Abstract
Rheumatoid arthritis (RA) is a prevalent inflammatory joint disease that imposes a significant medical burden and morbidity. Recent scientific evidence suggests that dietary components and patterns could be associated with RA risk. In this study, we aim to investigate the possible relationship between dietary fiber intake and RA risk. We included 15,114 participants from the 2010 to 2020 National Health and Nutrition Examination Survey database in our study. Participants aged 20 or above were categorized into those with and without RA. Univariate logistic regression analysis and multivariate regression models were used to test the association between dietary fiber intake, high-sensitivity C-reactive protein, and RA. Out of all the participants, 1053 were diagnosed with RA (6.97%). Multivariate logistic regression analysis indicated that fiber intake was negatively associated with high-sensitivity c-reactive protein (−0.09 [−0.18, −0.02]) and RA risk (0.99 [0.98, 0.99]). Furthermore, our sensitivity analysis suggested that individuals with higher fiber intake (>19.1 g/day) had a 25% lower risk of developing RA than those with lower fiber intake [0.75 (0.63, 0.88)]. Our findings suggest that higher dietary fiber intake is associated with a reduced risk of RA and may help reduce systemic inflammation, thereby potentially slowing down RA progression.
Keywords: cross-sectional study, fiber, NHANES, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a complex systemic autoimmune disease characterized by symmetrical inflammatory polyarthritis, which may ultimately result in debilitating joint damage and disability.[1,2] Global incidence rates of RA have increased by roughly 8.2% over the past 3 decades, posing a significant challenge to public health given the aging of populations worldwide.[3] Early identification and prevention of RA are therefore of utmost importance. While the etiology of RA remains elusive, dietary factors have been increasingly recognized as non-negligible contributors to disease pathogenesis.[4]
Dietary fiber, a complex carbohydrate comprised of soluble and insoluble components, exerts beneficial effects through modulation of intestinal microbiota and the ensuing metabolites.[5,6] Accumulating evidence suggests that dietary fiber intake may confer protection against various diseases.[7–15] Moreover, epidemiological investigations have linked fiber consumption to osteoporosis and osteoarthritis.[16,17] However, research investigating the relationship between fiber intake and RA prevalence is scarce in population-based studies, and the underlying mechanisms through which fiber may modulate RA development remain poorly understood. Given the well-documented anti-inflammatory effects of dietary fiber and its plausible involvement in the pathogenesis of RA, we hypothesize that increased fiber intake may reduce the prevalence of RA.
Thus, we conducted a cross-sectional study utilizing data from the National Health and Nutrition Examination Survey (NHANES) spanning 2010 to 2020 to investigate the relationship between dietary fiber intake and RA prevalence.
2. Methods
2.1. Study population
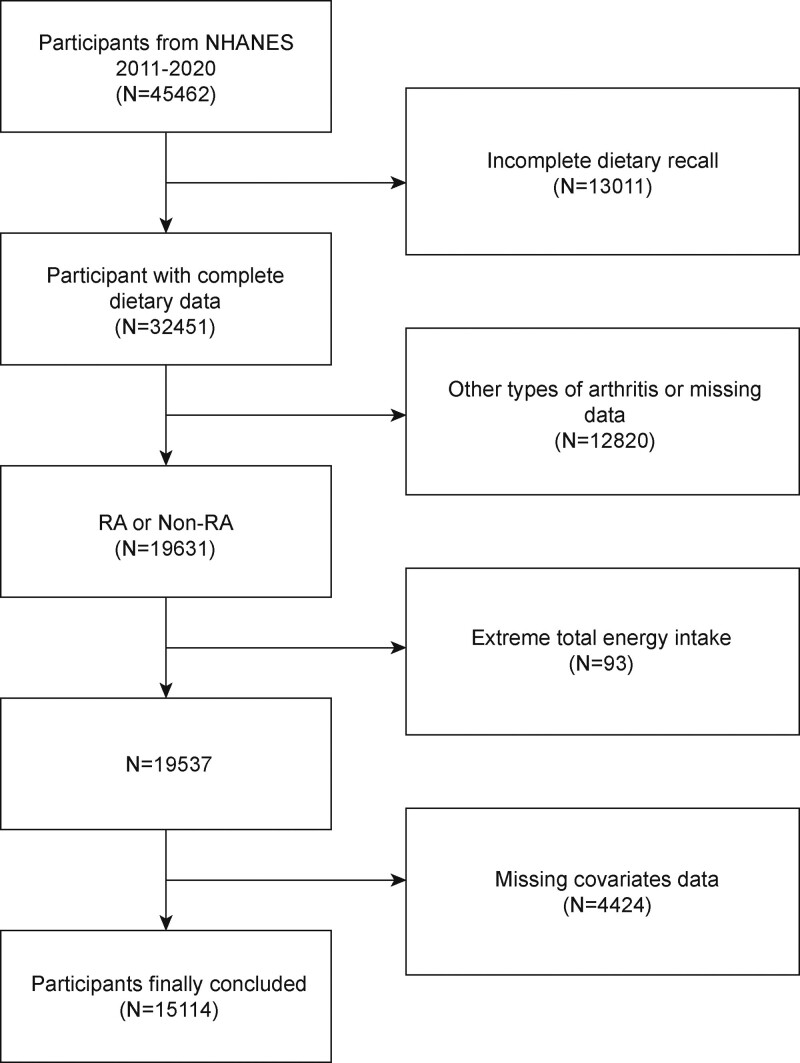
