Abstract
To evaluate the effects of various patient characteristics on vessel enhancement on arterio-venous fistula (AVF) computed tomography (CT) angiography (AVF-CT angiography). A total of 127 patients with suspected or confirmed shunt stenosis and internal AVF complications were considered for inclusion in a retrospective cohort study. The tube voltage was 120 kVp, and the tube current was changed from 300 to 770 mA to maintain the image quality (noise index: 14) using automatic tube current modulation. To evaluate the effects of age, sex, body size, and scan delay on the CT number of the brachial artery or vein, we used correlation coefficients and multivariate regression analyses. There was a significant positive correlation between the CT number of the brachial artery or vein and age (R = 0.21 or 0.23, P < .01). The correlations were inverse with the height (r = −0.45 or −0.42), total body weight (r = −0.52 or −0.50), body mass index (r = −0.21 or −0.23), body surface area (body surface area [BSA]; r = −0.56 or −0.54), and lean body weight (r = −0.55 or −0.53) in linear regression analysis (P < .01 for all). There was a significant correlation between the CT number of the brachial artery or vein and scan delay (R = 0.19 or 01.9, P < .01). Only the BSA had significant effects on the CT number in multivariate regression analysis (P < .01). The BSA was significantly correlated with the CT number of the brachial artery or vein on AVF-CT angiography.
Keywords: arterio-venous fistula, CECT, contrast material, CT angiography, MDCT
1. Introduction
In cases of chronic renal failure, patients depend on a well-functioning arterio-venous fistula (AVF) for hemodialysis treatment.[1] Even if an AVF matures, late fistula failure and other complications might occur in the long term.[2,3] The complications associated with access to hemodialysis account for a sizable proportion of these costs and provide challenges for physicians involved in their management of hemodialysis. Vascular access complications lead to substantial morbidity and high rates of hospitalization in these patients.[4] The patency and function of an AVF are related to the prognosis and quality of life of a patient[5]; therefore, early diagnosis and salvage before failure are important.[6]
The most commonly used diagnostic imaging methods for AVF evaluation are digital subtraction angiography, color Doppler ultrasonography, magnetic resonance angiography, and computed tomography (CT) angiography (CTA). Digital subtraction angiography is considered the gold standard for AVF assessment and, if necessary, may be combined with angiographic intervention. Color Doppler ultrasonography is user-dependent and often requires experienced operators and interpreters.[7] The disadvantage of magnetic resonance imaging is its limited availability. Multi-detector CT is a fast, noninvasive examination with confirmed accuracy,[8–10] and the clinical experience in many medical centers is also encouraging.[11,12]
A fixed contrast material dose and fixed rate of contrast material administration are widely used for AVF-CTA. However, contrast enhancement is affected by patient characteristics, including age, sex, body size, and other pathological conditions.[13] Acquisition parameters, such as the table movement speed and circulating time, must be considered. Therefore, the effect of patient characteristics on vessel enhancement may differ between AVF-CTA and other CTA studies.
The purpose of this study was to evaluate the effects of various patient characteristics on vessel enhancement on AVF-CTA.
2. Materials and method
This retrospective study was approved by our institutional review board, and informed consent was waived.
2.1. Patients receiving dialysis
Between January 2015 and December 2017, 148 consecutive patients with suspected or confirmed shunt stenosis and internal AVF complications were considered for inclusion in our study. We excluded 17 patients with allergic reactions to iodinated contrast (n = 6) and when a leg vein was the injection location (n = 15). Finally, 127 patients were enrolled in this study.
2.2. CT protocol and image reconstruction
A 64-detector row CT scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI) was used to scan all patients receiving dialysis. Internal AVF-CTA was performed from the neck to the palm in the craniocaudal direction. The tube voltage was 120 kVp, and the tube current was changed from 300 to 770 mA to maintain the image quality (noise index: 14) using automatic tube current modulation. The scanning parameters were as follows: 0.5-second rotation, 1.25-mm detector row width, 0.986 helical pitch, and 50-cm scan field of view. The scanning time varied from 17.0 seconds to 24.0 seconds.
