Abstract
A well-established model for how plants start the process of flowering in periods of cold weather may need revisiting.
Research organism: A. thaliana
Related research article Jeon M, Jeong G, Yang Y, Luo X, Jeong D, Kyung J, Hyun Y, He Y, Lee I. 2023. Vernalization-triggered expression of the antisense transcript COOLAIR is mediated by CBF genes. eLife 12:e84594. doi: 10.7554/eLife.84594.
The timing of flowering is one of the most important transitions during the life of a plant. Synchronising the flowering period with warmer temperatures enhances the reproductive success of a plant (and its impact on pollinators). To do so, plants must be able to sense cold temperatures, distinguish between short- and long-term periods of cold, and remember when these changes take place. This requires a complex interplay between internal regulators and environmental cues (Simpson and Dean, 2002; Wellmer and Riechmann, 2010).
In some plants, such as the model plant Arabidopsis thaliana, prolonged periods of cold lasting several weeks are required to initiate flowering via a process known as vernalisation. During this time, a gene called Flowering Locus C (FLC), which acts as a brake to flowering, gets switched off by epigenetic processes that stably hold the gene in the off position long after the vernalisation period (Michaels and Amasino, 1999; Sheldon et al., 1999; Michaels and Amasino, 2000; Whittaker and Dean, 2017).
Several decades of intensive research using a wide range of techniques has led to a well-established model for how the flowering block imposed by FLC is regulated. A long, non-coding RNA, called COOLAIR, is thought to play an important role in this model. Since COOLAIR is an antisense RNA with a complementary sequence of nucleotides to FLC, it is assumed to be an ideal candidate for suppressing the expression of this gene. Now, in eLife, Ilha Lee and colleagues – including Myeongjune Jeon and Goowon Jeong as joint first authors – report new results that challenge this theory (Jeon et al., 2023).
To better understand the role of COOLAIR in vernalisation, the researchers – who are based at the Seoul National University, the Chinese Academy of Sciences, and the Peking University Institute of Advanced Agricultural Sciences – did a combination of molecular, genetic and physiology experiments before, during and after vernalisation in A. thaliana plants and cells to explore the role of COOLAIR and some DNA transcription factors, known as CBFs, in vernalisation.
In a search for factors involved in the early stages of the vernalisation process, Jeon et al. focussed on the cold signal transduction that regulates the transcription of COOLAIR. The results revealed that the CBFs are required for COOLAIR transcription (Figure 1). CBF proteins have been known to play a central role for plants to increase their tolerance to freezing but they had not been linked to the vernalisation response before. Moreover, the CBF-binding DNA sequence in the COOLAIR promoter (a critical sequence for transcription) is conserved amongst different plant species, suggesting the CBF-COOLAIR regulator mechanism evolved some significant time ago.
Figure 1. Vernalisation in Arabidopsis thaliana.
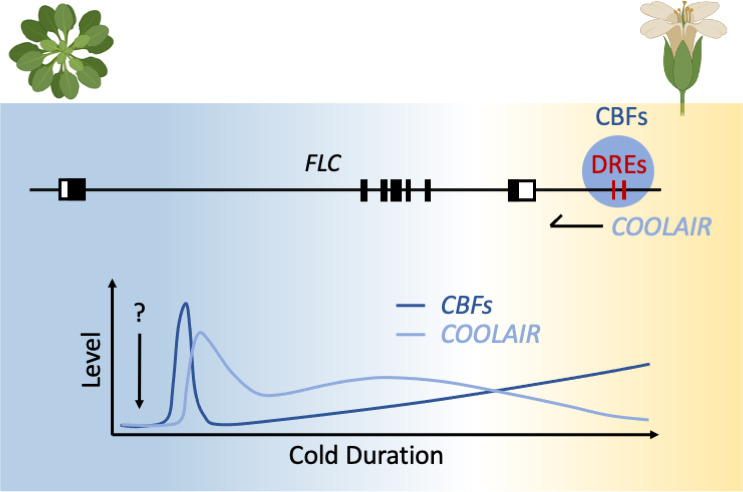
Some plants need a prolonged period of cold lasting several weeks to permit flowering in a process known as vernalisation. In A. thaliana, a gene called FLC stops plants from flowering during winter. During this time, FLC is highly expressed (black bars), but this activity is reduced after vernalisation. Jeong et al. show that early in the vernalisation process, CBF proteins (dark blue) bind to conserved DNA sequences (DREs) at the end of FLC to transcribe a long non-coding RNA, called COOLAIR (light blue). During vernalisation, the amount of CBFs increases, while COOLAIR levels decrease. The upstream regulator of CBF transcription remains unknown (shown as question mark).