The current study utilizes data from the NHANES, a nationwide survey aimed at investigating the nutrition and health status of individuals in the United States.[18–20] The NHANES is authorized by the National Center for Health Statistics Research Ethics Review Board and all participants provided written consent at the time of recruitment.[21] The study analyzed data from the most recent 5 survey cycles conducted over the past decade. To investigate the association between dietary fiber intake and RA prevalence, the study excluded 12,820 participants without self-reported RA data, 13,011 participants with missing or incomplete dietary intake data, 4424 participants with missing covariates data, and 93 participants with extreme total energy intakes. The final study population included 15,114 participants (Fig. 1).
Figure 1.
Flow chart of participants selection. NHANES = National Health and Nutrition Examination Survey, RA = Rheumatoid arthritis.
2.2. Fiber intake
Dietary fiber intake was determined by 2 24-hour food recall interviews conducted by a qualified dietitian, with the second interview conducted by phone 3 to 10 days later. Fiber intake was calculated as an average of 2 days, adjusted for body weight.[22]
2.3. Rheumatoid arthritis
RA prevalence was assessed by 2 medical condition questionnaires and participants were considered to have RA if they answered “yes” to the question “Has your doctor ever told you have arthritis?” and identified it as rheumatoid arthritis. Self-reported RA has high accuracy and is acceptable in large studies, according to a meta-analysis of 16 epidemiological studies.[23]
2.4. Covariables
Covariates, including age, gender, race, body mass index, family income-to-poverty ratio, moderate activities, waist circumference, energy intake, drinking alcohol status, education level, and smoke status were chosen based on prior knowledge as factors associated with fiber intake and RA. The NHANES Survey Methods and Analysis Guide provides detailed information on variable collection methods.
2.5. Statistical analysis
Statistical analyses were conducted using R (version 4.2) or Empowerstats (version 4.1). The chi-square test and t test were used to assess the demographic characteristics of the samples by RA status. Univariate and multivariate logistic regression analyses were performed to investigate the association between fiber intake, inflammation, and RA prevalence. Generalized additive models and smoothed curve fits were also used to examine the nonlinear association between fiber intake and RA prevalence. Subgroup analyses and interaction effects were implemented to investigate the heterogeneity of the associations between subgroups.[24,25]
3. Results
3.1. Baseline characteristics
At the time of assessment, the mean (SD) age of the 15,114 participants was 46.37 (16.89) years and a total of 1053 participants (6.97%) were diagnosed with RA. A total of 42.26% of participants with RA were male, 35.52% were non-Hispanic white, and 34.57% were non-Hispanic black. and 11.97% were Mexican American. The median fiber intake was 13.6 g/day (range 0–97.4 g/day) for participants with RA and 14.7 g/day (range 0–134.8 g/day) for those without RA. In comparison to the non-RA group, participants with RA are more likely to be females and older; in terms of socioeconomic status, RA participants were more likely to have lower educational attainment and lower income; in terms of lifestyle, a higher proportion of RA participants smoked and drank alcohol, while a lower proportion exercised. In addition, RA participants were more likely to have higher BMI and waist circumference in terms of body size, and lower calorie and dietary fiber intake than non-RA participants (Table 1).
Table 1.