2.3. Injection protocols
Using a power injector (Dual Shot; Nemoto-Kyorindo, Tokyo, Japan), we delivered contrast material (Omnipaque-300; Daiichi-Sankyo, Tokyo, Japan) via a 22-gauge catheter into the antecubital vein without the shunt side. For AVF-CTA, 100 mL of contrast material was intravenously administered at an injection rate of 3.3 mL/second. This was followed by 20 mL of saline solution delivered at the same injection rate. We used a computer-assisted bolus tracking technique to synchronize the initiation of scanning with the arrival of contrast material at the brachial vein at the level of the elbow. To monitor the arrival, we performed axial scans of the brachial vein at the elbow level 15 seconds after the initiation of contrast material injection. Scanning was initiated automatically after enhancement reached 200 Hounsfield units (HU) in a region of interest (ROI) within the brachial vein.
2.4. Data analysis
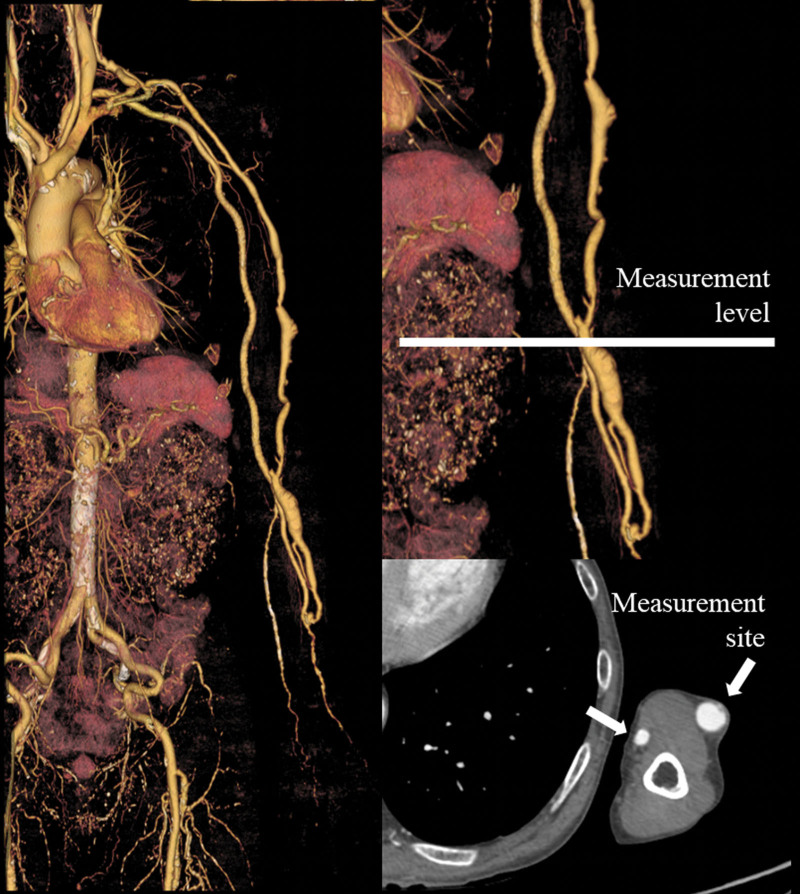
The ROI of the brachial artery or vein was determined by 1 observer. However, this was determined by 1 observer thrice. We measured the CT number of the brachial artery or vein at the elbow level thrice on 3 consecutive slices for all patients, and all CT numbers were averaged (Fig. 1). The mean CT number and scan delay at the brachial artery or vein were recorded for 127 patients undergoing AVF-CTA. An attempt was made to maintain a constant ROI area of approximately 7.0 mm2. The ROI range was 3.1 to 12.6 mm2. We acquired the patients age and sex from their electronic health records. Their total body weight (TBW) and height (HT) were measured prior to CTA scanning. Others[14,15] have suggested that protocols tailored to the body surface area (BSA) and lean body weight (LBW) reduce the effect of body size on vessel enhancement; therefore, we also evaluated the body mass index (BMI), BSA, and LBW. We used the Mosteller formula[16] to calculate the BSA [BSA (m2) = (square root of the product of the weight (kg) × height (cm)/ 60]. The LBW[17,18] was calculated using the formula (1.10 × W)–[128 (W2/(100 × H)[2]] for men and (1.07 × W)–[148 (W2/(100 × H)[2]] for women, where W is the weight in kilograms and H is the height in meters.
Figure 1.