In genetically modified plants that only had inactive forms of the genes coding for CBFs, cold-induced expression of COOLAIR was severely impaired. In modified plants with overactive CBF-coding genes, COOLAIR levels were high even when temperatures were warm. Unexpectedly, Jeon et al. found that these genetically modified plants were still able to go through vernalisation, even though they were unable to produce COOLAIR during the cold period. In addition, well described epigenetic histone modifications responsible for switching off the FLC gene remained unchanged before and after vernalisation in the genetically modified plants. This suggest that neither COOLAIR, nor the CBF transcription factors that transcribe COOLAIR, are required to induce vernalisation.
Jeon et al. used an elegant combination of genetic and molecular experiments to paint a compelling picture that suggests that the previously well-established vernalisation model requires updating. This is also in accordance with recent research, suggesting a similar theory (Helliwell et al., 2011; Luo et al., 2019). There are only a few genes where an in-depth analysis of how they are controlled has improved our overall knowledge of gene regulation. These include the trp operon genes in Escherichia coli, which regulate synthesis of the essential amino acid tryptophan and the beta-globin locus in mammals, famous for their involvement in the transport of oxygen (Yanofsky, 1981; Myers et al., 1986). It is clear the FLC locus also deserves a spot on this list.
Biographies
Vy Nguyen is in the Department of Molecular and Biomedical Sciences, School of Biological Sciences, The University of Adelaide, Adelaide, Australia
Iain Searle is in the Department of Molecular and Biomedical Sciences, School of Biological Sciences, The University of Adelaide, Adelaide, Australia
Competing interests
No competing interests declared.
References
- Helliwell CA, Robertson M, Finnegan EJ, Buzas DM, Dennis ES. Vernalization-repression of Arabidopsis FLC requires promoter sequences but not antisense transcripts. PLOS ONE. 2011;6:e21513. doi: 10.1371/journal.pone.0021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon M, Jeong G, Yang Y, Luo X, Jeong D, Kyung J, Hyun Y, He Y, Lee I. Vernalization-triggered expression of the antisense transcript COOLAIR is mediated by CBF genes. eLife. 2023;12:e84594. doi: 10.7554/eLife.84594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Chen T, Zeng X, He D, He Y. Feedback regulation of FLC by flowering locus T (FT) and FD through a 5’ FLC promoter region in Arabidopsis. Molecular Plant. 2019;12:285–288. doi: 10.1016/j.molp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Flowering locus C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Memories of winter: vernalization and the competence to flower. Plant, Cell and Environment. 2000;23:1145–1153. doi: 10.1046/j.1365-3040.2000.00643.x. [DOI] [Google Scholar]
- Myers RM, Tilly K, Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986;232:613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. The Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Wellmer F, Riechmann JL. Gene networks controlling the initiation of flower development. Trends in Genetics. 2010;26:519–527. doi: 10.1016/j.tig.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Whittaker C, Dean C. The FLC locus: a platform for discoveries in epigenetics and adaptation. Annual Review of Cell and Developmental Biology. 2017;33:555–575. doi: 10.1146/annurev-cellbio-100616-060546. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981;289:751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]