Basic characteristics of participants by RA among U.S. adults.
| Characteristics | RA (1053) | non-RA (14,061) | P value |
|---|---|---|---|
| Age (yr) | 60.51 ± 12.92 | 45.31 ± 16.67 | <.001 |
| Sex, n (%) | <.001 | ||
| Male | 445 (42.26) | 7136 (50.75) | |
| Female | 608 (57.74) | 6925 (49.25) | |
| Race/ethnicity, n (%) | <.001 | ||
| Non-Hispanic white | 374 (35.52) | 4893 (34.80) | |
| Non-Hispanic black | 364 (34.57) | 3400 (24.18) | |
| Mexican American | 126 (11.97) | 1933 (13.75) | |
| Other race/multiracial | 189 (17.94) | 3835 (27.27) | |
| Education level, n (%) | <.001 | ||
| Less than high school | 294 (27.92) | 2528 (17.98) | |
| High school | 262 (24.88) | 3092 (21.99) | |
| More than high school | 497 (47.20) | 8441 (60.03) | |
| Moderate activities, n (%) | <.001 | ||
| Yes | 355 (33.71) | 6266 (44.56) | |
| No | 698 (66.29) | 7795 (55.44) | |
| Smoking status, n (%) | <.001 | ||
| Never | 496 (47.10) | 8635 (61.41) | |
| Ever | 557 (52.90) | 5426 (38.59) | |
| Drinking alcohol status, n (%) | <.001 | ||
| Never | 734 (69.71) | 109,67 (78.00) | |
| Ever | 319 (30.29) | 3094 (22.00) | |
| Family PIR | 2.11 ± 1.55 | 2.59 ± 1.65 | <.001 |
| BMI (kg/m2) | 31.44 ± 7.92 | 28.93 ± 6.91 | <.001 |
| Waist circumference (cm) | 105.65 ± 16.64 | 98.24 ± 16.38 | <.001 |
| Energy intake (kcal/day) | 1817.14 ± 824.21 | 1979.06 ± 943.68 | |
| hs-CRP (mg/L) | 4.34 ± 2.69 | 2.79 ± 2.03 | <.001 |
| Dietary fiber intake (g/day) | 15.37 ± 9.84 | 17.12 ± 11.37 | <.001 |
Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model.
(%) for categorical variables: the P value was calculated by the weighted chi-square test.
BMI = body mass index, Family PIR = the ratio of family income-to poverty, hs-CRP = high-sensitivity c-reactive protein, RA = rheumatoid arthritis.
3.2. Relationship between fiber intake and RA prevalence
Table 2 indicates the results of univariate logistic regression analysis, where all variables were significantly associated with RA prevalence. Among them, there was a negative relationship between fiber intake and RA prevalence (0.98 [0.98, 0.99]). We further converted fiber intake from a continuous variable to a categorical variable (tertile) for sensitivity analysis. Participants in the highest tertile of fiber intake had a statistically significant 27% increased prevalence of RA compared with those in the lowest tertile of fiber intake (0.73 [0.62, 0.85]).
Table 2.
Results of the weighted univariate logistic regression analysis of factors associated with RA.
| Exposure | Statistics | OR (95% CI) |
|---|---|---|
| Dietary fiber intake (g/d) | 16.99 ± 11.28 | 0.98 (0.98, 0.99) |
| Dietary fiber intake tertile | ||
| Tertile 1 (<10.9 g/d) | 5034 (33.31%) | 1.0 (ref) |
| Tertile 2 (11.0–19.1 g/d) | 5036 (33.32%) | 0.85 (0.73, 0.99) |
| Tertile 3 (>19.1 g/d) | 5044 (33.37%) | 0.73 (0.62, 0.85) |
| Sex, n (%) | ||
| Male | 7581 (50.16%) | 1.0 (ref) |
| Female | 7533 (49.84%) | 1.41 (1.24, 1.60) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 5267 (34.85%) | 1.0 (ref) |
| Non-Hispanic black | 3764 (24.90%) | 1.40 (1.20, 1.63) |
| Mexican American | 2059 (13.62%) | 0.85 (0.69, 1.05) |
| Other race/multiracial | 4024 (26.62%) | 0.64 (0.54, 0.77) |
| BMI, n (%) | ||
| <24.9 | 4447 (29.67%) | 1.0 (ref) |
| 25–29.9 | 4885 (32.59%) | 1.60 (1.33, 1.92) |
| ≥30 | 5657 (37.74%) | 2.23 (1.88, 2.64) |
| Age, n (%) | ||
| 20–59 | 11,222 (74.25%) | 1.0 (ref) |
| ≥60 | 3892 (25.75%) | 4.54 (3.99, 5.16) |
| Education, n (%) | ||
| Less than high school | 2822 (18.67%) | 1.0 (ref) |
| High school | 3354 (22.19%) | 0.73 (0.61, 0.87) |
| More than high school | 8938 (59.14%) | 0.50 (0.43, 0.59) |
| Family PIR, n (%) | ||
| <1.2 | 3737 (27.22%) | 1.0 (ref) |
| ≥1.2 | 9993 (72.78%) | 0.59 (0.52, 0.68) |
| Moderate activities, n (%) | ||
| Yes | 6621 (43.81%) | 1.0 (ref) |
| No | 8493 (56.19%) | 0.56 (0.49, 0.63) |
| Smoking status, n (%) | ||
| Never | 9131 (60.41%) | 1.0 (ref) |
| Ever | 5983 (39.59%) | 1.58 (1.39, 1.80) |
| Drinking alcohol status, n (%) | ||
| Never | 11,701 (77.42%) | 1.0 (ref) |
| Ever | 3413 (22.58%) | 0.50 (0.43, 0.59) |
Mean ± standard error (SE) for continuous variables, Percentage (%) for categorical variables. P value was calculated via logistic regression analysis.