CT attenuation measurement on AVF-CTA. (A) Volume-rendering image of AVF-CTA in a 74-year-old man. (B) Measurement sites of the volume-rendering image of the brachial artery or vein at the level of the elbow. (C) Measurement sites of axial images of the brachial artery or vein at the level of the elbow. AVF-CTA = arterio-venous fistula computed tomography angiography, CT = computed tomography.
2.5. Statistical analysis
All continuous variables are presented as means ± standard deviations. We used Student t test to compare continuous variables and the chi-square and Fisher exact tests to compare categorical and skewed variables. The relationships among the patients age, sex, TBW, BMI, BSA, LBW, shunt site, scan delay, and CT number of the brachial artery or vein were investigated using correlation coefficients. We calculated the Pearson product-moment correlation coefficient (r) to assess the strength of the associations. One representative index (TBW, BMI, BSA, or LBW) was then selected for multivariate regression analysis using the Pearson product-moment correlation coefficient because the correlation coefficient between these factors tends to be high, and multicollinearity may result in wide confidence intervals and anomalous P values for independent variables. Welch t test was used to compare the mean CT number of the brachial artery or vein on AVF-CTA of men and women. To determine independent factors (i.e., age, sex, BSA, shunt site, and scan delay) with an effect on the enhancement and CT number of the brachial artery or vein, we performed multivariate linear regression analysis. We used the standardized coefficient (β) to assess the strength of associations in the multivariate linear regression analysis.
Variables with P values <.05 were considered statistically significant. Statistical analyses were performed using R software (version 3.2.2; The R Project for Statistical Computing; http://www.r-project.org/). A nomogram was formulated based on the results of the binomial logistic model using the “RMS” package in “R.”
3. Results
This retrospective study was approved by our institutional review board.
Informed patient consent was waived.
A summary of the patient characteristics is presented in Table 1. Of the 127 patients, 44 were female and 83 were male; their age ranged from 38 to 95 years (mean, 72.3 ± 12.3 years). Their HT ranged from 115.0 to 178.2 cm (mean, 157.2 ± 11.5 cm) and TBW from 32.0 to 88.0 kg (mean, 53.0 ± 11.1 kg). Their BMI ranged from 13.5 to 34.8 kg/m2 (mean, 21.5 ± 4.0 kg/m2), BSA from 2.0 to 3.5 m2 (mean, 2.7 ± 0.3 m2), and LBW from 28.5 to 59.9 kg (mean, 43.3 ± 7.2 kg). The scan delay ranged from 23.0 to 65.0 seconds (mean, 36.6 ± 10.2 seconds).
Table 1.
Patient characteristics.
| Patient characteristics | n = 127 |
|---|---|
| Female/ male | 44/ 83 |
| Age (yr) | 72.3 ± 12.3 |
| HT (cm) | 157.2 ± 11.5 |
| TBW (kg) | 53.0 ± 11.1 |
| BMI (kg/m2) | 21.5 ± 4.0 |
| BSA (m2) | 2.7 ± 0.3 |
| LBW (kg) | 43.3 ± 7.2 |
| Scan delay (s) | 36.6 ± 10.2 |
BMI = body mass index, BSA = body surface area, HT = height, LBW = lean body weight, TBW = total body weight.
