar]
- 30.Uslu G, Tanyol M.. Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead (II) and copper (II) ions onto Pseudomonas putida: effect of temperature. J Hazard Mater. 2006;135(1–3):87–93. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen PV, Hlaváček O, Maršíková J, et al. . Cyc8p and Tup1p transcription regulators antagonistically regulate Flo11p expression and complexity of yeast colony biofilms. PLoS Genet. 2018;14(7):e1007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parvathi K, Kumar RN, Nagendran R.. Biosorption of manganese by Aspergillus niger and Saccharomyces cerevisiae. World J Microbiol Biotechnol. 2007;23(5):671–676. [Google Scholar]
- 33.Ruas FAD, Amorim SS, Leão VA, et al. . Rhodotorula mucilaginosa isolated from the manganese mine water in Minas Gerais, Brazil: potential employment for bioremediation of contaminated water. Water, Air, Soil Pollut. 2020;231:1–14. [Google Scholar]
- 34.Silva RMP, Rodríguez AÁ, De Oca JMGM, et al. . Biosorption of chromium, copper, manganese and zinc by Pseudomonas aeruginosa AT18 isolated from a site contaminated with petroleum. Bioresour Technol. 2009;100:1533–1538. [DOI] [PubMed] [Google Scholar]
- 35.Ianis M, Tsekova K, Vasileva S.. Copper biosorption by Penicillium cyclopium: equilibrium and modelling study. Biotechnol Biotechnol Equip. 2006;20(1):195–201. [Google Scholar]
- 36.Bağ H, Lale M, Türker AR.. Determination of Cu, Zn and cd in water by FAAS after preconcentration by baker’s yeast (Saccharomyces cerevisiae) immobilized on sepiolite. Fresenius J Anal Chem. 1999;363(3):224–230. [Google Scholar]
- 37.Huang H, Zhao Y, Xu Z, et al. Biosorption characteristics of a highly Mn(II)-resistant Ralstonia pickettii strain isolated from Mn ore. PLoS One. 2018;13(8):e0203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanamarlapudi S, Chintalpudi VK, Muddada S.. Application of biosorption for removal of heavy metals from wastewater. Biosorption. 2018;18:69. [Google Scholar]
- 39.Febrianto J, Kosasih AN, Sunarso J, et al. . Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater. 2009;162(2-3):616–645. [DOI] [PubMed] [Google Scholar]
- 40.Reddi AR, Jensen LT, Naranuntarat A, et al. . The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med. 2009;46(2):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Wang R, Zhan J, et al. . High levels of copper retard the growth of Saccharomyces cerevisiae by altering cellular morphology and reducing its potential for ethanolic fermentation. Int J Food Sci Technol. 2021;56(6):2720–2731. [Google Scholar]
- 42.Dönmez G, Aksu Z.. Bioaccumulation of copper(II) and nickel(II) by the non-adapted and adapted growing Candida sp. Water Res. 2001;35(6):1425–1434. [DOI] [PubMed] [Google Scholar]
- 43.Zhenggang X, Yi D, Huimin H, et al. . Biosorption characteristics of Mn (II) by Bacillus cereus strain HM-5 isolated from soil contaminated by manganese ore. Pol J Environ Stud. 2018;28(1):463–472. [Google Scholar]
- 44.Çolak F, Olgun A, Atar N, et al. . Heavy metal resistances and biosorptive behaviors of Paenibacillus polymyxa: batch and column studies. J Ind Eng Chem. 2013;19(3):863–869. [Google Scholar]
- 45.Dursun AY, Uslu G, Cuci Y, et al. . Bioaccumulation of copper (II), lead (II) and chromium (VI) by growing Aspergillus niger. Process Biochem. 2003;38(12):1647–1651. [Google Scholar]
- 46.Ha J, Gélabert A, Spormann AM, et al. . Role of extracellular polymeric substances in metal ion complexation on Shewanella oneidensis: batch uptake, thermodynamic modeling, ATR-FTIR, and EXAFS study. Geochim Cosmochim Acta. 2010;74(1):1–15. [Google Scholar]
- 47.Nguyen N-T, Sekhon SS, Yoon J, et al. . Effect of heavy metals, pesticides and pharmaceuticals on yeast’s vacuoles as a biomarker for toxic detection. Mol Cell Toxicol. 2017;13(3):287–294. [Google Scholar]