PIR = the ratio of family income-to poverty, RA = rheumatoid arthritis.
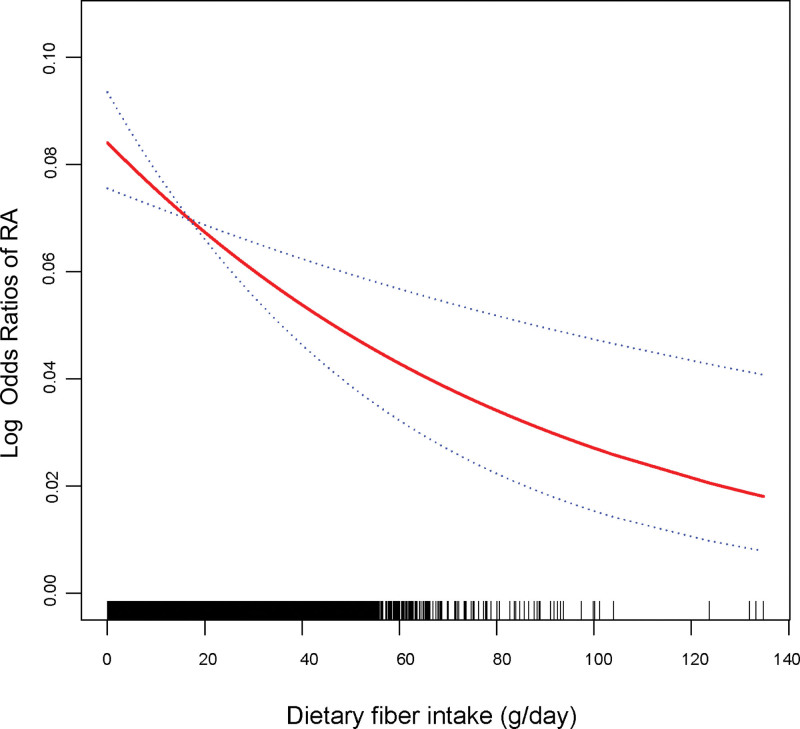
In addition, higher fiber intake remained significantly associated with lower prevalence of RA in multivariate logistic regression analysis (Table 3). In partially adjusted model, participants in the highest tertile had a 21% lower prevalence of RA than lowest (0.79 [0.67, 0.93]). When adjusted for all covariates, the difference in prevalence of RA between the highest tertile and the lowest tertile was 25% (0.75 [0.63, 0.88]). In addition, generalized additive models and smoothed curve fitting were used to further examine the nonlinear relationship between fiber intake and RA prevalence, and the results showed that fiber intake was negatively associated with RA prevalence (Fig. 2). Finally, we further investigated the relationship between fiber intake and high-sensitivity C-reactive protein (hs-CRP), a Inflammatory marker of RA, using multiple logistic regression (Table 4). The results showed a negative correlation between fiber intake and hs-CRP, with a subsequent decrease in hs-CRP of 0.09 mg/L for each 1 g/day increase in fiber intake (−0.09 [−0.18, −0.02]).
Table 3.