3.1. Univariate analysis
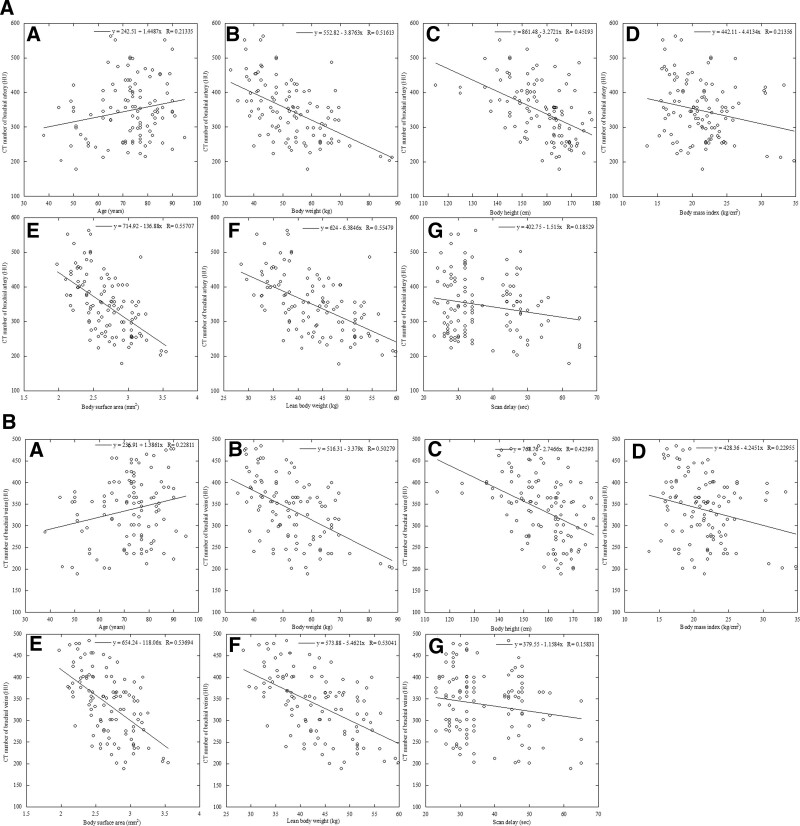
Univariate linear regression was used to assess the association between the CT number of the brachial artery or vein (dependent variable) and all contrast enhancement parameters (independent variables), except the scan delay (Fig. 2A–B). There was a significant positive correlation between the CT number of the brachial artery or vein and age (R = 0.21 or 0.23, P < .01). The correlations were inverse with the HT (r = −0.45 or −0.42), TBW (r = −0.52 or −0.50), BMI (r = −0.21 or −0.23), BSA (r = −0.56 or −0.54), and LBW (r = −0.55 or −0.53) in linear regression analysis (P < .01 for all). There was a significant correlation between the CT number of the brachial artery or vein and scan delay (R = 0.19 or 01.9, P < .01) (Table 2). The mean CT number of the brachial artery or vein was significantly higher in women than in men (345.7 ± 71.9 vs 315.1 ± 76.7 HU, P < .01). The mean CT number of the brachial artery or vein exhibited no significant differences in the shunt site, such as the right shunt or left shunt (337.2 ± 77.8 vs 322.3 ± 75.6 HU, P = .56). We used the BSA as a representative index of body size for multivariate regression analysis because its correlation coefficient was higher than those of the TBW, BMI, and LBW.
Figure 2.
Relationships between brachial artery and vein enhancement and patient characteristics. Scattergrams of the relationship between arterial enhancement obtained using the protocol involving a fixed dose of iodinated contrast material and the patients age (A), height (B), total body weight (C), body mass index (D), body surface area (E), lean body weight (F), and scan delay (G). There was a significant positive correlation between the CT number of the brachial artery or vein and age (R = 0.21 or 0.23, P < .01). The correlations were inverse with the height (r = −0.45 or −0.42), total body weight (r = −0.52 or −0.50), body mass index (r = −0.21 or −0.23), body surface area (r = −0.56 or −0.54), and lean body weight (r = −0.55 or −0.53) in linear regression analysis (P < .01 for all). There was a significant correlation between the CT number of the brachial artery or vein and scan delay (R = 0.19 or 01.9, P < .01). CT = computed tomography.
Table 2.
Effect of age, HT, TBW, BMI, BSA, LBW, and scan delay on the CT number of the brachial artery and vein.
| Patient index used to adjust the iodine concentration with the brachial artery | Correlation coefficient (r) |
|---|---|
| BSA (m2) | 0.56 |
| LBW (kg) | 0.55 |
| TBW (kg) | 0.52 |
| HT (cm) | 0.45 |
| BMI (kg/m2) | 0.21 |
| Age (yr) | 0.21 |
| Scan delay (s) | 0.19 |
| Patient index used to adjust the iodine concentration with the brachial vein | Correlation coefficient (r) |
| BSA (m2) | 0.54 |
| LBW (kg) | 0.53 |
| TBW (kg) | 0.50 |
| HT (cm) | 0.42 |
| BMI (kg/m2) | 0.23 |
| Age (yr) | 0.23 |
| Scan delay (s) | 0.19 |
BMI = body mass index, BSA = body surface area, CT = computed tomography, HT = height, LBW = lean body weight, TBW = total body weight.