- 48.Machado MD, Soares EV.. Modification of cell volume and proliferative capacity of Pseudokirchneriella subcapitata cells exposed to metal stress. Aquat Toxicol. 2014;147:1–6. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa K, Yamakoshi Y, Uemura I, et al. . Ultrastructural changes in Chlamydomonas acidophila (chlorophyta) induced by heavy metals and polyphosphate metabolism. FEMS Microbiol Ecol. 2003;44(2):253–259. [DOI] [PubMed] [Google Scholar]
- 50.Hasan HA, Abdullah SRS, Kofli NT, et al. . Biosorption of manganese in drinking water by isolated bacteria. J Appl Sci. 2010;10(21):2653–2657. [Google Scholar]
- 51.Ezzouhri L, Ruiz E, Castro E, et al. . Mechanisms of lead uptake by fungal biomass isolated from heavy metals habitats. Afinidad. 2010;67:269007. [Google Scholar]
- 52.Dursun AY, Uslu G, Tepe O, et al. . A comparative investigation on the bioaccumulation of heavy metal ions by growing Rhizopus arrhizus and Aspergillus niger. Biochem Eng J. 2003;15(2):87–92. [Google Scholar]
- 53.Taştan BE, Ertuğrul S, Dönmez G.. Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Bioresour Technol. 2010;101(3):870–876. [DOI] [PubMed] [Google Scholar]
- 54.Dönmez G, Aksu Z.. The effect of copper (II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem. 1999;35(1–2):135–142. [Google Scholar]
- 55.Do TA, Sakai T, Kishida M, et al. . Isolation and characterization of a variant manganese resistant strain of Saccharomyces cerevisiae. Biocontrol Sci. 2016;21(4):253–260. [DOI] [PubMed] [Google Scholar]
- 56.Batic M, Raspor P.. Uptake and bioaccumulation of Cr (III) in yeast Saccharomyces cerevisiae. Food Technol Biotechnol. 1998;36:291–297. [Google Scholar]
- 57.Das D, Charumathi D, Das N.. Bioaccumulation of the synthetic dye basic violet 3 and heavy metals in single and binary systems by Candida tropicalis grown in a sugarcane bagasse extract medium: modelling optimal conditions using response surface methodology (RSM) and inhibition kinetics. J Hazard Mater. 2011;186(2–3):1541–1552. [DOI] [PubMed] [Google Scholar]
- 58.Khan Z, Rehman A, Hussain SZ.. Resistance and uptake of cadmium by yeast, Pichia hampshirensis 4Aer, isolated from industrial effluent and its potential use in decontamination of wastewater. Chemosphere. 2016;159:32–43. [DOI] [PubMed] [Google Scholar]
- 59.Kaduková J, Virčíková E.. Comparison of differences between copper bioaccumulation and biosorption. Environ Int. 2005;31(2):227–232. [DOI] [PubMed] [Google Scholar]
- 60.Barboza NR, Guerra ‐SR, Leão VA.. Mechanisms of manganese bioremediation by microbes: an overview. J Chem Technol Biotechnol. 2016;91(11):2733–2739. [Google Scholar]
- 61.Vijayaraghavan K, Winnie HYN, Balasubramanian R.. Biosorption characteristics of crab shell particles for the removal of manganese (II) and zinc (II) from aqueous solutions. Desalination. 2011;266(1–3):195–200. [Google Scholar]
- 62.Han R, Li H, Li Y, et al. . Biosorption of copper and lead ions by waste beer yeast. J Hazard Mater. 2006;137(3):1569–1576. [DOI] [PubMed] [Google Scholar]
- 63.Wang J-Y, Cui H, Cui C-W, et al. . Biosorption of copper (II) from aqueous solutions by Aspergillus niger-treated rice straw. Ecol Eng. 2016;95:793–799. [Google Scholar]
- 64.Luk CHJ, Yip J, Yuen CWM, et al. Biosorption performance of encapsulated Candida krusei for the removal of copper(II)). Sci Rep. 2017;7(1):2159–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Tian Z, Cheng H, et al. . Adsorption process and mechanism of heavy metal ions by different components of cells, using yeast (Pichia pastoris) and Cu 2+ as biosorption models. RSC Adv. 2021;11(28):17080–17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh S, Kumar V, Datta S, et al. . Current advancement and future prospect of biosorbents for bioremediation. Sci Total Environ. 2020;709:135895. [DOI] [PubMed] [Google Scholar]
- 67.Wierzba S. Biosorption of nickel (II) and zinc (II) from aqueous solutions by the biomass of yeast Yarrowia lipolytica. Polish J Chem Technol. 2017;19(1):1–10. [Google Scholar]