Results of the multivariate logistic regression analysis of association between fiber intake and RA.
| Exposure | Model 1 [OR (95%)] | Model 2 [OR (95%)] | Model 3 [OR (95%)] |
|---|---|---|---|
| Dietary fiber intake (g/d) | 0.98 (0.98, 0.99) | 0.99 (0.98, 0.99) | 0.99 (0.98, 0.99) |
| Dietary fiber intake Tertile | |||
| Tertile 1 (<10.9 g/d) | 1.0 | 1.0 | 1.0 |
| Tertile 2 (11.0–19.1 g/day) | 0.85 (0.73, 0.99) | 0.80 (0.69, 0.94) | 0.85 (0.72, 0.99) |
| Tertile 3 (>19.1 g/d) | 0.73 (0.62, 0.85) | 0.79 (0.67, 0.93) | 0.75 (0.63, 0.88) |
Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, educational level, BMI, waist circumference, Energy intake, family income-to-poverty ratio, moderate activities, smoking status and drinking alcohol status were adjusted.
RA = rheumatoid arthritis.
Figure 2.
The association between fiber intake and RA prevalence. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. RA = rheumatoid arthritis.
Table 4.
Results of the multivariate logistic regression analysis of association between fiber intake and Hs-CRP.
| Exposure | Model 1[β (95%CI)] | Model 2[β (95%CI)] | Model 3[β (95%CI)] |
|---|---|---|---|
| Fiber intake (g/d) | −0.12 (−0.22, −0.03) | −0.10 (−0.20, −0.03) | −0.09 (−0.18, −0.02) |
| Categories | |||
| Tertile 1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Tertile 2 | −0.28 (−0.32, −0.23) | −0.25 (−0.34, −0.16) | −0.25 (−0.32, −0.18) |
| Tertile 3 | −0.51 (−0.68, −0.34) | −0.46 (−0.61, −0.30) | −0.50 (−0.66, −0.34) |
Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, educational level, BMI, waist circumference, Energy intake, family income-to-poverty ratio, moderate activities, smoking status and drinking alcohol status were adjusted.
hs-CRP = high-sensitivity c-reactive protein.
3.3. Subgroup analysis
Subgroup analysis was performed to analyze the consistency of the association between fiber intake and RA prevalence in different groups (Table 5). For subgroups by sex, age, BMI, family income-to-poverty ratio, physical activity, smoking status, and alcohol status, a significant association between fiber intake and RA prevalence was detected in each subgroup. In contrast, for subgroups stratified by race and education, no statistically significant associations were found among non-Hispanic blacks, Mexican Americans, and participants with a high school degree. Interaction tests showed that the association between fiber intake and RA prevalence was not significantly different across subgroups, indicating no significant dependence of all covariates on this positive association.
Table 5.
Subgroup analysis of the association between fiber intake and RA.
| Subgroup | Statistics | OR (95% CI) | P value for interaction |
|---|---|---|---|
| Sex, n (%) | .658 | ||
| Male | 7581 (50.16%) | 0.99 (0.98, 1.00) | |
| Female | 7533 (49.84%) | 0.98 (0.97, 0.99) | |
| Age, n (%) | .845 | ||
| 20–59 | 11,222 (74.25%) | 0.98 (0.97, 0.99) | |
| ≥60 | 3892 (25.75%) | 0.98 (0.97, 0.99) | |
| Race/ethnicity, n (%) | .972 | ||
| Non-Hispanic white | 5267 (34.85%) | 0.97 (0.96, 0.99) | |
| Non-Hispanic black | 3764 (24.90%) | 1.00 (0.99, 1.01) | |
| Mexican American | 2059 (13.62%) | 1.00 (0.98, 1.01) | |
| Other race/multiracial | 4024 (26.62%) | 0.98 (0.97, 1.00) | |
| BMI, n (%) | .289 | ||
| <24.9 | 4447 (29.67%) | 0.98 (0.96, 0.99) | |
| 25–29.9 | 4885 (32.59%) | 0.99 (0.98, 1.00) | |
| ≥30 | 5657 (37.74%) | 0.99 (0.98, 1.00) | |
| Family PIR, n (%) | .859 | ||
| <1.2 | 3737 (27.22%) | 0.99 (0.98, 1.00) | |
| ≥1.2 | 9993 (72.78%) | 0.98 (0.97, 0.99) | |
| Education, n (%) | .467 | ||
| Less than high school | 2822 (18.67%) | 0.99 (0.98, 1.00) | |
| High school | 3354 (22.19%) | 1.00 (0.99, 1.01) | |
| More than high school | 8938 (59.14%) | 0.98 (0.97, 0.99) | |
| Smoking status, n (%) | .365 | ||
| Never | 9131 (60.41%) | 0.99 (0.98, 1.00) | |
| Ever | 5983 (39.59%) | 0.98 (0.98, 0.99) | |
| Moderate activities, n (%) | .951 | ||
| Yes | 6621 (43.81%) | 0.99 (0.98, 1.00) | |
| No | 8493 (56.19%) | 0.99 (0.98, 0.99) | |
| Drinking alcohol status, n (%) | .749 | ||
| Never | 11,701 (77.42%) | 0.99 (0.97, 1.00) | |
| Ever | 3413 (22.58%) | 0.99 (0.98, 1.00) |
PIR = the ratio of family income-to poverty, RA = rheumatoid arthritis.