3.2. Multivariate linear regression analysis
Using multivariate linear regression analysis, the patient’s sex, age, BSA, shunt site, and scan delay were the independent variables to avoid overestimating potentially linked variables. As shown in Table 3, which shows a multivariate linear regression analysis, only the BSA maintained the independent predictive values (BSA, P < .01). Standardized regressions (BSA, 0.79) suggested that the effect of the BSA was higher than that of the other variables.
Table 3.
Multivariate linear regression analysis of the effect of patient characteristics on the mean CT number of the brachial artery and vein.
| Multivariate regression analysis | |||
|---|---|---|---|
| Estimate (CI) | std. Beta | P value | |
| (Intercept) | 584.51 (226.38 to 890.21) | <.001 | |
| Sex (m/f) | 3.71 (−0.14 to 0.24) | −0.09 | .795 |
| Age (yr) | 0.46 (−0.09 to 0.24) | −0.16 | .380 |
| BSA (m2) | −96.13 (−0.61 to −0.14) | 0.79 | <.001 |
| Shunt side | −0.60 (−0.17 to 0.15) | 0.02 | .996 |
| Scan delay (s) | −0.96 (−0.28 to 0.04) | 0.26 | .116 |
| Observations | 127 | ||
BSA = body surface area, CI = confidence interval, CT = computed tomography, f = female, m = male, std = standardized.
4. Discussions and conclusion
This study suggested that based on correlation coefficients, the patients age, HT, TBW, BMI, BSA, and LBW were significantly related to the CT number of the brachial artery or vein on AVF-CTA. Likewise, multivariate linear regression analysis demonstrated that the patients BSA significantly affected the enhancement of the brachial vessel on AVF-CTA.
The BSA was the body size index most strongly correlated with brachial vessels on AVF-CTA. According to Bae et al,[19] for cardiovascular CT, the BSA allows for better adjustment of the iodine dose across a wide range of body sizes than that associated with the BW. Onishi et al[20] and Yanaga et al[14] found that determining the contrast material dose based on the BSA reduces patient-to-patient variability in aortic enhancement. Kidoh et al[21] reported that among various body size indices, the BSA is most strongly correlated with aortic attenuation during the arterial phase. We think that using the BSA may help identify patients with poor vessel enhancement on AVF-CTA. In similar peripheral blood vessels, the BSA, cardiac output (CO), and age are significantly correlated with the CT number of the popliteal artery on lower-extremity CT angiography.[22] A key physiological parameter that affects arterial enhancement is the CO,[22–25] which is closely related to the BSA.[23,26,27] Bae[13] suggested that the blood volume and CO directly affect vessel enhancement by contrast material and that there are complex relationships among the body size, age, and sex on the 1 hand and blood volume and CO on the other. They stated that these relationships are indirectly involved in changes in vessel enhancement. We have yet to confirm the contrast enhancement of AVF and CO levels, however, it is imperative to authenticate these measures in future investigations.
To our knowledge, this is the first clinical study to evaluate AVF-CTA images. The global prevalence of end-stage renal disease treated with maintenance dialysis exceeds 2 million individuals.[28] Recognition of these limitations has prompted a search for alternatives to conventional thrice-weekly hemodialysis that may improve patient-centered clinical outcomes, including survival, treatment burden, and quality of life. Accumulating evidence suggests that longer and/or more frequent dialysis may benefit patients through improvements in metabolic parameters, reduced left ventricular mass, and greater blood pressure control.[29–34] The most common complications include infection, stenosis, thrombosis, arterial steal syndrome, aneurysms, and pseudoaneurysms. Diagnostic imaging plays a critical role in the management of hemodialysis access and promotes the early management of complications. Therefore, it is necessary to establish diagnoses with the optimum contrast effect for AVF-CTA images.
This study had some limitations. The range and mean BSA of the Japanese patients were lower than those of North American and European individuals. This was a single-center study with a small sample size. Lastly, we did not investigate the relationship between contrast enhancement and image quality. In addition, we did not evaluate the image quality with respect to the severity of AVF on CTA, such as obstruction, severe narrowing, or mild narrowing.
In conclusion, a patient’s BSA significantly affects the CT number of the brachial artery or vein on AVF-CTA.
Author contributions
Conceptualization: Takanori Masuda, Takeshi Nakaura, Yoshinori Funama, Tomoyasu Sato, Keiko Arao, Hiromasa Imaizumi, Shinichi Arao.