- 68.Salvadori MR, Ando RA, Oller do Nascimento CA, et al. . Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian amazonia. PLoS One. 2014;9(1):e87968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahan T, Ceylan H, Sahiner N, et al. . Optimization of removal conditions of copper ions from aqueous solutions by trametes versicolor. Bioresour Technol. 2010;101(12):4520–4526. [DOI] [PubMed] [Google Scholar]
- 70.Omar HH. Biosorption of copper, nickel and manganese using non-living biomass of marine alga, ulva lactuca. Pak J Biol Sci. 2008;11(7):964–973. [DOI] [PubMed] [Google Scholar]
- 71.Dutta A, Zhou L, Castillo-Araiza CO, et al. . Cadmium (II), lead (II), and copper (II) biosorption on baker’s yeast (Saccharomyces cerevesiae). J Environ Eng. 2016;142:C6015002. [Google Scholar]
- 72.Chang J-S, Law R, Chang C-C.. Biosorption of lead, copper and cadmium by biomass of Pseudomonas aeruginosa PU21. Water Res. 1997;31(7):1651–1658. [Google Scholar]
- 73.Amirnia S, Ray MB, Margaritis A.. Heavy metals removal from aqueous solutions using Saccharomyces cerevisiae in a novel continuous bioreactor–biosorption system. Chem Eng J. 2015;264:863–872. [Google Scholar]
- 74.Hou Y, Cheng K, Li Z, et al. . Biosorption of cadmium and manganese using free cells of Klebsiella sp. isolated from waste water. PLoS One. 2018;13(5):e0198309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sayyadi S, Ahmady-Asbchin S, Kamali K. Biosorption of Cd(II) and Cs(I) from aqueous solution by live and dead cells of Saccharomyces carlsbergensis PTCC 5051. Environ Technol. 2018;39(4):450–456. [DOI] [PubMed] [Google Scholar]
- 76.Torab-Mostaedi M, Asadollahzadeh M, Hemmati A, et al. . Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. J Taiwan Inst Chem Eng. 2013;44(2):295–302. [Google Scholar]
- 77.Savastru E, Zamfir C-I, Diaconu M, et al. Biosorption of Cu (II) Ions from aqueous solution on Saccharomyces cerevisiae biomass: isotherm and kinetics modelling. 2019 E-Health Bioeng Conf. IEEE; 2019. p. 1–4. [Google Scholar]
- 78.Kareem SO, Omeike SO, Balogun SA, et al. . Removal of Mn (II) and Fe (II) by Aspergillus sp. TU-GM14 immobilized on Detarium microcarpum matrix. Glob Nest J. 2014;16:597–608. [Google Scholar]
- 79.Khalilnezhad R, Olya ME, Khosravi M, et al. . Manganese biosorption from aqueous solution by Penicillium camemberti biomass in the batch and fix bed reactors: a kinetic study. Appl Biochem Biotechnol. 2014;174(5):1919–1934. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Wu S, Liu G, et al. . Simultaneous biosorption of cadmium (II) and lead (II) ions by pretreated biomass of Phanerochaete chrysosporium. Sep Purif Technol. 2004;34(1–3):135–142. [Google Scholar]
- 81.Li C-C, Chung H-P, Wen H-W, et al. . The radiation resistance and cobalt biosorption activity of yeast strains isolated from the lanyu low-level radioactive waste repository in Taiwan. J Environ Radioact. 2015;146:80–87. [DOI] [PubMed] [Google Scholar]
- 82.Li C, Yu J, Wang D, et al. . Efficient removal of zinc by multi-stress-tolerant yeast Pichia kudriavzevii A16. Bioresour Technol. 2016;206:43–49. [DOI] [PubMed] [Google Scholar]
- 83.Honfi K, Tálos K, Kőnig-Péter A, et al. . Copper (II) and phenol adsorption by cell surface treated Candida tropicalis cells in aqueous suspension. Water Air Soil Pollut. 2016;227:61. [Google Scholar]
- 84.Van Nguyen P, Plocek V, Váchová L, et al. . Glucose, Cyc8p and Tup1p regulate biofilm formation and dispersal in wild Saccharomyces cerevisiae. NPJ Biofilms Microbiomes. 2020;6(1):7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirayama T, Miyazaki T, Ito Y, et al. . Virulence assessment of six major pathogenic Candida species in the mouse model of invasive candidiasis caused by fungal translocation. Sci Rep. 2020;10(1):3814–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.