4. Discussion
The present investigation has revealed a discernible linkage between heightened consumption of dietary fiber and reduced levels of the inflammatory marker hs-CRP in individuals with RA, alongside a negative correlation between fiber intake and RA prevalence. These empirical findings proffer the proposition that dietary fiber might potentially influence the progression of RA by mitigating systemic inflammation.
A growing body of data shows that individual dietary components and dietary patterns may have a role in the development of RA.[26] Diet, as an important environmental factor, mediates pathological changes in RA by affecting the gut microbiota, antigen expression, inflammatory and antioxidant defense systems.[27,28] In addition, diet has a significant impact on RA comorbidities such as atherosclerosis,[29] metabolic syndrome[30] and insulin resistance,[31] which can be improved by an appropriate diet. Patients with RA are often recommended to increase their intake of anti-inflammatory nutrients,[32] such as dietary fiber and long-chain fatty acids,[33] which have anti-inflammatory and antioxidant properties and can mitigate the progression of inflammation.[34]
Unfortunately, the quality of diet in RA patients is currently unsatisfactory, and our results found that RA patients had significantly higher DII scores than the average participant alone. Two studies assessing the diet quality of RA patients by the Healthy Eating Index also coincide with our results.[35,36] And dietary fiber intake in RA patients is even worse, with most Americans consuming <50% of the recommended daily level of dietary fiber and even less in RA patients, despite nutritional guidelines encouraging increased dietary fiber intake.[37] Poor dietary quality is associated with the duration and severity of RA symptoms and may also contribute to an increased prevalence of RA.[28,35]
In the past, increased dietary fiber intake was often thought to be beneficial in relieving the symptoms of RA.[38] However, studies on the association between dietary fiber and susceptibility to RA are scarce and controversial. In the EPIC-Norfolk study, a lower intake of fiber-containing foods such as fruits and vegetables was associated with an increased risk of inflammatory polyarthritis,[39] while the results of a case-control study from Greece showed that consumption of cooked vegetables and olive oil were independently and negatively associated with the risk of RA.[40] Some of the past epidemiological studies have been weak and controversial in some aspects of the evidence due to small sample sizes and poor reproducibility, and the present study circumvents these shortcomings in terms of sample size and variable adjustment.
The mechanisms underlying the association between dietary fiber intake and RA prevalence are currently not fully identified. However, evidence from both basic and epidemiological studies suggests that inflammation is a factor that cannot be ignored. Both high-fiber diets, Mediterranean diets and vegan diets as dietary regimens for RA patients have been shown to be strongly associated with reduced levels of inflammation.[38,41] Several large population-based observational studies have discovered a connection between fiber consumption and general indicators of inflammation in both healthy individuals and individuals with particular metabolic diseases.[42–44] In addition, the gut-joint axis in rheumatoid arthritis may explain the association between fiber intake and susceptibility to RA.[45] Intestinal bacteria mediate their beneficial effects through the fermentation of dietary fiber, thereby tilting the metabolic group of the intestinal flora toward an anti-inflammatory pattern.[46] More importantly, dysbiosis may be mediated by affecting tight junction turnover on the intestinal surface, which is considered an important intervention step in the prevention of RA, as disruption of barrier function occurs prior to the onset of arthritis in mice and humans.[47–49]
Our study has some limitations. First, due to the design of the cross-sectional study, we were unable to determine the causal relationship between fiber intake and RA prevalence.[50,51] In addition, self-reported RA diagnoses may lead to data bias and affect the accuracy of conclusions. Despite these shortcomings, our study has several advantages. This study includes data from a large and representative cross-sectional survey, as well as data on dietary intake. What is more, this study confirms the previously observed association between dietary fiber and RA prevalence.
5. Conclusion
In conclusion, our results present a negative association between fiber intake and prevalence of RA. Increasing fiber intake in the daily diet may be beneficial in preventing RA.
Acknowledgements
We thank the National Health and Nutrition Examination Surveys for providing the data.
Author contributions
Conceptualization: Li Liu, Songlin Xie.