Data curation: Takanori Masuda, Takeshi Nakaura, Yoshinori Funama, Shouko Masuda, Takayuki Yoshiura, Rumi Gotanda, Hiromasa Imaizumi.
Formal analysis: Takanori Masuda, Takeshi Nakaura, Takayuki Yoshiura, Rumi Gotanda.
Investigation: Takanori Masuda, Yoshinori Funama, Takayuki Yoshiura, Rumi Gotanda, Keiko Arao, Hiromasa Imaizumi, Kazuo Awai.
Methodology: Takanori Masuda, Takeshi Nakaura, Takayuki Yoshiura.
Project administration: Takanori Masuda, Takeshi Nakaura, Yoshinori Funama, Tomoyasu Sato, Takayuki Yoshiura, Keiko Arao, Junichi Hiratsuka, Atsushi Ono.
Resources: Takanori Masuda.
Software: Takanori Masuda, Takeshi Nakaura, Tomoyasu Sato, Shinichi Arao, Junichi Hiratsuka.
Supervision: Takanori Masuda, Tomoyasu Sato.
Validation: Takanori Masuda, Tomoyasu Sato.
Writing – original draft: Takanori Masuda, Takeshi Nakaura, Shouko Masuda, Kazuo Awai.
Writing – review & editing: Takanori Masuda, Takeshi Nakaura, Shouko Masuda, Kazuo Awai.
Abbreviations:
- AVF
- arterio-venous fistula
- BMI
- body mass index
- BSA
- body surface area
- CO
- cardiac output
- CT
- computed tomography
- CTA
- CT angiography
- HT
- height
- HU
- Hounsfield units
- LBW
- lean body weight
- ROI
- region of interest
- TBW
- total body weight
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Masuda T, Nakaura T, Funama Y, Sato T, Masuda S, Yoshiura T, Gotanda R, Arao K, Imaizumi H, Arao S, Ono A, Hiratsuka J, Awai K. Effect of patient characteristics on vessel enhancement on arterio-venous fistula CT angiography in a retrospective cohort study. Medicine 2023;102:12(e33328).
Contributor Information
Takeshi Nakaura, Email: kff00712@nifty.com.
Yoshinori Funama, Email: funama@kumamoto-u.ac.jp.
Tomoyasu Sato, Email: tomoyasu_satou@yahoo.co.jp.
Shouko Masuda, Email: syoukomasuda@yahoo.co.jp.
Takayuki Yoshiura, Email: momotaro137koko@yahoo.co.jp.
Rumi Gotanda, Email: gotandar@mw.kawasaki-m.ac.jp.
Keiko Arao, Email: arao@jc.kawasaki-m.ac.jp.
Hiromasa Imaizumi, Email: imaizumi@mw.kawasaki-m.ac.jp.
Shinichi Arao, Email: arao@jc.kawasaki-m.ac.jp.
Atsushi Ono, Email: a_ono@mw.kawasaki-m.ac.jp.
Junichi Hiratsuka, Email: hiratuka@med.kawasaki-m.ac.jp.
Kazuo Awai, Email: awai@hiroshima-u.ac.jp.
References
- [1].Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4, Supplement 2):S1–S164. [DOI] [PubMed] [Google Scholar]
- [2].Mercado C, Salman L, Krishnamurthy G, et al. Early and late fistula failure. Clin Nephrol. 2008;69:77–83. [DOI] [PubMed] [Google Scholar]
- [3].Salahi H, Fazelzadeh A, Mehdizadeh A, et al. Complications of arteriovenous fistula in dialysis patients. Transplant Proc. 2006;38:1261–4. [DOI] [PubMed] [Google Scholar]
- [4].Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S248–273. [DOI] [PubMed] [Google Scholar]
- [5].Lorenzo V, Martn M, Rufino M, et al. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. Am J Kidney Dis. 2004;43:999–1007. [DOI] [PubMed] [Google Scholar]
- [6].Allon M, Robbin ML. Hemodialysis vascular access monitoring: current concepts. Hemodial Int. 2009;13:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Visciano B, Riccio E, De Falco V, et al. Complications of native arteriovenous fistula: the role of color doppler ultrasonography. Ther Apher Dial. 2014;18:155–61. [DOI] [PubMed] [Google Scholar]
- [8].Heye S, Maleux G, Claes K, et al. Stenosis detection in native hemodialysis fistulas with MDCT angiography. AJR Am J Roentgenol. 2009;192:1079–84. [DOI] [PubMed] [Google Scholar]
- [9].Ko SF, Huang CC, Ng SH, et al. MDCT angiography for evaluation of the complete vascular tree of hemodialysis fistulas. AJR Am J Roentgenol. 2005;185:1268–74. [DOI] [PubMed] [Google Scholar]
- [10].Karadeli E, Tarhan NC, Ulu EM, et al. Evaluation of failing hemodialysis fistulas with multidetector CT angiography: comparison of different 3D planes. Eur J Radiol. 2009;69:184–92. [DOI] [PubMed] [Google Scholar]