Data curation: Li Liu, Songlin Xie.
Formal analysis: Li Liu, Songlin Xie.
Investigation: Li Liu, Songlin Xie.
Methodology: Li Liu, Songlin Xie.
Project administration: Songlin Xie.
Resources: Li Liu, Songlin Xie.
Software: Li Liu.
Supervision: Songlin Xie.
Validation: Songlin Xie.
Visualization: Li Liu.
Writing – original draft: Li Liu.
Writing – review & editing: Songlin Xie.
Abbreviations:
- hs-CRP
- high-sensitivity c-reactive protein
- NHANES
- National Health and Nutrition Examination Survey
- RA
- rheumatoid arthritis
The datasets generated during and/or analyzed during the current study are publicly available.
The studies involving human participants were reviewed and approved by NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
The survey data are publicly available on the internet for data users and researchers throughout the world (www.cdc.gov/nchs/nhanes/).
This study was funded by the Clinical Research Center of Hand and Foot Wound Repair and Functional Reconstruction in Hunan province (2021SK4030); Special Project of Hunan Provincial Health and Family Planning Commission (20201906); and Scientific Research Project of Hunan Health and Family Planning Commission (A2017018).
The authors have no conflicts of interest to disclose.
How to cite this article: Liu L, Xie S. Dietary fiber intake associated with risk of rheumatoid arthritis among U.S. adults: NHANES 2010-2020. Medicine 2023;102:12(e33357).
References
- [1].England BR, Thiele GM, Anderson DR, et al. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320:1360–72. [DOI] [PubMed] [Google Scholar]
- [3].Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. 2019;78:1463–71. [DOI] [PubMed] [Google Scholar]
- [4].Philippou E, Nikiphorou E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun Rev. 2018;17:1074–7. [DOI] [PubMed] [Google Scholar]
- [5].Swann OG, Kilpatrick M, Breslin M, et al. Dietary fiber and its associations with depression and inflammation. Nutr Rev. 2020;78:394–411. [DOI] [PubMed] [Google Scholar]
- [6].Häger J, Bang H, Hagen M, et al. The role of dietary fiber in rheumatoid arthritis patients: a feasibility study. Nutrients. 2019;11:2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soliman GA. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients. 2019;11:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dong W, Yang Z. Association of dietary fiber intake with myocardial infarction and stroke events in US adults: a cross-sectional study of NHANES 2011-2018. Front Nutr. 2022;9:936926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Day AS, Davis R, Costello SP, et al. The adequacy of habitual dietary fiber intake in individuals with inflammatory bowel disease: a systematic review. J Acad Nutr Diet. 2021;121:688–708.e3. [DOI] [PubMed] [Google Scholar]
- [11].Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Anderson JW, Midgley WR, Wedman B. Fiber and diabetes. Diabetes Care. 1979;2:369–77. [DOI] [PubMed] [Google Scholar]
- [13].Sun L, Zhang Z, Xu J, et al. Dietary fiber intake reduces risk for Barrett’s esophagus and esophageal cancer. Crit Rev Food Sci Nutr. 2017;57:2749–57. [DOI] [PubMed] [Google Scholar]
- [14].Yu EYW, Wesselius A, Mehrkanoon S, et al. Grain and dietary fiber intake and bladder cancer risk: a pooled analysis of prospective cohort studies. Am J Clin Nutr. 2020;112:1252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng B, Shen H, Han H, et al. Dietary fiber intake and reduced risk of ovarian cancer: a meta-analysis. Nutr J. 2018;17:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dai Z, Niu J, Zhang Y, et al. Dietary intake of fibre and risk of knee osteoarthritis in two US prospective cohorts. Ann Rheum Dis. 2017;76:1411–9. [DOI] [PubMed] [Google Scholar]
- [17].Zhou T, Sun D, Li X, et al. Genetically determined SCFA concentration modifies the association of dietary fiber intake with changes in bone mineral density during weight loss: the preventing overweight using novel dietary strategies (POUNDS LOST) trial. Am J Clin Nutr. 2021;114:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Y, Xie R, Ou J. A U-shaped association between serum albumin with total triiodothyronine in adults. J Clin Lab Anal. 2022;36:e24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ouyang Y, Quan Y, Guo C, et al. Saturation effect of body mass index on bone mineral density in adolescents of different ages: a population-based study. Front Endocrinol (Lausanne). 2022;13:922903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xie R, Zhang Y, Yan T, et al. Relationship between nonalcoholic fatty liver disease and bone mineral density in adolescents. Medicine (Baltim). 2022;101:e31164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xie R, Liu M. Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol (Lausanne). 2022;13:857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xie R, Liu Y, Wang J, et al. Race and gender differences in the associations between cadmium exposure and bone mineral density in US adults. Biol Trace Elem Res. 2022:3521–5. [DOI] [PubMed] [Google Scholar]
- [23].Xie R, Zhang Y. Association between 19 dietary fatty acids intake and rheumatoid arthritis: results of a nationwide survey. Prostaglandins Leukot Essent Fatty Acids. 2022;188:102530. [DOI] [PubMed] [Google Scholar]
- [24].Xie R, Zhang Y. Index-based calculation or transient elastography to assess the degree of hepatic steatosis and fibrosis. J Nutr. 2022. [DOI] [PubMed] [Google Scholar]
- [25].Xie R, Zhang Y. Is assessing the degree of hepatic steatosis and fibrosis based on index calculations the best choice for epidemiological studies? Environ Pollut. 2022;317:120783. [DOI] [PubMed] [Google Scholar]
- [26].Rondanelli M, Perdoni F, Peroni G, et al. Ideal food pyramid for patients with rheumatoid arthritis: a narrative review. Clin Nutr. 2021;40:661–89. [DOI] [PubMed] [Google Scholar]
- [27].MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].de Hair MJ, Landewé RB, van de Sande MG, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72:1654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- [30].Ferraz-Amaro I, González-Juanatey C, López-Mejias R, et al. Metabolic syndrome in rheumatoid arthritis. Mediators Inflamm. 2013;2013:710928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Castillo-Hernandez J, Maldonado-Cervantes MI, Reyes JP, et al. Obesity is the main determinant of insulin resistance more than the circulating pro-inflammatory cytokines levels in rheumatoid arthritis patients. Rev Bras Reumatol Engl Ed. 2017;57:320–9. [DOI] [PubMed] [Google Scholar]
- [32].Hagfors L, Leanderson P, Sköldstam L, et al. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J. 2003;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gioia C, Lucchino B, Tarsitano MG, et al. Dietary habits and nutrition in rheumatoid arthritis: can diet influence disease development and clinical manifestations? Nutrients. 2020;12:1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Berube LT, Kiely M, Yazici Y, et al. Diet quality of individuals with rheumatoid arthritis using the healthy eating index (HEI)-2010. Nutr Health. 2017;23:17–24. [DOI] [PubMed] [Google Scholar]
- [36].Grimstvedt ME, Woolf K, Milliron BJ, et al. Lower healthy eating index-2005 dietary quality scores in older women with rheumatoid arthritis v. healthy controls. Public Health Nutr. 2010;13:1170–7. [DOI] [PubMed] [Google Scholar]
- [37].Evans CEL. Dietary fibre and cardiovascular health: a review of current evidence and policy. Proc Nutr Soc. 2020;79:61–7. [DOI] [PubMed] [Google Scholar]
- [38].King DE, Egan BM, Woolson RF, et al. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–6. [DOI] [PubMed] [Google Scholar]
- [39].Pattison DJ, Silman AJ, Goodson NJ, et al. Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case-control study. Ann Rheum Dis. 2004;63:843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Linos A, Kaklamani VG, Kaklamani E, et al. Dietary factors in relation to rheumatoid arthritis: a role for olive oil and cooked vegetables? Am J Clin Nutr. 1999;70:1077–82. [DOI] [PubMed] [Google Scholar]
- [41].Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Krishnamurthy VM, Wei G, Baird BC, et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Qi L, van Dam RM, Liu S, et al. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–11. [DOI] [PubMed] [Google Scholar]
- [45].Hvatum M, Kanerud L, Hällgren R, et al. The gut-joint axis: cross reactive food antibodies in rheumatoid arthritis. Gut. 2006;55:1240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hills RD, Jr., Pontefract BA, Mishcon HR, et al. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zaiss MM, Joyce Wu HJ, Mauro D, et al. The gut-joint axis in rheumatoid arthritis. Nat Rev Rheumatol. 2021;17:224–37. [DOI] [PubMed] [Google Scholar]
- [48].Pianta A, Arvikar S, Strle K, et al. Evidence of the immune relevance of prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xie R, Xiao M, Li L, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. 2022;13:925690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Xie R, Huang X, Zhang Y, et al. High low-density lipoprotein cholesterol levels are associated with osteoporosis among adults 20–59 years of age. Int J Gen Med. 2022;15:2261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]