- [11].Neyman EG, Johnson PT, Fishman EK. Hemodialysis fistula occlusion: demonstration with 64-slice CT angiography. J Comput Assist Tomogr. 2006;30:157–9. [DOI] [PubMed] [Google Scholar]
- [12].Dohan A, Dautry R, Guerrache Y, et al. Three-dimensional MDCT angiography of splanchnic arteries: pearls and pitfalls. Diagn Interventional Imaging. 2015;96:187–200. [DOI] [PubMed] [Google Scholar]
- [13].Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256:32–61. [DOI] [PubMed] [Google Scholar]
- [14].Yanaga Y, Awai K, Nakaura T, et al. Contrast material injection protocol with the dose adjusted to the body surface area for MDCT aortography. AJR Am J Roentgenol. 2010;194:903–8. [DOI] [PubMed] [Google Scholar]
- [15].Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:F632–6. [DOI] [PubMed] [Google Scholar]
- [16].Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- [17].Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19:389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hallynck TH, Soep HH, Thomis JA, et al. Should clearance be normalized to body surface or to lean body mass? Br J Clin Pharmacol. 1981;11:523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bae KT, Seeck BA, Hildebolt CF, et al. Contrast enhancement in cardiovascular MDCT: effect of body weight, height, body surface area, body mass index, and obesity. AJR Am J Roentgenol. 2008;190:777–84. [DOI] [PubMed] [Google Scholar]
- [20].Onishi H, Murakami T, Kim T, et al. Abdominal multi-detector row CT: effectiveness of determining contrast medium dose on basis of body surface area. Eur J Radiol. 2011;80:643–7. [DOI] [PubMed] [Google Scholar]
- [21].Kidoh M, Nakaura T, Oda S, et al. Contrast enhancement during hepatic computed tomography: effect of total body weight, height, body mass index, blood volume, lean body weight, and body surface area. J Comput Assist Tomogr. 2013;37:159–64. [DOI] [PubMed] [Google Scholar]
- [22].Masuda T, Nakaura T, Funama Y, et al. Effect of patient characteristics on vessel enhancement at lower extremity CT angiography. Korean J Radiol. 2018;19:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bae KT, Heiken JP, Brink JA. Aortic and hepatic contrast medium enhancement at CT. Part II. Effect of reduced cardiac output in a porcine model. Radiology. 1998;207:657–62. [DOI] [PubMed] [Google Scholar]
- [24].Makita O, Yamashita Y, Arakawa A, et al. Diffuse perfusion abnormality of the liver parenchyma on angiography-assisted helical CT in relation to cirrhosis and previous treatments: a potential diagnostic pitfall for detecting hepatocellular carcinoma. Clin Imaging. 2000;24:292–7. [DOI] [PubMed] [Google Scholar]
- [25].Masuda T, Nakaura T, Funama Y, et al. Aortic and hepatic contrast enhancement during hepatic-arterial and portal venous phase computed tomography scanning: multivariate linear regression analysis using age, sex, total body weight, height, and cardiac output. J Comput Assist Tomogr. 2017;41:309–14. [DOI] [PubMed] [Google Scholar]
- [26].Sawyer M, Ratain MJ. Body surface area as a determinant of pharmacokinetics and drug dosing. Invest New Drugs. 2001;19:171–7. [DOI] [PubMed] [Google Scholar]
- [27].de Simone G, Devereux RB, Daniels SR, et al. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95:1837–43. [DOI] [PubMed] [Google Scholar]
- [28].Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–9. [DOI] [PubMed] [Google Scholar]
- [31].Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61:2235–9. [DOI] [PubMed] [Google Scholar]
- [32].Johansen KL, Zhang R, Huang Y, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int. 2009;76:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lacson E, Jr., Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]